In the title pyridazinone derivative, the unsubstituted phenyl ring and the pyridazine ring are inclined to each other, making a dihedral angle of 17.41 (13)°, whereas the Cl-substituted phenyl ring is nearly orthogonal to the pyridazine ring [88.19 (13)°], C21H19ClN2O3, contains one independent molecule. C—H⋯O hydrogen bonds, weak C—H⋯π and weak offset π–π stacking interactions stabilize the packing.
Keywords: crystal structure, pyridazine, Hirshfeld surface analysis
Abstract
The title pyridazinone derivative, C21H19ClN2O3, is not planar. The unsubstituted phenyl ring and the pyridazine ring are inclined to each other, making a dihedral angle of 17.41 (13)° whereas the Cl-substituted phenyl ring is nearly orthogonal to the pyridazine ring [88.19 (13)°]. In the crystal, C—H⋯O hydrogen bonds generate dimers with R 2 2(10) and R 2 2(24) ring motifs which are linked by C—H⋯O interactions, forming chains extending parallel to the c-axis direction. The intermolecular interactions were investigated using Hirshfeld surface analysis and two-dimensional fingerprint plots, revealing that the most significant contributions to the crystal packing are from H⋯H (44.5%), C⋯H/H⋯C (18.5%), H⋯O/H⋯O (15.6%), Cl⋯H/H⋯Cl (10.6%) and C⋯C (2.8%) contacts.
Chemical context
Pyridazines are an important family of six-membered aromatic heterocycles (Akhtar et al., 2016 ▸). The related compound pyridazinone is an important pharmacophore with a wide range of biological applications (Asif, 2015 ▸), and its chemistry has been studied for several decades. Pyridazinones are used as scaffolds for potential drug candidates (Dubey & Bhosle, 2015 ▸; Thakur et al., 2010 ▸) because of their significant potential as antimicrobial (Sönmez et al., 2006 ▸), antidepressant (Boukharsa et al., 2016 ▸), anti-inflammatory (Barberot et al., 2018 ▸), antihypertensive (Siddiqui et al., 2011 ▸), analgesic (Gökçe et al., 2009 ▸), anti-HIV (Livermore et al., 1993 ▸), anticonvulsant (Partap et al., 2018 ▸; Sharma et al., 2014 ▸), cardiotonic (Wang et al., 2008 ▸), antihistaminic (Tao et al., 2012 ▸), glucan synthase inhibitors (Zhou et al., 2011 ▸), phosphodiesterase (PDE) inhibitors (Ochiai et al., 2012 ▸) and herbicidal (Asif, 2013 ▸) agents.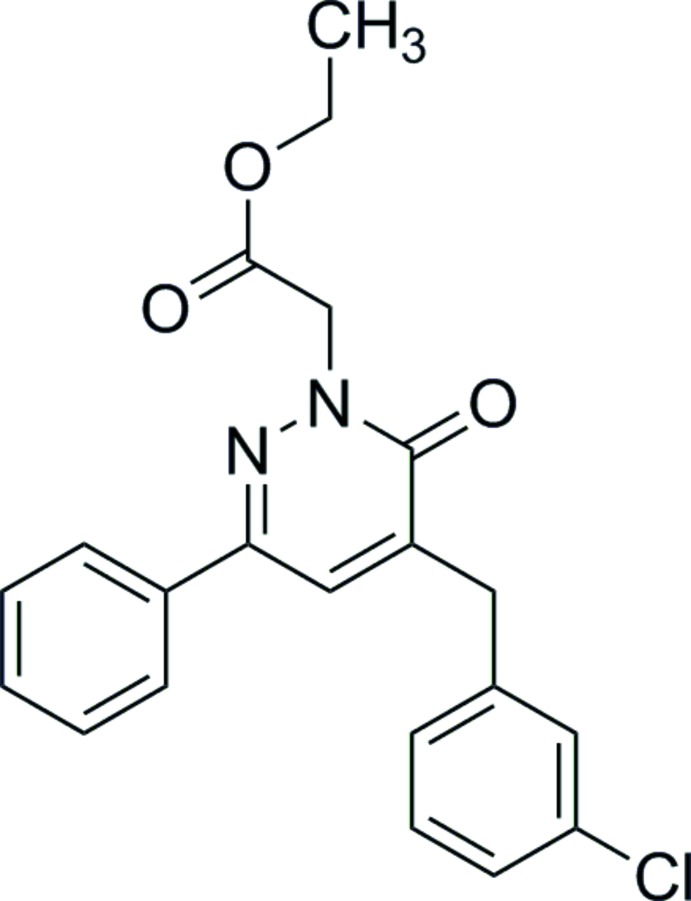
In this context and in a continuation of our work in this field (Chkirate et al., 2019a ▸,b ▸; Karrouchi et al., 2015 ▸, 2016a ▸,b ▸), we report herein the synthesis and the molecular and crystal structures of the title pyridazinone derivative, together with its Hirshfeld surface analysis.
Structural commentary
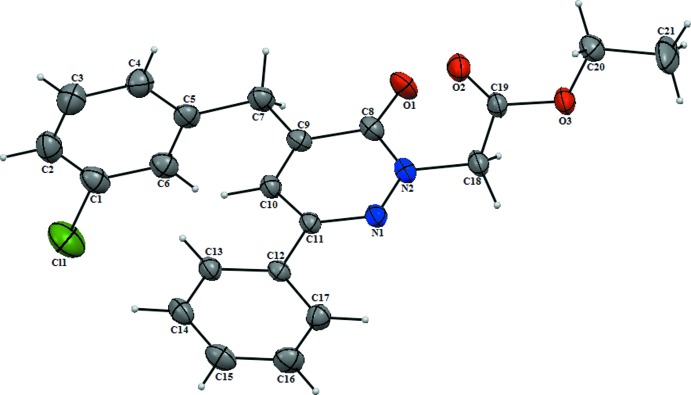
The molecule of the title compound is not planar (Fig. 1 ▸). The unsubstituted phenyl ring (C12–C17) and the pyridazine ring (C8–C11/N1/N2) are twisted relative to each other, making a dihedral angle of 17.41 (13)°; the chloro-substituted phenyl ring (C1–C6) is inclined to the pyridazine ring by 88.19 (13)°. Atoms C8 and N2 of the pyridazine ring show the largest deviations from planarity (root-mean-square deviation = 0.0236 Å) in positive and negative directions [C8 = 0.0357 (15) Å; N2 = −0.0319 (14) Å]. The O1=C8 bond length of the pyridazinone carbonyl function is 1.230 (3) Å, and the N1—N2 bond length in the pyridazine ring is 1.362 (2) Å, both in accordance with values for related pyridazinones.
Figure 1.
The molecular structure of the title compound with displacement ellipsoids drawn at the 50% probability level.
Supramolecular features
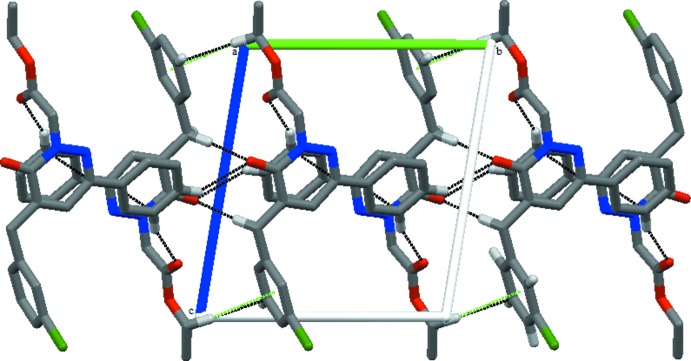
The crystal packing exhibits C—H⋯O hydrogen bonds between aryl or methylene groups and carbonyl O atoms (Table 1 ▸), as well as C—H⋯π interactions and van der Waals contacts. Intermolecular C7—H7B⋯O1 and C14—H14⋯O2 hydrogen bonds produce 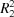 (10) and
(10) and 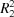 (24) motif rings (Fig. 2 ▸), supplemented by C15—H15⋯O1 contacts, forming chains extending parallel to the c axis (Fig. 2 ▸). A weak C20—H20B⋯Cg2 (−x + 1, −y, −z + 1; Cg2 is the centroid of the C1–C6 phenyl ring) contact is also present in this chain (Table 1 ▸; Fig. 2 ▸). Weak aromatic π–π stacking interactions between adjacent pyridazine rings [Cg1⋯Cg1(−x + 1, −y + 1, −z + 1) = 3.8833 (13) Å, where Cg1 is the centroid of the C8–C11/N1/N2 ring] along the a axis lead to the formation of a three-dimensional network.
(24) motif rings (Fig. 2 ▸), supplemented by C15—H15⋯O1 contacts, forming chains extending parallel to the c axis (Fig. 2 ▸). A weak C20—H20B⋯Cg2 (−x + 1, −y, −z + 1; Cg2 is the centroid of the C1–C6 phenyl ring) contact is also present in this chain (Table 1 ▸; Fig. 2 ▸). Weak aromatic π–π stacking interactions between adjacent pyridazine rings [Cg1⋯Cg1(−x + 1, −y + 1, −z + 1) = 3.8833 (13) Å, where Cg1 is the centroid of the C8–C11/N1/N2 ring] along the a axis lead to the formation of a three-dimensional network.
Table 1. Hydrogen-bond geometry (Å, °).
Cg2 is the centroid of the C1–C6 phenyl ring
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C14—H14⋯O2i | 0.93 | 2.53 | 3.416 (3) | 160 |
| C7—H7B⋯O1ii | 0.97 | 2.54 | 3.485 (3) | 164 |
| C15—H15⋯O1iii | 0.93 | 2.66 | 3.474 (3) | 147 |
| C20—H20B⋯Cg2iv | 0.97 | 2.81 | 3.759 (3) | 165 |
Symmetry codes: (i) 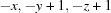 ; (ii)
; (ii) 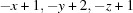 ; (iii)
; (iii) 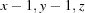 ; (iv)
; (iv) 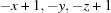 .
.
Figure 2.
A view along the a axis of the crystal structure of the title compound. Black dashed lines symbolize intermolecular C—H⋯O hydrogen bonds; C—H⋯π interactions are shown as green dashes lines.
Database survey
A search of the Cambridge Structural Database (CSD, version 5.40, update November 2018; Groom et al., 2016 ▸) revealed two structures containing a similar pyridazinone moiety as in the title structure but with different substituents, viz. 4-benzyl-6-p-tolylpyridazin-3(2H)-one (YOTVIN; Oubair et al., 2009 ▸) and ethyl 3-methyl-6-oxo-5-(3-(trifluoromethyl)phenyl)-1,6-dihydro-1-pyridazineacetate (QANVOR; Xu et al., 2005 ▸). In the crystal structure of YOTVIN, the molecules are connected two-by-two through N—H⋯O hydrogen bonds with an 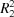 (8) graph-set motif, forming dimers arranged around an inversion center. Weak C—H⋯O hydrogen bonds and weak offset π–π stacking stabilize the packing. In the crystal structure of QANVOR, the phenyl and pyridazinone rings are approximately co-planar, making a dihedral angle of 4.84 (13)°. Centrosymmetrically related molecules form dimers through non-classical intermolecular C—H⋯O hydrogen bonds.
(8) graph-set motif, forming dimers arranged around an inversion center. Weak C—H⋯O hydrogen bonds and weak offset π–π stacking stabilize the packing. In the crystal structure of QANVOR, the phenyl and pyridazinone rings are approximately co-planar, making a dihedral angle of 4.84 (13)°. Centrosymmetrically related molecules form dimers through non-classical intermolecular C—H⋯O hydrogen bonds.
Hirshfeld surface analysis
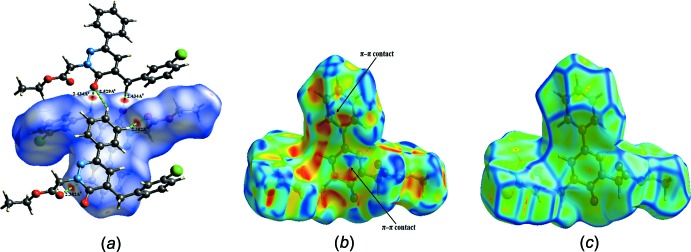
A Hirshfeld surface analysis (Spackman & Jayatilaka, 2009 ▸) and the associated two-dimensional fingerprint plots (McKinnon et al., 2007 ▸) were performed with CrystalExplorer17 (Turner et al., 2017 ▸), using a standard (high) surface resolution with the three-dimensional d norm surfaces plotted over a fixed colour scale of −0.1647 (red) to 1.1730 (blue) a.u. The three-dimensional d norm surface of the title molecule is illustrated in Fig. 3 ▸ a. The pale-red spots symbolize short contacts and negative d norm values on the surface and correspond to the C—H⋯O interactions (Table 1 ▸).
Figure 3.
(a) d norm mapped on the Hirshfeld surface for visualizing the intermolecular interactions, (b) shape-index map of the title compound and (c) curvedness map of the title compound.
The shape-index map of the title molecule was generated in the range −1 to 1 Å (Fig. 3 ▸ b). The convex blue regions symbolize hydrogen-donor groups and the concave red regions hydrogen-acceptor groups. π–π interactions are generally indicated by adjacent red and blue triangles in the shape-index map, as is the case for the title molecule.
The curvedness map of the title complex was generated in the range −4.0 to 0.4 Å (Fig. 3 ▸ c). The curvedness plot of the title complex shows large regions of green with a relatively flat (i.e. planar) surface area, indicating the presence of π–π stacking interactions, while the blue regions demonstrate areas of curvature.
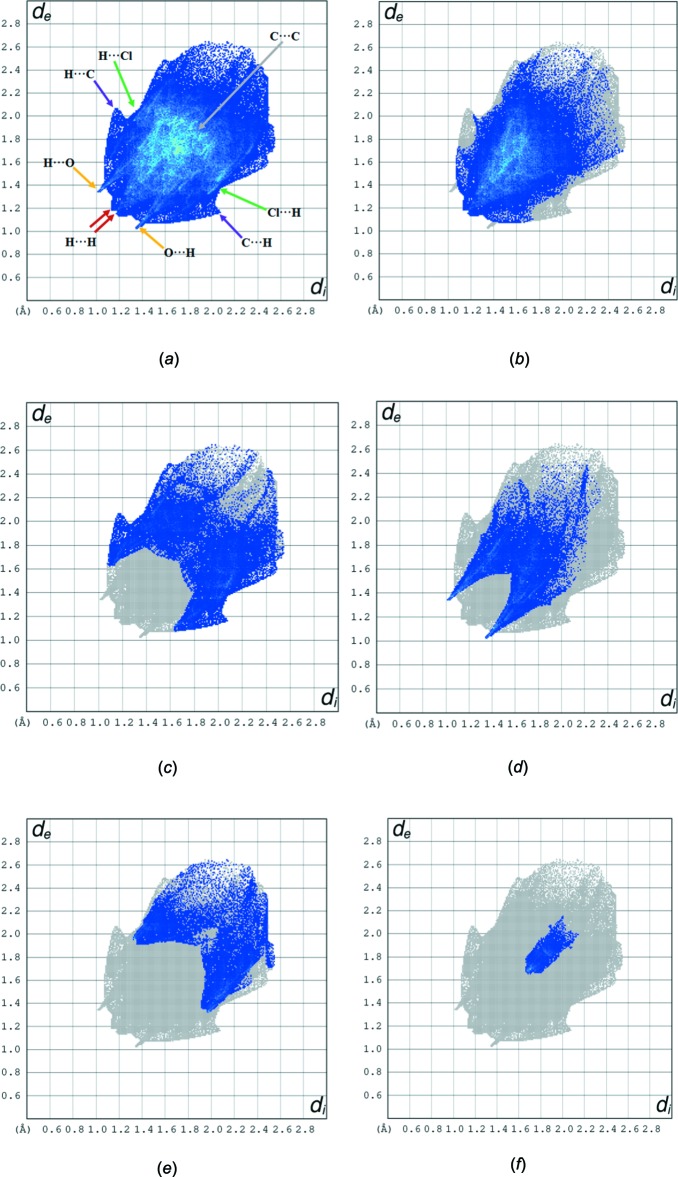
The overall two-dimensional fingerprint plot is illustrated in Fig. 4 ▸ a, delineated into H⋯H, H⋯C/ C⋯H, H⋯O/O⋯H, H⋯Cl/Cl⋯H, C⋯C contacts associated with their relative contributions to the Hirshfeld surface in Fig. 4 ▸ b–f, respectively. The most important intermolecular interaction is H⋯H, contributing 44.5% to the overall crystal packing, with the centre of the peak d e = d i = 1.18 Å (Fig. 4 ▸ b). H⋯C/ C⋯H contacts, with a 18.5% contribution to the Hirshfeld surface, indicate the presence of the weak C—H⋯π interaction (Table 1 ▸). Two pairs of characteristic wings in the fingerprint plot with pairs of tips at d e + d i ∼2.8 Å are present (Fig. 4 ▸ c). H⋯O/O⋯H contacts arising from intermolecular C—H⋯O hydrogen bonding make a 15.6% contribution to the Hirshfeld surface and are represented by a pair of sharp spikes in the region d e + d i ∼2.35 Å The C⋯C contacts are a measure of π–\p stacking interactions and contribute 2.8% of the Hirshfeld surface. They appear as an arrow-shaped distribution at d e + d i ∼3.3 Å. Another contact to the Hirshfeld surface is from H⋯Cl/Cl⋯H interactions (10.6%).
Figure 4.
(a) The overall two-dimensional fingerprint plot, and delineated into (b) H⋯H, (c) H⋯C/C⋯H, (d) H⋯O/O⋯H, (e) H⋯Cl/Cl⋯H and (f) C⋯C interactions.
Synthesis and crystallization
To a solution (0.99 g, 3 mmol) of 4-(3-dichlorobenzyl)-6-phenylpyridazin-3(2H)-one in 30 ml of tetrahydrofuran (THF), potassium carbonate (0.5 g, 3.5 mmol) was added. The mixture was refluxed for 1 h. After cooling, ethyl bromoacetate (0.66 g, 4 mmol) was added and the mixture was refluxed for 8 h. The precipitated material was removed by filtration and the solvent evaporated under vacuum. The residue was purified through silica gel column chromatography using hexane/ethyl acetate (4:6 v/v). Slow evaporation at room temperature led to formation of single crystals with a yield of 70%.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. Hydrogen atoms were fixed geometrically and treated as riding, with C—H = 0.97 Å for methyl [U iso(H) = 1.2U eq(C)], C—H = 0.96 Å for methylene [U iso(H) = 1.5U eq(C)], C—H = 0.93 Å for aromatic [U iso(H) = 1.2U eq(C)] and C—H = 0.98 Å for methine [U iso(H) = 1.2U eq(C)] H atoms.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C21H19ClN2O3 |
| M r | 382.83 |
| Crystal system, space group | Triclinic, P
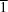
|
| Temperature (K) | 296 |
| a, b, c (Å) | 8.8410 (11), 10.3043 (12), 11.3610 (12) |
| α, β, γ (°) | 94.801 (9), 103.596 (9), 106.905 (9) |
| V (Å3) | 949.6 (2) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.23 |
| Crystal size (mm) | 0.88 × 0.53 × 0.19 |
| Data collection | |
| Diffractometer | Stoe IPDS 2 |
| Absorption correction | Integration (X-RED32; Stoe & Cie, 2002 ▸) |
| T min, T max | 0.876, 0.960 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 9612, 3716, 2058 |
| R int | 0.031 |
| (sin θ/λ)max (Å−1) | 0.617 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.047, 0.127, 0.91 |
| No. of reflections | 3716 |
| No. of parameters | 245 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.26, −0.34 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989019007424/wm5505sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989019007424/wm5505Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989019007424/wm5505Isup3.cml
CCDC reference: 1917654
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors acknowledge the Faculty of Arts and Sciences, Ondokuz Mayıs University, Turkey, for the use of the Stoe IPDS 2 diffractometer (purchased under grant F.279 of the University Research Fund).
supplementary crystallographic information
Crystal data
| C21H19ClN2O3 | Z = 2 |
| Mr = 382.83 | F(000) = 400 |
| Triclinic, P1 | Dx = 1.339 Mg m−3 |
| a = 8.8410 (11) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 10.3043 (12) Å | Cell parameters from 11025 reflections |
| c = 11.3610 (12) Å | θ = 3.0–31.4° |
| α = 94.801 (9)° | µ = 0.23 mm−1 |
| β = 103.596 (9)° | T = 296 K |
| γ = 106.905 (9)° | Prism, yellow |
| V = 949.6 (2) Å3 | 0.88 × 0.53 × 0.19 mm |
Data collection
| Stoe IPDS 2 diffractometer | 2058 reflections with I > 2σ(I) |
| Detector resolution: 6.67 pixels mm-1 | Rint = 0.031 |
| rotation method scans | θmax = 26.0°, θmin = 3.0° |
| Absorption correction: integration (X-RED32; Stoe & Cie, 2002) | h = −10→10 |
| Tmin = 0.876, Tmax = 0.960 | k = −12→12 |
| 9612 measured reflections | l = −14→14 |
| 3716 independent reflections |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.047 | H-atom parameters constrained |
| wR(F2) = 0.127 | w = 1/[σ2(Fo2) + (0.0666P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.91 | (Δ/σ)max < 0.001 |
| 3716 reflections | Δρmax = 0.26 e Å−3 |
| 245 parameters | Δρmin = −0.34 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.29831 (14) | 0.53925 (11) | −0.10674 (8) | 0.1264 (4) | |
| O3 | 0.72303 (19) | 0.86361 (16) | 0.93869 (13) | 0.0654 (5) | |
| O2 | 0.4976 (2) | 0.87323 (18) | 0.80559 (15) | 0.0704 (5) | |
| O1 | 0.6649 (2) | 0.89797 (17) | 0.57180 (15) | 0.0725 (5) | |
| N1 | 0.4130 (2) | 0.57611 (18) | 0.60996 (15) | 0.0503 (5) | |
| N2 | 0.5368 (2) | 0.69655 (18) | 0.62523 (15) | 0.0542 (5) | |
| C12 | 0.1658 (3) | 0.4089 (2) | 0.49033 (17) | 0.0463 (5) | |
| C11 | 0.3033 (3) | 0.5381 (2) | 0.50375 (17) | 0.0462 (5) | |
| C19 | 0.6111 (3) | 0.8315 (2) | 0.83046 (19) | 0.0536 (6) | |
| C10 | 0.3151 (3) | 0.6166 (2) | 0.40745 (18) | 0.0507 (5) | |
| H10 | 0.238158 | 0.583782 | 0.331377 | 0.061* | |
| C9 | 0.4352 (3) | 0.7377 (2) | 0.42376 (19) | 0.0528 (6) | |
| C13 | 0.0216 (3) | 0.3784 (2) | 0.39727 (19) | 0.0556 (6) | |
| H13 | 0.012681 | 0.437926 | 0.340794 | 0.067* | |
| C8 | 0.5539 (3) | 0.7869 (2) | 0.5427 (2) | 0.0557 (6) | |
| C5 | 0.3334 (3) | 0.7688 (2) | 0.2046 (2) | 0.0602 (6) | |
| C17 | 0.1752 (3) | 0.3177 (2) | 0.5723 (2) | 0.0617 (6) | |
| H17 | 0.270130 | 0.335520 | 0.636054 | 0.074* | |
| C18 | 0.6531 (3) | 0.7357 (2) | 0.7459 (2) | 0.0618 (6) | |
| H18A | 0.762208 | 0.780076 | 0.737838 | 0.074* | |
| H18B | 0.654649 | 0.653680 | 0.781667 | 0.074* | |
| C14 | −0.1095 (3) | 0.2608 (3) | 0.3867 (2) | 0.0659 (7) | |
| H14 | −0.206077 | 0.242734 | 0.324506 | 0.079* | |
| C6 | 0.3669 (3) | 0.6883 (3) | 0.1175 (2) | 0.0693 (7) | |
| H6 | 0.465811 | 0.669538 | 0.134709 | 0.083* | |
| C15 | −0.0967 (4) | 0.1717 (3) | 0.4678 (3) | 0.0735 (7) | |
| H15 | −0.183752 | 0.091911 | 0.460255 | 0.088* | |
| C7 | 0.4550 (3) | 0.8283 (2) | 0.3280 (2) | 0.0659 (7) | |
| H7A | 0.565102 | 0.846300 | 0.318471 | 0.079* | |
| H7B | 0.444144 | 0.915570 | 0.356666 | 0.079* | |
| C20 | 0.6983 (3) | 0.9534 (3) | 1.0330 (2) | 0.0697 (7) | |
| H20A | 0.592464 | 0.912468 | 1.048073 | 0.084* | |
| H20B | 0.702185 | 1.041629 | 1.007747 | 0.084* | |
| C16 | 0.0439 (4) | 0.1999 (3) | 0.5596 (3) | 0.0749 (8) | |
| H16 | 0.051884 | 0.138934 | 0.614912 | 0.090* | |
| C1 | 0.2528 (4) | 0.6351 (3) | 0.0037 (2) | 0.0752 (8) | |
| C4 | 0.1844 (4) | 0.7928 (3) | 0.1767 (2) | 0.0752 (8) | |
| H4 | 0.160060 | 0.847345 | 0.234301 | 0.090* | |
| C2 | 0.1061 (4) | 0.6593 (3) | −0.0218 (3) | 0.0839 (9) | |
| H2 | 0.030067 | 0.622809 | −0.097796 | 0.101* | |
| C3 | 0.0711 (4) | 0.7371 (3) | 0.0646 (3) | 0.0902 (9) | |
| H3 | −0.029726 | 0.752677 | 0.047719 | 0.108* | |
| C21 | 0.8330 (5) | 0.9709 (4) | 1.1455 (3) | 0.1279 (16) | |
| H21A | 0.936252 | 1.019422 | 1.131745 | 0.192* | |
| H21B | 0.834403 | 0.882260 | 1.164831 | 0.192* | |
| H21C | 0.815587 | 1.022374 | 1.212596 | 0.192* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.1545 (9) | 0.1266 (8) | 0.0925 (6) | 0.0370 (7) | 0.0468 (6) | −0.0221 (5) |
| O3 | 0.0619 (10) | 0.0768 (11) | 0.0468 (8) | 0.0251 (9) | −0.0023 (8) | −0.0084 (7) |
| O2 | 0.0597 (11) | 0.0818 (12) | 0.0620 (10) | 0.0286 (9) | 0.0007 (8) | −0.0056 (8) |
| O1 | 0.0687 (11) | 0.0536 (10) | 0.0731 (11) | −0.0058 (9) | 0.0156 (9) | −0.0070 (8) |
| N1 | 0.0517 (11) | 0.0514 (11) | 0.0437 (10) | 0.0153 (9) | 0.0090 (8) | 0.0008 (8) |
| N2 | 0.0504 (11) | 0.0545 (11) | 0.0460 (10) | 0.0091 (10) | 0.0048 (8) | −0.0036 (8) |
| C12 | 0.0519 (13) | 0.0456 (12) | 0.0411 (11) | 0.0144 (10) | 0.0158 (10) | 0.0004 (9) |
| C11 | 0.0502 (13) | 0.0478 (12) | 0.0386 (11) | 0.0162 (11) | 0.0100 (10) | 0.0005 (9) |
| C19 | 0.0475 (13) | 0.0558 (13) | 0.0452 (12) | 0.0093 (11) | 0.0012 (10) | 0.0008 (9) |
| C10 | 0.0563 (14) | 0.0501 (13) | 0.0401 (11) | 0.0141 (11) | 0.0086 (10) | 0.0009 (9) |
| C9 | 0.0594 (14) | 0.0458 (12) | 0.0505 (12) | 0.0126 (11) | 0.0175 (11) | 0.0015 (9) |
| C13 | 0.0589 (14) | 0.0537 (13) | 0.0475 (12) | 0.0142 (12) | 0.0098 (11) | −0.0004 (10) |
| C8 | 0.0573 (15) | 0.0511 (13) | 0.0525 (13) | 0.0119 (12) | 0.0145 (11) | −0.0035 (10) |
| C5 | 0.0752 (17) | 0.0482 (13) | 0.0541 (13) | 0.0100 (12) | 0.0218 (12) | 0.0158 (10) |
| C17 | 0.0624 (16) | 0.0636 (15) | 0.0562 (13) | 0.0174 (13) | 0.0130 (12) | 0.0126 (11) |
| C18 | 0.0541 (14) | 0.0672 (15) | 0.0505 (12) | 0.0165 (12) | −0.0014 (11) | −0.0082 (11) |
| C14 | 0.0514 (15) | 0.0637 (16) | 0.0668 (15) | 0.0067 (13) | 0.0084 (12) | −0.0105 (13) |
| C6 | 0.0742 (18) | 0.0634 (15) | 0.0690 (16) | 0.0162 (13) | 0.0246 (14) | 0.0100 (12) |
| C15 | 0.0699 (18) | 0.0566 (16) | 0.0838 (18) | 0.0018 (13) | 0.0295 (15) | −0.0022 (14) |
| C7 | 0.0793 (18) | 0.0524 (14) | 0.0592 (14) | 0.0114 (12) | 0.0179 (13) | 0.0110 (11) |
| C20 | 0.0812 (19) | 0.0734 (17) | 0.0524 (13) | 0.0277 (14) | 0.0147 (13) | −0.0022 (12) |
| C16 | 0.091 (2) | 0.0592 (16) | 0.0750 (17) | 0.0149 (16) | 0.0318 (16) | 0.0214 (13) |
| C1 | 0.095 (2) | 0.0646 (16) | 0.0622 (16) | 0.0137 (16) | 0.0299 (15) | 0.0051 (12) |
| C4 | 0.089 (2) | 0.0779 (18) | 0.0639 (16) | 0.0308 (16) | 0.0244 (15) | 0.0151 (13) |
| C2 | 0.092 (2) | 0.085 (2) | 0.0601 (16) | 0.0142 (17) | 0.0099 (16) | 0.0108 (14) |
| C3 | 0.088 (2) | 0.101 (2) | 0.083 (2) | 0.0356 (18) | 0.0183 (17) | 0.0193 (17) |
| C21 | 0.156 (3) | 0.171 (4) | 0.0489 (16) | 0.091 (3) | −0.0186 (19) | −0.0337 (18) |
Geometric parameters (Å, º)
| Cl1—C1 | 1.724 (3) | C17—H17 | 0.9300 |
| O3—C19 | 1.331 (2) | C18—H18A | 0.9700 |
| O3—C20 | 1.454 (3) | C18—H18B | 0.9700 |
| O2—C19 | 1.187 (3) | C14—C15 | 1.362 (4) |
| O1—C8 | 1.230 (3) | C14—H14 | 0.9300 |
| N1—C11 | 1.304 (2) | C6—C1 | 1.391 (4) |
| N1—N2 | 1.362 (2) | C6—H6 | 0.9300 |
| N2—C8 | 1.378 (3) | C15—C16 | 1.359 (4) |
| N2—C18 | 1.450 (3) | C15—H15 | 0.9300 |
| C12—C17 | 1.383 (3) | C7—H7A | 0.9700 |
| C12—C13 | 1.385 (3) | C7—H7B | 0.9700 |
| C12—C11 | 1.487 (3) | C20—C21 | 1.484 (4) |
| C11—C10 | 1.420 (3) | C20—H20A | 0.9700 |
| C19—C18 | 1.502 (3) | C20—H20B | 0.9700 |
| C10—C9 | 1.347 (3) | C16—H16 | 0.9300 |
| C10—H10 | 0.9300 | C1—C2 | 1.360 (4) |
| C9—C8 | 1.447 (3) | C4—C3 | 1.378 (4) |
| C9—C7 | 1.500 (3) | C4—H4 | 0.9300 |
| C13—C14 | 1.386 (3) | C2—C3 | 1.363 (4) |
| C13—H13 | 0.9300 | C2—H2 | 0.9300 |
| C5—C6 | 1.378 (3) | C3—H3 | 0.9300 |
| C5—C4 | 1.380 (4) | C21—H21A | 0.9600 |
| C5—C7 | 1.503 (3) | C21—H21B | 0.9600 |
| C17—C16 | 1.384 (4) | C21—H21C | 0.9600 |
| C19—O3—C20 | 116.13 (18) | C13—C14—H14 | 120.1 |
| C11—N1—N2 | 116.83 (18) | C5—C6—C1 | 120.0 (3) |
| N1—N2—C8 | 126.86 (17) | C5—C6—H6 | 120.0 |
| N1—N2—C18 | 114.58 (19) | C1—C6—H6 | 120.0 |
| C8—N2—C18 | 118.35 (19) | C16—C15—C14 | 119.7 (2) |
| C17—C12—C13 | 117.8 (2) | C16—C15—H15 | 120.1 |
| C17—C12—C11 | 121.31 (19) | C14—C15—H15 | 120.1 |
| C13—C12—C11 | 120.81 (19) | C9—C7—C5 | 114.18 (19) |
| N1—C11—C10 | 121.6 (2) | C9—C7—H7A | 108.7 |
| N1—C11—C12 | 116.04 (18) | C5—C7—H7A | 108.7 |
| C10—C11—C12 | 122.40 (17) | C9—C7—H7B | 108.7 |
| O2—C19—O3 | 125.0 (2) | C5—C7—H7B | 108.7 |
| O2—C19—C18 | 126.11 (19) | H7A—C7—H7B | 107.6 |
| O3—C19—C18 | 108.9 (2) | O3—C20—C21 | 106.6 (2) |
| C9—C10—C11 | 121.50 (19) | O3—C20—H20A | 110.4 |
| C9—C10—H10 | 119.3 | C21—C20—H20A | 110.4 |
| C11—C10—H10 | 119.3 | O3—C20—H20B | 110.4 |
| C10—C9—C8 | 118.4 (2) | C21—C20—H20B | 110.4 |
| C10—C9—C7 | 125.0 (2) | H20A—C20—H20B | 108.6 |
| C8—C9—C7 | 116.5 (2) | C15—C16—C17 | 121.1 (3) |
| C12—C13—C14 | 121.3 (2) | C15—C16—H16 | 119.4 |
| C12—C13—H13 | 119.4 | C17—C16—H16 | 119.4 |
| C14—C13—H13 | 119.4 | C2—C1—C6 | 120.6 (3) |
| O1—C8—N2 | 120.3 (2) | C2—C1—Cl1 | 119.6 (2) |
| O1—C8—C9 | 125.2 (2) | C6—C1—Cl1 | 119.8 (3) |
| N2—C8—C9 | 114.5 (2) | C3—C4—C5 | 120.9 (3) |
| C6—C5—C4 | 118.5 (2) | C3—C4—H4 | 119.6 |
| C6—C5—C7 | 121.1 (3) | C5—C4—H4 | 119.6 |
| C4—C5—C7 | 120.4 (2) | C1—C2—C3 | 119.7 (3) |
| C12—C17—C16 | 120.2 (2) | C1—C2—H2 | 120.1 |
| C12—C17—H17 | 119.9 | C3—C2—H2 | 120.1 |
| C16—C17—H17 | 119.9 | C2—C3—C4 | 120.3 (3) |
| N2—C18—C19 | 112.24 (19) | C2—C3—H3 | 119.9 |
| N2—C18—H18A | 109.2 | C4—C3—H3 | 119.9 |
| C19—C18—H18A | 109.2 | C20—C21—H21A | 109.5 |
| N2—C18—H18B | 109.2 | C20—C21—H21B | 109.5 |
| C19—C18—H18B | 109.2 | H21A—C21—H21B | 109.5 |
| H18A—C18—H18B | 107.9 | C20—C21—H21C | 109.5 |
| C15—C14—C13 | 119.8 (2) | H21A—C21—H21C | 109.5 |
| C15—C14—H14 | 120.1 | H21B—C21—H21C | 109.5 |
Hydrogen-bond geometry (Å, º)
Cg2 is the centroid of the C1–C6 phenyl ring
| D—H···A | D—H | H···A | D···A | D—H···A |
| C14—H14···O2i | 0.93 | 2.53 | 3.416 (3) | 160 |
| C7—H7B···O1ii | 0.97 | 2.54 | 3.485 (3) | 164 |
| C15—H15···O1iii | 0.93 | 2.66 | 3.474 (3) | 147 |
| C20—H20B···Cg2iv | 0.97 | 2.81 | 3.759 (3) | 165 |
Symmetry codes: (i) −x, −y+1, −z+1; (ii) −x+1, −y+2, −z+1; (iii) x−1, y−1, z; (iv) −x+1, −y, −z+1.
References
- Akhtar, W., Shaquiquzzaman, M., Akhter, M., Verma, G., Khan, M. F. & Alam, M. M. (2016). Eur. J. Med. Chem. 123, 256–281. [DOI] [PubMed]
- Asif, M. (2013). Mini-Rev. Org. Chem. 10, 113–122.
- Asif, M. (2015). Mini Rev. Med. Chem. 14, 1093–1103. [DOI] [PubMed]
- Barberot, C., Moniot, A., Allart-Simon, I., Malleret, L., Yegorova, T., Laronze-Cochard, M., Bentaher, A., Médebielle, M., Bouillon, J. P., Hénon, E., Sapi, J., Velard, F. & Gérard, S. (2018). Eur. J. Med. Chem. 146, 139–146. [DOI] [PubMed]
- Boukharsa, Y., Meddah, B., Tiendrebeogo, R. Y., Ibrahimi, A., Taoufik, J., Cherrah, Y., Benomar, A., Faouzi, M. E. A. & Ansar, M. (2016). Med. Chem. Res. 25, 494–500.
- Chkirate, K., Kansiz, S., Karrouchi, K., Mague, J. T., Dege, N. & Essassi, E. M. (2019a). Acta Cryst. E75, 154–158. [DOI] [PMC free article] [PubMed]
- Chkirate, K., Kansiz, S., Karrouchi, K., Mague, J. T., Dege, N. & Essassi, E. M. (2019b). Acta Cryst. E75, 33–37. [DOI] [PMC free article] [PubMed]
- Dubey, S. & Bhosle, P. A. (2015). Med. Chem. Res. 24, 3579–3598.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Gökçe, M., Utku, S. & Küpeli, E. (2009). Eur. J. Med. Chem. 44, 3760–3764. [DOI] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Karrouchi, K., Ansar, M., Radi, S., Saadi, M. & El Ammari, L. (2015). Acta Cryst. E71, o890–o891. [DOI] [PMC free article] [PubMed]
- Karrouchi, K., Radi, S., Ansar, M. H., Taoufik, J., Ghabbour, H. A. & Mabkhot, Y. N. (2016a). Z. Kristallogr. New Cryst. Struct. 231, 883–886.
- Karrouchi, K., Radi, S., Ansar, M. H., Taoufik, J., Ghabbour, H. A. & Mabkhot, Y. N. (2016b). Z. Kristallogr. New Cryst. Struct. 231, 839–841.
- Livermore, D., Bethell, R. C., Cammack, N., Hancock, A. P., Hann, M. M., Green, D., Lamont, R. B., Noble, S. A., Orr, D. C. & Payne, J. J. (1993). J. Med. Chem. 36, 3784–3794. [DOI] [PubMed]
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- McKinnon, J. J., Jayatilaka, D. & Spackman, M. A. (2007). Chem. Commun. pp. 3814–3816. [DOI] [PubMed]
- Ochiai, K., Takita, S., Eiraku, T., Kojima, A., Iwase, K., Kishi, T., Fukuchi, K., Yasue, T., Adams, D. R., Allcock, R. W., Jiang, Z. & Kohno, Y. (2012). Bioorg. Med. Chem. 20, 1644–1658. [DOI] [PubMed]
- Oubair, A., Daran, J.-C., Fihi, R., Majidi, L. & Azrour, M. (2009). Acta Cryst. E65, o1350–o1351. [DOI] [PMC free article] [PubMed]
- Partap, S., Akhtar, M. J., Yar, M. S., Hassan, M. Z. & Siddiqui, A. A. (2018). Bioorg. Chem. 77, 74–83. [DOI] [PubMed]
- Sharma, B., Verma, A., Sharma, U. K. & Prajapati, S. (2014). Med. Chem. Res. 23, 146–157.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Siddiqui, A. A., Mishra, R., Shaharyar, M., Husain, A., Rashid, M. & Pal, P. (2011). Bioorg. Med. Chem. Lett. 21, 1023–1026. [DOI] [PubMed]
- Sönmez, M., Berber, I. & Akbaş, E. (2006). Eur. J. Med. Chem. 41, 101–105. [DOI] [PubMed]
- Spackman, M. A. & Jayatilaka, D. (2009). CrystEngComm, 11, 19–32.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Stoe & Cie (2002). X-AREA and X-RED32. Stoe & Cie GmbH, Darmstadt, Germany.
- Tao, M., Aimone, L. D., Gruner, J. A., Mathiasen, J. R., Huang, Z., Lyons, J., Raddatz, R. & Hudkins, R. L. (2012). Bioorg. Med. Chem. Lett. 22, 1073–1077. [DOI] [PubMed]
- Thakur, A. S., Verma, P. & Chandy, A. (2010). Asian. J. Res. Chem, 3, 265–271.
- Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D. & Spackman, M. A. (2017). CrystalExplorer17. University of Western Australia. http://hirshfeldsurface.net.
- Wang, T., Dong, Y., Wang, L.-C., Xiang, B.-R., Chen, Z. & Qu, L.-B. (2008). Arzneimittelforschung, 58, 569–573. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Xu, H., Song, H.-B., Yao, C.-S., Zhu, Y.-Q., Hu, F.-Z., Zou, X.-M. & Yang, H.-Z. (2005). Acta Cryst. E61, o1561–o1563.
- Zhou, G., Ting, P. C., Aslanian, R., Cao, J., Kim, D. W., Kuang, R., Lee, J. F., Schwerdt, J., Wu, H., Jason Herr, R., Zych, A. J., Yang, J., Lam, S., Wainhaus, S., Black, T. A., McNicholas, P. M., Xu, Y. & Walker, S. S. (2011). Bioorg. Med. Chem. Lett. 21, 2890–2893. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989019007424/wm5505sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989019007424/wm5505Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989019007424/wm5505Isup3.cml
CCDC reference: 1917654
Additional supporting information: crystallographic information; 3D view; checkCIF report