Abstract
Background
Pain modulation is a critical function of the nociceptive system that includes the ability to engage descending pain control systems to maintain a functional balance between facilitation and inhibition of incoming sensory stimuli. Dysfunctional pain modulation is associated with increased risk for chronic pain and is characteristic of fibromyalgia (FM). Catastrophizing is also common in FM. However, its influence on pain modulation is poorly understood.
Objective
To determine the role of catastrophizing on central nervous system processing during pain modulation in FM via examining brain responses and pain sensitivity during an attention-distraction paradigm.
Methods
Twenty FM patients and 18 healthy controls (CO) underwent functional magnetic resonance imaging while receiving pain stimuli, administered alone and during distracting cognitive tasks. Pain ratings were assessed after each stimulus. Catastrophizing was assessed with the Pain Catastrophizing Scale (PCS).
Results
The ability to modulate pain during distraction varied among FM patients and was associated with catastrophizing. This was demonstrated by significant positive relationships between PCS scores and pain ratings (P < 0.05) and brain responses in the dorsolateral prefrontal cortex (P < 0.01). Relationships between catastrophizing and pain modulation did not differ between FM and CO (P > 0.05).
Conclusions
FM patients with higher levels of catastrophizing were less able to distract themselves from pain, indicative of catastrophizing-related impairments in pain modulation. These results suggest that the tendency to catastrophize interacts with attention-resource allocation and may represent a mechanism of chronic pain exacerbation and/or maintenance. Reducing catastrophizing may improve FM symptoms via improving central nervous system regulation of pain.
Keywords: Chronic Pain, Pain Inhibition, Functional Neuroimaging, Psychobiology, Fibromyalgia, Brain
Introduction
Fibromyalgia is a chronic musculoskeletal pain condition with a host of related symptoms including sleep disturbance, fatigue, and cognitive complaints [1,2]. Recent prevalence estimates suggest that FM affects approximately 1.75% of the US population and is about twice as common in women as men [3]. There is no distinct biomarker for FM, making diagnosis and treatment challenging. However, mounting evidence demonstrates that these individuals tend to have impairments in pain modulation [4–9] and FM is considered a central sensitivity or functional pain disorder [2].
The experience of pain in FM is influenced by a complex interplay of psychobiological, behavioral, cognitive, and social factors [10]. One such factor, pain catastrophizing, is a maladaptive cognitive and emotional response to pain that involves a propensity to ruminate about, magnify the threat associated with, and feel helpless in the face of pain [11]. Clinically, catastrophizing is related to symptom severity, disability, distress, and poor prognoses [12,13]. Experimentally, it is associated with heightened anticipation and attention, greater sensitivity, and exaggerated brain responses to experimental pain stimuli [14–17]. To date, relationships between catastrophizing and pain modulation in FM have not been examined.
Pain modulation refers to a change in pain sensitivity that is centrally or peripherally mediated by both internal and external factors (e.g., mood, distraction, exercise) [18]. Modulation can refer to both facilitation and inhibition, and a variety of models have been used to study pain modulation in fibromyalgia patients. These include pharmacologic manipulations [6], diffuse noxious inhibitory control [4,19], vibratory stimulation [19], hypnotic and nonhypnotic suggestion [20], emotional context [21–23], and cognitive distraction [24,25]. Regardless of the type of modulation and the assessment method, research has demonstrated that the ability to modulate pain is crucial for maintaining a functional balance between facilitation and inhibition of sensory stimuli and that dysregulations in pain modulation can influence quality of life, disability, and the development of chronic pain [26]. Thus the impaired pain modulation often observed in patients with fibromyalgia (FM) likely plays a critical role in chronic pain maintenance. Gaining a better understanding of factors that influence the ability to modulate pain is important for both determining mechanisms of chronic pain maintenance and for the development of effective therapies.
Although catastrophizing and dysfunctional pain modulation are common in FM, there are individual differences in these characteristics. Understanding the relationship between pain catastrophizing and pain modulation in FM is important for examining a potential mechanism underlying the maintenance of pain as well as determining a potential behavioral target for improving symptoms and quality of life. Our purpose was to examine the relationship between catastrophizing and brain responses during cognitive distraction from pain in FM patients and healthy controls. In this scenario, pain modulation is demonstrated when pain ratings are lower for pain stimuli delivered during distraction as compared with stimuli delivered alone. We hypothesized that catastrophizing would be positively associated with activity in brain regions involved in attention and affective responses during cognitive modulation of pain. As catastrophizing has been previously associated with pain processing in both FM patients and controls [16,27], we did not hypothesize group differences.
Methods
Participants
Female FM patients and healthy controls (CO) were recruited from the community via advertisements and screened for eligibility. For patients, inclusion criteria were physician-confirmed diagnosis of FM and being between the ages of 18 and 65 years. Confirmation of FM status was obtained from each patient’s care provider [28]. For controls, inclusion criteria were age (18–65 years) and absence of chronic pain symptoms. Exclusion criteria for all participants were current diagnosis of Axis I psychiatric disorders, being left-handed, contraindications for the magnetic resonance environment (e.g., presence of ferrous metal), and regular use of opioids, cardiovascular medications, anticonvulsants (e.g., gabapentin, pregabalin), or high-dose antidepressant medications; continuation of low-dose antidepressants was permitted. Participants taking low-dose antidepressants (four FM patients) were instructed to maintain their current dosage throughout the course of the study.
Twenty female FM patients and 20 age-matched female CO met criteria and were enrolled. Prior to testing, participants were asked to abstain from 1) exercise for 48 hours, 2) alcohol for 24 hours, 3) pain medications for 24 hours, 4) caffeine for four hours, and 5) smoking for two hours. A compliance check was conducted at the beginning of each session, and all participants indicated that they had followed instructions. Participants were recruited as part of a larger study investigating brain responses to pain and were paid $200.
Procedures
The institutional review board at the University of Wisconsin-Madison approved the procedures of this study, and written consent was obtained from each participant. Testing took place on two days separated by approximately one week. To characterize the sample, participants completed a screening questionnaire asking about current and past pain symptoms, a demographic questionnaire, the Beck Depression Inventory (BDI), the State-Trait Anxiety Inventory (STAI), the Profile of Mood States (POMS), the Short-Form of the McGill Pain Questionnaire (SF-MPQ), and the Fibromyalgia Impact Questionnaire (FIQ; FM patients only) [29–33].
To assess pain catastrophizing, participants completed the Pain Catastrophizing Scale (PCS) [34]. This scale was developed to assess pain-related catastrophic thinking in three dimensions (helplessness, rumination, and magnification) and asks respondents to indicate to what degree they have specific thoughts and feelings when they are experiencing pain. Example items include “It’s terrible and I think it’s never going to get better” (helplessness), “I anxiously want the pain to go away” (rumination), and “I become afraid that the pain will get worse” (magnification). The PCS has been shown to be internally consistent for the total score (α = 0.87) as well as for the three subscales (helplessness, α = 0.79; rumination, α = 0.87; magnification, α = 0.60) [34]. There is also evidence that PCS scores are stable over time, with a test-retest reliability of r = 0.75 over a six-week period and r = 0.70 over 10 weeks [34]. This scale is widely used cross-culturally and has been validated in populations with and without chronic pain [35–37].
Testing – Mock–Magnetic Resonance Imaging
On the first day of testing, participants completed a simulated magnetic resonance imaging (MRI) session to familiarize themselves with the scanning environment and procedures. This included thorough instructions and practice of the distracting cognitive task detailed below, as well as practice and experience with the heat pain stimuli and rating procedures. For the cognitive task, training included verbal instructions as well as sufficient practice in front of a computer and in the mock-MRI unit to ensure proficiency and avoid learning effects during the subsequent functional MRI.
Psychophysical assessment of pain sensitivity was also conducted during the simulated MRI to determine the temperature for the individualized pain stimulus used during scanning. This involved exposing participants to 14 thermal stimuli ranging from 43°C to 49°C in 1°C increments; all participants tolerated the full range of temperatures. Each temperature was administered twice in random sequence and separated by one minute. Thermal stimuli were presented via a PATHWAY Pain and Sensory Evaluation System with a 900-mm2 Peltier thermode (Medoc Advanced Medical Systems, Durham, NC, USA) and delivered to the thenar eminence of the participant’s left hand. The baseline temperature for thermal testing procedures was maintained at 35°C and increased to the target temperature at a rate of 8°C/second. This temperature was maintained for eight seconds before returning to baseline. Immediately following each stimulus presentation, participants were asked to rate the pain intensity and unpleasantness using the 0–20 Gracely Box Scales [38] viewed with a set of MRI-compatible goggles (Avotec, Inc., Stuart, FL, USA) and presented with the use of E-Prime software (Psychology Software Tools, Pittsburg, PA, USA). Pain intensity and unpleasantness ratings were made using a scanner-compatible button-press response unit (Current Designs, Philadelphia, PA, USA). Linear regression of pain intensity ratings and stimulus temperatures was calculated to predict the temperature each individual would rate as “slightly intense” pain, or a 13 on the 0–20 rating scale. This temperature was used for all remaining pain testing procedures. For FM patients, the average temperature used for further testing was 47.2°C ± 1.1°C, and for healthy controls the average temperature was 47.9°C ± 1.1°C. Our choice of a perceptually relative pain stimulus helped to ensure that sensitivity to peripheral pain stimuli was controlled for and thus allowed for a more specific test of the relationship between pain catastrophizing and brain and perceptual responses to stimuli [39]. It also allowed us to the use ratings during the distracting cognitive task in comparison with ratings to stimuli delivered alone as evidence of pain modulation.
Cognitive Modulation of Pain
Though pain modulation can be investigated in multiple ways, we focused on cognitive modulation. Cognitive modulation can refer to one of several paradigms including distraction, reappraisal, and anticipation. For the purposes of this study, cognitive modulation is operationally defined as the ablity to shift attention away from pain during a cognitive task using an attention-distraction paradigm that has been employed in previous studies [40,41]. Pain was delivered alone and during the performance of the Stroop color-word task. The Stroop is a sustained attention task that presents the words “red,” “green,” “yellow,” and “blue” in the colors red, green, yellow, and blue [42]. Words are presented in either congruent (e.g., the word “red” appears in red font) or incongruent (e.g., the word “red” appears in a font color that is not red) fashion. The incongruent version of the Stroop requires greater attention and consequently has been shown to be more distracting [43]. The Stroop has previously been shown to be an effective cognitive distraction in both behavioral and neuroimaging pain studies [40,44]. Participants were instructed to press a button corresponding to the color of the word and ignore the word itself. Pain modulation was defined as a reduction in pain sensitivity (i.e., pain ratings) during cognitive distraction (i.e., performance of the Stroop) when compared with delivering pain alone.
Functional Neuroimaging
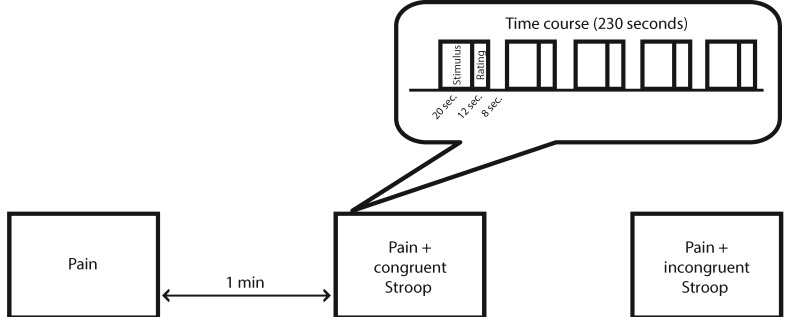
The following week, participants returned for their functional MRI session. This session was designed to determine the perceptual and brain responses during cognitive modulation of pain. Scanning included three functional runs, each lasting three minutes and 50 seconds, presented in a pseudo-randomized, counterbalanced order (Figure 1). Each functional run started with a 30-second off-period and included 20-second interstimulus intervals. Runs included pain alone and pain delivered during the congruent and incongruent Stroop. During each run, five perceptually relative stimuli were administered for 20 seconds each, and pain intensity and unpleasantness ratings were collected immediately following each stimulus. The rating period for each stimulus was a maximum of 12 seconds. For the runs involving the Stroop task, nine word-color combinations were presented during each of the pain stimuli. Each word was presented for 1,500 ms, with 500 ms between words. Participants had two seconds to respond, starting when the word first appeared. In order to avoid potential confounds due to expectations, participants were not told when each run-type would occur during the scan. Thus, prior to the start of each run and regardless of its type, participants were instructed to focus on the cognitive task, respond as fast and accurately as possible, and rate the pain stimuli when prompted by the rating scales.
Figure 1.
Overview of the scanning protocol. The three experimental runs were presented in a counterbalanced order and separated by one-minute intervals.
Magnetic Resonance Imaging Acquisition
Functional and anatomical magnetic resonance images were collected on a 3-Tesla GE SIGNA MRI scanner (GE Health Systems, Waukesha, WI, USA). Anatomical acquisitions (EFGRE3D) consisted of 124 1.2-mm-thick, T1-weighted (TR: 8.4 ms, TE: 1.7 ms, FOV: 240 mm, flip angle: 10°) axial images with a matrix of 256 × 256. To correct inhomogeneity-induced distortions in the images, a set of 2D gradient echo fieldmaps was collected in the sagittal direction with the following parameters: three 4-mm-thick slices with a 1-mm gap (TR: 700 ms, TE: 7 and 10 ms, flip angle: 60°, FOV = 240 mm, acquisition matrix: 256 × 256). Functional MRI acquisitions were obtained with a gradient echo EPI sequence (TR: 2,000 ms, TE: 30 ms, flip angle: 90°) and consisted of 30 4-mm-thick slices collected in the sagittal direction with a 1-mm gap. The acquisition matrix was 64 × 64, and the FOV was 240 mm, delivering an in-plane voxel resolution of 3.75 × 3.75 × 5 mm.
Image Processing
Image processing was conducted using Analysis of Functional Neuroimaging (AFNI) software [45]. Anatomical images were registered to the MNI-152 template [46] using an affine transformation. For functional data, the initial three sets of functional images were discarded from functional analyses due to saturation effects. Data were despiked to reduce the influence of outlier time points, then slice-time-, motion-, and fieldmap-corrected, aligned to the MNI-152 template with an affine warp, iteratively blurred to a smoothness of 8-mm full-width, half-maximum, and converted to percent signal change. AFNI’s 3dREMLfit program was then used to perform linear regression on each participant’s data, including separate regressors for the prestimulus countdown, heat stimulus, and rating period. The duration of the rating periods was modeled based on the actual time participants took to rate the intensity and unpleasantness of each stimulus. Excessive head movement (>2 mm) during a run resulted in exclusion of that participant from the group-level analyses.
Statistical Analyses
Statistical analyses for demographic and behavioral data were performed using SPSS, version 22 (IBM, Armonk, NY, USA). Functional neuroimaging data were analyzed using AFNI software [45]. For all statistical analyses, the α level was set to 0.05, and corrections for multiple comparisons were applied where appropriate, as described below.
Participant Characteristics
Demographics were compared between FM and CO using independent-samples t tests for continuous measures and chi-square tests for categorical measures; t tests were also employed to compare FM and CO with respect to symptoms of depression (BDI), pain symptoms (SF-MPQ), and pain catastrophizing (PCS). To assess the relationship between catastrophizing and FM symptoms, correlations were calculated between PCS scores and scores on the FIQ, SF-MPQ, STAI, POMS, and BDI.
Cognitive Task Performance and Pain Modulation
To confirm the anticipated degree of distraction provided by the different versions of the Stroop task and to assess group differences in performance, Group by Run (2 × 2) repeated-measures analyses of variance (ANOVAs) were conducted with Stroop accuracy and reaction time as dependent variables. To determine whether catastrophizing impacted cognitive performance, correlation coefficients (Pearson’s r) were calculated between PCS scores and Stroop performance for FM and CO.
To assess the degree of pain modulation induced by the Stroop task, pain ratings and brain responses for pain stimuli delivered alone were compared with those during the congruent and incongruent versions of the cognitive task. For ratings, within- and between-group comparisons were made using Group by Run (2 × 3) repeated-measures ANOVAs. Follow-up contrasts and effect size calculations (Cohen’s d) were conducted to examine differences in pain intensity and unpleasantness ratings among the runs within each group. For brain responses, within- and between-group comparisons were made using Mixed Effects Meta Analyses in AFNI’s 3dMEMA program [47] comparing brain responses during pain alone with responses during the Stroop tasks. Additionally, group (FM and CO) images for each experimental run are included in the Supplementary Data for descriptive purposes, in accordance with recent recommendations [48].
Catastrophizing and Pain Modulation – Pain Ratings
To address our primary hypotheses, relationships between catastrophizing and pain ratings and brain data were examined within and between groups. Partial correlations (Pearson’s r) between pain intensity and unpleasantness ratings for pain stimuli during the congruent and incongruent versions of the Stroop task were analyzed for FM and CO separately. Because of the documented relationship between catastrophizing and depression [12], scores on the BDI were controlled for in these analyses. Correlation coefficients for individual groups were also compared with one another using Fisher’s r-to-z transformation to assess the specificity of observed relationships for FM compared with CO.
Catastrophizing and Pain Modulation – Brain Responses
Relationships between catastrophizing and brain responses to pain were analyzed using a region of interest approach [45]. Regions of interest (ROIs) were selected based on current research documenting brain responses to pain in patients with FM and healthy controls [49]. Each ROI was defined using the Harvard-Oxford cortical and subcortical structural probabilistic atlases developed at the Centre for Morphometric Analysis at Harvard University, Boston, Massachusetts (www.cma.mgh.harvard.edu). Region boundaries were defined using a probability threshold of 25%. Regions included pre- and postcentral gyri, superior parietal lobule, anterior, posterior, and paracingulate cortices, brainstem, frontal medial, and orbital cortices, frontal and parietal opercula, frontal pole including the dorsal and ventrolateral prefrontal cortices, insula, thalamus, caudate, putamen, globus pallidus, amygdala, hippocampus, middle frontal gyrus, and superior temporal gyrus.
To examine the relationships between pain catastrophizing and brain responses during pain processing and distraction from pain, comparisons were made using Mixed Effects Meta Analyses from AFNI’s 3dMEMA program [47]. Relationships between scores on the PCS and brain responses during pain alone and pain during the congruent and incongruent versions of the Stroop task were analyzed between and within groups. As with the pain ratings data, scores on the BDI were statistically controlled for in all analyses. Within each analysis, we thresholded the voxel-wise data at P < 0.05 and corrected for multiple comparisons using a cluster-size threshold of 73 voxels. This threshold was determined by Monte Carlo simulations using AFNI’s 3dClustSim and was based on the smoothness of the data (8 mm FWHM) and the size and shape of the ROI mask. These data were then visualized using a dual coding approach, as recommended by Allen and colleagues [50]. This display format allows for the presentation of both the robust outcomes of statistical interest and the level of uncertainty in the data set (i.e., pattern of whole-brain activity). Analyses were performed at 4 × 4 × 4 mm resolution using a mask of regions determined a priori from the hypotheses. For visualization purposes only, the mask has been omitted and the data have been interpolated to 1 × 1 × 1 mm resolution; all statistical inference was made at the cluster level.
To further explicate the neuroimaging results, functional data for each participant were extracted from brain regions showing significant associations in the 3dMEMA analyses within each group. These data were then correlated with PCS scores, pain ratings, and Stroop accuracy and reaction time. To determine the specificity of the relationship between distraction from pain and catastrophizing, the occipital pole was employed as a control region. The coordinates for the center of this region were 18.5, 89.5, 0.5. This region was active during the viewing of our pain rating scale and the cognitive task, though it is not typically considered a pain-relevant brain region. Thus, we considered this region to be a reasonable control.
For exploratory purposes, our primary analyses were also conducted controlling for the STAI. Results similar to those observed when controlling for BDI were observed in all cases. Because the BDI and STAI were highly correlated in our sample (r = 0.83, P < 0.001) and the results were qualitatively the same when controlling for either variable, the STAI was not retained as a covariate for either behavioral or brain imaging analyses.
Results
Participant Characteristics
Twenty female patients with a physician-confirmed diagnosis of FM and 20 age- and sex-matched CO completed all testing procedures (see Table 1 for participant characteristics). Patients received their diagnosis approximately 11.8 ± 7.5 years earlier and reported pain in all four quadrants of their body and along the axial skeleton at the time of testing. Fifty percent of the FM participants reported being “unemployed due to health,” and the remainder reported that FM “substantially” interfered wth their job. During screening, patients also reported that, on average, their pain and other symptoms had a substantial impact on their personal lives by limiting their daily activities over the past six months, rating the impact as 48.8 ± 23.1 on a scale of 0 (no limitations) to 100 (totally disabled). FM patients averaged 51.2 ± 15.2 on the Fibromyalgia Impact Questionnaire, which is similar to the average patient score of 50 [33].
Table 1.
Participant characteristics
| Fibromyalgia Patients (N = 20) | Controls (N = 18) | Group Comparison P Value (t Test and χ2) | ||
|---|---|---|---|---|
| Age | 42.3 (11.3) | 40.7 (9.3) | 0.64 | |
| Height, m | 1.67 (0.1) | 1.64 (0.1) | 0.14 | |
| Weight, kg | 75.0 (16.3) | 66.4 (10.9) | 0.07 | |
| Body mass index, kg/m2 | 26.8 (5.3) | 24.7 (3.8) | 0.17 | |
| Race/ethnicity, % white | 95 | 89 | 0.49 | |
| Marital status, % married | 45 | 56 | 0.64 | |
| Education, % w/ college degree | 60 | 94 | 0.08 | |
| Employment, % employed at least part time | 50 | 89 | 0.03 | |
| Fibromyalgia Impact Questionnaire Score | 51.2 (15.2) | NA | NA | |
| Beck Depression Inventory | 9.2 (8.1) | 2.3 (2.8) | 0.002 | |
| POMS: Total Mood Disturbance | 133.7 (30.4) | 97.6 (8.3) | <0.001 | |
| STAI: Trait Anxiety | 38.2 (10.6) | 29.8 (5.6) | 0.005 | |
| McGill Pain Questionnaire | Total | 11.1 (6.8) | 0.6 (0.9) | <0.001 |
| VAS | 38.3 (17.0) | 3.1 (6.1) | <0.001 | |
| Pain Catastrophizing Scale | Helplessness | 5.5 (3.0) | 1.8 (2.2) | <0.001 |
| Rumination | 5.1 (4.3) | 3.4 (2.1) | 0.15 | |
| Magnification | 2.4 (1.9) | 1.2 (1.2) | 0.026 | |
| Total | 12.9 (7.5) | 6.4 (4.1) | 0.003 | |
POMS = Profile of Mood States; STAI = State-Trait Anxiety Inventory.
FM patients were not significantly different from CO with respect to total PCS scores (P > 0.06). However, two controls had PCS scores that were more than two standard deviations above the mean for that group, which significantly impacted the interpretation of the primary results for controls. Thus, all analyses were conducted with and without these individuals. Herein we present the results with the outliers removed (CO, N = 18). Results for the primary outcomes with outliers included can be found in the Supplementary Data associated with this study.
For FM patients, PCS scores were significantly and positively related to the impact of FM on participants’ lives, measured with the FIQ (r = 0.45, P = 0.047). However, PCS scores were not significantly related to symptoms of depression measured with the BDI (r = 0.26, P = 0.28), current levels of pain as measured by the SF-MPQ visual analog scale (r = 0.11, P = 0.64), or the SF-MPQ total (r = −0.15, P = 0.52).
Brain Responses to Pain and Cognitive Tasks
Of the original sample, four FM patients and two CO had excessive head motion (>2 mm) during scanning and were excluded from these analyses. For both FM and CO, brain responses were significantly different within each group when comparing pain delivered alone with pain delivered during the both the congruent and incongruent Stroop tasks. Brain responses were also significantly different between pain delivered during the congruent vs incongruent Stroop for both groups, demonstrating the greater neural activation required to perform the more challenging incongruent version. These results are detailed in the Supplementary Data. There were no significant group differences in brain responses to pain delivered alone or pain delivered during the cognitive tasks or the interaction between group and task (pain alone vs pain during the Stroop; P > 0.05).
Cognitive Task Performance and Pain Modulation
Accuracy and reaction time data for the Stroop tasks are presented in Table 2. For both FM and CO, participants’ reaction times were significantly faster for the congruent vs the incongruent Stroop task (P < 0.001). There were not group differences in reaction time for the congruent Stroop (P > 0.05). Controls were siginificantly faster on the incongruent Stroop (P = 0.04). For accuracy, there was a significant group by task interaction (P = 0.031), showing that while both groups decreased their accuracy on the incongruent Stroop in comparison with the congruent version, FM patients showed a greater decline in performance.
Table 2.
Group differences in performance (accuracy and reaction time) on the Stroop Color Word Task
| Fibromyalgia (N = 20) | Controls (N = 18) | Group Differences P Value (t Test) | ||
|---|---|---|---|---|
| Congruent Stroop | Accuracy, % correct | 95.2 (5.4) | 98.0 (3.4) | 0.06 |
| Reaction time, ms | 799.8 (109.6) | 741.6 (114.3) | 0.12 | |
| Incongruent Stroop | Accuracy, % correct | 84.2 (23.9) | 97.5 (3.6) | 0.03 |
| Reaction time, ms | 977.7 (133.1) | 885.8 (131.4) | 0.04 | |
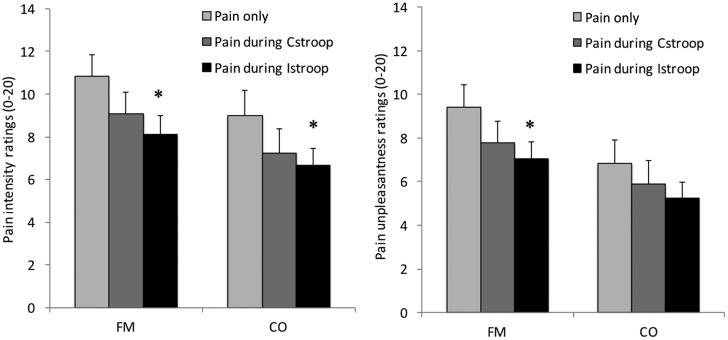
Pain ratings for FM and CO during the three experimental runs are shown in Figure 2. Analysis of group differences demonstrated that FM patients rated the pain stimuli as significantly more unpleasant than CO (P = 0.019). For both FM and CO, there were significant differences in pain intensity (PI; P < 0.01) and unpleasantness ratings (PU; P < 0.01) across experimental runs, demonstrating that participants were successfully distracted from pain during performance of the cognitive task (i.e., exhibited pain inhibition). Follow-up contrasts showed that FM patients had significantly lower intensity and unpleasantness ratings for pain delivered during the incongruent version of the Stroop task as compared with pain delivered alone (PI: P = 0.01, d = 0.63; PU: P = 0.02, d = 0.60). Controls also had significantly lower pain intensity (P = 0.02, d = 0.56), but not pain unpleasantness ratings (P > 0.05, d = 0.41) during the incongruent Stroop task. Pain intensity and unpleasantness ratings were not significantly lower during the congruent Stroop task for either FM (P > 0.05, pain intensity: d = 0.36, pain unpleasantness: d = 0.36) or CO (P > 0.05, pain intensity: d = 0.20, pain unpleasantness: d = 0.42). However, the degree of modulation varied widely among participants, suggesting that there were individuals who modulated during the congruent version of the task. Changes in pain intensity and unpleasantness ratings between pain alone and pain delivered during the Stroop task ranged from a decrease of 15.6 points to an increase of 11.8 points on the Gracely 0–20 scale.
Figure 2.
Fibromyalgia patients had significantly higher pain intensity (PI) and pain unpleasantness (PU) ratings during pain delivered alone as compared with pain delivered during the incongruent version of the Stroop task (P < 0.05). Controls had significantly lower PI ratings (P < 0.05), but not PU ratings (P > 0.05) during the incongruent Stroop task. *Significantly different from pain alone, P < 0.05. CO = controls; FM = fibromyalgia.
Catastrophizing and Pain Modulation
Pain intensity and unpleasantness ratings during the cognitive tasks were significantly related to catastrophizing (Table 3). FM patients showed significant positive relationships that were moderate to large (rrange = 0.53–0.79, P < 0.05) between PCS scores and pain intensity and unpleasantness ratings for pain presented during both the congruent and incongruent versions of the Stroop task. For CO, correlations were smaller and nonsignificant (rrange = 0.32–0.42, P > 0.05). Comparisons between groups showed that there were no significant group differences in the magnitude of the relationships between pain ratings and catastrophizing during any of the experimental runs (P > 0.05).
Table 3.
Relationships Between Pain Ratings and Pain Catastrophizing
| Pain Alone |
Pain During CStroop |
Pain During IStroop |
||||
|---|---|---|---|---|---|---|
| PI | PU | PI | PU | PI | PU | |
| Fibromyalgia (N = 20) | 0.28 | 0.28 | 0.58 | 0.57 | 0.72 | 0.79 |
| P = 0.25 | P = 0.24 | P = 0.009 | P = 0.011 | P = 0.001 | P < 0.001 | |
| Control (N = 18) | −0.19 | −0.33 | 0.37 | 0.32 | 0.42 | 0.36 |
| P = 0.46 | P = 0.23 | P = 0.14 | P = 0.22 | P = 0.087 | P = 0.15 | |
| Group comparisons (z-scores) | 1.4 | 1.84 | 0.8 | 0.92 | 1.34 | 2.02 |
| P = 0.16 | P = 0.06 | P = 0.42 | P = 0.36 | P = 0.18 | P = 0.04 | |
Upper rows show partial correlations between pain intensity and unpleasantness ratings and scores on the Pain Catastrophizing Scale, controlling for Scores on the Beck Depression Inventory for FM patients and controls during each of the three experimental runs. The bottom row shows group differences in correlation coefficients (z-scores) associated with each experimental run.
CStroop = congruent Stroop; IStroop = incongruent Stroop; PI = pain intensity; PU = pain unpleasantness.
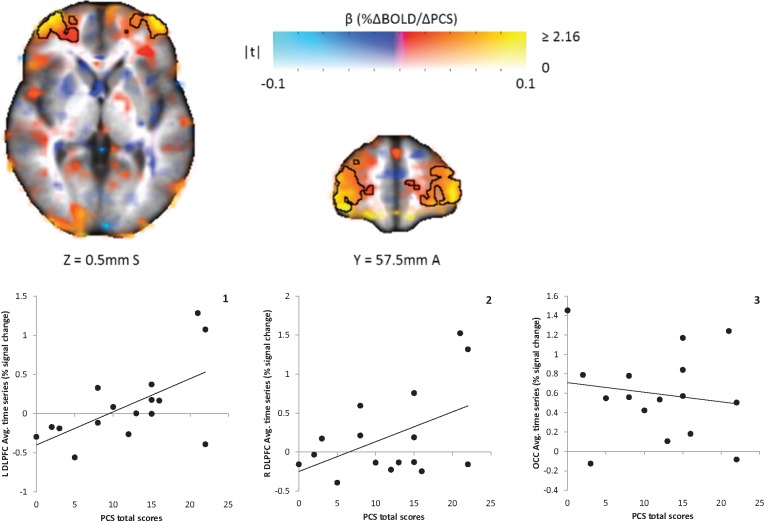
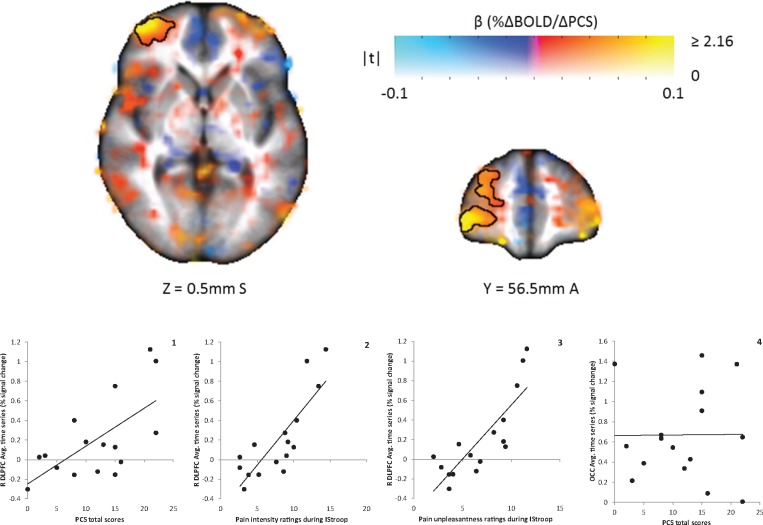
PCS scores were significantly correlated with brain responses during cognitive modulation of pain for FM patients (P < 0.05) (see Table 4 for list of brain regions and coordinates). Significant positive relationships indicative of greater brain activity for those who were higher in catastrophizing were found bilaterally in the dorsolateral prefrontal cortex (DLPFC) for pain during the congruent Stroop task (Figure 3). Illustrative of this, activity in both these regions was positively related to pain intensity (right [R] DLPFC: r = 0.38; left [L] DLPFC: r = 0.44) and unpleasantness ratings (R DLPFC: r = 0.32; L DLPFC: r = 0.41). For pain during the incongruent Stroop task, FM patients had a significant positive relationship in the R DLPFC (Figure 4), again showing that brain activity in this region was greater in those who were higher in catastrophizing. Greater activity in this region was also positively related to higher ratings of pain intensity (r = 0.82) and unpleasantness (r = 0.83). For both FM patients and controls, measures of Stroop performance (accuracy and reaction time) were unrelated to brain activity in these regions for either version of the Stroop task (P > 0.05). No significant relationships were observed between PCS scores and brain responses in the experimental control region, the occipital pole (P > 0.05). There were also no significant group differences with respect to relationships between catastrophizing and brain responses during the Stroop tasks (P > 0.05).
Table 4.
Results from the Mixed Effects Meta Analyses examining the relationships between scores on the Pain Catastrophizing Scale and brain responses during pain processing and cognitive modulation of pain for FM (N = 16)
| Pain During Congruent Stroop | ||||
|---|---|---|---|---|
| Group | Brain Regions | Peak X, Y, X | Volume, mm3 | Peak t-Statistic |
| FM | L PFC | 40, −56, 6 | 9,984 | 6.38 |
| FM | R PFC | −36, −60, 10 | 7,360 | 5.97 |
|
Pain During Incongruent Stroop | ||||
| Group | Brain Regions | Peak X, Y, X | Volume, mm3 | Peak t-Statistic |
| FM | R PFC | −40, −60, 2 | 6,592 | 5.63 |
Included in the table are clusters showing significant relationships between total scores on the PCS and brain responses associated with cognitive modulation of pain during the Stroop color-word tasks, controlling for scores on the Beck Depression Inventory. All clusters represent positive relationships. For each analysis, multiple comparisons were corrected for using a cluster-size threshold of 65 voxels or 4,570 mm3. There were no significant regions for CO (N = 15).
FM = fibromyalgia; L = left; PFC = prefrontal cortex; R = right.
Figure 3.
Map of association between Pain Catastrophizing Scale (PCS) total scores and BOLD responses to cognitive modulation of pain during the congruent Stroop task in 16 fibromyalgia patients. Color represents the β coefficient, and opacity represents t-statistic, with full opacity at a voxelwise t corresponding to P < 0.05. The two clusters are significant at α < 0.05 (corresponding to a cluster size threshold of 73 voxels); these are outlined. Positive correlations were found in the right and left dorsolateral prefrontal cortices (DLPFC; graphs 1 and 2). There was not a significant relationship between PCS Scores and brain responses in the occipital lobe (OCC; control region, graph 3). Average cluster values (percent signal change) for each individual were extracted and are shown plotted against PCS Scores and pain ratings. L = left; R = right.
Figure 4.
Map of association between PCS total scores and BOLD responses to cognitive modulation of pain during the incongruent Stroop task in 16 fibromyalgia patients. Color represents the β coefficient, and opacity represents the t-statistic, with full opacity at a voxelwise t corresponding to P < 0.05. The single cluster significant at α < 0.05 (corresponding to a cluster size threshold of 73 voxels) is outlined. A significant positive correlation was found in the right dorsolateral prefrontal cortex (DLPFC; graph 1). Graphs 2 and 3 show the relationships between the brain response in the right DLPFC and pain intensity and unpleasantness ratings to pain stimuli delivered during the incongruent Stroop task. There was not a significant relationship between PCS scores and brain responses in the occipital lobe (OCC; control region, graph 4). Average cluster values (percent signal change) for each individual were extracted and are shown plotted against PCS scores and pain ratings. L = left; R = right.
Discussion
Our results show that the tendency to catastrophize about pain interferes with the neural processes involved in pain modulation in FM, specifically in the dorsolateral prefrontal cortex. Patients who reported greater levels of catastrophizing engaged this region to a greater degree. Further, activity in the DLPFC was positively associated with pain ratings and was not significantly related to performance on the Stroop task, suggesting the specificity of its involvement in pain modulation. These neuroimaging findings are supported by behavioral data showing that patients who were higher in catastrophizing found the pain stimuli more intense and unpleasant during the cognitive tasks than those lower in catastrophizing. Thus, catastrophizing appeared to weaken the potentially beneficial effects of cognitive distraction on descending inhibitory processes involved in pain modulation.
Previous research has demonstrated significant relationships between catastrophizing and brain responses during pain processing in both FM patients and healthy individuals [16,17,27]. Gracely and colleagues [16] found that in FM patients catastrophizing was related to activity in several pain-relevant brain regions, although, paradoxically, catastrophizing was not significantly related to pain ratings. Seminowicz and Davis [27] found that catastrophizing was predictive of brain responses associated with pain in healthy individuals. More recently, Loggia and collegues [17] reported that catastrophizing was related to pain anticipation in the lateral prefrontal cortex (PFC) in patients with FM. These studies show that catastrophizing is related to central nervous system processing of pain but do not directly assess its influence on pain modulation. The present study addresses this critical gap by demonstrating that the tendency to catastrophize interferes with the neural processes associated with cognitive distraction—a crucial pain inhibitory function.
This study also adds to the small and equivocal body of work examining catastrophizing and pain modulation in patients with chronic pain conditions. Schreiber and colleagues [51] compared pain modulation between chronic back pain patients with higher and lower levels of catastrophizing and found that those who were higher in catastrophizing had greater pain facilitation, but comparable levels of inhibition. Owens and colleagues [52] also examined the association between pain modulation and catastrophizing in patients with chronic low back pain and found that the tendency to catastrophize was not significantly related to pain facilitation or pain inhibition. The methods used to assess modulation varied in these studies and were different from the attention-distraction paradigm that was used in the present investigation. Further, there is inconsistent evidence regarding whether patients with chronic low back pain experience the central abnormalities associated with pain modulation that are seen consistently in patients with FM [53]. As such, it is difficult to draw any conclusions regarding the generalizability of the observed relationships between catastrophizing and pain modulation to all patients with chronic pain. Further research is needed using multiple methods of assessing pain modulation and directly comparing chronic pain patients with different conditions to understand the consistency of these relationships across pain conditions.
The relationships we observed with catastrophizing were located within the DLPFC. Previous research has demonstrated involvement of this region in aspects of executive control associated with affective and cognitive pain processing [54,55]. Atlas and colleagues [54] identified a group of regions including the lateral PFC where activity predicted pain perception but was not associated with stimulus intensity. They hypothesized that these regions contribute to pain perception through their role in attention and cognitive processes involved in the evaluation of pain. The results from the present study support this by demonstrating that activity in the DLPFC is associated with catastrophizing as well as pain ratings during distraction. From a resource allocation perspective, FM patients with higher tendencies to catastrophize may be less able to shift attention resources away from pain or require greater resources to evaluate pain.
The PFC is also involved in the integration of cognition and emotion [56,57]. For example, Peers and colleagues [58] found that for individuals who have difficulties with attentional control, the PFC was involved in regulation of attentional bias toward stimuli with a higher level of perceived threat in dual-attention tasks. The present data suggest that patients with higher levels of catastrophizing may have a bias toward attending to pain stimuli, resulting in greater activity in the DLPFC, heightened perception of pain, and a lesser ability to distract themselves away from pain. How these processes contribute to chronic pain is not fully understood, but maintenance of symptoms through central dysregulation of pain modulation is plausible. Reduced ability to distract from pain could result in a vicious cycle of augmented sensory processing of afferent signaling combined with a reduced ability to inhibit incoming sensory information. Alternatively, poor pain regulation could result in greater attention to pain or hypervigilance, both of which are associated with poor clinical outcomes in chronic pain patients [59].
It has also been suggested that activity in the PFC during a dual task reflects psychological load, or the amount of top-down activity that is needed to maintain cognitive performance [60]. Patients commonly report cognitive difficulties, and there is evidence showing deficits in performance in several cognitive domains [61]. The present data demonstrated that patients were moderately slower and significantly less accurate than controls during the more difficult, incongruent version of the Stroop task. However, task performance was not related to catastrophizing or to activity in the PFC during distraction. Perhaps abnormal processing in the PFC underlies several characteristics of FM, including dysfunction in pain modulation and cognitive difficulties. A reduced ability to distract from pain or hypervigilance toward painful stimuli could also directly interfere with cognitive performance by occupying attention resources needed for efficient cognitive processing. This may be particularly relevant for more demanding cognitive tasks (i.e., incongruent Stroop).
Consistent with previous research on patients with FM, the participants in our study reported that their pain and associated symptoms substantially impacted their quality of life and decreased their physical function. The results showed that catastrophizing exacerbated this impact, as demonstrated by significant positive relationships between PCS scores and scores on the FIQ. However, catastrophizing was not significantly related to the intensity of pain symptoms as measured with the SF-MPQ. This suggests that individuals who are higher in catastrophizing do not necessarily experience more pain, but the pain they do experience has a greater impact on their quality of life. These findings are consistent with data demonstrating that catastrophizing influences depression, physical function, disability, treatment efficacy, and chronicity of disease in chronic pain and highlight the importance of developing and implementing interventions to reduce catastrophizing in patients with FM [12,62].
Our lack of group differences between FM patients and controls was not unexpected, but nonetheless warrants comment. As the temperatures delivered during scanning were relativized based on each individual’s range of pain sensitivity during the mock-MRI session, the lack of differences in brain or rating responses to pain was anticipated [63]. However, the absence of significant differences with respect to relationships between cognitive modulation of pain and catastrophizing suggests that this is not necessarily a phenomenon that is specific to FM. Rather, as seen for relationships between catastrophizing and pain processing [27], it suggests that catastrophizing may interfere with pain modulation regardless of health status. However, as FM patients encounter pain more frequently and have a greater propensity to catastrophize, it may have a greater impact on their lives. Future studies are needed that include healthy individuals with higher levels of catastrophizing to more definitively examine the nature of this phenomenon.
Although there are effective treatment strategies for FM (e.g., pharmaceuticals, exercise), improvements in symptoms are typically modest and the long-term prognosis remains poor or is poorly understood. Thus, identifying potentially modifiable factors that influence FM is warranted. We previously demonstrated that higher levels of physical activity and lower levels of sedentary behavior were associated with an improved ability to cognitively modulate pain in FM [24]. There is also evidence that reducing pain catastrophizing results in improvements in health outcomes for chronic pain patients. For example, George and colleagues [64] compared exercise training with exposure therapy on outcomes related to pain and disability in patients with chronic low back pain and found that both interventions led to statistically significant improvements in pain and disability and reductions in catastrophizing. Moreover, reductions in catastrophizing were significantly related to improvements in disability, suggesting that catastrophizing has implications for the daily functioning of patients. More recently, it was demonstrated that a cognitive-behavioral intervention significantly increased gray matter in the PFC in FM patients and that this increase was related to a decrease in catastrophizing [65]. These studies suggest that catastrophizing is modifiable and may be an important moderator of improvements in symptoms, neurobiology, and physical function.
There were several limitations in this study. Our patient population reported relatively low levels of catastrophizing, when compared with normative data [34]. However, patients with FM in the current study reported that their pain had a “substantial impact” on their lives and their FIQ scores were representative of the “typical” FM patient [33]. Because we did not specifically recruit participants based on catastrophizing, we were unable to directly determine whether high and low catastrophizers differed in their neural responses across conditions or between groups. Further, as our study was focused on pain modulation and not pain sensitivity, we only used thermal stimuli at one individualized temperature. This limited our ability to address relationships between catastrophizing and pain sensitivity across multiple intensities, as has been done previously [16,27]. Additionally, our sample size was relatively small, potentially reducing the generalizability of the results, and we did not control for the potential confounding effects of the menstrual cycle on pain modulation. Lastly, the lack of catastrophizing in our sample of healthy controls limited our ability to examine whether the observed relationships between pain modulation and catastrophizing are specific to FM or a more general phenomenon.
The study provides evidence that an FM patient’s tendency toward catastrophizing influences the ability to actively engage the central nervous system to inhibit pain during distraction. Thus, catastrophizing may be disrupting the balance between facilitation and inhibition of pain processing and acting, in part, to maintain pain and associated symptoms. Therapies to reduce catastrophizing including cognitive-behavioral therapy and exercise may be effective for improving symptoms in FM patients via improvements in pain modulation. Interventions designed to reduce catastrophizing and determine the effects on central nervous system processing of pain modulation are needed.
Authors’ Contributions
Drs Ellingson, Stegner, and Cook conceived of this project and worked on data collection, analysis, and the drafting and editing of this manuscript. Isaac Schwabacher was primarily responsible for processing the neuroimaging data, including performing the final analyses and creating figures for the manuscript submission. All authors discussed the results have read and approved this manuscript.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
Supplementary Material
Funding sources: This research was supported by a grant from National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institute of Health (AR50969).
Conflicts of interest: There are no conflicts of interest for any of the authors.
Disclaimer: The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
References
- 1. Wolfe F, Clauw DJ, Fitzcharles M-A, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010;62(5):600–10. 10.1002/acr.20140 [DOI] [PubMed] [Google Scholar]
- 2. Boomershine CS. Fibromyalgia: The prototypical central sensitivity syndrome. Curr Rheumatol Rev 2015;11(2):131–45. 10.2174/1573397111666150619095007 [DOI] [PubMed] [Google Scholar]
- 3. Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F.. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS One 2015;10(9):e0138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lautenbacher S, Rollman GB.. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain 1997;13(3):189–96. 10.1097/00002508-199709000-00003 [DOI] [PubMed] [Google Scholar]
- 5. Vierck CJ Jr, Staud R, Price DD, et al. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain 2001;2(6):334–44. 10.1054/jpai.2001.25533 [DOI] [PubMed] [Google Scholar]
- 6. Price DD, Staud R, Robinson ME, et al. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 2002;99(1–2):49–59. 10.1016/S0304-3959(02)00053-2 [DOI] [PubMed] [Google Scholar]
- 7. Julien N, Goffaux P, Arsenault P, Marchand S.. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 2005;114(1–2):295–302. 10.1016/j.pain.2004.12.032 [DOI] [PubMed] [Google Scholar]
- 8. Jensen KB, Kosek E, Petzke F, et al. Evidence of dysfunctional pain inhibition in fibromyalgia reflected in rACC during provoked pain. Pain 2009;144(1):95–100. 10.1016/j.pain.2009.03.018 [DOI] [PubMed] [Google Scholar]
- 9. Potvin S, Marchand S.. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia. Pain 2016;157(8):1704–10. 10.1097/j.pain.0000000000000573 [DOI] [PubMed] [Google Scholar]
- 10. Clauw DJ. Fibromyalgia: A clinical review. JAMA 2014;311(15):1547–55. 10.1001/jama.2014.3266 [DOI] [PubMed] [Google Scholar]
- 11. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17(1):52–64. 10.1097/00002508-200103000-00008 [DOI] [PubMed] [Google Scholar]
- 12. Edwards RR, Cahalan C, Calahan C, et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 2011;7(4):216–24. 10.1038/nrrheum.2011.2 [DOI] [PubMed] [Google Scholar]
- 13. Giesecke T, Williams DA, Harris RE, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum 2003;48(10):2916–22. 10.1002/art.11272 [DOI] [PubMed] [Google Scholar]
- 14. Burgmer M, Petzke F, Giesecke T, et al. Cerebral activation and catastrophizing during pain anticipation in patients with fibromyalgia. Psychosom Med 2011;73(9):751–9. 10.1097/PSY.0b013e318236588a [DOI] [PubMed] [Google Scholar]
- 15. Geisser ME, Casey KL, Brucksch CB, et al. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: Association with mood, somatic focus, and catastrophizing. Pain 2003;102(3):243–50. 10.1016/S0304-3959(02)00417-7 [DOI] [PubMed] [Google Scholar]
- 16. Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain J Neurol 2004;127(4):835–43. 10.1093/brain/awh098 [DOI] [PubMed] [Google Scholar]
- 17. Loggia ML, Berna C, Kim J, et al. The lateral prefrontal cortex mediates the hyperalgesic effects of negative cognitions in chronic pain patients. J Pain 2015;16(8):692–9. 10.1016/j.jpain.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yarnitsky D, Granot M, Granovsky Y.. Pain modulation profile and pain therapy: Between pro- and antinociception. Pain 2014;155(4):663–5. 10.1016/j.pain.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 19. Kosek E, Hansson P.. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain 1997;70(1):41–51. 10.1016/S0304-3959(96)03295-2 [DOI] [PubMed] [Google Scholar]
- 20. Derbyshire SWG, Whalley MG, Oakley DA.. Fibromyalgia pain and its modulation by hypnotic and non-hypnotic suggestion: An fMRI analysis. Eur J Pain 2009;13(5):542–50. 10.1016/j.ejpain.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 21. Montoya P, Sitges C, García-Herrera M, et al. Abnormal affective modulation of somatosensory brain processing among patients with fibromyalgia. Psychosom Med 2005;67(6):957–63. [DOI] [PubMed] [Google Scholar]
- 22. Kamping S, Bomba IC, Kanske P, Diesch E, Flor H.. Deficient modulation of pain by a positive emotional context in fibromyalgia patients. Pain 2013;154(9):1846–55. 10.1016/j.pain.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 23. Rhudy JL, DelVentura JL, Terry EL, et al. Emotional modulation of pain and spinal nociception in fibromyalgia. Pain 2013;154(7):1045–56. 10.1016/j.pain.2013.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellingson LD, Shields MR, Stegner AJ, Cook DB.. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain 2012;13(2):195–206. 10.1016/j.jpain.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellingson LD, Stegner AJ, Schwabacher IJ, Koltyn KF, Cook DB.. Exercise strengthens central nervous system modulation of pain in fibromyalgia. Brain Sci 2016;6(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ossipov MH, Morimura K, Porreca F.. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014;8(2):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seminowicz DA, Davis KD.. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 2006;120(3):297–306. 10.1016/j.pain.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 28. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33(2):160–72. [DOI] [PubMed] [Google Scholar]
- 29. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J.. An inventory for measuring depression. Arch Gen Psychiatry 1961;4(6):561–71. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 30. Spielberger CD, Gorsuch RL, Luschene RE.. Manual for the State-Trait Anxiey Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1970. [Google Scholar]
- 31. McNair DM, Lorr M, Droppleman LF.. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 32. Melzack R. The short-form McGill Pain Questionnaire. Pain 1987;30(2):191–7. 10.1016/0304-3959(87)91074-8 [DOI] [PubMed] [Google Scholar]
- 33. Burckhardt CS, Clark SR, Bennett RM.. The fibromyalgia impact questionnaire: Development and validation. J Rheumatol 1991;18(5):728–33. [PubMed] [Google Scholar]
- 34. Sullivan M, Bishop SR, Pivik J.. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;7(4):524–32. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 35. D’Eon JL, Harris CA, Ellis JA.. Testing factorial validity and gender invariance of the pain catastrophizing scale. J Behav Med 2004;27(4):361–72. [DOI] [PubMed] [Google Scholar]
- 36. Osman A, Barrios FX, Kopper BA, et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 1997;20(6):589–605. 10.1023/A:1025570508954 [DOI] [PubMed] [Google Scholar]
- 37. Osman A, Barrios FX, Gutierrez PM, et al. The Pain Catastrophizing Scale: Further psychometric evaluation with adult samples. J Behav Med 2000;23(4):351–65. 10.1023/A:1005548801037 [DOI] [PubMed] [Google Scholar]
- 38. Gracely RH, McGrath F, Dubner R.. Ratio scales of sensory and affective verbal pain descriptors. Pain 1978;5(1):5–18. 10.1016/0304-3959(78)90020-9 [DOI] [PubMed] [Google Scholar]
- 39. McLoughlin MJ, Stegner AJ, Cook DB.. The relationship between physical activity and brain responses to pain in fibromyalgia. J Pain 2011;12(6):640–51. 10.1016/j.jpain.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bantick SJ, Wise RG, Ploghaus A, et al. Imaging how attention modulates pain in humans using functional MRI. Brain J Neurol 2002;125(2):310–9. 10.1093/brain/awf022 [DOI] [PubMed] [Google Scholar]
- 41. Valet M, Sprenger T, Boecker H, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain 2004;109(3):399–408. [DOI] [PubMed] [Google Scholar]
- 42. Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18(6):643–62. 10.1037/h0054651 [DOI] [Google Scholar]
- 43. MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychol Bull 1991;109(2):163–203. 10.1037/0033-2909.109.2.163 [DOI] [PubMed] [Google Scholar]
- 44. Peyron R, Laurent B, García-Larrea L.. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 2000;30(5):263–88. [DOI] [PubMed] [Google Scholar]
- 45. Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29(3):162–73. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- 46. Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J.. A probabilistic atlas of the human brain: Theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 1995;2(2):89–101. [DOI] [PubMed] [Google Scholar]
- 47. Chen G, Saad ZS, Nath AR, Beauchamp MS, Cox RW.. FMRI group analysis combining effect estimates and their variances. Neuroimage 2012;60(1):747–65. 10.1016/j.neuroimage.2011.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dehghan M, Schmidt-Wilcke T, Pfleiderer B, et al. Coordinate-based (ALE) meta-analysis of brain activation in patients with fibromyalgia. Hum Brain Mapp 2016;37(5):1749–58. 10.1002/hbm.23132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bingel U, Tracey I.. Imaging CNS modulation of pain in humans. Physiology 2008;23:371–80. 10.1152/physiol.00024.2008 [DOI] [PubMed] [Google Scholar]
- 50. Allen EA, Erhardt EB, Calhoun VD.. Data visualization in the neurosciences: Overcoming the curse of dimensionality. Neuron 2012;74(4):603–8. 10.1016/j.neuron.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schreiber KL, Campbell C, Martel MO, et al. Distraction analgesia in chronic pain patients: The impact of catastrophizing. Anesthesiology 2014;121(6):1292–301. 10.1097/ALN.0000000000000465 [DOI] [PubMed] [Google Scholar]
- 52. Owens MA, Bulls HW, Trost Z, et al. An examination of pain catastrophizing and endogenous pain modulatory processes in adults with chronic low back pain. Pain Med 2016;17(8):1452–64. 10.1093/pm/pnv074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roussel NA, Nijs J, Meeus M, et al. Central sensitization and altered central pain processing in chronic low back pain: Fact or myth? Clin J Pain 2013;29(7):625–38. [DOI] [PubMed] [Google Scholar]
- 54. Atlas LY, Lindquist MA, Bolger N, Wager TD.. Brain mediators of the effects of noxious heat on pain. Pain 2014;155(8):1632–48. 10.1016/j.pain.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lorenz J, Minoshima S, Casey KL.. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain J Neurol 2003;126(Pt 5):1079–91. [DOI] [PubMed] [Google Scholar]
- 56. Cromheeke S, Mueller SC.. Probing emotional influences on cognitive control: An ALE meta-analysis of cognition emotion interactions. Brain Struct Funct 2014;219(3):995–1008. 10.1007/s00429-013-0549-z [DOI] [PubMed] [Google Scholar]
- 57. Gray JR, Braver TS, Raichle ME.. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci U S A 2002;99(6):4115–20. 10.1073/pnas.062381899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peers PV, Simons JS, Lawrence AD.. Prefrontal control of attention to threat. Front Hum Neurosci 2013;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vlaeyen JWS, Linton SJ.. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 2012;153(6):1144–7. 10.1016/j.pain.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 60. Bunge SA, Klingberg T, Jacobsen RB, Gabrieli JDE.. A resource model of the neural basis of executive working memory. Proc Natl Acad Sci U S A 2000;97(7):3573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bertolucci PHF, de Oliveira FF.. Cognitive impairment in fibromyalgia. Curr Pain Headache Rep 2013;17(7):344. 10.1007/s11916-013-0344-9 [DOI] [PubMed] [Google Scholar]
- 62. Velly AM, Look JO, Carlson C, et al. The effect of catastrophizing and depression on chronic pain—a prospective cohort study of temporomandibular muscle and joint pain disorders. Pain 2011;152(10):2377–83. [DOI] [PubMed] [Google Scholar]
- 63. Cook DB, Lange G, Ciccone DS, et al. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol 2004;31(2):364–78. [PubMed] [Google Scholar]
- 64. George SZ, Wittmer VT, Fillingim RB, Robinson ME.. Comparison of graded exercise and graded exposure clinical outcomes for patients with chronic low back pain. J Orthop Sports Phys Ther 2010;40(11):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seminowicz DA, Shpaner M, Keaser ML, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 2013;14(12):1573–84. 10.1016/j.jpain.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.