Abstract
Air pollutant exposures are linked to cardiopulmonary diseases, diabetes, metabolic syndrome, neurobehavioral conditions, and reproductive abnormalities. Significant effort is invested in understanding how pollutants encountered by the lung might induce effects in distant organs. The role of circulating mediators has been predicted; however, their origin and identity have not been confirmed. New evidence has emerged which implicates the role of neuroendocrine sympathetic-adrenal-medullary (SAM) and hypothalamic-pituitary-adrenal (HPA) stress axes in mediating a wide array of systemic and pulmonary effects. Our recent studies using ozone exposure as a prototypical air pollutant demonstrate that increases in circulating adrenal-derived stress hormones (epinephrine and cortisol/corticosterone) contribute to lung injury/inflammation and metabolic effects in the liver, pancreas, adipose, and muscle tissues. When stress hormones are depleted by adrenalectomy in rats, most ozone effects including lung injury/inflammation are diminished. Animals treated with antagonists for adrenergic and glucocorticoid receptors show inhibition of the pulmonary and systemic effects of ozone, whereas treatment with agonists restore and exacerbate the ozone-induced injury/inflammation phenotype, implying the role of neuroendocrine activation. The neuroendocrine system is critical for normal homeostasis and allostatic activation; however, chronic exposure to stressors may lead to increases in allostatic load. The emerging mechanisms by which circulating mediators are released and are responsible for producing multiorgan effects of air pollutants insists upon a paradigm shift in the field of air pollution and health. Moreover, since these neuroendocrine responses are linked to both chemical and nonchemical stressors, the interactive influence of air pollutants, lifestyle, and environmental factors requires further study.
Keywords: air pollution, neuroendocrine, hypothalamus, autonomic, stress hormones, ozone
Physical and psychosocial stress are associated with a wide variety of metabolic, cardiovascular, neurodegenerative, and pulmonary diseases (Black, 2003; Chen and Miller, 2007; Dimsdale, 2008). Although these diseases are unique and diverse, development and exacerbation of symptoms typically involve the neuroendocrine stress response system that includes the hypothalamic-pituitary-adrenal (HPA) and sympathetic-adrenal-medullary (SAM) axes. Furthermore, maternal stress during fetal development leads to reprogramming of these axes resulting in increased risk of disease as the offspring age (Cottrell and Seckl, 2009; Kapoor et al., 2006). Steroidal and adrenergic medication that targets the receptors associated with stress hormones are commonly administered to alleviate inflammatory and other disease conditions (Barnes, 1998; Nelson, 1995).
In addition to increasing the risk of disease, stress is also associated with the exacerbation of adverse effects following exposure to inhaled environmental pollutants (Clougherty and Kubzansky, 2009; O’Neill et al., 2003). For instance, Rosa et al. (2017) have demonstrated that the encounter of stressors during pregnancy increases the risk of asthma in children exposed to air pollution. In children living in an urban environment, exposure to violence (a chronic stressor) elevated the risk of asthma associated with traffic-related air pollution (Clougherty et al., 2007). Children from high-stress households were also at an increased risk of lung function deficits when exposed to traffic pollution (Islam et al., 2011). These epidemiological studies suggest the involvement of the neuroendocrine stress pathways in mediating air pollution health effects; however, there are few experimental studies to demonstrate the mechanisms behind these adverse outcomes.
Annually, over 3 million people die by direct consequence of ambient outdoor air pollution world-wide demonstrating a significant health burden (World Health Organization, 2016). Exposure to air pollution has been linked to respiratory, cardiovascular, metabolic, neuronal, and developmental diseases. The evidence gathered from epidemiological and toxicological studies show that in addition to the lung, air pollution affects multiple extrapulmonary organs of the body. The mechanisms by which inhaled pollutants affect extrapulmonary organs remains an area of research interest. It is postulated that the extrapulmonary effects of lung-encountered pollutants are mediated by 3 potential mechanisms (Donaldson et al., 2001; Kodavanti et al., 2011): (1) once deposited in the lung, soluble gases/vapors, and leachable/insoluble nano-sized particulate components can be absorbed into the circulation and distributed to extrapulmonary organs where they mediate effects, (2) injury to local lung cellular milieu or lung lining structures produce bioactive and vasoactive components and cytokines that subsequently circulate and encounter extrapulmonary tissues where they produce effects, and (3) air pollutants interact with pulmonary nerves and receptors that activate the autonomic nervous system leading to systemic alterations. Since air pollution is composed of numerous chemically and physically heterogeneous substances, none of these theories can be discounted. Rather, using individual components of air pollutants, experimental studies have proved that all these mechanisms can be supported (Carll et al., 2013; Chen et al., 2018; Wallenborn et al., 2007). However, regardless of the specific differences in physicochemical characteristics, one can presume that the initial cellular interaction upon encountering pollutants and the associated irritancy might induce a rapid and generalized neural stress response that affects all organs.
In this review, we will discuss the following: (1) the potential role of autonomic sensory pathways in triggering central neuroendocrine stress response, (2) how air pollution health effects are comparable to stress response mechanisms involving homeostatic balance and allostasis, and (3) the evidence of pollution-induced adverse effects on the brain, lung, and metabolic organs through activation of neuroendocrine stress pathways. Although neuroendocrine mechanisms have been widely implicated in health and disease, the role of stress pathways in mediating pulmonary and extrapulmonary health effects of air pollutants has been overlooked in experimental studies. The emerging mechanisms by which air pollutants activate the neuroendocrine system and release stress hormones into the circulation to produce multiorgan effects insists upon a paradigm shift in the field of air pollution and health, chronic disease susceptibility, and developmental impairment.
AUTONOMIC REGULATION OF CARDIOPULMONARY FUNCTIONAL ALTERATIONS
Studies using gaseous air pollutants have implicated the role of the autonomic nervous system in pulmonary and cardiovascular physiological effects (Alarie, 1973; Godleski et al., 2000; Nielsen et al., 2007; Widdicombe and Lee, 2001). Numerous intervention and physiological experiments have shown that within minutes after inhalation of gaseous pollutants, animals react by producing reflex bronchoconstriction, apnea, cough, and/or sensory irritation depending on the chemical nature of inhalant. Based on the solubility and chemical nature of the pollutant, Alarie (1973) classified the respiratory irritants as follows: (1) sensory irritants, which deposit in the nasal cavity and stimulate the trigeminal nerve to induce irritation, coughing, and bronchoconstriction (acrolein, ammonia, and sulfur dioxide); (2) pulmonary irritants, which deposit in bronchiolar and parenchymal regions and cause bronchoconstriction or apnea (ozone, nitrogen dioxide, and phosgene); and (3) respiratory irritants, which induce irritation across the entire respiratory tract (chlorine, particulate matter [PM], and chloropicrin). These inhaled pollutants, depending on their concentration, solubility, and/or size, stimulate trigeminal and vagal sensory neurons to induce reflexively mediated cardiopulmonary changes.
A large body of epidemiology and human clinical studies support the role of the autonomic nervous system in cardiovascular electrophysiological changes due to high levels of particulate and gaseous pollutants (Magari et al., 2002; Sun et al., 2010; Zanobetti et al., 2014). Acute vasoconstriction in human subjects exposed to diesel exhaust was presumed to involve nociception and renin-angiotensin systems (reviewed in Sack et al., 2016). Clinical studies involving exposure to air pollutants at ambient levels have also demonstrated alterations in heart rate variability, autonomic tone, and cardiovascular physiology (Cascio, 2016; Gold et al., 2000; Huang et al., 2012). A number of animal studies have corroborated the findings that changes in parasympathetic and sympathetic tone are involved in cardiac electrophysiological changes following exposure to gaseous and particulate pollutants (Carll et al., 2013; Perez et al., 2015). Along the same line, several studies have shown decreased heart rate, cardiac depression, and bradycardia as a consequence of exposure to air pollutants in animals likely involving parasympathetic influence (Gordon et al., 2014; Uchiyama et al., 1986; Wagner et al., 2014). These studies support the role of the autonomic nervous system in mediating cardiac electrophysiological changes after air pollution exposure.
The autonomic sensory neurons innervate the upper (including nasal cavity) and lower respiratory tract, and relay perceived information about chemical, odor, irritant, and mechanical stimuli to the brain centers. Once processed, the responses are sent to the effector site through motor neurons producing rapid reflex reactions including cough, apnea, bronchoconstriction, and/or pain sensation that are important in protecting the host from the damaging influence of external stimuli or internal change (Alarie, 1973; Mazzone and Undem, 2016).
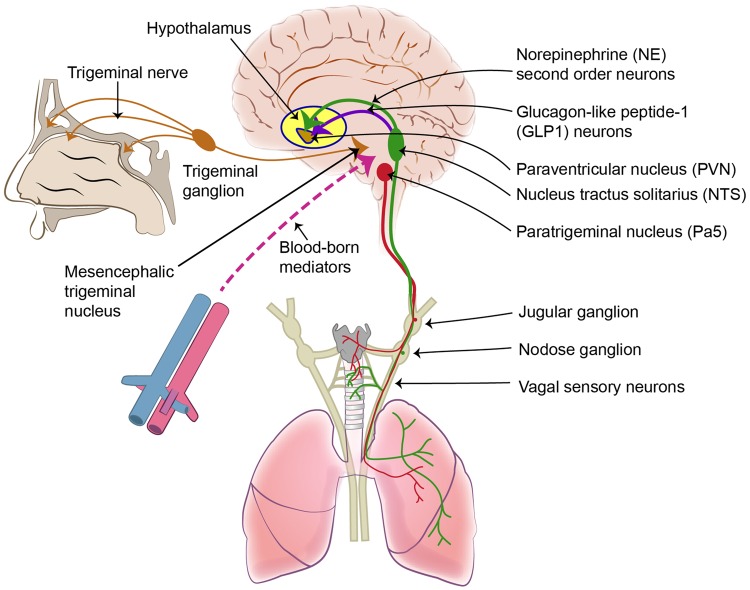
Trigeminal sensory neurons innervate nasal cavity and facial tissues, and relay the information to external trigeminal ganglion and the central nervous system (CNS) mesencephalic trigeminal nucleus (Figure 1; Lazarov, 2012). The motor neurons travelling through the trigeminal ganglion relay the information back to the nasal upper respiratory tract. Vagal sensory neurons innervating the upper and lower respiratory tract includes ∼20% of the total sensory innervation of the body (Mazzone and Undem, 2016; McGovern and Mazzone, 2014). The fibers originating from nodose ganglion include myelinated rapidly adapting stretch receptors (RARs; Aβ fibers), slowly adapting stretch receptors (SARs), relatively faster acting Aδ fibers, and bronchopulmonary C-fibers. Those originating from the jugular ganglion are slow acting nociceptor C-fibers and some of the nociceptor Aδ fibers (Mazzone and Undem, 2016). Pulmonary C-fibers originating from jugular ganglion express neuropeptides, substance P, neurokinin A, vasoactive intestinal polypeptide (VIP), calcitonin gene-related peptide (CGRP), and receptors, such as transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential cation channel, subfamily A, member 1 (TRPA1) that respond to chemical and electrophilic stimuli (Mazzone and Canning, 2013). Those neural terminals originating from nodose ganglion are closely associated with neuroendocrine bodies (ie, neuroendocrine cells) that express ATP, serotonin, and various other neuropeptides. The distribution of these terminals along the airways and alveoli differ between different laboratory animals (Mazzone and Undem, 2016). The wide distribution of these C-fibers is critical in sensing the changes in the chemical composition of the airway lining, epithelium, and interstitial tissues within the lung. For instance, a number of studies have shown the role of pulmonary C-fibers and TRPA1/TRPV1 activation in mediating cardiovascular and respiratory physiological changes following exposure to air pollutants (Deering-Rice et al., 2012; Kurhanewicz et al., 2017).
Figure 1.
Autonomic sensory innervation of the respiratory system. Portions of this figure were recreated based on the information published in Herman (2018) and Mazzone and Undem (2016).
The signals originating from the respiratory tract traversing through the jugular and nodose ganglia are processed and integrated in the brainstem resulting in reflex cardiopulmonary responses, and are also sent to the higher order brain centers for further integration (Mazzone and Undem, 2016). The neural projections to the jugular ganglion are confined primarily to the paratrigeminal nucleus (Pa5) whereas those to the nodose ganglion are confined to the nucleus tractus solitarius (NTS) within the brainstem (Figure 1). Integration at each stage generates defensive responses which are relayed back to effector organs via efferent sympathetic (nor-adrenergic) and parasympathetic (cholinergic) outflow (Pereira and Leite, 2016). These neural terminals innervate part of the respiratory tract in most laboratory animals (McGovern and Mazzone, 2014). In humans, the sympathetic innervation to airway smooth muscles is modest, and the bronchial responses are primarily regulated by circulating catecholamines and noncholinergic parasympathetic innervation (McGovern and Mazzone, 2014).
The roles of specific mediators released at the nerve terminals in the lung, the specificity of neuronal bundles conveying distinct pollutant signals through activation of these receptors to the brain, and how the information is processed to activate hypothalamic stress pathways have not been fully understood. Examining the distinct roles of jugular nerve fibers at the respiratory nerve terminals versus nodose fibers which are in close association with neuroendocrine bodies in mediating specific brain reflex responses and activation of the neuroendocrine system will provide insights into the mechanism by which the brain regulates air pollution-induced pulmonary and systemic responses.
NEUROENDOCRINE REGULATION OF HOMEOSTASIS UNDER STRESS, ADAPTATION, AND ALLOSTATIC LOAD
During the process of evolution, organisms have developed complex autonomic and neuroendocrine systems to recognize danger signals or threatening situations, interpret the information, and initiate an appropriate and immediate survival response. The physiological and biochemical characteristics of this response have been studied for decades in relation to various kinds of physical and perceived stress encounters. The most prominent manifestations of this response are physiological changes such as increased heart rate, dilated pupils, and changes in blood flow. These rapid, adaptive responses accomplish 2 fundamental survival needs of an organism: (1) protect potential injured tissues and prepare the body to fight possible invading pathogens by generating a protective immune response, and (2) ensure adequate availability of nutrients by conserving and channeling energy resources to different areas of the body based on need (Ulrich-Lai and Herman, 2009). Once the perceived threat is eliminated or avoided, the organism’s biology returns to the state of normality. Homeostasis is a condition of stability in the internal environment of an organism despite changes in the external environment. The term “homeostasis” has been defined, discussed, and debated in many excellent reviews (Goldstein and Kopin, 2017; McEwen, 2016). The exposure to stressors such as sudden environmental change, exposure to noxious agents or pathogens, lack of food, predator fear, socially demeaning situations, or a disturbed biological process in the body are rapidly recognized by the sensory autonomic system and processed through the activation of the neuroendocrine system (McEwen, 2016; McEwen et al., 2015). This autonomic neuroendocrine response involving the activation of the sympathetic and parasympathetic nervous systems brings about organ-specific changes in metabolic and immune processes to protect the affected organ(s).
Alternatively, the term “allostasis” was coined in several review papers by McEwen and collaborators as “maintaining stability through change, as a fundamental process through which organisms actively adjust to both predictable and unpredictable events” (McEwen, 2016; McEwen and Wingfield, 2010). Allostasis also incorporates the brain programming that is linked to adaptation/learning upon repeated encounters of a stressor. However, in some chronic stress conditions, adaptability can be compromised through defects in biological pathways of the neuroendocrine system leading to allostatic overload (Ramsay and Woods, 2014). McEwen and collaborators (2015) explained 2 types of allostatic overload situations: (1) when there is a limited supply of food, the organism will adapt a lifestyle that requires less energy and once the food supply is adequate, the normality is reestablished, and (2) in many circumstances, the stressful situation is not related to the shortage of energy supply in humans, but rather involves other types of stressors, including psychosocial. Over time, these stressors are likely to lead to allostatic load (McEwen and Stellar, 1993), and upon repeated encounters, is associated with accelerated aging, diabetes, and neurodegenerative and cardiovascular diseases.
A fight-or-flight response is rapidly orchestrated through activation of sympathetic nor-adrenergic, HPA, SAM, hypothalamic-pituitary-thyroid (HPT), and hypothalamic-pituitary-gonadal (HPG) axes to affect visceral effector organs (Kozlowska et al., 2015). The stress hormones and neurotransmitters released by the motor sympathetic and parasympathetic activation play a key role in mediating tissue specific responses largely governed by differential patterns of receptor subtypes, distribution, and specificity for stress hormones or neurotransmitters. The homeostatic responses they generate are reversible once the stressor is removed and homeostasis is reestablished (Herman, 2018). However, the magnitude of this response likely determines if the homeostatic balance is achievable or if the lingering effect of stressors culminates in allostatic load over time.
A large number of air pollution studies involving animal models and humans have demonstrated that cardiac and pulmonary physiological changes are mediated through the activation of the autonomic nervous system (Carll et al., 2013; Gold et al., 2000; Perez et al., 2015). It has been recognized that these adaptive and reversible physiological changes induced after an acute air pollution exposure (ie, irritation, hypothermia, changes in respiratory, and cardiac physiological rhythms) involve multiple organs (reviewed in Alarie, 1973; Gordon et al., 2008; Pauluhn, 2004). However, neither the mechanistic pathways, immunological, and metabolic consequences of these changes, nor their relation to the neuroendocrine system have been established in the field of air pollution toxicology until recently (Kodavanti, 2016). New evidence supports a paradigm by which inhaled pollutants activate the neuroendocrine system and can impact physiological processes that involve homeostasis/allostasis and allostatic overload. Furthermore, it has been established that upon encountering a stressor, neurons in the NTS expressing norepinephrine and glucagon-like peptide-1 (GLP1) that project to the paraventricular nucleus (PVN; Figure 1), play a major role in integrating acute HPA activation with other stress pathways to generate a systemic adaptive response (Herman, 2018). However, the involvement of this pathway and how the lung communicates with stress responsive regions in the brain during an exposure to air pollutants is not well understood.
THE EVIDENCE OF IRRITANT AIR POLLUTANT-INDUCED NEUROENDOCRINE STRESS RESPONSES
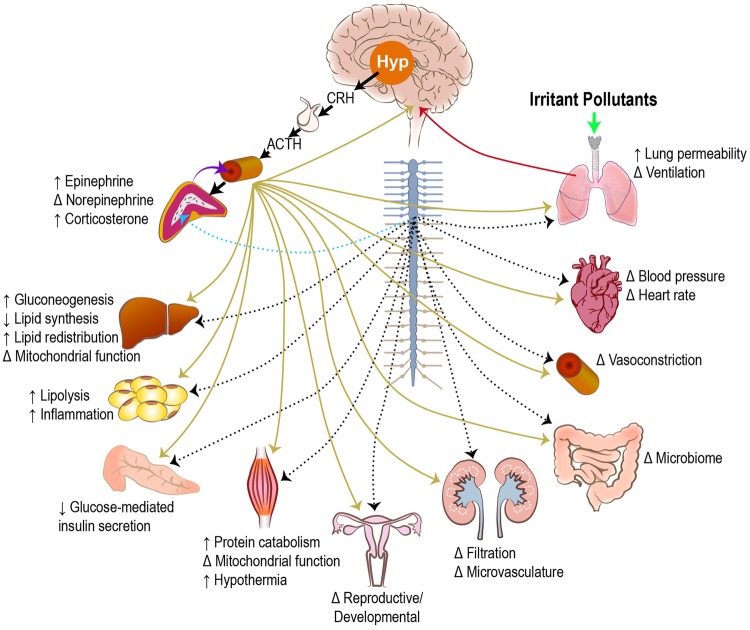
Adaptive stress responses are conserved from an evolutionary point of view and are present in all living organisms (Fink, 2010). The activation of SAM and HPA axes produce behavioral and physiological changes in the host (Figure 2). Biogenic or physical stress bypasses interpretative mechanisms and promotes the neuroendocrine changes (Everly and Lating, 2013). SAM axis activation is controlled by the direct sympathetic innervation of the adrenal medulla, which causes the release of epinephrine and norepinephrine into the bloodstream (Ranabir and Reetu, 2011). Although epinephrine is primarily made in the adrenal medulla, only a small portion (∼10–20%) of circulating norepinephrine is derived from here. Sympathetic nerve endings distributed in all organs of the body produce norepinephrine locally, which in a paracrine manner induce rapid local responses through binding of norepinephrine to adrenergic receptors.
Figure 2.
Organ-specific metabolic effects of neuroendocrine stress response induced by inhaled irritants. Irritant pollutants interacting with the lung (solid green arrow) result in sensory signals and pulmonary mediators that cause neuroendocrine activation in the brain (solid red arrow). Activation of the hypothalamic-pituitary-adrenal (HPA; solid black arrows) and sympathetic-adrenal-medullary (SAM; dotted blue arrow) axes lead to the release of stress hormones from the adrenal gland into the circulation (solid purple arrow). These stress hormones can mediate organ-specific effects through activation of adrenergic and glucocorticoid receptors (solid gold arrows). In addition, nerves originating from the spinal ganglion innervate the visceral organs and mediate direct sympathetic activation (dotted black arrows). ↑, increase in response; ↓, decrease in response; Δ, change in response depending on irritant pollutant; Hyp, hypothalamus; CRH, corticotropin releasing hormone; ACTH, adrenocorticotropic hormone.
HPA axis activation starts with the release of corticotropin releasing hormone (CRH) from the PVN of the hypothalamus, which activates the pituitary gland to release adrenocorticotropic hormone (ACTH) into the circulation, which in turn binds to melanocortin receptor type 2 located in the adrenal cortex (Figure 2). This promotes the release of glucocorticoids from the adrenal cortex. In humans this is primarily cortisol while in rodents it is corticosterone (Koren et al., 2012). Epinephrine and corticosterone/cortisol, through their action on the adrenergic and steroidal receptors in various tissues, cause mobilization of energy sources from their depots, induce immune surveillance, redirect blood flow to areas in need, and generate many other changes associated with a fight-or-flight response (Figure 2; Everly and Lating, 2013). This neuroendocrine activation response initiates changes in virtually all organ systems that varies based on the type of stressor and the distribution of receptor subtypes.
Inhalation of a prototypic air pollutant, ozone, has been associated with the activation of stress responsive regions in the brain as determined by increased c-fos staining (Gackiere et al., 2011). We and others have recently observed that acute exposure to irritant air pollutants increased levels of ACTH, corticosterone, and epinephrine in rodents, as well as cortisol and corticosterone in humans (Bass et al., 2013; Martrette et al., 2011; Miller et al., 2016a,b,c; Snow et al., 2017; Thomson et al., 2013). A 4-month exposure to concentrated ambient PM was also associated with the inhibition of HPG axis in male mice (Qiu et al., 2017a). The evidence presented below supports the involvement of neuroendocrine stress pathways in systemic and pulmonary health effects of air pollutants.
Effects of Air Pollution Exposure on the Brain
There are numerous studies implicating air pollution to a variety of neurological and neurobehavioral abnormalities. A number of recent reviews have summarized studies which show an association of air pollution with autism spectrum disorders (Bilbo et al., 2018), Parkinson’s disease (Palacios et al., 2017), anxiety and depression (Vert et al., 2017), and neurodegenerative disorders (Heusinkveld et al., 2016). Multiple experimental studies have also emerged demonstrating effects of air pollutants on the brain. Acute exposure to PM and ozone have been shown to impair microvascular integrity and blood-brain barrier in animals (Suwannasual et al., 2018). Diesel exhaust exposure is associated with microglial inflammation in different areas of rat brains (Levesque et al., 2011). In rats, ozone inhalation promoted hippocampal neurodegeneration and reduced repair (Rivas-Arancibia et al., 2010). The CNS effects including neuroinflammation, oxidative stress, and autonomic processes following ozone exposure have been recently reviewed (Martinez-Lazcano et al., 2013). Collectively, these studies demonstrate that acute and subacute air pollution exposure induces a variety of adverse responses in different areas of the brain.
The mechanisms of how these changes occur and relay signals to the periphery after pollutant inhalation remains unclear. An emerging mechanism proposing the formation of circulating bioactive factors derived from the lung that disrupt the blood-brain barrier and interact with brain glial cells to cause injury and inflammation is one possibility. Recent studies demonstrating activation of microglial following ozone exposure (Mumaw et al., 2016) as well as disruption in the blood-brain barrier and neuroinflammation following pulmonary exposure to multiwalled carbon nanotubes (Aragon et al., 2017) support this notion. The evidence from ozone inhalation studies may also provide some insights. Changes in catecholaminergic neurons within the locus coeruleus, striatum, and NTS, which process chemosensory signals, were noted after ozone exposure (Cottet-Emard et al., 1997; Soulage et al., 2004). Ozone was also able to increase catecholamine turnover in sympathetic and central neurons located in the cervical ganglia, NTS, and cortex (Soulage et al., 2004). In infant primates, episodic ozone exposure triggered neuroplasticity in the NTS neurons (Chen et al., 2003). As mentioned earlier, Gackiere and colleagues (2011) demonstrated that ozone inhalation caused a time- and dose-dependent activation of stress-responsive regions, specifically the PVN and dorsolateral regions of the NTS overlapping terminal fields of lung vagal afferents, thus providing a possible mechanism by which ozone-induced lung responses are communicated to the hypothalamus. Chounlamountry et al. (2015) described how ozone promotes remodeling of glutamatergic synapses in the NTS in rats and concluded that “ozone-induced pulmonary inflammation results in a specific activation of vagal lung afferents rather than nonspecific overall brain alterations mediated by blood-borne agents”. These studies support the evidence that neural mechanisms might be involved in air pollution-induced neuroendocrine activation.
Lung Injury/Inflammation Following Air Pollution Exposure
One of the survival processes induced during a fight-or-flight response involves immune surveillance to recognize and remove any foreign substances or damaged host molecules during injury or infection. This process also assures that adequate mechanisms are in place to be more effective during potential subsequent encounters of the same insult by influencing the adaptive immune response (Cain and Cidlowski, 2015; Verburg-van Kemenade et al., 2017). Lymphoid organs such as the spleen, thymus, lymph nodes, and bone marrow are essential for leukocyte maturation and release. Thymus atrophy (Dziedzic and White, 1986) and reduction in number of total thymocytes and splenocytes (Li and Richters, 1991) were noted after ozone exposure in animals. Chronic exposure to ozone has also been shown to induce splenomegaly (Hassett et al., 1985) and alter maturation of myelopoietic progenitors in the spleen (Goodman et al., 1989). It was recently demonstrated that the spleen is a source of lung inflammatory macrophages after ozone exposure, and splenectomized rats exhibited decreased levels of ozone-induced pulmonary vascular leakage and cellular infiltration (Francis et al., 2017). In addition, bone marrow-derived neutrophils were shown to be recruited into the lungs following ozone inhalation (Kenyon et al., 2006). Although immune processes are tightly regulated by sympathetic adrenergic and steroidal mechanisms (Cain and Cidlowski, 2015; Verburg-van Kemenade et al., 2017), these studies did not examine the neuroendocrine stress response as a contributing factor for these multi immune-organ effects.
The role of neuroendocrine stress pathways in inflammatory mechanisms derives from studies assessing the effects of psychosocial stresses. Several studies conducted by Dhabhar and colleagues (Dhabhar, 2014; Dhabhar et al., 2012) using a rat model of restraint-induced stress have characterized the dynamic relationships between increases in stress hormones and immune responses occurring minutes and hours after the encounter of stressful stimuli. These increases were first associated with the mobilization of lymphocytes, monocytes, and neutrophils from reservoirs such as bone marrow, spleen, or lymph nodes to the circulation, and then a reduction due to their extravasation to peripheral organs. These changes were confirmed by examining the effects of stress hormones injection in rats (Dhabhar, 2014; Dhabhar et al., 2012). Moreover, in a study conducted by Clougherty et al. (2010), a rat model of social stress was found to have higher circulating levels of markers of inflammation (TNF-α and white blood cell count) and injury (C-reactive protein) as well as altered respiratory parameters following exposure to fine concentrated ambient particles. These data suggest that chronic stress may enhance adverse air pollution health effects.
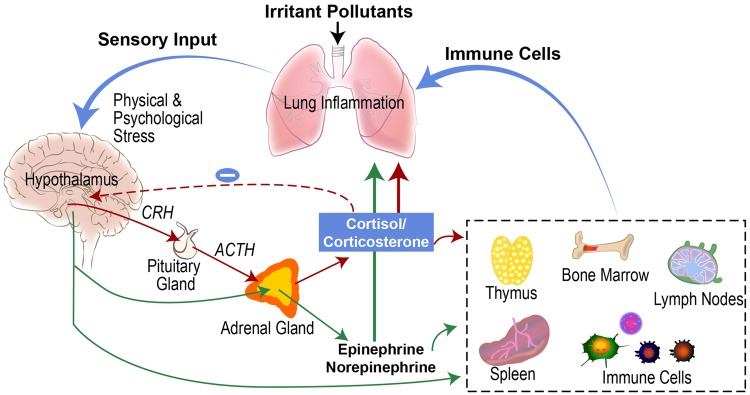
Air pollutants are known to induce lung injury and inflammation. The role of epinephrine and corticosterone in pollutant-induced lung injury and inflammation was confirmed when ozone-induced lung damage and neutrophilic inflammation were diminished in adrenalectomized rats with near zero levels of these circulating stress hormones (Miller et al., 2016c). This accompanied reversal of ozone-induced leukopenia. Adrenalectomy was also associated with reversal of lung transcriptional changes and induction of glucocorticoid responsive genes involved in inflammatory processes induced by ozone (Henriquez et al., 2017). The cytokine profile of the lung lavage fluid in rats that underwent sham surgeries indicated an increased IL-4 to interferon-γ ratio (a Th2 phenotype linked to asthma) after ozone exposure that was not present in adrenalectomized rats. Moreover, when adrenergic and glucocorticoid receptors were antagonized by pharmacological intervention, ozone-induced lung protein leakage, neutrophilic inflammation, and changes in inflammatory cytokines were selectively prevented thus confirming specific roles of these stress hormone receptors in mediating lung inflammation (Henriquez et al., 2018a). It was apparent that blocking glucocorticoid receptors reversed leukopenia whereas blocking β-adrenergic receptors was associated with diminished extravasation of neutrophils in the lung suggesting specific actions of different stress hormones and their receptors on different immune cell populations. We also demonstrated that the protection offered against ozone exposure by adrenalectomy can be reversed by treating rats with stress hormone receptor agonists (Henriquez et al., 2018b, manuscript under review). Furthermore, agonists of β-adrenergic receptors have been shown to exacerbate inflammatory cytokines and thrombogenic effects following exposure to PM (Chiarella et al., 2014). Collectively, these results indicate that neuroendocrine activation of the SAM and HPA axes are critical in mediating air pollution-induced lung injury and inflammation (Figure 3).
Figure 3.
Neuroendocrine involvement in air pollution-induced lung inflammation and injury. Sympathetic-adrenal-medullary (SAM) axis depicted with green arrows. Hypothalamic-pituitary-adrenal (HPA) axis depicted with red arrows. CRH, corticotropin releasing hormone; ACTH, adrenocorticotropic hormone.
Metabolic Effects of Air Pollution Exposure
A number of studies have shown that concentrated PM and diesel exhaust inhalation impairs glucose metabolism, insulin signaling, and induces adipose inflammation (Liu et al., 2017; Xu et al., 2011). The interactive effects between obesity and how these metabolic processes are impacted by air pollution exposure has also been examined (Pardo et al., 2018; Qiu et al., 2017b). However, the metabolic changes have not been linked to the activation of the neuroendocrine pathways. Our recent series of studies involving ozone and acrolein exposure in rodents and humans show widespread metabolic effects that are similar to those induced by a fight-or-flight response involving adrenal-derived stress hormones (Bass et al., 2013; Miller et al., 2015, 2016a,b,c; Snow et al., 2017). We have demonstrated that acute ozone exposure produces fasting hyperglycemia, glucose intolerance, and increases hepatic gluconeogenesis that is associated with increases in circulating stress hormones (Bass et al., 2013; Miller et al., 2015, 2016a,c). A recent study by Thomson and collaborators also supports the role of corticosterone in mediating ozone-induced glucose metabolic effects (Thomson et al., 2018). Although glucose metabolism was altered after ozone exposure, we have shown that acute or repeated subchronic ozone exposure does not induce peripheral insulin resistance as determined by an insulin tolerance test and assessment of insulin signaling in the liver and muscle; however, it did attenuate glucose-mediated pancreatic β-cell insulin secretion (Miller et al., 2016b). On the contrary, a study by Vella et al. (2015) involving 16 h of continuous ozone exposure demonstrated muscle insulin resistance in rats, likely due to the high concentration exposure over a long period of time. Epinephrine increases hepatic gluconeogenesis that contributes to hyperglycemia (Dufour et al., 2009), whereas glucocorticoids regulate a wide array of metabolic processes including glucose metabolism through its effects on insulin production and action as well as adipose and muscle tissue metabolic processes (Fichna and Fichna, 2017), and thus may contribute to ozone-induced metabolic changes. Catecholamines, such as epinephrine, have also been proposed to act through different members of the α-adrenergic family of receptors and impair insulin secretion through changes in cAMP and/or Ca2+ levels, hyperpolarization of the pancreatic β-cells, or glucagon release from pancreatic α-cells (Peterhoff et al., 2003). Incidentally, ambient ozone has been associated with type 1 diabetes in pregnant women and children (Beyerlein et al., 2015; Hathout et al., 2006). Altogether, these studies implicate liver, adipose, pancreas, and muscle tissues as potential targets in ozone-induced glucose metabolic alterations through the action of circulating stress hormones.
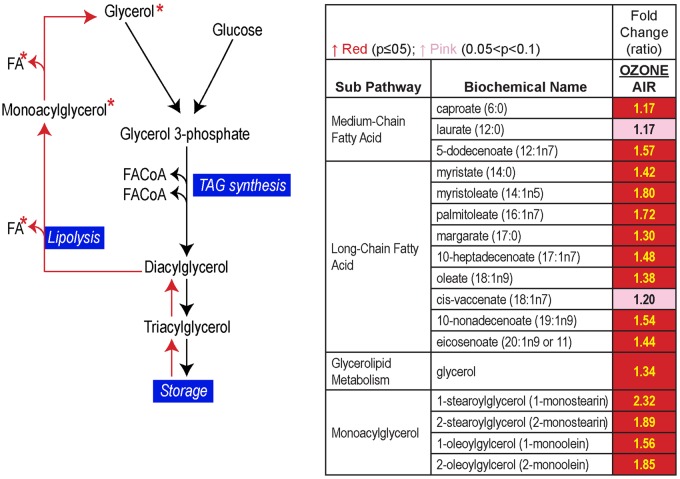
Using a serum metabolomics approach together with liver transcriptome analysis, we noted that the pattern of metabolite changes after ozone exposure reflected those that are induced in a classical flight-or-fight hormonal stress response. These approaches revealed adipose lipolysis, muscle protein catabolism, increased gluconeogenesis, and major alterations in liver lipid metabolism involving changes in mitochondrial bioenergetics (Miller et al., 2015). Another irritant air pollutant, acrolein, which primarily deposits in the nasal airways, induced similar metabolic effects in terms of glucose intolerance and increases in circulating free fatty acids that were accompanied by changes in circulating stress hormones (Snow et al., 2017). A metabolomic assessment of human serum samples obtained from a clinical study involving controlled air or ozone exposure also provided evidence of increased adipose lipolysis and changes in lipid metabolic processes (Figure 4; Miller et al., 2016a). Importantly, increases in circulating cortisol without changes in cortisone, which if increased can contribute to circulating cortisol, accompanied metabolic alterations, supporting the role of neuroendocrine stress axes activation (Miller et al., 2016a). Wide-spread changes in lipid metabolites including increases in lysolipids, a variety of phospholipid metabolites, and β-oxidation byproducts in humans indicated an interactive effect of ozone and intermittent exercise. Some of these interactive effects of ozone stress and exercise may be more detrimental to health than the effect of ozone without concurrent exercise.
Figure 4.
Ozone exposure in exercising humans led to increases in circulating free fatty acids and glycerol indicating adipose lipolysis. FA, fatty acid; FACoA, fatty-acyl-CoA; TAG, triglyceride. Reproduced with permission from Miller et al. (2016b).
Similar to our findings with ozone-induced lung injury and inflammation described earlier, the contribution of circulating stress hormones in mediating systemic metabolic effects was confirmed by demonstrating that adrenalectomy prevented virtually all metabolic effects induced by ozone (Miller et al., 2016c). Multiorgan metabolic effects of ozone in rats have been recently demonstrated in other studies using transcriptomic assessment of different organs (Thomson et al., 2016). Thus, it is likely that similar to a single or persistent stress event, an acute or chronic exposure to air pollutants may contribute to metabolic disease. Collectively, these data confirm the link between neuroendocrine activation and metabolic impairment, and emphasize the process-specific changes that might or might not have lasting effects if the stressors are removed.
CONCLUSION AND FUTURE PERSPECTIVES
Although the mechanism(s) by which the lung communicates with the brain following air pollutant exposure remains to be fully elucidated, new studies demonstrate that air pollutants activate the neuroendocrine stress pathways involving the SAM and HPA axes, which produce widespread metabolic and innate immune changes in multiple organs. If air pollution exposures occur over a long period of time, the impairment in these processes can contribute to diabetes, obesity, cardiometabolic diseases, neurodegenerative diseases, and developmental abnormalities. Chronic inflammation is linked to recurrent stressors and is associated with persistent activation of the SAM and HPA axes as well as disease exacerbation through alteration in homeostatic balance and allostatic overload (Verburg-van Kemenade et al., 2017). The modeling of environmental disease burdens incorporates allostatic load from a variety of stressors, thus, incorporating air pollution as an additional stressor can better inform relative disease risk and regulation strategies.
Observational studies in humans have linked increased levels of circulating stress hormones with exposure to air pollutants (Jia et al., 2018; Miller et al., 2016a), which can be reduced following air purification (Li et al., 2017). Psychosocial stresses have been shown to potentiate these adverse responses triggered by air pollution exposure (Clougherty et al., 2010). For instance, stressful conditions during pregnancy were shown to increase air pollution health effects in children (Brunst et al., 2018). Developmental abnormalities in the hypothalamus and neuroendocrine system have been presumed to be linked to heightened sensitivity of children to air pollution health effects and chronic pulmonary disease susceptibility (Cowell and Wright, 2017). High levels of cortisol/corticosterone have also been linked to chronic stress, PTSD, and depression (Gelman et al., 2015; Hensler et al., 2013). Therefore, the evidence of air pollutant activation of the SAM and HPA axes provides potential mechanistic commonalities between chemical and nonchemical psychosocial stressors. Better understanding of interactive effects of various stressors in peripheral chronic disease susceptibility will be useful in the development of preventive strategies and potential therapeutic interventions.
Exposure to some air pollutants (eg, ozone) lead to adaptation and diminished responses from subsequent exposures; however, the mechanisms are not well understood. Since a variety of brain centers involved in integrating adaptive and reversible homeostatic processes are induced after encounter with stress and air pollution exposure, it is likely that brain centers may play a role in this adaptation phenomenon. Understanding the role of neuroendocrine axes in adaptation and its failure in certain circumstances can allow for appropriate intervention strategies to counter air pollution effects.
The role of the autonomic nervous system in regulating reflex cardiopulmonary mechanisms has been primarily characterized using irritant air pollutants. Along those same lines, the bulk of the data presented here focuses on studies using the irritant air pollutants ozone and acrolein to demonstrate the role of the neuroendocrine system in mediating adverse pulmonary and systemic effects. It is therefore important to also determine whether particulate air pollutants, which induce less respiratory irritation and are cleared effectively by alveolar macrophages, can activate these same neuroendocrine responses.
The complex interplay of neural and endocrine systems responding to basic homeostatic mechanisms will be best addressed using an integrated systems approach and comprehensive evaluation strategies involving transcriptomic, metabolomic, proteomic, and epigenetic techniques. Manipulating neuroendocrine pathways represents a logical follow-up approach to understanding how air pollutants and other stressors might contribute to adaptability, allostatic load, and chronic disease. Major stress hormone receptors and their subtypes, which have been extensively manipulated therapeutically for chronic illnesses, are the key players in survival homeostatic processes. Incorporating cell signaling mechanisms and downstream cellular changes involving these adrenergic and glucocorticoid receptors will lead to the development of new adverse outcome pathways (AOPs). The creation of these AOPs in conjunction with understanding the complex interplay of environmental and psychosocial factors as well as the neural and endocrine pathways can provide a framework for future predictive modeling. As policy decision making gradually embraces more predictive approaches, real gains in public health outcomes will emerge, especially in those groups impacted by a spectrum of stressors.
ACKNOWLEDGMENTS
The authors thank Drs Michael Madden, Andrew Ghio, and Ian Gilmour of the US EPA for their critical review of the manuscript. This work was supported by Oak Ridge Institute for Science and Education and EPA Co-Operative agreement (A.R.H.).
REFERENCES
- Alarie Y. (1973). Sensory irritation by airborne chemicals. CRC Crit. Rev. Toxicol. 2, 299–363. [DOI] [PubMed] [Google Scholar]
- Aragon M. J., Topper L., Tyler C. R., Sanchez B., Zychowski K., Young T., Herbert G., Hall P., Erdely A., Eye T. et al. , . (2017). Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood-brain barrier impairment. Proc. Natl. Acad. Sci. U. S. A. 114, E1968–E1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. (1998). Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin. Sci. (Lond) 94, 557–572. [DOI] [PubMed] [Google Scholar]
- Bass V., Gordon C. J., Jarema K. A., MacPhail R. C., Cascio W. E., Phillips P. M., Ledbetter A. D., Schladweiler M. C., Andrews D., Miller D. et al. , . (2013). Ozone induces glucose intolerance and systemic metabolic effects in young and aged brown norway rats. Toxicol. Appl. Pharmacol. 273, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyerlein A., Krasmann M., Thiering E., Kusian D., Markevych I., D’Orlando O., Warncke K., Jochner S., Heinrich J., Ziegler A.-G. (2015). Ambient air pollution and early manifestation of type 1 diabetes. Epidemiology 26, e31–e32. [DOI] [PubMed] [Google Scholar]
- Bilbo S. D., Block C. L., Bolton J. L., Hanamsagar R., Tran P. K. (2018). Beyond infection – maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 299, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. H. (2003). The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type ii diabetes and metabolic syndrome x. Brain Behav. Immun. 17, 350–364. [DOI] [PubMed] [Google Scholar]
- Brunst K. J., Sanchez-Guerra M., Chiu Y. M., Wilson A., Coull B. A., Kloog I., Schwartz J., Brennan K. J., Bosquet Enlow M., Wright R. O. et al. , . (2018). Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: Effect modification by maternal lifetime trauma and child sex. Environ. Int. 112, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain D. W., Cidlowski J. A. (2015). Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 29, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carll A. P., Hazari M. S., Perez C. M., Krantz Q. T., King C. J., Haykal-Coates N., Cascio W. E., Costa D. L., Farraj A. K. (2013). An autonomic link between inhaled diesel exhaust and impaired cardiac performance: Insight from treadmill and dobutamine challenges in heart failure-prone rats. Toxicol. Sci. 135, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio W. E. (2016). Proposed pathophysiologic framework to explain some excess cardiovascular death associated with ambient air particle pollution: Insights for public health translation. Biochim. Biophys. Acta 1860, 2869–2879. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Bonham A. C., Plopper C. G., Joad J. P. (2003). Neuroplasticity in nucleus tractus solitarius neurons after episodic ozone exposure in infant primates. J. Appl. Physiol. (1985) 94, 819–827. [DOI] [PubMed] [Google Scholar]
- Chen E., Miller G. E. (2007). Stress and inflammation in exacerbations of asthma. Brain Behav. Immun. 21, 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Li H., Cai J., Wang C., Lin Z., Liu C., Niu Y., Zhao Z., Li W., Kan H. (2018). Fine particulate air pollution and the expression of micrornas and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ. Health Perspect. 126, 017007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarella S. E., Soberanes S., Urich D., Morales-Nebreda L., Nigdelioglu R., Green D., Young J. B., Gonzalez A., Rosario C., Misharin A. V. et al. . (2014). Beta(2)-adrenergic agonists augment air pollution-induced il-6 release and thrombosis. J Clin Invest. 124, 2935–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chounlamountry K., Boyer B., Penalba V., Francois-Bellan A. M., Bosler O., Kessler J. P., Strube C. (2015). Remodeling of glial coverage of glutamatergic synapses in the rat nucleus tractus solitarii after ozone inhalation. J. Neurochem. 134, 857–864. [DOI] [PubMed] [Google Scholar]
- Clougherty J. E., Kubzansky L. D. (2009). A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ. Health Perspect. 117, 1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty J. E., Levy J. I., Kubzansky L. D., Ryan P. B., Suglia S. F., Canner M. J., Wright R. J. (2007). Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ. Health Perspect. 115, 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty J. E., Rossi C. A., Lawrence J., Long M. S., Diaz E. A., Lim R. H., McEwen B., Koutrakis P., Godleski J. J. (2010). Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ. Health Perspect. 118, 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottet-Emard J. M., Dalmaz Y., Pequignot J., Peyrin L., Pequignot J. M. (1997). Long-term exposure to ozone alters peripheral and central catecholamine activity in rats. Pflugers Arch. 433, 744–749. [DOI] [PubMed] [Google Scholar]
- Cottrell E. C., Seckl J. R. (2009). Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 3, 19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell W. J., Wright R. J. (2017). Sex-specific effects of combined exposure to chemical and non-chemical stressors on neuroendocrine development: A review of recent findings and putative mechanisms. Curr. Environ. Health Rep. 4, 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering-Rice C. E., Johansen M. E., Roberts J. K., Thomas K. C., Romero E. G., Lee J., Yost G. S., Veranth J. M., Reilly C. A. (2012). Transient receptor potential vanilloid-1 (trpv1) is a mediator of lung toxicity for coal fly ash particulate material. Mol. Pharmacol. 81, 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F. S. (2014). Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 58, 193–210. [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S., Malarkey W. B., Neri E., McEwen B. S. (2012). Stress-induced redistribution of immune cells–from barracks to boulevards to battlefields: A tale of three hormones–curt richter award winner. Psychoneuroendocrinology 37, 1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale J. E. (2008). Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol. 51, 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K., Stone V., Seaton A., MacNee W. (2001). Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ. Health Perspect. 109, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour S., Lebon V., Shulman G. I., Petersen K. F. (2009). Regulation of net hepatic glycogenolysis and gluconeogenesis by epinephrine in humans. Am. J. Physiol. Endocrinol. Metab. 297, E231–E235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic D., White H. J. (1986). Thymus and pulmonary lymph node response to acute and subchronic ozone inhalation in the mouse. Environ. Res. 41, 598–609. [DOI] [PubMed] [Google Scholar]
- Everly G. S., Lating J. M. (2013). The Anatomy and Physiology of the Human Stress Response. Springer, New York, NY. [Google Scholar]
- Fichna M., Fichna P. (2017). Glucocorticoids and beta-cell function. Endokrynol. Pol. 68, 568–573. [DOI] [PubMed] [Google Scholar]
- Fink G. (2010). Stress Science: Neuroendocrinology. Academic Press, Cambridge, MA. [Google Scholar]
- Francis M., Sun R., Cervelli J. A., Choi H., Mandal M., Abramova E. V., Gow A. J., Laskin J. D., Laskin D. L. (2017). Role of spleen-derived macrophages in ozone-induced lung inflammation and injury. Toxicol. Sci. 155, 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackiere F., Saliba L., Baude A., Bosler O., Strube C. (2011). Ozone inhalation activates stress-responsive regions of the cns. J. Neurochem. 117, 961–972. [DOI] [PubMed] [Google Scholar]
- Gelman P. L., Flores-Ramos M., Lopez-Martinez M., Fuentes C. C., Grajeda J. P. (2015). Hypothalamic-pituitary-adrenal axis function during perinatal depression. Neurosci. Bull. 31, 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godleski J. J., Verrier R. L., Koutrakis P., Catalano P., Coull B., Reinisch U., Lovett E. G., Lawrence J., Murthy G. G., Wolfson J. M. et al. , . (2000). Mechanisms of morbidity and mortality from exposure to ambient air particles. Res. Rep. Health Eff. Inst. 5–88, discussion 91, 89–103. [PubMed] [Google Scholar]
- Gold D. R., Litonjua A., Schwartz J., Lovett E., Larson A., Nearing B., Allen G., Verrier M., Cherry R., Verrier R. (2000). Ambient pollution and heart rate variability. Circulation 101, 1267–1273. [DOI] [PubMed] [Google Scholar]
- Goldstein D. S., Kopin I. J. (2017). Homeostatic systems, biocybernetics, and autonomic neuroscience. Auton. Neurosci. 208, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. W., Peter-Fizaine F. E., Shinpock S. G., Hall E. A., Fahmie D. J. (1989). Immunologic and hematologic consequences in mice of exposure to ozone. J. Environ. Pathol. Toxicol. Oncol. 9, 243–252. [PubMed] [Google Scholar]
- Gordon C. J., Johnstone A. F., Aydin C., Phillips P. M., MacPhail R. C., Kodavanti U. P., Ledbetter A. D., Jarema K. A. (2014). Episodic ozone exposure in adult and senescent brown norway rats: Acute and delayed effect on heart rate, core temperature and motor activity. Inhal. Toxicol. 26, 380–390. [DOI] [PubMed] [Google Scholar]
- Gordon C. J., Spencer P. J., Hotchkiss J., Miller D. B., Hinderliter P. M., Pauluhn J. (2008). Thermoregulation and its influence on toxicity assessment. Toxicology 244, 87–97. [DOI] [PubMed] [Google Scholar]
- Hassett C., Mustafa M. G., Coulson W. F., Elashoff R. M. (1985). Splenomegaly in mice following exposure to ambient levels of ozone. Toxicol. Lett. 26, 139–144. [DOI] [PubMed] [Google Scholar]
- Hathout E. H., Beeson W. L., Ischander M., Rao R., Mace J. W. (2006). Air pollution and type 1 diabetes in children. Pediatr. Diabetes 7, 81–87. [DOI] [PubMed] [Google Scholar]
- Henriquez A., House J., Miller D. B., Snow S. J., Fisher A., Ren H., Schladweiler M. C., Ledbetter A. D., Wright F., Kodavanti U. P. (2017). Adrenal-derived stress hormones modulate ozone-induced lung injury and inflammation. Toxicol. Appl. Pharmacol. 329, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez A. R., Snow S. J., Schladweiler M. C., Miller C. N., Dye J. A., Ledbetter A. D., Richards J. E., Mauge-Lewis K., McGee M. A., Kodavanti U. P. (2018a). Adrenergic and glucocorticoid receptor antagonists reduce ozone-induced lung injury and inflammation. Toxicol. Appl. Pharmacol. 339, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez A. R., Snow S. J., Schladweiler M. C., Miller C. N., Dye J. D., Ledbetter A. D., Richards J. E., Hargrove M. M., Williams W. C., Kodavanti U. P. (2018b). Beta-2 adrenergic and glucocorticoid receptor agonists modulate ozone-induced pulmonary protein leakage and inflammation in healthy and adrenalectomized rats. Toxicol. Sci. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler J. G., Artigas F., Bortolozzi A., Daws L. C., De Deurwaerdere P., Milan L., Navailles S., Koek W. (2013). Catecholamine/serotonin interactions: Systems thinking for brain function and disease. Adv. Pharmacol. 68, 167–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. P. (2018). Regulation of hypothalamo-pituitary-adrenocortical responses to stressors by the nucleus of the solitary tract/dorsal vagal complex. Cell. Mol. Neurobiol. 38, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusinkveld H. J., Wahle T., Campbell A., Westerink R. H. S., Tran L., Johnston H., Stone V., Cassee F. R., Schins R. P. F. (2016). Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 56, 94–106. [DOI] [PubMed] [Google Scholar]
- Huang W., Zhu T., Pan X., Hu M., Lu S. E., Lin Y., Wang T., Zhang Y., Tang X. (2012). Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: Interactions of systemic inflammation, overweight, and gender. Am. J. Epidemiol. 176, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam T., Urman R., Gauderman W. J., Milam J., Lurmann F., Shankardass K., Avol E., Gilliland F., McConnell R. (2011). Parental stress increases the detrimental effect of traffic exposure on children’s lung function. Am. J. Respir. Crit. Care Med. 184, 822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Wei Y., Li X., Yang L., Liu H., Guo C., Zhang L., Li N., Guo S., Qian Y. et al. , . (2018). Exposure to ambient air particles increases the risk of mental disorder: Findings from a natural experiment in beijing. Int. J. Environ. Res. Public Health 15, 160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Dunn E., Kostaki A., Andrews M. H., Matthews S. G. (2006). Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J. Physiol. 572, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon N. J., Last M. S., Eiserich J. P., Morrissey B. M., Temple L. M., Last J. A. (2006). Differentiation of the roles of no from airway epithelium and inflammatory cells in ozone-induced lung inflammation. Toxicol. Appl. Pharmacol. 215, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti U. P. (2016). Stretching the stress boundary: Linking air pollution health effects to a neurohormonal stress response. Biochim. Biophys. Acta. 2880–2890. [DOI] [PubMed] [Google Scholar]
- Kodavanti U. P., Chen L. C., Costa D. L. (2011). Experimental Studies in Animals. John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- Koren L., Whiteside D., Fahlman S., Ruckstuhl K., Kutz S., Checkley S., Dumond M., Wynne-Edwards K. (2012). Cortisol and corticosterone independence in cortisol-dominant wildlife. Gen. Comp. Endocrinol. 177, 113–119. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Walker P., McLean L., Carrive P. (2015). Fear and the defense cascade: Clinical implications and management. Harv. Rev. Psychiatry 23, 263–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurhanewicz N., McIntosh-Kastrinsky R., Tong H., Ledbetter A., Walsh L., Farraj A., Hazari M. (2017). Trpa1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol. Appl. Pharmacol. 324, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov N. E. (2012). The Neurochemical Anatomy of Trigeminal Primary Afferent Neurons. InTech, New York, NY. [Google Scholar]
- Levesque S., Taetzsch T., Lull M. E., Kodavanti U., Stadler K., Wagner A., Johnson J. A., Duke L., Kodavanti P., Surace M. J. et al. , . (2011). Diesel exhaust activates and primes microglia: Air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ. Health Perspect. 119, 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. F., Richters A. (1991). Ambient level ozone effects on subpopulations of thymocytes and spleen t lymphocytes. Arch. Environ. Health 46, 57–63. [DOI] [PubMed] [Google Scholar]
- Li H., Cai J., Chen R., Zhao Z., Ying Z., Wang L., Chen J., Hao K., Kinney P. L., Chen H. et al. , . (2017). Particulate matter exposure and stress hormone levels: A randomized, double-blind, crossover trial of air purification. Circulation 136, 618–627. [DOI] [PubMed] [Google Scholar]
- Liu C., Xu X., Bai Y., Zhong J., Wang A., Sun L., Kong L., Ying Z., Sun Q., Rajagopalan S. (2017). Particulate air pollution mediated effects on insulin resistance in mice are independent of ccr2. Part Fibre Toxicol. 14, 6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magari S. R., Schwartz J., Williams P. L., Hauser R., Smith T. J., Christiani D. C. (2002). The association between personal measurements of environmental exposure to particulates and heart rate variability. Epidemiology 13, 305–310. [DOI] [PubMed] [Google Scholar]
- Martinez-Lazcano J. C., Gonzalez-Guevara E., del Carmen Rubio M., Franco-Perez J., Custodio V., Hernandez-Ceron M., Livera C., Paz C. (2013). The effects of ozone exposure and associated injury mechanisms on the central nervous system. Rev. Neurosci. 24, 337–352. [DOI] [PubMed] [Google Scholar]
- Martrette J. M., Thornton S. N., Trabalon M. (2011). Prolonged ozone exposure effects behaviour, hormones and respiratory muscles in young female rats. Physiol. Behav. 103, 302–307. [DOI] [PubMed] [Google Scholar]
- Mazzone S. B., Canning B. J. (2013). Autonomic neural control of the airways. Handb. Clin. Neurol. 117, 215–228. [DOI] [PubMed] [Google Scholar]
- Mazzone S. B., Undem B. J. (2016). Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 96, 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. (2016). In pursuit of resilience: Stress, epigenetics, and brain plasticity. Ann. N. Y. Acad. Sci. 1373, 56–64. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Bowles N. P., Gray J. D., Hill M. N., Hunter R. G., Karatsoreos I. N., Nasca C. (2015). Mechanisms of stress in the brain. Nat. Neurosci. 18, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., Stellar E. (1993). Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 153, 2093–2101. [PubMed] [Google Scholar]
- McEwen B. S., Wingfield J. C. (2010). What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 57, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern A. E., Mazzone S. B. (2014). Neural regulation of inflammation in the airways and lungs. Auton. Neurosci. 182, 95–101. [DOI] [PubMed] [Google Scholar]
- Miller D. B., Ghio A. J., Karoly E. D., Bell L. N., Snow S. J., Madden M. C., Soukup J., Cascio W. E., Gilmour M. I., Kodavanti U. P. (2016a). Ozone exposure increases circulating stress hormones and lipid metabolites in humans. Am. J. Respir. Crit. Care Med. 193, 1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. B., Snow S. J., Henriquez A., Schladweiler M. C., Ledbetter A. D., Richards J. E., Andrews D. L., Kodavanti U. P. (2016b). Systemic metabolic derangement, pulmonary effects, and insulin insufficiency following subchronic ozone exposure in rats. Toxicol. Appl. Pharmacol. 306, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. B., Snow S. J., Schladweiler M. C., Richards J. E., Ghio A. J., Ledbetter A. D., Kodavanti U. P. (2016c). Acute ozone-induced pulmonary and systemic metabolic effects are diminished in adrenalectomized rats. Toxicol. Sci. 150, 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. B., Karoly E. D., Jones J. C., Ward W. O., Vallanat B. D., Andrews D. L., Schladweiler M. C., Snow S. J., Bass V. L., Richards J. E. et al. , . (2015). Inhaled ozone (o3)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicol. Appl. Pharmacol. 286, 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumaw C. L., Levesque S., McGraw C., Robertson S., Lucas S., Stafflinger J. E., Campen M. J., Hall P., Norenberg J. P., Anderson T. et al. , . (2016). Microglial priming through the lung-brain axis: The role of air pollution-induced circulating factors. FASEB J. 30, 1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H. S. (1995). Beta-adrenergic bronchodilators. N. Engl. J. Med. 333, 499–506. [DOI] [PubMed] [Google Scholar]
- Nielsen G. D., Wolkoff P., Alarie Y. (2007). Sensory irritation: Risk assessment approaches. Regul. Toxicol. Pharmacol. 48, 6–18. [DOI] [PubMed] [Google Scholar]
- O’Neill M. S., Jerrett M., Kawachi I., Levy J. I., Cohen A. J., Gouveia N., Wilkinson P., Fletcher T., Cifuentes L., Schwartz J. et al. , . (2003). Health, wealth, and air pollution: Advancing theory and methods. Environ. Health Perspect. 111, 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios N., Fitzgerald K. C., Hart J. E., Weisskopf M., Schwarzschild M. A., Ascherio A., Laden F. (2017). Air pollution and risk of Parkinson’s disease in a large prospective study of men. Environ. Health Perspect. 125, 087011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M., Kuperman Y., Levin L., Rudich A., Haim Y., Schauer J. J., Chen A., Rudich Y. (2018). Exposure to air pollution interacts with obesogenic nutrition to induce tissue-specific response patterns. Environ. Pollut. 239, 532–543. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. (2004). Acute inhalation studies with irritant aerosols: Technical issues and relevance for risk characterization. Arch. Toxicol. 78, 243–251. [DOI] [PubMed] [Google Scholar]
- Pereira M. R., Leite P. E. (2016). The involvement of parasympathetic and sympathetic nerve in the inflammatory reflex. J. Cell. Physiol. 231, 1862–1869. [DOI] [PubMed] [Google Scholar]
- Perez C. M., Hazari M. S., Farraj A. K. (2015). Role of autonomic reflex arcs in cardiovascular responses to air pollution exposure. Cardiovasc. Toxicol. 15, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhoff M., Sieg A., Brede M., Chao C. M., Hein L., Ullrich S. (2003). Inhibition of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor knockout mice. Eur. J. Endocrinol. 149, 343–350. [DOI] [PubMed] [Google Scholar]
- Qiu L., Chen M., Wang X., Qin X., Chen S., Qian Y., Liu Z., Cao Q., Ying Z. (2017a). Exposure to concentrated ambient pm2.5 compromises spermatogenesis in a mouse model: Role of suppression of hypothalamus-pituitary-gonads axis. Toxicol. Sci. 1621:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Zheng Z., Kim H., Yang Z., Zhang G., Shi X., Sun F., Peng C., Ding Y., Wang A. et al. , . (2017b). Inhalation exposure to pm2.5 counteracts hepatic steatosis in mice fed high-fat diet by stimulating hepatic autophagy. Sci. Rep. 7, 16286.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay D. S., Woods S. C. (2014). Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol. Rev. 121, 225–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranabir S., Reetu K. (2011). Stress and hormones. Indian J. Endocrinol. Metab. 15, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Arancibia S., Guevara-Guzman R., Lopez-Vidal Y., Rodriguez-Martinez E., Zanardo-Gomes M., Angoa-Perez M., Raisman-Vozari R. (2010). Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. 113, 187–197. [DOI] [PubMed] [Google Scholar]
- Rosa M. J., Just A. C., Kloog I., Pantic I., Schnaas L., Lee A., Bose S., Chiu Y. M., Hsu H. L., Coull B. et al. , . (2017). Prenatal particulate matter exposure and wheeze in mexican children: Effect modification by prenatal psychosocial stress. Ann. Allergy Asthma Immunol. 119, 232–237 e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack C. S., Jansen K. L., Cosselman K. E., Trenga C. A., Stapleton P. L., Allen J., Peretz A., Olives C., Kaufman J. D. (2016). Pretreatment with antioxidants augments the acute arterial vasoconstriction caused by diesel exhaust inhalation. Am. J. Respir. Crit. Care Med. 193, 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow S. J., McGee M. A., Henriquez A., Richards J. E., Schladweiler M. C., Ledbetter A. D., Kodavanti U. P. (2017). Respiratory effects and systemic stress response following acute acrolein inhalation in rats. Toxicol. Sci. 158, 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulage C., Perrin D., Cottet-Emard J. M., Pequignot J., Dalmaz Y., Pequignot J. M. (2004). Central and peripheral changes in catecholamine biosynthesis and turnover in rats after a short period of ozone exposure. Neurochem. Int. 45, 979–986. [DOI] [PubMed] [Google Scholar]
- Sun Q., Hong X., Wold L. E. (2010). Cardiovascular effects of ambient particulate air pollution exposure. Circulation 121, 2755–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwannasual U., Lucero J., McDonald J. D., Lund A. K. (2018). Exposure to traffic-generated air pollutants mediates alterations in brain microvascular integrity in wildtype mice on a high-fat diet. Environ. Res. 160, 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E. M., Pal S., Guenette J., Wade M. G., Atlas E., Holloway A. C., Williams A., Vincent R. (2016). Ozone inhalation provokes glucocorticoid-dependent and -independent effects on inflammatory and metabolic pathways. Toxicol. Sci. 152, 17–28. [DOI] [PubMed] [Google Scholar]
- Thomson E. M., Pilon S., Guenette J., Williams A., Holloway A. C. (2018). Ozone modifies the metabolic and endocrine response to glucose: Reproduction of effects with the stress hormone corticosterone. Toxicol. Appl. Pharmacol. 342, 31–38. [DOI] [PubMed] [Google Scholar]
- Thomson E. M., Vladisavljevic D., Mohottalage S., Kumarathasan P., Vincent R. (2013). Mapping acute systemic effects of inhaled particulate matter and ozone: Multiorgan gene expression and glucocorticoid activity. Toxicol. Sci. 135, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama I., Simomura Y., Yokoyama E. (1986). Effects of acute exposure to ozone on heart rate and blood pressure of the conscious rat. Environ. Res. 41, 529–537. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y. M., Herman J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella R. E., Pillon N. J., Zarrouki B., Croze M. L., Koppe L., Guichardant M., Pesenti S., Chauvin M. A., Rieusset J., Geloen A. et al. , . (2015). Ozone exposure triggers insulin resistance through muscle c-jun n-terminal kinase activation. Diabetes 64, 1011–1024. [DOI] [PubMed] [Google Scholar]
- Verburg-van Kemenade B. M. L., Cohen N., Chadzinska M. (2017). Neuroendocrine-immune interaction: Evolutionarily conserved mechanisms that maintain allostasis in an ever-changing environment. Dev. Comp. Immunol. 66, 2–23. [DOI] [PubMed] [Google Scholar]
- Vert C., Sanchez-Benavides G., Martinez D., Gotsens X., Gramunt N., Cirach M., Molinuevo J. L., Sunyer J., Nieuwenhuijsen M. J., Crous-Bou M. et al. , . (2017). Effect of long-term exposure to air pollution on anxiety and depression in adults: A cross-sectional study. Int. J. Hyg. Environ. Health 220, 1074–1080. [DOI] [PubMed] [Google Scholar]
- Wagner J. G., Allen K., Yang H. Y., Nan B., Morishita M., Mukherjee B., Dvonch J. T., Spino C., Fink G. D., Rajagopalan S. et al. , . (2014). Cardiovascular depression in rats exposed to inhaled particulate matter and ozone: Effects of diet-induced metabolic syndrome. Environ. Health Perspect. 122, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenborn J. G., McGee J. K., Schladweiler M. C., Ledbetter A. D., Kodavanti U. P. (2007). Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicol. Sci. 98, 231–239. [DOI] [PubMed] [Google Scholar]
- Widdicombe J., Lee L. Y. (2001). Airway reflexes, autonomic function, and cardiovascular responses. Environ. Health Perspect. 109, 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2016). Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease. World Health Organization, Geneva, Switzerland.
- Xu X., Liu C., Xu Z., Tzan K., Zhong M., Wang A., Lippmann M., Chen L. C., Rajagopalan S., Sun Q. (2011). Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol. Sci. 124, 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A., Luttmann-Gibson H., Horton E. S., Cohen A., Coull B. A., Hoffmann B., Schwartz J. D., Mittleman M. A., Li Y., Stone P. H. et al. , . (2014). Brachial artery responses to ambient pollution, temperature, and humidity in people with type 2 diabetes: A repeated-measures study. Environ. Health Perspect. 122, 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]