Abstract
CXCR4 is a pleiotropic chemokine receptor which acts through its ligand CXCL12 to regulate diverse physiological processes. CXCR4/CXCL12 axis plays a pivotal role in proliferation, invasion, dissemination and drug resistance in multiple myeloma (MM). Apart from its role in homing, CXCR4 also affects MM cell mobilization and egression out of the bone marrow (BM) which is correlated with distant organ metastasis. Aberrant CXCR4 expression pattern is associated with osteoclastogenesis and tumor growth in MM through its cross talk with various important cell signalling pathways. A deeper insight into understanding of CXCR4 mediated signalling pathways and its role in MM is essential to identify potential therapeutic interventions. The current therapeutic focus is on disrupting the interaction of MM cells with its protective tumor microenvironment where CXCR4 axis plays an essential role. There are still multiple challenges that need to be overcome to target CXCR4 axis more efficiently and to identify novel combination therapies with existing strategies. This review highlights the role of CXCR4 along with its significant interacting partners as a mediator of MM pathogenesis and summarizes the targeted therapies carried out so far.
Abbreviations: AMC, Angiogenic monomuclear cells; BM, Bone marrow; BMSC, Bone marrow stromal cells; CAM-DR, Cell adhesion‐mediated drug resistance; CNS, Central nervous system; CSCs, Cancer stem cells; CL—CC, Chemokine ligand; CCR–CC, Chemokine receptor; CCX–CKR, Chemo Centryx–chemokine receptor; CD4, Cluster of differentiation 4; CTAP-III, Connective tissue-activating peptide-III; CXCL, CXC chemokine ligand; CXCR, CXC chemokine receptor; EPC, Endothelial progenitor cells; EPI, Endogenous peptide inhibitor; EGF, Epidermal growth factor; EMD, Extramedullary disease; ERK, Extracellular signal related kinase; FGF, Fibroblast growth factor; GPCRs, G protein-coupled chemokine receptors; G-CSF, Granulocyte colony-stimulating factor; HIF1α, Hypoxia-inducible factor-1 alpha; HPV, Human papillomavirus; HIV, Human Immunodeficiency Virus; HCC, Hepatocellular carcinoma; HD, Hodgkin's disease; HGF, Hepatocyte growth factor; HMGB1, High Mobility Group Box 1; HSC, Hematopoietic stem cells; IGF, Insulin-like growth factor; JAK/STAT, Janus Kinase signal transducer and activator of transcription; JAM-A, Junctional adhesion molecule-A; JNK, Jun N-terminal kinase; MAPK, Mitogen Activated Protein Kinase; MM, Multiple myeloma; MMP, Matrix metalloproteinases; MIF, Macrophage migration inhibitory factor; MRD, Minimal residual disease; NHL, Non-Hodgkin's lymphoma; OCL, Octeoclast; OPG, Osteoprotegerin; PLC, Phospholipase C; PKA, protein kinase A; PKC, Protein kinase C; PI3K, phosphoinositide-3 kinase; Pim, Proviral Integrations of Moloney virus; RANKL, Receptor activator of nuclear factor kappa-Β ligand; RRMM, Relapsed/refractory multiple myeloma; SFM-DR, Soluble factor mediated drug resistance; VEGF, Vascular endothelial growth factor; VHL, Von Hippel-Lindau; WHIM, Warts, Hypogammaglobulinemia, Infections, and Myelokathexis; WM, Waldenström macroglobulinemia
1. Introduction
Chemokines are a family of low molecular weight (8–11 kDa) secreted proteins which function as leukocyte-specific chemoattractants. With more than 50 members, vertebrate G protein-coupled chemokine receptor (GPCR) family is subdivided into four sub-families, of which CXC chemokine receptors, named CXCR1 through CXCR7, is one of the largest chemokine families involved in various physiological and pathological conditions [1]. CXC chemokine receptors can be grossly classified based on their function as inflammatory, homeostatic and dual-role subtypes. Detailed structural and functional difference of the CXC chemokine receptors are described elsewhere [2], [3], [4], [5].
CXCR4 (C-X-C motif chemokine receptor 4) is a widely studied chemokine receptor due to its significant role in immune response, hematopoiesis, developmental processes as well as in pro-tumorigenic functions. CXCR4 expression is ubiquitous in different hematopoietic cells [6]. CXCR4 is also expressed in different non-hematopoietic cells [4], [7], [8], [9]. CXCR4 can bind to CXCL12, CD4 and CD74, among which CXCL12 or stromal cell-derived factor 1α (SDF-1α), acts as an exclusive endogenous ligand for CXCR4. Apart from CXCR4, CXCL12 can also bind to its second receptor CXCR7 [6], [7]. Contrary CXCR4, CXCL12 is not expressed in hematopoietic cells but rather it is expressed and secreted in different non-hematopoietic tissue sites, most prominently in brain, lung, liver, stromal, endothelial cells and BM where it chemo attracts CXCR4-expressing hematopoietic stem cells (HSCs), thus playing a critical role in the homing of these cells in the BM microenvironment. The expression of CXCL12 in BM ensures the retention of the hematopoietic stem cells until they are needed elsewhere in the body. Therefore, inhibiting CXCL12-CXCR4 interaction can liberate HSCs from the BM niche into circulation [3], [6], [9], [10].
CXCR4-CXCL12 interaction activates a variety of extra and intracellular signalling pathways, thus contributing to different vital biological processes. Upon agonist stimulation, CXCR4 is rapidly phosphorylated on serine and threonine residues in its C-terminal [11]. This is then followed by the activation of some major signalling processes such as phospholipase C (PLC)/ Protein kinase C (PKC)-dependent increase in intracellular calcium level [12]; NF-κB, Ca2+-sensitive protein tyrosine kinase PYK2 and phosphoinositide-3 kinase (PI3K)-Akt pathways [13]; MAPK1/MAPK3 (Mitogen Activated Protein Kinases), JNK (Jun N-terminal Kinases) and PI3K (Phosphoinositide-3 Kinase) activation-dependent processes [14]; Janus kinase signal transducer and activator of transcription (JAK/STAT) pathway [15], [16]; extracellular signal-regulated kinases 1 and 2 (ERK1/2), and Ras/Raf pathways [17]. Other CXCR4 regulatory pathways include Wnt/β-catenin, Sonic hedgehog (SHH)-GLI-NANOG and Notch [[12], [18], [19], [20]. These pathways are all involved in cell differentiation, survival, migration, proliferation and chemotaxis. Arrestins play significant role in the regulation of CXCR4/CXCL12 signaling through internalization and desensitization process of CXCR4 [7], [12], [21].
Due to its involvement in multiple divergent pathways, CXCR4/CXCL12 cascade plays significant role in malignancies including multiple myeloma (MM) which are discussed in the following sections.
2. Role of CXCR4 in malignancies
CXCR4 is known as an independent prognostic biomarker of cancer. CXCR4 expression has been shown to be associated with oral squamous cell carcinoma [22], oesophageal [23], gastric [24], colon [25], liver [26], pancreas [27], thyroid [28], ovary [29], [30], prostate [31], lung [32], kidney [33], [34], breast [35], brain [36], [37], melanoma [38] and leukemia [39]. CXCR4 axis activates the major physiological processes associated with tumor growth, survival, invasion, and homing which involves epidermal growth factor receptor (EGFR), mitogen-activated protein kinase (MAPK), PI3K/AKT, Wnt/ β-catenin and NF-κB mediated pathways. CXCL12 recruit CXCR4-positive inflammatory, vascular and stromal cells to tumor microenvironment and together their signaling leads to aggressive tumor growth and stemness by the secretion of different cytokines, chemokines and growth factors [40], [41].
CXCR4/CXCL12 also has significant role in providing survival advantage and drug resistance of cancer cells through integrin signaling and adhesion to the ECM which leads to secretion of growth factors and activation of anti-apoptotic signaling pathways [42], [43]. As for angiogenesis, pro-angiogenic factors like VEGF, fibroblast growth factor (FGF), matrix metalloproteinases (MMPs), TNF-α plays a pivotal role in angiogenesis through CXCR4 signaling effects [17]. It was recently demonstrated that laminins, a family of basement membrane glycoproteins can initiate cross-talk between VEGF, integrin α2β1 and CXCR4 to promote tumor growth and angiogenesis in colorectal cancer [44]. According to a study by Zhang et al., it was revealed that in pancreatic cancer, VEGF and CXCR4 acts collaboratively to promote angiogenesis and invasion.
CXCL12/CXCR4 signaling is also involved in metastasis. It was shown that this axis can stimulate the activation of a small GTPase RhoA, which is required for remodeling of the extracellular matrix and directional cell migration [45]. As CXCL12 expression is highest in common metastatic sites, CXCR4+ normal or cancer stem cells (CSCs) are chemo attracted in a CXCL12 dependent manner through the circulation [46], [47]. CXCL12 expression on the target organ can lead to the activation of adhesion molecules and MMP secretion that assists in metastasis [48]. Another evidence for CXCR4/CXCL12 involvement in metastatic process is its linkage with CD44, a transmembrane glycoprotein which has tight interaction with the ECM [33]. Different growth factors like fibroblast growth factor, VEGF, epidermal growth factor (EGF) and hypoxic conditions in tumor microenvironment assist in the up-regulation of CXCR4 that further leads to cancer invasion [6], [49], [50], [51], [52]. CXCR4 activity in CSCs when continuously sustained by endogenous CXCL12, is associated with CSC self-renewal, metastatic potential and surviving capacity [53].
CXCR4 was found to promote tumor invasion and metastasis in aggressive cancer cells via downregulating Forkhead Box Class O protein (FoxO3), a tumor suppressor which acts through the inhibition of PI3K/AKT signaling pathway [54]. In vitro and in vivo experiments in lung cancer have shown that, CXCR4/CXCL12 mediated ERK/ AKT pathway activation is associated with the invasion and migration of the cancer cells [55]. English et al. has recently shown that CXCR4-mediated AKT signaling is associated with endocytosis where the inhibition of endocytosis leads to an attenuated AKT signaling [56].
MicroRNAs and long noncoding RNAs (lncRNA) can also target CXCR4 to regulate cancerous pathways. miRNAs were shown to be involved in inhibition of cancer metastasis by down regulating CXCR4 [57], [58]. A recent study in breast cancer has shown that a peptide derived from viral macrophage inflammatory protein called NT21MP can have anticancer effects through targeting miRNAs via CXCR4 pathway [59]. It was observed in osteosarcoma that lncRNA UCA1 is involved in cell migration and invasion, the inhibition of which can have anti-tumor effects. However, this inhibitory effects are reversed when miR-301a is overexpressed as it is involved in the activation of Wnt/ß-catenin pathways via CXCR4 expression regulation [60].
3. CXCR4 and pathogenesis of multiple myeloma
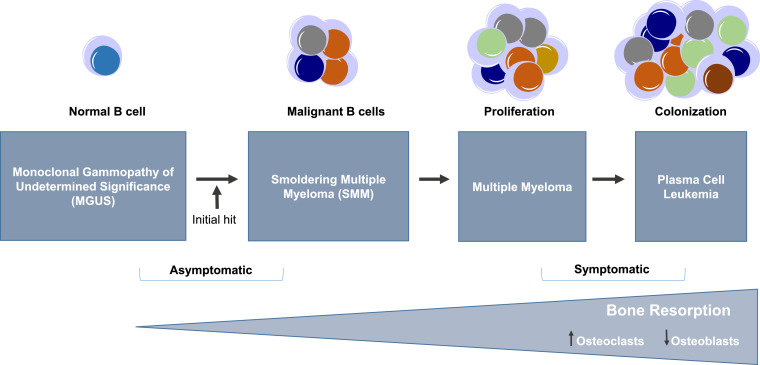
Multiple myeloma (MM) is the second most common form and accounts for 10% of all hematologic malignancies [61]. In spite of significant advances in treatment strategies, MM remains incurable [62]. It is a heterogeneous plasma cell neoplasm characterized by the clonal proliferation and accumulation of malignant plasma cells or excessive production of monoclonal myeloma proteins in the BM compartment and sometimes extra-medullary tissues resulting in osteolytic lesions and thus osteopenia, renal diseases, hypercalcemia and anemia. Fig. 1 highlights the progression of multiple myeloma [1], [21].
Fig. 1.
Stages of Multiple myeloma (MM) progression. The malignant transformation of B cells occurs through a stepwise process involving multiple genetic aberrations and interaction of the B cells with the BM microenvironment. MM evolves from being asymptomatic to symptomatic through acquisition of a fundamental genetic instability followed by further genetic and epigenetic changes to develop a diverse plasma cell clone with the oncogenic potential that enhances bone resorption process throughout the disease progression stages. MM progresses from MGUS (Monoclonal gammopathy of undetermined significance) to SMM (smoldering multiple myeloma) followed by medullary and extramedullary MM to plasma cell leukemia.
MM is thought to initiate from long lived plasma cells which develop in germinal center of lymphoid tissues. The myeloma plasma cells attain oncogenic potential, home to the BM and due to the support in microenvironment niche, survive for a long time [63], [64]. MM can progress from asymptomatic to symptomatic stages ranging from monoclonal gammopathy of unknown significance (MGUS) to smoldering multiple myeloma (SMM) to MM or plasma cell leukemia [61], [63].
The association between CXCR4 and MM has been implicated in different studies [65]. Due to its involvement in normal plasma cell development, CXCR4/CXCL12 axis also plays significant role in MM progression [21], [62]. CXCR4 has role in the expansion and colonization of MM plasma cells in the bone [1]. CXCR4/CXCL12 axis can also regulate homing, adhesion, invasion, migration and mobilization of MM cells out of the BM [66]. It was observed that Persistent chemo-resistant minimal residual disease (MRD) plasma cell clones in MM express high levels of CXCR4, integrins (CD11a/CD11c/CD29/CD49d/CD49e) and adhesion molecules (CD44/CD54) [67], while abrogation of CXCR4/CXCL12 pathway can deregulate BM colonization by hematopoietic cells [68]. Elevated CXCR4 expression in MM is induced by different factors present in the malignant cells. For instance, hypoxia [69], [70], different proinflammatory cytokines like TNF-α,TGF-β, and VEGF [71] were noted to induce CXCR4 expression in MM. It was shown that down-regulation of HIF1α decreased CXCR4 expression and reversed the migration and homing of MM cells into the BM [72]. Some biological roles of CXCR4 in MM is partly played by its interaction with a pleiotropic inflammatory cytokine called macrophage migration inhibitory factor (MIF) which subsequently leads to receptor activation and promotion of chemotaxis [73], [74], [75]. The involvement of CXCR4 in MM pathogenesis as discussed in the following sections.
3.1. CXCR4, carcinogenesis and tumour growth in MM
CXCL12-expressing BM stromal cells (BMSCs) recruit CXCR4-expressing B cells, eosinophils and monocytes that are required for their retention in the BM [76]. It was suggested that CXCR7 itself also has a vital role in cell adhesion, angiogenesis and tumor progression in MM where it indirectly interacts with CXCR4/CXCL12 axis [6], [7], [65], [77]. CXCR7 serves in modulating the function of CXCR4 by forming heterodimeric receptor unit with CXCR4 for CXCL12 signal transduction as well as recruitment of the tumorigenic monocytes [68], [78].
In a similar way as the normal plasma cells, myeloma cells utilizing CXCR4 interact with CXCL12-expressing BMSCs and migrate across the endothelium lining the BM for homing and localization through chemotaxis [62], [79]. Myeloma-BM stroma is rich in integrins like VLA-4 (Very-late-antigen-4, α4β1 integrin, CD49d/CD29) ligands that has significant role in cell adhesion-mediated drug resistance (CAM-DR) [21], [80].
When MM cells adhere to BMSCs, CXCL12 up regulates its own secretion, which further up regulates VEGF and IL-6 secretion and thus promote enhanced homing through further expression of integrins [1], [81]. Thus, trans-endothelial migration, homing, adhesion and localization of MM cells in the BM microenvironment to form tumor niche is promoted by the up-regulation of integrins through CXCL12 [21], [82]. An increase in intracellular cAMP in association with the activation of protein kinase A (PKA) downregulates CXCL12 mediated α4ß1-dependent cell adhesion and induces apoptosis in MM [82], [83]. It was shown that MM cells express high levels of tumor promoter heparanase enzyme which promotes MM invasion and angiogenesis mediated by VLA-4 [84]. Again, MIF has a role in MM cell adhesion to the BMSCs through regulating the expression of adhesion molecules via its receptor CXCR4. MIF mediated B-cell chemotaxis is abrogated when CXCR4 is inhibited [73]. MIF-CXCR4 interaction leads to activation of adhesion molecules in MM cells whereas abrogating MIF made MM cells more chemotherapy-sensitive when co-cultured with BMSCs in vivo [75]. MIF can also bind to CD74 and MM cells were shown to express not only CXCR4 but also CD74. It was evident that MIF-deficient MM cells had aberrant tumor growth [75].
Localization and interaction of MM cells in the BM microenvironment leads to the activation of osteoclasts and suppression of osteoblasts which associates with MM progression, metastasis and drug resistance. Hyperactivated Notch signaling is an important mediator of this unbalanced osteoclast and osteoblast activity [85]. It was shown that CXCL12-CXCR4 interaction is pro-osteolytic. Disruption of CXCR4 enhances osteoclast activation and enhances tumor growth in bone [65], [86]. CXCL12 can promote migration of osteoclast precursors and up regulate several pro-osteoclastic genes [65]. Osteoclast precursors express Bruton's tyrosine kinase (BTK), a MM stem cell marker which is involved in the generation of osteoclasts and their migration towards CXCL12. BTK expression is associated with CXCR4 expression in primary myeloma cells [87]. BTK is also involved in myeloma cell homing to the bone [88]. BM-stromal cells secrete different factors along with CXCL12 such as IL6, insulin-like growth factor 1 (IGF1), VEGF, TNFα and osteoprotegerin which are even more upregulated as MM cells localize in the stromal cells. The interaction of MM cells with these factors is associated with osteoclastogenesis. The activity of Osteopontin (OPN), a matrix protein that plays a dual role in MM as a marker for osteoclastic activity and also angiogenesis, has been linked to CXCR4/CXCL12 [89].
The NF-κB (RANK)/RANK ligand (RANKL) signaling pathway is another crucial regulatory system of bone remodeling. RANKL is over-expressed in MM cells and the ratio of RANK/osteoprotegerin regulates the level OCL activity [65], [88], [90]. It was suggested that CXCL12 can enhance the pro-resorptive effects of RANKL [91].
Previous evidence shows that CXCR4 overexpression is associated with poor disease prognosis [65]. Abrogation of this signaling axis can down regulate BM colonization by hematopoietic cells [68]. Interestingly in a controversial study, it was demonstrated that CXCR4 expression and disease activity is inversely correlated. MM cells express CXCR4 in high levels in the peripheral blood but in low levels in the BM. CXCR4 expression is down-regulated in MM cells from the BM in response to high levels of CXCL12. High CXCL12 in the BM mediates internalization of the CXCR4 receptor from the surface to the intracellular compartment in MM cells. This subcellular location of CXCR4 in MM can activate different downstream signaling pathways, like PI3K and ERK/MAPK pathways [40], [66].
This is supported by the observation that, extramedullary homing of MM cells in the skin showed reduced CXCR4 surface expression [92].
It has been proven that even though CXCR4 is a membrane receptor, it can be internalized by CXCL12 from the membrane to the cytoplasm. High CXCR4 expression in the cytoplasm indicates poor prognosis in contrast to a better prognosis evident when CXCR4 is highly expressed in the nucleus [40]. MM plasma cells have the potential for phenotypic plasticity and have different subpopulations existing simultaneously that can affect disease initiation and progression. A proposed model by Shmuel Yaccoby showed that a subpopulation of MM stem-like cells, called quiescent MM stem cells act as tumor-initiators. Quiescent MM stem cells show elevated expression of adhesion molecules and CXCR4, which help in MM cell motility, migration and adherence to BMSCs. However, for proliferative MM stem cells, which is responsible for disease progression and emergence of evolved subclones, the expression of CXCR4 is minimal [87]. This supports the notion that CXCR4 expression is down-regulated in more advanced stage of MM. Since MRD expressed high levels of CXCR4, integrins and adhesion molecules, they supposedly belong to the tumor initiating quiescent stem cells [87].
3.2. Role of CXCR4 in progression of Myeloma and distant metastasis
Metastasis for cancer is a stepwise process where cancerous cells migrate from their initial establishment site to invade and spread to specific tissues resulting in the formation of new ‘foci’. This process is well described as Stephen Paget's “seed and soil” hypothesis-where metastatic primary tumor cells as the “seed” interact with their preferred organ microenvironment or the “soil” and colonize there, as they egress and home to the new sites [93].
In MM, primary tumor without distant metastasis is represented as plasmocytomas. This leads to micrometastasis in MGUS through local invasion, followed by macrometastasis or distant colonization of the small number of carcinogenic MM cells [94]. Some MM features include extramedullary disease (EMD), a metastasis prone phenotype where the tumor cells home to BM niches and can also infiltrate in other organs [95]. Extramedullary spread of MM involves altered expression of different adhesion molecules by MM cells [92].
CXCR4 is known as the marker for bone metastatic signature as it is universally up regulated in cancer cells metastasizing in the bone [65]. As CXCL12 is expressed by mesenchymal stromal cells in different organs like liver, lungs and BM, CXCR4 expressing MM plasma cells are recruited to these organs and can metastasize there [40]. The preferential homing of MM cells to the BM requires rolling of the MM cells along the endothelium by binding to selectin and CXCR4/CXCL12. This binding process leads to the activation of MM cells for adhesion and transmigration at a later stage [64], [96].
Again, Notch signaling system is known to control the expression and function of CXCR4/CXCL12 axis and therefore, MM metastatic pathway. Increased Notch signaling involves the imbalance in osteoclast and osteoblast activity and is associated with continuous homing and egression of MM cells leading to tumor infiltration in different bone locations [85].
For MM, dysregulation of RANK/RANKL/OPG is associated with cancer invasion and metastasis. This signaling also is osteolytic [88], [97], [98]. The role of OPG in bone metastatic process has been known to involve CXCR4/CXCL12 axis [65], [99]. The osteolytic process in MM creates a favorable microenvironment for MM growth, which supports the fertile soil hypothesis by Paget. It also leads to osteoclast activation through the release of different growth factors-further promoting bone resorption by osteoclast activation in a positive feedback loop [65]. MM cells interact with different cellular components, extracellular matrix proteins, cytokines, chemokines, proteolytic enzymes and growth factors in the BM microenvironment which assist in osteoclastogenesis, angiogenesis and MM metastatic process. IL-6, TNFα and NF-κB are significant myeloma-growth promoting lymphokines. IL-6 and TNFα are associated with rapid growth and spread of MM cells to other bones while NF-κB mainly plays growth-inducing and anti-apoptotic roles. CXCR4/CXCL12 axis was shown to induce NF-κB activation in MM and increased secretion of VEGF and IL-6 in BMSCs to promote MM growth, survival and migration [1], [100].
Some metastatic cells can remain dormant for a long time in MM with decreased expression of proliferation marker Ki67 before emerging back from the dormant stage and producing overt metastasis. The dormancy is associated with hypoxia and cellular interaction with BMSCs [101], [102]. It was discerned that activation of osteoclasts can facilitate the reactivation of the dormant MM cells based on favorable bone microenvironment [103]. MM progression and metastasis requires continuous egression of MM cells into the circulation and continuous homing of MM cells to new BM sites, where the mobilization and extravasation is regulated by CXCR4/CXCL12 signaling [64], [68]. CXCR4/CXCL12 interaction mediated MMP synthesis degrades extracellular matrix (ECM). This facilitates detachment of MM cells from primary tumor sites, egression into the blood steam and distant organ migration [6], [48], [104]. The initial step for metastasis involves an epithelial to mesenchymal transition (EMT) phenotype of the transformed malignant cells [94]. CXCR4 is a regulator of the EMT-like transcriptional modulation that characterizes EMD phenotype. EMD development, tumor growth and further metastasis is associated with the acquisition of EMT-like signature in MM cells [95]. In mouse MM models CXCR4/CXCL12 downregulation is associated with EMD in MM, which is due to cell-adhesion disruption process [94], [105], [106], [107]. It was observed in vivo that CXCR4 overexpressing MM cells with enhanced invasive properties changed actin cytoskeleton organization and up regulates EMT related genes as Twist, Slug and Snail while down regulates E-cadherin [108]. Both EMD and EMT show the properties of decreased adhesion and enhanced egression into the circulation [69].
As previously mentioned, hypoxia is a critical regulator of cancer metastasis. The BM micro- environment is very hypoxic with around 1−2% O2 [64]. The hypoxic microenvironment of BM promotes de-adhesion, increased chemotaxis and homing of MM cells to new BM niches through acquisition of EMT phenotype regulated by CXCR4 [69]. Pim (Proviral Integrations of Moloney virus) kinases, which are upregulated in hematological malignancies, are known to have oncogenic potential as they mediate MM cell migration and homing. Hypoxia in BM promotes Pim activity which also correlates with CXCR4 upregulation [109].
It was evident that, in addition to CXCR4, CXCR7 might additionally work in the MM metastatic process [65]. Angiogenic monomuclear cells (AMC) have significant role in MM metastasis as they can migrate from BM involving chemotaxis, adhesion and invasion processes. Azab et al. demonstrated that CXCR7 plays an indirect role in MM progression and metastasis. It was confirmed in MM mouse model that CXCR7 is highly expressed on AMCs and enhances metastatic effects complementary to CXCR4. Both in vitro and in vivo studies confirmed that CXCR7 inhibition abrogated trafficking of AMCs and decreased MM progression [78].
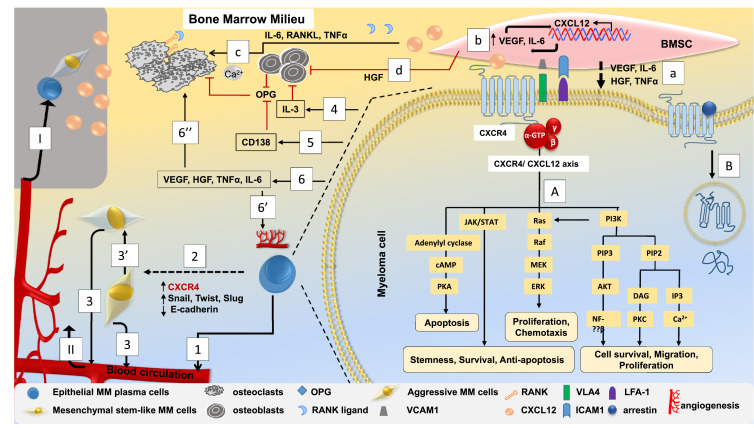
It was recently shown that protein junctional adhesion molecule-A (JAM-A), a new biomarker in MM, is associated with CXCR4. High JAM-A expression is associated with poor clinical prognosis in MM patients due to its role in invasion and metastasis [110], [111]. Blocking JAM-A activity has shown to impair MM viability, migration, proliferation and spread in MM mice [110], [111]. Fig. 2 illustrates how CXCR4-CXCL12 axis can have a role in the complex signalling process in multiple myeloma.
Fig. 2.
Role of CXCR4-CXCL12 axis in the possible signalling events in multiple myeloma (MM). MM cells express high levels of CXCR4 and interact with CXCL12 expressing BMSCs and localize across the endothelium lining the BM. This interaction upregulates cytokine secretion from both BMSCs and MM cells and upregulates the expression of adhesion molecules like VLA-4 and LFA-1 which are present on the surface of MM cells to VCAM-1 and ICAM-1, respectively, which are expressed on BMSCs. A. CXCR4-CXCL12 interaction leads to receptor internalization from the surface to the subcellular location that can activate different signaling cascades that can be associated with MM cell stemness, survival, proliferation, migration and metastasis. B. CXCR4 is desensitized through arrestin mediated internalization and lysosomal degradation followed by ubiquitination. MM cell adherence and localization to BMSCs upregulates the expression of a. VEGF, HGF, IL-6, TNFα all of which along with other cytokines and growth factors associate both homing and proliferation of MM cells through promoting the expression of integrin molecules; b. CXCL12 is also upregulated which leads to more VEGF and IL-6 expression to further promote enhanced CXCL12 expression by BMSCs and enhanced homing process. c. High CXCL12 and its associated cytokines and growth factors lead to overproduction of osteoclasts, where the process to inhibit osteoclastogenesis by OPG secreted from both osteoblasts and BMSCs is downregulated. Also, CXCR4-CXCL12 interaction through promoting MM cell adherence to BMSCs, enhances RANKL production which further suppress OPG production. d. Osteoblastogenesis is inhibited due to secretion of HGF from BMSCs. 1. Imbalanced osteoclast and osteoblast activity leads to the continuous homing-egression of MM cells into the circulation which is regulated by CXCR4 signaling. 2. Hypoxic BM microenvironment in association with CXCR4 over-expression by the MM cells lead to enhanced expression of EMT related genes (Twist, Slug, Snail) and reduced E-cadherin expression that further enhance de-adhesion and egression of MM cells into circulation through acquisition of 3. EMT phenotype followed by 3′. aggressive MM cell features with enhanced metastatic potential. 4. MM cells secrete IL-3 that also inhibit osteoblastogenesis. 5. CD138 expressed on the surface off MM cells can bind OPG to prevent its inhibitory effect on RANKL function. This higher RANKL/OPG ratio leads to osteoclast differentiation that promotes osteolysis and hypercalcemia. 6. MM cell interaction with BMSCs leads to VEGF, HGF, IL-6, TNFα overexpression by MM cells which are involved in both 6′′. osteoclastogenesis and 6′. angiogenesis. The complex interaction of MM cells with different cytokines, cellular components, extracellular matrix proteins along with MMPs can promote both, angiogenesis and aggressive metastatic behavior. I. Expansion and colonization of aggressive MM cells to secondary metastatic sites is associated by higher CXCL12 gradient that promotes CXCR4-positive MM cell migration and II. homing from the primary tumor sites. Overall, the net result of all these complex interactions is tumor expansion and MM progression.
4. CXCR4 and therapeutic resistance in MM
One of the biggest challenges associated with MM is acquired drug resistance and disease relapse, making MM a yet incurable disease [112]. CXCR4 is not only involved in MM cell homing, retention in BM, growth, invasion, angiogenesis and metastasis, but also is associated with resistance and relapse process. Different drugs and treatment strategies are often not effective enough due to relapsed/refractory MM (RRMM) which indicates non-responsiveness and progression on therapy.
CXCR4 signaling is protective for MM cells as it prevents spontaneous and chemotherapy-induced apoptosis for MM cells via their retention in protective BM environment. This protective effect further promotes therapeutic resistance in MRD [113]. It was investigated by Kim et al. that dexamethasone enhanced intracellular and surface CXCR4 expression in MM cell lines while decreasing CXCL12 level in BMSC [70]. Some studies have showed that certain chemotherapeutic agents and radiation can activate CXCR4/CXCL12 pathway and this can be associated with therapeutic resistance [40]. In another study, low CXCR4 expression was implicated to be the biomarker of Bortezomib resistance. This is due to the effect that Bortezomib-resistant MM cells were found to express less CXCR4, leading to escape of PCs from BM extramedullary metastasis in MM mouse model [106]. This was further confirmed in another study in MM patient sample [114].
Hypoxic environment can facilitate MM cells to acquire dormancy and RRMM phenotype via the interaction with BMSCs [115]. Also, MM cell adhesion helps in sustaining the expression of anti-apoptotic genes to promote chemo-resistance in MM, implying that CAM-DR is an important feature of RRMM. CXCR4 and CXCL12 interaction is known to directly promote MM cell survival and CAM-DR [66], [80], [95]. Growth factors like IGF and Hepatocyte growth factor (HGF) promote migration of MM cells synergizing with CXCL12 [116]. Inhibition of IGF pathway reversed CAM-DR both in vivo and in vitro [117]. Again, RANK-RANKL signaling system has been associated with MM chemo-resistance and CAM-DR through the activation of multiple signal transduction pathways [118].
The binding of the MM cells to BMSCs upregulate secretion of adhesion molecules and cytokines which further promote migration, MM cell growth and therapeutic resistance in a positive feedback pattern [80], [95], [117]. Integrin-α8 (ITGA8) is highly expressed in MM patients with early relapse and activates genes like VEGF, HIF1α, cadherin, Slug, Snail and CXCR4 which are associated to stemness and EMT. In a study by Ryu eta al in relapsed MM patients, integrin-α8 was highly expressed and induced EMT features with the upregulation of CXCR4- ultimately leading to MM migration, invasion and drug resistance [119]. According to Yaccoby et al., of the two stem cell populations in MM cells, quiescent MM stem cells are detected during remission, while proliferative MM stem cells are associated with relapse [87]. Moreover, TNF-α, VEGF and IL-6 activation is associated with CAM-DR as all of these factors are concurrently expressed by the MM cells-BMSCs adhesion stimuli [120]. Enhanced activation of Notch pathway, linked to CXCR4/CXCL12 axis also leads to an increased secretion of IL-6, IGF-1, and VEGF [120] and intrinsic and acquired pharmacological resistance in MM. It was shown that Notch blockade can improve MM cell sensitivity towards standard chemotherapeutic drugs both in vivo and in vitro [121].
Apart from EMT phenotype, epigenetic changes are also often responsible for MM progression and drug resistance. Many studies have shown an increase in hypermethylation status in different MM regulatory genes with MM progression. Abdi et al. showed low frequency of methylated CXCR4 genes in MM patients and higher rate of progression free survival [122].
4.1. CXCR4/CXCL12-targeted therapy in MM
CXCR4 represents a valuable target for development of novel therapeutics due to its critical role in the crosstalk of MM pathogenesis. The CXCR4 targeting agents include CXCR4 antagonists, small synthetic or natural molecules and peptides. These agents can affect the activity of many genes and proteins via multiple pathways. However, inspite of being broad spectrum anticancer agents, different clinical trials have shown the efficacy of these agents can be linked to targeting CXCR4/CXCL12 axis. It is often speculated that CXCR4 inhibitors combined with chemotherapy exert additive antitumor effects [40].
Plerixafor was the first FDA-approved (2008) selective CXCR4 antagonist which competitively inhibits CXCL12 binding to its receptor. This antagonist inhibited MM cells’ migration in vitro and their homing in vivo through interfering with PI3K/AKT and ERK pathways [66], [68], [95], [123]. It also helps with egressing and mobilizing MM cells into the peripheral blood circulation by disrupting their adhesion to the BM microenvironment. It can be used either alone or in combination with granulocyte colony-stimulating factor (G-CSF) to mobilize HSCs to the peripheral blood [6]. This effect was investigated in a phase II clinical trial which showed that Plerixafor combined with G-CSG caused enhanced peripheral blood stem cell mobilization and higher expression of genes associated with superior engraftment than G-CSF alone [124], [125], [126]. It was shown that Plerixafor can induce chemosensitization in MM cells [68], [127], [128], [129]. Several studies have shown that it can induce chemosensitivity to the proteasome inhibitor Bortezomib in MM cells [80], [129]. AMD3465, a monocyclam analog of plerixafor, also shows antitumor effect [40]. High-affinity CXCR4 antagonist BKT140 in addition to inducing MM cell apoptosis, was also shown to mobilize hematopoietic stem cells for autologous transplantation when combined with G-CSF [6], [130], [131], [132]. Panobinostat, another FDA approved CXCR4 antagonist was used in several clinical trials for RRMM either alone or in combination with other agents which demonstrated antimyeloma activity (PANORAMA trials) [133]. One such phase I/II clinical study for panobinostat in combination with melphalan, showed lack of toxicity effects and low disease progression [134]. Another phase III clinical trial has shown that in RRMM, higher overall survival and progression free survival benefit can be induced with Panobinostat, Bortezomib and Dexamethasone combination [135], [136], [137].
Of the anti-CXCR4 antibodies, Ulocuplumab was developed by Kuhne et al. and showed efficacy in inducing apoptosis in vitro and antitumor activity in xenograft models [138]. A phase I clinical trial for MDX-1338 has been conducted for the treatment of RRMM either alone or in combination with lenalidomide/dexamethasone or bortezomib/dexamethasone [21], [139]. MDX-1338 was shown to affect survival and adhesion of MM cells in a dose-dependent manner along with inhibition of MM cell proliferation in vivo in xenograft mice models [140]. Roccaro et al. tested the effect of MDX-1338 both in vivo and in vitro and identified that it can inhibit MM cell bone-to-bone dissemination by suppressing EMT phenotype [62], [108], [120]. Another humanized monoclonal IgG1 anti-CXCR4 antibody F50067 showed MM anti-tumor activity through competing for CXCL12 binding, inhibiting G-protein activation and CXCL12 induced downstream signaling pathways [141]. A phase I clinical trial for F50067 alone and in combination with lenalidomide and low dose dexamethasone (Len-Dex) in RRMM showed promising effects in egression of MM cells to the circulation, though the study was terminated due to hematological toxicity [142]. LY2624587 is another potent anti-CXCR4 antibody which showed dose dependent inhibition in tumor growth in hematological malignancies particularly in human leukemia and lymphoma [143], showing potentials for its investigation in future MM trials.
It was recently shown that CXCR4 targeted endo-radiotherapy, which represents an alternative therapeutic mode could be effective in treating MM as it showed to induce better response even in relapsed MM patients [144]. Recent studies further showed CXCR4/CXCL12 axis mediated macrophage polarization phase can be a potential new MM treatment strategy [21].
Apart from targeting CXCR4, targeting its ligand CXCL12 has been brought under focus. Olaptesed pegol (Ola-PEG) and Spiegelmer / NOX-A12 (Noxxon Pharma) can specifically bind to CXCL12. In one B-cell line, Ola-PEG inhibited CXCL12 mediated internalization of the CXCR4 receptor and inhibited chemotaxis in a dose-dependent manner [128]. Ola-PEG also blocked CXCL-12-dependent activation of its second receptor CXCR7 [123]. Ola-PEG was shown to be effective than Plerixafor in suppressing tumor growth and metastasis in a xenograft MM model [128]. It also was shown to act synergistically with Bortezomib and associate in MM cell mobilization to the circulation [123]. Also, CTCE-9908 can target CXCL12 and show clinical activity against MM [40], [145]. It was shown that thalidomide, which is used for MM treatment can downregulate both CXCR4 and CXCL12 [128], [146]. Tymoquinone and Sorafenib both could also target CXCR4/CXCL12 axis to promote anti-apoptotic effects and blocked chemotaxis of MM cells [147], [148].
Other CXCR4/CXCL12 targeting compounds include Carfilzomib, a second-generation proteasome inhibitor which interfere CXCR4/CXCL12 mediated CAM-DR by inhibiting CXCR4 phosphorylation [80]. Interestingly, though carfilzomib can target CXCR4, it was observed that CXCR7 is unaffected by it, warranting a possible independent role of CXCR7 from CXCR4 [78], [80]. EPI-X4 is a naturally occurring endogenous CXCR4 antagonist has implications in cancer metastasis [149]. Some synthetic EPI-X4 derivative showed greater potential in blocking CXCR4 signaling than AMD3100, hence its therapeutic potential to treat MM can be evaluated [150]. The other agents which can also target CXCR4/CXCL12 axis in MM include TG-0054, POL6326, BKT-140, Sorafenib and NOX-A12.
The functional effects of some important CXCR4/CXCL12 targeting compounds which were used in clinical trials against MM are summarized in Table 1.
Table 1.
Overview of some CXCR4/CXCL12 targeting anti-MM compounds.
| Compound | Secondary names | Mechanism of action | Significant clinical trial identifiers | References |
|---|---|---|---|---|
| Plerixafor | AMD3100/ mozobil | CXCR4 antagonist; abrogates CXCL12 induced receptor internalization | NCT00322842 | [6], [66], [68], [95], [123], [124], [125], [126], [127], [128], [129], [151] |
| NCT00322387 | ||||
| Ulocuplumab | BMS-936564/MDX-1338 | Anti-CX43 antibody that affects survival and adhesion of MM cells | NCT01359657 | [21], [139] |
| Burixafor | TG-0054 | CXCR4 antagonist that can block CXCL12 binding; stem cell mobilizer | NCT02104427 | [68], [152], [153] |
| Balixafortide | Polyphor (POL6326) | Inhibits CXCR4-CXCL12 interaction; hematopoietic stem cell mobilizer | NCT01105403 | [68], [127], [153], [154] |
| BKT140 | BL8040/TN14003 | CXCR4 antagonist; induce MM cell apoptosis | NCT01010880 | [6], [132] |
| Panobinostat | LBH589 | CXCR4 inverse antagonist; affect MM cell viability and osteoclast formation | NCT00743288 | [133], [134], [135], [136], [137], [155] |
| NCT00532675 | ||||
| NCT01023308 |
4.2. CXCR4-targeted therapy: current challenges
Due to its immense roles in progression of MM, CXCR4 is considered as one of the best potential targets for inhibition of disease growth, dissemination and drug resistance and optimization of current anti-MM treatment strategies. However, certain limitations and challenges of CXCR4 targeting strategies should be noted.
Successful treatment of MM by CXCR4 inhibitors is challenging as CXCR4 is ubiquitously expressed in healthy normal cells as well as in cancer cells. Interfering with CXCR4 signaling can affect hematopoiesis and organ development along with other essential physiological effects like cardiovascular functions, development and apoptosis. Thus, even though CXCR4 inhibition can have positive anti-MM effect, it can also negate immunological and physiological responses. Whether CXCR4 antagonists can affect cell cycle should be explored further. The side effects or off-tumor cytotoxicity of CXR4 inhibitor upon non-MM cells may impede the use of CXCR4 targeted therapies in translational research schemes. Therefore, a balance between its positive effects on disease outcome and negative effects on normal biological functions should be maintained. In addition, differential expression of CXCR4 in blood and BM as well as its actual correlation with MM progression and dissemination should be investigated in thoroughly before designing a treatment scheme.
Plerixafor has shown to have minimal side effects in clinical studies. However, plerixafor and many other CXCR4 modulators used in clinical trials show hematological toxicity and poor pharmacological profile. Small molecules and small peptides which target CXCR4/CXCL12 axis pose several challenges in MM treatment; this is due to the unwanted side effects of the small molecules due to ubiquitous CXCR4 expression in different sites and the later often shows poor selectivity [68], [156]. So, the current challenge to overcome in clinical translation is to find more potent, effective and safer CXCR4 modulators with synergistic activity when used with combination drugs for long term use. Combinatorial strategies rather than monotherapy can be beneficial to prevent relapse which can target both cancer stem cells as well as bulk tumor. Complexity of genetic events and tumor microenvironment associated interactions suggest that combination therapies will be required to increase cytotoxic effects, overcome drug resistance and improve patient outcome.
In terms of hematopoiesis, as CXCR4/CXCL12 signaling associates in HSC quiescence and retention within the BM, whether CXCR4/CXCL12 antagonism can perturb hematopoiesis should be studied in further details for more rational therapeutic strategies. CXCR4/CXCL12 disruption can affect HSC long-term maintenance as this axis was shown to be involved in the protection of HSCs against oxidative stress [157]. Although mobilizing HSCs or metastatic cancer cells is induced by CXCR4 targeted drugs, this process can act as a dual-faced sword-firstly as mobilized MM cells can initiate distant metastasis. In addition, healthy stem cells can also be mobilized during the mobilization process of HSCs or metastatic cancer cells by CXCR4 treatment. Considering the possible effects of these agents on healthy stem cells, this may mediate drug toxicity. Angiogenesis may also be affected since CXCR4 antagonists may recruit endothelial progenitor cells (EPCs) which secrete large quantities of angiogeneic factors leading to vascular growth [68], [156], [158].
Determination of the timing to initiate MM treatment is crucial as even best combinations of drugs can still lead to relapse due to late initiation of treatment when the macrometastasis has already started [94]. As CXCR4 has its role from initiation to invasion and distant metastasis in MM, the best timing to initiate CXCR4 antagonist-based treatment has to be further evaluated. Gene and protein profiling before initiating therapy and at relapse along with personalized combinatorial drugs can be effective. It is also essential to evaluate the relation between CXCR4 and tumor initiating cells and how differential expression of CXCR4 in MM cells correlates to MM pathogenesis. Thus, systematic studies and rational design of combined therapeutic strategies will be pivotal.
High risk MM cell lines has the potential for transition from either quiescent to proliferative MM stem cells through extrinsic and intrinsic mechanisms. MM stem cell plasticity and heterogeneity is another challenge to overcome therapeutic limitations as it makes CXCR4+ cancer cell tracking a difficult task. Defining each stem cell subpopulation with identification of their biomarkers that are predictive of response is essential to target the MM cells effectively [43], [87].
To attain effective treatment strategies for MM, it is essential to elucidate the complete picture of how CXCR4 are associated with different counterparts in the complex signaling process of BMSC and egress/homing of MM cells in mediating CAM-DR, which is followed by further drug resistance due to secretion of different cytokines and growth factors by the BM microenvironment. MM cells are protected due to the supportive role of the BM niche. Thus, a better treatment strategy might be targeting both MM cells and BM microenvironment along with a broader understanding of how CXCR4 associates with the surrounding environment. Although cell signaling targeted and tumor microenvironment targeted therapies have yielded initial promising results in preclinical or clinical studies for RRMM [68], [159], these findings need to be further confirmed and expanded by evaluating the relation between CXCR4 and cancer initiating cells.
Based on the current evidence, the sole blockage of CXCR4 does not seem sufficient for restriction of the effects mediated by CXCL12 since CXCR7 also acts as an alternative receptor for CXCL12 in cancer and stromal cells to affect MM pathogenesis. It is also speculated that CXCR7 is the functional modulator of CXCR4 [40], [160]. Thus, it can be assumed that simultaneous blocking of both CXCR4 and CXCR7 rather than selectively blocking CXCR4 could be a much more efficient anti-MM treatment strategy [80]. Anti-CXCR4 therapy usually requires its combination with other chemotherapeutics or radiotherapy due to more pronounced effects of other effectors [Tables 1–3] [68]. It was agreed from different pre-clinical studies that simply blocking CXCR4 would not be beneficial for established tumors and it cannot prevent metastasis [156]. Thus, not only CXCR4/CXCL12 axis, but also the role of CXCR7 has to be studied. This will help to define how inhibition of MM cells’ interaction with the relevant ligand can interfere with MM progression, thus identification of new therapeutics combination.
Dormancy poses major clinical problem for MM treatment. MM Cells often harbor dormant cells as MRD that are associated with remission and relapse after their reactivation mediated by osteoclasts engagement with the endothelial niche [103]. Since CXCR4/CXCL12 axis is linked to osteoclast activation, a better understanding of the molecular mechanism of CXCR4/CXCL12 axis mediated osteoclast activation is required to target RRMM.
5. Conclusion
Collective evidence from an abundance of studies support the pivotal role of the biological involvement of CXCR4 in MM. CXCR4 binding with its chemokine receptor can traffic immune and cancer cells, as well as affect the properties of tumor microenvironment to promote disease progression. Thus, with its diverse functional properties and complex signaling crosstalk, CXCR4 can have major pathologic role in different stages of MM and the patients’ drug resistance. The evaluation of CXCR4 expression and signaling pathway along with its ligand and other interacting partners can have significant prognostic value for MM. Therapeutic interventions targeting single receptor or its ligand is not efficient enough. A better understanding of the role of CXCR4 pathway can provide answer on how MM progresses and relapses and how drug resistant MM cell clones persist, which will further provide with the framework to design efficient therapeutic strategies to impair MM tumor dissemination in combined therapies.
Conflict of interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.100253.
Appendix. Supplementary material
References
- 1.Aggarwal R., Ghobrial I.M., Roodman G.D. Chemokines in multiple myeloma. Exp. Hematol. 2006;34(10):1289–1295. doi: 10.1016/j.exphem.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachelerie F., Ben-Baruch A., Burkhardt A.M., Combadiere C., Farber J.M., Graham G.J. International union of basic and clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2014;66(1):1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen K., Bao Z., Tang P., Gong W., Yoshimura T., Wang J.M. Chemokines in homeostasis and diseases. Cell Mol. Immunol. 2018;15(4):324–334. doi: 10.1038/cmi.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han X. Constitutively active chemokine CXC receptors. Adv. Pharmacol. 2014;70:265–301. doi: 10.1016/B978-0-12-417197-8.00009-2. [DOI] [PubMed] [Google Scholar]
- 5.Martins-Green M., Petreaca M., Wang L. Chemokines and their receptors are key players in the orchestra that regulates wound healing. Adv. Wound Care (New Rochelle) 2013;2(7):327–347. doi: 10.1089/wound.2012.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee S., Behnam Azad B., Nimmagadda S. The intricate role of CXCR4 in cancer. Adv. Cancer Res. 2014;124:31–82. doi: 10.1016/B978-0-12-411638-2.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nazari A., Khorramdelazad H., Hassanshahi G. Biological/pathological functions of the CXCL12/CXCR4/CXCR7 axes in the pathogenesis of bladder cancer. Int. J. Clin. Oncol. 2017;22(6):991–1000. doi: 10.1007/s10147-017-1187-x. [DOI] [PubMed] [Google Scholar]
- 8.Rossi D., Zlotnik A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 9.Murphy P.M., Heusinkveld L. Multisystem multitasking by CXCL12 and its receptors CXCR4 and ACKR3. Cytokine. 2018;109:2–10. doi: 10.1016/j.cyto.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kucia M., Jankowski K., Reca R., Wysoczynski M., Bandura L., Allendorf D.J. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004;35(3):233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 11.Busillo J.M., Armando S., Sengupta R., Meucci O., Bouvier M., Benovic J.L. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J. Biol. Chem. 2010;285(10):7805–7817. doi: 10.1074/jbc.M109.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu C., Zhao H., Chen H., Yao Q. CXCR4 in breast cancer: oncogenic role and therapeutic targeting. Drug Des. Devel Ther. 2015;9:4953–4964. doi: 10.2147/DDDT.S84932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurth R., Bajetto A., Harrison J.K., Barbieri F., Florio T. CXCL12 modulation of CXCR4 and CXCR7 activity in human glioblastoma stem-like cells and regulation of the tumor microenvironment. Front Cell Neurosci. 2014;8:144. doi: 10.3389/fncel.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandis A.Z., Prasad A., Band H., Klosel R., Ganju R.K. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23(1):157–167. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- 15.Vila-Coro A.J., Rodriguez-Frade J.M., Martin De Ana A., Moreno-Ortiz M.C., Martinez A.C., Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. Faseb. J. 1999;13(13):1699–1710. [PubMed] [Google Scholar]
- 16.Soldevila G., Licona I., Salgado A., Ramirez M., Chavez R., Garcia-Zepeda E. Impaired chemokine-induced migration during T-cell development in the absence of JAK 3. Immunology. 2004;112(2):191–200. doi: 10.1111/j.1365-2567.2004.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng Y.M., Liang J., Wu C., Xu J., Zeng D.N., Yu X.J. Monocytes/Macrophages promote vascular CXCR4 expression via the ERK pathway in hepatocellular carcinoma. Oncoimmunology. 2018;7(3) doi: 10.1080/2162402X.2017.1408745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Cai J., Han F., Yang C., Tong Q., Cao T. Silencing of CXCR4 blocks progression of ovarian cancer and depresses canonical WNT signaling pathway. Int. J. Gynecol. Cancer. 2011;21(6):981–987. doi: 10.1097/IGC.0b013e31821d2543. [DOI] [PubMed] [Google Scholar]
- 19.Fareh M., Turchi L., Virolle V., Debruyne D., Almairac F., de-la-Forest Divonne S. The miR 302-367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG network. Cell Death Differ. 2012;19(2):232–244. doi: 10.1038/cdd.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Z., Zhao C., Han X., Han Y. Wnt5a promotes ewing sarcoma cell migration through upregulating CXCR4 expression. BMC Cancer. 2012;12:480. doi: 10.1186/1471-2407-12-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teixido J., Martinez-Moreno M., Diaz-Martinez M., Sevilla-Movilla S. The good and bad faces of the CXCR4 chemokine receptor. Int. J. Biochem. Cell Biol. 2018;95:121–131. doi: 10.1016/j.biocel.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Meng X., Wuyi L., Yuhong X., Xinming C. Expression of CXCR4 in oral squamous cell carcinoma: correlations with clinicopathology and pivotal role of proliferation. J. Oral Pathol. Med. 2010;39(1):63–68. doi: 10.1111/j.1600-0714.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 23.Kodama J., Hasengaowa K.T., Seki N., Matsuo T., Ojima Y. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann. Oncol. 2007;18(1):70–76. doi: 10.1093/annonc/mdl342. [DOI] [PubMed] [Google Scholar]
- 24.Han M., Lv S., Zhang Y., Yi R., Huang B., Fu H. The prognosis and clinicopathology of CXCR4 in gastric cancer patients: a meta-analysis. Tumour Biol. 2014;35(5):4589–4597. doi: 10.1007/s13277-013-1603-4. [DOI] [PubMed] [Google Scholar]
- 25.Lv S., Yang Y., Kwon S., Han M., Zhao F., Kang H. The association of CXCR4 expression with prognosis and clinicopathological indicators in colorectal carcinoma patients: a meta-analysis. Histopathology. 2014;64(5):701–712. doi: 10.1111/his.12321. [DOI] [PubMed] [Google Scholar]
- 26.Ghanem I., Riveiro M.E., Paradis V., Faivre S., de Parga P.M., Raymond E. Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. Am. J. Transl. Res. 2014;6(4):340–352. [PMC free article] [PubMed] [Google Scholar]
- 27.Liang J.J., Zhu S., Bruggeman R., Zaino R.J., Evans D.B., Fleming J.B. High levels of expression of human stromal cell-derived factor-1 are associated with worse prognosis in patients with stage II pancreatic ductal adenocarcinoma. Cancer Epidemiol. Biomarkers Prev. 2010;19(10):2598–2604. doi: 10.1158/1055-9965.EPI-10-0405. [DOI] [PubMed] [Google Scholar]
- 28.Werner T.A., Forster C.M., Dizdar L., Verde P.E., Raba K., Schott M. CXCR4/CXCR7/CXCL12-axis in follicular thyroid carcinoma. J. Cancer. 2018;9(6):929–940. doi: 10.7150/jca.23042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obermajer N., Muthuswamy R., Odunsi K., Edwards R.P., Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71(24):7463–7470. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Y., Shi X., Shu Z., Xie T., Huang K., Wei L. Stromal cell-derived factor-1 (SDF-1)/CXCR4 axis enhances cellular invasion in ovarian carcinoma cells via integrin beta1 and beta3 expressions. Oncol. Res. 2013;21(4):217–225. doi: 10.3727/096504014X13907540404879. [DOI] [PubMed] [Google Scholar]
- 31.Hirata H., Hinoda Y., Kikuno N., Kawamoto K., Dahiya A.V., Suehiro Y. CXCL12 G801A polymorphism is a risk factor for sporadic prostate cancer susceptibility. Clin. Cancer Res. 2007;13(17):5056–5062. doi: 10.1158/1078-0432.CCR-07-0859. [DOI] [PubMed] [Google Scholar]
- 32.Gangadhar T., Nandi S., Salgia R. The role of chemokine receptor CXCR4 in lung cancer. Cancer Biol. Ther. 2010;9(6):409–416. doi: 10.4161/cbt.9.6.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corro C., Moch H. Biomarker discovery for renal cancer stem cells. J. Pathol. Clin. Res. 2018;4(1):3–18. doi: 10.1002/cjp2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan J., Mestas J., Burdick M.D., Phillips R.J., Thomas G.V., Reckamp K. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol. Cancer. 2006;5:56. doi: 10.1186/1476-4598-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu F., Lang R., Wei J., Fan Y., Cui L., Gu F. Increased expression of SDF-1/CXCR4 is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Histopathology. 2009;54(6):741–750. doi: 10.1111/j.1365-2559.2009.03289.x. [DOI] [PubMed] [Google Scholar]
- 36.Moosavi S.R., Khorramdelazad H., Amin M., Fatahpoor S., Moogooei M., Karimabad M.N. The SDF-1 3′A genetic variation is correlated with elevated intra-tumor tissue and circulating concentration of CXCL12 in glial tumors: a study on Iranian anaplastic astrocytoma and glioblastoma multiforme patients. J. Mol. Neurosci. 2013;50(2):298–304. doi: 10.1007/s12031-013-9954-2. [DOI] [PubMed] [Google Scholar]
- 37.Ping Y.F., Yao X.H., Chen J.H., Liu H., Chen D.L., Zhou X.D. The anti-cancer compound nordy inhibits CXCR4-mediated production of IL-8 and VEGF by malignant human glioma cells. J. Neurooncol. 2007;84(1):21–29. doi: 10.1007/s11060-007-9349-8. [DOI] [PubMed] [Google Scholar]
- 38.Scala S., Ottaiano A., Ascierto P.A., Cavalli M., Simeone E., Giuliano P. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin. Cancer Res. 2005;11(5):1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 39.Konoplev S., Rassidakis G.Z., Estey E., Kantarjian H., Liakou C.I., Huang X. Overexpression of CXCR4 predicts adverse overall and event-free survival in patients with unmutated FLT3 acute myeloid leukemia with normal karyotype. Cancer. 2007;109(6):1152–1156. doi: 10.1002/cncr.22510. [DOI] [PubMed] [Google Scholar]
- 40.Guo F., Wang Y., Liu J., Mok S.C., Xue F., Zhang W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2016;35(7):816–826. doi: 10.1038/onc.2015.139. [DOI] [PubMed] [Google Scholar]
- 41.Espinoza-Sanchez N.A., Enciso J., Pelayo R., Fuentes-Panana E.M. An NFkappaB-dependent mechanism of tumor cell plasticity and lateral transmission of aggressive features. Oncotarget. 2018;9(42):26679–26700. doi: 10.18632/oncotarget.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domanska U.M., Kruizinga R.C., Nagengast W.B., Timmer-Bosscha H., Huls G., de Vries E.G. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur. J. Cancer. 2013;49(1):219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Cojoc M., Peitzsch C., Trautmann F., Polishchuk L., Telegeev G.D., Dubrovska A. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. Oncol. Targets Ther. 2013;6:1347–1361. doi: 10.2147/OTT.S36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mammadova-Bach E., Rupp T., Spenle C., Jivkov I., Shankaranarayanan P., Klein A. Laminin alpha1 orchestrates VEGFA functions in the ecosystem of colorectal carcinoma. Biol. Cell. 2018 doi: 10.1111/boc.201800007. [DOI] [PubMed] [Google Scholar]
- 45.Scarlett K.A., White E.Z., Coke C.J., Carter J.R., Bryant L.K., Hinton C.V. Agonist-induced CXCR4 and CB2 heterodimerization inhibits Galpha13/rhoa-mediated migration. Mol. Cancer Res. 2018;16(4):728–739. doi: 10.1158/1541-7786.MCR-16-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kucia M., Reca R., Miekus K., Wanzeck J., Wojakowski W., Janowska-Wieczorek A. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7):879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 47.Furusato B., Mohamed A., Uhlen M., Rhim J.S. CXCR4 and cancer. Pathol. Int. 2010;60(7):497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J., Liu C., Mo X., Shi H., Li S. Mechanisms by which CXCR4/CXCL12 cause metastatic behavior in pancreatic cancer. Oncol. Lett. 2018;15(2):1771–1776. doi: 10.3892/ol.2017.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo M., Cai C., Zhao G., Qiu X., Zhao H., Ma Q. Hypoxia promotes migration and induces CXCR4 expression via HIF-1alpha activation in human osteosarcoma. PLoS One. 2014;9(3):e90518. doi: 10.1371/journal.pone.0090518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh Y.S., Kim H.Y., Song I.C., Yun H.J., Jo D.Y., Kim S. Hypoxia induces CXCR4 expression and biological activity in gastric cancer cells through activation of hypoxia-inducible factor-1alpha. Oncol. Rep. 2012;28(6):2239–2246. doi: 10.3892/or.2012.2063. [DOI] [PubMed] [Google Scholar]
- 51.Phillips R.J., Mestas J., Gharaee-Kermani M., Burdick M.D., Sica A., Belperio J.A. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J. Biol. Chem. 2005;280(23):22473–22481. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- 52.Zhao X.P., Huang Y.Y., Huang Y., Lei P., Peng J.L., Wu S. Transforming growth factor-beta1 upregulates the expression of CXC chemokine receptor 4 (CXCR4) in human breast cancer MCF-7 cells. Acta Pharmacol. Sin. 2010;31(3):347–354. doi: 10.1038/aps.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gatti M., Pattarozzi A., Bajetto A., Wurth R., Daga A., Fiaschi P. Inhibition of CXCL12/CXCR4 autocrine/paracrine loop reduces viability of human glioblastoma stem-like cells affecting self-renewal activity. Toxicology. 2013;314(2–3):209–220. doi: 10.1016/j.tox.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Kim C.G., Lee H., Gupta N., Ramachandran S., Kaushik I., Srivastava S. Role of Forkhead Box Class O proteins in cancer progression and metastasis. Semin. Cancer Biol. 2018;50:142–151. doi: 10.1016/j.semcancer.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng Y., Wang X., Yin B., Xia G., Shen Z., Gu W. Role of the stromal cell derived factor-1/CXC chemokine receptor 4 axis in the invasion and metastasis of lung cancer and mechanism. J. Thorac. Dis. 2017;9(12):4947–4959. doi: 10.21037/jtd.2017.10.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.English E.J., Mahn S.A., Marchese A. Endocytosis is required for CXC chemokine receptor type 4 (CXCR4)-mediated AKT activation and antiapoptotic signaling. J. Biol. Chem. 2018;293(29):11470–11480. doi: 10.1074/jbc.RA118.001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang Z., Bian X., Shim H. Inhibition of breast cancer metastasis with microRNA-302a by downregulation of CXCR4 expression. Breast Cancer Res. Treat. 2014;146(3):535–542. doi: 10.1007/s10549-014-3053-0. [DOI] [PubMed] [Google Scholar]
- 58.Shen P.F., Chen X.Q., Liao Y.C., Chen N., Zhou Q., Wei Q. MicroRNA-494-3p targets CXCR4 to suppress the proliferation, invasion, and migration of prostate cancer. Prostate. 2014;74(7):756–767. doi: 10.1002/pros.22795. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Yan L., Zhang L., Xu H., Chen T., Li Y. NT21MP negatively regulates paclitaxel-resistant cells by targeting miR1553p and miR155-5p via the CXCR4 pathway in breast cancer. Int. J. Oncol. 2018 doi: 10.3892/ijo.2018.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu G., Liu X., Su Y., Kong F., Hong X., Lin Z. Knockdown of urothelial carcinoma associated 1 suppressed cell growth and migration through regulating miR-301a and CXCR4 in osteosarcoma MHCC97 cells. Oncol. Res. 2018 doi: 10.3727/096504018X15201143705855. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Willenbacher W., Seeber A., Steiner N., Willenbacher E., Gatalica Z., Swensen J. Towards molecular profiling in multiple myeloma: a literature review and early indications of its efficacy for informing treatment strategies. Int. J. Mol. Sci. 2018;19(7) doi: 10.3390/ijms19072087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peled A., Klein S., Beider K., Burger J.A., Abraham M. Role of CXCL12 and CXCR4 in the pathogenesis of hematological malignancies. Cytokine. 2018;109:11–16. doi: 10.1016/j.cyto.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 63.Anderson K.C., Carrasco R.D. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249–274. doi: 10.1146/annurev-pathol-011110-130249. [DOI] [PubMed] [Google Scholar]
- 64.Manier S., Sacco A., Leleu X., Ghobrial I.M., Roccaro A.M. Bone marrow microenvironment in multiple myeloma progression. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/157496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coniglio S.J. Role of tumor-derived chemokines in osteolytic bone metastasis. Front Endocrinol. (Lausanne) 2018;9:313. doi: 10.3389/fendo.2018.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alsayed Y., Ngo H., Runnels J., Leleu X., Singha U.K., Pitsillides C.M. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109(7):2708–2717. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paiva B., Corchete L.A., Vidriales M.B., Puig N., Maiso P., Rodriguez I. Phenotypic and genomic analysis of multiple myeloma minimal residual disease tumor cells: a new model to understand chemoresistance. Blood. 2016;127(15):1896–1906. doi: 10.1182/blood-2015-08-665679. [DOI] [PubMed] [Google Scholar]
- 68.K Pandey M. Targeting CXCL12/CXCR4 axis in multiple myeloma. J. Hematol. Thromboembol. Diseases. 2014;02(05) [Google Scholar]
- 69.Azab A.K., Hu J., Quang P., Azab F., Pitsillides C., Awwad R. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119(24):5782–5794. doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim S.W., Kim H.Y., Lee H.J., Yun H.J., Kim S., Jo D.Y. Dexamethasone and hypoxia upregulate CXCR4 expression in myeloma cells. Leuk. Lymphoma. 2009;50(7):1163–1173. doi: 10.1080/10428190902893801. [DOI] [PubMed] [Google Scholar]
- 71.Kim S.-W., Kim H.-Y., Lee H.-J., Yun H.-J., Kim S., Jo D.-Y. Mechanisms regulating CXCR4 expression in myeloma cells. Blood. 2007;110(11) 47–5154. [Google Scholar]
- 72.Vandyke K., Zeissig M.N., Hewett D.R., Martin S.K., Mrozik K.M., Cheong C.M. HIF-2alpha promotes dissemination of plasma cells in multiple myeloma by regulating CXCL12/CXCR4 and CCR1. Cancer Res. 2017;77(20):5452–5463. doi: 10.1158/0008-5472.CAN-17-0115. [DOI] [PubMed] [Google Scholar]
- 73.Klasen C., Ohl K., Sternkopf M., Shachar I., Schmitz C., Heussen N. MIF promotes b cell chemotaxis through the receptors CXCR4 and CD74 and ZAP-70 signaling. J. Immunol. 2014;192(11):5273–5284. doi: 10.4049/jimmunol.1302209. [DOI] [PubMed] [Google Scholar]
- 74.Rajasekaran D., Groning S., Schmitz C., Zierow S., Drucker N., Bakou M. Macrophage migration inhibitory factor-CXCR4 receptor Interactions: evidence for partial allosteric agonism in comparison with CXCL12 chemokine. J. Biol. Chem. 2016;291(30):15881–15895. doi: 10.1074/jbc.M116.717751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng Y., Wang Q., Li T., Qian J., Lu Y., Li Y. Role of myeloma-derived MIF in myeloma cell adhesion to bone marrow and chemotherapy response. J. Natl. Cancer Inst. 2016;108(11) doi: 10.1093/jnci/djw131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conley-LaComb M.K., Semaan L., Singareddy R., Li Y., Heath E.I., Kim S. Pharmacological targeting of CXCL12/CXCR4 signaling in prostate cancer bone metastasis. Mol. Cancer. 2016;15(1):68. doi: 10.1186/s12943-016-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balabanian K., Lagane B., Infantino S., Chow K.Y., Harriague J., Moepps B. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 2005;280(42):35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 78.Azab A.K., Sahin I., Moschetta M., Mishima Y., Burwick N., Zimmermann J. CXCR7-dependent angiogenic mononuclear cell trafficking regulates tumor progression in multiple myeloma. Blood. 2014;124(12):1905–1914. doi: 10.1182/blood-2014-02-558742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kometani K., Kurosaki T. Differentiation and maintenance of long-lived plasma cells. Curr. Opin. Immunol. 2015;33:64–69. doi: 10.1016/j.coi.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 80.Waldschmidt J.M., Simon A., Wider D., Muller S.J., Follo M., Ihorst G. CXCL12 and CXCR7 are relevant targets to reverse cell adhesion-mediated drug resistance in multiple myeloma. Br. J. Haematol. 2017;179(1):36–49. doi: 10.1111/bjh.14807. [DOI] [PubMed] [Google Scholar]
- 81.Hideshima T., Chauhan D., Hayashi T., Podar K., Akiyama M., Gupta D. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol. Cancer Ther. 2002;1(7):539–544. [PubMed] [Google Scholar]
- 82.Parmo-Cabanas M., Bartolome R.A., Wright N., Hidalgo A., Drager A.M., Teixido J. Integrin alpha4beta1 involvement in stromal cell-derived factor-1alpha-promoted myeloma cell transendothelial migration and adhesion: role of cAMP and the actin cytoskeleton in adhesion. Exp. Cell Res. 2004;294(2):571–580. doi: 10.1016/j.yexcr.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Follin-Arbelet V., Hofgaard P.O., Hauglin H., Naderi S., Sundan A., Blomhoff R. Cyclic AMP induces apoptosis in multiple myeloma cells and inhibits tumor development in a mouse myeloma model. BMC Cancer. 2011;11:301. doi: 10.1186/1471-2407-11-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jung O., Trapp-Stamborski V., Purushothaman A., Jin H., Wang H., Sanderson R.D. Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins. Oncogenesis. 2016;5:e202. doi: 10.1038/oncsis.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colombo M., Galletti S., Garavelli S., Platonova N., Paoli A., Basile A. Notch signaling deregulation in multiple myeloma: a rational molecular target. Oncotarget. 2015;6(29):26826–26840. doi: 10.18632/oncotarget.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirbe A.C., Rubin J., Uluckan O., Morgan E.A., Eagleton M.C., Prior J.L. Disruption of CXCR4 enhances osteoclastogenesis and tumor growth in bone. Proc. Natl. Acad. Sci. USA. 2007;104(35):14062–14067. doi: 10.1073/pnas.0705203104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yaccoby S. Two states of myeloma stem cells. Clin. Lymph. Myeloma. Leuk. 2018;18(1):38–43. doi: 10.1016/j.clml.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Terpos E., Ntanasis-Stathopoulos I., Gavriatopoulou M., Dimopoulos M.A. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8(1):7. doi: 10.1038/s41408-017-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Samant S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014;37:131–141. doi: 10.1016/j.matbio.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giuliani N., Colla S., Sala R., Moroni M., Lazzaretti M., La Monica S. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa b ligand (RANKL) in t lymphocytes: a potential role in multiple myeloma bone disease. Blood. 2002;100(13):4615–4621. doi: 10.1182/blood-2002-04-1121. [DOI] [PubMed] [Google Scholar]
- 91.Ooi L.L., Dunstan C.R. CXCL12/CXCR4 axis in tissue targeting and bone destruction in cancer and multiple myeloma. J. Bone Miner Res. 2009;24(7):1147–1149. doi: 10.1359/jbmr.090503. [DOI] [PubMed] [Google Scholar]
- 92.Marchica V., Accardi F., Storti P., Mancini C., Martella E., Dalla Palma B. Cutaneous localization in multiple myeloma in the context of bortezomib-based treatment: how do myeloma cells escape from the bone marrow to the skin? Int. J. Hematol. 2016;105(1):104–108. doi: 10.1007/s12185-016-2104-1. [DOI] [PubMed] [Google Scholar]
- 93.Langley R.R., Fidler I.J. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer. 2011;128(11):2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghobrial I.M. Myeloma as a model for the process of metastasis: implications for therapy. Blood. 2012;120(1):20–30. doi: 10.1182/blood-2012-01-379024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roccaro A.M., Mishima Y., Sacco A., Moschetta M., Tai Y.T., Shi J. CXCR4 regulates extra-medullary myeloma through epithelial-mesenchymal-transition-like transcriptional activation. Cell Rep. 2015;12(4):622–635. doi: 10.1016/j.celrep.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azab A.K., Quang P., Azab F., Pitsillides C., Thompson B., Chonghaile T. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood. 2012;119(6):1468–1478. doi: 10.1182/blood-2011-07-368050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sisay M., Mengistu G., Edessa D. The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: potential targets for anticancer therapy. Onco. Targets Ther. 2017;10:3801–3810. doi: 10.2147/OTT.S135867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zdzisinska B., Kandefer-Szerszen M. The role of RANK/RANKL and OPG in multiple myeloma. Postepy Hig. Med. Dosw. (Online) 2006;60:471–482. [PubMed] [Google Scholar]
- 99.Benslimane-Ahmim Z., Pereira J., Lokajczyk A., Dizier B., Galy-Fauroux I., Fischer A.M. Osteoprotegerin regulates cancer cell migration through SDF-1/CXCR4 axis and promotes tumour development by increasing neovascularization. Cancer Lett. 2017;395:11–19. doi: 10.1016/j.canlet.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 100.Reisenbuckler C. Multiple myeloma and diagnostic imaging. Radiol. Technol. 2014;85(4):391–410. quiz 1-3. [PubMed] [Google Scholar]
- 101.Gomis R.R., Gawrzak S. Tumor cell dormancy. Mol. Oncol. 2016 doi: 10.1016/j.molonc.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kikuchi J., Kuroda Y., Koyama D., Osada N., Izumi T., Yasui H. Myeloma cells are activated in bone marrow microenvironment by the CD180/MD-1 complex, which senses lipopolysaccharide. Cancer Res. 2018;78(7):1766–1778. doi: 10.1158/0008-5472.CAN-17-2446. [DOI] [PubMed] [Google Scholar]
- 103.Lawson M.A., McDonald M.M., Kovacic N., Hua Khoo W., Terry R.L., Down J. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 2015;6:8983. doi: 10.1038/ncomms9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gelmini S., Mangoni M., Serio M., Romagnani P., Lazzeri E. The critical role of SDF-1/CXCR4 axis in cancer and cancer stem cells metastasis. J. Endocrinol. Invest. 2008;31(9):809–819. doi: 10.1007/BF03349262. [DOI] [PubMed] [Google Scholar]
- 105.Blade J., Fernandez de Larrea C., Rosinol L. Extramedullary disease in multiple myeloma in the era of novel agents. Br. J. Haematol. 2015;169(6):763–765. doi: 10.1111/bjh.13384. [DOI] [PubMed] [Google Scholar]
- 106.Stessman H.A., Mansoor A., Zhan F., Janz S., Linden M.A., Baughn L.B. Reduced CXCR4 expression is associated with extramedullary disease in a mouse model of myeloma and predicts poor survival in multiple myeloma patients treated with bortezomib. Leukemia. 2013;27(10):2075–2077. doi: 10.1038/leu.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuji Mishima M.M., Reagan M.R., Zhang Y., Sahin I., Maulkhan N., Roccaro A.M., Ghobrial I.M. CXCR4 is a regulator of disease involvement of extramedullary myeloma confirmed by a novel mouse model for extramedullary disease. Blood. 2013;122(5320) [Google Scholar]
- 108.Roccaro A.M., Sacco A., Moschetta M., Mishima Y., Maiso P., Huynh D., Chiarini M., Cardarelli P.M., Cohen L., Kuhne M., Ghobrial I.M. Novel CXCR4-Targeted therapy to inhibit multiple myeloma bone dissemination. Blood. 2014;124:4709. [Google Scholar]
- 109.Keane N.A., Reidy M., Natoni A., Raab M.S., O'Dwyer M. Targeting the PIM kinases in multiple myeloma. Blood Cancer J. 2015;5:e325. doi: 10.1038/bcj.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Solimando A.G., Brandl A., Mattenheimer K., Graf C., Ritz M., Ruckdeschel A. JAM-A as a prognostic factor and new therapeutic target in multiple myeloma. Leukemia. 2018;32(3):736–743. doi: 10.1038/leu.2017.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang C.H., Hale S.J., Cox C.V., Blair A., Kronsteiner B., Grabowska R. Junctional adhesion molecule – a is highly expressed on human hematopoietic repopulating cells and associates with the key hematopoietic chemokine receptor CXCR4. Stem Cells. 2016;34(6):1664–1678. doi: 10.1002/stem.2340. [DOI] [PubMed] [Google Scholar]
- 112.Turner J.G., Dawson J.L., Grant S., Shain K.H., Dalton W.S., Dai Y. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J. Hematol. Oncol. 2016;9(1):73. doi: 10.1186/s13045-016-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu S.H., Gu Y., Pascual B., Yan Z., Hallin M., Zhang C. A novel CXCR4 antagonist IgG1 antibody (PF-06747143) for the treatment of hematologic malignancies. Blood Adv. 2017;1(15):1088–1100. doi: 10.1182/bloodadvances.2016003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Valentina Marchica F.A., Storti P., Mancini C., Martella E., Bolzoni M., Todoerti K., Schifano C., Bonomini S., Palma B.D., Marcatti M., Ponzoni M., Neri A., Aversa F., Giuliani N. The myeloma cells escape from bone marrow to skin extramedullary localization upon bortezomib resistance: role of CXCR4. Blood. 2015;126(5315) [Google Scholar]
- 115.Nass J., Efferth T. Drug targets and resistance mechanisms in multiple myeloma. Cancer Drug Resist. 2018 [Google Scholar]
- 116.Ro T.B., Holien T., Fagerli U.M., Hov H., Misund K., Waage A. HGF and IGF-1 synergize with SDF-1alpha in promoting migration of myeloma cells by cooperative activation of p21-activated kinase. Exp. Hematol. 2013;41(7):646–655. doi: 10.1016/j.exphem.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 117.Furukawa Y., Kikuchi J. Epigenetic mechanisms of cell adhesion-mediated drug resistance in multiple myeloma. Int. J. Hematol. 2016;104(3):281–292. doi: 10.1007/s12185-016-2048-5. [DOI] [PubMed] [Google Scholar]
- 118.Tsubaki M., Takeda T., Yoshizumi M., Ueda E., Itoh T., Imano M. RANK-RANKL interactions are involved in cell adhesion-mediated drug resistance in multiple myeloma cell lines. Tumour Biol. 2016;37(7):9099–9110. doi: 10.1007/s13277-015-4761-8. [DOI] [PubMed] [Google Scholar]
- 119.Ryu J., Koh Y., Park H., Kim D.Y., Kim D.C., Byun J.M. Highly expressed Integrin-alpha8 induces epithelial to mesenchymal transition-like features in multiple myeloma with early relapse. Mol. Cells. 2016;39(12):898–908. doi: 10.14348/molcells.2016.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Di Marzo L., Desantis V., Solimando A.G., Ruggieri S., Annese T., Nico B. Microenvironment drug resistance in multiple myeloma: emerging new players. Oncotarget. 2016;7(37):60698–60711. doi: 10.18632/oncotarget.10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nefedova Y., Sullivan D.M., Bolick S.C., Dalton W.S., Gabrilovich D.I. Inhibition of notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008;111(4):2220–2229. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 122.Abdi J., Chen G., Chang H. Drug resistance in multiple myeloma: latest findings and new concepts on molecular mechanisms. Oncotarget. 2013;4(12):2186–2207. doi: 10.18632/oncotarget.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roccaro A.M., Sacco A., Purschke W.G., Moschetta M., Buchner K., Maasch C. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 2014;9(1):118–128. doi: 10.1016/j.celrep.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fruehauf S., Ehninger G., Hubel K., Topaly J., Goldschmidt H., Ho A.D. Mobilization of peripheral blood stem cells for autologous transplant in non-Hodgkin's lymphoma and multiple myeloma patients by plerixafor and G-CSF and detection of tumor cell mobilization by PCR in multiple myeloma patients. Bone Marrow Transplant. 2010;45(2):269–275. doi: 10.1038/bmt.2009.142. [DOI] [PubMed] [Google Scholar]
- 125.Fruehauf S., Seeger T., Maier P., Li L., Weinhardt S., Laufs S. The CXCR4 antagonist AMD3100 releases a subset of G-CSF-primed peripheral blood progenitor cells with specific gene expression characteristics. Exp. Hematol. 2006;34(8):1052–1059. doi: 10.1016/j.exphem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 126.Fruehauf S., Veldwijk M.R., Seeger T., Schubert M., Laufs S., Topaly J. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11(8):992–1001. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- 127.Debnath B., Xu S., Grande F., Garofalo A., Neamati N. Small molecule inhibitors of CXCR4. Theranostics. 2013;3(1):47–75. doi: 10.7150/thno.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bouyssou J.M., Ghobrial I.M., Roccaro A.M. Targeting SDF-1 in multiple myeloma tumor microenvironment. Cancer Lett. 2016;380(1):315–318. doi: 10.1016/j.canlet.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 129.Azab A.K., Runnels J.M., Pitsillides C., Moreau A.S., Azab F., Leleu X. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kashyap M.K., Amaya-Chanaga C.I., Kumar D., Simmons B., Huser N., Gu Y. Targeting the CXCR4 pathway using a novel anti-CXCR4 IgG1 antibody (PF-06747143) in chronic lymphocytic leukemia. J. Hematol. Oncol. 2017;10(1):112. doi: 10.1186/s13045-017-0435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peled A., Abraham M., Avivi I., Rowe J.M., Beider K., Wald H. The high-affinity CXCR4 antagonist BKT140 is safe and induces a robust mobilization of human CD34+ cells in patients with multiple myeloma. Clin. Cancer Res. 2014;20(2):469–479. doi: 10.1158/1078-0432.CCR-13-1302. [DOI] [PubMed] [Google Scholar]
- 132.Beider K., Begin M., Abraham M., Wald H., Weiss I.D., Wald O. CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple myeloma tumor growth. Exp. Hematol. 2011;39(3):282–292. doi: 10.1016/j.exphem.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 133.Pilar de la Puente B.M., Azab F., Luderer M., Azab A.K. Molecularly targeted therapies in multiple myeloma. Leuk. Res. Treatment. 2014;2014(976567):1–8. doi: 10.1155/2014/976567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berenson J.R., Hilger J.D., Yellin O., Boccia R.V., Matous J., Dressler K. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Ann. Hematol. 2014;93(1):89–98. doi: 10.1007/s00277-013-1910-2. [DOI] [PubMed] [Google Scholar]
- 135.Richardson P.G., Hungria V.T., Yoon S.S., Beksac M., Dimopoulos M.A., Elghandour A. Panobinostat plus bortezomib and dexamethasone in previously treated multiple myeloma: outcomes by prior treatment. Blood. 2016;127(6):713–721. doi: 10.1182/blood-2015-09-665018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.San-Miguel J.F., Hungria V.T., Yoon S.S., Beksac M., Dimopoulos M.A., Elghandour A. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 137.San-Miguel J.F., Hungria V.T., Yoon S.S., Beksac M., Dimopoulos M.A., Elghandour A. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): a randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 2016;3(11):e506–ee15. doi: 10.1016/S2352-3026(16)30147-8. [DOI] [PubMed] [Google Scholar]
- 138.Kuhne M.R., Mulvey T., Belanger B., Chen S., Pan C., Chong C. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin. Cancer Res. 2013;19(2):357–366. doi: 10.1158/1078-0432.CCR-12-2333. [DOI] [PubMed] [Google Scholar]
- 139.Lonial S., Durie B., Palumbo A., San-Miguel J. Monoclonal antibodies in the treatment of multiple myeloma: current status and future perspectives. Leukemia. 2016;30(3):526–535. doi: 10.1038/leu.2015.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cardarelli M., Ghobrial I.M., Roccaro A.M. CXCR4 monoclonal antibody, BMS-936564 (MDX-1338), modulates epithelial to mesenchymal transition (EMT) in multiple myeloma cells. Blood. 2012;120(4009) [Google Scholar]
- 141.Broussas M., Boute N., Akla B., Berger S., Beau-Larvor C., Champion T. A new Anti-CXCR4 antibody that blocks the CXCR4/SDF-1 axis and mobilizes effector cells. Mol. Cancer Ther. 2016;15(8):1890–1899. doi: 10.1158/1535-7163.MCT-16-0041. [DOI] [PubMed] [Google Scholar]
- 142.Fouquet G., Guidez S., Richez V., Stoppa A.M., Le Tourneau C., Macro M. Phase i dose-escalation study of F50067, a humanized anti-CXCR4 monoclonal antibody alone and in combination with lenalidomide and low-dose dexamethasone, in relapsed or refractory multiple myeloma. Oncotarget. 2018;9(35):23890–23899. doi: 10.18632/oncotarget.25156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sheng-Bin Peng X.Z., Paul D., Kays L.M., Ye M., Vaillancourt P., Dowless M., Stancato L.F., Stewart J., Uhlik M.T., Long H., Chu S., Obungu V.H. Inhibition of CXCR4 by LY2624587, a fully humanized anti-CXCR4 antibody induces apoptosis of hematologic malignancies. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lapa C., Herrmann K., Schirbel A., Hanscheid H., Luckerath K., Schottelius M. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed multiple myeloma. Theranostics. 2017;7(6):1589–1597. doi: 10.7150/thno.19050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xue L.-J., Mao X.-B., Ren L.-L., Chu X.-Y. Inhibition of CXCL12/CXCR4 axis as a potential targeted therapy of advanced gastric carcinoma. Cancer Med. 2017;6(6):1424–1436. doi: 10.1002/cam4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oliveira A.M., M D., Metzger M., Linardi C., Giorgi R.R., Moura F., Martinez G.A., Bydlowski S.P., Novak E.M. Thalidomide treatment down-regulates SDF-1alpha and CXCR4 expression in multiple myeloma patients. Leuk Res. 2009;33(7):970–973. doi: 10.1016/j.leukres.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 147.Udi J., Schüler J., Wider D., Ihorst G., Catusse J., Waldschmidt J. Potentin vitroandin vivoactivity of sorafenib in multiple myeloma: induction of cell death, CD138-downregulation and inhibition of migration through actin depolymerization. Br. J. Haematol. 2013;161(1):104–116. doi: 10.1111/bjh.12226. [DOI] [PubMed] [Google Scholar]
- 148.Badr G., Lefevre E.A., Mohany M. Thymoquinone inhibits the CXCL12-induced chemotaxis of multiple myeloma cells and increases their susceptibility to Fas-mediated apoptosis. PLoS One. 2011;6(9):e23741. doi: 10.1371/journal.pone.0023741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zirafi O., Hermann P., Münch J. Proteolytic processing of human serum albumin generates EPI-X4, an endogenous antagonist of CXCR4. J. Leukoc. Biol. 2016;99(6):863–868. doi: 10.1189/jlb.2MR1115-521RR. [DOI] [PubMed] [Google Scholar]
- 150.Buske C., Kirchhoff F., Münch J. EPI-X4, a novel endogenous antagonist of CXCR4. Oncotarget. 2015;6(34):35137–35138. doi: 10.18632/oncotarget.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dugan M.J., Maziarz R.T., Bensinger W.I., Nademanee A., Liesveld J., Badel K. Safety and preliminary efficacy of plerixafor (Mozobil) in combination with chemotherapy and G-CSF: an open-label, multicenter, exploratory trial in patients with multiple myeloma and non-Hodgkin's lymphoma undergoing stem cell mobilization. Bone Marrow Transplant. 2010;45(1):39–47. doi: 10.1038/bmt.2009.119. [DOI] [PubMed] [Google Scholar]
- 152.Gayatri Setia N.H., Jalilizeinali B., Funkhouser S., Pierzchanowski L., Lan F., Gabig T.G., Kiner-Strachan B., Kelleher K., Hsu M.-C., Chang L.-W., Schuster M.W. A phase II, open-label pilot study to evaluate the hematopoietic stem cell mobilization of TG-0054 combined with G-CSF in 12 patients with multiple myeloma, Non-Hodgkin Lymphoma or Hodgkin Lymphoma – an Interim Analysis. Blood. 2015;126(515) [Google Scholar]
- 153.Rettig M.P., Ansstas G., DiPersio J.F. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26(1):34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Angelopoulou M.K., Tsirkinidis P., Boutsikas G., Vassilakopoulos T.P., Tsirigotis P. New insights in the mobilization of hematopoietic stem cells in lymphoma and multiple myeloma patients. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/835138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Imai Y., Ohta E., Takeda S., Sunamura S., Ishibashi M., Tamura H., Wang Y.H., Deguchi A., Tanaka J., Maru Y., Motoji T. Histone deacetylase inhibitor panobinostat induces calcineurin degradation in multiple myeloma. JCI Insight. 2016;21(5):e85061. doi: 10.1172/jci.insight.85061. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de Nigris F., Schiano C., Infante T., Napoli C. CXCR4 inhibitors: tumor vasculature and therapeutic challenges. Recent Pat. Anticancer Drug Discov. 2012;7(3):251–264. doi: 10.2174/157489212801820039. [DOI] [PubMed] [Google Scholar]
- 157.Zhang Y., Depond M., He L., Foudi A., Kwarteng E.O., Lauret E. CXCR4/CXCL12 axis counteracts hematopoietic stem cell exhaustion through selective protection against oxidative stress. Sci. Rep. 2016;6:37827. doi: 10.1038/srep37827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Karpova D., Bonig H. Concise Review: CXCR4/CXCL12 signaling in immature hematopoiesis–lessons from pharmacological and genetic models. Stem Cells. 2015;33(8):2391–2399. doi: 10.1002/stem.2054. [DOI] [PubMed] [Google Scholar]
- 159.de la Puente P., Muz B., Azab F., Luderer M., Azab A.K. Molecularly targeted therapies in multiple myeloma. Leuk. Res. Treatment. 2014;2014 doi: 10.1155/2014/976567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sierro F., Biben C., Martinez-Munoz L., Mellado M., Ransohoff R.M., Li M. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc. Natl. Acad. Sci. USA. 2007;104(37):14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.