Abstract
SOD1 is commonly known for its ROS scavenging activity, but recent work has uncovered additional roles in modulating metabolism, maintaining redox balance, and regulating transcription. This new paradigm of expanded SOD1 function raises questions regarding the regulation of SOD1 and the cellular partitioning of its biological roles. Despite decades of research on SOD1, much of which focuses on its pathogenic role in amyotrophic lateral sclerosis, relatively little is known about its regulation by post-translational modifications (PTMs). However, over the last decade, advancements in mass spectrometry have led to a boom in PTM discovery across the proteome, which has also revealed new mechanisms of SOD1 regulation by PTMs and an array of SOD1 PTMs with high likelihood of biological function. In this review, we address emerging mechanisms of SOD1 regulation by post-translational modifications, many of which begin to shed light on how the various functions of SOD1 are regulated within the cell.
Keywords: SOD1, Post-translational modification, Phosphorylation, Acylation, Ubiquitination
1. Introduction
The enzymatic function of Cu/Zn Superoxide Dismutase (SOD1), previously known as hemocuprein, was first characterized in 1969 [1]. Decades of research that followed revealed several key aspects of SOD1 biology, including the structure of SOD1 as an active dimer, its essential metal cofactors (copper and zinc), and its ability to convert superoxide radicals into molecular oxygen and hydrogen peroxide—referred to as dismutation. In disease contexts, SOD1 is best-known for its role in a familial form of amyotrophic lateral sclerosis (fALS) [2], in which a wide variety of SOD1 mutations increase the propensity of SOD1 to aggregate, which is thought to ultimately induce motor neuron death. In addition, SOD1 is overexpressed in numerous cancer types, including lung adenocarcinoma [3], non-small-cell lung cancer [4], and 70% of primary breast cancers [5]. SOD1 overexpression in cancer likely confers a growth advantage by providing protection against oxidative damage [6]. In support of this idea, genomic deletion of SOD1 in mammalian cells induces cell death due to the buildup of oxidative stress [7].
Studies in the model eukaryote Saccharomyces cerevisiae (Baker's yeast) suggest that the vast majority of the total SOD1 pool, estimated to represent nearly 1% of total protein in some cells [8], is dispensable for protection of cells from oxidative stress [[9], [10], [11]]. Although SOD1 is abundant in the cytosol, the pro-survival effect of SOD1 appears to be most critical within the intermembrane space (IMS) of mitochondria, as an IMS-targeted SOD1 is sufficient to protect cells from oxidative stress [11]. These findings suggest that the large majority of SOD1 (>99%) may be required for functions outside its canonical role in dismutating superoxide. Indeed, SOD1 is reported to function in cellular zinc [12] and copper buffering [13], as a nuclear transcription factor to control the expression of antioxidant genes [14,15] and for peroxide-mediated redox signaling [11].
In addition, SOD1 was shown to be a metabolic focal point in yeast for integrating nutrient availability to regulate a switch between respiration and fermentation [9]. This mechanism of SOD1-mediated control of metabolism involves a glucose- and O2-dependent signal that triggers SOD1-mediated stabilization of two yeast casein kinase 1-gamma (CK1γ) isoforms, which, in turn, suppresses mitochondrial respiration. In addition, our recent work in mammalian cells demonstrated a similar role for SOD1 in suppressing mitochondrial metabolism, potentially via suppression of complex I of the electron transport chain (ETC) [16]. Furthermore, mutants of SOD1 that are defective in suppressing respiration, but capable of dismutation, do not fully rescue cell survival in SOD1-null cells, suggesting that this metabolic function of SOD1 may represent an additional facet of SOD1-mediated antioxidant defense—potentially via suppression of ROS formation at the ETC [16]. Thus, this new complexity of SOD1 function provides fresh context in which we can examine how PTMs may control the various roles of SOD1.
In this review, we compile a comprehensive list of SOD1 PTMs and draw from recent literature to discuss how these PTMs regulate the canonical ROS-scavenging function of SOD1, in addition to emerging SOD1 functions and SOD1 aggregation. Furthermore, we examine SOD1 PTMs that are predicted to impact SOD1 function and merit future study.
2. Phosphorylation
At least 12 phosphorylation sites have been discovered (phosphosite.org) on SOD1 and a few have been implicated in regulating the role of SOD1 in ROS-scavenging, possible cytoskeletal maintenance and transcription (Fig. 1 and Table 1) [14,17,18]. Early mass spectrometry work on SOD1 identified a granulocyte-colony stimulating factor (GCSF)-induced phosphorylated species of SOD1 [19]. Although at the time, no specific site of phosphorylation was discovered, the authors suspected the phosphorylation may control SOD1 degradation based on their observation that GCSF treatment triggered a decrease in SOD1 protein levels that correlated with increased phosphorylation. Hjornevik et al. [17] later found that treatment of primary hepatocytes with nodularin, a Ser/Thr phosphatase inhibitor, increased SOD1 phosphorylation levels (detected by two-dimensional gel electrophoresis—no specific sites detected) and disrupted SOD1 colocalization with the cytoskeleton without any change in SOD1 ROS scavenging activity—suggesting that SOD1 phosphorylation may alter the subcellular localization of SOD1. They posited that the increased phosphorylation and loss of colocalization may contribute to cytoskeletal rearrangement in the early stages of apoptotic budding after nodularin treatment [17]. While these early studies hinted at some interesting modes of SOD1 regulation by phosphorylation, the absence of identified sites makes it impossible to rule out other mechanisms of SOD1 regulation that have contributed to their observations.
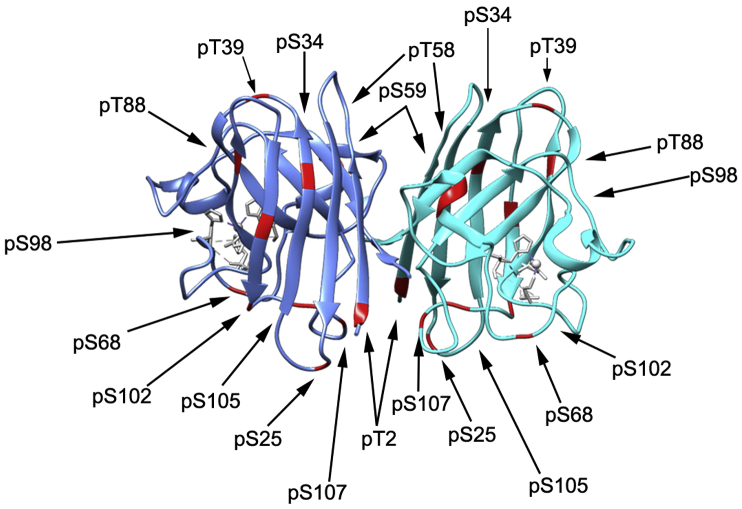
Fig. 1.
SOD1 sites of phosphorylation. Crystal structure of mouse SOD1 (PDB: 3GTV) with known human SOD1 sites of phosphorylation highlighted in red (sites compiled from papers discussed in this review and from www.phosphosite.org). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
SOD1 phosphorylation sites. Summary of SOD1 phosphorylation sites described in this paper.
| Species | Residue | Modification | Modifying Enzyme (if known); Potential Location of PTM | Significance | Reference |
|---|---|---|---|---|---|
| human | Thr2 | phosphorylation | unknown | Wilcox, 2009 | |
| phospho-mimetic stabilizes SOD1 dimer, even when combined with fALS A4V mutation | Fay, 2016 | ||||
| human | Thr39 | phosphorylation | mTORC1; cytoplasm, lysosome, organelle membranes | phosphorylated by mTORC1 during nutrient rich conditions to inhibit ROS-scavenging activity | Tsang, 2018 |
| human | Thr58 | phosphorylation | unknown | Wilcox, 2009 | |
| human | Ser59 | phosphorylation | unknown | Wilcox, 2009 | |
| human | unknown | phosphorylation | may cause degradation because decreased levels of SOD1 observed | Csar, 2001 | |
| human | unknown | phosphorylation | decreased SOD1 colocalization with actin filaments, may be implicated in cytoskeletal rearrangement in the early stages of apoptotic budding | Hjornevik, 2012 | |
| yeast | Ser38 | phosphorylation | phosphorylation stimulated upon low oxygen conditions, postulated that this may tag unfolded SOD1 for fast activation by CCS or for binding to other partners | Leitch, 2012 | |
| TORC1; membranes, vacuole | phosphorylation turns off ROS scavenging activity; phosphorylated by TORC1 during nutrient rich conditions | Tsang, 2018 | |||
| yeast | Ser59, Ser98 | phosphorylation | Dun1; nucleus | phosphorylated by kinase, Dun1, which promotes SOD1 nuclear localization to maintain genomic stability | Tsang, 2014 |
Phosphorylations at Thr2 and either Thr58 or Ser59 were initially identified in human erythrocytes, but neither showed any evidence of regulating canonical SOD1 enzymatic activity [20]. Rather, Thr2 was linked to a possible rescue phenotype for an fALS mutant of SOD1. Indeed, Fay et al. [21] found that a phospho-mimicking aspartic acid substitution at Thr2, which sits at the SOD1 dimer interface, stabilizes the SOD1 dimer even when combined with the fALS-linked, SOD1-destabilizing A4V mutation. However, the mechanism by which Thr2 phosphorylation is regulated and stabilizes SOD1 is still unknown.
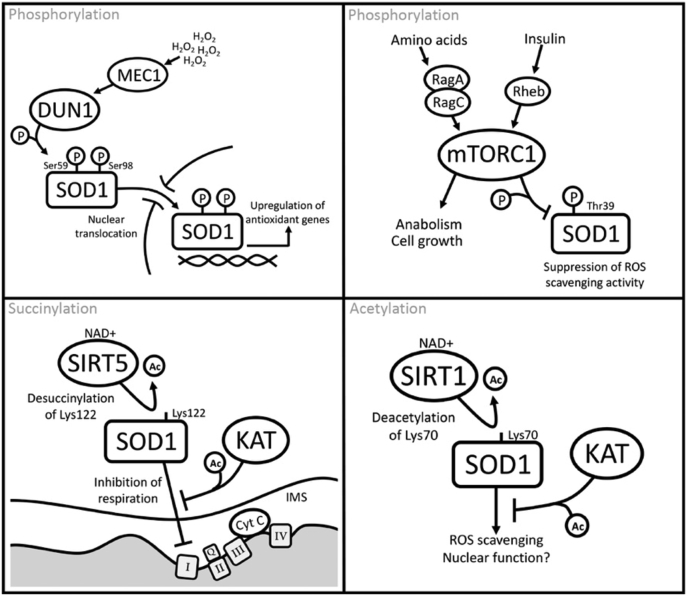
In yeast, phosphorylations at Ser59* and Ser98* are proposed to regulate a non-canonical function of SOD1 in transcription. Tsang et al. [14] found that in response to toxic levels of oxidative stress (0.4 mM H2O2), SOD1 translocates to the nucleus to transcriptionally upregulate antioxidant genes. In addition, this new nuclear function of SOD1 was recently shown to involve direct SOD1-DNA interactions with specific DNA sequence preferences [15]. The nuclear translocation of SOD1 is dependent on activation of ATM/Mec1 by ROS, which promotes SOD1 binding to the Chk2-related kinase, Dun1. Dun1 then phosphorylates SOD1 at Ser59 and Ser98 and promotes its translocation to the nucleus to maintain genomic stability. Mutation of these serine residues to alanine abrogates SOD1 nuclear localization, suggesting that they may play a role in mediating SOD1 interactions with nuclear transport machinery [14]. These data raise a number of questions, including whether SOD1 functions as a nuclear transcription factor in response to chemotherapeutics or other means of activating the DNA damage response via ATM. Furthermore, Dun1-mediated phosphorylation of SOD1 was observed with apoptosis-inducing levels of oxidative stress, so whether these phosphorylations are involved in normal homeostatic SOD1 signaling or only under extreme oxidative stress or DNA damage is unknown.
Interestingly, an analysis of all known SOD1 PTMs by SAPH-ire, a machine learning approach aimed at predicting functional PTMs, scored Ser98 as the most likely SOD1 PTM for biological function [16,22,23]. Indeed, unmodified Ser98 may play a role in maintaining the structural integrity of SOD1 by H-bonding with adjacent residues, as demonstrated in crystal structures of apo-SOD1 [24]. Thus, the sphere of negative charge conferred by phosphorylation at Ser98 would likely impose structural change on SOD1, potentially leading to changes in interacting partners (e.g., nuclear chaperones) or enzymatic activity, which could begin to explain the observation that the S98A SOD1 mutant fails to move to the nucleus during oxidative stress [14].
Leitch et al. first studied a phosphorylation on yeast SOD1 at Ser38* [25], which sits at the entry site of a positively charged tunnel within the SOD1 structure that is thought to guide negatively charged superoxide to the active site for dismutation [26]. Recent evidence suggests that phosphorylation at Ser38 (Thr39 in humans) may act as a TORC1/mTORC1-governed switch to control SOD1 activity in yeast and mammalian cells. Specifically, Tsang et al. [18] found that under full glucose conditions in yeast, TORC1 directly phosphorylates SOD1 at Ser38, which results in suppression of SOD1 enzymatic activity. Conversely, inhibition of TORC1, by incubation of cells in glycerol or the TOR inhibitor rapamycin, resulted in dephosphorylation of SOD1 and increased ROS scavenging activity. In human cells, Thr39 was similarly regulated by mTORC1. Furthermore, tumor cells expressing the SOD1 T39E phospho-mimic were impaired for survival and tumor growth, whereas the phospho-null SOD1 Thr39A showed the opposite—increased survival and tumor growth in mouse xenograft models [18].
Previous work in yeast suggested that Ser38 phosphorylation increases dramatically under low oxygen conditions, in which mTORC1 is presumably less active. Leitch also found that Ser38 phosphorylation correlates strongly with a decrease in SOD1 activity during hypoxia [25]. These results raise the possibility that other kinases may target this site to inhibit SOD1 activity in hypoxia. Ser38 sits adjacent to a proline (AGNpSPNA), which makes it a candidate substrate for MAPKs and other proline-directed kinases. Thus, p38 MAPK, or hypoxia-activated proline-directed kinases may target Ser38 to suppress SOD1 activity. Furthermore, the hypoxia-induced phosphorylation of Ser38 may help explain why hypoxic conditions result in a loss of antioxidant defense, which returns promptly when cells/tissues are returned to full-oxygen conditions [27].
3. Lysine modifications
Acylation. Lysine acylation is a reversible modification that can vary in carbon length, with two of the most frequently observed acylations being acetylation (1 carbon) and succinylation (2 carbons). Acylation is typically regulated by lysine acyl transferases (KATs) and lysine deacylases (KDACs), but can also be added and removed non-enzymatically [[28], [29], [30]]. Acylation is thought to impact protein function by changing the net charge of lysine from +1 to 0 (acetylation) or −1 (succinylation). High throughput mass spectrometry studies have identified a variety of acetylation and succinylation sites on SOD1, but only a few have been investigated for their impact on SOD1 function.
SOD1 was initially identified as a substrate of the Sirtuin family deacylase, SIRT1, in Xenopus egg extracts [31]. Later work revealed that SIRT1 regulates an acetylation at Lys70* [32], which sits within a solvent-exposed flexible loop in the SOD1 structure (Fig. 2). Acetylation at Lys70 is proposed to inactivate SOD1 activity by disrupting SOD1 binding to the copper chaperone for superoxide dismutase (CCS) and, in turn, inhibiting the formation of SOD1 homodimers [32].
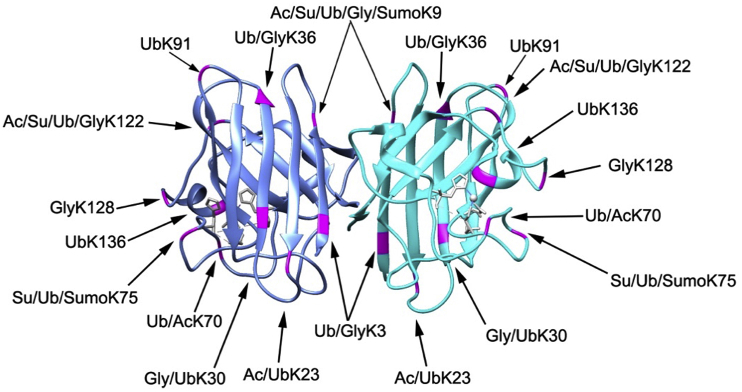
Fig. 2.
SOD1 sites of lysine modification. Crystal structure of mouse SOD1 (PDB: 3GTV) with known human SOD1 sites of lysine-modifications highlighted in magenta (sites compiled from papers discussed in this review and from www.phosphosite.org).
Given that Sirtuins are linked to metabolism by their dependence on NAD+ for catalytic activity, these data suggest that SOD1 activity, in turn, may rise and fall with NAD+ levels. Furthermore, SIRT1 is primarily considered a nuclear deacetylase. Thus, SIRT1 may serve to maintain nuclear SOD1 in an active, deacetylated state via Lys70 deacetylation, thereby promoting the previously reported antioxidant and transcriptional functions of SOD1 [14]. In support of this idea, high levels of Lys70 acetylation are correlated with sensitivity to genotoxic agents [32].
Another Sirtuin-governed acylation on SOD1 with known function occurs at Lys122*, which is both acetylated and succinylated [[33], [34], [35], [36], [37], [38]]. Lys122 sits within the electrostatic loop, a region thought to participate in shuttling superoxide radicals toward the SOD1 active site [26,39]. Lin et al. reported that the lysine desuccinylase SIRT5 interacts with SOD1 and desuccinylates Lys122 and that SIRT5 depletion reduces SOD1 enzymatic activity [40]. However, we found that neither acetylation nor succinylation-mimicking mutations (Gln (Q) and Glu (E), respectively) at Lys122 affect the ROS scavenging activity of SOD1 [16]. In an effort to investigate other functions of SOD1 that may be impacted by Lys122 acylation, we found that SOD1 K122Q or E mutants were unable to suppress mitochondrial respiration [16], a recently reported SOD1 function [9]. Depletion of SIRT5 increased SOD1 succinylation at Lys122 , which, in turn, suppressed SOD1-mediated inhibition of complex I of the electron transport chain (ETC), resulting in an increase in mitochondrial respiration. Importantly, the increase in respiration in SIRT5-depleted cells was rescued to near-normal levels by expressing an acylation-refractory K122R mutant of SOD1, which suggests that SIRT5 controls mitochondrial respiration by maintaining SOD1 in a desuccinylated state, capable of suppressing respiration.
The precise mechanism by which acylation at Lys122 inhibits the ability of SOD1 to suppress respiration remains an open question. In yeast, Reddi et al. found that SOD1 suppresses respiration by interacting with and stabilizing two casein kinase 1-gamma (CK1γ) isoforms, which, in turn, inhibits mitochondrial respiration [9]. This suggests that acylation of Lys122 may disrupt the SOD1-CK1γ interaction. However, it is not yet clear whether mammalian SOD1 acts through the same mechanism. Furthermore, Lys122 is only conserved in higher eukaryotes, which suggests that the ability to toggle SOD1-mediated suppression of respiration on or off via acylation may only be critical in the context of more complex multicellular organisms [16].
The evolutionary conservation of SOD1-mediated suppression of respiration from yeast to human and its link to Sirtuin activity beg the question of its biological significance. Could suppression of respiration be an additional part of the expanding antioxidant program governed by SOD1? In support of this idea, the K122E mutant of SOD1, which is enzymatically active but defective in suppressing respiration, is unable to fully rescue SOD1 KO cells from oxidative stress-induced death [16], suggesting that the inhibition of respiration contributes to SOD1-mediated antioxidant defense. Exactly how this occurs is still unclear. Decades of research have shown that e− leakage from the ETC, generally attributed to complex III, is a major source of ROS in the cell [41,42]. Thus, SOD1-mediated inhibition of respiration may directly inhibit ROS formation by suppressing e− flux through the ETC. In support of this idea, genetic inhibition of respiration partially rescues the loss of viability caused by SOD1 deletion in yeast (known to be caused by excessive oxidative stress) [43]. Furthermore, metformin, which, like SOD1, inhibits complex I, reduces ROS formation via inhibition of ETC activity [[44], [45], [46]].
In vitro studies of SOD1 aggregation have also revealed a role for lysine acylation in controlling SOD1 aggregation and the prion-like self-propagation of SOD1 oligomers. Abdolvahabi et al. [47] found that aspirin-induced acetylation on lysines of purified SOD1 impeded amyloidogenesis caused by the fALS-linked A4V mutation. This effect does not appear to be specific to any particular lysine position and can be accomplished with as few as three acylated lysines, suggesting that simply increasing the net negative charge of SOD1 effectively brings it back from the tipping point of aggregation [48]. Therefore, the development of small molecules that selectively increase the net negative charge of SOD1 may be a promising therapeutic avenue for SOD1-driven ALS [48].
Sumoylation and ubiquitination. SOD1 lysines are also modified by SUMO-1 at Lys9 and Lys75 [49,50]. SUMO-1 modification at Lys75 increases the aggregation propensity of fALS-linked SOD1 mutants and increases in sumoylation are also observed in SOD1 aggregates [49]. Niikura et al. confirmed this site of sumoylation and found that Lys75 could also be modified by SUMO-2/3 [50]. Similar to the effect of SUMO-1 modification, they observed that the addition of SUMO-3 to Lys75 increases the stabilization of an fALS-SOD1 mutant and accelerates its aggregation. It is unclear whether SOD1 sumoylation directly causes aggregation by changing physical properties of the protein, or is simply blocking the proteasomal degradation of SOD1, thereby promoting aggregation by increasing SOD1 concentration.
SOD1 aggregates can be degraded through proteasomal or lysosomal (autophagy) degradation pathways, both of which are thought to contribute substantially to reducing the SOD1 aggregate burden [51]. Clinical fALS patient samples, as well as G85R and G93A SOD1 transgenic mice, show colocalization of ubiquitin with SOD1 [[52], [53], [54]], which could reflect the cell's failed attempt at degrading SOD1 through a ubiquitin-dependent mechanism. Further, in an A4V SOD1 mutant fALS cell model, the distribution and homeostasis of ubiquitination was altered [55]. The ubiquitination of SOD1 depends on several ubiquitin ligases, including Dorfin, NEDL1, and MITOL [[56], [57], [58]], and can occur at Lys136 [59], as well as other lysines identified in high throughput studies (see phosphosite.org). Biosensor imaging [60] of intact single neuronal cells demonstrated that mutant SOD1, G93A and G85R, have increased ubiquitination and chaperone interaction (Hsp70) over WT SOD1, but do not cause general proteasomal dysfunction. This suggests the possibility that fALS-linked SOD1 mutants exceed the cell's chaperone capacity (i.e., chaperone depletion). In support of this idea, other studies have observed an inhibition of chaperone activity caused by mutant SOD1 expression [61,62].
Glycation. Glycation of erythrocyte SOD1 has been shown to inactivate SOD1 enzymatic activity in vitro [63]. The sites of glycation were lysines 3, 9, 30, 36, 122, and 128, although glycation at Lys122 and Lys128 appeared to be the most critical for enzymatic deactivation of SOD1 [63]. The effect of glycation on mutant SOD1 in fALS has been reviewed in Ref. [64]. However, more recently, Sirangelo et al. [65] suggested that glycation of SOD1 does not promote amyloid formation in fALS, but may cause cytotoxicity through a yet undetermined pathway. The known SOD1 lysine modification sites are summarized in Table 2 and are indicated in the SOD1 crystal structure in Fig. 2 (human sites only).
Table 2.
SOD1 lysine-modified sites. Summary of SOD1 lysine-modified sites described in this paper, including acetylation, succinylation, sumoylation, ubiquitination, and glycation.
| Species | Residue | Modification | Modifying Enzyme (if known); Potential Location of PTM | Significance | Reference |
|---|---|---|---|---|---|
| human | Lys70 | acetylation | SIRT1; nucleus, cytoplasm, mitochondrion | inactivates ROS scavenging activity, deacetylated by SIRT1, may help sensitize cancer cells to genotoxic agents | Lin, 2015 |
| human | Lys122 | succinylation | SIRT5; mitochondrion, cytoplasm, nucleus | inactivates ROS scavenging activity, desuccinylated by SIRT5 | Lin, 2013 |
| human | Lys122 | acetylation/succinylation | SIRT5 (desuccinylation); mitochondrion, cytoplasm, nucleus | inhibits SOD1's anti-respiratory activity, succinyl-mimetics cause decreased growth and less healthy mitochondria in HCT116 cells, does not affect ROS scavenging activity | Banks, 2017 |
| human | Lys122 | acetylation | acetylated SOD1 found in distinct regions of adult central nervous system | Kaliszewski, 2016 | |
| human | general | acetylation | treatment with aspirin increases SOD1 acetylation and decreases A4V SOD1 amyloidogenesis | Abdolvahabi, 2015 | |
| human | Lys9 | sumoylation | SUMO-1 modification observed but function not yet known | Niikura, 2014 | |
| human | Lys75 | sumoylation | SUMO-1 modification increases SOD1 stability and propensity to aggregate; sumoylation increases further after aggregation of SOD1 | Fei, 2006 | |
| SUMO-3 modification also increases SOD1 stability and propensity to aggregate | Niikura, 2014 | ||||
| yeast | Lys18, Lys69 | sumoylation | unknown | Zhou, 2004 | |
| human | Lys136 | ubiquitination | ubiquitination occurs after formation of the aggregates and may occur on additional lysines as well | Basso, 2006 | |
| human | unknown | ubiquitination | colocalizes with SOD1 aggregates in patients with fALS | Kato, 1997 | |
| human | unknown | ubiquitination | colocalizes with SOD1 aggregates in G85R and G93A SOD1 transgenic mice; Stieber et al. postulates this may be a result of the ubiquitin-proteasome pathway being unable to handle degradation of the aggregates | Bruijn, 1997; Stieber, 2000 | |
| human | unknown | ubiquitination | intact single neuronal cells demonstrated that G93A and G85R SOD1 had increased ubiquitination and colocalization with Hsp70; did not cause proteasomal dysfunction so chaperone depletion may be a cause of mutant SOD1 toxicity | Ganesan, 2008 | |
| human | Lys3, Lys9, Lys30, Lys36, Lys122, Lys128 | glycation | Lys122, Lys128 most critical for enzymatic deactivation | Fujii, 1996 | |
| human | unknown | glycation | does not promote amyloid formation in fALS but may cause cytotoxicity through another pathway | Sirangelo, 2016 |
4. Redox modifications
Oxidation and glutathionylation. Oxidation may be the best understood SOD1 PTM to date. When WT SOD1 is oxidized to sulfonic acid at Cys111, it obtains similar properties to fALS mutants of SOD1—a propensity to misfold and inhibit kinesin-based fast axonal transport [66]. This draws a striking connection between fALS and sporadic forms of ALS (sALS) and may explain at least a subset of sALS cases, in which aggregates of WT SOD1 are found. Interestingly, Xu et al. found that pathological concentrations of H2O2 cause sulfenic acid modifications at C111 and fibrillization of SOD1, which self-propagates in a prion-like manner [67]. In comparing the cerebrospinal fluid of patients with or without sALS, sulfenic acid modified SOD1 was significantly increased in disease-positive patients. To further support the idea that SOD1 oxidation may lead to sporadic forms of ALS, Martins et al. [68] found that SOD1 oxidation at Cys146, His71, and His120 was enriched in high molecular weight, aggregated forms of yeast SOD1, raising the possibility that oxidation of SOD1 leads to its misfolding and aggregation. Trp32 can also be oxidized and has been shown to cause aggregation of human SOD1 [69].
Another form of redox modification that occurs during oxidative stress is glutathionylation, which occurs on SOD1 at Cys111, a residue that is critical for maintaining SOD1 stability. Glutathionylation at Cys111 destabilizes SOD1 and promotes monomer formation, which is the initiating step for SOD1 aggregation [20]. Therefore, among PTMs associated with SOD1 stability, oxidation of SOD1 is a relatively well-established PTM with potential links to SOD1 misfolding in ALS [[70], [71], [72]]. The sites of oxidation and glutathionylation are summarized in Table 3 and their positions in the SOD1 crystal structure are shown in Fig. 3 (human sites only).
Table 3.
SOD1 redox-modified sites. Summary of SOD1 redox-modified sites described in this paper, including oxidation, glutathionylation, and cysteinylation.
| Species | Residue | Modification | Modifying Enzyme (if known); Potential Location of PTM | Significance | Reference |
|---|---|---|---|---|---|
| human | Trp32 | oxidation | increases aggregation of SOD1 | Zhang, 2003 | |
| human | Cys111 | oxidation | increases SOD1 propensity to misfold and inhibit kinesin-based fast axonal transport | Bosco, 2010 | |
| yeast | Cys146, His71, His120 | oxidation | speculated that oxidation leads to SOD1 misfolding and aggregation | Martins, 2014 | |
| human | Cys111 | glutathion-ylation | destabilizes SOD1 and promotes monomer formation which is the initiating step for SOD1 aggregation | Wilcox, 2009 | |
| human | Cys111 | cysteinylation | protects SOD1 from oxidation; slight conformational change at the dimer interface and electrostatic loop | Auclair, 2013a; Auclair, 2013b |
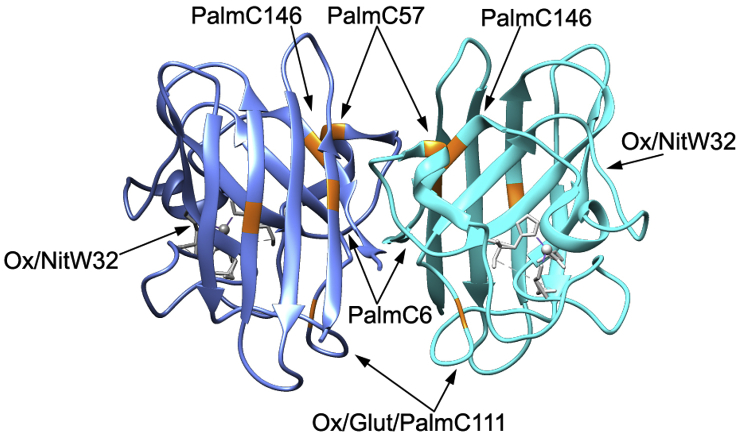
Fig. 3.
SOD1 sites of redox, palmitoylation, and nitration modifications. Crystal structure of mouse SOD1 (PDB: 3GTV) with known human SOD1 sites of redox, palmitoylation, and nitration modifications highlighted in orange (sites compiled from papers discussed in this review). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Cysteinylation. In support of the idea that oxidation may cause WT SOD1 to aggregate, cysteinylation at Cys111 protects SOD1 from oxidation and aggregation. Auclair et al. [73] observed cysteinylation and oxidation modifications on SOD1 from post-mortem human nervous tissue. In vitro cysteinylation offered almost full protection against peroxide-induced oxidation of other regions of SOD1. Thus, SOD1 may use cysteinylation as a defense mechanism against the destabilizing effects of oxidation. The crystal structure of cysteinylated SOD1, while very similar to native SOD1, shows a slight conformational change at the dimer interface (loop VI) and the electrostatic loop (loop VII) [74]. Based on data collected previously for a crystal structure of 2-mercaptoethanol-modified SOD1 [75,76], which also showed conformational changes when Cys111 was modified, Auclair et al. [74] predict that loops VI and VII are important for SOD1 stability. The cysteinylation site is shown in Fig. 3 and summarized in Table 3.
5. S-acylation
Another reversible PTM that occurs on cysteine side chains is S-acylation, in which a lipid is attached via a thioester bond. Taking advantage of acyl-biotin exchange (ABE), click chemistry, and a mass spectrometry approach, Marin et al. and Antinone et al. discovered that SOD1 is S-acylated via palmitoylation [77,78]. This modification commonly serves as a means to target and anchor proteins to cellular membranes [78].
The first studies on SOD1 palmitoylation discovered the modification at Cys6 and subsequent mutation of this cysteine to serine (C6S) reduced enzymatic activity (~30%), and impaired SOD1 nuclear localization [77]. An analysis of SOD1 by resin-assistant capture (acyl-RAC) suggested palmitoylation of SOD1 at Cys111, Cys57, and/or Cys146, as well [79]. Antinone et al. found fALS mutants of SOD1 in cell culture and in transgenic mice spinal cords displayed increased palmitoylation over WT SOD1 [78]. Furthermore, the authors suggested that SOD1 is palmitoylated prior to full maturation, the modification decreases with overexpression of CCS, and palmitoylation may play a role in SOD1 maturation by anchoring SOD1 and/or CCS to cell membranes [78,79]. The palmitoylation sites are shown in Fig. 3 and summarized in Table 4.
Table 4.
SOD1 palmitoylation and nitration sites. Summary of SOD1 palmitoylation and nitration sites described in this paper.
| Species | Residue | Modification | Modifying Enzyme (if known); Potential Location of PTM | Significance | Reference |
|---|---|---|---|---|---|
| human | Cys6 | palmitoylation | mutation to C6S (preventing palmitoylation) reduces enzymatic activity and nuclear transport | Marin, 2012 | |
| palmitoylation levels correlate with membrane-bound levels; likely palmitoylated prior to maturation | Antinone, 2013 | ||||
| human | Cys6, Cys111, Cys57 and/or Cys146 | palmitoylation | trend of higher palmitoylation on SOD1 in ALS patients (although not significant); hypothesized to play a role in maturation by anchoring SOD1 to the membrane | Antinone, 2017 | |
| human | Trp32 | nitration | partial loss of dismutase activity | Yamakura, 2001; Yamakura, 2005 | |
| human | Trp32 | substitution of Trp-32 with Phenylalanine decreases cytotoxicity and aggregation propensity of fALS mutant of SOD1 | Taylor, 2007 | ||
| bovine | unknown | nitration | no effect on dismutase activity | Ischiropoulos, 1992 |
6. Nitration
Similar to ROS, reactive nitrogen species (RNS) play a role in degenerative diseases associated with oxidative stress [[80], [81]]. Nitration of SOD1, specifically, has been reviewed elsewhere [80]. In brief, bovine SOD1 has been shown to contain 3-nitrotyrosine residues after reaction with peroxynitrite (a reactive nitrogen species), but this modification appears to have no effect on SOD1 enzymatic activity [81]. Human SOD1 does not contain any tyrosine residues but has the potential to be nitrated at Trp32 to 6-nitrotryptophan which demonstrates a partial loss of dismutase activity [82,83]. In another study, peroxynitrite reaction with human SOD1 led to inactivation of its enzymatic activity, but also histidinyl radical formation [84]. Further, substitution of Trp32 with a phenylalanine decreased the cytotoxicity and aggregation propensity of a fALS mutant of SOD1 [85]. As mentioned earlier, Trp32 can also be oxidized and has been shown to cause aggregation of SOD1 [69]. The known SOD1 nitration sites are shown in Fig. 3 (human sites only) and summarized in Table 4.
7. Exploring SOD1 PTMS of unknown function
To prioritize SOD1 PTMs for future study, we used the tool SAPH-ire, which was developed by the Torres laboratory to predict which domains and residues on SOD1 would most likely contain biologically relevant PTMs [16,22,23]. SAPH-ire is a machine learning approach that takes into account a variety of predictive elements around experimentally-identified PTMs, ultimately ranking PTMs in order of likelihood for biological function [22,23,86]. SAPH-ire gives each modified amino acid a FPx score that indicates its predicted likelihood for affecting protein function. We discovered that a relatively small region of SOD1, encompassing 31 amino acids between Ser98 and Lys128, included the seven PTM sites with the highest FPx scores (highlighted in Fig. 4; described in more detail in Ref. [16]).
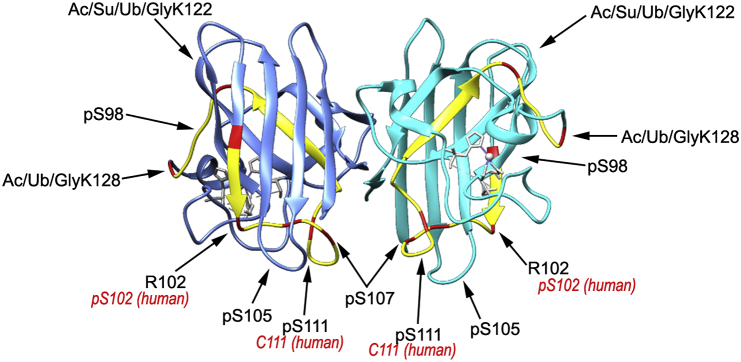
Fig. 4.
Highest ranked PTM ‘hotspots’ identified by SAPH-ire. Crystal structure of mouse SOD1 (PDB: 3GTV) with highest ranked PTM hotspots identified by SAPH-ire highlighted in red and the region between S98–K128 (which contains the seven highest ranked PTM hotspots) highlighted in yellow. Residues labeled in black are the mouse sites with the corresponding human site labeled in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Furthermore, this region contains part of the dimer interface (loop VI; residues 102–115) [76] and the electrostatic loop (loop VII; residues 122–143) [87]. The importance of part of the electrostatic loop is supported by recent work from Mojumdar et al., in which they used optical tweezers to measure transition states during SOD1 folding and unfolding [88]. They discovered that SOD1 maturation starts with a stable-core and misfolding is mainly centered around loops IV and VII. Additionally, they proposed that strand 8 (part of the dimer interface) and the electrostatic loop are the first to unfold and the last to refold. Thus, the electrostatic loop and dimer interface likely form last around the stable core [88]. Interestingly, loop VI encompasses Cys111, which as discussed earlier is critical for preserving the dimer interface and SOD1 stability [73,74]. Destabilization of the dimer interface and the electrostatic loop have been implicated in fALS [71,[89], [90], [91]]. Thus, even small charge perturbations in this region may alter SOD1 structure/function and stability [74]. The residues in this region that were highlighted by SAPH-ire are shown with their respective FPx ranking in Table 5.
Table 5.
Highest ranked SAPH-ire PTM sites. The highest ranked SAPH-ire PTM sites along with the predominant PTMs identified and what is known about these PTMs from the literature.
| SAPH-ire FPx RANK | MAP ID (IPR001424) | Residue in Mouse (P08228) | Predominant PTM | SAPH-ire Score (rel to max) | What is Already Known about this PTM in Different Species | Reference |
|---|---|---|---|---|---|---|
| 1 | 129 | Ser98 | Phos. | 100 | phosphorylation promotes SOD1 nuclear localization to maintain genomic stability; kinase is Dun1 | Tsang, 2014 |
| 2 | 142 | Ser111 | Phos. | 35 | ||
| 3 | 138 | Ser107 | Phos. | 24 | ||
| 4 | 167 | Lys128 | Ac./Ub./Gly. | 19 | glycation causes enzymatic deactivation | Fujii, 1996 |
| 5 | 133 | Arg102 (human Ser102) | Phos. | 18 | ||
| 6 | 155 | Lys122 | Ac./Su./Ub./Gly. | 16 | succinylation inactivates ROS scavenging activity; desuccinylated by SIRT5 | Lin, 2013 |
| acetylation/succinylation inhibit SOD1's anti-respiratory activity, succinyl-mimetics cause decreased growth and less healthy mitochondria in HCT116 cells, does not affect ROS scavenging activity | Banks, 2017 | |||||
| acetylated SOD1 found in distinct regions of adult central nervous system | Kaliszewski, 2016 | |||||
| 7 | 136 | Ser105 | Phos. | 14 |
8. Conclusion
At least 75 of the 154 amino acids that comprise SOD1 are sites of point mutations in patients with ALS, and many of these mutations cause SOD1 destabilization and amyloidogenesis [92]. Accordingly, the bulk of research on post-translational mechanisms of SOD regulation focuses on the effect of PTMs on SOD1 destabilization and aggregation propensity [[70], [71], [72],93,94]. Work from Shaw et al. and others supports the idea that SOD1 sits on a precipice of aggregation, upon which even small increases in net charge can push SOD1 toward aggregation [47,48]. Conversely, relatively small decreases in net charge—caused by acetylation of lysine or serine, for example—reduce SOD1 aggregation and the prion-like propagation of SOD1 fibrils [47]. Furthermore, there appears to be a variety of SOD1 intermediates, representing different folding states, all separated by small energy barriers [88]. Thus, it is not surprising that many net charge-altering SOD1 PTMs, including acetylation and redox-regulated PTMs (see above), alter the tendency of SOD1 to aggregate. Future therapeutic efforts may take advantage of this charge-dependency of SOD1 by designing therapeutics that either boost the stoichiometry of PTM-modified SOD1 (e.g., by targeting the cellular machinery that regulates a particular PTM) or target SOD1 directly to decrease its net charge [47].
In recent years, our understanding of SOD1 has expanded beyond its enzymatic ROS scavenging activity to new roles for SOD1 in the regulation of transcriptional pathways and mitochondrial metabolism. Furthermore, the essential role of SOD1 in promoting tumor cell survival has marked SOD1 as a potential therapeutic target in cancer [4,6,16,95]. In parallel to these discoveries, advancements in mass spectrometry-based PTM discovery revealed new complexity across the PTM landscape, which is also reflected in the array of PTMs now known to occur on SOD1. These include around 38 SOD1 PTMs catalogued on phosphosite.org (e.g., phosphorylation, ubiquitination and acylation), in addition to known sites of lipidation, nitration, cysteinylation, glutathionylation, and glycation, as discussed above.
The SOD1 PTMs presented here provide a hint of the complexity of SOD1 regulation and are beginning to reveal how the various functions of SOD1, including its non-canonical roles, are regulated within the cell. For example, a Dun1 (yeast homolog of CHK2)-mediated phosphorylation at Ser59 and Ser98 in yeast promotes translocation of SOD1 to the nucleus where it upregulates genes that combat oxidative stress [14]. Acylation of Lys122 inhibits SOD1-mediated suppression of mitochondrial respiration and links SOD1 to NAD+ levels via Sirtuin activity [16]. In addition, a TOR-mediated phosphorylation at Ser38 in yeast (Thr39 in humans) suppresses SOD1 enzymatic activity, which suggests a mechanism whereby SOD1 ROS scavenging is linked to nutrient levels via the mTORC1 complex [18]. Some of these PTM-driven mechanisms of SOD1 regulation are highlighted in Fig. 5. These new data place SOD1 at the center of a stress-responsive nexus with inputs from DNA damage, amino acid starvation, and general metabolic fitness, all of which may tune SOD1 activity (via PTMs) to fit the needs of the cell (Fig. 5).
Fig. 5.
PTM-driven mechanisms of SOD1 regulation.
PTMs often occur at low stoichiometry, on just a fraction of the total protein, which presents challenges to understanding PTM function. Given the complexity of SOD1 biology and the multiple signaling inputs that regulate SOD1 function, future work should carefully dissect the subcellular compartments where SOD1 PTMs occur and make use of improved tools—specific PTM probes, biochemical fractionation, and high-resolution imaging. In addition, a historical reliance on overexpression studies has likely led to misinterpretation of some PTM-null/mimic mutant phenotypes. Thus, we hope future PTM studies will rely more heavily on manipulation of endogenous SOD1 via CRISPR/Cas9 knock-in mutagenesis or knock-out and stable reconstitution approaches.
The SAPH-ire data presented in Table 5 provide a potential starting point for work on the numerous SOD1 PTMs of unknown function. We were surprised to find that the top seven SAPH-ire-predicted PTMs were within a small region of SOD1 between Ser98 and Lys128 [16]. This region is particularly interesting because it contains part of the dimer interface, Cys111, and the electrostatic loop—all of which have been implicated in maintaining SOD1 stability [71,73,74,76,87,[89], [90], [91]]. Although functions for a few of these PTMs are beginning to emerge, most are still uncharacterized in the literature. For example, a notable cluster of phosphorylations at Ser105, Ser107 and Ser111 (mouse numbering) has, to our knowledge, no known function. Future studies that reveal the impact and regulation of these and other SOD1 PTMs will expand the picture of SOD1 as a focal point for diverse stress signaling.
Note
Some studies include the start codon of SOD1 in residue numbering and some do not. An asterisk indicates that the numbering of the residue from this study has been changed from the original publication to make the numbering consistent across this manuscript (start codon not included in numbering).
Acknowledgements
We thank Dr. Matt Torres for help with SAPH-ire and insight into SOD1 PTMs. JLA is supported by a National Institutes of Health grant R15CA202619, a Research Scholar Grant, RSG-19-006-01-CCG, from the American Cancer Society and grants from The Fritz B. Burns Foundation and Simmons Center for Cancer Research.
References
- 1.McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 2.Kruman ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp. Neurol. 1999;160(1):28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- 3.Somwar R. Superoxide dismutase 1 (SOD1) is a target for a small molecule identified in a screen for inhibitors of the growth of lung adenocarcinoma cell lines. Proc. Natl. Acad. Sci. U. S. A. 2011;108(39):16375–16380. doi: 10.1073/pnas.1113554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glasauer A. Targeting SOD1 reduces experimental non-small-cell lung cancer. J. Clin. Investig. 2014;124(1):117–128. doi: 10.1172/JCI71714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papa L. SOD2 to SOD1 switch in breast cancer. J. Biol. Chem. 2014;289(9):5412–5416. doi: 10.1074/jbc.C113.526475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papa L., Manfredi G., Germain D. SOD1, an unexpected novel target for cancer therapy. Genes Canc. 2014;5:15–21. doi: 10.18632/genesandcancer.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue E. SOD1 is essential for the viability of DT40 cells and nuclear SOD1 functions as a guardian of genomic DNA. J. Nucleic Acids. 2010;2010 doi: 10.4061/2010/795946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardo C.A. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc. Natl. Acad. Sci. U. S. A. 1995;92(4):954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddi A.R., Culotta V.C. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell. 2013;152(1–2):224–235. doi: 10.1016/j.cell.2012.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corson L.B. Chaperone-facilitated copper binding is a property common to several classes of familial amyotrophic lateral sclerosis-linked superoxide dismutase mutants. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6361–6366. doi: 10.1073/pnas.95.11.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montllor-Albalate C. Extra-mitochondrial Cu/Zn superoxide dismutase (Sod1) is dispensable for protection against oxidative stress but mediates peroxide signaling in Saccharomyces cerevisiae. Redox Biol. 2019;21:101064. doi: 10.1016/j.redox.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei J.P. Evidence for a novel role of copper-zinc superoxide dismutase in zinc metabolism. J. Biol. Chem. 2001;276(48):44798–44803. doi: 10.1074/jbc.M104708200. [DOI] [PubMed] [Google Scholar]
- 13.Culotta V.C. A physiological role for Saccharomyces cerevisiae copper/zinc superoxide dismutase in copper buffering. J. Biol. Chem. 1995;270:29991–29997. doi: 10.1074/jbc.270.50.29991. [DOI] [PubMed] [Google Scholar]
- 14.Tsang C.K. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014;5:3446. doi: 10.1038/ncomms4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X. A new function of copper zinc superoxide dismutase: as a regulatory DNA-binding protein in gene expression in response to intracellular hydrogen peroxide. Nucleic Acids Res. 2019;47(10):5074–5085. doi: 10.1093/nar/gkz256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks C.J., Rodriguez N.W., Gashler K.R., Pandya R., Mortenson J.B., Whited M.D., Soderblom E.J., Thompson J.W., Moseley M.A., Reddi A.R., Tessem J.S., Torres M.P., Bikman B.T., Andersen J.L. Acylation of superoxide dismutase 1 (SOD1) at K122 governs SOD1-mediated inhibition of mitochondrial respiration. Mol. Cell Biol. 2017;37(20) doi: 10.1128/MCB.00354-17. e00354-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjornevik L.V. Nodularin exposure induces SOD1 phosphorylation and disrupts SOD1 Co-localization with actin filaments. Toxins. 2012;4(12):1482–1499. doi: 10.3390/toxins4121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsang C.K. SOD1 phosphorylation by mTORC1 couples nutrient sensing and redox regulation. Mol. Cell. 2018;70(3):502–515 e8. doi: 10.1016/j.molcel.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csar X.F. Copper/zinc superoxide dismutase is phosphorylated and modulated specifically by granulocyte-colony stimulating factor in myeloid cells. Proteomics. 2001;1(3):435–443. doi: 10.1002/1615-9861(200103)1:3<435::AID-PROT435>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox K.C. Modifications of superoxide dismutase (SOD1) in human erythrocytes a possible role in amyotrophic lateral sclerosis. J. Biol. Chem. 2009;284(20):13940–13947. doi: 10.1074/jbc.M809687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fay J.M. A phosphomimetic mutation stabilizes SOD1 and rescues cell viability in the context of an ALS-associated mutation. Structure. 2016;24(11):1898–1906. doi: 10.1016/j.str.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewhurst H.M., Choudhury S., Torres M.P. Structural analysis of PTM hotspots (SAPH-ire)--A quantitative informatics method enabling the discovery of novel regulatory elements in protein families. Mol. Cell. Proteom. 2015;14(8):2285–2297. doi: 10.1074/mcp.M115.051177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres M.P., Dewhurst H., Sundararaman N. Proteome-wide structural analysis of PTM hotspots reveals regulatory elements predicted to impact biological function and disease. Mol. Cell. Proteom. 2016;15(11):3513–3528. doi: 10.1074/mcp.M116.062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banci L. Structural and dynamic aspects related to oligomerization of apo SOD1 and its mutants. Proc. Natl. Acad. Sci. U. S. A. 2009;106(17):6980–6985. doi: 10.1073/pnas.0809845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitch J.M. Post-translational modification of Cu/Zn superoxide dismutase under anaerobic conditions. Biochemistry. 2012;51(2):677–685. doi: 10.1021/bi201353y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Getzoff E.D. Electrostatic recognition between superoxide and copper, zinc superoxide dismutase. Nature. 1983;306(5940):287–290. doi: 10.1038/306287a0. [DOI] [PubMed] [Google Scholar]
- 27.Coimbra-Costa D. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017;12:216–225. doi: 10.1016/j.redox.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner G.R., Hirschey M.D. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol. Cell. 2014;54(1):5–16. doi: 10.1016/j.molcel.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner G.R., Payne R.M. Widespread and enzyme-independent Nε-acetylation and Nε-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J. Biol. Chem. 2013;288(40):29036–29045. doi: 10.1074/jbc.M113.486753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinert B.T. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol. Syst. Biol. 2015;11(10):833. doi: 10.15252/msb.156513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen J.L. A biotin switch-based proteomics approach identifies 14-3-3ζ as a target of Sirt1 in the metabolic regulation of caspase-2. Mol. Cell. 2011;43(5):834–842. doi: 10.1016/j.molcel.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin C. Acetylation at lysine 71 inactivates superoxide dismutase 1 and sensitizes cancer cells to genotoxic agents. Oncotarget. 2015;6(24):20578–20591. doi: 10.18632/oncotarget.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaliszewski M. SOD1 lysine 123 acetylation in the adult central nervous system. Front. Cell. Neurosci. 2016;10:287. doi: 10.3389/fncel.2016.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinert B.T. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013;4(4):842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Mertins P. Integrated proteomic analysis of post-translational modifications by serial enrichment. Nat. Methods. 2013;10(7):634–637. doi: 10.1038/nmeth.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beli P. Proteomic investigations reveal a role for RNA processing factor THRAP3 in the DNA damage response. Mol. Cell. 2012;46(2):212–225. doi: 10.1016/j.molcel.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao S. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choudhary C. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 39.Getzoff E.D. Faster superoxide dismutase mutants designed by enhancing electrostatic guidance. Nature. 1992;358(6384):347–351. doi: 10.1038/358347a0. [DOI] [PubMed] [Google Scholar]
- 40.Lin Z.F. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem. Biophys. Res. Commun. 2013;441(1):191–195. doi: 10.1016/j.bbrc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Takeshige K., Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem. J. 1979;180(1):129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raha S., Robinson B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000;25(10):502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 43.Longo V.D., Gralla E.B., Valentine J.S. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J. Biol. Chem. 1996;271(21):12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 44.Ouslimani N. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. 2005;54(6):829–834. doi: 10.1016/j.metabol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Kelly B. Metformin inhibits the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of interleukin-1beta (IL-1beta) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J. Biol. Chem. 2015;290(33):20348–20359. doi: 10.1074/jbc.M115.662114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marycz K. Metformin decreases reactive oxygen species, enhances osteogenic properties of adipose-derived multipotent mesenchymal stem cells in vitro, and increases bone density in vivo. Oxid. Med. Cell Longev. 2016;2016:9785890. doi: 10.1155/2016/9785890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdolvahabi A. Arresting amyloid with coulomb's law: acetylation of ALS-linked SOD1 by aspirin impedes aggregation. Biophys. J. 2015;108(5):1199–1212. doi: 10.1016/j.bpj.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasouli S. Lysine acylation in superoxide dismutase-1 electrostatically inhibits formation of fibrils with prion-like seeding. J. Biol. Chem. 2017;292(47):19366–19380. doi: 10.1074/jbc.M117.805283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fei E. SUMO-1 modification increases human SOD1 stability and aggregation. Biochem. Biophys. Res. Commun. 2006;347(2):406–412. doi: 10.1016/j.bbrc.2006.06.092. [DOI] [PubMed] [Google Scholar]
- 50.Niikura T., Kita Y., Abe Y. SUMO3 modification accelerates the aggregation of ALS-linked SOD1 mutants. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kabuta T., Suzuki Y., Wada K. Degradation of amyotrophic lateral sclerosis-linked mutant Cu,Zn-superoxide dismutase proteins by macroautophagy and the proteasome. J. Biol. Chem. 2006;281(41):30524–30533. doi: 10.1074/jbc.M603337200. [DOI] [PubMed] [Google Scholar]
- 52.Kato S. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am. J. Pathol. 1997;151(2):611–620. [PMC free article] [PubMed] [Google Scholar]
- 53.Stieber A., Gonatas J.O., Gonatas N.K. Aggregation of ubiquitin and a mutant ALS-linked SOD1 protein correlate with disease progression and fragmentation of the Golgi apparatus. J. Neurol. Sci. 2000;173(1):53–62. doi: 10.1016/s0022-510x(99)00300-7. [DOI] [PubMed] [Google Scholar]
- 54.Bruijn L.I. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18(2):327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 55.Farrawell N.E. SOD1(A4V) aggregation alters ubiquitin homeostasis in a cell model of ALS. J. Cell Sci. 2018;131(11) doi: 10.1242/jcs.209122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niwa J. Dorfin ubiquitylates mutant SOD1 and prevents mutant SOD1-mediated neurotoxicity. J. Biol. Chem. 2002;277(39):36793–36798. doi: 10.1074/jbc.M206559200. [DOI] [PubMed] [Google Scholar]
- 57.Miyazaki K. NEDL1, a novel ubiquitin-protein isopeptide ligase for dishevelled-1, targets mutant superoxide dismutase-1. J. Biol. Chem. 2004;279(12):11327–11335. doi: 10.1074/jbc.M312389200. [DOI] [PubMed] [Google Scholar]
- 58.Yonashiro R. Mitochondrial ubiquitin ligase MITOL ubiquitinates mutant SOD1 and attenuates mutant SOD1-induced reactive oxygen species generation. Mol. Biol. Cell. 2009;20(21):4524–4530. doi: 10.1091/mbc.E09-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basso M. Insoluble mutant SOD1 is partly oligoubiquitinated in amyotrophic lateral sclerosis mice. J. Biol. Chem. 2006;281(44):33325–33335. doi: 10.1074/jbc.M603489200. [DOI] [PubMed] [Google Scholar]
- 60.Ganesan S. Mutant SOD1 detoxification mechanisms in intact single cells. Cell Death Differ. 2008;15(2):312–321. doi: 10.1038/sj.cdd.4402262. [DOI] [PubMed] [Google Scholar]
- 61.Bruening W. Up-regulation of protein chaperones preserves viability of cells expressing toxic Cu/Zn-superoxide dismutase mutants associated with amyotrophic lateral sclerosis. J. Neurochem. 1999;72(2):693–699. doi: 10.1046/j.1471-4159.1999.0720693.x. [DOI] [PubMed] [Google Scholar]
- 62.Tummala H. Inhibition of chaperone activity is a shared property of several Cu,Zn-superoxide dismutase mutants that cause amyotrophic lateral sclerosis. J. Biol. Chem. 2005;280(18):17725–17731. doi: 10.1074/jbc.M501705200. [DOI] [PubMed] [Google Scholar]
- 63.Arai K. Glycation and inactivation of human Cu-Zn-superoxide dismutase. Identification of the in vitro glycated sites. J. Biol. Chem. 1987;262(35):16969–16972. [PubMed] [Google Scholar]
- 64.Fujii J. Oxidative stress caused by glycation of Cu,Zn-superoxide dismutase and its effects on intracellular components. Nephrol. Dial. Transplant. 1996;11(Suppl 5):34–40. doi: 10.1093/ndt/11.supp5.34. [DOI] [PubMed] [Google Scholar]
- 65.Sirangelo I. Glycation in demetalated superoxide dismutase 1 prevents amyloid aggregation and produces cytotoxic ages adducts. Front Mol. Biosci. 2016;3:55. doi: 10.3389/fmolb.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bosco D.A. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat. Neurosci. 2010;13(11):1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu W.C. Pathological hydrogen peroxide triggers the fibrillization of wild-type SOD1 via sulfenic acid modification of Cys-111. Cell Death Dis. 2018;9(2):67. doi: 10.1038/s41419-017-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martins D., English A.M. SOD1 oxidation and formation of soluble aggregates in yeast: relevance to sporadic ALS development. Redox Biol. 2014;2:632–639. doi: 10.1016/j.redox.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H. Bicarbonate-dependent peroxidase activity of human Cu,Zn-superoxide dismutase induces covalent aggregation of protein: intermediacy of tryptophan-derived oxidation products. J. Biol. Chem. 2003;278(26):24078–24089. doi: 10.1074/jbc.M302051200. [DOI] [PubMed] [Google Scholar]
- 70.Rakhit R. Oxidation-induced misfolding and aggregation of superoxide dismutase and its implications for amyotrophic lateral sclerosis. J. Biol. Chem. 2002;277(49):47551–47556. doi: 10.1074/jbc.M207356200. [DOI] [PubMed] [Google Scholar]
- 71.Rakhit R. Monomeric Cu,Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J. Biol. Chem. 2004;279(15):15499–15504. doi: 10.1074/jbc.M313295200. [DOI] [PubMed] [Google Scholar]
- 72.Kabashi E. Oxidized/misfolded superoxide dismutase-1: the cause of all amyotrophic lateral sclerosis? Ann. Neurol. 2007;62(6):553–559. doi: 10.1002/ana.21319. [DOI] [PubMed] [Google Scholar]
- 73.Auclair J.R. Post-translational modification by cysteine protects Cu/Zn-superoxide dismutase from oxidative damage. Biochemistry. 2013;52(36):6137–6144. doi: 10.1021/bi4006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Auclair J.R. Structural consequences of cysteinylation of Cu/Zn-superoxide dismutase. Biochemistry. 2013;52(36):6145–6150. doi: 10.1021/bi400613h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujiwara N. Oxidative modification to cysteine sulfonic acid of Cys111 in human copper-zinc superoxide dismutase. J. Biol. Chem. 2007;282(49):35933–35944. doi: 10.1074/jbc.M702941200. [DOI] [PubMed] [Google Scholar]
- 76.Ihara K. Structural switching of Cu,Zn-superoxide dismutases at loop VI: insights from the crystal structure of 2-mercaptoethanol-modified enzyme. Biosci. Rep. 2012;32(6):539–548. doi: 10.1042/BSR20120029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marin E.P. Endothelial cell palmitoylproteomic identifies novel lipid-modified targets and potential substrates for protein acyl transferases. Circ. Res. 2012;110(10):1336–1344. doi: 10.1161/CIRCRESAHA.112.269514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antinone S.E. Palmitoylation of superoxide dismutase 1 (SOD1) is increased for familial amyotrophic lateral sclerosis-linked SOD1 mutants. J. Biol. Chem. 2013;288(30):21606–21617. doi: 10.1074/jbc.M113.487231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antinone S.E., S-acylation of SOD1 CCS, and a stable SOD1-CCS heterodimer in human spinal cords from ALS and non-ALS subjects. Sci. Rep. 2017;7:41141. doi: 10.1038/srep41141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamakura F., Kawasaki H. Post-translational modifications of superoxide dismutase. Biochim. Biophys. Acta Protein Proteonomics. 2010;1804(2):318–325. doi: 10.1016/j.bbapap.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Ischiropoulos H. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 82.Yamakura F. Modification of a single tryptophan residue in human Cu,Zn-superoxide dismutase by peroxynitrite in the presence of bicarbonate. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2001;1548(1):38–46. doi: 10.1016/s0167-4838(01)00212-6. [DOI] [PubMed] [Google Scholar]
- 83.Yamakura F. Nitrated and oxidized products of a single tryptophan residue in human Cu,Zn-superoxide dismutase treated with either peroxynitrite-carbon dioxide or myeloperoxidase-hydrogen peroxide-nitrite. J. Biochem. 2005;138(1):57–69. doi: 10.1093/jb/mvi095. [DOI] [PubMed] [Google Scholar]
- 84.Alvarez B. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic. Biol. Med. 2004;37(6):813–822. doi: 10.1016/j.freeradbiomed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 85.Taylor D.M. Tryptophan 32 potentiates aggregation and cytotoxicity of a copper/zinc superoxide dismutase mutant associated with familial amyotrophic lateral sclerosis. J. Biol. Chem. 2007;282(22):16329–16335. doi: 10.1074/jbc.M610119200. [DOI] [PubMed] [Google Scholar]
- 86.Dewhurst H.M., Torres M.P. Systematic analysis of non-structural protein features for the prediction of PTM function potential by artificial neural networks. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rakhit R., Chakrabartty A. Structure, folding, and misfolding of Cu,Zn superoxide dismutase in amyotrophic lateral sclerosis. Biochim. Biophys. Acta. 2006;1762(11–12):1025–1037. doi: 10.1016/j.bbadis.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Sen Mojumdar S. Partially native intermediates mediate misfolding of SOD1 in single-molecule folding trajectories. Nat. Commun. 2017;8(1):1881. doi: 10.1038/s41467-017-01996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Molnar K.S. A common property of amyotrophic lateral sclerosis-associated variants: destabilization of the copper/zinc superoxide dismutase electrostatic loop. J. Biol. Chem. 2009;284(45):30965–30973. doi: 10.1074/jbc.M109.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hornberg A. The coupling between disulphide status, metallation and dimer interface strength in Cu/Zn superoxide dismutase. J. Mol. Biol. 2007;365(2):333–342. doi: 10.1016/j.jmb.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 91.Rakhit R. An immunological epitope selective for pathological monomer-misfolded SOD1 in ALS. Nat. Med. 2007;13(6):754–759. doi: 10.1038/nm1559. [DOI] [PubMed] [Google Scholar]
- 92.Saccon R.A. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136(Pt 8):2342–2358. doi: 10.1093/brain/awt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bredesen D.E. Do posttranslational modifications of CuZnSOD lead to sporadic amyotrophic lateral sclerosis? Ann. Neurol. 1997;42(2):135–137. doi: 10.1002/ana.410420202. [DOI] [PubMed] [Google Scholar]
- 94.Choi J. Oxidative modifications and aggregation of Cu,Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J. Biol. Chem. 2005;280(12):11648–11655. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- 95.Huang P. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407(6802):390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]