Abstract
Evidence indicates that complex gene-environment interactions underlie the incidence and progression of Parkinson’s disease (PD). Neuroinflammation is a well-characterized feature of PD widely believed to exacerbate the neurodegenerative process. Environmental toxicants associated with PD, such as pesticides and heavy metals, can cause cellular damage and stress potentially triggering an inflammatory response. Toxicant exposure can cause stress and damage to cells by impairing mitochondrial function, deregulating lysosomal function, and enhancing the spread of misfolded proteins. These stress-associated mechanisms produce sterile triggers such as reactive oxygen species (ROS) along with a variety of proteinaceous insults that are well documented in PD. These associations provide a compelling rationale for analysis of sterile inflammatory mechanisms that may link environmental exposure to neuroinflammation and PD progression. Intracellular inflammasomes are cytosolic assemblies of proteins that contain pattern recognition receptors, and a growing body of evidence implicates the association between inflammasome activation and neurodegenerative disease. Characterization of how inflammasomes may function in PD is a high priority because the majority of PD cases are sporadic, supporting the widely held belief that environmental exposure is a major factor in disease initiation and progression. Inflammasomes may represent a common mechanism that helps to explain the strong association between exposure and PD by mechanistically linking environmental toxicant-driven cellular stress with neuroinflammation and ultimately cell death.
Keywords: Parkinson’s disease, inflammasome, environment, toxicant, neuroinflammation, neurodegeneration
The clinical progression of Parkinson’s disease (PD) is well-documented but we still do not understand how PD is initiated and what factors modify the disease course. The vast majority of PD occurs sporadically with no evidence of a causal genetic lesion (Rajput et al., 1984) and epidemiologic and experimental evidence strongly support a role for environmental exposure in the incidence and progression of idiopathic PD (for review [Cannon and Greenamyre, 2013; Fleming, 2017; Hatcher et al., 2008; Tanner et al., 2011]). Despite these findings, the molecular basis of increased risk resulting from environmental exposure remains poorly characterized. Toxicants released into the environment as the result of human activity accumulate in the air, water, and soil and consist of a variety of synthetic chemical pollutants such as detergents, solvents, and pesticides as well as other industrial by-products such as carbon-dioxide, various particulates, and heavy metals (Zhuang et al., 2015). These pollutants can persist and pose a challenge to host organisms because the immune system has not evolved mechanisms for managing exposure to newly emerging man-made pollutants. This is highly relevant to progressive age-related neurologic disorders because many toxicants are central nervous system (CNS) permeant, readily crossing the blood-brain barrier (BBB), potentially impacting the health and function of CNS cells over an extended period of time. Based on the likelihood that a high percentage of PD cases are associated with environmental exposure (Costello et al., 2009; Klein and Westenberger, 2012; Liou et al., 1997; Tanner et al., 2011; Wang et al., 2014), characterization of cellular mechanisms engaged by environmental toxicants is expected to reveal key aspects of initiation and progression, improving our ability to diagnose, monitor, and treat the disease.
The mechanisms by which environmental toxicants activate specific inflammatory processes range from the induction of acute necrosis to more discrete cellular pathophysiologies including oxidative stress, protein misfolding, and programmed cell death (Cannon and Greenamyre, 2013). Inflammasomes can mediate the cytosolic response to sterile inflammatory triggers and the induction of inflammasome activity by PD-associated toxicant-driven cellular stress is a rational means by which the CNS might translate environmental toxicant exposure into neuroinflammation. Supporting this premise are recent reports of toxicant-based animal models of PD indicating that genetic inactivation of the inflammasomes suppresses neuroinflammation and is neuroprotective (Lee et al., 2018; Martinez et al., 2017; Yan et al., 2015). Here, we discuss mechanisms of inflammasome activity that are relevant to known cellular pathologies observed in PD. We highlight recent findings related to inflammasomes in the response to PD-associated toxicants, providing a platform for addressing longstanding questions related to the impact of environmental pollution on the initiation and progression of PD.
MECHANISMS AND ORIGINS OF INFLAMMASOME ACTIVITY
Inflammasomes are multi-protein cytosolic complexes that contain pattern recognition receptors (PRRs) that sense both viral and microbial pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Inflammasomes are organized into three sub-families based on their protein composition: nucleotide-binding domain-like receptors (NLRs), absent in melanoma 2-like receptors (ALRs), and the more recently discovered pyrin family. These core proteins drive caspase-1 catalysis and thereby initiate cytokine maturation, and potentially pyroptosis, an inflammatory caspase-1-dependent subcategory of programmed cell death (Chen et al., 1996). Among the inflammasome subfamilies, the NLRs have been reported in association with sterile inflammation and disorders of the CNS. NLR proteins are defined by three conserved domains: a central nucleotide-binding and oligomerization (NACHT) domain, a C-terminal leucine-rich repeats (LRRs), and either a pyrin domain (PYD) or a caspase activation and recruitment domain (CARD) at the N-terminus and are encoded by 25 currently annotated genes (Ting et al., 2008). Among the NLRs, NLRP3 is the most widely studied in the context of sterile inflammation and neurologic disease and therefore will be the principal complex discussed throughout this review (Figure 1). For further details, excellent reviews are available describing the cellular and molecular biology of the inflammasomes (He et al., 2016; Hoffman and Brydges, 2011; Schroder and Tschopp, 2010; Sharma and Kanneganti, 2016).
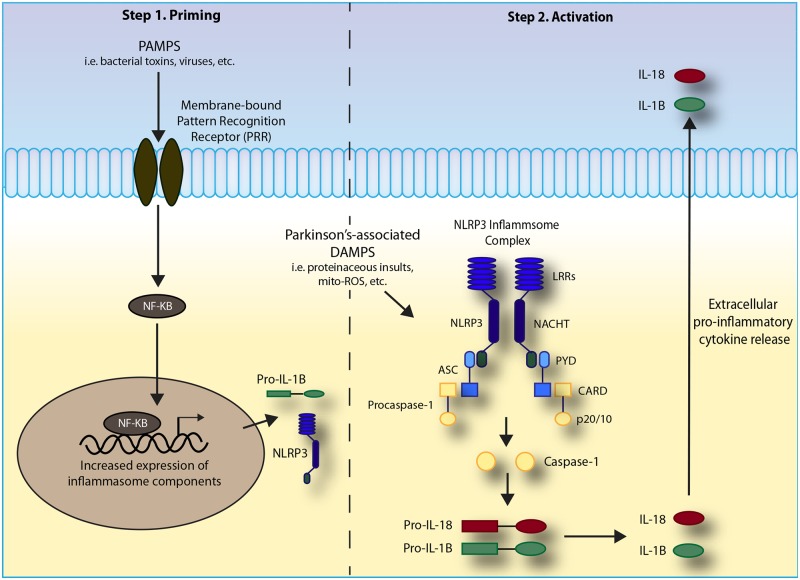
Figure 1.
Mechanism of NLRP3 inflammasome assembly. Two major steps are required for the formation of the NLRP3 inflammasome complex, (1) priming and (2) activation. Priming begins when a pro-inflammatory stimulus, such as a pathogen-associated molecular pattern (PAMP) or damage-associated molecular pattern (DAMP), interacts with a pattern recognition receptor (PRR) resulting in alterations in transcriptional programs, including NF-kB activation. Upon activation, NF-kB translocates into the nucleus where it increases expression of key inflammasome-related proteins including pro-IL-1B and NLRP3. A second stimulus is required for formation of the NLRP3 inflammasome complex. Stimuli have recently been identified to include the Parkinson’s-associated sterile trigger mitochondrial ROS, among others (see Figure 3). Structurally, the NLRP3 protein is comprised of three main domains—an amino-terminal pyrin (PYD) effector domain, a central nucleotide binding domain (NACHT), and a carboxy-terminal leucine-rich repeat (LRR) motif. Upon activation, the monomeric intracellular NLRP3 proteins interact through the homophilic NACHT domain. Once formed, this complex can interact with the PYD of the adaptor protein apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC). The N-terminal CARD domain of ASC then recruits pro-caspase-1 through its CARD domain to form the complete inflammasome complex. Pro-caspase-1 undergoes auto-activation when it binds to oligomerized NLRP3, resulting in cleavage into the p10 and p20 fragments of caspase-1. These active fragments cleave and subsequently activate the pro-inflammatory cytokines pro-IL-1B and pro-IL-18. Once activated, these cytokines are transported out of the cell where they can act in a pro-inflammatory manner.
Environmental toxicant exposure causes intracellular damage and stress leading to the elaboration of DAMPs. Here, we will consider the impact of PD-associated environmental toxicants on inflammasome activity within the framework of three well-supported models of inflammasome activation: the channel model (Kahlenberg and Dubyak, 2004), the lysosome rupture model (Hornung et al., 2008), and the mitochondrial-derived ROS (mito-ROS) model (Shimada et al., 2012). The channel model is based on the observation that sterile activating agents gain access to the intracellular complex via translocation into the cytoplasm through the formation of non-selective pores in the cytoplasmic membrane. In these studies, the ATP-gated P2XZ ion channel is modified by Pannexin 1 resulting in the formation of large non-selective pores that are required for caspase-1 activation and IL-1B maturation in the context of ATP-exposed LPS-primed macrophages (Pelegrin and Surprenant, 2006). In more recent studies, this model has been refined through the comparison of multiple inflammasome stimuli. Munoz-Planillo et al. identify K+ efflux as the common requirement of inflammasome activation by multiple agonists and determine that whereas non-selective pore formation is a component of the response to certain stimuli, these pores are not required for others (Munoz-Planillo et al., 2013). The lysosomal rupture model of inflammasome activation accounts for larger particulate toxicant activators of the inflammasomes and is also highly relevant to proteinopathies like PD where toxicants themselves and misfolded proteins can contribute to lysosomal damage (Halle et al., 2008). Lysosome rupture releases a host of proteases into the cytoplasm including cathepsin B. Cathepsin B is able to activate the NLRP3 inflammasome and cathepsin B inhibitors, such as Ca-074-me, can impede NLRP3 activation in human cells exposed to silica (Hornung et al., 2008). Likewise, evidence indicates that PD-associated heavy metals like manganese can cause lysosomal stress and death in glia (Gorojod et al., 2017). Elevated ROS is a major indicator of neuronal distress in PD and a common feature of channelopathies, lysosome distress, and toxicant exposure. ROS has been found to drive an interaction between the LRRs of NLRP3 and the thioredoxin (TRX)-interacting protein (TXNIP), to regulate autophagy and impact PD-associated synucleinopathy through the inhibition of ATP13A2 (Su et al., 2017). In the presence of ROS, TXNIP can become unbound and associate with NLRP3 (Zhou et al., 2010) resulting in the activation of the NLRP3 inflammasome. In TXNIP-deficient animals, both caspase-1 activation and IL-1B secretion are decreased in macrophages following exposure to multiple toxicants associated with NLRP3 activation (Zhou et al., 2011). Indicating that similar processes unfold in the CNS, recent studies in the mouse microglial BV2 cell line model of thrombin-induced inflammation suggest that dampening of the TXNIP/ROS pathway with the ROS scavenger N-acetylcysteine both reduces NLRP3 activation and ameliorates apoptosis (Ye et al., 2017). Independent of the mode of activation, NLRP3 inflammasome formation results in the cleavage of procaspase-1 into its active form. Active caspase-1 can cleave pro-inflammatory cytokines, including IL-1B and IL-18, and/or trigger other caspase-1-dependent processes, such as pyroptosis.
CELLULAR ORIGINS OF INFLAMMASOME ACTIVITY IN THE CNS
Although inflammasomes are best characterized in the peripheral innate immune system, increasing evidence indicates that CNS cells also harbor inflammasome activity. Microglia provide substantive innate immune monitoring and inflammatory mediation in the CNS and are capable of secreting cytokines to influence local CNS cells and the peripheral immune system (Wolf et al., 2017). Originally characterized in peripheral monocytes, the canonical inflammasome pathway that results in the maturation and secretion of IL-1B is now well-described in microglia indicating that they represent the major contributor of inflammasome-dependent inflammatory activity in the CNS (Halle et al., 2008; Sarkar et al., 2017a; Scheiblich et al., 2017). Astroglia have also an extensively characterized role in inflammation during neurodegeneration, injury, and repair. The role of astroglia in the inflammatory response in PD is complex and manifests as either neuroprotective or detrimental depending on the trigger, timing, and anatomic context of the response. Whether astroglia express functional inflammasomes remains the subject of debate. Whereas studies have indicated inflammasome activity in astroglia (Johann et al., 2015), other studies focused on Nlrp3 have concluded that astrocytes do not express functional inflammasomes (Gustin et al., 2015; Martinez et al., 2017). Oftentimes, studies have been conducted in primary mixed glial cultures where the isolation of astrocytes without microglial contamination remains a technical challenge. What is clear is that astroglia respond to microglia-derived stimulus including IL-1B (Moynagh, 2005) and participate in the inflammatory response in the nigrostriatal system in PD (Booth et al., 2017). In addition to glia, recent evidence indicates that other CNS lineages may also utilize the inflammasome danger-sensing platform. Neurons are capable of directly modulating the inflammatory response through the release of electrochemicals and neuropeptides in a process described as neurogenic inflammation (Richardson and Vasko, 2002). Whereas the function of inflammasomes in neurogenic inflammation is poorly understood, expression of the NLRP1 (de Rivero Vaccari et al., 2008), AIM2 (Adamczak et al., 2014), and NLRP3 (Zhang et al., 2016)NLRs has been detected in neurons, with our laboratory recently reporting elevated NLRP3 expression in pigmented neurons of the PD mesencephalon (von Herrmann et al., 2018). These findings suggest that inflammasomes may function in neurons providing a novel mechanism of neurogenic inflammation capable of initiating or influencing neuroinflammation through a mechanism potentially culminating in pyroptotic cell death. In addition, peripheral monocytes exhibiting canonical inflammasome activity enter and exit the CNS microenvironment monitoring the state of CNS tissues during the neurodegenerative processes occurring in PD (Grozdanov et al., 2014) and cerebral endothelial cells are perturbed in PD and express multiple NLRs, along with caspase-1 and IL-1B whose expression can be induced using LPS (Nagyőszi et al., 2015). In summary, whereas microglia are clearly a major source of inflammasome activity in the CNS, emerging evidence suggests that these cytosolic danger sensors may function in multiple cell types. Given that inflammasome-competent cells exist throughout the CNS, and at multiple interfaces between the CNS and the environment, continued experiments to understand whether inflammasomes function as a core environmental stress response pathway in the CNS are warranted.
INFLAMMASOMES IN PARKINSON’S DISEASE
The evidence for neuroinflammation occurring in association with PD is well-established and has been extensively reviewed by others (Hirsch et al., 2012; Tansey and Goldberg, 2010). In PD, sterile cellular stress can manifest in several ways including metabolic and oxidative stress (Bosco et al., 2006), dysregulation of autophagy and prion-like propagation of misfolded proteins (Luk et al., 2012; Mao et al., 2016), and the accumulation of toxic DA metabolites (Burbulla et al., 2017). Here, we discuss evidence supporting a role for inflammasomes in the response to these PD-associated manifestations of cellular stress that provides mechanistic clues as to how toxicant exposure may interact with and exacerbate these cellular pathologies thereby impacting PD progression. The activity of inflammasomes has been linked to synuclein pathology, widely believed to be modulated by environmental toxicant exposure (Liu and Yang, 2005; Pan-Montojo et al., 2012; Villar-Piqué et al., 2016). Monocytes isolated from healthy donors are capable of phagocytosing fibrillary α-synuclein, and pathologic α-synuclein multimers more robustly induce NLRP3-associated caspase-1-dependent IL-1B maturation as compared with α-synuclein in monomeric form (Codolo et al., 2013). It is important to note that these studies in monocytes are conducted in the context of LPS exposure, a bacterial endotoxin that is sufficient to induce a robust IL-1B response in monocytes without requiring an additional inflammasome activation step. It remains to be seen whether α-synuclein can act as an inflammasome stimulus in microglia under sterile conditions. In cultured neuroblastoma cells, activation of the inflammasome results in caspase-1-dependent cleavage of α-synuclein. Caspase-1 truncation of α-synuclein increased protein aggregation and could be inhibited using small molecule caspase-1 inhibitors (Wang et al., 2016). In a related study, inhibition of caspase-1 using the brain permeant pro-drug VX-765 mitigated synucleinopathy and nigral cell loss in a mouse model of multiple system atrophy (Bassil et al., 2016). Many environmental toxicants target mitochondrial pathways and mitochondrial dysfunction is well-characterized in idiopathic PD (Langston et al., 1983) with numerous genes associated with hereditary PD also impacting mitochondrial homeostasis and the cellular redox state (Vives-Bauza et al., 2010). The PINK1/Parkin pathway is central to mitochondrial quality control (for review [Pickrell and Youle, 2015]) and inactivation of either of these genes causes rare hereditary forms of PD (Kitada et al., 1998; Valente et al., 2004). The NLRP3 inflammasome relies on mitochondria for assembly and function and mitochondrial deregulation can result in inflammasome activation (Elliott et al., 2018; Sadatomi et al., 2017; Zhou et al., 2011). Deregulation of PINK1/Parkin-mediated mitophagy results in the accumulation of impaired mitochondria (Geisler et al., 2010; Matsuda et al., 2010). Loss of PINK1/Parkin function exacerbates Nlrp3 inflammasome activity in primary microglia in a manner that can be mitigated by the small molecule Nlrp3 inflammasome inhibitor MCC950 (Mouton-Liger et al., 2018) consistent with findings that impaired mitophagy and subsequent generation of ROS activated the NLRP3 inflammasome in bone marrow-derived macrophages (Zhou et al., 2011). Mutations in LRRK2 are prevalent in PD having been identified in both idiopathic and autosomal dominant disease. PD-associated mutations in LRRK2 result in loss-of-function which has been subsequently associated with elevated oxidative stress (Angeles et al., 2011) and mitochondrial fragmentation (Wang et al., 2012). In Lrrk2−/− macrophages, an Lrrk2/Nlrc4 interaction is disrupted resulting in reduced phosphorylation of Nlrc4, impaired inflammasome activation, and deficiencies in the host-pathogen response (Liu et al., 2017). Dopamine can modulate the immune response (Besser et al., 2005; Farber et al., 2005) and recent reports indicate that dopamine regulates the activity of the Nlrp3 inflammasome in bone marrow-derived macrophages and that this function relies on the presence of the DRD1 receptor (Yan et al., 2015). In the same report, Nlrp3−/− mice resisted MPTP-mediated nigral degeneration whereas similarly treated Drd1−/− mice were more sensitive to the toxin and had enhanced inflammasome activity. These findings provide strong support for the authors conclusion that dopamine functions to inhibit inflammasome activity and that suppression of Nlrp3-driven neuroinflammation may be neuroprotective for PD. Dopamine metabolism results in the generation of toxic by-products that are posited to underlie dopamine neuron susceptibility in PD (Burbulla et al., 2017). In addition to increasing oxidative stress, dopamine metabolism by-products accumulate in the form of neuromelanin granules reminiscent of the crystalline activators of inflammasomes observable in inflammatory disorders such as gout (Yagnik et al., 2000). Although research in inflammasomes and dopamine neuron susceptibility is in its early stages, our recent report (von Herrmann et al., 2018), and the potential that CNS permeant environmental toxicants may enter neurons suggests that dopamine neurons may be a cell type of interest in the initiation and propagation of inflammasome-driven neuroinflammation in PD. Taken together, it is noteworthy that well-characterized aspects of PD-related cell stress and death either have already been linked to inflammasome activity or are readily predicted based on the known mechanisms of inflammasome activation as identified in other systems. Whereas not unique to PD, the overwhelming evidence of an environmental component to the disease has prompted intense study to determine how potentially deleterious exposures can be detected and prevented. In the following section, we describe evidence supporting a role for inflammasomes at the intersection between environmental toxicants and aforementioned cellular pathologies, so well characterized in PD.
INFLAMMASOMES IN THE CELLULAR RESPONSE TO PARKINSON’S-ASSOCIATED TOXICANTS
Pesticides
Pesticide exposure has been identified as a risk factor for the development of PD (Costello et al., 2009; Hatcher et al., 2008; Liou et al., 1997; Tanner et al., 2011; Wang et al., 2014) and multiple brain-permeant pesticides have been either directly linked with inflammasome activation or have been characterized as invoking cellular stress known to be associated with inflammasome activation. Among the best characterized in the context of inflammasome activation is rotenone, a broad-spectrum organic pesticide used in agriculture and to cull unwanted fish populations in water management projects (Krumholz, 1948). Rotenone is lipophilic, easily crossing the BBB (Figure 2), and exposure has been associated with the development of PD in agricultural workers [7]. Chronic exposure in rodents recapitulates some of the symptomology of PD, resulting in nigrostriatal degeneration, the accumulation of α-synuclein positive inclusions, motor impairment, and neuroinflammation (Cannon et al., 2009; Martinez et al., 2017). Rotenone toxicity results from inhibition of mitochondrial complex I, implicating rotenone as a potentiator of mito-ROS-mediated inflammasome activation (Figure 3) (Sherer et al., 2003). In support of this premise, the Nlrp3 inflammasome responds to mitochondrial damage, ROS, and proteinopathy and rotenone activates the Nlrp3 inflammasome within both peripheral and CNS-derived cells in vitro (Lawana et al., 2017; Won et al., 2015). In mice, Nlrp3 loss prevents the degeneration of nigral dopaminergic neurons resulting from chronic rotenone exposure (Martinez et al., 2017). Microglial inflammasome activity has been associated with rotenone-based neurodegeneration in vitro where studies indicate that the amplification of Nlrp3 signaling caused by rotenone-dependent mitochondrial impairment augments dopaminergic neuron degeneration (Sarkar et al., 2017b).
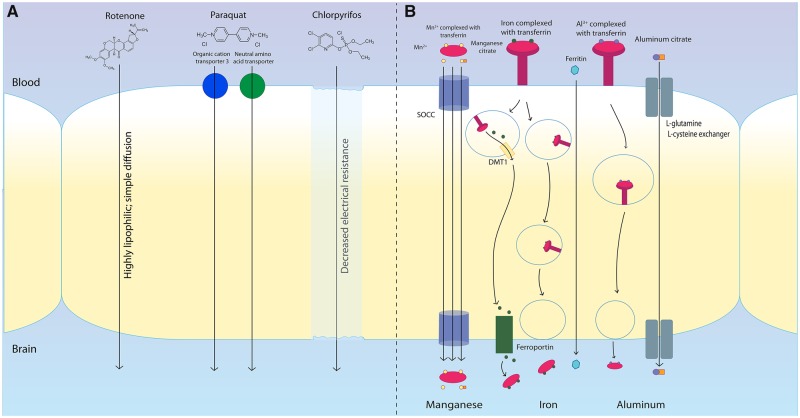
Figure 2.
Mechanisms of toxicant transportation across the blood-brain barrier. Many environmental toxicants associated with inflammasome activity are able to bypass the blood-brain barrier (BBB). The pesticide rotenone is highly lipophilic, easily crossing the BBB through simple, passive diffusion. The herbicide paraquat enters the brain through neutral amino acid transporters. The organophosphate pesticide chlorpyrifos crosses the BBB by altering electrical resistance resulting in decreased membrane integrity. The heavy metal manganese has been shown to cross the BBB as the Mn2+ ion alone or in complex with transferrin or citrate. Each of these three forms crosses the BBB through a store-operated calcium channel (SOCC). Iron crosses the BBB through three different methods: endocytosis of ferritin and its receptor coupled by a release into the cytoplasm by DMT1 and transport into the brain through ferroportin, endocytosis of ferritin and its receptor for release into the brain, or direct transport of ferritin across the BBB. Aluminum crosses the BBB largely in complex with transferrin or citrate, likely through the l-glutamine, l-cysteine exchanger.
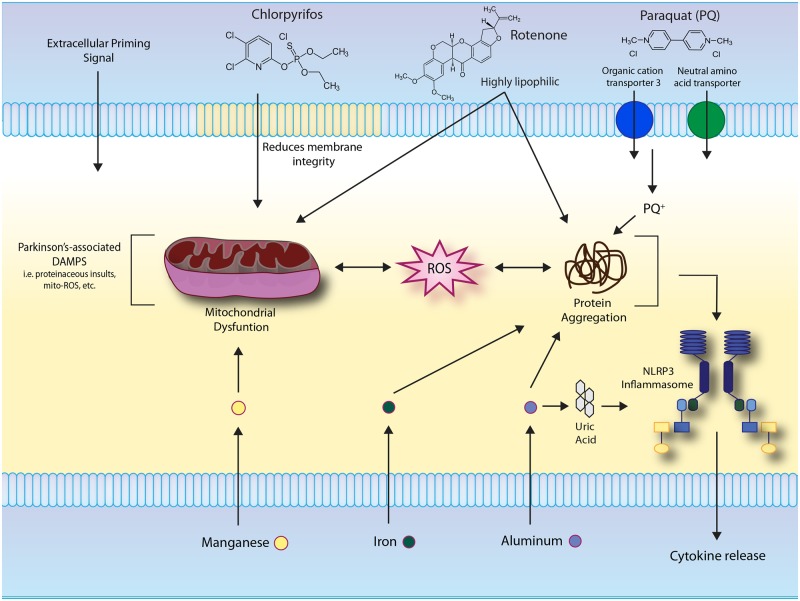
Figure 3.
Environmental toxicants exacerbate Parkinson’s-associated sterile molecular patterns associated with activation the NLRP3 inflammasome. Parkinson’s-associated cytopathologies, such as the aggregation of misfolded proteins and generation of mitochondrial ROS, can stimulate inflammasome activity. Rotenone inhibits mitochondrial complex I decreasing synthesis of ATP and deregulating electron transfer, leading to generation of ROS. Paraquat exposure elicits inflammasome-related cellular stressors such as α-synuclein protein aggregation. Chlorpyrifos exposure elevates ROS production in association with mitochondrial dysfunction. IL-1B cytokine release is increased as a result of manganese accumulation in LPS-primed glial cells, consistent with canonical two-step inflammasome activation. In addition, manganese exposure increases mitochondrial and oxidative stress. Buildup of labile iron results in protein aggregation. Free ferrous iron can react to produce ROS, resulting in activation of the NLRP3 inflammasome. Aluminum exposure also propagates the misfolding of proteins, including pathologic α-synuclein, and contributes to ROS production and the formation of uric acid, all of which can activate the NLRP3 inflammasome.
Whereas rotenone-mediated inflammasome activation has been most widely studied, evidence suggests that inflammasomes may respond similarly to other PD-associated pesticides. Paraquat is a widely used, non-selective, contact-based herbicide also linked to an increased risk of PD (Tanner et al., 2011). Differing from rotenone, paraquat’s chemical structure is similar to that of MPP+, the causal metabolite of the PD-associated drug-of-abuse MPTP (Wang, Ren, et al., 2017). Initially, paraquat was thought to be unable to cross the BBB (Naylor et al., 1995) but more recent studies suggest that it can in fact bypass the BBB and directly enter the brain (Figure 2) (Shimizu et al., 2001, 2003). Once in the CNS, paraquat can be reduced to the monovalent cation PQ+ by NADPH oxidase in microglia and enter other CNS cells including neurons by co-opting the dopamine transporter, organic cation transporter 3, and neutral amino acid transporters (Shimizu et al., 2003). Systemic administration of paraquat results in degeneration of dopaminergic neurons of the substantia nigra pars compacta (Manning-Boğ et al., 2003; McCormack et al., 2002; Ossowska et al., 2006) and the combination of paraquat and maneb is widely used to model PD in rodents (Cicchetti et al., 2005). Direct evidence of inflammasome activity in response to paraquat in the CNS is limited. However, paraquat exposure in animal models elicits inflammasome-related cellular stressors including mitochondrial ROS (McCarthy et al., 2004) and α-synuclein aggregation (Manning-Bog et al., 2002) (Figure 3). Among these pathologies, evidence has emerged to support a prediction that mito-ROS-based inflammasome activation may occur in paraquat-based models of PD. In support of this prediction, detoxification of mitochondrial ROS suppressed NLRP3 activation and the inflammatory response in a paraquat-based mouse model of Alzheimer’s disease (Chen et al., 2015). Paraquat-induced cytotoxicity, ROS generation, and resultant NLRP3 activation were reduced in monocytes pre-treated with the anti-oxidant silymarin (Liu et al., 2018). Indicating a potential role for inflammasomes in paraquat-driven dopaminergic neurodegeneration, nigral cell loss resulting from paraquat exposure is enhanced by an endotoxin priming step highly suggestive of the canonical two-step model of inflammasome activation (Purisai et al., 2007) (Figure 1). Although direct characterization of the mechanism by which paraquat may interact with inflammasomes in PD has not been completed, the currently available database provides ample support for the pursuit of such studies.
Mechanistic data related to the organophosphate chlorpyrifos (CPF) is limited, but this pesticide bears mention because it is widely used, entering the environment at an estimated 3.2–4.1 million kilograms annually in the U.S. alone (Solomon et al., 2014), and persists in soil and water (Mackay et al., 2014). Epidemiological studies have found a link between CPF exposure and an increased risk of PD (Dhillon et al., 2008; Gatto et al., 2009; Manthripragada et al., 2010). Parran and others reported that CPF and its metabolites were able to cross a membrane co-culture of bovine microvascular endothelial cells and neonatal rat astrocytes occurring in association with a concentration-dependent decrease in electrical resistance (Parran et al., 2005) (Figure 2). Supporting this finding, CPF transiently altered the expression of proteins important for BBB integrity, such as claudin5, ZO1, and TRPC4 (Li and Ehrich, 2013) and following systemic administration in mice, CPF and its metabolites are identifiable within CNS tissues (Kopjar et al., 2018; Williamson Leah et al., 2006). CPF and its metabolites act through the inhibition of acetylcholinesterase (AchE) (Colombo et al., 2005) and CPF-induced neuronal stress and apoptosis have been identified in a variety of models including zebrafish (Garcia-Reyero et al., 2016), rat primary cortical neurons (Caughlan et al., 2004), and the human catecholaminergic neuroblastoma cell line SH-SY5Y (Eun et al., 2014; Ki et al., 2013; Raszewski et al., 2015). Experimental evidence of neurodegeneration resulting from chronic CPF exposure is limited. However, dopaminergic cell loss was observed in CPF-exposed neonatal rats (Zhang et al., 2015). Suggestive of a role for inflammasomes in the response to CPF, studies identify oxidative stress within the CNS (Figure 3) and CNS-derived cell lines (Verma et al., 2007) and in oligodendrocyte progenitors upon CPF exposure (Saulsbury et al., 2009). In human keratinoctyes, CPF-induced ROS was associated with elevated levels of NLRP3 and subsequent apoptosis (Jang et al., 2015). Further studies need to be done to characterize the effect of CPF exposure on inflammasome activation in the context of the CNS.
Heavy Metals
In addition to pesticides, heavy metal toxicants have been linked with the development of parkinsonism (for review [Caudle et al., 2012]). Manganese (Mn) is an essential nutrient but it is also released into the environment through industrial activities and has been widely studied in the context of PD and inflammasome activation. In both the occupational and laboratory setting, Mn exposure has been implicated in the development of inflammation (Kresovich et al., 2018; Santos et al., 2013), manganism, and progressive parkinsonism (Racette et al., 2017). Mn-associated PD symptomology is distinct from idiopathic PD (Olanow, 2004), however, evidence indicates that the dopamine system is impacted by Mn exposure in non-human primates (Guilarte et al., 2008) and rodent models (Sanchez-Betancourt et al., 2012; Zhao et al., 2009) making the understanding of Mn toxicity highly relevant to PD and similar disorders. Mn crosses the BBB as Mn2+, in complex with transferrin, or in complex with citrate (Figure 2). Each of these forms enters the brain via a store-operated calcium channel (SOCC). Passage into the brain via the SOCC has been shown to be more rapid than simple diffusion from the brain, the primary method of exit, likely underlying the observation of Mn accumulation in brain tissue (Antonini et al., 2009; Jenkitkasemwong et al., 2018; Uchino et al., 2007; Yokel, 2009). Several lines of evidence support indirectly a role for microglial inflammasomes in Mn toxicity. Mn accumulation results in microglial activation and exacerbates LPS-mediated IL-1B production, suggestive of two-step inflammasome activation (Filipov et al., 2005). Mechanistically, evidence from MitoPark mice indicates that Mn exposure can exacerbate mitochondrial stress suggesting the potential for mito-ROS-mediated inflammasome activation (Langley et al., 2018) (Figure 3). Beyond circumstantial evidence of Mn-induced mito-ROS-mediated inflammasome activation, subacute Mn exposure resulted in microglial NLRP3 inflammasome activation associated with dysregulation of autophagy and lysosomal stress (Wang, Zhang, et al., 2017). Authors conclude that Mn-driven assembly of the autophagosome and elevated cathepsin B are required for Nlrp3 inflammasome activation supporting a role for the lysosomal rupture model of inflammasome activation and reminiscent of the related stress outcomes associated with proteinopathy.
Other PD-implicated heavy metals may also interact with the inflammasomes. Iron deposition in the substantia nigra has been long recognized (Hallgren and Sourander, 1958), is considered a potential imaging biomarker of PD (Bartzokis et al., 1999), and nigral iron levels are correlated with PD severity (Martin-Bastida et al., 2017). Mechanisms for iron to cross the BBB include direct transport of ferritin across the barrier and endocytosis of transferrin and its receptor for release into the brain. Once iron enters the CNS it can be stored in ferritin or released via ferroportin (Duck et al., 2017) (Figure 2). Rodent models of PD indicate that iron has a broad role in increasing the permeability of the BBB suggesting the potential for a more generalizable role for iron in the exacerbation of neuroinflammation and toxicity (Olmedo-Diaz et al., 2017). Monocytes traffic across the BBB into the CNS (Depboylu et al., 2012) and have an underappreciated potential for driving both systemic and neuroinflammation in PD (Grozdanov et al., 2014). Monocytes play a key role in iron homeostasis and recent evidence indicates the labile iron activates the Nlrp3 inflammasome through a ROS-dependent mechanism (Nakamura et al., 2016). Such an activity supports an intriguing model in which inflammasome activity in monocytes may provide insight into inflammatory processes in the CNS detectable in the periphery. Direct evidence is sparse but studies indicate that iron-mediated inflammasome activation may also occur in CNS microglia (Figure 3). Microglia function in iron homeostasis (Rathore et al., 2012) and rodent and human studies identify a correlation between microglial activation and increased iron levels (Olmedo-Diaz et al., 2017). Mito-ROS-mediated inflammasome activation is likely given that microglia are hypothesized to produce ROS whereas simultaneously promoting iron uptake. Over time, the ROS generated leads to release of stored iron in the microglia, which can further increase ROS production (Garrido-Gil et al., 2013) and impact neuronal iron homeostasis and death (Wang et al., 2013; Zhang et al., 2014).
Studies of aluminum (Al) toxicity in PD are limited, but noteworthy, due to potential associations between Al exposure and related neurodegenerative disorders (Mirza et al., 2017). Al enters the brain in a similar mechanism to iron with 90% of Al complexed with transferrin and endocytosed at the BBB, whereas the remainder is complexed with citrate and likely taken up by a l-glutamine/l-cysteine exchanger (Nagasawa et al., 2005) (Figure 2). Similar to iron, mito-ROS-mediated inflammasome activation appears most likely. In a comparative study that included multiple common metals, Al exposure resulted in the most robust induction of ROS in glial cells (Pogue et al., 2012). Interestingly, Al exposure may also be related to other potential inflammasome triggers, including pathologic α-synuclein, as observed in an in vitro neuronal cell model (Saberzadeh et al., 2016). Perhaps the most indicative of Al’s potential to activate the inflammasomes, Al exposure has been demonstrated to generate uric acid in exposed tissues, a well-known activator of the NLRP3 inflammasome (Kool et al., 2008) (Figure 3).
FUTURE DIRECTIONS
The study of inflammasomes in PD follows extensive characterization of these intracellular mediators in the context of innate immunity. The recent interest in evaluating inflammasomes in PD stems from important and longstanding questions surrounding the cellular mechanisms underlying neuroinflammation and environmental risk, two well-known but incompletely understood aspects of PD. Fueling excitement in exploring the inflammasomes in PD is the potential that these pathways may be broadly employed mechanisms linking environmental insult with multiple aspects of PD-associated cellular stress. Moreover, inflammasomes are enzymatically active multi-protein complexes that can be modulated pharmacologically. Based on the accumulating data reviewed herein, the interest in inflammasomes in PD is certainly warranted, however, many open questions remain related to the functioning of these danger-sensing platforms in the CNS.
Core pathologies evident in PD, namely mitochondrial stress, proteinopathy, and cell death, are intuitive modulators of inflammasome activity and it is likely that the ongoing evaluation of inflammasomes in the context of these pathologies will continue to reveal new roles for inflammasomes in PD. One open question surrounds the concept of a ‘two-hit’ hypothesis for inflammasome activation. In many model systems, an initial priming trigger likely operating through toll-like receptors (TLRs) upregulates the components and targets of the inflammasomes and a subsequent stimulus triggers the catalytic events resulting in cytokine maturation and possible pyroptosis. Whether such a priming step is required for PD-associated inflammasome activity is unclear. One possibility is that indeed a pathogenic insult may prime the CNS for inflammasome activation. In support of this model, viral infections have been posited as having a role in the etiology of PD (Jang et al., 2009). Alternatively, in the absence of pathogenic priming, neuronal death could produce both the priming and activating stimuli based on the release of intracellular contents including pathologic α-synuclein, known to activate microglial TLR4 (Fellner et al., 2013), along with other canonical inflammasome stimulators such as ATP (Schroder and Tschopp, 2010). These findings are important because they begin to delineate the means by which the neurodegenerative microenvironment may engage canonical inflammasome activation through sterile mechanisms. However, it is likely that additional non-canonical inflammasome activation mechanisms also exist. As noted in previous sections, inroads toward understanding non-canonical inflammasome signaling in PD have been made in monocytes where synuclein priming is sufficient for assembly of the intracellular inflammasome in the absence of an activating stimulus (Codolo et al., 2013). Intriguingly, aging itself has been posited as a priming factor (Bauernfeind et al., 2016). Youm and others report that inactivation of Nlrp3 suppresses the development of age-related neuroinflammatory changes and functional decline in mice (Youm et al., 2013). Looking forward, we are in the early stages of understanding how inflammasomes may impact age-related neurologic disorders and how these processes may be exacerbated by environmental toxicants.
Pyroptosis is an intriguing area for exploration in the CNS, particularly in the context of cytotoxic inflammatory toxicants. Unlike classical apoptosis, pyroptotic cell death is pro-inflammatory, characterized by the formation of pores in the cytoplasmic membrane, cellular lysis, and significant release of intracellular material [19]. Such a mechanism is intriguing and suggests that inflammasome activation in CNS cells leading to pyroptosis may not only continually exacerbate co-occurring neuroinflammation throughout the disease course, but could also result in the release of CNS-specific biomolecules into the interstitium and possibly into systemic circulation. Although we do not fully understand how such a process could be initiated and unfold in the context of toxicant exposure in PD, evidence of neurologic disease-associated intracellular proteins in the cerebrospinal fluid (Irwin et al., 2018; Wang et al., 2018) and plasma (Lin et al., 2017) provide a strong rationale for further investigation of the pyroptotic process. Going forward, systemic analysis of inflammasome proteins including caspase-1 and IL-1B along with other indicators such as cleaved gasdermin D, recently discovered to be required to drive pyroptosis and associated IL-1B release (Liu et al., 2016), will be of great interest. Although initially characterized in macrophages (Brennan and Cookson, 2000), this concept is now supported by evidence of pyroptosis in the CNS, with hippocampal neurons (Fann et al., 2013) and retinal photoreceptor cells (Viringipurampeer et al., 2016) demonstrated to undergo NLRP3-dependent pyroptosis. Findings from ongoing studies in this area are awaited with great anticipation because they have the potential to identify not only novel intracellular mechanisms of cell death associated with environmental toxicants and PD, but may also reveal unexpected biologic indicators of ongoing neurodegenerative and neuroinflammatory processes relevant to a panoply of neurologic disorders.
A key finding in our review of current literature was the preponderance of studies focused on the NLRs; particularly NLRP3. In these early stages, evaluation of NLRP3 is appropriate based on its recognition as a mediator of sterile inflammation, as compared with other inflammasome-related NLRs whose activities are primarily characterized in the response to biological toxins and intracellular pathogens. Based on unexpected findings from studies of NLRP3 in the CNS, it stands to reason that continued analyses of all inflammasome proteins with an eye toward the identification of non-canonical signaling mechanisms will identify novel pathways of interest with appreciable impact on our understanding of health and disease. Another key aspect of the inflammasomes is their potential as therapeutic targets. These cytosolic multi-protein complexes rely on cell surface receptors, inducible protein interactions, and catalytic events whose interruption with small molecules is already under intense evaluation as noted by many recent reviews (Lopez-Castejon and Pelegrin, 2012; Ozaki et al., 2015). These translational efforts will be catapulted forward by further characterization of the basic mechanisms of inflammasome activity and the identification of new protein interactions, biologic indicators of inflammasome activity, and the development of high throughput methods for screening potential anti-inflammasome compounds. Given the growing body of evidence that modulation of inflammasomes may impact the progression of human diseases and the relative manageability of side-effects associated with anti-inflammatories, anti-inflammasome drugs have a reasonable potential for utility in the clinic, especially in age-related, slowly progressive disorders like PD.
FUNDING
This work is supported by the National Institute for Environmental Health Sciences (NIEHS) 1R01ES024745-01A1 (MCH) and the Michael J. Fox Foundation (MCH).
REFERENCES
- Adamczak S. E., de Rivero Vaccari J. P., Dale G., Brand F. J. 3rd, Nonner D., Bullock M. R., Dahl G. P., Dietrich W. D., Keane R. W. (2014). Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J. Cereb. Blood Flow Metab. 34, 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles D. C., Gan B. H., Onstead L., Zhao Y., Lim K. L., Dachsel J., Melrose H., Farrer M., Wszolek Z. K., Dickson D. W., et al. (2011). Mutations in LRRK2 increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Hum. Mutat. 32, 1390–1397. [DOI] [PubMed] [Google Scholar]
- Antonini J. M., Sriram K., Benkovic S. A., Roberts J. R., Stone S., Chen B. T., Schwegler-Berry D., Jefferson A. M., Billig B. K., Felton C. M., et al. (2009). Mild steel welding fume causes manganese accumulation and subtle neuroinflammatory changes but not overt neuronal damage in discrete brain regions of rats after short-term inhalation exposure. Neurotoxicology 30, 915–925. [DOI] [PubMed] [Google Scholar]
- Bartzokis G., Cummings J. L., Markham C. H., Marmarelis P. Z., Treciokas L. J., Tishler T. A., Marder S. R., Mintz J. (1999). MRI evaluation of brain iron in earlier- and later-onset Parkinson's disease and normal subjects. Magn. Reson. Imaging 17, 213–222. [DOI] [PubMed] [Google Scholar]
- Bassil F., Fernagut P. O., Bezard E., Pruvost A., Leste-Lasserre T., Hoang Q. Q., Ringe D., Petsko G. A., Meissner W. G. (2016). Reducing C-terminal truncation mitigates synucleinopathy and neurodegeneration in a transgenic model of multiple system atrophy. Proc. Natl. Acad. Sci. U.S.A. 113, 9593–9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F., Niepmann S., Knolle P. A., Hornung V. (2016). Aging-associated TNF production primes inflammasome activation and NLRP3-related metabolic disturbances. J. Immunol. 197, 2900–2908. [DOI] [PubMed] [Google Scholar]
- Besser M. J., Ganor Y., Levite M. (2005). Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. J. Neuroimmunol. 169, 161–171. [DOI] [PubMed] [Google Scholar]
- Booth H. D. E., Hirst W. D., Wade-Martins R. (2017). The role of astrocyte dysfunction in Parkinson's disease pathogenesis. Trends Neurosci. 40, 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco D. A., Fowler D. M., Zhang Q., Nieva J., Powers E. T., Wentworth P. Jr, Lerner R. A., Kelly J. W. (2006). Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat. Chem. Biol. 2, 249–253. [DOI] [PubMed] [Google Scholar]
- Brennan M. A., Cookson B. T. (2000). Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38, 31–40. [DOI] [PubMed] [Google Scholar]
- Burbulla L. F., Song P., Mazzulli J. R., Zampese E., Wong Y. C., Jeon S., Santos D. P., Blanz J., Obermaier C. D., Strojny C., et al. (2017). Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson's disease. Science 357, 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. R., Greenamyre J. T. (2013). Gene-environment interactions in Parkinson's disease: Specific evidence in humans and mammalian models. Neurobiol. Dis. 57, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. R., Tapias V., Na H. M., Honick A. S., Drolet R. E., Greenamyre J. T. (2009). A highly reproducible rotenone model of Parkinson's disease. Neurobiol. Dis. 34, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle W. M., Guillot T. S., Lazo C. R., Miller G. W. (2012). Industrial toxicants and Parkinson's disease. Neurotoxicology 33, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughlan A., Newhouse K., Namgung U., Xia Z.. 2004. Chlorpyrifos induces apoptosis in rat cortical neurons that is regulated by a balance between p38 and ERK/JNK MAP kinases. Toxicol Sci. 134, 125–134. [DOI] [PubMed] [Google Scholar]
- Chen L., Na R., Boldt E., Ran Q. (2015). NLRP3 inflammasome activation by mitochondrial reactive oxygen species plays a key role in long-term cognitive impairment induced by paraquat exposure. Neurobiol. Aging 36, 2533–2543. [DOI] [PubMed] [Google Scholar]
- Chen Y., Smith M. R., Thirumalai K., Zychlinsky A. (1996). A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15, 3853–3860. [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F., Lapointe N., Roberge-Tremblay A., Saint-Pierre M., Jimenez L., Ficke B. W., Gross R. E. (2005). Systemic exposure to paraquat and maneb models early Parkinson's disease in young adult rats. Neurobiol. Dis. 20, 360–371. [DOI] [PubMed] [Google Scholar]
- Codolo G., Plotegher N., Pozzobon T., Brucale M., Tessari I., Bubacco L., de Bernard M. (2013). Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS One 8, e55375.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo A., Orsi F., Bonfanti P. (2005). Exposure to the organophosphorus pesticide chlorpyrifos inhibits acetylcholinesterase activity and affects muscular integrity in Xenopus laevis larvae. Chemosphere 61, 1665–1671. [DOI] [PubMed] [Google Scholar]
- Costello S., Cockburn M., Bronstein J., Zhang X., Ritz B. (2009). Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am. J. Epidemiol. 169, 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depboylu C., Stricker S., Ghobril J. P., Oertel W. H., Priller J., Hoglinger G. U. (2012). Brain-resident microglia predominate over infiltrating myeloid cells in activation, phagocytosis and interaction with T-lymphocytes in the MPTP mouse model of Parkinson disease. Exp. Neurol. 238, 183–191. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari J. P., Lotocki G., Marcillo A. E., Dietrich W. D., Keane R. W. (2008). A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 28, 3404–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon A. S., Tarbutton G. L., Levin J. L., Plotkin G. M., Lowry L. K., Nalbone J. T., Shepherd S. (2008). Pesticide/environmental exposures and Parkinson's disease in East Texas. J. Agromed. 13, 37–48. [DOI] [PubMed] [Google Scholar]
- Duck K. A., Simpson I. A., Connor J. R. (2017). Regulatory mechanisms for iron transport across the blood-brain barrier. Biochem. Biophys. Res. Commun. 494, 70–75. [DOI] [PubMed] [Google Scholar]
- Elliott E. I., Miller A. N., Banoth B., Iyer S. S., Stotland A., Weiss J. P., Gottlieb R. A., Sutterwala F. S., Cassel S. L. (2018). Cutting edge: Mitochondrial assembly of the NLRP3 inflammasome complex is initiated at priming. J. Immunol. 200, 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun J., Sun M., Hyeon J., Hwan C., Chul H. (2014). NeuroToxicology nuclear NF-kB contributes to chlorpyrifos-induced apoptosis through p53 signaling in human neural precursor cells. NeuroToxicology 42, 58–70. [DOI] [PubMed] [Google Scholar]
- Fann D. Y., Lee S. Y., Manzanero S., Tang S. C., Gelderblom M., Chunduri P., Bernreuther C., Glatzel M., Cheng Y. L., Thundyil J., et al. (2013). Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 4, e790.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber K., Pannasch U., Kettenmann H. (2005). Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol. Cell. Neurosci. 29, 128–138. [DOI] [PubMed] [Google Scholar]
- Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G. K., Stefanova N. (2013). Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 61, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipov N. M., Seegal R. F., Lawrence D. A. (2005). Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol. Sci. 84, 139–148. [DOI] [PubMed] [Google Scholar]
- Fleming S. M. (2017). Mechanisms of gene-environment interactions in Parkinson's disease. Curr. Environ. Health Rep. 4, 192–199. [DOI] [PubMed] [Google Scholar]
- Garcia-Reyero N., Escalon L., Prats E., Faria M., Soares A. M. V. M., Raldúa D. (2016). Targeted gene expression in zebrafish exposed to chlorpyrifos-oxon confirms phenotype-specific mechanisms leading to adverse outcomes. Bull. Environ. Contam. Toxicol. 96, 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Gil P., Rodriguez-Pallares J., Dominguez-Meijide A., Guerra M. J., Labandeira-Garcia J. L. (2013). Brain angiotensin regulates iron homeostasis in dopaminergic neurons and microglial cells. Exp. Neurol. 250, 384–396. [DOI] [PubMed] [Google Scholar]
- Gatto N. M., Cockburn M., Bronstein J., Manthripragada A. D., Ritz B. (2009). Well-water consumption and Parkinson's disease in rural California. Environ. Health Perspect. 117, 1912–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S., Holmstrom K. M., Treis A., Skujat D., Weber S. S., Fiesel F. C., Kahle P. J., Springer W. (2010). The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy 6, 871–878. [DOI] [PubMed] [Google Scholar]
- Gorojod R. M., Alaimo A., Porte Alcon S., Saravia F., Kotler M. L. (2017). Interplay between lysosomal, mitochondrial and death receptor pathways during manganese-induced apoptosis in glial cells. Arch. Toxicol. 91, 3065–3078. [DOI] [PubMed] [Google Scholar]
- Grozdanov V., Bliederhaeuser C., Ruf W. P., Roth V., Fundel-Clemens K., Zondler L., Brenner D., Martin-Villalba A., Hengerer B., Kassubek J., et al. (2014). Inflammatory dysregulation of blood monocytes in Parkinson's disease patients. Acta Neuropathol. 128, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte T. R., Burton N. C., McGlothan J. L., Verina T., Zhou Y., Alexander M., Pham L., Griswold M., Wong D. F., Syversen T., et al. (2008). Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): Implications to manganese-induced parkinsonism. J. Neurochem. 107, 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin A., Kirchmeyer M., Koncina E., Felten P., Losciuto S., Heurtaux T., Tardivel A., Heuschling P., Dostert C. (2015). NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS One 10, e0130624.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. (2008). The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9, 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B., Sourander P. (1958). The effect of age on the non-haemin iron in the human brain. J. Neurochem. 3, 41–51. [DOI] [PubMed] [Google Scholar]
- Hatcher J. M., Pennell K. D., Miller G. W. (2008). Parkinson's disease and pesticides: A toxicological perspective. Trends Pharmacol. Sci. 29, 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Hara H., Nunez G. (2016). Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41, 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E. C., Vyas S., Hunot S. (2012). Neuroinflammation in Parkinson's disease. Parkinsonism Relat. Disord. 18(Suppl. 1), S210–S212. [DOI] [PubMed] [Google Scholar]
- Hoffman H. M., Brydges S. D. (2011). Genetic and molecular basis of inflammasome-mediated disease. J. Biol. Chem. 286, 10889–10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008). Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D. J., Xie S. X., Coughlin D., Nevler N., Akhtar R. S., McMillan C. T., Lee E. B., Wolk D. A., Weintraub D., Chen-Plotkin A., et al. (2018). CSF tau and beta-amyloid predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 90, e1038–e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H., Boltz D., Sturm-Ramirez K., Shepherd K. R., Jiao Y., Webster R., Smeyne R. J. (2009). Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 106, 14063–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y., Young A., Jeong S.-H., Park K.-H., Paik M.-K., Cho N.-J., Kim J.-E., Cho M.-H. (2015). Chlorpyrifos induces NLRP3 in fl ammasome and pyroptosis/apoptosis via mitochondrial oxidative stress in human keratinocyte HaCaT cells. Toxicology 338, 37–46. [DOI] [PubMed] [Google Scholar]
- Jenkitkasemwong S., Akinyode A., Paulus E., Weiskirchen R., Hojyo S., Fukada T., Giraldo G., Schrier J., Garcia A., Janus C., et al. (2018). SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc. Natl. Acad. Sci. U.S.A. 115, E1769–E1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johann S., Heitzer M., Kanagaratnam M., Goswami A., Rizo T., Weis J., Troost D., Beyer C. (2015). NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 63, 2260–2273. [DOI] [PubMed] [Google Scholar]
- Kahlenberg J. M., Dubyak G. R. (2004). Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 286, C1100–C1108. [DOI] [PubMed] [Google Scholar]
- Ki Y.-W., Park J. H., Lee J. E., Shin I. C., Koh H. C. (2013). JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol. Lett. 218, 235–245. [DOI] [PubMed] [Google Scholar]
- Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. (1998). Mutations in the Parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608. [DOI] [PubMed] [Google Scholar]
- Klein C., Westenberger A. (2012). Genetics of Parkinson's disease. Cold Spring Harb. Perspect. Med. 2, a008888.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M., Soullie T., van Nimwegen M., Willart M. A., Muskens F., Jung S., Hoogsteden H. C., Hammad H., Lambrecht B. N. (2008). Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 205, 869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopjar N., Žunec S., Mendaš G., Micek V., Kašuba V., Mikolić A., Lovaković B. T., Milić M., Pavičić I., Čermak A. M. M., et al. (2018). Evaluation of chlorpyrifos toxicity through a 28-day study: Cholinesterase activity, oxidative stress responses, parent compound/metabolite levels, and primary DNA damage in blood and brain tissue of adult male Wistar rats. Chem. Biol. Interact. 279, 51–63. [DOI] [PubMed] [Google Scholar]
- Kresovich J. K., Bulka C. M., Joyce B. T., Vokonas P. S., Schwartz J., Baccarelli A. A., Hibler E. A., Hou L. (2018). The inflammatory potential of dietary manganese in a cohort of elderly men. Biol. Trace Elem. Res. 183, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz L. A. (1948). The use of rotenone in fisheries research. J. Wildl. Manag. 12, 305–317. [Google Scholar]
- Langley M. R., Ghaisas S., Ay M., Luo J., Palanisamy B. N., Jin H., Anantharam V., Kanthasamy A., Kanthasamy A. G. (2018). Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson's disease: Relevance to gene and environment interactions in metal neurotoxicity. Neurotoxicology 64, 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. (1983). Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980. [DOI] [PubMed] [Google Scholar]
- Lawana V., Singh N., Sarkar S., Charli A., Jin H., Anantharam V., Kanthasamy A. G., Kanthasamy A. (2017). Involvement of c-Abl kinase in microglial activation of NLRP3 inflammasome and impairment in autolysosomal system. J. Neuroimmune Pharmacol. 12, 624–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Hwang I., Park S., Hong S., Hwang B., Cho Y., Son J., Yu J. W. (2018). MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ehrich M. (2013). Transient alterations of the blood-brain barrier tight junction and receptor potential channel gene expression by chlorpyrifos. J. Appl. Toxicol. 33, 1187–1191. [DOI] [PubMed] [Google Scholar]
- Lin C. H., Yang S. Y., Horng H. E., Yang C. C., Chieh J. J., Chen H. H., Liu B. H., Chiu M. J. (2017). Plasma alpha-synuclein predicts cognitive decline in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 88, 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou H. H., Tsai M. C., Chen C. J., Jeng J. S., Chang Y. C., Chen S. Y., Chen R. C. (1997). Environmental risk factors and Parkinson's disease: A case-control study in Taiwan. Neurology 48, 1583–1588. [DOI] [PubMed] [Google Scholar]
- Liu W., Liu X., Li Y., Zhao J., Liu Z., Hu Z., Wang Y., Yao Y., Miller A. W., Su B., et al. (2017). LRRK2 promotes the activation of NLRC4 inflammasome during Salmonella typhimurium infection. J. Exp. Med. 214, 3051–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Sun M., Wang Y., Zhang L., Zhao H., Zhao M. (2018). Silymarin attenuated paraquat-induced cytotoxicity in macrophage by regulating Trx/TXNIP complex, inhibiting NLRP3 inflammasome activation and apoptosis. Toxicol. In Vitro 46, 265–272. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang H. (2005). Environmental toxins and alpha-synuclein in Parkinson's disease. Mol. Neurobiol. 31, 273–282. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V. G., Wu H., Lieberman J. (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castejon G., Pelegrin P. (2012). Current status of inflammasome blockers as anti-inflammatory drugs. Expert Opin. Investig. Drugs 21, 995–1007. [DOI] [PubMed] [Google Scholar]
- Luk K. C., Kehm V., Carroll J., Zhang B., O'Brien P., Trojanowski J. Q., Lee V. M. (2012). Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D., Giesy J. P., Solomon K. R. (2014). Fate in the environment and long-range atmospheric transport of the organophosphorus insecticide, chlorpyrifos and its oxon. Rev. Environ. Contam. Toxicol. 231, 35–76. [DOI] [PubMed] [Google Scholar]
- Manning-Bog A. B., McCormack A. L., Li J., Uversky V. N., Fink A. L., Di Monte D. A. (2002). The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: Paraquat and alpha-synuclein. J. Biol. Chem. 277, 1641–1644. [DOI] [PubMed] [Google Scholar]
- Manning-Boğ A. B., McCormack A. L., Purisai M. G., Bolin L. M., Di Monte D. A. (2003). Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J. Neurosci. 23, 3095–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthripragada A. D., Costello S., Cockburn M. G., Bronstein J. M., Ritz B. (2010). Paraoxonase 1, agricultural organophosphate exposure, and Parkinson disease. Epidemiology 21, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Ou M. T., Karuppagounder S. S., Kam T. I., Yin X., Xiong Y., Ge P., Umanah G. E., Brahmachari S., Shin J. H., et al. (2016). Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353, aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bastida A., Lao-Kaim N. P., Loane C., Politis M., Roussakis A. A., Valle-Guzman N., Kefalopoulou Z., Paul-Visse G., Widner H., Xing Y., et al. (2017). Motor associations of iron accumulation in deep grey matter nuclei in Parkinson's disease: A cross-sectional study of iron-related magnetic resonance imaging susceptibility. Eur. J. Neurol. 24, 357–365. [DOI] [PubMed] [Google Scholar]
- Martinez E. M., Young A. L., Patankar Y. R., Berwin B. L., Wang L., von Herrmann K. M., Weier J. M., Havrda M. C. (2017). Editor's highlight: Nlrp3 is required for inflammatory changes and nigral cell loss resulting from chronic intragastric rotenone exposure in mice. Toxicol. Sci. 159, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C. A., Sou Y. S., Saiki S., Kawajiri S., Sato F., et al. (2010). PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S., Somayajulu M., Sikorska M., Borowy-Borowski H., Pandey S. (2004). Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol. Appl. Pharmacol. 201, 21–31. [DOI] [PubMed] [Google Scholar]
- McCormack A. L., Thiruchelvam M., Manning-Bog A. B., Thiffault C., Langston J. W., Cory-Slechta D. A., Di Monte D. A. (2002). Environmental risk factors and Parkinson's disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol. Dis. 10, 119–127. [DOI] [PubMed] [Google Scholar]
- Mirza A., King A., Troakes C., Exley C. (2017). Aluminium in brain tissue in familial Alzheimer's disease. J. Trace Elem. Med. Biol. 40, 30–36. [DOI] [PubMed] [Google Scholar]
- Mouton-Liger F., Rosazza T., Sepulveda-Diaz J., Ieang A., Hassoun S. M., Claire E., Mangone G., Brice A., Michel P. P., Corvol J. C., et al. (2018). Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop. Glia. 66, 1736–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynagh P. N. (2005). The interleukin-1 signalling pathway in astrocytes: A key contributor to inflammation in the brain. J. Anat. 207, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Planillo R., Kuffa P., Martinez-Colon G., Smith B. L., Rajendiran T. M., Nunez G. (2013). K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa K., Ito S., Kakuda T., Nagai K., Tamai I., Tsuji A., Fujimoto S. (2005). Transport mechanism for aluminum citrate at the blood-brain barrier: Kinetic evidence implies involvement of system Xc− in immortalized rat brain endothelial cells. Toxicol. Lett. 155, 289–296. [DOI] [PubMed] [Google Scholar]
- Nagyőszi P., Nyúl-Tóth Á., Fazakas C., Wilhelm I., Kozma M., Molnár J., Haskó J., Krizbai I. A. (2015). Regulation of NOD-like receptors and inflammasome activation in cerebral endothelial cells. J. Neurochem. 135, 551–564. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kawakami T., Yamamoto N., Tomizawa M., Fujiwara T., Ishii T., Harigae H., Ogasawara K. (2016). Activation of the NLRP3 inflammasome by cellular labile iron. Exp. Hematol. 44, 116–124. [DOI] [PubMed] [Google Scholar]
- Naylor J. L., Widdowson P. S., Simpson M. G., Farnworth M., Ellis M. K., Lock E. A. (1995). Further evidence that the blood/brain barrier impedes paraquat entry into the brain. Hum. Exp. Toxicol. 14, 587–594. [DOI] [PubMed] [Google Scholar]
- Olanow C. W. (2004). Manganese-induced parkinsonism and Parkinson's disease. Ann. N.Y. Acad. Sci. 1012, 209–223. [DOI] [PubMed] [Google Scholar]
- Olmedo-Diaz S., Estevez-Silva H., Oradd G., Af Bjerken S., Marcellino D., Virel A. (2017). An altered blood-brain barrier contributes to brain iron accumulation and neuroinflammation in the 6-OHDA rat model of Parkinson's disease. Neuroscience 362, 141–151. [DOI] [PubMed] [Google Scholar]
- Ossowska K., Śmiałowska M., Kuter K., Wierońska J., Zięba B., Wardas J., Nowak P., Dąbrowska J., Bortel A., Biedka I., et al. (2006). Degeneration of dopaminergic mesocortical neurons and activation of compensatory processes induced by a long-term paraquat administration in rats: Implications for Parkinson's disease. Neuroscience 141, 2155–2165. [DOI] [PubMed] [Google Scholar]
- Ozaki E., Campbell M., Doyle S. L. (2015). Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 8, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Montojo F., Schwarz M., Winkler C., Arnhold M., O'Sullivan G. A., Pal A., Said J., Marsico G., Verbavatz J. M., Rodrigo-Angulo M., et al. (2012). Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep. 2, 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parran D. K., Magnin G., Li W., Jortner B. S., Ehrich M. (2005). Chlorpyrifos alters functional integrity and structure of an in vitro BBB model: Co-cultures of bovine endothelial cells and neonatal rat astrocytes. NeuroToxicology 26, 77–88. [DOI] [PubMed] [Google Scholar]
- Pelegrin P., Surprenant A. (2006). Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell A. M., Youle R. J. (2015). The roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue A. I., Jones B. M., Bhattacharjee S., Percy M. E., Zhao Y., Lukiw W. J. (2012). Metal-sulfate induced generation of ROS in human brain cells: Detection using an isomeric mixture of 5- and 6-carboxy-2′,7′-dichlorofluorescein diacetate (carboxy-DCFDA) as a cell permeant tracer. Int. J. Mol. Sci. 13, 9615–9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purisai M. G., McCormack A. L., Cumine S., Li J., Isla M. Z., Di Monte D. A. (2007). Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol. Dis. 25, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette B. A., Searles Nielsen S., Criswell S. R., Sheppard L., Seixas N., Warden M. N., Checkoway H. (2017). Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology 88, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput A. H., Offord K. P., Beard C. M., Kurland L. T. (1984). Epidemiology of parkinsonism: Incidence, classification, and mortality. Ann. Neurol. 16, 278–282. [DOI] [PubMed] [Google Scholar]
- Raszewski G., Lemieszek Marta K., Łukawski K., Juszczak M., Rzeski W. (2015). Chlorpyrifos and cypermethrin induce apoptosis in human neuroblastoma cell line SH‐SY5Y. Basic Clin. Pharmacol. Toxicol. 116, 158–167. [DOI] [PubMed] [Google Scholar]
- Rathore K. I., Redensek A., David S. (2012). Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-alpha and TGF-beta1. Glia 60, 738–750. [DOI] [PubMed] [Google Scholar]
- Richardson J. D., Vasko M. R. (2002). Cellular mechanisms of neurogenic inflammation. J. Pharmacol. Exp. Ther. 302, 839–845. [DOI] [PubMed] [Google Scholar]
- Saberzadeh J., Arabsolghar R., Takhshid M. A. (2016). Alpha synuclein protein is involved in aluminum-induced cell death and oxidative stress in PC12 cells. Brain Res. 1635, 153–160. [DOI] [PubMed] [Google Scholar]
- Sadatomi D., Nakashioya K., Mamiya S., Honda S., Kameyama Y., Yamamura Y., Tanimura S., Takeda K. (2017). Mitochondrial function is required for extracellular ATP-induced NLRP3 inflammasome activation. J. Biochem. 161, 503–512. [DOI] [PubMed] [Google Scholar]
- Sanchez-Betancourt J., Anaya-Martinez V., Gutierrez-Valdez A. L., Ordonez-Librado J. L., Montiel-Flores E., Espinosa-Villanueva J., Reynoso-Erazo L., Avila-Costa M. R. (2012). Manganese mixture inhalation is a reliable Parkinson disease model in rats. Neurotoxicology 33, 1346–1355. [DOI] [PubMed] [Google Scholar]
- Santos D., Batoreu M. C., Tavares de Almeida I., Davis Randall L., Mateus M. L., Andrade V., Ramos R., Torres E., Aschner M., Marreilha dos Santos A. P. (2013). Evaluation of neurobehavioral and neuroinflammatory end-points in the post-exposure period in rats sub-acutely exposed to manganese. Toxicology 314, 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Malovic E., Harishchandra D. S., Ghaisas S., Panicker N., Charli A., Palanisamy B. N., Rokad D., Jin H., Anantharam V., et al. (2017a). Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson's disease. NPJ Parkinsons Dis. 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Malovic E., Harishchandra D. S., Ghaisas S., Panicker N., Charli A., Palanisamy B. N., Rokad D., Jin H., Anantharam V., et al. (2017b). Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. NPJ Parkinson's Dis. 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulsbury M., Heyliger S., Kaiyu W., Johnson D. J.. 2009. Chlorpyrifos induces oxidative stress in oligodendrocyte progenitor cells. Toxicology. 259(1–2), 1–9. [DOI] [PubMed] [Google Scholar]
- Scheiblich H., Schlutter A., Golenbock D. T., Latz E., Martinez-Martinez P., Heneka M. T. (2017). Activation of the NLRP3 inflammasome in microglia: The role of ceramide. J. Neurochem. 143, 534–550. [DOI] [PubMed] [Google Scholar]
- Schroder K., Tschopp J. (2010). The inflammasomes. Cell 140, 821–832. [DOI] [PubMed] [Google Scholar]
- Sharma D., Kanneganti T. D. (2016). The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 213, 617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer T. B., Betarbet R., Testa C. M., Seo B. B., Richardson J. R., Kim J. H., Miller G. W., Yagi T., Matsuno-Yagi A., Greenamyre J. T. (2003). Mechanism of toxicity in rotenone models of Parkinson's disease. J. Neurosci. 23, 10756–10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Crother T. R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V. K., Wolf A. J., Vergnes L., Ojcius D. M., et al. (2012). Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Matsubara K., Ohtaki K., Fujimaru S., Saito O., Shiono H. (2003). Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res. 976, 243–252. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Ohtaki K., Matsubara K., Aoyama K., Uezono T., Saito O., Suno M., Ogawa K., Hayase N., Kimura K., et al. (2001). Carrier-mediated processes in blood-brain barrier penetration and neural uptake of paraquat. Brain Res. 906, 135–142. [DOI] [PubMed] [Google Scholar]
- Solomon K. R., Williams W. M., Mackay D., Purdy J., Giddings J. M., Giesy J. P. (2014). Properties and uses of chlorpyrifos in the United States. Rev. Environ. Contam. Toxicol. 231, 13–34. [DOI] [PubMed] [Google Scholar]
- Su C. J., Feng Y., Liu T. T., Liu X., Bao J. J., Shi A. M., Hu D. M., Liu T., Yu Y. L. (2017). Thioredoxin-interacting protein induced alpha-synuclein accumulation via inhibition of autophagic flux: Implications for Parkinson's disease. CNS Neurosci. Ther. 23, 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., Marras C., Bhudhikanok G. S., Kasten M., Chade A. R., et al. (2011). Rotenone, paraquat, and Parkinson's disease. Environ. Health Perspect. 119, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey M. G., Goldberg M. S. (2010). Neuroinflammation in Parkinson's disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 37, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J. P., Lovering R. C., Alnemri E. S., Bertin J., Boss J. M., Davis B. K., Flavell R. A., Girardin S. E., Godzik A., Harton J. A., et al. (2008). The NLR gene family: A standard nomenclature. Immunity 28, 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino A., Noguchi T., Nomiyama K., Takase Y., Nakazono T., Nojiri J., Kudo S. (2007). Manganese accumulation in the brain: MR imaging. Neuroradiology 49, 715–720. [DOI] [PubMed] [Google Scholar]
- Valente E. M., Abou-Sleiman P. M., Caputo V., Muqit M. M., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A. R., Healy D. G., et al. (2004). Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160. [DOI] [PubMed] [Google Scholar]
- Verma R. S., Mehta A., Srivastava N. (2007). In vivo chlorpyrifos induced oxidative stress: Attenuation by antioxidant vitamins. Pest. Biochem. Physiol. 88, 191–196. [Google Scholar]
- Villar-Piqué A., Lopes da Fonseca T., Sant’Anna R., Szegö É. M., Fonseca-Ornelas L., Pinho R., Carija A., Gerhardt E., Masaracchia C., Abad Gonzalez E., et al. (2016). Environmental and genetic factors support the dissociation between alpha-synuclein aggregation and toxicity. Proc. Natl. Acad. Sci. U.S.A. 113, E6506–E6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viringipurampeer I. A., Metcalfe A. L., Bashar A. E., Sivak O., Yanai A., Mohammadi Z., Moritz O. L., Gregory-Evans C. Y., Gregory-Evans K. (2016). NLRP3 inflammasome activation drives bystander cone photoreceptor cell death in a P23H rhodopsin model of retinal degeneration. Hum. Mol. Genet. 25, 1501–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C., Zhou C., Huang Y., Cui M., de Vries R. L., Kim J., May J., Tocilescu M. A., Liu W., Ko H. S., et al. (2010). PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. U.S.A. 107, 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Herrmann K., Lucas S., Eileen M., Alison Y., Joseph H., Mary F., Brock C., Owen W., Stephen L., William H., et al. (2018). NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson’s disease. NPJ Parkinson’s 4, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Cockburn M., Ly T. T., Bronstein J. M., Ritz B. (2014). The association between ambient exposure to organophosphates and Parkinson's disease risk. Occup. Environ. Med. 71, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Nguyen L. T., Burlak C., Chegini F., Guo F., Chataway T., Ju S., Fisher O. S., Miller D. W., Datta D., et al. (2016). Caspase-1 causes truncation and aggregation of the Parkinson's disease-associated protein alpha-synuclein. Proc. Natl. Acad. Sci. U.S.A. 113, 9587–9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Ren N., Cai Z., Lin Q., Wang Z., Zhang Q., Wu S., Li H. (2017). Paraquat and MPTP induce neurodegeneration and alteration in the expression profile of microRNAs: The role of transcription factor Nrf2. NPJ Parkinsons Dis. 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Song N., Jiang H., Wang J., Xie J. (2013). Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim. Biophys. Acta 1832, 618–625. [DOI] [PubMed] [Google Scholar]
- Wang H., Stewart T., Toledo J. B., Ginghina C., Tang L., Atik A., Aro P., Shaw L. M., Trojanowski J. Q., Galasko D. R., et al. (2018). A longitudinal study of total and phosphorylated alpha-synuclein with other biomarkers in cerebrospinal fluid of Alzheimer's disease and mild cognitive impairment. J. Alzheimers Dis. 61, 1541–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yan M. H., Fujioka H., Liu J., Wilson-Delfosse A., Chen S. G., Perry G., Casadesus G., Zhu X. (2012). LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet. 21, 1931–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhang J., Jiang W., Cao Z., Zhao F., Cai T., Aschner M., Luo W. (2017). The role of NLRP3-CASP1 in inflammasome-mediated neuroinflammation and autophagy dysfunction in manganese-induced, hippocampal-dependent impairment of learning and memory ability. Autophagy 13, 914–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson Leah N., Terry Alvin V., Bartlett Michael G. (2006). Determination of chlorpyrifos and its metabolites in rat brain tissue using coupled‐column liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 20, 2689–2695. [DOI] [PubMed] [Google Scholar]
- Wolf S. A., Boddeke H. W., Kettenmann H. (2017). Microglia in physiology and disease. Annu. Rev. Physiol. 79, 619–643. [DOI] [PubMed] [Google Scholar]
- Won J. H., Park S., Hong S., Son S., Yu J. W. (2015). Rotenone-induced impairment of mitochondrial electron transport chain confers a selective priming signal for NLRP3 inflammasome activation. J. Biol. Chem. 290, 27425–27437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagnik D. R., Hillyer P., Marshall D., Smythe C. D., Krausz T., Haskard D. O., Landis R. C. (2000). Noninflammatory phagocytosis of monosodium urate monohydrate crystals by mouse macrophages. Implications for the control of joint inflammation in gout. Arthritis Rheum. 43, 1779–1789. [DOI] [PubMed] [Google Scholar]
- Yan Y., Jiang W., Liu L., Wang X., Ding C., Tian Z., Zhou R. (2015). Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73. [DOI] [PubMed] [Google Scholar]
- Ye X., Zuo D., Yu L., Zhang L., Tang J., Cui C., Bao L., Zan K., Zhang Z., Yang X., et al. (2017). ROS/TXNIP pathway contributes to thrombin induced NLRP3 inflammasome activation and cell apoptosis in microglia. Biochem. Biophys. Res. Commun. 485, 499–505. [DOI] [PubMed] [Google Scholar]
- Yokel R. A. (2009). Manganese flux across the blood-brain barrier. Neuromol. Med. 11, 297–310. [DOI] [PubMed] [Google Scholar]
- Youm Y. H., Grant R. W., McCabe L. R., Albarado D. C., Nguyen K. Y., Ravussin A., Pistell P., Newman S., Carter R., Laque A., et al. (2013). Canonical NLRP3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Dai H., Deng Y., Tian J., Zhang C., Hu Z., Bing G., Zhao L. (2015). Neonatal chlorpyrifos exposure induces loss of dopaminergic neurons in young adult rats. Toxicology 336, 17–25. [DOI] [PubMed] [Google Scholar]
- Zhang P., Shao X. Y., Qi G. J., Chen Q., Bu L. L., Chen L. J., Shi J., Ming J., Tian B. (2016). Cdk5-dependent activation of neuronal inflammasomes in Parkinson's disease. Mov. Disord. 31, 366–376. [DOI] [PubMed] [Google Scholar]
- Zhang W., Yan Z. F., Gao J. H., Sun L., Huang X. Y., Liu Z., Yu S. Y., Cao C. J., Zuo L. J., Chen Z. J., et al. (2014). Role and mechanism of microglial activation in iron-induced selective and progressive dopaminergic neurodegeneration. Mol. Neurobiol. 49, 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Cai T., Liu M., Zheng G., Luo W., Chen J. (2009). Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol. Sci. 107, 156–164. [DOI] [PubMed] [Google Scholar]
- Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. (2010). Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140. [DOI] [PubMed] [Google Scholar]
- Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225. [DOI] [PubMed] [Google Scholar]
- Zhuang J., Yu H. Q., Henry T. B., Sayler G. S. (2015). Fate and toxic effects of environmental stressors: Environmental control. Ecotoxicology 24, 2043–2048. [DOI] [PubMed] [Google Scholar]