Abstract
Objective
This study investigates the characteristics and trends of medication errors involving analgesic medications.
Design and Methods
A retrospective analysis was conducted of analgesic-related medication errors reported to the National Poison Data System (NPDS) from 2000 through 2012.
Results
From 2000 through 2012, the NPDS received 533,763 reports of analgesic-related medication errors, averaging 41,059 medication errors annually. Overall, the rate of analgesic-related medication errors reported to the NPDS increased significantly by 82.6% from 2000 to 2009, followed by a 5.7% nonsignificant decrease from 2009 to 2012. Among the analgesic categories, rates of both acetaminophen-related and opioid-related medication errors reported to the NPDS increased during 2000–2009, but the opioid error rate leveled off during 2009–2012, while the acetaminophen error rate decreased by 17.9%. Analgesic-related medication errors involved nonsteroidal anti-inflammatory drugs (37.0%), acetaminophen (35.5%), and opioids (23.2%). Children five years or younger accounted for 38.8% of analgesics-related medication errors. Most (90.2%) analgesic-related medication errors were managed on-site, rather than at a health care facility; 1.6% were admitted to a hospital, and 1.5% experienced serious medical outcomes, including 145 deaths. The most common type of medication error was inadvertently taking/given the medication twice (26.6%).
Conclusion
Analgesic-related medication errors are common, and although most do not result in clinical consequences, they can have serious adverse outcomes. Initiatives associated with the decrease in acetaminophen-related medication errors among young children merit additional research and potential replication as a model combining government policy and multisectoral collaboration.
Keywords: Analgesic, Acetaminophen, Opioid, Ibuprofen, Poisoning, Poison Control Center
Introduction
Analgesics, including both nonprescription medications (such as acetaminophen, ibuprofen, and aspirin) and prescription medications (such as opioids), are the most frequently used medications in the United States among both children and adults [1,2]. According to a 2006 survey, acetaminophen is the most frequently utilized over-the-counter (OTC) product in the United States, used by 19% of adults in any given week [2]. While the use of analgesics is generally considered safe, exceeding the maximum recommended dose, due to either unintentional therapeutic error or intentional overdose, can result in serious injury or death [3,4]. From 2000 through 2012, almost 3 million calls involving unintentional therapeutic errors were made to US Poison Control Centers (PCCs), and approximately one-fifth of these involved analgesic medications [5]. In addition, analgesic medications had the greatest increase in the rate of unintentional therapeutic errors reported to PCCs among all drug classes during this period [6,7].
Previous epidemiologic studies characterizing medication errors associated with analgesics have often concentrated only on a single substance [8–11] or specific age groups [12,13] or have been limited to emergency department visits [14–17]. More recently, a six-year retrospective study of National Poison Data System (NPDS) data and a prospective observational study of out-of-hospital medication errors indicated that analgesics are the leading drug class associated with out-of-hospital medication errors [18,19]. Medication errors are a major source of morbidity and mortality and a large contributor to increasing health care costs [20]. A more comprehensive analysis of unintentional analgesic medication errors may improve our understanding of the origin of these errors and serve as a guide in prevention.
The objective of this study is to conduct an in-depth analysis of analgesic-related medication errors reported to the NPDS over a 13-year period. To our knowledge, this is the first study to describe and compare trends of unintentional medication errors associated with specific categories of analgesic medications.
Methods
Study Design and Data Source
This study retrospectively analyzes data from the NPDS to evaluate characteristics and trends of unintentional therapeutic errors involving analgesics occurring between January 1, 2000 and December 31, 2012. The NPDS is a proprietary database maintained by the American Association of Poison Control Centers (AAPCC) and consists of all informational and poison exposure calls received by regional PCCs in the United States and its territories. The NPDS is the only comprehensive poison exposure surveillance database in the United States, and it has extensive internal quality control measures to help ensure accuracy and completeness.
Study Population
The study population was limited to exposure calls from within the 50 US states and the District of Columbia that involved an unintentional therapeutic error associated with an analgesic medication. The AAPCC defines an unintentional therapeutic error as “an unintentional deviation from a proper therapeutic regimen that results in the wrong dose, incorrect route of administration, administration to the wrong person, or administration of the wrong substance” [6]. These types of errors are referred to as “medication errors” in this study. Intentional misuse, abuse, and exploratory, suicidal, and malicious exposures to analgesics were excluded. Exposures with a medical outcome of “confirmed nonexposure” or “unrelated effect” were also excluded.
Analgesics included in this study were opioids, acetaminophen, acetylsalicylic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), and their respective combinations (Appendix A). Although the NPDS classifies colchicine as an analgesic, it was not included in this study. The analgesic category was determined by the AAPCC generic coding system, which is maintained by Micromedex Poisindex System (Micromedex Healthcare Series [Internet database]; Truven Health Analytics, Inc., Greenwood Village, CO, USA). For unintentional therapeutic error-related exposures involving multiple medications, the medication determined by the PCC specialist as the primary contributor to the observed clinical effects was used to categorize the exposure.
Study Variables
Variables included in this study were age, gender, analgesic category, number of substances involved, type of therapeutic error, management site, level of health care received, and medical outcome. Analgesics were classified into the following categories: opioids, acetaminophen, aspirin (acetylsalicylic acid), acetaminophen/aspirin (combinations of acetaminophen and aspirin), NSAIDs, and other (Appendix A).
Medical outcomes (minor effect, moderate effect, major effect, and death) were classified by PCC specialists in accordance with standard NPDS definitions. “Minor effect” indicates minimally bothersome symptoms that generally resolve rapidly with no residual disability or disfigurement; “moderate effect” indicates more prolonged, pronounced, or systemic symptoms, usually requiring medical intervention; and “major effect” signifies life-threatening symptoms or significant residual disability or disfigurement [6]. “Serious medical outcomes” are defined in this study to include cases with moderate effects, major effects, or who died. Health care facility (HCF) admissions include admissions to a critical care unit (CCU), noncritical care unit (non-CCU), or psychiatric facility. Ages were grouped into the following categories: five years or younger, six to 19 years, 20 to 49 years, and 50 years or older.
Data Analysis
Data were analyzed using IBM SPSS Statistics 21.0 (IBM Corp., Armonk, NY, USA) and SAS Enterprise Guide 7.11 HF3 (SAS Institute, Cary, NC, USA) statistical software. Overall and age group–specific rates of medication errors were calculated using July 1 US intercensal and postcensal US resident population estimates for 2000 through 2012 [21, 22]. Trend analyses for rates of medication errors reported to the NPDS were performed using simple linear regression or piecewise regression based on whether there was a shift in directionality of the trends (Figures 1–4). The estimated annual rate of change from the regression model (m) is reported, along with the associated P values. Statistical significance was determined at α = 0.05. This study was deemed exempt by the Institutional Review Board at Nationwide Children Hospital.
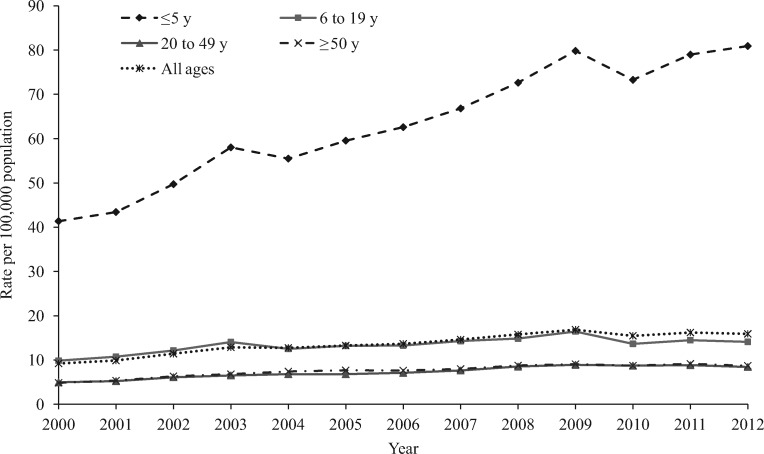
Figure 1.
Annual rate per 100,000 population of analgesic-related medication errors reported to US Poison Control Centers by age group, National Poison Data System 2000–2012.
Results
General Characteristics
From 2000 through 2012, PCCs in the United States received 533,763 calls about unintentional analgesic medication errors, averaging 41,059 medication errors annually or one error every 13 minutes. The majority (87.7%) of these exposures involved a single substance. NSAIDs (37.0%), acetaminophen (35.5%), and opioids (23.2%) accounted for 95.7% of reported analgesic medication errors (Table 1). Most (95.9%) analgesic medication errors occurred at the caller’s own residence, and only 0.5% occurred at an HCF. Almost all (99.1%) analgesic medication errors were associated with ingestion as the route of exposure. Females accounted for 56.8% of analgesic medication errors, and the mean age of all exposed individuals was 22.3 years (SD = 24.5 years) with median age of 11 years (interquartile range = 2 to 38 years). Children five years or younger accounted for 38.8% of the errors, and adults age 20 to 49 years accounted for 23.5%. The rate of analgesic medication errors per 100,000 population was more than four times higher among children five years or younger (63.50) than among children age six to 19 years (13.37), and more than eight times higher than that among adults age 20 to 49 years (7.28) and adults age 50 years or older (7.69).
Table 1.
Characteristics of analgesic-related medication errors by analgesic category, NPDS 2000–2012
| Characteristics | Acetaminophen, No. (%)* | Opioids, No. (%)* | NSAIDs, No. (%)* | Aspirin, No. (%)* | Acetaminophen/Aspirin Combinations, No. (%)* | Other†, No. (%)* | Total, No. (%)* |
|---|---|---|---|---|---|---|---|
| Age, y | |||||||
| 0–5 | 99,062 (54.0) | 13,140 (11.6) | 82,594 (43.7) | 1,756 (13.7) | 134 (2.0) | 111 (5.1) | 196,797 (38.8) |
| 6–19 | 37,353 (20.4) | 16,153 (14.2) | 42,240 (22.3) | 3,425 (26.7) | 1,809 (27.7) | 385 (17.6) | 101,365 (20.0) |
| 20–49 | 28,527 (15.6) | 43,869 (38.6) | 38,999 (20.6) | 3,199 (25.0) | 3,845 (58.8) | 901 (41.3) | 119,340 (23.5) |
| ≥50 | 18,474 (10.1) | 40,419 (35.6) | 25,309 (13.4) | 4,436 (34.6) | 754 (11.5) | 787 (36.0) | 90,179 (17.8) |
| Unknown‡ | 6,074 | 10,032 | 8,370 | 794 | 581 | 231 | 26,082 |
| Gender | |||||||
| Male | 85,767 (45.4) | 48,721 (39.5) | 87,144 (44.2) | 5,662 (41.7) | 2,048 (28.8) | 596 (24.7) | 229,938 (43.2) |
| Female | 103,342 (54.6) | 74,687 (60.5) | 110,084 (55.8) | 7,919 (58.3) | 5,072 (71.2) | 1,815 (75.3) | 302,919 (56.8) |
| Unknown‡ | 381 | 205 | 284 | 29 | 3 | 4 | 906 |
| Acuity/chronicity | |||||||
| Acute | 165,649 (87.6) | 85,766 (69.6) | 172,485 (87.5) | 10,770 (79.4) | 6,139 (86.3) | 1,940 (80.5) | 442,749 (83.1) |
| Acute-on-chronic | 16,124 (8.5) | 33,006 (26.8) | 19,682 (10.0) | 1,942 (14.3) | 555 (7.8) | 388 (16.1) | 71,697 (13.5) |
| Chronic | 7,415 (3.9) | 4,400 (3.6) | 5,060 (2.6) | 852 (6.3) | 420 (5.9) | 82 (3.4) | 18,229 (3.4) |
| Unknown‡ | 302 | 441 | 285 | 46 | 9 | 5 | 1,088 |
| Management site | |||||||
| Managed on-site at non-HCF | 174,309 (92.2) | 97,074 (79.1) | 189,109 (96.0) | 11,007 (81.3) | 6,300 (88.9) | 2,073 (86.3) | 479,872 (90.2) |
| Individual already in/enroute to HCF when PCC called | 9,130 (4.8) | 11,594 (9.4) | 4,225 (2.1) | 1,670 (12.3) | 370 (5.2) | 175 (7.3) | 27,164 (5.1) |
| Individual referred by PCC to a HCF | 4,238 (2.2) | 12,476 (10.2) | 1,972 (1.0) | 712 (5.3) | 343 (4.8) | 126 (5.2) | 19,867 (3.7) |
| Other | 1,344 (0.7) | 1,640 (1.3) | 1,652 (0.8) | 151 (1.1) | 75 (1.1) | 29 (1.2) | 4,891 (0.9) |
| Unknown‡ | 469 | 829 | 554 | 70 | 35 | 12 | 1,969 |
| HCF level of care | |||||||
| No HCF treatment received | 176,122 (92.9) | 99,543 (80.5) | 191,315 (96.9) | 11,228 (82.5) | 6,410 (90.0) | 2,114 (87.5) | 486,732 (91.2) |
| Treated/evaluated and released | 7,470 (3.9) | 12,039 (9.7) | 4,187 (2.1) | 1,045 (7.7) | 399 (5.6) | 166 (6.9) | 25,306 (4.7) |
| Admitted to critical care unit | 1,035 (0.5) | 1,971 (1.6) | 97 (0.0) | 456 (3.4) | 25 (0.4) | 24 (1.0) | 3,608 (0.7) |
| Admitted to noncritical care unit | 2,060 (1.1) | 2,019 (1.6) | 241 (0.1) | 354 (2.6) | 44 (0.6) | 26 (1.1) | 4,744 (0.9) |
| Admitted to psychiatric facility | 68 (0.0) | 103 (0.1) | 22 (0.0) | 15 (0.1) | 5 (0.1) | 0 (0.0) | 213 (0.0) |
| Other§ | 2,735 (1.4) | 7,938 (6.4) | 1,650 (0.8) | 512 (3.8) | 240 (3.4) | 85 (3.5) | 13,160 (2.5) |
| Medical outcome | |||||||
| Death | 74 (0.0) | 61 (0.0) | 0 (0.0) | 8 (0.1) | 2 (0.0) | 0 (0.0) | 145 (0.0) |
| Major effect | 349 (0.2) | 505 (0.4) | 24 (0.0) | 106 (0.8) | 7 (0.1) | 13 (0.5) | 1,004 (0.2) |
| Moderate effect | 1,616 (0.9) | 4,050 (3.3) | 509 (0.3) | 655 (4.8) | 177 (2.5) | 70 (2.9) | 7,077 (1.3) |
| Minor effect | 4,476 (2.4) | 20,096 (16.3) | 5,829 (3.0) | 1,028 (7.6) | 857 (12.0) | 285 (11.8) | 32,571 (6.1) |
| No effect | 25,863 (13.6) | 21,732 (17.6) | 29,417 (14.9) | 2,081 (15.3) | 790 (11.1) | 353 (14.6) | 80,236 (15.0) |
| Not followed¶ | 154,598 (81.6) | 70,517 (57.0) | 160,558 (81.3) | 9,337 (68.6) | 5,114 (71.8) | 1,627 (67.4) | 401,751 (75.3) |
| Unable to follow‖ | 2,514 (1.3) | 6,652 (5.4) | 1,175 (0.6) | 395 (2.9) | 176 (2.5) | 67 (2.8) | 10,979 (2.1) |
| Total, row %** | 189,490 (35.5) | 123,613 (23.2) | 197,512 (37.0) | 13,610 (2.5) | 7,123 (1.3) | 2,415 (0.5) | 533,763 (100.0) |
HCF = heath care facility; NPDS = National Poison Data System; NSAID = nonsteroidal anti-inflammatory drug; PCC = poison control center.
Column and **row percentages may not sum to 100.0% due to rounding error.
Includes nonaspirin salicylates (excluding topicals and/or gastrointestinal drugs), other analgesics, phenacetin, phenazopyridine, salicylamide, and unknown analgesics.
Not included in percentage calculations.
Includes lost to follow-up/left against medical advice and refused referral/did not arrive at HCF.
Includes not followed, minimal clinical effects possible (no more than minor effects possible) and not followed, judged as nontoxic exposure (clinical effects not expected).
Unable to follow, judged as a potentially toxic exposure.
Most (90.2%) analgesic medication errors were managed on-site (i.e., not at an HCF), while 4.7% were treated at an HCF and released, and another 1.6% were admitted to an HCF (Table 1). Serious medical outcomes occurred in 1.5% of medication error-related exposures, including 145 deaths. Most of the deaths were among adults age 20–49 years (29.7%) and 50 years or older (52.4%), and were associated with acetaminophen (51.0%) or opioid (42.1%) medication errors. The proportions of exposures resulting in HCF admission and serious outcomes increased with increasing age, with children five years or younger having the lowest proportions (0.5% HCF admission and 0.2% serious outcomes) and adults age 50 years or older having the highest proportions (3.7% HCF admission and 3.4% serious outcomes). Higher proportions of serious outcomes were observed among exposures to aspirin (5.7%), opioids (3.7%), and “other analgesics” (3.4%) (Table 2). These three analgesic categories also had the highest rates of HCF admission.
Table 2.
Frequency (and percentage) of medication errors resulting in health care facility admission or a serious medical outcome for selected analgesic categories, NPDS 2000–2012
| Admitted | Serious Outcome | Total | |
|---|---|---|---|
| Type of Analgesic* | No. (Row)† | No. (Row %)† | No. (Col.%)‡ |
| Acetaminophen | |||
| Acetaminophen alone, pediatric | 520 (0.5) | 158 (0.2) | 102,662 (54.2) |
| Acetaminophen alone, adult | 1,781 (3.2) | 1,148 (2.0) | 56,187 (29.7) |
| Acetaminophen in combination with other drugs, adult formulations | 261 (1.4) | 349 (1.9) | 18,630 (9.8) |
| Acetaminophen alone, unknown if adult or pediatric | 599 (5.3) | 376 (3.3) | 11,383 (6.0) |
| Acetaminophen in combination with other drugs, pediatric formulations | — | — | 512 (0.3) |
| Acetaminophen with diphenhydramine | 2 (1.7) | 8 (6.9) | 116 (0.1) |
| Subtotal | 3,163 (1.7) | 2,039 (1.1) | 189,490 |
| Opioids | |||
| Acetaminophen with hydrocodone | 762 (2.3) | 846 (2.5) | 33,581 (27.2) |
| Oxycodone alone or in combination§ | 485 (3.7) | 515 (3.9) | 13,044 (10.6) |
| Acetaminophen with oxycodone | 308 (2.5) | 335 (2.7) | 12,320 (10.0) |
| Other or unknown narcotics | 463 (4.1) | 655 (5.7) | 11,405 (9.2) |
| Acetaminophen with codeine | 138 (1.4) | 189 (1.9) | 10,060 (8.1) |
| Tramadol | 248 (2.5) | 412 (4.2) | 9,823 (7.9) |
| Codeine | 35 (0.4) | 53 (0.7) | 8,139 (6.6) |
| Morphine | 628 (8.4) | 570 (7.6) | 7,470 (6.0) |
| Acetaminophen with propoxyphene | 167 (2.5) | 200 (3.1) | 6,553 (5.3) |
| Methadone | 597 (11.9) | 507 (10.1) | 5,024 (4.1) |
| Acetaminophen with other narcotics or narcotic analogs | 25 (2.0) | 23 (1.9) | 1,226 (1.0) |
| Hydrocodone alone or in combination¶ | 14 (1.2) | 39 (3.4) | 1,160 (0.9) |
| Meperidine | 68 (8.1) | 63 (7.5) | 835 (0.7) |
| Hydromorphone | 32 (6.6) | 28 (5.8) | 485 (0.4) |
| Buprenorphine | 36 (7.7) | 47 (10.1) | 467 (0.4) |
| Propoxyphene | 6 (1.5) | 15 (3.7) | 402 (0.3) |
| Oxymorphone | 14 (5.7) | 14 (5.7) | 246 (0.2) |
| Tapentadol | 12 (5.0) | 24 (10.0) | 240 (0.2) |
| Pentazocine | 3 (1.3) | 12 (5.1) | 235 (0.2) |
| Ibuprofen with hydrocodone | 2 (0.9) | 8 (3.4) | 234 (0.2) |
| Fentanyl | 33 (14.3) | 43 (18.7) | 230 (0.2) |
| Acetylsalicylic acid with codeine | 8 (5.9) | 7 (5.1) | 136 (0.1) |
| Acetylsalicylic acid with oxycodone | 2 (1.5) | 2 (1.5) | 133 (0.1) |
| Butorphanol | — | 4 (6.5) | 62 (0.1) |
| Acetylsalicylic acid with other narcotics or narcotic analogs | 1 (2.0) | 2 (4.0) | 50 (0.0) |
| Acetylsalicylic acid with propoxyphene | 1 (2.7) | — | 37 (0.0) |
| Nalbuphine | 5 (41.7) | 3 (25.0) | 12 (0.0) |
| Levorphanol | — | — | 2 (0.0) |
| Dihydrocodeine | — | — | 1 (0.0) |
| Sufentanil | — | — | 1 (0.0) |
| Subtotal | 4,093 (3.3) | 4,616 (3.7) | 123,613 |
| NSAIDs | |||
| Ibuprofen | 169 (0.1) | 258 (0.2) | 150,284 (76.1) |
| Naproxen | 59 (0.3) | 125 (0.5) | 23,196 (11.7) |
| Other types of nonsteroidal anti-inflammatory drug | 106 (0.7) | 90 (0.6) | 15,981 (8.1) |
| Cyclooxygenase-2 inhibitors | 12 (0.2) | 38 (0.6) | 6,459 (3.3) |
| Indomethacin | 11 (0.9) | 19 (1.5) | 1,262 (0.6) |
| Ketoprofen | 2 (0.7) | 2 (0.7) | 295 (0.1) |
| Ibuprofen with diphenhydramine | 1 (4.8) | 1 (4.8) | 21 (0.0) |
| Unknown types of nonsteroidal anti-inflammatory drug | — | — | 14 (0.0) |
| Subtotal | 360 (0.2) | 533 (0.3) | 197,512 |
| Aspirin | |||
| Acetylsalicylic acid alone, unknown if adult or pediatric formulations | 502 (9.5) | 455 (8.6) | 5,312 (39.0) |
| Acetylsalicylic acid alone, adult formulations | 212 (4.4) | 195 (4.1) | 4,805 (35.3) |
| Acetylsalicylic acid in combination with other drugs, adult formulations | 99 (4.1) | 105 (4.3) | 2,442 (17.9) |
| Acetylsalicylic acid alone, pediatric formulations | 5 (0.6) | 5 (0.6) | 852 (6.3) |
| Acetylsalicylic acid with carisoprodol | 7 (5.5) | 8 (6.3) | 127 (0.9) |
| Acetylsalicylic acid in combination with other drugs, pediatric formulations | — | 1 (1.4) | 72 (0.5) |
| Subtotal | 825 (6.1) | 769 (5.7) | 13,610 |
NPDS = National Poison Data System; NSAID = nonsteroidal anti-inflammatory drug.
“Acetaminophen/aspirin” and “other” analgesic categories are not included in this table.
Percent of each substance.
Percent of each analgesic category.
Excluding combination products with acetaminophen or acetylsalicylic acid.
Excluding combination products with acetaminophen, acetylsalicylic acid, or ibuprofen.
The overall annual rate of analgesic medication errors reported to the NPDS per 100,000 population increased significantly by 82.6% (m = 0.76, P < 0.001) from 9.23 in 2000 to 16.9 in 2009, followed by a nonsignificant decrease of 5.7% (m = –0.20, P = 0.294) from 2009 to 2012 (Figure 1). For each age group, the annual rate of analgesic medication errors reported to the NPDS increased significantly by more than 60.0% from 2000 to 2009. While the rate of analgesic medication errors reported to the NPDS remained constant for children age zero to five years (1.4%, m = 1.33, P = 0.191) from 2009 to 2012, there was a nonsignificant decrease in the rate among individuals age six to 19 years (–14.2%, m = –0.57, P = 0.089), 20–49 years (–5.4%, m = –0.06, P = 0.526), and 50 years and older (–4.0%, m = –0.12, P = 0.295) during this period. The annual rate of analgesic medication errors reported to the NPDS was consistently higher among children age five years or younger than among other age groups throughout the study period (Figure 1).
Type of Medication Error
Medication errors attributed to “inadvertently given/taken medication twice” or “medication doses given/taken too close together” accounted for a large proportion of errors in all age groups, ranging from 28.3% of medication errors among children age six to 19 years to 49.4% of medication errors among adults age 50 years or older (Table 3). Analgesic medication errors attributed to “confused units of measure,” “incorrect formulation/concentration given,” and “dispensing cup error” were most common among children five years or younger (together accounting for 21.2% of errors) and decreased with increasing age group. Although “health professional/iatrogenic error” (0.9%) and “10-fold dosing error” (0.3%) each accounted for less than 1% of all errors, they had the highest proportions of serious outcomes (10.6% and 9.0%, respectively) and HCF admission (19.8% and 13.9%, respectively).
Table 3.
Type of medication error by age group for all analgesics, NPDS 2000–2012
| 0–5 y | 6–19 y | 20–49 y | ≥50 y | Unknown Age | Total | |
|---|---|---|---|---|---|---|
| Type of Medication Error | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) |
| Inadvertently took/given medication twice | 58,094 (29.5) | 17,568 (17.3) | 27,492 (23.0) | 31,745 (35.2) | 7,048 (27.0) | 141,947 (26.6) |
| Other incorrect dose | 38,650 (19.6) | 21,976 (21.7) | 23,064 (19.3) | 13,898 (15.4) | 4,061 (15.6) | 101,649 (19.0) |
| Medication doses given/taken too close together | 24,600 (12.5) | 11,149 (11.0) | 18,782 (15, 7) | 12,806 (14.2) | 4,138 (15.9) | 71,475 (13.4) |
| Wrong medication given/taken | 12,207 (6.2) | 12,862 (12.7) | 17,404 (14.6) | 13,934 (15.5) | 3,952 (15.2) | 60,359 (11.3) |
| Incorrect formulation or concentration given | 14,532 (7.4) | 7,273 (7.2) | 2,912 (2.4) | 1,408 (1.6) | 561 (2.2) | 26,686 (5.0) |
| Inadvertently took/given someone else’s medication | 6,564 (3.3) | 6,897 (6.8) | 5,871 (4.9) | 5,573 (6.2) | 1,466 (5.6) | 26,371 (4.9) |
| Confused units of measure | 15,596 (7.9) | 6,123 (6.0) | 2,373 (2.0) | 947 (1.1) | 345 (1.3) | 25,384 (4.8) |
| More than 1 product containing same ingredient | 2,910 (1.5) | 4,971 (4.9) | 8,910 (7.5) | 2,941 (3.3) | 1,629 (6.2) | 21,361 (4.0) |
| Dispensing cup error | 11,670 (5.9) | 3,743 (3.7) | 830 (0.7) | 435 (0.5) | 130 (0.5) | 16,808 (3.1) |
| Health professional/iatrogenic error* | 1,757 (0.9) | 746 (0.7) | 1,041 (0.9) | 1,012 (1.1) | 335 (1.3) | 4,891 (0.9) |
| Incorrect formulation or concentration dispensed | 2,449 (1.2) | 1,179 (1.2) | 608 (0.5) | 322 (0.4) | 183 (0.7) | 4,741 (0.9) |
| Drug interaction | 420 (0.2) | 499 (0.5) | 1,761 (1.5) | 732 (0.8) | 409 (1.6) | 3,821 (0.7) |
| Incorrect dosing route | 654 (0.3) | 301 (0.3) | 478 (0.4) | 510 (0.6) | 207 (0.8) | 2,150 (0.4) |
| 10-fold dosing error | 745 (0.4) | 200 (0.2) | 306 (0.3) | 267 (0.3) | 51 (0.2) | 1,569 (0.3) |
| Exposure through breast milk | 262 (0.1) | 2 (0.0) | 9 (0.0) | 1 (0.0) | 11 (0.0) | 285 (0.1) |
| Other/unknown therapeutic error | 12,194 (6.2) | 9,517 (9.4) | 12,296 (10.3) | 7,333 (8.1) | 2,569 (9.8) | 43,909 (8.2) |
| Study Total | 196,797 | 101,365 | 119,340 | 90,179 | 26,082 | 533,763 |
Column percentages may sum to more than 100.0% because more than one type of error may be involved.
NPDS = National Poison Data System.
Including pharmacist/nurse/physician.
Acetaminophen
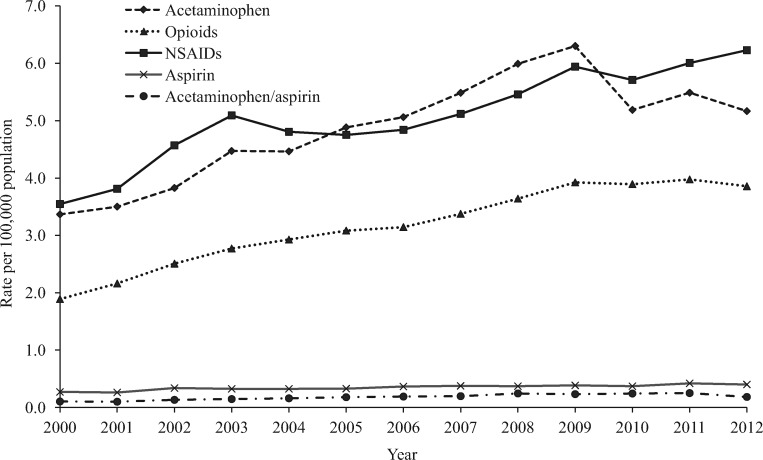
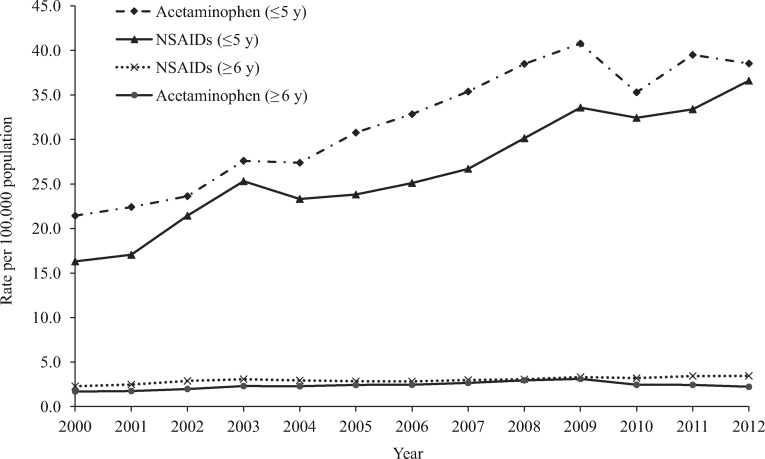
More than half (54.0%) of acetaminophen-related medication errors were observed among children age five years or younger (Table 1). The annual rate per 100,000 population of acetaminophen-related errors reported to the NPDS increased significantly by 86.9% (m = 0.31, P < 0.001) from 3.37 in 2000 to 6.30 in 2009, followed by a significant decrease of 17.9% (m = –0.35, P = 0.002) between 2009 and 2012 (5.17 per 100,000) (Figure 2). The annual rate of acetaminophen medication errors reported to the NPDS was consistently higher for children age five years and younger than for individuals age six years and older (Figure 3). Most (92.2%) acetaminophen-related medication errors were managed on-site, 1.1% were associated with a serious medical outcome, and 1.7% resulted in HCF admission (Table 1).
Figure 2.
Annual rate per 100,000 population of analgesic-related medication errors reported to US Poison Control Centers by selected analgesic category, National Poison Data System 2000–2012. NSAID = nonsteroidal anti-inflammatory drug.
Figure 3.
Annual rate per 100,000 population of acetaminophen-related and NSAID-related medication errors reported to US Poison Control Centers by age group, National Poison Data System 2000–2012. NSAID = nonsteroidal anti-inflammatory drug.
Acetaminophen alone (pediatric formulation) was the most common (54.2%) preparation involved in acetaminophen-related medication errors, followed by acetaminophen alone (adult formulation; 29.7%) (Table 2). Acetaminophen with diphenhydramine was associated with the highest proportion of serious outcomes (6.9%). Among the 74 acetaminophen-related deaths, 61 (82.4%) were among adults, 52 (70.3%) involved chronic exposures, and 48 (64.9%) involved acetaminophen alone (adult formulation). The types of medication errors most commonly involved in these deaths were “other incorrect dose” (39.2%), “medication doses given/taken too close together” (20.3%), or “more than one product containing same ingredient” (8.1%).
Opioids
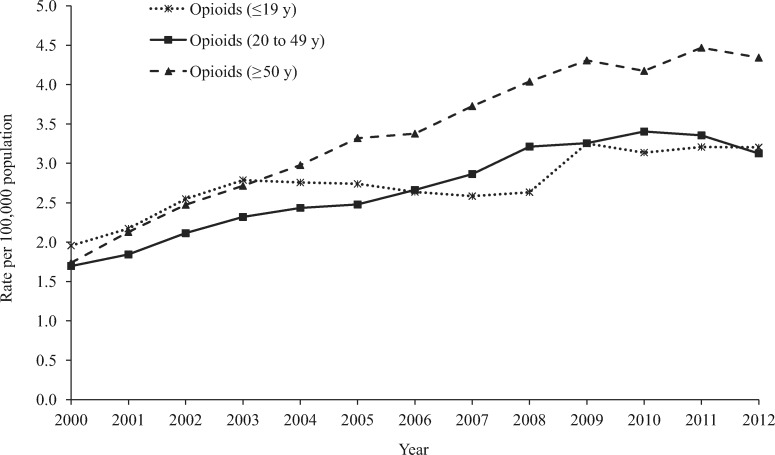
Adults age 20 to 49 years and age 50 years or older accounted for 38.6% and 35.6% of opioid-related medication errors, respectively (Table 1). Three-fifths (60.5%) of opioid-related errors were observed among females. The annual rate of opioid-related medication errors reported to the NPDS per 100,000 population more than doubled (107.4%, m = 0.21, P < 0.001) from 1.89 in 2000 to 3.92 in 2009, before leveling off (1.5%, m = 0.01, P = 0.828) from 2009 through 2012 (3.86 per 100,000) (Figure 2). The greatest increase in the rate of opioid-related errors reported to the NPDS was observed among adults age 50 years or older; the annual rate increased by 150.0% (m = 0.23, P < 0.001) from 2000 to 2012 (Figure 4). Among opioid-related medication errors, 3.3% were admitted to an HCF and 3.7% resulted in serious outcomes, including 61 deaths (Table 1).
Figure 4.
Annual rate per 100,000 population of opioid-related medication errors reported to US Poison Control Centers by age group, National Poison Data System 2000–2012.
Acetaminophen with hydrocodone accounted for 27.2% of the opioid-related medication errors, followed by oxycodone alone or in combination (excluding acetaminophen or acetylsalicylic acid) (10.6%) and acetaminophen with oxycodone (10.0%) (Table 2). Although nalbuphine, with only 12 errors, had the highest proportion of serious outcomes (25.0%), among opioids with at least 100 errors, fentanyl had the highest proportion of serious outcomes (18.7%), followed by methadone (10.1%) and buprenorphine (10.1%).
Nonsteroidal Anti-inflammatory Drugs
Children five years or younger (43.7%) and children age six to 19 years (22.3%) accounted for the majority of NSAID-related medication errors (Table 1). The annual rate of NSAID-related medication errors reported to the NPDS increased significantly by 75.5% (m = 0.20, P < 0.001) between 2000 (3.55 per 100,000) and 2012 (6.23 per 100,000) (Figure 2). During 2000–2012, the rate of NSAID medication errors reported to the NPDS increased by 124.6% (m = 1.59, P < 0.001) among children age five years and younger and 50.7% (m = 0.08, P < 0.001) among individuals age six years and older (Figure 3). NSAIDs was the analgesic category with the lowest proportions of serious outcomes (0.3%) and admissions to an HCF (0.2%) and had no associated fatalities (Table 1). Most NSAID-related medication errors involved ibuprofen (76.1%) or naproxen (11.7%) (Table 2).
Acetylsalicylic Acid
Aspirin-related medication errors were more common among adults age 50 years or older, accounting for about one-third (34.6%) of these errors (Table 1). The annual rate per 100,000 population of medication errors reported to the NPDS attributed to aspirin increased 48.1% (m = 0.01, P < 0.001) during the study period, from 0.27 in 2000 to 0.40 in 2012, with a peak of 0.42 in 2011 (Figure 2). Among the analgesic categories, aspirin-related medication errors had the highest proportions of serious outcomes (5.7%) and admission to an HCF (6.1%), but only accounted for eight deaths (Table 2).
Acetaminophen/Acetylsalicylic Acid Combination
Adults age 20 to 49 years accounted for the majority (58.8%) of acetaminophen/aspirin-related medication errors (Table 1). The annual rate of acetaminophen/aspirin-related medication errors reported to the NPDS increased 150.0% (m = 0.01, P < 0.001) during 2000 to 2011, with the rate per 100,000 population increasing from 0.10 in 2000 to 0.25 in 2011, before dropping to 0.18 in 2012 (Figure 2). Serious outcomes occurred in 2.6% of these exposures, and 1.1% were admitted to an HCF.
Discussion
From 2000 through 2012, there were more than 500,000 calls to US PCCs for analgesic-related medication errors, accounting for almost one-fifth of all unintentional medication errors reported to the NPDS during that period [5]. Although the rate of medication errors was highest among children age five years or younger, this age group had the lowest proportions of serious outcomes and HCF admissions compared with other age groups. Most errors among this young age group were associated with the acetaminophen and NSAID analgesic categories, and most were attributed to “inadvertently giving or taking the medication twice.” This double-dosing may result from parental distraction or poor interparent communication about when a dose was given. “Smart” medication containers and apps may help prevent this type of error in the future. The large number of analgesic-related medication errors among young children is, in part, attributable to the frequent use of acetaminophen and ibuprofen as antipyretics in this age group. “Fever phobia” has been well described in the literature, and the potential for unintentional therapeutic errors is a valid reason to promote more judicious use of these medications [23,24].
Poor consumer understanding of dosing directions and active ingredients in medications is a contributing factor to unintentional medication errors. A 2011 study focusing on consumer knowledge of acetaminophen medications indicated that less than 50% of participants routinely examine product label information, and 44% of participants read at or below a sixth grade level [25]. In addition, errors associated with measurement of medication were most common among children age five years or younger. Because young children are frequently given liquid formulations, use of inappropriate dispensing devices, such as a teaspoon instead of the metric dosing device provided with the medication, is a preventable source of these errors [26]. Additional efforts to implement current and new strategies for promoting consumer awareness, health literacy and numeracy, and use of appropriate dispensing devices are needed to help prevent analgesic-related and other medication errors.
In this study, the overall rate of analgesic-related medication errors reported to the NPDS increased significantly by more than 80.0% from 2000 through 2009 before a nonsignificant decline of 5.7% from 2009 through 2012. The increased use of analgesics, especially opioids, to treat pain in the United States has likely been a contributor to part of this observed overall increase in analgesic-related medication errors reported to the NPDS. Between 1991 and 2013, prescriptions for opioids increased from 76 million to nearly 207 million, with the United States being the biggest consumer of opioid analgesics worldwide [27]. Mirroring the overall trend, the rate of opioid-related medication errors reported to the NPDS increased between 2000 and 2009 and then leveled off between 2009 and 2012. This plateau in opioid-related medication errors may reflect federal and state efforts to curb opioid use and abuse, including the institution of prescription drug monitoring programs, consumer awareness campaigns, and educational and legal initiatives to prevent inappropriate prescribing practices [11,28–30].
There was a significant decrease observed in this study in medication errors associated with acetaminophen products beginning in 2009, which is consistent with previous research [31]. A national initiative that may be influencing this trend is the Know Your Dose campaign sponsored by the Acetaminophen Awareness Coalition, which is comprised of leading health, health care provider, and consumer organizations and is advised by the American Academy of Pediatrics, Centers for Disease Control and Prevention (CDC), and US Food and Drug Administration (FDA). This campaign educates consumers on the importance of knowing the ingredients in their medicines and following label directions to prevent unintentional acetaminophen overdose [32]. Another initiative that may be contributing to the decline is the CDC’s Prevention of Overdoses and Treatment Errors in Children Taskforce (PROTECT) initiative, which was started in 2008 and convenes a large coalition of experts and stakeholders to prevent medication overdoses among children. PROTECT activities include efforts to promote the use of milliliters as the standard dosing unit for liquid medications, use of appropriate dosing devices, improved labeling and packaging to reduce potential errors, and consumer educational campaigns [33]. In addition, FDA action in collaboration with manufacturers may have contributed to the decline in acetaminophen-related medication errors. In 2009, the FDA provided guidance to manufacturers of OTC analgesics containing acetaminophen or ibuprofen to display warning messages regarding liver injury associated with acetaminophen and bleeding risk associated with NSAIDs in both adult and child formulations [34]. This built on FDA actions dating back to 1998 to conduct educational programs on NSAID and acetaminophen safety and to request voluntary labeling changes [34–36]. In 2011, the FDA and OTC drug manufacturers agreed to phase out concentrated infant acetaminophen drops and to restrict liquid acetaminophen products for children to a single concentration (160 mg/5 mL) to reduce dosing confusion by parents and child caregivers [37].
Our study did not show a concomitant decrease in NSAID-related medication errors. One possible explanation is the difference in the wording of warning labels for acetaminophen products compared with that for NSAID products, which may have influenced consumer behavior differently. NSAID labeling changes highlighted the potential for gastrointestinal bleeding among individuals older than 60 years of age and other population subgroups, whereas the acetaminophen labeling changes emphasized the risk of liver toxicity among individuals of all ages [34]. Further, there was significant attention from major media outlets in 2009 regarding the risks associated with acetaminophen; however, the same coverage did not extend to NSAIDs [38].
Study Limitations
This study has several limitations. Exposure calls to PCCs are voluntary; therefore, NPDS data underestimate the true incidence of analgesic medication errors because they do not capture errors for which individuals did not seek advice and errors treated by health care professionals without reporting to a PCC. NPDS data may not be representative of the entire spectrum of analgesic medication errors because of potential reporting bias. Because the NPDS is a passive surveillance system, the rates and trends reported in this study are those of analgesic medication errors reported to PCCs and are not necessarily representative of the rates and trends of analgesic medication errors in the US population. Information received by PCCs is self-reported and cannot be completely verified by the PCCs and AAPCC. Exposures do not necessarily represent a poisoning or overdose. We only had access to generic analgesic product codes and could not evaluate specific products. For medication error-related exposures involving multiple medications, we relied on the PCC specialist’s ranking of the primary substance responsible for the observed clinical effects; however, exposures to multiple medications may increase the risk of adverse effects due to potential drug interactions and may contribute to mischaracterization of the effects of single-substance exposures. Despite these limitations, the NPDS is recognized as a source of high-quality national data appropriate for analyses such as in this study.
Conclusions
Analgesics are the most commonly used class of medications in the United States, and unintentional therapeutic errors associated with these medications are common and can have serious adverse outcomes. This study provides a detailed description and comparison of trends of unintentional medication errors associated with specific categories of analgesic medications reported to NPDS over a 13-year period. There was an average of more than 41,000 analgesic-related medication errors annually or one error every 13 minutes. In addition, there was an average of 660 admissions to an HCF and 11 deaths annually. Although this represents an important and preventable public health burden, most analgesic-related medication errors did not have clinical consequences. There was a decrease in acetaminophen-related medication errors reported to PCCs after 2009, especially among children age five years or younger, that was likely attributable to policy changes by the FDA and collaborative prevention efforts among government, health care, manufacturer, and consumer organizations. This success merits additional research and potential adoption as a framework for future public health endeavors combining government policy and multisectoral collaboration.
Author Contributions
Madhulika Eluri: Ms. Eluri conducted data analysis, drafted and revised the manuscript, and approved the final manuscript. Henry Spiller: Mr. Spiller contributed to conceptualization of the study, assisted in data analysis, critically reviewed the manuscript, and approved the final manuscript. Marcel Casavant: Dr. Casavant contributed to conceptualization of the study, assisted in data analysis, critically reviewed the manuscript, and approved the final manuscript. Thitphalak Chounthirath: Mr. Chounthirath assisted in data analysis, revised the manuscript, and approved the final manuscript. Kristen Conner: Ms. Conner assisted in data analysis, revised the manuscript, and approved the final manuscript. Gary Smith: Dr. Smith contributed to the conceptualization of the study, assisted in data analysis, critically reviewed the manuscript, and approved the final manuscript.
Appendix A Analgesic categories included in this study with their American Association of Poison Control Centers’ generic code names
| Analgesic Category and Generic Code Name |
| Acetaminophen |
| Acetaminophen alone, adult |
| Acetaminophen alone, pediatric |
| Acetaminophen alone, unknown if adult or pediatric |
| Acetaminophen in combination with other drugs, adult formulations |
| Acetaminophen in combination with other drugs, pediatric formulations |
| Acetaminophen with diphenhydramine |
| NSAIDs |
| Cyclooxygenase-2 (COX-2) inhibitors |
| Ibuprofen |
| Ibuprofen with diphenhydramine |
| Indomethacin |
| Ketoprofen drugs, adult formulations |
| Naproxen |
| Other types of nonsteroidal anti-inflammatory drug |
| Unknown types of nonsteroidal anti-inflammatory drug |
| Aspirin |
| Acetylsalicylic acid alone, adult |
| Acetylsalicylic acid alone, pediatric formulations |
| Acetylsalicylic acid alone, unknown if adult or pediatric formulations |
| Acetylsalicylic acid in combination with other drugs, adult formulations |
| Acetylsalicylic acid in combination with other drugs, pediatric formulations |
| Acetylsalicylic acid with carisoprodol |
| Acetaminophen and aspirin |
| Acetaminophen and acetylsalicylic acid with other ingredients |
| Acetaminophen and acetylsalicylic acid without other ingredients |
| Opioids |
| Acetaminophen with codeine |
| Acetaminophen with hydrocodone |
| Acetaminophen with other narcotics or narcotic analogs |
| Acetaminophen with oxycodone |
| Acetaminophen with propoxyphene |
| Acetylsalicylic acid with codeine |
| Acetylsalicylic acid with other narcotics or narcotic analogs |
| Acetylsalicylic acid with oxycodone |
| Acetylsalicylic acid with propoxyphene |
| Ibuprofen with hydrocodone |
| Buprenorphine |
| Butorphanol |
| Codeine |
| Alfentanil |
| Difenoxin |
| Dihydrocodeine |
| Fentanyl |
| Hydrocodone alone or in combination (excluding combination products with acetaminophen, acetylsalicylic acid, or ibuprofen) |
| Hydromorphone |
| Levorphanol |
| Meperidine |
| Methadone |
| Morphine |
| Nalbuphine |
| Oxycodone alone or in combination (excluding combination products with acetaminophen or acetylsalicylic acid) |
| Oxymorphone |
| Pentazocine |
| Propoxyphene |
| Remifentanil |
| Sulfentanil |
| Tapentadol |
| Tramadol |
| Other or unknown narcotics |
| Other |
| Nonaspirin salicylates (excluding topicals and/or gastrointestinal drugs) |
| Other analgesics |
| Phenacetin |
| Phenazopyridine |
| Salicylamide |
| Unknown analgesics |
Funding sources: Author Madhulika Eluri received a research stipend from the National Student Injury Research Training Program at the Nationwide Children’s Hospital, funded by the Centers for Disease Control and Prevention (grant #1R49CE002106), and a research stipend from the Child Injury Prevention Alliance while she worked on this study. The authors have no other financial disclosures relevant to this study.
Disclaimer: The inferences and conclusions expressed by the authors of this study do not necessarily represent those of the American Association of Poison Control Centers or its members, the Centers for Disease Control and Prevention, or the Child Injury Prevention Alliance.
Conflicts of interest: The authors have no conflicts of interest to report.
References
- 1. Paulose-Ram R, Hirsch R, Dillon C, et al. Prescription and non-prescription analgesic use among the US adult population: Results from the third National Health and Nutrition Examination Survey (NHANES III). Pharmacoepidemiol Drug Saf 2003;12(4):315–26. 10.1002/pds.755 [DOI] [PubMed] [Google Scholar]
- 2. Slone Epidemiology Center Boston University. Patterns of medication use in the United States. Available at: http://www.bu.edu/slone/files/2012/11/SloneSurveyReport2006.pdf (accessed May 2017).
- 3. Bjarnason I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int J Clin Pract Suppl 2013;(178):37–42. [DOI] [PubMed] [Google Scholar]
- 4. Black M. Acetaminophen hepatotoxicity. Annu Rev Med 1984;35:577–93. 10.1146/annurev.me.35.020184.003045 [DOI] [PubMed] [Google Scholar]
- 5. Brophy TJ, Spiller HA, Casavant MJ, et al. Medication errors reported to U.S. poison control centers, 2000-2012. Clin Toxicol (Phila) 2014;52(8):880–8. 10.3109/15563650.2014.953168 [DOI] [PubMed] [Google Scholar]
- 6. Mowry JB, Spyker DA, Cantilena LR Jr, Bailey JE, Ford M.. 2012 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th annual report. Clin Toxicol (Phila) 2013;51(10):949–1229. [DOI] [PubMed] [Google Scholar]
- 7. Warner M CL, Makuck DM, Anderson RN, Mineno AM.. Drug poisoning deaths in the United States, 1980–2008. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control, National Center for Health Statistics; 2011. Available at: http://www.cdc.gov/nchs/data/databriefs/db81.pdf (accessed May 2017). [Google Scholar]
- 8. Nourjah P, Ahmad SR, Karwoski C, Willy M.. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf 2006;15(6):398–405. 10.1002/pds.1191 [DOI] [PubMed] [Google Scholar]
- 9. Ogilvie JD, Rieder MJ, Lim R.. Acetaminophen overdose in children. CMAJ 2012;184(13):1492–6. 10.1503/cmaj.111338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med 2011;12(suppl 2):S26–35. [DOI] [PubMed] [Google Scholar]
- 11. Paulozzi LJ, Budnitz DS, Xi Y.. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf 2006;15(9):618–27. 10.1002/pds.1276 [DOI] [PubMed] [Google Scholar]
- 12. Miech R, Bohnert A, Heard K, Boardman J.. Increasing use of nonmedical analgesics among younger cohorts in the United States: A birth cohort effect. J Adolesc Health 2013;52(1):35–41. 10.1016/j.jadohealth.2012.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maaskant J, Bosman D, van Rijn-Bikker P, van Aalderen W, Vermeulen H.. Preventable errors with nonopioid analgesics and antiemetic drugs may increase burden in surgical pediatric patients. Eur J Pediatr Surg 2014;24(5):381–8. [DOI] [PubMed] [Google Scholar]
- 14. Zed PJ, Haughn C, Black KJ, et al. Medication-related emergency department visits and hospital admissions in pediatric patients: A qualitative systematic review. J Pediatr 2013;163(2):477–83. 10.1016/j.jpeds.2013.01.042 [DOI] [PubMed] [Google Scholar]
- 15. Li C, Martin BC.. Trends in emergency department visits attributable to acetaminophen overdoses in the United States: 1993-2007. Pharmacoepidemiol Drug Saf 2011;20(8):810–8. 10.1002/pds.2103 [DOI] [PubMed] [Google Scholar]
- 16. Manthripragada AD, Zhou EH, Budnitz DS, Lovegrove MC, Willy ME.. Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol Drug Saf 2011;20(8):819–26. [DOI] [PubMed] [Google Scholar]
- 17. Willy M, Kelly JP, Nourjah P, et al. Emergency department visits attributed to selected analgesics, United States, 2004-2005. Pharmacoepidemiol Drug Saf 2009;18(3):188–95. 10.1002/pds.1691 [DOI] [PubMed] [Google Scholar]
- 18. Shah K, Barker KA.. Out-of-hospital medication errors: A 6-year analysis of the national poison data system. Pharmacoepidemiol Drug Saf 2009;18(11):1080–5. 10.1002/pds.1823 [DOI] [PubMed] [Google Scholar]
- 19. Lavon O, Ben-Zeev A, Bentur Y.. Medication errors outside healthcare facilities: A national poison centre perspective. Basic Clin Pharmacol Toxicol 2014;114(3):288–92. 10.1111/bcpt.12150 [DOI] [PubMed] [Google Scholar]
- 20. Kohn LT, Corrigan J, Donaldson MS.. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 21. United States Census Bureau. Intercensal estimates of the resident population by sex and age for the United States: April 1, 2000 to July 1, 2010. Available at: https://www.census.gov/data/tables/time-series/demo/popest/intercensal-2000-2010-national.html (accessed May 2017).
- 22. United States Census Bureau. Annual estimates of the resident population by single year of age and sex: April 1, 2010 to July 1, 2012. Available at: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml? src=bkmk (accessed May 2017).
- 23. Schmitt BD. Fever phobia: Misconceptions of parents about fevers. Am J Dis Child 1980;134(2):176–81. 10.1001/archpedi.1980.02130140050015 [DOI] [PubMed] [Google Scholar]
- 24. Wallenstein MB, Schroeder AR, Hole MK, et al. Fever literacy and fever phobia. Clin Pediatr (Phila) 2013;52(3):254–9. 10.1177/0009922812472252 [DOI] [PubMed] [Google Scholar]
- 25. King JP, Davis TC, Bailey SC, et al. Developing consumer-centered, nonprescription drug labeling a study in acetaminophen. Am J Prev Med 2011;40(6):593–8. 10.1016/j.amepre.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 26. Budnitz DS, Lovegrove MC, Rose KO.. Adherence to label and device recommendations for over-the-counter pediatric liquid medications. Pediatrics 2014;133(2):e283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Volkow ND. Prescription opioid and heroin abuse. Available at: https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/prescription-opioid-heroin-abuse (accessed May 2017).
- 28. Johnson HPL, Porucznik C, Mack K, Herter B.. Decline in drug overdose deaths after state policy changes—Florida, 2010-2012. MMWR 2014;63(26):569–74. [PMC free article] [PubMed] [Google Scholar]
- 29. Paulozzi LJ, Kilbourne EM, Desai HA.. Prescription drug monitoring programs and death rates from drug overdose. Pain Med 2011;12(5):747–54. 10.1111/j.1526-4637.2011.01062.x [DOI] [PubMed] [Google Scholar]
- 30. Paulozzi LJ, Weisler RH, Patkar AA.. A national epidemic of unintentional prescription opioid overdose deaths: How physicians can help control it. J Clin Psychiatry 2011;72(5):589–92. 10.4088/JCP.10com06560 [DOI] [PubMed] [Google Scholar]
- 31. Major JM, Zhou EH, Wong HL, et al. Trends in rates of acetaminophen-related adverse events in the United States. Pharmacoepidemiol Drug Saf 2016;25(5):590–8. 10.1002/pds.3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Acetaminophen Awareness Coalition. Know Your Dose campaign. http://www.knowyourdose.org/ (accessed May 2017).
- 33. Centers for Disease Control and Prevention. The PROTECT initiative: Advancing children’s medication safety. Available at: http://www.cdc.gov/medicationsafety/protect/protect_initiative.html (accessed May 2017).
- 34. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for industry: Organ-specific warnings: Internal analgesic, antipyretic, and antirheumatic drug products for over-the-counter human use—labeling for products that contain acetaminophen. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm310477.pdf (accessed May 2017).
- 35. FDA drug safety communication: Addition of another concentration of liquid acetaminophen marketed for infants. US Food and Drug Administration. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm284741.htm (accessed May 2014).
- 36. US Food and Drug Administration. FDA drug safety communication: Prescription acetaminophen products will be limited to 325 mg per dosage unit; boxed warning will highlight potential for severe liver failure. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm239821.htm (acessed May 2017).
- 37. Consumer Healthcare Products Association. OTC industry announces voluntary transition to one concentration of single-ingredient pediatric liquid acetaminophen medicines [Press Release]. Available at: www.chpa.org/05_04_11_PedAcet.aspx (accessed May 2017).
- 38. Childs D. FDA scrutinizes acetaminophen’s liver risk. abc NEWS. Available at: http://abcnews.go.com/Health/PainManagement/story? id=7955370&page=1 (accessed May 2017).