Abstract
Objective
To determine the effect of remote ischemic preconditioning (RIPC) on dynamic cerebral autoregulation (dCA) and various blood biomarkers in healthy adults.
Methods
A self-controlled interventional study was conducted. Serial measurements of dCA were performed at 7 time points (7, 9, and 11 am; 2, 5, and 8 pm, and 8 am on the next day) without or with RIPC, carried out at 7:20 to 8 am. Venous blood samples were collected at baseline (7 am) and 1 hour after RIPC, and blood biomarkers, including 5 neuroprotective factors and 25 inflammation-related biomarkers, were measured with a quantitative protein chip.
Results
Fifty participants were enrolled (age 34.54 ± 12.01 years, 22 men). Compared with the results on the day without RIPC, dCA was significantly increased at 6 hours after RIPC, and the increase was sustained for at least 24 hours. After RIPC, 2 neuroprotective factors (glial cell-derived neurotrophic factor and vascular endothelial growth factor-A) and 4 inflammation-related biomarkers (transforming growth factor-β1, leukemia inhibitory factor, matrix metallopeptidase-9, and tissue inhibitor of metalloproteinase-1) were significantly elevated compared with their baseline levels. Conversely, monocyte chemoattractant protein-1 was significantly lower compared with its baseline level.
Conclusions
RIPC induces a sustained increase of dCA from 6 to at least 24 hours after treatment in healthy adults. In addition, several neuroprotective and inflammation-related blood biomarkers were differentially regulated shortly after RIPC. The increased dCA and altered blood biomarkers may collectively contribute to the beneficial effects of RIPC on cerebrovascular function.
ClinicalTrials.gov identifier:
Remote ischemic preconditioning (RIPC), defined as brief transient episodes of ischemia/reperfusion applied in distant tissues or organs, renders remote tissues and organs resistant to a subsequent prolonged ischemia insult.1 Studies of cardiovascular diseases have repeatedly shown that RIPC could significantly reduce infarct size after myocardial ischemia in both animals and human patients.1–4 Recently, several animal and clinical studies demonstrated a similar beneficial role of RIPC during cerebral ischemia/reperfusion injury and cerebral small vessel disease.5–10 It has been shown that RIPC activates both neuronal signals and humoral factors to confer its protective effects on remote tissues and organs,1 but the underlying mechanisms, especially in the brain, remain unclear.
Dynamic cerebral autoregulation (dCA) is a unique function of the cerebrovasculature and is critical to the regulation of cerebral hemodynamics.11 dCA predicts the occurrence and prognosis of cerebrovascular disease in the clinic.12 Previous studies showed that RIPC can regulate several blood biomarkers such as adenosine,13 bradykinin,1 and nitric oxide or nitrite.14 Several of these biomarkers are vasoactive, so they may affect dCA.15,16 Nevertheless, it remains unknown whether RIPC can regulate dCA in humans. Moreover, recent studies have shown that RIPC may have neuroprotective and inflammation regulatory functions in animal models.9,17,18 However, whether neuroprotective and inflammation-related blood biomarkers are regulated by RIPC in humans is unknown.
In the present study, we hypothesize that RIPC improves dCA and affects neuroprotective and inflammation-related blood biomarkers, and we test this using the following approaches. First, we continuously monitored the changes of dCA in healthy adults at 7 time points (baseline and 1, 3, 6, 9, 12, and 24 hours after RIPC). Second, we assessed the effect of RIPC on 30 biomarkers in venous blood, including 5 neuroprotective factors and 25 inflammation-related biomarkers. We demonstrated that RIPC can persistently improve dCA and differentially regulate a series of neuroprotective and inflammation-related biomarkers in the blood.
Methods
Standard protocol approvals, registrations, and patient consents
This prospective study was approved by the ethics committee of the First Hospital of Jilin University. Written informed consent was obtained from all participants. The participants had the right to withdraw at any time point during the procedure. This trial is registered at ClinicalTrials.gov (NCT02965547).
Participants
Fifty healthy adult volunteers (age 18–70 years, men and women, Asian) were included in the present study from January 2017 to July 2017. The exclusion criteria included (1) currently experiencing or having a history of chronic physical or mental diseases (including generalized anxiety disorder, depression, insomnia, hypertension, diabetes mellitus and chronic heart disease), (2) having an infectious disease in the past month, (3) being pregnant or lactating (women), (4) smoking or heavy drinking (formerly or currently), and (5) being unable to cooperate sufficiently to complete the dCA examination. Each participant received a comprehensive physical examination by a physician to exclude potential disease before inclusion in the study.
Study design
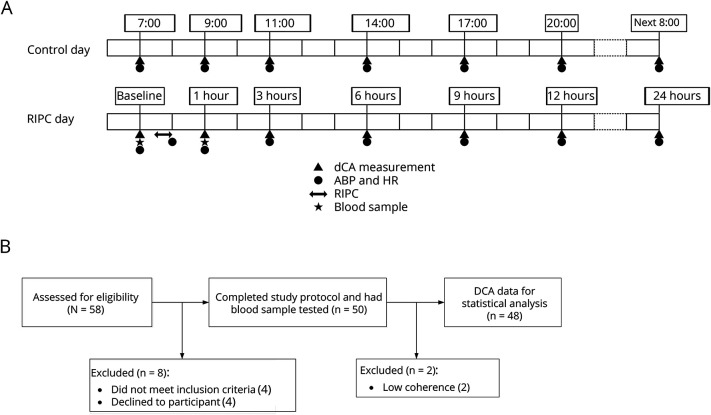
Each participant received two 24-hour monitoring sessions. The first 24-hour session was defined as the control day, and the second 24-hour session was the RIPC day. The control day and the RIPC day were consecutive days (control day: day 1 and 2; RIPC day: day 3 and 4). Serial measurements of dCA were performed at 7 time points on both the control day and the RIPC day. On the control day, the 7 time points were 7, 9, and 11 am; 2, 5, and 8 pm; and 8 am on the next day. On the RIPC day, the RIPC was carried out at 7:20 to 8 am, and the 7 time points were 7 (baseline), 9 (1 hour after RIPC), and 11 (3 hours after RIPC) am; 2 (6 hours after RIPC), 5 (9 hours after RIPC), and 8 pm (12 hours after RIPC); and 8 am on the next day (24 hours after RIPC, figure 1A). Blood samples were collected from the cubital vein of each participant before and 1 hour after RIPC intervention for further quantitative protein chip testing.
Figure 1. Flowcharts and protocols of the study.
(A) Flowcharts of the study. (B) Study protocols. ABP = arterial blood pressure; DCA = dynamic cerebral autoregulation; HR = heart rate; RIPC = remote ischemic preconditioning.
Intervention
The RIPC was performed by an automatic device (BB-RIC-D1/LAPUL Medical Devices Co, Ltd, China). The whole intervention process consisted of 4 cycles of extremity ischemia: 5 minutes of blood pressure cuff inflation to 200 mm Hg, followed by 5 minutes of cuff deflation; the whole process took 40 minutes. Tourniquets were applied to 1 upper arm and 1 thigh. This intervention was undertaken only once in each participant. Measurement of arterial blood pressure (ABP) was performed in the brachial artery by an automatic blood pressure monitor (Omron 711, Tokyo, Japan) immediately after RIPC.
dCA measurement and data analysis
All dCA measurements were performed in a specific, quiet examination room with a controlled temperature of 20°C to 24°C to minimize confounding stimuli. Participants were asked to adopt a relaxed supine position for 10 minutes, and ABP was measured at the brachial artery by an automatic blood pressure monitor (Omron 711). Continuous ABP was measured noninvasively with a servo-controlled plethysmograph (Finometer model 1, FMS, Rotterdam, the Netherlands) on the middle finger. Two 2-MHz transcranial Doppler probes (MultiDop X2, DWL, Sipplingen, Germany) were used to simultaneously measure continuous cerebral blood flow velocity (CBFV) in the left and right middle cerebral arteries at a depth of 45 to 60 mm. The probes were placed over temporal windows and fixed with a customized head frame. CBFV and continuous ABP were recorded simultaneously from each participant in the supine position for 10 minutes. All data were stored for offline assessment and analysis.
Continuous recordings of ABP and CBFV were processed by MATLAB (MathWorks, Inc, Natick, MA) using scripts developed by the research team. The ABP and CBFV signals in each participant were aligned by a cross-correlation function. The resultant signals were then down-sampled to 1 Hz after application of an antialias filter with a cutoff frequency at 0.5 Hz. The dynamic relationship between ABP and CBFV was assessed by transfer function analysis (TFA)19 with an algorithm used in previous studies.20 TFA was thus calculated in the frequency domain as the quotient of the cross-spectrum of the 2 signals and the autospectrum of ABP. Phase difference (PD), gain, and coherence function within a low-frequency range, 0.06 to 0.12 Hz, were then derived from TFA to evaluate dCA. A low value of PD indicated that CBFV followed the changes of ABP passively, whereas a high value of PD suggested that CBFV was actively regulated to counteract the fluctuations of ABP. Because estimates of TFA are unreliable when coherence between the signals is low, recordings with low coherence between ABP and CBFV (≤0.40) were not included in the later statistical analysis.
Blood samples and quantitative protein chip testing
Blood samples were collected from the cubital vein of each participant before and 1 hour after RIPC. The volume of each sample was ≈6 mL. All blood samples were centrifuged, and the supernatant was stored in separate vials at −80°C for batch serum analysis.
Differential protein screening was conducted with the RayBiotech Human Custom Antibody Array (RayBiotech, Inc. Norcross, GA; catalog No. QAH-CUST-H12), which consists of 16 subarrays in 1 slide and allows the interrogation of 1 sample per subarray (8 for standard and 8 for the samples) according to the manufacturer's instructions. Briefly, monoclonal antibodies against various proteins were printed on the slides as bait to capture the corresponding proteins in serum, incubated with a mixture of biotinylated secondary antibodies, and then detected with Cy3-labeled streptavidin. Each analyte was assayed in quadruplicate. The slides were then scanned with a Mapix scanner (InnoScan 300 Microarray Scanner, Innopsys, France) and further processed by the Mapix software. In the array, positive control spots composed of standardized amounts of biotinylated immunoglobulin G were printed directly onto the array. All other variables being equal, the positive control intensities should be the same for each subarray. This allows for normalization of results from different subarrays (or samples). The array also included negative control spots consisting of buffer alone (used to dilute antibodies printed on the array).
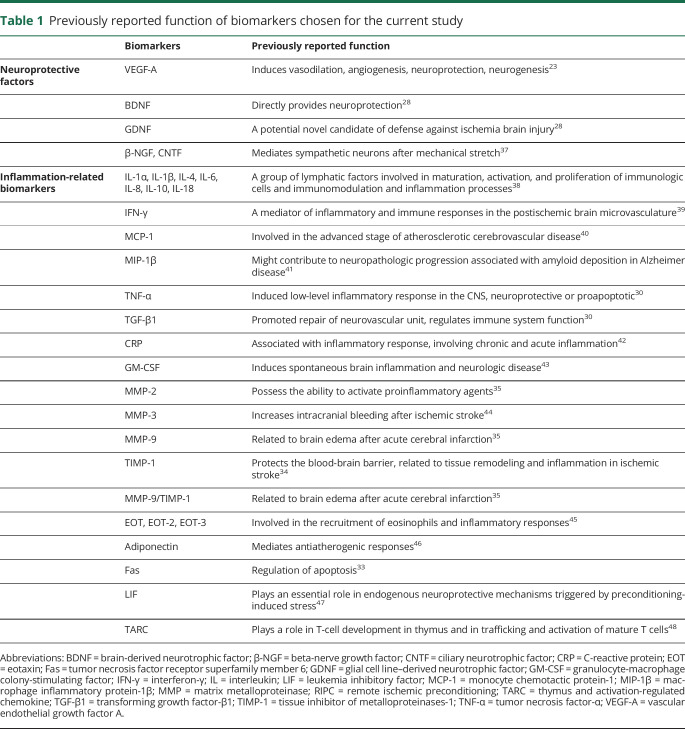
Because several previous studies showed that RIPC induced changes of serum biomarkers rapidly (within 30 minutes to 1 hour) after preconditioning,21 we compared cubital vein blood components before and 1 hour after RIPC using the quantitative protein chip to identify the biomarkers altered by RIPC. According to previous studies of RIPC in animal models,9,17,18 30 biomarkers were chosen on the basis of their previously reported neuroprotective function or their regulation of inflammatory responses. The reasons why each biomarker was chosen are listed in table 1. The 5 neuroprotective factors included brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), β-nerve growth factor, ciliary neurotrophic factor, and vascular endothelial growth factor-A (VEGF-A; also a potent vasoactive factor). The 25 inflammation-related biomarkers included interleukin (IL)-1α, IL-1β, IL-4, IL-6, IL-8, IL-18, IL-10, interferon-γ, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1β, matrix metalloproteinase (MMP)-2 , MMP-3, MMP-9, tissue inhibitor of metalloproteinases-1 (TIMP-1), tumor necrosis factor-α (TNF-α), transforming growth factor-β1 (TGF-β1), adiponectin, C-reactive protein, granulocyte-macrophage colony-stimulating factor, eotaxin (EOT), EOT-2, EOT-3, adiponectin, tumor necrosis factor receptor superfamily member 6, leukemia inhibitory factor (LIF), and thymus and activation-regulated chemokine.
Table 1.
Previously reported function of biomarkers chosen for the current study
Statistical analysis
The data were analyzed with the Statistical Program for Social Sciences, version 22.0 (SPSS; IBM, West Grove, PA). Continuous variables were described as mean ± SD or median (interquartile range), depending on the distribution of the variable. The Shapiro-Wilk test was used to test the normality of data. A paired t test was used to compare the difference between the 2 groups if they were in normal distributions. Alternatively, the Wilcoxon signed-rank test was used if the data distribution was not normal. Categorical variables were described as absolute values and percentages. To compare PD, gain, mean arterial pressure, and heart rate between RIPC and different time points, a mixed linear model for repeated measurements was used. Both of the 2 factors (RIPC and time) that were included in the mixed linear model were considered to be the factor of repeated measurement. Multiple biomarkers were compared between baseline and 1 hour after RIPC, so Bonferroni correction for multiple comparison between groups was applied. The adjusted p value was obtained by multiplying the crude p value by the number of multiple comparisons (6 times). All tests were 2 tailed, and values of p < 0.05 were considered statistically significant.
Data availability
The deidentified data generated and analyzed in the current study will be available and shared by request from any qualified investigator for purposes of replicating procedures and results.
Results
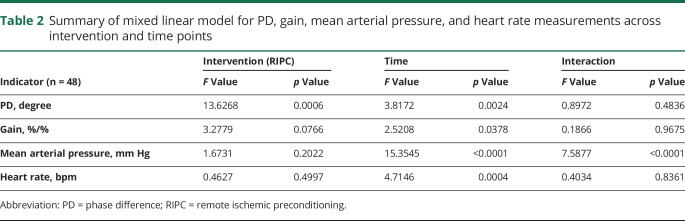
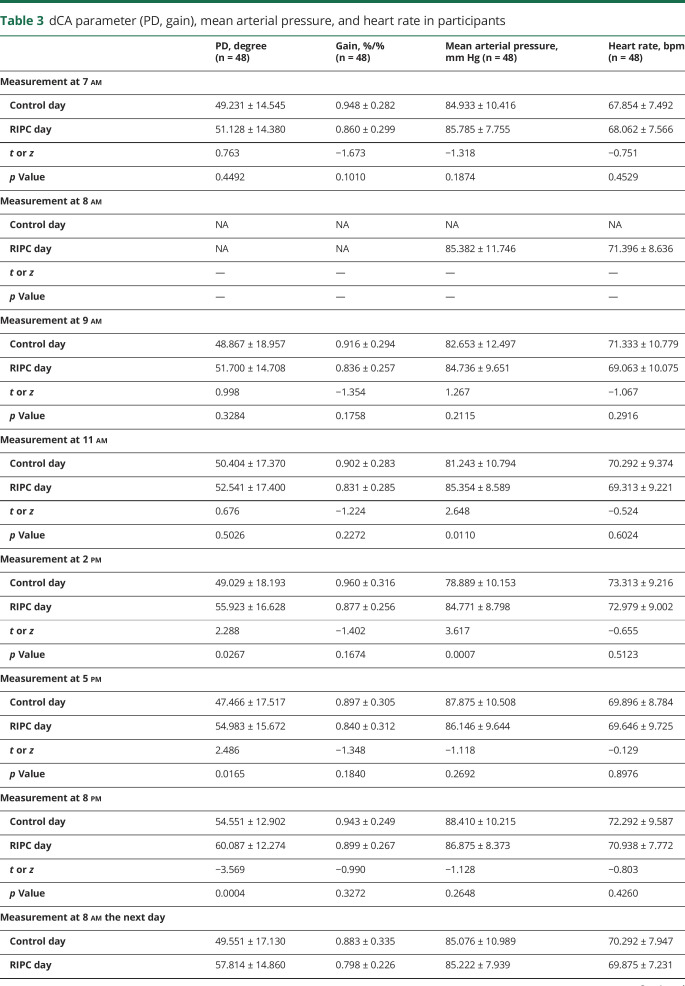
Fifty-eight healthy adult volunteers were assessed for eligibility, and 8 volunteers who did not meet the inclusion criteria or declined to participate were excluded. In the current study, we enrolled 50 healthy adults (age 34.54 ± 12.01 years, 22 men [44%], all Asian). Data from 2 participants were excluded due to low coherence. Thus, the study included 48 participants in total for dCA analysis. A summary of the mixed linear model for PD, gain, mean arterial pressure, and heart rate measurements across intervention and time points is presented in table 2. Mean arterial pressure and heart rate of serial measurements are presented in table 3 and figure 2.
Table 2.
Summary of mixed linear model for PD, gain, mean arterial pressure, and heart rate measurements across intervention and time points
Table 3.
dCA parameter (PD, gain), mean arterial pressure, and heart rate in participants
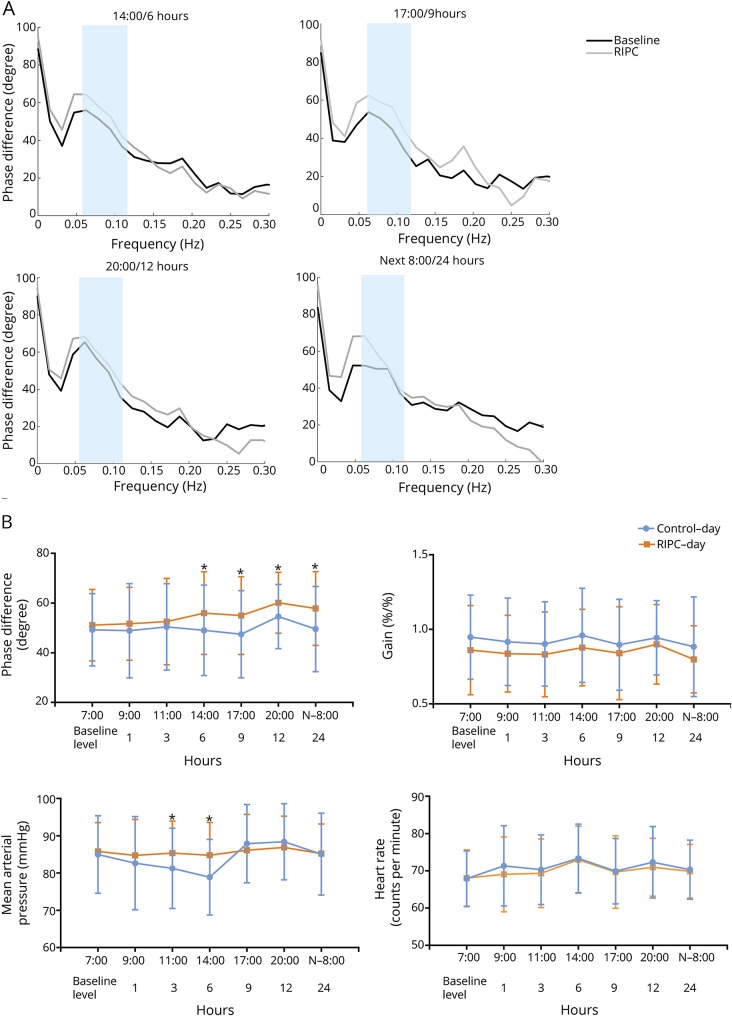
Figure 2. Autoregulatory parameter and statistical analysis of dCA, MAP, and HR.
(A) Autoregulatory parameter derived from the transfer function analysis. (B) Statistical analysis of dynamic cerebral autoregulation (dCA), mean arterial pressure (MAP), and heart rate (HR) by serial measurements. *p < 0.05 for comparison between the control day and remote ischemic preconditioning (RIPC) day.
Dynamic cerebral autoregulation
The mixed linear model identified the highly significant effects of intervention (p = 0.0006) and time points (p = 0.0024) on PD but did not identify the interaction effect of them (p = 0.4836) (table 2). Compared with the PD values at the same time points on the control day and RIPC day, the PD was not significantly altered within 3 hours after RIPC. However, the PD value significantly increased starting from 6 hours after RIPC, and the increase was sustained for at least 18 hours until 24 hours after RIPC (table 3 and figure 2). The gain did not differ significantly between the control day and the RIPC day across all study time points.
Blood biomarkers
Neuroprotective factors
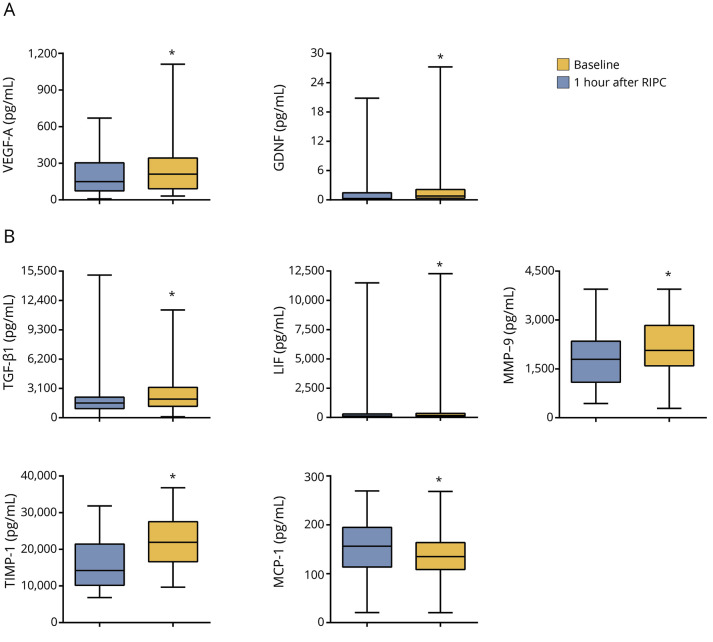
One hour after RIPC, VEGF-A and GDNF in venous blood serum increased significantly compared to their baseline levels (figures 3 and 4A). BDNF, ciliary neurotrophic factor, and β-nerve growth factor in venous blood serum at 1 hour after RIPC were not significantly different from their baseline levels (figure 3).
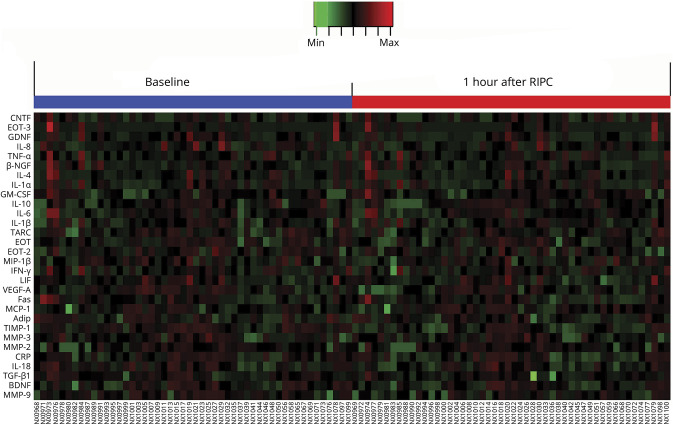
Figure 3. Heat map of quantitative protein chip of 30 biomarkers.
Adip = adiponectin; BDNF = brain-derived neurotrophic factor; β-NGF = β-nerve growth factor; CNTF = ciliary neurotrophic factor; CRP = C-reactive protein; EOT = eotaxin; Fas = tumor necrosis factor receptor superfamily member 6; GDNF = glial cell line–derived neurotrophic factor; GM-CSF = granulocyte-macrophage colony-stimulating factor; IFN-γ= interferon-γ; IL = interleukin; LIF = leukemia inhibitory factor; MCP-1 = monocyte chemotactic protein-1; MIP-1β = macrophage inflammatory protein-1β; MMP, matrix metalloproteinase; NX = blood sample number; RIPC = remote ischemic preconditioning; TARC = thymus and activation-regulated chemokine; TGF-β1= transforming growth factor-β1; TIMP-1 = tissue inhibitor of metalloproteinases-1; TNF-α= tumor necrosis factor-α; VEGF-A = vascular endothelial growth factor A.
Figure 4. Statistical distributions of 7 biomarkers with significant differences.
(A) Statistical distributions of the neuroprotective factors vascular endothelial growth factor A (VEGF-A) and glial cell line–derived neurotrophic factor (GDNF) at baseline and 1 hour after remote ischemic preconditioning (RIPC) in each group. (B) Statistical distributions of inflammation-related biomarkers transforming growth factor-β1 (TGF-β1), leukemia inhibitory factor (LIF), matrix metalloproteinase-9 (MMP-9), tissue inhibitor of metalloproteinases-1 (TIMP-1), and monocyte chemotactic protein-1 (MCP-1) at baseline and 1 hour after RIPC in each group. Whiskers represent highest and lowest values; middle square represents interquartile values; and middle line indicates median. *Adjusted p < 0.05 for comparison with the baseline level.
Inflammation-related biomarkers
One hour after RIPC, the levels of TGF-β1, LIF, MMP-9, and TIMP-1 were significantly higher than the baseline levels of these biomarkers (figures 3 and 4B). In contrast, the level of MCP-1 was significantly lower than the baseline level (figures 3 and 4B).
The IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-18, interferon-γ, macrophage inflammatory protein-1β, MMP-2, MMP-3, TNF-α, C-reactive protein, granulocyte-macrophage colony-stimulating factor, adiponectin, EOT, EOT-2, EOT-3, tumor necrosis factor receptor superfamily member 6, thymus and activation-regulated chemokine, and MMP-9/TIMP-1 ratio levels in venous blood serum at 1 hour after RIPC were not significantly different from their baseline levels (figure 3).
Discussion
In the present study, we found that after RIPC dCA improved from 6 to 24 hours after the intervention in healthy adults. RIPC was also associated with changes in some neuroprotective and inflammation-related biomarkers in blood. The increased dCA and altered blood biomarkers may contribute at least partially to the beneficial effects of RIPC on cerebrovascular function.
Previous studies suggested that RIPC can protect the target organ or tissue by inducing ischemic tolerance, which includes early ischemic tolerance (from 30 to 60 minutes after RIPC),21 intermediate tolerance (12 hours after RIPC),22 and delayed ischemic tolerance (from 24 hours after RIPC and lasts for days).18 In our study, we found that dCA was not immediately modulated by RIPC (no significant changes within 3 hours after RIPC) but started to elevate significantly from 6 hours after RIPC, implying that the intermediate tolerance after RIPC may be earlier than previously noted.
Several previous studies have reported that RIPC can induce, for example, adenosine,13 bradykinin,1 and nitric oxide or nitrite.14 Many of these substances are vasoactive and, when carried to the brain, could regulate dCA by changing the diameter of microcerebral arteries.16 In the current study, we found that a series of additional blood biomarkers were regulated by RIPC, which might also positively regulate the dCA function. For example, we found that the level of circulating VEGF-A increased significantly 1 hour after RIPC. VEGF-A, a potent vasodilator and proangiogenic factor,23 has been reported not only to induce neuroprotection directly in ischemic disorders but also to improve dCA through hypoxia-inducible transcription factor-1–mediated pathways.24 In addition, GDNF can act upstream of VEGF25 and hence may improve dCA by enhancing the VEGF signaling pathways.26 Further studies are warranted to dissect the relative contributions of these biomarkers to improved dCA after RIPC.
Neuroprotection is an important function of RIPC in animal and clinical studies.9,17,18,27 In our study, we found that the neurotrophic factor GDNF was significantly elevated after RIPC. This factor can directly provide neuroprotection not only in cerebrovascular diseases such as stroke and subarachnoid hemorrhage28 but also in other neuropathy such as Parkinson disease and epilepsy.29 These results suggest that RIPC induces neuroprotective biomarkers in humans and may be beneficial in the prevention of various neurologic diseases.
Previous studies have reported that RIPC results in the release of proinflammatory and anti-inflammatory cytokines and chemokines that orchestrate the neuroinflammatory response, resolution of inflammation, and transition to neurologic recovery and regeneration.9,17,30 In our study, we found that the TGF-β1, LIF, MMP-9, TIMP-1, and MCP-1 levels were significantly changed compared to their baseline levels. Among these biomarkers, both anti-inflammatory biomarkers (TGF-β1, LIF, and TIMP-1) and a proinflammatory factor (MMP-9) underwent significant changes. Similar to our study, previous studies have shown differential regulation of inflammation-related factors by RIPC. For example, a study found that the serum level of macrophage migration inhibitory factor was increased, whereas no difference was found in IL-6, IL-8, and IL-10 serum levels between the RIPC group and a control group in patients undergoing cardiac surgery.31 Another study reported increased blood levels of TNF-α, IL-6, IL-8, and IL-10 in 5 healthy volunteers after RIPC.32 TNF-α and IL-6 play major roles in initiating and amplifying the postischemic inflammatory response, whereas IL-10 is mainly an anti-inflammatory factor.33 Thus, these studies and our own indicate an effect of RIPC on the inflammatory profile, although there are some differences in biomarkers tested and affected; differences in experimental protocols and measurement points may explain some variations in results. However, it must be pointed out that whether a factor participates in the proinflammatory function or anti-inflammatory function is cell and tissue context dependent. The changes of the aforementioned inflammation-related biomarkers in our study could explain the effect of RIPC on inflammatory regulation; however, we do not currently know how these factors regulate the inflammatory system. The exact roles and mechanisms of these (or other) factors in the regulation of dCA need further study. Further evaluation is required to determine whether the overall effects of these inflammation-related biomarkers are beneficial to cerebrovascular diseases.
In this study, we found that RIPC increased the blood level of TIMP-1 significantly. TIMP-1 is an endogenous inhibitor of MMP-9, which is related to tissue remodeling and inflammation. TIMP-1 has been reported to protect the blood-brain barrier and to play an important role in ischemic stroke.34 However, we also found an increased level of MMP-9 after RIPC. A previous study suggested that MMP-9 levels and the MMP-9/TIMP-1 ratio in serum are related to brain edema after acute cerebral infarction.35 However, we did not find a significant change in this ratio after RIPC.
Neuronal, humoral, and immunologic mediators may all play roles in the transduction of protective signals generated from limbs to the targeted organs.1,7,9 A previous review suggested that both early ischemic tolerance and delayed ischemic tolerance induced the attenuation or prevention of ischemic injury.18 RIPC is associated with both local and systemic mechanisms (i.e., circulating hormones, cytokines, and growth factors) that contribute to improvement in vascular function or structure of targeted organs. It was rational that the biomarkers were produced in the preconditioning location and then transported by the circulatory system to the brain and thus affect the function of the cerebrovasculature directly or indirectly (humoral signal transduction).1 It is worth noting that these biomarkers have various half-lives in blood, ranging from minutes to several days. In addition, the time it takes their biological effects to occur varies substantially. For example, the vessel genesis effects of VEGF-A would be quite long (weeks), while the neuroprotective effects of BDNF would be quite limited in time, occurring quickly over days. Further studies are needed to determine the temporal profiles of each biomarkers in blood and to clarify how these biomarkers contribute to the improved dCA.
Circadian rhythm changes mean arterial pressure over the course of a day.36 In our study, it was interesting to find that RIPC seemed to have an effect on the circadian rhythm of mean arterial pressure, which was significantly stabilized by RIPC. Future studies are needed to explore the underlying mechanism and potential applications.
We found that after RIPC the levels of 2 neuroprotective factors and several inflammation-related biomarkers in serum increased significantly compared to the baseline levels in healthy participants. Thus, in the future, we can choose more biomarkers that are related to neuroprotection and inflammation to study RIPC. Furthermore, it will be worth investigating other biomarkers that are related to these biomarkers identified or factors/pathways that act upstream and downstream of these biomarkers to further elucidate the mechanism of RIPC. In the current study, we collected and analyzed only serum samples. Other biological samples, including brain parenchyma, urine, and the expression and genomic data in different individuals or in animals, would further elaborate the mechanism of RIPC in dCA improvement. Besides, it would be important in the future to investigate whether these benefits are still apparent at time points consistent with other stroke studies, including 30 days, 90 days, and 1 year.
We acknowledge limitations in this study. First, we could not collect blood samples at multiple time points due to difficulties of obtaining the consent of participants and approval of the ethics committee. In the present study, we chose 1 hour after RIPC as the measurement time point of blood sample collection on the basis of previous studies in which serum levels of proteins were altered during or rapidly after RIPC.3 Although it is possible that normal circadian fluctuations may account for the differences in serum biomarker levels, we firmly believe that the changes of serum biomarkers between baseline and 1 hour after RIPC can be attributed to the RIPC on the basis of numerous previous studies that have demonstrated that RIPC could rapidly induce changes in the levels of hundreds of serum proteins, including the biomarkers measured in our study.3 Further research into the related biomarker concentrations at longer time points such as 6 and 12 hours and their dynamic changes is needed. The second limitation of this study was that the sample size was relatively small and the participants were healthy adults. With the results from this study, we can safely conclude only that the present conclusion is applicable to healthy adults without the conditions described in the Methods. More significantly, it is necessary to investigate whether RIPC can also improve dCA in patients with various cerebrovascular and neurologic diseases such as ischemic stroke, depression, anxiety disorders, and migraine.
Overall, our results suggested that RIPC improves dCA from at least 6 to 24 hours after RIPC in healthy adults and that RIPC plays neuroprotective and inflammation regulatory roles in humans by altering various blood biomarkers. Our study provides evidence of RIPC inducing neuroprotection and a new approach to improve the cerebrovascular function in terms of dCA.
Acknowledgment
The authors thank all the volunteers for their contributions to the study.
Glossary
- ABP
arterial blood pressure
- BDNF
brain-derived neurotrophic factor
- CBFV
cerebral blood flow velocity
- dCA
dynamic cerebral autoregulation
- EOT
eotaxin
- GDNF
glial cell line–derived neurotrophic factor
- IL
interleukin
- LIF
leukemia inhibitory factor
- MCP-1
monocyte chemotactic protein 1
- MMP
matrix metalloproteinase
- PD
phase difference
- RIPC
remote ischemic preconditioning
- TFA
transfer function analysis
- TGF-β1
transforming growth factor-β1
- TIMP-1
tissue inhibitor of metalloproteinases-1
- TNF-α
tumor necrosis factor-α
- VEGF-A
vascular endothelial growth factor-A
Appendix. Authors
Footnotes
Editorial, page 15
Study funding
This article was supported by the National Key R&D Program of China (2016YFC1301600) and Program for JLU Science and Technology Innovative Research Team (2017TD-12) to Yi Yang.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White SK, Frohlich GM, Sado DM, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2015;8:178–188. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht M, Zitta K, Bein B, et al. Remote ischemic preconditioning regulates HIF-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol 2013;108:314. [DOI] [PubMed] [Google Scholar]
- 4.Verouhis D, Sörensson P, Gourine A, et al. Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J 2016;181:66–73. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Meng R, Song H, et al. Remote ischemic conditioning may improve outcomes of patients with cerebral small-vessel disease. Stroke 2017;48:3064–3072. [DOI] [PubMed] [Google Scholar]
- 6.Jensen HA, Loukogeorgakis S, Yannopoulos F, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation 2011;123:714–721. [DOI] [PubMed] [Google Scholar]
- 7.Hu S, Dong H, Zhang H, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res 2012;1459:81–90. [DOI] [PubMed] [Google Scholar]
- 8.Blauenfeldt RA, Hougaard KD, Mouridsen K, Andersen G. High prestroke physical activity is associated with reduced infarct growth in acute ischemic stroke patients treated with intravenous tPA and randomized to remote ischemic perconditioning. Cerebrovasc Dis (Basel, Switzerland) 2017;44:88–95. [DOI] [PubMed] [Google Scholar]
- 9.Pan J, Li X, Peng Y. Remote ischemic conditioning for acute ischemic stroke: dawn in the darkness. Rev Neurosci 2016;27:501–510. [DOI] [PubMed] [Google Scholar]
- 10.Zhao W, Meng R, Ma C, et al. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof-of-concept, randomized controlled trial. Circulation 2017;135:1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong L, Liu X, Shang T, et al. Impaired cerebral autoregulation: measurement and application to stroke. J Neurol Neurosurg Psychiatry 2017;88:520–531. [DOI] [PubMed] [Google Scholar]
- 12.Reinhard M, Rutsch S, Lambeck J, et al. Dynamic cerebral autoregulation associates with infarct size and outcome after ischemic stroke. Acta Neurol Scand 2012;125:156–162. [DOI] [PubMed] [Google Scholar]
- 13.Randhawa PK, Jaggi AS. Unraveling the role of adenosine in remote ischemic preconditioning-induced cardioprotection. Life Sci 2016;155:140–146. [DOI] [PubMed] [Google Scholar]
- 14.Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 2014;114:1601–1610. [DOI] [PubMed] [Google Scholar]
- 15.Takada J, Ibayashi S, Nagao T, Ooboshi H, Kitazono T, Fujishima M. Bradykinin mediates the acute effect of an angiotensin-converting enzyme inhibitor on cerebral autoregulation in rats. Stroke 2001;32:1216–1219. [DOI] [PubMed] [Google Scholar]
- 16.Guo ZN, Shao A, Tong LS, Sun W, Liu J, Yang Y. The role of nitric oxide and sympathetic control in cerebral autoregulation in the setting of subarachnoid hemorrhage and traumatic brain injury. Mol Neurobiol 2016;53:3606–3615. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Liu C, Du X, et al. Hypoxia inducible factor 1alpha plays a key role in remote ischemic preconditioning against stroke by modulating inflammatory responses in rats. J Am Heart Assoc 2018;7;e007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durukan A, Tatlisumak T. Preconditioning-induced ischemic tolerance: a window into endogenous gearing for cerebroprotection. Exp Transl Stroke Med 2010;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claassen JA, Meel-van den Abeelen AS, Simpson DM, Panerai RB. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab 2016;36:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma H, Guo ZN, Liu J, Xing Y, Zhao R, Yang Y. Temporal course of dynamic cerebral autoregulation in patients with intracerebral hemorrhage. Stroke 2016;47:674–681. [DOI] [PubMed] [Google Scholar]
- 21.Meller R. The role of the ubiquitin proteasome system in ischemia and ischemic tolerance. Neuroscientist 2009;15:243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience 2008;151:1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling: in control of vascular function. Nat Rev Mol Cell Biol 2006;7:359–371. [DOI] [PubMed] [Google Scholar]
- 24.Sorond FA, Tan CO, LaRose S, et al. Deferoxamine, cerebrovascular hemodynamics, and vascular aging: potential role for hypoxia-inducible transcription factor-1-regulated pathways. Stroke 2015;46:2576–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SM, Chen TS, Chiu CM, et al. GDNF increases cell motility in human colon cancer through VEGF-VEGFR1 interaction. Endocr Relat Cancer 2014;21:73–84. [DOI] [PubMed] [Google Scholar]
- 26.Yang JP, Liu HJ, Liu XF. VEGF promotes angiogenesis and functional recovery in stroke rats. J Invest Surg 2010;23:149–155. [DOI] [PubMed] [Google Scholar]
- 27.England TJ, Hedstrom A, O'Sullivan S, et al. RECAST (Remote Ischemic Conditioning After Stroke Trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke 2017;48:1412–1415. [DOI] [PubMed] [Google Scholar]
- 28.Duarte EP, Curcio M, Canzoniero LM, Duarte CB. Neuroprotection by GDNF in the ischemic brain. Growth Factors 2012;30:242–257. [DOI] [PubMed] [Google Scholar]
- 29.Morcuende S, Muñoz-Hernández R, Benítez-Temiño B, Pastor AM, de la Cruz RR. Neuroprotective effects of NGF, BDNF, NT-3 and GDNF on axotomized extraocular motoneurons in neonatal rats. Neuroscience 2013;250:31–48. [DOI] [PubMed] [Google Scholar]
- 30.McDonough A, Weinstein JR. Neuroimmune response in ischemic preconditioning. Neurotherapeutics 2016;13:748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ney J, Hoffmann K, Meybohm P, et al. Remote ischemic preconditioning does not affect the release of humoral factors in propofol-anesthetized cardiac surgery patients: a secondary analysis of the RIPHeart study. Int J Mol Sci 2018;19:E1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimizu M, Saxena P, Konstantinov IE, et al. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res 2010;158:155–161. [DOI] [PubMed] [Google Scholar]
- 33.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011;17:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddahi A, Chen Q, Edvinsson L. Enhanced cerebrovascular expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 via the MEK/ERK pathway during cerebral ischemia in the rat. BMC Neurosci 2009;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li DD, Song JN, Huang H, et al. The roles of MMP-9/TIMP-1 in cerebral edema following experimental acute cerebral infarction in rats. Neurosci Lett 2013;550:168–172. [DOI] [PubMed] [Google Scholar]
- 36.Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med 2018;119:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rana OR, Schauerte P, Hommes D, et al. Mechanical stretch induces nerve sprouting in rat sympathetic neurocytes. Auton Neurosci 2010;155:25–32. [DOI] [PubMed] [Google Scholar]
- 38.Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer's disease. Curr Drug Targets Inflamm Allergy 2005;4:247–256. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 2006;113:2105–2112. [DOI] [PubMed] [Google Scholar]
- 40.Arakelyan A, Petrkova J, Hermanova Z, Boyajyan A, Lukl J, Petrek M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators Inflamm 2005;2005:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu M, Allard JS, Zhang Y, et al. Age-related brain expression and regulation of the chemokine CCL4/MIP-1beta in APP/PS1 double-transgenic mice. J Neuropathol Exp Neurol 2014;73:362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz S, Lüdike H, Lierath M, et al. C-reactive protein levels and genetic variants of CRP as prognostic markers for combined cardiovascular endpoint (cardiovascular death, death from stroke, myocardial infarction, and stroke/TIA). Cytokine 2016;88:71–76. [DOI] [PubMed] [Google Scholar]
- 43.Spath S, Komuczki J, Hermann M, et al. Dysregulation of the cytokine GM-CSF induces spontaneous phagocyte invasion and immunopathology in the central nervous system. Immunity 2017;46:245–260. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki Y, Nagai N, Umemura K, Collen D, Lijnen HR. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J Thromb Haemost 2007;5:1732–1739. [DOI] [PubMed] [Google Scholar]
- 45.Owczarek W, Paplińska M, Targowski T, et al. Analysis of eotaxin 1/CCL11, eotaxin 2/CCL24 and eotaxin 3/CCL26 expression in lesional and non-lesional skin of patients with atopic dermatitis. Cytokine 2010;50:181–185. [DOI] [PubMed] [Google Scholar]
- 46.Gairolla J, Kler R, Modi M, Khurana D. Leptin and adiponectin: pathophysiological role and possible therapeutic target of inflammation in ischemic stroke. Rev Neurosci 2017;28:295–306. [DOI] [PubMed] [Google Scholar]
- 47.Chollangi S, Wang J, Martin A, Quinn J, Ash JD. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol Dis 2009;34:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garlisi CG, Xiao H, Tian F, et al. The assignment of chemokine-chemokine receptor pairs: TARC and MIP-1 beta are not ligands for human CC-chemokine receptor 8. Eur J Immunol 1999;29:3210–3215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The deidentified data generated and analyzed in the current study will be available and shared by request from any qualified investigator for purposes of replicating procedures and results.