Abstract
Background
In a 2012 pilot, 9111 Mongolian girls aged 11–17 years received three doses of the quadrivalent (4vHPV) vaccine, Gardasil®. This is the first study to measure early vaccine effectiveness and assess knowledge and attitudes of young women in Mongolia in relation to the human papillomavirus (HPV), the vaccine and cervical cancer.
Methods
A cohort of women vaccinated in 2012 (n = 726) and an unvaccinated cohort (n = 790) provided self-administered vaginal swabs for detection of high-risk HPV genotypes 16, 18/45, 31, 33, 35, 39, 51, 52, 56, 58, 59, 66, 68 five years following vaccination. Participant knowledge and attitudes were assessed through a questionnaire.
Results
A total of 1882 questionnaires and 1516 self-administered vaginal swabs were analyzed. The prevalence of any HRHPV was 39.5% among both cohorts. The prevalence of vaccine-targeted HPV types was significantly lower in the vaccinated cohort than unvaccinated: 4.8% and 17.2% respectively. The 4vHPV was shown to be protective against HRHPV 16, 18/45 with 75% vaccine effectiveness. Participant knowledge was low.
Conclusions
This study demonstrates that the 4vHPV is associated with reduced vaccine-targeted HPV detection rates in young Mongolian women. The questionnaire results highlight a need for awareness-raising initiatives in Mongolia on HPV, the vaccine and cervical cancer.
Keywords: Human papillomavirus, HPV, HPV vaccine, Cervical cancer, Mongolia
1. Introduction
Globally, cervical cancer is the fourth most frequent cancer in women, yet it is also one of the most preventable and treatable forms of cancer if detected early [1]. This disparity is evident in low and middle-income countries where poor or no screening programs operate and thus approximately 90% of cervical cancer-related deaths occur [1]. In Mongolia, cancer is the second leading cause of mortality [2] and cervical cancer the primary cause of cancer-related deaths in women aged 15–44 years [3].
Persistent infection with high-risk genotypes of human papillomavirus (HRHPV) is a prerequisite for cervical cancer; ~70% of which are attributable to HRHPV 16 or 18 [1]. Since their introduction, the three internationally licensed HPV vaccines (bivalent, quadrivalent and nonavalent) have demonstrated high efficacy in preventing HPV 16/18 infection and thus, related diseases [4]. In May 2018, the Director-General of the World Health Organization (WHO) called for globally coordinated action to eliminate cervical cancer as a public health problem [5]. Accompanied by comprehensive screening and public health awareness, vaccination of girls, pre-sexual debut, is a key component of WHO's recommended approach to prevent and control cervical cancer [4]. Recent modelling from Australia, the first country to initiate a publicly-funded national HPV vaccination program, indicates cervical cancer elimination is both realistic and attainable [6]. Multiple studies have documented a significant decrease in vaccine-targeted HPV types since the introduction of the quadrivalent (4vHPV) vaccine in Australia [[7], [8], [9]] with some evidence of cross-protection against vaccine-related types [10].
Available data on HPV prevalence in Mongolia are limited. The most recent data is from a small-scale study to determine HPV prevalence in Mongolian women with prior cervical abnormalities. This study detected HPV in 47% of the 100 study participants. Of the HPV-positive participants, 68% were co-infected with multiple genotypes of HPV and the most prevalent was HRHPV 16 (21%) [11]. An earlier study reported the age-standardized prevalence of HPV among women to be 35% in Mongolia's capital city, Ulaanbaatar [12]. The study obtained 969 cervical specimens from women aged 15–59 years and HRHPV 16 was the most prevalent genotype with high-risk types detected in 24.5% of women [12]. This finding is similar to a study among 110 women attending an urban sexually transmitted infection (STI) clinic that indicated HPV prevalence at 36% [13]. Among the HPV-positive participants, high-risk genotypes were detected in 44% of women [13].
In view of the high burden of cervical cancer and limited available HPV genoprevalence data in Mongolia, 44,800 doses of the 4vHPV vaccine, Gardasil®, were donated by the Axios Foundation through the Millennium Challenge Account to the Mongolian Ministry of Health in 2012 [14]. The donation was intended to vaccinate 14,063 girls aged 11–17 years with three doses of vaccine at 0, 2 and 6 months. The campaign reached a total of 9111 girls (64.9% coverage of target population) with the complete three vaccine doses [15]. Coverage for the first and second doses reached 77.4% and 75.4% of the overall target population respectively [15]. The vaccination campaign did not reach full target coverage due to community resistance and the vaccine has not been available in-country following the pilot.
This study, conducted five years following the pilot vaccination campaign, aimed to demonstrate early effectiveness of the vaccine by comparing HPV detection rates between young, sexually active, Mongolian women vaccinated with three doses of 4vHPV, to rates in age-matched women never vaccinated against HPV. The study intended to answer whether vaccination with three doses of 4vHPV reduced vaccine-targeted HPV detection rates in young Mongolian women. The questionnaire intended to determine the knowledge and attitudes of young Mongolian women in relation to HPV, the vaccine and cervical cancer. The HPV vaccine is not currently included in the national immunization schedule of Mongolia. The results of this study therefore provide an important resource for future decision-makers on its implementation, both locally and in similar context settings.
2. Methods
2.1. Study design and participants
This retrospective paired cohort study was conducted between August 2017 and January 2018 across the Bayangol and Baganuur districts of Mongolia's capital city, Ulaanbaatar, and two outer provinces; Umnugobi and Selenge. Young women, aged 18–23 years, who received three doses of 4vHPV in the 2012 pilot were frequency matched to a cohort of vaccine-naïve women to ensure that the vaccinated and unvaccinated cohorts were balanced with respect to distributions of age and place of residence. The study included a self-administered swab for HPV detection among participants who reported prior sexual activity, accompanied by a questionnaire in the local language to determine participant knowledge and attitudes on HPV, the vaccine and cervical cancer. All participants were de-identified by a unique study ID related to their study site.
Participants were selected using a non-random, proportional sampling method. Approval was obtained to use national immunization data from the National Center for Communicable Diseases to recruit women from the original vaccination cohort. The vaccination status of unvaccinated women was cross-checked against this data. The age of participants (18–23 years) was selected based on the assumption it would increase the incidence of participants past first sexual debut at the time of the study. Recruitment of unvaccinated participants was conducted using peer-referral from participants in the vaccine cohort and by approaching health centers in the selected districts and universities. Peer-referral was the preferred method of recruitment on the assumption it would increase comparable environmental, lifestyle and behavioral factors. University recruitment was also justified on the basis that the prominent demographic of vaccinated participants was university students. Sampling for the unvaccinated cohort was proportional to the number of HPV-vaccinated girls in each district.
The required sample size of 961 women per vaccinated and unvaccinated cohort was calculated to have >90% power and >5% significance for measuring vaccine effectiveness and HPV prevalence. This was calculated on the assumption that HRHPV 16/18 prevalence would be 7.2% in unvaccinated women and HRHPV 18/45 would be 2.4% and 4.8% respectively. A participant information sheet and consent form was provided in the local language and individual written informed consent was received by all participants. Women unable to consent due to any intellectually or physically decreased capacity were excluded from participating. All participants received a nominal fee to compensate their time and travel costs incurred.
2.2. Ethical considerations
This study was approved by the Ethical Review Board of the Ministry of Health, Mongolia and the Royal Children's Hospital, Melbourne (RCH) Human Research Ethics Committee (HREC). Respective reference numbers are MOH #3, 25 Nov 2016, and RCH HREC 36270A.
2.3. Study rationale and objectives
The primary objective of the study was to compare prevalence of HRHPV 16 and 18/45 in sexually active Mongolian women aged 18–23 years who had; a) received three doses of 4vHPV vaccine and, b) received no HPV vaccination. The secondary objective was to compare HPV detection rates of 11 other HRHPV genotypes (31, 33, 35, 39, 51, 52, 56, 58, 59, 66, 68) in the same cohorts. The tertiary objective was to determine knowledge and attitudes of young Mongolian women in relation to HPV, the vaccine and cervical cancer. These objectives supported the aim of identifying whether the 4vHPV, when given five years previous, was associated with reduced HPV detection rates in young Mongolian women aged 18–23 years.
To complement the study objectives, demographic and disease-associated characteristics between participants who tested positive and participants who tested negative for HRHPV were analyzed. The vaccine effectiveness of 4vHPV was determined in cases with HRHPV 16 and 18/45 compared with women who tested negative.
2.4. Questionnaire methods
The survey questionnaire combined closed-ended and semi-open-ended questions to collect data on participants' demographics, sexual and reproductive history, relevant lifestyle factors, as well as knowledge and attitudes on HPV, the vaccine and cervical cancer. The questionnaire was delivered to participants from both cohorts and was available via an online or paper-based form. Knowledge-based questions comprised answers of either ‘true, false, don't know’ or ‘yes, no, don't know’ and earned an assigned score for each correct answer. Knowledge scales of low, medium and high were constructed for each category (HPV, HPV vaccine and cervical cancer) based on the final score. The knowledge scale (0–6) covered low (0–2), moderate (3–4) and high (5–6) knowledge. Results with decimal places were assigned to the closest score with a 0.5 cut off. Attitudes-based questions allowed for multiple responses.
2.5. Laboratory methods
Self-administered vaginal swabs were requested from participants if they had engaged in previous sexual activity, specifically, sexual intercourse. A detailed description, with pictorial instructions, on how to perform the swab was provided by research assistants who labeled and stored samples according to the de-identified participant codes. Cervical specimens were stored in Preserv Cyt solution (ThinPrep Pap Test, Hologic Inc, Malborough, MA, USA) at room temperature until they were transferred to Onoshmed laboratory in Ulaanbaatar. One milliliter of each Preserv Cyt sample was tested at the laboratory using Xpert HPV Assay (Cepheid Inc, Sunnyvale, CA, USA), a real-time PCR system with integrated automated sample processing, cell lysis, purification, nucleic acid amplification and qualitative E6/E7 region detection of the viral DNA genome of HRHPV types. The system identifies HRHPV 16 and HRHPV 18/45 types in two distinct detection channels and reports 11 other high risk types (P3 channel: 31, 33, 35, 52, 58; P4 channel: 51, 59; P5 channel: 39, 56, 66 and 68) in a pooled result. As the laboratory assay is unable to distinguish between genotypes 18 and 45, the prevalence of genotype 45 was expected to be unaffected by 4vHPV and remain at 2.4%. As a quality control measure, 130 randomly identified samples were selected for repeat analysis. Of these, 53 (9 negative, 35 positive, 9 invalid) were repeated in Onoshmed laboratory and 77 (23 negative, 37 positive, 17 invalid) in the National Center for Communicable Diseases laboratory in Ulaanbaatar.
2.6. Statistical analysis
Statistical analyses were performed using SPSS version 20 [16] and STATA version 15.0 [17]. The primary and secondary endpoints utilized 95% CIs for detection rates of HRHPV and 2-sided p-values for prevalence comparison between the vaccinated and unvaccinated cohorts. Tertiary endpoints were evaluated using a Chi-square test and were grouped as; knowledge and attitudes on HPV, the HPV vaccine and cervical cancer. In all analyses, a 2-sided p-value of <0.05 was defined as statistically significant.
In order to account for potential confounders and identify associated risk-factors, a univariate regression model and separate unconditional multivariate logistic regression models were added to the primary and secondary outcomes. This compared demographic and disease-associated characteristics between participants who tested positive and participants who tested negative for HRHPV 16, 18/45 (primary) and the 11 other tested HRHPV genotypes (secondary). Vaccine effectiveness was calculated from this subset as . Variables that were predicted to impact the likelihood of HPV positivity included age, residential area, education, employment, current smoking and alcohol use, age of sexual debut, partner's age of sexual debut, number of sexual partners and history of pregnancy. Due to their nature, questions on sexual history returned a high non-response rate; however no pattern was detectable among non-responses. Non-responses were excluded from analysis and reported findings are therefore calculated on total number of respondents to each question. The supplementary flowchart (Supp. 1) provides an overview of results included for analysis.
3. Results
3.1. Demographic characteristics
From the 1909 young women screened for the study, two were ineligible due to decreased capacity to consent and four did not consent. A total of 1903 participants were recruited into the study. From these, 43 participants had received fewer than three doses of 4vHPV and five were under the age of 18 (Supp. 1). A resulting 1855 participants are reported on (Table 1); 878 in the vaccinated cohort (47.3%) and 977 in the unvaccinated cohort (52.7%). Of these, 1834 (98.9%) completed the questionnaire and 1587 (85.6%) provided self-administered vaginal swabs. From all reported-on study participants (n = 1855), Selenge Province was the highest represented location (30.1%), followed by Bayangol district (27%), Umnugobi province (26%) and Baganuur district (16.9%). The mean age at recruitment of all study participants was 20.4. The majority of participants were students (54.8%), 11.2% employed (includes employed but away from work and herders) and 26.3% unemployed (includes unemployed and looking for work) at the time of the survey. Recruitment of the unvaccinated cohort was primarily achieved by peer-referral and through universities (80%), with the remainder recruited through district health centers.
Table 1.
Demographic characteristics of participants according to vaccination status (n = 1855); with questionnaire completed (n = 1834).
| Characteristic | Variable | Vaccinated cohort |
Unvaccinated cohort |
p value |
|---|---|---|---|---|
| n (%) | n (%) | |||
| All participants (N) | 878 (47.3) | 977 (52.7) | ||
| Age (years) | 18–19 | 352 (40.1) | 288 (29.5) | <0.001 |
| 20–21 | 518 (59.0) | 615 (62.9) | ||
| 22–23 | 8 (0.9) | 74 (7.6) | ||
| Mean age at recruitment (years) | 20.3 (18.0–22.6) | 20.6 (18.0–23.9) | <0.001 | |
| Place of residence | Bayangol district | 196 (21.4) | 317 (32.5) | <0.001 |
| Baganuur district | 146 (15.9) | 175 (17.9) | ||
| Umnugobi province | 260 (28.4) | 232 (23.7) | ||
| Selenge province |
315 (34.3) |
253 (25.9) |
||
| Participants completed questionnaires (N) | 870 | 964 | ||
| Relationship status | Single | 390 (44.8) | 446 (46.3) | 0.02 |
| Couple, not living together | 178 (20.4) | 219 (22.7) | ||
| Couple living together | 118 (13.6) | 106 (11.0) | ||
| Divorced | 12 (1.4) | 22 (2.3) | ||
| Separated but not divorced | 9 (1.0) | 14 (1.5) | ||
| Married | 144 (16.6) | 120 (12.4) | ||
| No answer | 19 (2.2) | 37 (3.8) | ||
| Highest level of education | Did not graduate from junior school | 7 (0.8) | 7 (0.7) | 0.06 |
| Junior school | 16 (1.8) | 11 (1.1) | ||
| High school | 657 (75.5) | 783 (81.3) | ||
| College/university | 184 (21.1) | 159 (16.5) | ||
| No answer | 6 (0.7) | 4 (0.4) | ||
| Employment status | Employed1 | 125 (14.4) | 76 (7.9) | <0.001 |
| Unemployed2 | 216 (24.8) | 268 (27.8) | ||
| Student | 451 (51.8) | 557 (57.8) | ||
| Caring for a child | 72 (8.3) | 54 (5.6) | ||
| Prefer not to answer/no answer | 6 (0.7) | 9 (0.9) | ||
| Income (monthly average after tax) | 0₮-200000₮ (lowest livelihood level) | 405 (46.6) | 491 (50.9) | 0.01 |
| 200,001₮ −500000₮ | 108 (12.4) | 90 (9.4) | ||
| 500,001₮ or more | 41 (4.7) | 26 (2.7) | ||
| No answer | 316 (36.3) | 357 (37.0) | ||
| Religion | None | 361 (41.5) | 346 (35.9) | <0.001 |
| Buddhism | 270 (31.0) | 382 (39.6) | ||
| Christianity | 65 (7.5) | 36 (3.7) | ||
| Muslim | 2 (0.2) | 10 (1.0) | ||
| Others | 82 (9.4) | 97 (10.1) | ||
| No answer | 81 (9.3) | 83 (8.6) | ||
| Smoking status | Yes | 67 (7.7) | 95 (9.9) | 0.10 |
| Alcohol Consumption | Yes | 102 (11.7) | 181 (18.8) | <0.001 |
1. Employed includes employed, but away from work (e.g. holiday, paid/maternity or child care leave) and herders.
2. Unemployed includes unemployed and looking for work.
Two participants had reading and writing difficulties and were consented and assisted with the questionnaire by their local medical professional. A representation of variables according to vaccination status is provided in Table 1. Cohort variables were most aligned on sexual and reproductive history. Respondents’ sexual and reproductive history characteristics are provided in Table 2. From the 1266 women who responded to the question on prior pregnancy, 565 (44.6%) answered that they had been pregnant before. The rate of pregnancies differed between the vaccinated (48.1%) and unvaccinated (41.4%) cohorts with slightly higher rates of pregnancy in the vaccinated cohort (p = 0.02). Overall, data collected on sexual experience and reproductive history remained consistent between the vaccinated and unvaccinated cohorts. The rate of engagement in sexual intercourse was 74.3% in the vaccinated and 73.5% in the unvaccinated cohort. The mean age of first sexual intercourse was 18.5 (95%CI 18.3–18.5) in vaccinated women and 18.7 (95%CI 18.5–18.7) in unvaccinated women. The greatest reported differences in sexual and reproductive variables appeared between urban and rural provinces, however this was not further explored as it was not an objective of the study.
Table 2.
Characteristics of sexual and reproductive experience among questionnaire-participants according to vaccination status, n = 1834.
| Characteristic | Variable | Vaccinated |
Unvaccinated |
p value |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Sexually active | Total responses | N = 821 | N = 919 | |
| Yes | 610 (74.3) | 675 (73.5) | 0.69 | |
| Age at first vaginal intercourse (years) | Total responses | N = 585 | N = 652 | |
| 13–15 | 16 (2.7) | 7 (1.1) | 0.02 | |
| 16–18 | 278 (47.5) | 282 (43.2) | ||
| 19–24 | 291 (49.7) | 363 (55.7) | ||
| Difference in partner age at debut (years) | Total responses | N = 579 | N = 645 | |
| <3 | 468 (80.8) | 541 (83.9) | 0.34 | |
| 3–7 | 103 (17.8) | 98 (15.2) | ||
| 8–17 | 8 (1.4) | 6 (0.9) | ||
| Number of sexual partners in past 12 months | Total responses | N = 563 | N = 622 | |
| 0 | 32 (5.7) | 42 (6.8) | 0.89 | |
| 1 | 478 (84.9) | 521 (83.8) | ||
| 2–4 | 48 (8.5) | 54 (8.7) | ||
| 5–10 | 5 (0.9) | 5 (0.8) | ||
| Number of lifetime sexual partners | Total responses | N = 529 | N = 592 | |
| 1 | 346 (65.4) | 377 (63.7) | 0.66 | |
| 2–4 | 165 (31.2) | 198 (33.4) | ||
| >5 | 18 (3.2) | 17 (2.9) | ||
| Prior pregnancy | Total responses | N = 609 | N = 657 | |
| Yes | 293 (48.1) | 272 (41.4) | 0.02 | |
| Number of pregnancies | Total responses | N = 278 | N = 263 | |
| 1 | 212 (76.3) | 207 (78.7) | 0.50 | |
| >2 | 66 (23.7) | 56 (21.3) | ||
| Number of births | Total responses | N = 250 | N = 231 | |
| 0 | 43 (17.2) | 40 (17.3) | 0.97 | |
| >=1 | 207 (82.8) | 191 (82.7) | ||
3.2. Laboratory results: primary and secondary endpoint
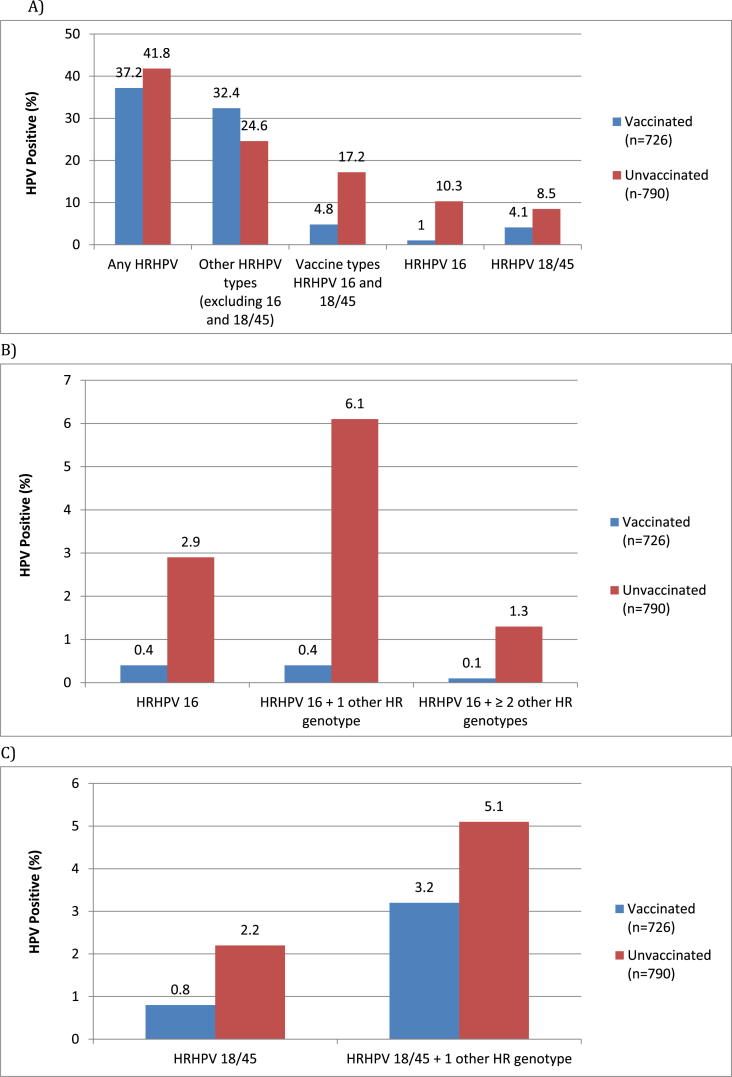
A total of 1587 women provided vaginal swabs for analysis. From these, 71 (4.5%) returned invalid error results and were excluded. The remaining 1516 were included for analysis (Supp. 1); 726 from vaccinated women and 790 from unvaccinated women. The 130 randomly identified samples sent for repeat analysis showed high concordance. No significant difference was demonstrated for the prevalence of any HRHPV genotype (Fig. 1); 37.2% in the vaccinated cohort (95%CI 33.7–40.8) and 41.8% in the unvaccinated cohort (95%CI 38.3–45.3). The prevalence of vaccine-targeted HPV types 16 and 18/45 was substantially lower in the vaccinated cohort; 4.8% (95% CI 3.4–6.6) compared with 17.2% (95% CI 14.6–20.0) in the unvaccinated (p=<0.001, Fig. 1). The greatest demonstrated difference between the cohorts was in prevalence of HRHPV 16; detected at 1% (95% CI 0.4–2.0) in the vaccinated women and 10.3% (95% CI 8.2–12.6) in the unvaccinated women (p=<0.001). Vaccinated participants were less likely to have multiple genotypes than single genotypes overall (Fig. 1).
Fig. 1.
Crude HPV genoprevalence among vaccinated and unvaccinated cohorts, n = 1516 Crude prevalence of: (A) any HRHPV type, other 11 HRHPV types excluding 16 and 18/45, any vaccine targeted HRHPV type, specific vaccine HRHPV types; (B) HRHPV16, co-infection of HRHPV16 with other HR genotypes; (C) HRHPV18/45, co-infection of HRHPV 18/45 with other HR genotypes.
At the time of request, following rationale for previous sexual activity, a greater number of women elected to provide self-administered vaginal swabs than those who reported sexual activity on their questionnaire. A total of 82 samples were provided by women who were non-responsive in the questionnaire regarding prior sexual engagement and 240 by women who answered ‘no’ to prior sexual engagement. Of the 82 non-responses, 23 (28%) were HPV positive and of the 240 ‘no’ answers, 43 were HPV positive (17.9%). Based on these results and the explanation provided at the time of specimen request, women who provided self-administered swabs for analysis were considered sexually active for the purpose of the laboratory analysis.
3.3. Complementary analysis and vaccine effectiveness
For the additional univariate and multivariate analyses, high-risk HPV positivity was associated with alcohol consumption and increased number of sexual partners. Specific to HRHPV 16 and 18/45 (Table 3) the odds of HPV positivity were 3.59 fold higher in women who reported 2–4 prior sexual partners (aOR 3.59 [95%CI 2.35–5.49]) and 4.51 higher in women who reported ≥5 prior sexual partners (aOR 4.51 [95%CI 1.66–12.26]) compared to those who reported one lifetime partner. Having received the 4vHPV was shown to be protective against HRHPV 16, 18/45. The odds of HPV positivity were 75% less in the vaccinated cohort (aOR 0.25 [95%CI 0.15–0.40]). Specific to all other high-risk HPV genotypes tested (Table 4), the odds of HPV positivity were higher in respondents who reported alcohol use (aOR 1.54 [95%CI 1.10–2.16]). The odds of HPV positivity were higher in women who reported 2–4 prior sexual partners (aOR 2.62 [95%CI 1.96–3.49]) and in women who reported ≥5 prior sexual partners (aOR 1.98 [95%CI 0.92–4.26]) compared to those who reported one lifetime partner.
Table 3.
Factors associated with positivity of HRHPV16 and 18/45.
| Characteristic | HPV positive |
HPV negative |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | p value | aOR (95% CI) | p value | |||
| Age groups | N = 171 | N = 917 | ||||||
| 18–19 years | 38 (22) | 304 (33) | Ref | |||||
| 20–21 years | 126 (74) | 576 (63) | 1.75 (1.19–2.58) | 0.005 | ||||
| 22–23 years | 7 (4) | 37 (4) | 1.51 (0.63–3.63) | 0.35 | ||||
| Site | N = 171 | N = 917 | ||||||
| Bayangol District | 49 (29) | 238 (26) | Ref | |||||
| Baganuur District | 31 (18) | 173 (19) | 0.87 (0.53–1.42) | 0.58 | ||||
| Selenge Province | 50 (29) | 255 (28) | 0.95 (0.62–1.47) | 0.83 | ||||
| Umnugobi Province | 41 (24) | 251 (27) | 0.79 (0.51–1.25) | 0.32 | ||||
| Relationship | N = 166 | N = 878 | ||||||
| Single | 62 (37) | 358 (41) | Ref | |||||
| Couple, not cohabitating | 47 (28) | 203 (23) | 1.34 (0.88–2.03) | 0.17 | ||||
| Couple, cohabitating | 27 (16) | 128 (15) | 1.22 (0.74–1.99) | 0.44 | ||||
| Divorced | 7 (4) | 19 (2) | 2.13 (0.86–5.27) | 0.10 | ||||
| Separated | 6 (4) | 10 (1) | 3.46 (1.22–9.87) | 0.02 | ||||
| Married | 17 (10) | 160 (18) | 0.61 (0.35–1.08) | 0.09 | ||||
| Education | N = 170 | N = 915 | ||||||
| Primary | 5 (3) | 22 (2) | Ref | |||||
| Secondary | 149 (88) | 751 (82) | 0.87 (0.32–2.34) | 0.79 | 0.58 (0.17–1.98) | 0.38 | ||
| Tertiary | 16 (9) | 142 (16) | 0.50 (0.17–1.49) | 0.21 | 0.26 (0.07–1.02) | 0.05 | ||
| Employment | N = 170 | N = 910 | ||||||
| Employed | 15 (9) | 86 (9) | Ref | |||||
| Unemployed | 32 (19) | 166 (18) | 1.11 (0.57–2.15) | 0.77 | ||||
| Student | 116 (68) | 583 (64) | 1.14 (0.64–2.04) | 0.66 | ||||
| Childcare | 8 (5) | 81 (9) | 0.57 (0.23–1.41) | 0.22 | ||||
| Smokes tobacco | N = 168 | N = 894 | ||||||
| Yes | 18 (11) | 79 (9) | 1.24 (0.72–2.13) | 0.44 | ||||
| Consumes alcohol | N = 170 | N = 888 | ||||||
| Yes | 47 (28) | 124 (14) | 2.35 (1.60–3.46) | <0.001 | ||||
| Age at sexual debut | N = 154 | N = 652 | ||||||
| <18 years | 18 (12) | 114 (18) | Ref | Ref | ||||
| >=18 years | 136 (88) | 538 (82) | 1.60 (0.94–2.73) | 0.08 | 2.80 (1.50–5.23) | 0.001 | ||
| Age of firstsexualpartner | N = 154 | N = 647 | ||||||
| <18 years | 9 (6) | 49 (8) | Ref | |||||
| >=18 years | 145 (94) | 598 (92) | 1.32 (0.63–2.75) | 0.46 | ||||
| Age difference with firstsexualpartner | N = 153 | N = 645 | ||||||
| <3 years | 136 (89) | 527 (82) | Ref | |||||
| 3–7 years | 15 (10) | 111 (17) | 0.52 (0.30–0.93) | 0.03 | ||||
| 8–17 years | 2 (1) | 7 (1) | 1.11 (0.23–5.39) | 0.90 | ||||
| Number of lifetime sexual partners | N = 141 | N = 593 | ||||||
| 1 | 64 (45) | 435 (74) | Ref | Ref | ||||
| 2–4 | 69 (49) | 143 (24) | 3.28 (2.22–4.84) | <0.001 | 3.59 (2.35–5.49) | <0.001 | ||
| ≥5 | 8 (6) | 15 (2) | 3.63 (1.48–8.89) | 0.005 | 4.51 (1.66–12.26) | 0.003 | ||
| Prior pregnancy | N = 154 | N = 663 | ||||||
| Yes | 60 (39) | 319 (48) | 0.69 (0.48–0.98) | 0.04 | ||||
| Received 4vHPV | N = 171 | N = 916 | ||||||
| Yes | 35 (20) | 456 (50) | 0.26 (0.18–0.38) | <0.001 | 0.25 (0.15–0.40) | <0.001 | ||
Table 4.
Factors associated with positivity of other HRHPV types.
| Characteristic | HPV positive |
HPV negative |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | p value | aOR (95% CI) | p value | |||
| Age groups | N = 548 | N = 917 | ||||||
| 18–20 years | 372 (68) | 637 (69) | Ref | |||||
| 21–23 years | 176 (32) | 280 (31) | 1.08 (0.86–1.35) | 0.53 | ||||
| Site | N = 548 | N = 917 | ||||||
| Bayangol District | 139 (25) | 238 (26) | Ref | |||||
| Baganuur District | 91 (17) | 173 (19) | 0.90 (0.65–1.25) | 0.53 | ||||
| Selenge Province | 170 (31) | 255 (28) | 1.15 (0.86–1.52) | 0.36 | ||||
| Umnugobi Province | 148 (27) | 251 (27) | 1.01 (0.75–1.35) | 0.95 | ||||
| Relationship | N = 525 | N = 878 | ||||||
| Single | 209 (40) | 358 (41) | Ref | |||||
| Couple, not cohabitating | 142 (27) | 203 (23) | 1.20 (0.91–1.58) | 0.20 | ||||
| Couple, cohabitating | 79 (15) | 128 (15) | 1.06 (0.76–1.47) | 0.74 | ||||
| Divorced | 9 (2) | 19 (2) | 0.81 (0.36–1.83) | 0.61 | ||||
| Separated | 8 (1) | 10 (1) | 1.37 (0.53–3.53) | 0.51 | ||||
| Married | 78 (15) | 160 (18) | 0.84 (0.61–1.15) | 0.27 | ||||
| Education | N = 544 | N = 915 | ||||||
| Primary | 9 (2) | 22 (2) | Ref | |||||
| Secondary | 443 (81) | 751 (82) | 1.44 (0.66–3.16) | 0.36 | ||||
| Tertiary | 92 (17) | 142 (16) | 1.58 (0.70–3.59) | 0.27 | ||||
| Employment | N = 547 | N = 916 | ||||||
| Employed | 66 (12) | 86 (9) | Ref | |||||
| Unemployed | 99 (18) | 166 (18) | 0.78 (0.52–1.17) | 0.22 | ||||
| Student | 341 (62) | 583 (64) | 0.76 (0.54–1.08) | 0.13 | ||||
| Childcare | 41 (8) | 81 (9) | 0.66 (0.40–1.08) | 0.10 | ||||
| Smokes tobacco | N = 536 | N = 894 | ||||||
| Yes | 63 (12) | 79 (9) | 1.37 (0.97–1.95) | 0.08 | ||||
| Consumes alcohol | N = 536 | N = 888 | ||||||
| Yes | 119 (22) | 124 (14) | 1.76 (1.33–2.32) | <0.001 | 1.54 (1.10–2.16) | 0.01 | ||
| Age at sexual debut | N = 471 | N = 652 | ||||||
| <18 years | 68 (14) | 114 (17) | Ref | |||||
| >=18 years | 403 (86) | 538 (83) | 1.26 (0.91–1.74) | 0.17 | 1.68 (1.16–2.42) | 0.006 | ||
| Age of firstsexualpartner | N = 470 | N = 647 | ||||||
| <18 years | 35 (7) | 49 (8) | Ref | |||||
| >=18 years | 435 (93) | 598 (92) | 1.02 (0.65–1.60) | 0.94 | ||||
| Age difference with firstsexualpartner | N = 467 | N = 645 | ||||||
| <3 years | 387 (83) | 527 (82) | Ref | |||||
| 3–7 years | 74 (16) | 111 (17) | 0.91 (0.66–1.25) | 0.56 | ||||
| 8–17 years | 6 (1) | 7 (1) | 1.17 (0.39–3.50) | 0.78 | ||||
| Number of lifetime sexual partners | N = 421 | N = 593 | ||||||
| 1 | 220 (52) | 435 (73) | Ref | Ref | ||||
| 2–4 | 185 (44) | 143 (24) | 2.56 (1.95–3.36) | <0.001 | 2.62 (1.96–3.49) | <0.001 | ||
| > = 5 | 16 (4) | 15 (3) | 2.11 (1.02–4.35) | 0.04 | 1.98 (0.92–4.26) | 0.08 | ||
| Prior pregnancy | N = 476 | N = 663 | ||||||
| Yes | 193 (41) | 319 (48) | 0.74 (0.58–0.93) | 0.01 | ||||
| Received 4vHPV | N = 548 | N = 916 | ||||||
| Yes | 260 (47) | 456 (50) | 0.91 (0.74–1.13) | 0.39 | ||||
3.4. Questionnaire results: tertiary endpoint
Of the originally recruited 1903 participants, 1882 questionnaires were completed to assess knowledge and attitudes on HPV, the vaccine and cervical cancer among young Mongolian women. Non-responses were excluded. From the knowledge-based questions on HPV, 15% of respondents (n = 1882) achieved a score of zero; answering no questions correctly. No respondents achieved a full score where all 13 questions were answered correctly. The percentage of correct answers overall was 19.8% and the mean score was low at 1.31 (low knowledge score 0–2 [95%CI 1.28–1.33]). The majority of respondents were not aware of the link between HPV and anogenital warts (13.6% correct, n = 1856), oropharyngeal cancer (8.1% correct, n = 1861) or other, non-cervical, anogenital cancers (20.4% correct, n = 1859). Respondents incorrectly identified HPV as a genetic condition (23.8% incorrect, 68.8% ‘don't know’, n = 1861) specific to the elderly (42.7% incorrect, 55.6% ‘don't know’, n = 1858). The majority of respondents (61.2%, n = 1882) selected ‘don't know’ in regards to HPV symptoms and 15.2% abstained from responding. The question with the most correct answers was whether HPV can cause cancer of the cervix, where 41.8% (n = 1855) responded correctly. All questionnaire responses were consistent across vaccinated, unvaccinated, HPV-positive and HPV-negative respondents.
From the knowledge-based questions on the HPV vaccine, 87.5% of respondents (n = 1882) either did not know or responded incorrectly. The mean knowledge score was low at 1.17 (95%CI 1.15–1.19). The vaccinated cohort achieved a slightly higher mean knowledge score of 1.20 (95%CI 1.17–1.23) compared to 1.14 (95%CI 1.12–1.16) in the unvaccinated cohort. However, more respondents from the vaccinated cohort (38.8% vs. 27%, n = 1862, [p=<0.001]) incorrectly answered that a woman does not need any cervical cancer screening in the future if she has received the HPV vaccine.
From the knowledge-based questions on cervical cancer (n = 1844), the mean knowledge score was low at 2.34 (95%CI 2.31–2.79). This was consistent between both cohorts. When asked what health interventions they could take to reduce their future risk of developing cervical cancer, the majority of respondents (67.6%) correctly answered that the Papanicolaou (PAP) test reduces an individual's risk of cervical cancer; the PAP was understood to enable early detection of precancerous disease. Additionally, 88.5% correctly identified that exercise does not reduce the future risk of cervical cancer. The only difference observed between the vaccinated and unvaccinated cohorts was the understanding that the HPV vaccine reduces an individual's risk of cervical cancer (61.7% and 52.6% respectively [p=<0.001]).
The questions regarding personal attitudes revealed that 43.9% of questionnaire participants (n = 1882) would feel frightened if they tested positive for HPV. From all questionnaire participants, 54.8% would seek medical advice if they tested positive for HPV. Overall, 45.5% of participants were aware of a ‘cervical cancer vaccine’, this was higher among vaccinated participants (57%) than unvaccinated (34%). General practitioners and family physicians were the main source of this information (25.6%). Condoms were believed to be a protective factor against HPV infection by 44.9% of participants and 47.5% of participants would share their HPV-positive results with their partner. The majority of participants had not heard of cervical cancer screening or PAP tests (63.2%). Of the vaccinated women (n = 917), 58.9% identified protection against cervical cancer as a reason for receiving the vaccine, 59.8% on their doctor's recommendation and 46.3% because it was free. Of the unvaccinated women (n = 965), 22.2% identified disbelief in its efficacy as a reason for not receiving the vaccine and 40.8% selected they were not sure of its necessity.
4. Discussion
This study was designed to measure the early effectiveness of the 2012 pilot HPV vaccination campaign in Mongolia. This is the preliminary study to investigate HPV prevalence in women who received three doses of 4vHPV. While the prevalence of any tested HPV genotypes was comparable between the vaccinated and unvaccinated cohorts, vaccine-targeted types 16 and 18/45 were significantly lower in the vaccinated cohort. Vaccination with 4vHPV was shown to be protective against HRHPV 16, 18/45 with 75% vaccine effectiveness. This study demonstrates the 4vHPV has contributed to the reduction of cervical cancer-related HPV infections in Mongolia and has the potential to contribute to the reduction of cervical cancer-related disease.
Baseline data on HPV prevalence in Mongolian women is scarce and this study contributes to broadening context-specific evidence. The results of the study indicate prevalence of any HPV genotype across all sexually active study participants (both vaccinated and unvaccinated) at 39.5%. These results are higher than reported in the surrounding region of Asia [18]. Additionally, Mongolia has one of the highest age-standardized rates of cervical cancer in Asia [19]. Both of these factors highlight the importance of including a HPV-targeted response in the nation's cervical cancer agenda.
Sexual and reproductive behavior variables were consistent between the vaccinated and unvaccinated women. The results strengthen available data on age of first sexual debut in Mongolia (18.5 years in vaccinated, 18.7 years in unvaccinated women), contributing to future sexual health or HPV campaigns. Anecdotal reports of resistance to the 2012 pilot HPV vaccine campaign included a fear of infertility among vaccine recipients. The slightly higher pregnancy rate among the vaccinated women may provide a beneficial resource for future decision-makers on vaccine implementation. Though not an objective of the study, the greatest reported sexual and reproductive differences appeared between urban and rural settings. This identifies an important area of consideration for future HPV efforts.
Detection of HRHPV types was shown to have an association with alcohol consumption and increased number of sexual partners. These factors are supported in other studies on behavioral and sociodemographic, risk-taking behaviour factors for HPV infection [[20], [21], [22]] though do not account for additional documented risk factors such as contraceptive use, income and ethnicity [23,24].
The results from the questionnaire revealed an overall low level of knowledge among participants in both study cohorts on HPV, the vaccine and cervical cancer risks. Similar studies conducted among women 16–25 years in Japan and Australia demonstrated greater levels of participant knowledge across all areas [25]. Comparing answers with the highest correct response rates validates this. The question linking cervical cancer to HPV returned a correct response rate of 41.8% in this study compared to 81.2% and 92.4% in the Japanese and Australian studies respectively. The question identifying PAP tests as a method to reduce cervical cancer risks returned a correct response rate of 67.6% in this study compared to 98.7% and 91.4% in the Japanese and Australian studies respectively.
The highest reported reason for girls originally receiving the vaccine was on advice from a medical professional. General practitioners and family physicians were the main source of information among respondents who were aware of a ‘cervical cancer vaccine’. This identifies medical professionals as an important resource in any future HPV and cervical cancer initiatives. The predominant reasons selected by those who did not receive the vaccine in the 2012 pilot were a disbelief in its efficacy and being unaware if it were necessary. This, accompanied with the low knowledge scores, indicates a strong need for better HPV and cervical cancer awareness-raising initiatives in Mongolia.
4.1. Limitations and strengths
A recognized limitation of this study is the combined detection of HPV types 18 and 45 with the Xpert HPV assay; reducing the specificity of the 4vHPV impact on HRHPV 18 and the vaccine effectiveness overall. Another limitation of this study is the potential for herd protection among the unvaccinated cohort. Utilizing peer referral as the main method of recruitment for unvaccinated participants increases the likelihood of social similarity between the cohorts. Herd protection could have reduced vaccine-targeted HPV prevalence among the unvaccinated cohort leading to a smaller observed difference in vaccine-targeted HPV. Noted limitations of the questionnaire include the potential for recall bias among women who were teenagers at the time of vaccination. Additionally, the unvaccinated cohort may not have been eligible nor offered the 4vHPV in the original pilot campaign. As attitudes towards the HPV vaccine were assessed on participants’ reasons for either receiving or declining the vaccine, reported attitudes could be skewed by both factors.
This study is the first of its nature post-vaccination pilot in 2012. It is also the largest HPV prevalence study in the Mongolian context and therefore contributes important data for future vaccine policy decisions. Importantly, with the reliability of GeneXpert technology available and accessible, this study demonstrates Mongolia's capacity to transition to HPV testing for cervical screening. Another important demonstration for this transition is the acceptability of study participants to undertake a self-swabbing technique for HPV detection. An added benefit of this study is its potential public health impact; reaching ~2000 Mongolian women with an information sheet on HPV and notifying women of their HPV status with recommendations for future cervical screening.
5. Conclusion
This study demonstrates that the 4vHPV, when given five years previously, is associated with significantly reduced vaccine-targeted HPV detection rates in young Mongolian women aged 18–23 years. The study contributes important data on HPV prevalence among young Mongolian women, indicating relatively high rates of detection. Finally, the results highlight a significant need for better awareness-raising initiatives in Mongolia on HPV, the vaccine and cervical cancer. Following the 2012 vaccination pilot, the HPV vaccine was not included in the national immunization schedule. In 2018, the Mongolian Ministry of Health renewed discussions to implement a HPV vaccine from 2020. The first steering committee meeting was held in 2019 following approval by the National Immunization Technical Advisory Group, however further planning and recommendations are to be determined. The authors hope this data will contribute to these renewed discussions on the introduction of a HPV vaccine in Mongolia as well as other low and middle-income contexts.
Authors’ contributions
TB and KM designed the study. KM, CvM and SMG provided scientific input. TB, BT, JE and US collected or generated study data. ST, CvM and MTD provided technical statistical input. KT provided technical laboratory support. TB, CvM, SMG and MTD interpreted the data. MTD drafted the first edition of the paper. All authors reviewed and approved the final version for submission. All authors attest they meet the ICMJE criteria for authorship.
Conflicts of interest
Professor Suzanne Garland (SMG) is a member of 9vHPV Advisory Board, Merck Scientific and Global Advisory Boards. SMG has received grants through her institution for an Investigator Initiated grant from Merck to measure vaccine effectiveness for a young women's study and personally for lectures delivered in her own time.
All other authors have no conflicts of interest to declare.
Role of the funding source
Co-funding was provided by the Australian Department of Foreign Affairs and Trade Direct Aid Program (Grant ID: ULNB/2016-2017/003), the Murdoch Children's Research Institute and the World Health Organization, Mongolia office (Grant ID: 201594897, WPMNG1611473). The Australian Department of Foreign Affairs and Trade and the World Health Organization had no involvement in the study process. Researchers at the Murdoch Children's Research Institute provided support on study design, data analysis and interpretation.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all study participants as well as the support of the Embassy of Australia in Mongolia, the Australian Consulate in Hong Kong, the World Health Organization, Copan Italy, Cepheid France and the following organisations and institutions within Mongolia; Ministry of Health, National Center for Communicable Diseases, National Center for Public Health, Hospital of Civil Servants, Bayangol and Baganuur District Health Centers, Umnugobi Province Health Department, Selenge Province Health Department, Sukhbaatar District Hospital, Onoshmed Laboratory, Itgel Hospital, Happy Veritas laboratory and participating medical general practices.
The authors are also grateful for the contributions of the following staff and researchers; A/Prof Fiona Russell, A/Prof Paul Licciardi and Dr Zhen Quan Toh of Murdoch Children's Research Institute, Melbourne; Lynette Smith, Tsetsegdary Gombodorj, Margad-Erdene Munkhsaikhan and Togsdelger Sovd of National Cancer Council of Mongolia; Dr. Gantuya Dorj, Mongolian National University of Medical Sciences; Enkhtsetseg Ariunaa, Itgel Hospital; Nyamsaikhan Tsatsral and Erdenee Shirchin, Onoshmed Laboratory; and Oyunbayar Bud, Bayangol Health Department.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2019.100175.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization Cervical cancer [Internet]. 2018. http://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/ [cited 2018 Oct 10]. Available from:
- 2.Chimed T., Sandagdorj T., Znaor A., Laversanne M., Tseveen B., Genden P., Bray F. Cancer incidence and cancer control in Mongolia: results from the national cancer registry 2008-12. Int. J. Cancer. 2017 Jan 15;140(2):302–309. doi: 10.1002/ijc.30463. [DOI] [PubMed] [Google Scholar]
- 3.HPV Information Centre Human papillomavirus and related diseases report - Mongolia [Internet] http://www.hpvcentre.net/statistics/reports/MNG.pdf 2017 [updated 2017 Jul 27; cited 2018 Oct 10]. Available from:
- 4.World Health Organization . vol. 92. 2017. pp. 241–268.http://apps.who.int/iris/bitstream/handle/10665/255353/WER9219.pdf;jsessionid=5BD7DC1811B47C014D67E6BAB7F20A11?sequence=1 (Human Papillomavirus Vaccines: WHO Position Paper, May 2017 [Internet]. No 19). [cited 2018 Oct 10]. Available from: [Google Scholar]
- 5.World Health Organization WHO Director-General calls for all countries to take action to help end the suffering caused by cervical cancer [Internet] May 19, 2018. http://www.who.int/reproductivehealth/call-to-action-eliminationcervical- cancer/en/ [cited 2018 Oct 10]. Available from:
- 6.Hall M.T., Simms K.T., Lew J.B. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2018 Oct 1 doi: 10.1016/S2468-2667(18)30183-X. ([Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 7.Machalek D.A., Garland S.M., Brotherton J.M.L. Very low prevalence of vaccine human papillomavirus types among 18- to 35-year old Australian women 9 years following implementation of vaccination. J. Infect. Dis. 2018;217:1590–1600. doi: 10.1093/infdis/jiy075. [DOI] [PubMed] [Google Scholar]
- 8.Brotherton J.M., Gertig D.M., May C., Chappell G., Saville M. HPV vaccine impact in Australian women: ready for an HPV-based screening program. Med. J. Aust. 2016;204:184. doi: 10.5694/mja15.01038. [DOI] [PubMed] [Google Scholar]
- 9.Canfell K., Caruana M., Gebski V. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi: 10.1371/journal.pmed.1002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garland Suzanne M., Cornall Alyssa M., Brotherton Julia M.L., Wark John D., Malloy Michael J., Tabrizi Sepehr N. Final analysis of a study assessing genital human papillomavirus genoprevalence in young Australian women, following eight years of a national vaccination program. Vaccine. 2018 May 31;36(23):3221–3230. doi: 10.1016/j.vaccine.2018.04.080. [DOI] [PubMed] [Google Scholar]
- 11.Batchimeg T., Tomomi Bayarmaa E. Human papillomavirus genotyping among women with cervical abnormalities in Ulaanbaatar, Mongolia. Int. J. Infect. Dis. 2018 doi: 10.1016/j.ijid.2018.09.018. ([Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 12.Dondog B., Clifford G.M., Vaccarella S. Human papillomavirus infection in Ulaanbaatar, Mongolia: a population-based study. Cancer Epidemiol. Biomark. Prev. 2008;17(7):1731–1738. doi: 10.1158/1055-9965.EPI-07-2796. [DOI] [PubMed] [Google Scholar]
- 13.Garland S.M., Tabrizi S.N., Chen S., Byambaa C., Davaajav K. Prevalence of sexually transmitted infections (Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis and human papillomavirus) in female attendees of a sexually transmitted diseases clinic in Ulaanbaatar, Mongolia. Infect. Dis. Obstet. Gynecol. 2001;9(3):143–146. doi: 10.1155/S1064744901000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boguslavsky V., Caitlin M. University Research Co; LLC. Unpublished: Feb 2013. The Evaluation and Scale up of HPV Vaccine Program in Mongolia. [Google Scholar]
- 15.Ministry of Health . 2013. Mongolia. Report on the Implementation of Human Papillomavirus Vaccine Introduction. Ulaanbaatar, Mongolia. [Google Scholar]
- 16.IBM Corp Released . IBM Corp; Armonk, NY: 2011. IBM SPSS Statistics for Windows. [Google Scholar]
- 17.StataCorp . StataCorp LP; College Station, TX: 2015. STata Statistical Software: Release 14. [Google Scholar]
- 18.HPV Information Centre . 2017. Human Papillomavirus and Related Diseases Report - World [Internet]http://www.hpvcentre.net/statistics/reports/XWX.pdf [updated 2017 Jul 27; cited 2018 Oct 10]. Available from: [Google Scholar]
- 19.Toh Z.Q., Licciardi P.V., Russell F.M., Garland S.M., Batmunkh T., Mulholland E.K. Cervical cancer prevention through HPV vaccination in low-and middle-income countries in Asia. Asian Pac. J. Cancer Prev. APJCP. 2017 Sep 27;18(9):2339–2343. doi: 10.22034/APJCP.2017.18.9.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiley D.J., Masongsong E.V., Lu S., Heather L.S., Salem B., Guiliano A.R., Ault K.A., Haupt R.M., Brown D.R. Behavioural and sociodemographic risk factors for serological and DNA evidence of HPV6, 11, 16, 18 infections. Cancer Epidemiol. 2012;36(3):e183–e189. doi: 10.1016/j.canep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Cotton S.C., Sharp L., Seth R., Masson L.F., Little J., Cruickshank M.E., Neal K., Waugh N., on behalf of the TOMBOLA Group Lifestyle and socio-demographic factors associated with high-rsik HPV infection in UK women. Br. J. Canc. 2007 Jul 2;97(1):133–139. doi: 10.1038/sj.bjc.6603822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dempsey A. Human papillomavirus: the usefulness of risk factors in determining who should get vaccinated. Rev. Obstet. Gynecol. 2008;1(3):122–128. [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell S.M., Sekikubo M., Biryabarema C., Byamugisha J.J., Steinberg M., Jeronimo J., Money D.M., Christilaw J., Ogilvie G.S. Factors associated with high-risk HPV positivity in a low-resource setting in sub-Saharan Africa. Am. J. Obstet. Gynecol. 2014;210(1):81. doi: 10.1016/j.ajog.2013.08.038. e1-7. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell S.M., Pedersen H.N., Evelyn E.S., Sekikubo M., Moses E., Mwesigwa D. Self-collection based HPV testing for cervical cancer screening among women living with HIV in Uganda: a descriptive analysis of knowledge, intentions to screen and factors associated with HPV positivity. BMC Women's Health. 2017:17. doi: 10.1186/s12905-016-0360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyagi E., Motoki Y., Asai-Sato M., Taguri M., Morita S., Hirahara F., Wark J.D., Garland S.M. Web-based recruiting for a survey on knowledge and awareness of cervical cancer prevention among young women living in kanagawa prefecture, Japan. Int. J. Gynecol. Cancer. 2014;24(7):1347–1355. doi: 10.1097/IGC.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.