Abstract
Objective
Examine the longitudinal association between knee pain and prefrailty/frailty.
Design
Longitudinal study.
Setting
Five clinical centers across the United States.
Subject
Data from 3,053 nonfrail participants aged 45–79 years at baseline from the Osteoarthritis Initiative.
Methods
According to self-reported knee pain at baseline, the participants were placed into three groups: no knee pain (N = 1,600), unilateral knee pain (N = 822), and bilateral knee pain (N = 631). Frailty status was assessed over time using the five frailty indicators (unintentional weight loss, exhaustion, weak energy, slow gait speed, and little physical activity). Based on the number of frailty indicators present, prefrailty (1–2) and frailty (≥3) were diagnosed. Generalized estimating equations logistic regression analyses were conducted to examine the relationship between knee pain status and prefrailty/frailty.
Results
After adjusting for age, sex, race, education, marital status, smoking status, comorbidities, and body mass index, unilateral knee pain at baseline was associated with an increased odds of developing prefrailty (odds ratio [OR] = 1.14, 95% confidence interval [CI] = 1.01–1.27) and frailty (OR = 1.89, 95% CI = 1.38–2.62), and bilateral knee pain at baseline was also associated with an increased risk of prefrailty (OR = 1.41, 95% CI = 1.24–1.62) and frailty (OR = 2.21, 95% CI = 1.63–3.01) over time in comparison with no knee pain. The interaction of knee pain status by time was not significantly associated with either prefrailty or frailty.
Conclusions
Knee pain (particularly bilateral knee pain) is associated with an increased risk of developing prefrailty and frailty over time.
Keywords: Knee Pain, Frailty, Geriatrics, Longitudinal Study
Introduction
Knee pain (KP) is the most common symptom of knee osteoarthritis (OA), affecting more than 100 million individuals worldwide [1,2]. There is evidence that the major risk factor for KP is obesity, and given the rising prevalence of obesity and an aging population, the incidence of KP has also increased [3,4]. Several studies have found that KP leads to functional limitation, disability, and falls in older adults [5–9]. Other studies have demonstrated that KP is the most common predictor of future knee OA [10].
Frailty is a geriatric syndrome that is strongly associated with disability and other adverse outcomes [11–14]. The prevalence of frailty differs geographically (6.9%), with a range from 4% to 17% in older adults [15–18]. Thus, the increasing aging population with health conditions like KP and frailty poses a significant public health problem.
A number of studies have demonstrated a significant relationship between OA and frailty [19–21]. A population-based prospective cohort study investigated whether the presence of OA-related pain increased the risk of developing frailty compared with no OA-related pain in community-dwelling older adults [22]. The study demonstrated that people with knee OA-related pain had an increased risk of developing frailty. However, it is unclear if KP is associated with an increased risk of prefrailty and frailty compared with healthy cohorts (who have neither knee OA nor pain).
The purpose of the current study was to examine the longitudinal association between KP status and prefrailty/frailty over six years. We hypothesized that KP would be associated with an increased risk of prefrailty and frailty.
Methods
Data Source
The Osteoarthritis Initiative (OAI) is a publicly and privately funded multicenter, longitudinal, prospective observational study of knee OA. The study data were collected over time. The participants (N = 4,796) were between the ages of 45 and 79 years at baseline (2004–2006) and included the following three subcohorts: 1) the progression subcohort consisted of 1,390 subjects with symptomatic tibiofemoral knee OA in at least one native knee at baseline; 2) the incidence subcohort had 3,284 subjects with an elevated risk of developing symptomatic knee OA during the study; and 3) the control subcohort consisted of 122 subjects with no pain, aching, or stiffness, no radiographic findings of osteoarthritis, and no risk factors for OA in either knee in the past year.
The OAI study design and data collection have been described previously [23]. Access to the study database is available for free at http://www.oai.ucsf.edu. The study was approved (approval numbers: FWA00000068) by the Committee on Human Research at the University of California, San Francisco. Before enrollment into the study, all participants provided written informed consent.
Setting
The OAI study enrolled men and women from the following five clinical centers across the United States: 1) Ohio State University, University of Maryland School of Medicine, and Johns Hopkins University School of Medicine comprised a single recruitment center in Baltimore; 2) University of Pittsburgh School of Medicine; 3) Brown University School of Medicine and Memorial Hospital of Rhode Island; 4) Pawtucket University; and 5) University of California, San Francisco, School of Medicine. The enrollment was carried out between February 2004 and May 2006. At baseline, prior to the enrollment, initial eligibility of the individuals was assessed by staff via telephone, a screening clinic visit, and an enrollment clinic visit.
Participants
The data from 3,053 nonfrail participants aged 45–79 years at baseline were collected from the Osteoarthritis Initiative (OAI) database. The specific data sets used included the following version numbers: 0.2.2, 1.2.1, 3.2.1, 5.2.1, 6.2.1, and 8.2.1. According to self-reported KP status at baseline, the participants were placed into one of three groups: no KP, unilateral KP, or bilateral KP. Participants with missing information at any of the follow-ups were significantly more likely to be older, unmarried, obese, have less than a high school education, and have a higher score on the Physical Activity Scale for the Elderly (PASE) compared with those participants without missing information (P < 0.05).
Self-reported Knee Pain Status at Baseline
We defined participants as having unilateral KP if they answered “yes” to the question “During the past 12 months, have you had any pain, aching, or stiffness in or around your right (or left) knee?” and to the question “During the past 12 months, have you had any pain, aching, or stiffness in or around your right (or left) knee on most days for at least one month?”
Bilateral KP was considered to be present if participants answered “yes” to the question “During the past 12 months, have you had any pain, aching, or stiffness in or around your right (and left) knee?” and to the question “During the past 12 months, have you had any pain, aching, or stiffness in or around your right (and left) knee on most days for at least one month?” The follow-up question for each knee was used to assess the pain status at baseline.
The participants were determined to have no KP if they answered “no” to the question “During the past 12 months, have you had any pain, aching, or stiffness in or around your right (or left) knee?” No KP was used as the reference group. Similar questions have been used in other longitudinal and population-based studies [24,25].
Outcome
Frailty status was assessed over time (at baseline and at one, two, three, four, and six years) according to the frailty phenotype criteria (weight loss, exhaustion, weak energy, slow gait speed, and little physical activity) developed by Fried and colleagues [26]. Participants with unintentional weight loss of ≥5% from the previous visit were categorized as positive for weight loss (score = 1). For the baseline assessment, an unintentional weight loss of ≥5% from baseline to the subsequent follow-up visit was considered positive. Exhaustion was assessed using two items from the Center for Epidemiologic Studies–Depression (CES-D) scale: “How often have you felt that everything required considerable effort during the past week?” and “How often could you not get going during the past week?” The questions then asked, “Please tell me how often you felt that way,” with 0 = rarely or none of the time (less than one day), 1 = some of the time (one to two days), 2 = much of the time (three to four days), or 3 = most of the time (five to seven days) [27]. Subjects answering “2” or “3” to either of these two items were categorized as positive for the exhaustion criterion (score = 1). Weak energy was assessed using the 12-item short form health survey: “How often have you had a lot of energy in the past four weeks?” with answer options including 1 = all of the time, 2 = most of the time, 3 = some of the time, 4 = a little of the time, and 5 = none of the time [21]. Participants answering “4” or “5” were categorized as positive for the weak energy criterion (score = 1). Gait speed (meters/second) was assessed using a timed 20-meter walk test with a stopwatch. Participants walked a comfortable, self-directed pace in a corridor. Those who walked slower than 1 m/s or were unable to perform the test were categorized as positive for the low gait speed criterion (score = 1). Participants who scored in the lowest 20% of the PASE, adjusted for gender, were categorized as positive for the low physical activity criterion (score = 1). The overall frailty score ranged from 0 to 5. According to the frailty phenotype criteria score, either prefrailty (score = 1or 2) or frailty (score ≥ 3) was determined to be present.
Baseline Covariates
Baseline demographic, social, and health characteristics were assessed using self-reported questionnaires. Based on the literature and biologic plausibility, we included the following sociodemographic covariates: age (continuous), sex (female vs male), race (Caucasian vs African American or Asian), education (high school or less vs high school or more), marital status (married vs unmarried/divorced), and smoking status (current or former smoker vs nonsmoker). Medical comorbidities (>1 vs 0) were assessed using the Charlson Comorbidity Index [28], and body mass index (BMI; continuous) [29] was included.
Data Analysis
The three groups’ (no KP, unilateral KP, and bilateral KP) baseline descriptive statistics were summarized using mean ± standard deviation for continuous variables and percentage for categorical variables. We use a one-way analysis of variance for continuous variables and a chi-square test for categorical variables to examine the association between the three groups at baseline. According to KP status at baseline, the percentage (%) of individuals with prefrailty and frailty was plotted over time (at baseline and at one, two, three, four, and six years). To examine whether the KP status at baseline was associated with prefrailty and frailty over time, generalized estimating equations (GEEs) were fitted using the GENMOD procedure in statistical analysis software (SAS; SAS Institute, Inc., Cary, NC, USA) [30].
Two GEE logistic regression models for each group were used to examine the association between KP status and prefrailty/frailty as well as the interaction between KP status and time (representing the difference between the three groups and prefrailty/frailty over time). Model 1 in each cluster included KP status and interaction between KP status and time. Model 2 in each group was adjusted for model 1 plus age, sex, race, education, marital status, smoking status, comorbidities, and BMI. Odds ratios (ORs) and 95% confidence intervals (CIs) were presented for prefrailty and frailty at different levels of KP, using no KP as the reference group. Time was treated as a continuous variable. SAS [31] version 9.2 (SAS Institute, Inc., Cary, NC, USA) was used to perform all analyses.
Results
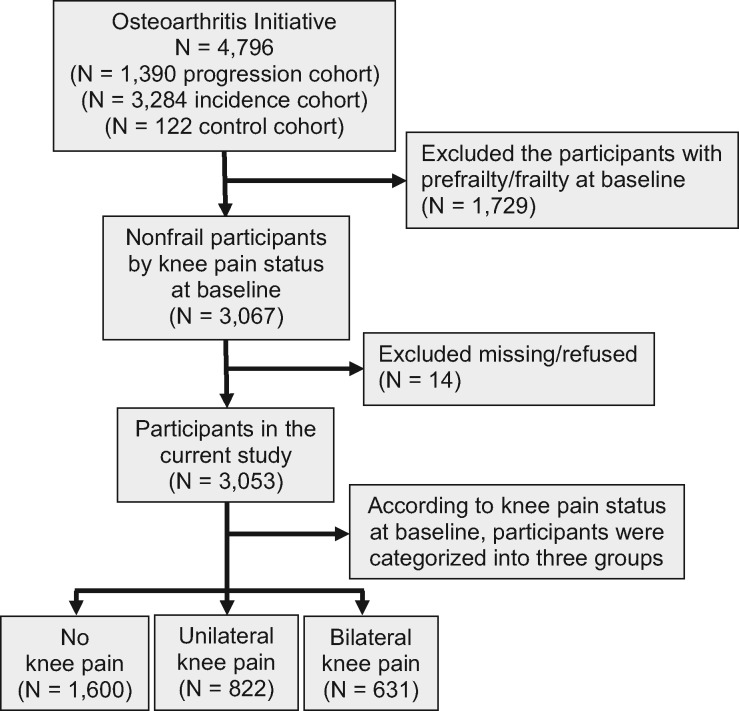
Figure 1 shows the flow chart of the study participants. Out of 4,796 participants at baseline, 3,053 nonfrail participants were included in the current study. Participants who were already frail (N = 1,729) or who had missing data (N = 14) were excluded from the analyses. Out of the 3,053 participants, 1,600 participants did not have KP (52%), 822 participants had unilateral KP (27%), and 631 participants had bilateral KP (21%).
Figure 1.
The flow chart of the study participants.
Table 1 shows the descriptive statistics of the study participants according to KP status (and for the overall sample). At baseline, participants with unilateral and bilateral KP were younger (P < 0.01 for trend), had a higher BMI (P < 0.01 for trend), were less educated, and were less likely to be Caucasian than those without knee pain (see Table 1). Participants without KP were more likely to have comorbidities than those with unilateral or bilateral KP.
Table 1.
Descriptive characteristics of the sample by knee pain status among nonfrail participants at baseline
| Characteristics | All (N = 3,053) | No KP (N = 1,600, 52%) | Unilateral KP (N = 822, 27%) | Bilateral KP (N = 631, 21%) | P |
|---|---|---|---|---|---|
| Age, mean ± SD, y | 59.9 ± 8.8 | 61.1 ± 8.9 | 59.7± 8.9 | 59.0 ± 8.5 | <0.001 |
| BMI, mean ± SD, kg/m2 | 28.3 ± 4.6 | 27.6 ± 4.5 | 28.6 ± 4.5 | 28.8 ± 4.7 | <0.001 |
| Female, No. (%) | 1,672 (55) | 897 (54) | 420 (25) | 355 (21) | 0.047 |
| Caucasians or white, No. (%) | 2,564 (84) | 1,424 (55) | 668 (26) | 472 (18) | <0.001 |
| High school or more, No. (%) | 2,673 (87) | 1,423 (53) | 716 (27) | 534 (20) | 0.019 |
| Married, No. (%) | 2,191 (72) | 1,183 (54) | 598 (27) | 410 (19) | <0.001 |
| Current or former smoker, No. (%) | 1,378 (45) | 700 (51) | 384 (28) | 294 (21) | 0.27 |
| Comorbidity >1, No. (%) | 427 (15) | 192 (45) | 132 (31) | 103 (24) | 0.002 |
| PASE, mean ± SD | 157.5 ± 83.5 | 156.7 ± 76.5 | 159.0 ± 85.3 | 156.7 ± 88.6 | 0.32 |
BMI = body mass index; KP = knee pain; PASE = physical activity scale for the elderly.
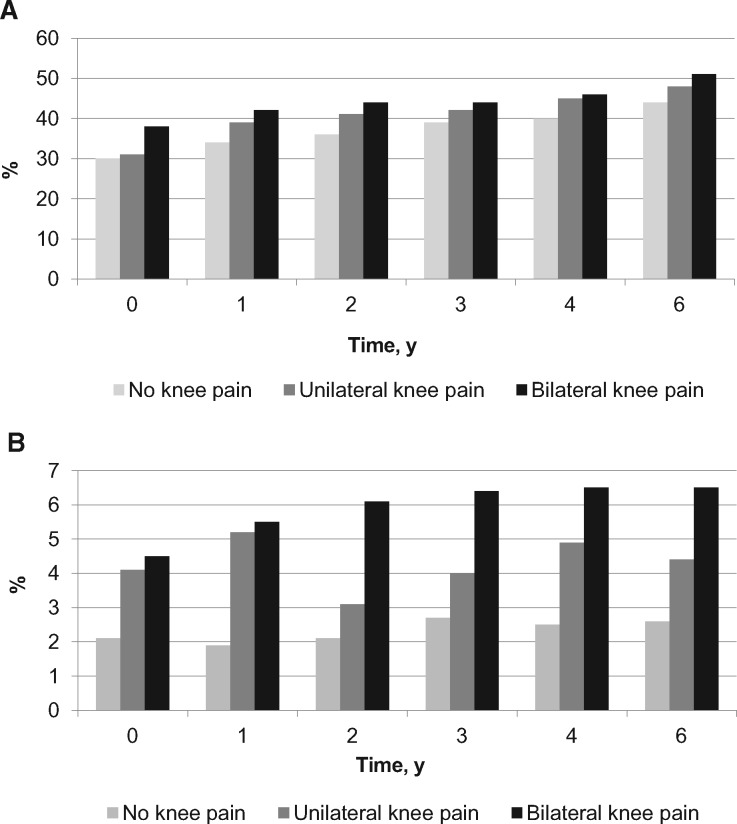
Figure 2 shows the percentage of individuals who developed prefrailty and frailty over time by baseline KP status. The frequency of prefrailty increased over time from 31% to 48% in those with unilateral KP, whereas it increased from 38% to 51% in those with bilateral KP over six years. Compared with those with no KP, the rate of frailty occurring per 1,000 person-years was increased by 0.4% (53 events per 1,000 person-years) in those with unilateral KP, whereas it was increased by 2% in those with bilateral KP (41 events per 1,000 person-years).
Figure 2.
Percentages of (A) prefrailty and (B) frailty over time by knee pain status at baseline.
Table 2 presents GEE models testing the relationship between KP status and odds of prefrailty and frailty over time. In model 1, participants with unilateral KP were significantly more likely to become prefrail (OR = 1.16, 95% CI = 1.01–1.31, P = 0.025) and frail (OR = 1.90, 95% CI = 1.37–2.63, P = 0.001), and participants with bilateral KP were also more likely to become prefrail (OR = 1.40, 95% CI = 1.23–1.60, P < 0.001) and frail (OR = 2.20, 95% CI = 1.62–3.00, P < 0.001) over time. In model 2, the association remained significant, indicating that the odds of becoming prefrail (OR = 1.14, 95% CI = 1.01–1.27, P = 0.022) and frail (OR = 1.89, 95% CI = 1.38–2.62, P = 0.001) with unilateral KP were higher, and the odds of becoming prefrail (OR = 1.41, 95% CI = 1.24–1.62, P < 0.001) and frail (OR = 2.21, 95% CI = 1.63–3.01, P < 0.001) with bilateral KP were also higher after adjusting for age, sex, race, education, marital status, smoking status, comorbidities, and BMI. There was no significant association between KP status at the different time points and prefrailty and frailty over time.
Table 2.
Generalized estimating equations logistic regression analyses of association between knee pain status among nonfrail participants at baseline and prefrailty/frailty over time
| Knee Pain, Past 12 Months | Prefrailty |
Frailty |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Model 1* | ||||
| No knee pain (reference) | 1.00 | 1.00 | ||
| Unilateral | 1.16 (1.01–1.31) | 0.025 | 1.90 (1.37–2.63) | 0.001 |
| Bilateral | 1.40 (1.23–1.60) | <0.001 | 2.20 (1.62–3.00) | <0.001 |
| Unilateral* time | 1.01 (0.98–1.03) | 0.40 | 0.96 (0.88–1.05) | 0.36 |
| Bilateral* time | 0.97 (0.94–0.99) | 0.54 | 1.00 (0.93–1.08) | 0.84 |
| Model 2† | ||||
| No knee pain (reference) | 1.00 | 1.00 | ||
| Unilateral | 1.14 (1.01–1.27) | 0.022 | 1.89 (1.38–2.62) | 0.001 |
| Bilateral | 1.41 (1.24–1.62) | <0.001 | 2.21 (1.63–3.01) | <0.001 |
| Unilateral* time | 0.95 (0.96–1.03) | 0.29 | 0.96 (0.87–1.03) | 0.37 |
| Bilateral* time | 0.97 (0.93–0.98) | 0.99 | 1.00 (0.93–1.08) | 0.86 |
OR = odds ratio; CI = confidence interval.
Model 1: levels of knee pain and interaction between levels of knee pain and time.
Model 2: adjusted for model 1 plus age, sex, race, education, marital status, smoking status, comorbidity, and body mass index.
Discussion
The current study examined the longitudinal association between KP status and prefrailty/frailty over six years of follow-up. Our findings suggest that the presence of either unilateral or bilateral KP was associated with an increased odds of prefrailty and frailty over a period of six years. Bilateral KP was more strongly associated with the development of frailty than was unilateral KP, and those with bilateral KP were more than two times more likely to develop frailty compared with those with no KP.
In this study, participants without KP were more likely to have medical comorbidities. Age likely contributed to this finding, as participants without KP were older than those with either unilateral or bilateral KP. The terms comorbidity, frailty, and disability are commonly used interchangeably to describe vulnerable older adults [32]. However, there is a growing consensus that these are distinct clinical entities that are causally related [33]. Thus, lifelong health improvement and disease prevention activities can prevent or slow the onset of comorbidities or frailty in aging people.
Our findings related to the incidence of frailty (4 to 50 cases per 1,000 person-years) agree with the small number of published longitudinal studies investigating knee OA and frailty published to date. For example, the combined Multicenter Osteoarthritis (MOST) and Osteoarthritis Initiative (OAI) study examined the association between knee OA and frailty over four years in community-dwelling older adults [21]. This study demonstrated that knee OA was associated with an increased risk of developing frailty. Other recent publications have also found an independent association between knee OA and prefrailty or frailty [20,34]. However, these studies did not specifically examine the association between KP and frailty, a relationship that has not been thoroughly explored.
Similar to our study, a population-based cohort study over 4.4 years demonstrated that OA-related pain influences the relationship between OA and the risk of frailty [9]. A cross-sectional analysis in the Canadian Study of Health and Aging-Wave 2 found that with the presence of KP compared with mild or no KP, the risk of developing prefrailty or frailty was higher by twofold and fivefold, respectively, in community-dwelling older adults [35]. Lee and colleagues examined the frailty status and associated factors in older Chinese adults [36]. The study suggested that age and knee OA were strongly associated with frailty status. Another recent study revealed that the incidence of frailty was significantly influenced by marital status [37]. However, our study differs from these studies. For example, our study examined the relationship between KP related to the onset (or elevated risk) of knee OA and prefrailty and frailty compared with the healthy cohort. Moreover, we attempted to delineate the specific impact of KP by stratifying our sample by no KP, unilateral KP, or bilateral KP. Also, we found a significant association between frailty and KP, age, sex, race, education, marital status, smoking status, comorbidities, and BMI. The relationship remained strong between bilateral KP and frailty, suggesting that this is an at-risk group.
The potential underlying mechanisms that may account for the relationship between KP and frailty have not yet been elucidated. KP is known to be associated with a reduction in physical activity levels [38], which may result in reduced muscle mass and increased susceptibility for an older person to developing disability [8] and difficulty with activities of daily living [39]. Sedentary behavior is also associated with a heightened inflammatory marker profile in older age [40], a factor that is associated with sarcopenia [41] and frailty [42]. Physical activity is known to be beneficial in reducing lower limb pain symptoms [43], inflammatory markers [44], and frailty [45]; thus, interventions helping people with KP to be more active may be a viable and important future research topic.
KP and frailty are two pervasive geriatric conditions that create an enormous burden in an expanding population of older adults [7,46]. Only a limited amount of research has attempted to address or prevent prefrailty or frailty in older adults with KP. Thus, the health care practitioner’s understanding of the relationship between these conditions is important in developing and promoting quality interdisciplinary geriatric clinical care to address the goals of Healthy People 2010 [47].
Our study had several strengths, including the use of data from a prospective, multicenter, publicly and privately funded ongoing osteoarthritis initiative study database, the study’s longitudinal design, the use of well-defined and validated assessments of frailty status, and the exploration of the potential role of KP status in increasing the risk of developing prefrailty and frailty. Our findings are generalizable because the participants included both older men and women with diverse ethnicities from different geographical regions in the United States.
This study also had limitations that should be acknowledged. First, KP status was defined using self-reported questionnaires, and this can introduce bias and lead to misclassification [48]. Second, we excluded the participants with frailty at baseline. This may have led to an underestimation of the relationship between KP status and odds of frailty. Third, the comorbidities score was based on self-report by the individuals. However, there was good agreement between the self-reported medical conditions and the medical diagnoses [49].
Conclusions
KP status was positively associated with an increased odds of prefrailty and frailty over a six-year period compared with lack of KP. Bilateral KP was more strongly associated with the development of frailty. Thus, health care systems should develop effective interventions directed toward these two common geriatric conditions, both for prevention and treatment purposes. Future studies should further explore the prevention of frailty (such as by increasing physical activity) to determine if improving KP symptoms can prevent prefrailty or frailty.
Author Contributions
SB: study concept and design; SB and VV: acquisition of data, analysis, interpretation of data, and drafting the manuscript; SB and BS: revised the manuscript for important intellectual content. All authors approved the final version for publication. All authors meet ICMJE criteria, and all those who fulfilled those criteria are listed as authors.
Acknowledgments
The OAI is a public-private partnership comprised of five contracts (N01-AR-2–2258; N01-AR-2–2259; N01-AR-2–2260; N01-AR-2–2261; N01-AR-2–2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer, Inc. The Foundation manages private sector financing for the OAI for the National Institutes of Health.
Funding sources: The authors extend their appreciations to the Deanship of Scientific Research at King Saud University for funding this work through Research Group No. RG-1438-085.
References
- 1. Guedes V, Castro JP, Brito I.. Topical capsaicin for pain in osteoarthritis: A literature review. Reumatol Clin In press. [DOI] [PubMed] [Google Scholar]
- 2. Bhatia D, Bejarano T, Novo M.. Current interventions in the management of knee osteoarthritis. J Pharm Bioallied Sci 2013;5(1):30–8. 10.4103/0975-7406.106561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marks R. Obesity profiles with knee osteoarthritis: Correlation with pain, disability, disease progression. Obesity (Silver Spring) 2007;15(7):1867–74. 10.1038/oby.2007.221 [DOI] [PubMed] [Google Scholar]
- 4. Blagojevic M, Jinks C, Jeffery A, Jordan KP.. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthritis Cartilage 2010;18(1):24–33. 10.1016/j.joca.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 5. Farrokhi S, Chen YF, Piva SR, et al. The influence of knee pain location on symptoms, functional status, and knee-related quality of life in older adults with chronic knee pain: Data from the osteoarthritis initiative. Clin J Pain 2016;32(6):463–70. 10.1097/AJP.0000000000000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vennu V, Bindawas SM.. Relationship between falls, knee osteoarthritis, and health-related quality of life: Data from the osteoarthritis initiative study. Clin Interv Aging 2014;9:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bindawas SM, Vennu V, Al Snih S.. Differences in health-related quality of life among subjects with frequent bilateral or unilateral knee pain: Data from the osteoarthritis initiative study. J Orthop Sports Phys Ther 2015;45(2):128–36. 10.2519/jospt.2015.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eggermont LH, Leveille SG, Shi L, et al. Pain characteristics associated with the onset of disability in older adults: The maintenance of balance, independent living, intellect, and zest in the Elderly Boston Study. J Am Geriatr Soc 2014;62(6):1007–16. 10.1111/jgs.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stubbs B, Binnekade T, Eggermont L, et al. Pain and the risk for falls in community-dwelling older adults: Systematic review and meta-analysis. Arch Phys Med Rehabil 2014;95(1):175–87.e9. [DOI] [PubMed] [Google Scholar]
- 10. Hannan MT, Felson DT, Pincus T.. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol 2000;27(6):1513–7. [PubMed] [Google Scholar]
- 11. Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A consensus report. J Am Geriatr Soc 2004;52(4):625–34. 10.1111/j.1532-5415.2004.52174.x [DOI] [PubMed] [Google Scholar]
- 12. De Rui M, Veronese N, Trevisan C, et al. Changes in frailty status and risk of depression: Results from the Progetto Veneto Anziani Longitudinal Study. Am J Geriatr Psychiatry 2017;25(2):190–7. 10.1016/j.jagp.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 13. Trevisan C, Veronese N, Maggi S, et al. Factors influencing transitions between frailty states in elderly adults: The Progetto Veneto Anziani Longitudinal Study. J Am Geriatr Soc 2017;65(1):179–84. 10.1111/jgs.14515 [DOI] [PubMed] [Google Scholar]
- 14. Veronese N, Stubbs B, Fontana L, et al. Frailty is associated with an increased risk of incident type 2 diabetes in the elderly. J Am Med Dir Assoc 2016;17(10):902–7. 10.1016/j.jamda.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 15. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC.. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc 2012;60(8):1487–92. 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 16. Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J.. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci 2009;64(6):675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avila-Funes JA, Helmer C, Amieva H, et al. Frailty among community-dwelling elderly people in France: The three-city study. J Gerontol A Biol Sci Med Sci 2008;63(10):1089–96. 10.1093/gerona/63.10.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Espinoza SE, Hazuda HP.. Frailty prevalence and neighborhood residence in older Mexican Americans: The San Antonio longitudinal study of aging. J Am Geriatr Soc 2015;63(1):106–11. 10.1111/jgs.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drey M, Wehr H, Wehr G, et al. The frailty syndrome in general practitioner care: A pilot study. Z Gerontol Geriatr 2011;44(1):48–54. 10.1007/s00391-010-0136-3 [DOI] [PubMed] [Google Scholar]
- 20. Miguel Rde C, Dias RC, Dias JM, et al. Frailty syndrome in the community-dwelling elderly with osteoarthritis. Rev Bras Reumatol 2012;52(3):331–47. [PubMed] [Google Scholar]
- 21. Misra D, Felson DT, Silliman RA, et al. Knee osteoarthritis and frailty: Findings from the multicenter osteoarthritis study and osteoarthritis initiative. J Gerontol A Biol Sci Med Sci 2015;70(3):339–44. 10.1093/gerona/glu102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Veronese N, Maggi S, Trevisan C, et al. Pain increases the risk of developing frailty in older adults with osteoarthritis. Pain Med 2017;18:414–27. [DOI] [PubMed] [Google Scholar]
- 23.The osteoarthritis initiative: Protocol for the cohort study. Available at: http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf (accessed February 2017).
- 24. Blake VA, Allegrante JP, Robbins L, et al. Racial differences in social network experience and perceptions of benefit of arthritis treatments among New York City Medicare beneficiaries with self-reported hip and knee pain. Arthritis Rheum 2002;47(4):366–71. 10.1002/art.10538 [DOI] [PubMed] [Google Scholar]
- 25. Creamer P, Lethbridge-Cejku M, Costa P, et al. The relationship of anxiety and depression with self-reported knee pain in the community: Data from the Baltimore Longitudinal Study of Aging. Arthritis Care Res 1999;12(1):3–7. [DOI] [PubMed] [Google Scholar]
- 26. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 27. Tomitaka S, Kawasaki Y, Furukawa T.. A distribution model of the responses to each depressive symptom item in a general population: A cross-sectional study. BMJ Open 2015;5(9):e008599.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong CH, Weiss D, Sourial N, et al. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: A cross-sectional study. Aging Clin Exp Res 2010;22(1):54–62. 10.1007/BF03324816 [DOI] [PubMed] [Google Scholar]
- 29. Garcia-Esquinas E, Jose Garcia-Garcia F, Leon-Munoz LM, et al. Obesity, fat distribution, and risk of frailty in two population-based cohorts of older adults in Spain. Obesity (Silver Spring) 2015;23(4):847–55. [DOI] [PubMed] [Google Scholar]
- 30. Chang YC. Residuals analysis of the generalized linear models for longitudinal data. Stat Med 2000;19(10):1277–93. [DOI] [PubMed] [Google Scholar]
- 31. By K, Qaqish BF, Preisser JS, Perin J, Zink RC.. ORTH: R and SAS software for regression models of correlated binary data based on orthogonalized residuals and alternating logistic regressions. Comput Methods Programs Biomed 2014;113(2):557–68. 10.1016/j.cmpb.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 32. van den Akker M, Buntinx F, Metsemakers JF, Roos S, Knottnerus JA.. Multimorbidity in general practice: Prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol 1998;51(5):367–75. [DOI] [PubMed] [Google Scholar]
- 33. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G.. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59(3):255–63. [DOI] [PubMed] [Google Scholar]
- 34. Castell MV, van der Pas S, Otero A, et al. Osteoarthritis and frailty in elderly individuals across six European countries: Results from the European Project on OSteoArthritis (EPOSA). BMC Musculoskelet Disord 2015;16(1):359.. 10.1186/s12891-015-0807-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shega JW, Dale W, Andrew M, et al. Persistent pain and frailty: A case for homeostenosis. J Am Geriatr Soc 2012;60(1):113–7. 10.1111/j.1532-5415.2011.03769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee JS, Auyeung TW, Leung J, Kwok T, Woo J.. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc 2014;15(4):281–6. 10.1016/j.jamda.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 37. Trevisan C, Veronese N, Maggi S, et al. Marital status and frailty in older people: Gender differences in the Progetto Veneto Anziani Longitudinal Study. J Womens Health (Larchmt) 2016;25(6):630–7. 10.1089/jwh.2015.5592 [DOI] [PubMed] [Google Scholar]
- 38. Stubbs B, Binnekade TT, Soundy A, et al. Are older adults with chronic musculoskeletal pain less active than older adults without pain? A systematic review and meta-analysis. Pain Med 2013;14(9):1316–31. 10.1111/pme.12154 [DOI] [PubMed] [Google Scholar]
- 39. Tak E, Kuiper R, Chorus A, Hopman-Rock M.. Prevention of onset and progression of basic ADL disability by physical activity in community dwelling older adults: A meta-analysis. Ageing Res Rev 2013;12(1):329–38. 10.1016/j.arr.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 40. Wirth K, Klenk J, Brefka S, et al. Biomarkers associated with sedentary behaviour in older adults: A systematic review. Ageing Res Rev 2017;35:87–111. 10.1016/j.arr.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 41. Bano G, Trevisan C, Carraro S, et al. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017;96:10–5. 10.1016/j.maturitas.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 42. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev 2016;31:1–8. 10.1016/j.arr.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 43. Uthman OA, van der Windt DA, Jordan JL, et al. Exercise for lower limb osteoarthritis: Systematic review incorporating trial sequential analysis and network meta-analysis. BMJ 2013;347:f5555.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kasapis C, Thompson PD.. The effects of physical activity on serum C-reactive protein and inflammatory markers. A systematic review. J Am Coll Cardiol 2005;45(10):1563–9. [DOI] [PubMed] [Google Scholar]
- 45. Tribess S, Virtuoso Junior JS, Oliveira RJ.. Physical activity as a predictor of absence of frailty in the elderly. Rev Assoc Med Bras (1992) 2012;58(3):341–7. 10.1016/S0104-4230(12)70205-1 [DOI] [PubMed] [Google Scholar]
- 46. Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009;57(3):492–8. 10.1111/j.1532-5415.2009.02137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. Obesity: preventing and managing the global epidemic. No. 894. World Health Organization, 2000. [PubMed] [Google Scholar]
- 48. Hughes SL, Edelman PL, Singer RH, Chang RW.. Joint impairment and self-reported disability in elderly persons. J Gerontol 1993;48(2):S84–92. [DOI] [PubMed] [Google Scholar]
- 49. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA.. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med 2007;69(5):483–9. [DOI] [PubMed] [Google Scholar]