Abstract
Objective
To examine epidural fat and its relationship to pain, physical function, and disability among older adults with chronic low back pain, chronic low back pain plus leg pain, and controls.
Design
Cross-sectional, comparative study.
Setting
Standardized examinations were conducted in a research laboratory, and magnetic resonance images were obtained.
Subjects
A total of 93 adults age 60 to 85 years (24 with chronic back pain, 25 with chronic back pain plus leg pain, and 44 controls).
Methods
Reliability for assessment of epidural fat diameter, averaged across spinal levels, was established (intraclass correlation coefficient = 0.95). Linear regression was used to explore how epidural fat diameter related to self-reported (Short Form-36 Health Survey: physical component summary score) and performance-based (stair climb performance) measures of physical function among adults with chronic back pain with and without leg pain, as compared with controls, while controlling for age, sex, and body mass index. Associations between epidural fat and pain intensity and low back pain–related disability were also explored (P ≤ 0.050).
Results
Epidural fat helped explain self-reported function (P < 0.001); adults with axial low back pain (LBP) may have a relationship between epidural fat and self-report function that is different from controls (P = 0.015). Relationships between epidural fat and stair performance were significantly different from controls for those with LBP (P = 0.000) but not for those with LBP plus leg pain (P = 0.366). Relationships between epidural fat and pain intensity and/or disability were not found.
Conclusions
Increased epidural fat may help explain better function among older adults with chronic axial back pain, but not among those who also report leg pain.
Keywords: Aged, Geriatric, Spine
Introduction
Epidural fat is adipose tissue located within the epidural space, that is, the area located between the dura mater and the vertebral wall of the spine. Epidural fat increases proximally to distally in the lumbar spinal canal [1,2]. Prior research has hypothesized that spinal epidural fat is not simply a space-occupying tissue as its anatomical location suggests a functional purpose [1]. Specifically, as epidural fat is found in the posterior aspect of the lumbar spine, it may buffer pulsatile dural sac movements, allow sliding of the dural sac along the surface of the vertebral arch during flexion and extension, and protect the dural sac during lashing and rotational motions [1,2]. The reduction in or absence of epidural fat has been associated with chronically painful spinal conditions, including lumbar stenosis [2,3]. Specifically, adults with long-standing lumbar spinal stenosis have less epidural fat than their peers with symptoms of shorter durations [4].
Emerging regenerative research in animal models suggests that reconstruction of epidural fat is possible [5,6]. Epidural fibrosis, which is common in failed back surgery syndrome, is thought to occur due to epidural fat destruction followed by invasion of fibroblasts [6]. In rabbits, adipose tissue engineered from adipose-derived stem cells has demonstrated promise for regenerating epidural fat to assist with epidural fibrosis prevention [6]. Adults with failed back surgery syndrome, pending future human subjects trials, may be one low back pain (LBP) subgroup who may benefit from regenerated epidural fat, while older adults with chronic LBP, including those with lumbar stenosis (who have reduced epidural fat) [2,4], may be another potential subgroup. That said, there has been little research evaluating relationships between epidural fat and clinically important outcomes, such as LBP intensity, LBP-related disability, and physical function among adults with chronic LBP. Establishing such relationships is a necessary step prior to human subjects trials that seek to restore epidural fat to improve pain, disability, and function in patients with chronic spinal conditions.
It is well-established that chronic LBP results in impaired physical function among older adults [7,8]. When chronic LBP is accompanied by leg pain, functional limitations and resultant disability increase. For example, prior research using the Medical Outcomes Short Form–36 Health Survey (SF-36) physical component score has shown that older adults with chronic LBP and leg pain (i.e., pain radiating into the hip, buttock, or lower extremity) are more likely to report daily activity limitations when compared with those reporting only chronic axial LBP [9]. Due to age-related changes, radicular symptoms in older adults commonly arise from irritation of neural structures at lower lumbar segments [10]. Consequently, adequate epidural fat in this region, given its proposed buffering, protective function [1,2], may be particularly important for older adults presenting with chronic LBP and radicular symptoms.
To our knowledge, there are no studies evaluating the relationship between epidural fat and physical function among older adults, nor are there studies evaluating the reliability of epidural fat quantification in older adults with chronic LBP. We sought to establish interexaminer measurement reliability for assessment of epidural fat at vertebral levels L2/3 through L5/S1 so that we could examine lumbar spine epidural fat and its relationship to pain, physical function, and LBP-related disability among older adults with chronic LBP (with and without leg pain) as compared with controls. We hypothesized that a novice examiner could reliably obtain measurements of epidural fat with minimal training. Based on the theory that epidural fat is protective, we hypothesized that associations between epidural fat and physical function would be different among older adults with chronic LBP (with or without leg pain) when compared with associations among controls without LBP, with less epidural fat being associated with worse physical function in those with chronic LBP. We also hypothesized that older adults with chronic LBP + leg pain would demonstrate stronger associations between epidural fat and physical function than individuals with chronic axial LBP only when both subgroups were compared with controls (without LBP). This hypothesis was based on prior studies reporting 1) epidural fat reduction with spinal stenosis (but no reports in axial LBP only conditions) and 2) greater impairments in physical function among adults with chronic LBP plus radicular symptoms when compared with adults with axial LBP only. Lastly, we sought to determine if associations between epidural fat and LBP intensity and/or LBP-related disability were present and if these relationships differed between adults with chronic LBP with and without leg pain. We hypothesized that the presence of leg symptoms would moderate this relationship among older adults with chronic LBP, such that higher levels of epidural fat would be related to even lower pain and disability levels in those with, as compared with those without, leg symptoms.
Methods
Participants
Older adults, age 60 to 85 years, were recruited through local physician offices, community centers, and print advertisements between May 2009 and December 2011. Individuals were excluded if they had a progressive neurological disorder, an acute or terminal illness, or if they had severely limited mobility (i.e., needed an assistive device for household ambulation). Older adults with chronic LBP were specifically recruited for a parent clinical trial [11]. Adults with LBP were excluded from the clinical trial if they had 1) a history of low back surgery, 2) received services for LBP (e.g., physical therapy, chiropractic care, injections) within the past six months to eliminate residual effects from prior treatment, or 3) experienced a recent traumatic event. The 12-week clinical trial compared trunk muscle training plus neuromuscular electrical stimulation to a passive therapeutic intervention [11]. To be included in the chronic LBP groups, participants had to have LBP of at least moderate intensity, that is, 3 or greater out of 10, on at least four out of seven days of the week, and for at least three months. Potential participants with chronic LBP were excluded if they had symptoms of nonmechanical LBP or a prominent component of radicular pain with symptoms below the knee as this subcategory of patients with chronic LBP may benefit from a different clinical intervention [12]. The Institutional Review Board for Human Subjects Research at the University of Delaware approved this study.
Data Collection and Analysis
After undergoing the informed consent process, participants rated their baseline pain intensity, completed self-report questionnaires, and participated in a standardized clinical examination conducted by a licensed physical therapist. Pain intensity was accessed on a scale of 0 to 10, where 0 represented “no pain” and 10 represented “worst possible pain.” A composite pain rating was obtained by averaging current, best, and worst pain in the past 24 hours [13]. Individuals with LBP with associated symptoms distal to the gluteal fold were considered to have LBP + leg pain.
Self-Reported Measures
Self-report questionnaires included the modified Oswestry Low Back Disability Questionnaire (mODQ) and the Medical Outcomes SF-36. The mODQ has established reliability and validity among older adults with LBP [14] and is divided into 10 areas that assess limitations in activities of daily living; each item is scored on a scale of 0 to 5, with 5 indicating greater LBP-related disability [15]. The sum of the items is multiplied by two and presented as a percentage, where higher scores indicate greater disability [15]. The SF-36 estimates disease burden and can be divided into two subscales: the physical component summary score (PCS) and the mental component summary score [16]. Each subscale has a maximum score of 100, with 50 representing “average” health and scores above 50 indicating better health status [16]. For these analyses, we used the PCS to represent self-reported physical function.
Performance-Based Measures
Older adults have rated stairs as one of the top five daily challenges due to advanced age [17,18]. Further, stair performance is a determinant of physical performance and disability that requires greater exertion than other measures of mobility, such as gait speed or chair rise [19]. Participants were asked to ascend 12 steps (17 cm in height) as “quickly as possible, but safely.” Participants were allowed to use a handrail if needed for safety. The average of two timed trials, with at least a one minute recovery between trials, was determined.
Epidural Fat Assessment
Individuals received magnetic resonance imaging on a 1.5 Tesla scanner (Siemens MAGNETOM, Erlangen, Germany) with a flexible spine coil at a nearby imaging facility. T1-weighted, spin-echo images were produced in the axial plane (repetition time/echo time = 879/13 ms; field of view = 230 mm × 230 mm; encoding matrix = 480 × 640; phase encoding direction = anterior to posterior; bandwidth = 180; flip angle = 150 degrees; slice thickness = 5 mm with 1.5 mm gap; acquisition time = ∼8 minutes). T1-weighted images allow for differentiation of epidural fat from dural tissue with a high degree of specificity [20]. For older adults with chronic LBP, magnetic resonance imaging (MRI) was completed prior to treatment in the clinical trial.
Sagittal images were utilized to determine the axial image that best represented the disc space for levels L2/3 through L5/S1; of the 102 possible participants, only those with data at each of the levels of interest were included in this analysis. Using ImageJ software (Bethesda, MD, USA), diameter measurements were taken by drawing a line from the posterior to the anterior portion of the epidural fat located in the posterior vertebral canal at each level. To establish interexaminer measurement reliability for assessment of epidural fat, two examiners took measurements of epidural fat diameter (EFD) on 20 individuals with chronic LBP, selected at random. The examiners, one with postdoctoral training in MRI data acquisition and analysis and the other with no prior MRI training, met for 30 minutes prior to data analysis to discuss and practice the measurement technique. Each examiner was blinded to the measurements of the other examiner.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics 24 (Armonk, NY, USA). After checking for parametric assumptions, means and standard deviations (and minimums and maximums) for continuous demographic variables were calculated for controls, older adults with chronic LBP, and older adults with chronic LBP plus leg pain. Analysis of variance (and covariance when necessary) were used to evaluate between-group differences in participant demographics (P ≤ 0.050).
Two-way intraclass correlation coefficients (ICCs) with 95% confidence intervals (CIs) were calculated to estimate interexaminer measurement reliability of assessments of a single slice, that is, a single disc level (model 3,1), and assessments of the average of the four slices, that is, the average across disc levels (model 3,4), for 20 participants with chronic LBP. Standard errors of measurements (SEMs) were calculated.
Linear regression modeling was used to evaluate relationships between the amount of epidural fat averaged across vertebral levels L2/3 through L5/S1 (obtained by the novice examiner) and pain, self-report and performance-based measures of physical function, and LBP-related disability. Age, sex, and BMI, as suspected covariates, were entered into the first block of each model for each dependent variable. Average EFD and the two groups were entered into the second block of each model, while the third and final block explored interactions (P ≤ 0.050). The percent of the variance for each block, that is, the amount of additional variance explained above and beyond the prior block(s), is reported as the change in R2. Associations are graphed to illustrate relationships between epidural fat and physical function after controlling for covariates. A priori power analyses were not conducted because this was a secondary analysis of data that was previously collected. A post hoc power analysis indicated that for 91 individuals, a power of 0.80, an alpha of 0.05, an R2 change of 0.09 would be required to find the interaction significant in Block 3.
Results
There were 93 participants with magnetic resonance images available that contained disc levels L2/3 through L5/S1 for analysis; participant demographics are provided in Table 1. Of those who reported chronic LBP (N = 49), there were 25 who also reported concurrent leg pain, that is, buttock and/or thigh symptoms. Data from 44 controls were available for comparison. There were no statistically significant differences among the three groups for sex or race (P > 0.050). Adults with chronic LBP plus leg pain were younger than those with chronic LBP only (P = 0.035) and controls (P = 0.025). Individuals with LBP plus leg pain, however, had greater BMIs when compared with controls (P = 0.011) but not when compared with those with axial LBP only (P = 0.109); BMI was similar between controls (N = 44) and older adults with axial LBP without leg pain (P = 0.458). Controlling for age, there were no statistically significant differences in pain intensity or LBP-related disability among older adults with and without leg pain (P > 0.050).
Table 1.
Participant demographics
| Controls (N = 44) |
LBP (N = 24) |
LBP + Leg Pain (N = 25) |
||||
|---|---|---|---|---|---|---|
| No. (%) or M (SD) | Min–Max | No. (%) or M (SD) | Min–Max | No. (%) or M (SD) | Min–Max | |
| Sex, females* | 32 (72.7%) | 13 (54.2%) | 15 (60.0%) | |||
| Race, Caucasian* | 38 (86.4%) | 22 (91.7%) | 23 (92.0%) | |||
| Age, y | 71.4 (6.5) | 60–85 | 71.7 (6.8) | 62–83 | 67.8 (5.7) | 60–82 |
| Body mass index, kg/m2 | 26.8 (4.4) | 20.5–38.4 | 27.6 (4.8) | 21.2–40.0 | 29.7 (4.5) | 21.1–39.7 |
| Average LBP intensity, 0–10 | – | – | 3.2 (1.5) | 1.0–6.0 | 3.9 (1.7) | 1.3–8.6 |
| mODQ, % | – | – | 32.2 (10.0) | 12–50 | 36.1 (11.1) | 14–64 |
| SF-36 PCS, percentile | 89.6 (10.6) | 35.6–100.0 | 62.8 (19.3) | 36.3–91.3 | 58.0 (21.4) | 15.6–91.3 |
| Stair climb performance, sec | 4.7 (1.2) | 2.6–8.4 | 6.1 (2.7) | 1.9–13.0 | 5.5 (1.8) | 2.4–10.7 |
| Epidural fat diameter, mm | 3.8 (1.1) | 1.8–7.1 | 4.8 (1.6) | 0.5–8.1 | 4.7 (1.6) | 1.6–8.3 |
LBP = low back pain; mODQ = modified Oswestry Low Back Disability Questionnaire; SF-36 PCS = Short Form–36 Physical Component Summary Score.
Data presented as number of participants (% of sample).
Interexaminer measurement reliability for EFD measurements for each disc level, as well as for the average across levels L2/3 through L5/S1, are provided in Table 2. ICC point estimates for assessment of EFD for individual levels ranged from 0.63 to 0.93 among older adults with chronic LBP. When EFD assessments were averaged across disc levels, reliability was improved, as noted by a higher ICC point estimate (ICC = 0.95) and a narrower confidence interval (95% CI = 0.87–0.98).
Table 2.
Interexaminer reliability for epidural fat diameter measurements at each disc level and averaged across disc levels
| Level | EFD Mean (SD) | ICC (95% CI) | SEM |
|---|---|---|---|
| L2/L3 | 5.53 (3.18) | 0.93 (0.83–0.97) | 0.84 |
| L3/L4 | 5.70 (2.17) | 0.85 (0.67–0.94) | 0.84 |
| L4/L5 | 4.44 (2.29) | 0.63 (0.27–0.83) | 1.39 |
| L5/S1 | 3.07 (2.59) | 0.82 (0.60–0.92) | 1.09 |
| Average | |||
| L2/3-L5/S1 | 4.69 (1.57) | 0.95 (0.87–0.98) | 0.35 |
CI = confidence interval; EFD = epidural fat diameter; ICC = intraclass correlation coefficient; SEM= standard error of measurement.
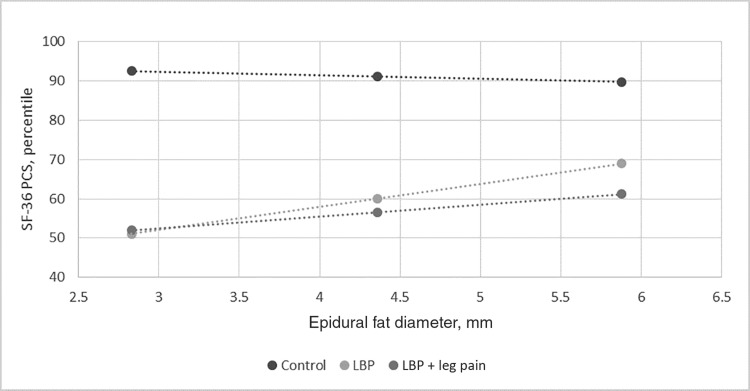
Table 3 shows how EFD relates to self-report physical function per the SF-36 PCS for each of the pain groups as compared with controls. Two controls were removed to meet the assumption of normality. Covariates collectively failed to explain any of the variance in Block 1 (although body mass index and age were significant: P = 0.029 and P = 0.036, respectively), while the addition of epidural fat in Block 2 explained 51% of the variance (per R2 change; P < 0.001) in self-reported function. Block 3, while nearly significant (P = 0.052), indicates that those with axial LBP only may have a relationship between epidural fat and self-report function that is statistically significantly different from controls (P = 0.015), while those with LBP plus leg pain do not (P = 0.170). Figure 1, which controls for covariates, illustrates that as EFD increases in those with chronic LBP, self-reported physical function may increase per the SF-36 PCS.
Table 3.
Model evaluating the contribution of epidural fat in explaining self-reported physical function for pain groups as compared with controls
| Short Form–36 PCS (N = 91) | Block Statistics |
Individual Predictor Statistics |
|||||
|---|---|---|---|---|---|---|---|
| R2 | AdjR2 | ΔR2 | P* | b | SE | P | |
| Block 1 | 0.07 | 0.04 | – | 0.109 | |||
| Age | –0.57 | 0.27 | 0.036 | ||||
| Male | 4.40 | 0.10 | 0.202 | ||||
| BMI | –0.76 | 0.34 | 0.029 | ||||
| Block 2 | 0.58 | 0.54 | 0.51 | <0.001 | |||
| EFD | –0.93 | 2.04 | 0.651 | ||||
| LBP | –60.76 | 12.31 | <0.001 | ||||
| LBP + leg pain | –51.71 | 13.05 | <0.001 | ||||
| Block 3 | 0.61 | 0.57 | 0.03 | 0.052 | |||
| LBP × EFD | 6.79 | 2.74 | 0.015 | ||||
| LBP + leg pain × EFD | 3.93 | 2.84 | 0.170 | ||||
Block 1: covariates; Block 2: Block 1 + EFD + pain groups; Block 3: Block 2 + interaction terms.
Adj = Adjusted; b = regression coefficient; EFD = epidural fat diameter; LBP = low back pain; PCS = physical component summary score.
*This P value is for the test in the change in R2, where the change in R2 represents the additional variance explained by a given block, above and beyond the prior block(s): .
Figure 1.
Self-reported physical function vs epidural fat diameter. LBP = low back pain; SF-36 PCS = Medical Outcomes Short Form–36 Health Survey physical component summary score.
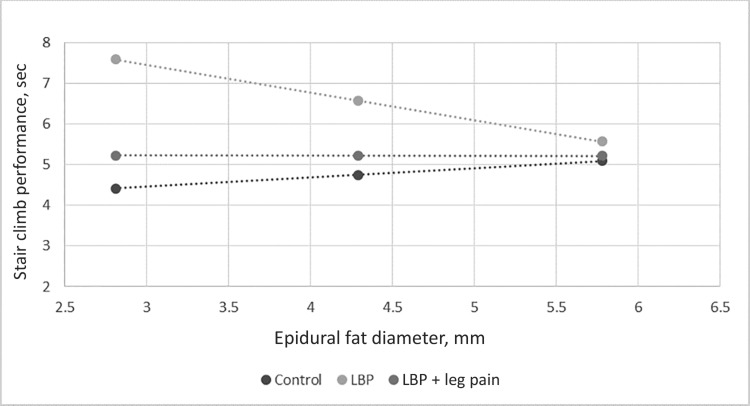
Table 4 shows how EFD relates to physical performance as assessed with stair climb performance for each of the pain groups as compared with controls. Two adults with LBP plus leg pain were removed to meet the assumption of normality. Covariates collectively explained 32% of the variance in Block 1 (with all covariates being independently statistically significant; P = 0.000 for all). In Block 2, epidural fat explained an additional 13% of the variance (P = 0.000), while interactions explained an additional 8% of the variance (P = 0.001). Relationships between stair climb performance and epidural fat were statistically significantly different from controls for those with LBP only (P = 0.000), but not for those with LBP plus leg pain (P = 0.366). Figure 2, which controls for covariates, shows that as EFD increases in older adults with chronic LBP, stair climb performance improves (as noted by decreased time).
Table 4.
Model evaluating the contribution of epidural fat in explaining physical performance for pain groups as compared with controls
| Stair Climb Performance (N = 91) | Block Statistics |
Individual Predictor Statistics |
|||||
|---|---|---|---|---|---|---|---|
| R2 | AdjR2 | ΔR2 | P* | b | SE | P | |
| Block 1 | 0.32 | 0.30 | – | <0.001 | |||
| Age | 0.113 | 0.02 | <0.001 | ||||
| Male | 4.40 | 0.10 | <0.001 | ||||
| BMI | –0.76 | 0.34 | <0.001 | ||||
| Block 2 | 0.46 | 0.42 | 0.14 | <0.001 | |||
| EFD | –0.93 | 0.18 | 0.212 | ||||
| LBP | 5.62 | 1.11 | <0.001 | ||||
| LBP + leg pain | 1.46 | 1.15 | 0.208 | ||||
| Block 3 | 0.54 | 0.50 | 0.08 | 0.001 | |||
| LBP × EFD | –0.91 | 0.25 | <0.001 | ||||
| LBP + leg pain × EFD | –0.23 | 0.25 | 0.366 | ||||
Block 1: covariates; Block 2: Block 1 + EFD + pain groups; Block 3: Block 2 + interaction terms.
Adj = Adjusted; b = regression coefficient; EFD = epidural fat diameter; LBP = low back pain.
*This P value is for the test in the change in R2, where the change in R2 represents the additional variance explained by given block, above and beyond the prior block(s): .
Figure 2.
Performance-based physical function vs epidural fat diameter. LBP = low back pain.
Models exploring the outcomes of average pain intensity and mODQ as they relate to epidural fat were not statistically significant for those with chronic LBP with or without leg pain (P > 0.050).
Discussion
Assessment of epidural fat was reliable between examiners for each of the vertebral levels based on ICC point estimates, but reliability improved and measurement error decreased when epidural fat was averaged across the vertebral levels. Results indicate that among older adults with chronic axial LBP, greater lumbar epidural fat is associated with better stair climb performance and may also be related to better self-reported physical function. Such relationships appear to differ from controls and may be unique to adults with chronic axial LBP as similar findings were not found in those with LBP plus leg pain. Epidural fat did not help to explain LBP intensity or LBP-related disability for older, community-dwelling adults with moderate, chronic LBP, regardless of leg pain presence. Results suggest that further longitudinal investigations of reduced epidural fat as it relates to physical function, particularly performance-based function, among older adults with chronic axial LBP (without leg pain) are warranted. Pending results of such investigations, clinical trials that seek to use regenerative techniques to restore spinal epidural fat may provide a means of improving clinical outcomes for older adults with chronic axial LBP.
The L4/5 disc level is prone to age-related, degenerative changes, such as narrowing of the spinal canal [10]. Structural spinal stenosis has been associated with decreased epidural fat [2,3]. Among our older adults with chronic LBP, epidural fat, which may help to protect the dural sac and spinal cord [1,2], was less at lower disc levels, that is, L4/5 and L5/S1, when compared with higher levels, that is, L2/3 and L3/4 (mean = 5.51 mm vs 3.75 mm). Age-related degenerative changes in spinal canal structure at L4/5, combined with reduced epidural fat, may make distinguishing small amounts of epidural fat from nearby structures more challenging than at adjacent levels with less degeneration and greater epidural fat; this may help to explain the lower reliability point estimates and greater SEMs for epidural fat measurements at lower lumbar levels.
Previous research has found that epidural fat is decreased with increased duration of LBP in middle-aged adults [4], but to our knowledge no studies have evaluated the relationships between epidural fat and LBP intensity, LBP-related disability, and/or physical function. LBP intensity and LBP-related disability were not significantly related to epidural fat for either LBP group in our study. Better self-reported and performance-based physical function was associated with increased epidural fat among those with chronic axial LBP (without leg pain), supporting the belief that epidural fat may be protective for this subgroup of patients [1,2].
Surprisingly, for those with chronic LBP with leg pain, epidural fat was not significantly related to physical function. In hindsight, we failed to consider that in some rare clinical conditions, such as spinal epidural lipomatosis (where there is excess adipose tissue deposition in the spinal canal, causing neural compression and perhaps radiculopathy, i.e., leg pain) [21], increased epidural fat may result in reduced physical function. Among those with buttock and thigh symptoms in our sample, it is also possible that leg symptoms were secondary to an undiagnosed hip condition [3,22] or other medical condition, for example, peripheral vascular disease, rather than related to the patient’s low back condition, particularly as those with prominent radicular presentation were excluded from the parent study. For example, hip osteoarthritis is known to cause symptoms in the buttocks and thighs [23]. As individuals in the study did not have hip radiographs (or receive ankle-brachial indices), we cannot exclude the possibility of hip osteoarthritis (or peripheral vascular disease) in these individuals with LBP plus leg pain.
Study Strengths and Limitations
A strength of the study was that the examiner who conducted MRI measurements was blinded to participant LBP intensity, leg pain status, and self-report and performance-based physical function data. This was, however, a secondary data analysis of available baseline MRI data from a clinical trial of older adults with nonspecific, chronic LBP; therefore, the pathoanatomical cause of LBP and/or leg pain is unknown (i.e., perhaps some participants had spinal epidural lipomatosis). Future studies may control for additional covariates that may impact epidural fat, such as comorbidities that result in endogenous steroid overproduction and long-term exogenous steroid use. Epidural fat measurements were conducted by the novice examiner, who was blinded to participant clinical data; it is possible that measurement precision would have been enhanced by using the more experienced examiner. We selected a single self-report measure and a single performance-based measure for this analysis, but future studies may evaluate additional measures to see if epidural fat helps to explain other measures of physical function. Inclusion of performance-based measures, which assess actual capacity, may be particularly important as our data suggest that relationships between epidural fat and physical performance may be stronger than relationships between epidural fat and self-perceived function, where individuals may over- or underestimate their capacity. Further, as this was a cross-sectional study, we are unable to determine if reduced epidural fat is predictive of reduced physical function in older adults with chronic axial LBP; this requires longitudinal investigation.
Conclusions
The average amount of epidural fat present in the posterior spinal canal can be reliably determined from T1-weighted lumbar spine MRIs in older adults with chronic LBP, even when measurements are taken by a novice examiner. The amount of epidural fat, averaged across vertebral levels, helps to explain stair climb performance and may help to explain self-reported physical function among older adults with chronic axial LBP without leg pain. Increased epidural fat is associated with better physical function among older adults with chronic LBP (without leg pain), supporting the theory that epidural fat may be protective in nature for this patient subgroup. No associations were found between epidural fat and LBP intensity and/or LBP-related disability for older adults with chronic LBP with or without leg pain. Future longitudinal studies may evaluate the relationships between epidural fat and physical function among patients with chronic axial LBP (without leg pain) to determine if reduced epidural fat is predictive of poorer outcomes, while controlling for potential confounding conditions. Such investigations are critical first steps before studies targeting regeneration of epidural fat are moved from animal to human models.
Funding sources: The work of Drs. Hicks and Sions was supported, in part, by R21 HD057274 (National Institute of Child Health and Human Development) and R01AG041202-01 (National Institute on Aging). The work of Christina Rodriguez was supported by the Delaware INBRE Program through P20 GM103446 (National Institute of General Medical Sciences). The work of Dr. Coyle was supported, in part, by T32 HD007490 (Eunice Kennedy Shriver National Institute of Child Health and Human Development).
Conflicts of interest: There are no conflicts of interest to declare related to this work.
Disclosure: This manuscript was submitted, in part, as C. Rodriguez’s thesis document at the University of Delaware in 2016. This work was presented, in part, as part of a platform presentation at the American Physical Therapy Association’s Combined Sections Meeting 2017 in San Antonio, Texas.
References
- 1. Beajeux R, Wolfram-Gabel R, Kehrli P, et al. Posterior lumbar epidural fat as a functional structure? Histologic specificities. Spine 1997;22:1264–8. [DOI] [PubMed] [Google Scholar]
- 2. Reina M, Franco C, Lopez A, De Andres J, Zundert A.. Clinical implications of epidural fat in the spinal canal. A scanning electron microscopic study. Acta Anaesthesiol Belg 2009;60:7–17. [PubMed] [Google Scholar]
- 3. Katz J, Harris M.. Lumbar spinal stenosis. N Engl J Med 2008;358:818–25. [DOI] [PubMed] [Google Scholar]
- 4. Prasartritha T, Suntisathaporn N, Vathana P, Sriphojanart C.. The size of the vertebral canal and the significance of epidural fat in lumbar spinal stenosis. J Med Assoc Thai 1997;80:247–56. [PubMed] [Google Scholar]
- 5. Lin C, Liu T, Chen M, Sun J, Chen M.. An injectable extracellular matrix for the reconstruction of epidural fat and the prevention of epidural fibrosis. Biomed Mater 2016;11:035010.. [DOI] [PubMed] [Google Scholar]
- 6. Xu J, Chen Y, Yue Y, Sun J, Cui L.. Reconstruction of epidural fat with engineered adipose tissue from adipose derived stem cells and PLGA in the rabbit dorsal laminectomy model. Biomaterials 2012;33:6965–73. [DOI] [PubMed] [Google Scholar]
- 7. Weiner K, Haggerty C, Kritchevsky B, et al. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC cohort and implications for the future. Pain Med 2003;4:311–20. [DOI] [PubMed] [Google Scholar]
- 8. Lavsky-Shulan M, Wallace R, Kohout F, et al. Prevalence and functional correlates of low back pain in the elderly: The Iowa 65+ Rural Health Study. J Am Geriatr Soc 2015;23:23–8. [DOI] [PubMed] [Google Scholar]
- 9. Vogt M, Lauerman W, Chirumbole M, Kuller L.. A community-based study of postmenopausal white women with back and leg pain: Health status and limitations in physical activity. J Gerontol A Biol Sci Med Sci 2002;57:M544–50. [DOI] [PubMed] [Google Scholar]
- 10. Kim K, Park J, Kuh S, et al. Changes in spinal canal diameter and vertebral body height with age. Yonsei Med J 2013;54:1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hicks GE, Sions JM, Velasco TO, Manal TJ.. Trunk muscle training augmented with neuromuscular electrical stimulation appears to improve function in older adults with chronic low back pain: A randomized preliminary trial. Clin J Pain 2016;3210:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delitto A, George S, Van Dillan L, et al. Low back pain. J Orthop Sports Phys Ther 2012;42:A1–A57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen MP, McFarland CA.. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 1993;55:195–203. [DOI] [PubMed] [Google Scholar]
- 14. Hicks G, Manal T.. Psychometric properties of commonly used low back disability questionnaires: Are they useful for older adults with low back pain? Pain Med 2009;10:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fritz J, Irrgang J.. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther 2001;81:776–88. [DOI] [PubMed] [Google Scholar]
- 16. Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders: Summary and general recommendations. Spine 2000;25:3100–3. [DOI] [PubMed] [Google Scholar]
- 17. Startzell J, Owens D, Mulfinger L, Cavanagh P.. Stair negotiation in older people: A review. J Am Geriatr Soc 2000;48:567–80. [DOI] [PubMed] [Google Scholar]
- 18. Williamson J, Fried L.. Characterization of older adults who attribute functional decrements to “old age.” J Am Geriatr Soc 1996;44:1429–34. [DOI] [PubMed] [Google Scholar]
- 19. Bean J, Kiely D, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc 2002;50:461–7. [DOI] [PubMed] [Google Scholar]
- 20. Fogel GR, Cunningham PY 3rd, Esses SI.. Spinal epidural lipomatosis: Case reports, literature review and meta-analysis. Spine J 2005;5:202–11. [DOI] [PubMed] [Google Scholar]
- 21. Al-Khawaja D, Seex K, Eslick GD.. Spinal epidural lipomatosis—a brief review. J Clin Neurosci 2008;15:1323–6. [DOI] [PubMed] [Google Scholar]
- 22. Rho M, Camacho-Soto A, Cheng A, et al. Deconstructing chronic low back pain in the older adult-step by step evidence and expert-based recommendations for evaluation and treatment. Part VIII: Lateral hip and thigh. Pain Med 2016;177:1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan A, McLoughlin E, Giannakas K, Hutchinson C, Andrew J.. Hip osteoarthritis: Where is the pain? Ann R Coll Surg Engl 2004;86:119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]