Abstract
We have shown that acute ozone inhalation activates sympathetic-adrenal-medullary and hypothalamus-pituitary-adrenal stress axes, and adrenalectomy (AD) inhibits ozone-induced lung injury and inflammation. Therefore, we hypothesized that stress hormone receptor agonists (β2 adrenergic-β2AR and glucocorticoid-GR) will restore the ozone injury phenotype in AD, while exacerbating effects in sham-surgery (SH) rats. Male Wistar Kyoto rats that underwent SH or AD were treated with vehicles (saline + corn oil) or β2AR agonist clenbuterol (CLEN, 0.2 mg/kg, i.p.) + GR agonist dexamethasone (DEX, 2 mg/kg, s.c.) for 1 day and immediately prior to each day of exposure to filtered air or ozone (0.8 ppm, 4 h/day for 1 or 2 days). Ozone-induced increases in PenH and peak-expiratory flow were exacerbated in CLEN+DEX-treated SH and AD rats. CLEN+DEX affected breath waveform in all rats. Ozone exposure in vehicle-treated SH rats increased bronchoalveolar lavage fluid (BALF) protein, N-acetyl glucosaminidase activity (macrophage activation), neutrophils, and lung cytokine expression while reducing circulating lymphocyte subpopulations. AD reduced these ozone effects in vehicle-treated rats. At the doses used herein, CLEN+DEX treatment reversed the protection offered by AD and exacerbated most ozone-induced lung effects while diminishing circulating lymphocytes. CLEN+DEX in air-exposed SH rats also induced marked protein leakage and reduced circulating lymphocytes but did not increase BALF neutrophils. In conclusion, circulating stress hormones and their receptors mediate ozone-induced vascular leakage and inflammatory cell trafficking to the lung. Those receiving β2AR and GR agonists for chronic pulmonary diseases, or with increased circulating stress hormones due to psychosocial stresses, might have altered sensitivity to air pollution.
Keywords: adrenalectomy, glucocorticoid receptor agonist, adrenergic receptor agonist, ozone, lung inflammation, lung edema
Chronic activation of neuroendocrine stress responses involving the sympathetic-adrenal-medullary (SAM) and hypothalamus-pituitary-adrenal (HPA) axes have been linked to development of asthma (Douwes et al., 2011), chronic cardiovascular diseases (An et al., 2016), obesity (Hewagalamulage et al., 2016; Hirotsu et al., 2015), and diabetes (Joseph and Golden, 2017). Individuals living in disadvantaged communities with psychosocial stressors have exacerbated air pollution effects including increased severity of asthma and chronic obstructive pulmonary disease (COPD) (Clark et al., 2015; Clougherty and Kubzansky, 2008; Shmool et al., 2014; Wright, 2011). Furthermore, maternal stress during pregnancy has been postulated to increase risk of developing asthma in children and COPD at an old age through reprogramming of the HPA stress axis (Awotidebe et al., 2017). Thus, neuroendocrine pathways involving SAM and HPA axes have been assumed to interactively enhance adverse health effects of air pollution.
The activation of neural SAM- and HPA-mediated stress responses increases the release of epinephrine and corticosterone/cortisol, respectively, from adrenal glands, which in turn, produces tissue-specific homeostatic changes in metabolic and immune processes associated with the fight-or-flight response (Gorman, 2013). Chronic elevations of stress hormones have been associated with neurological abnormalities including psychosocial disorders and systemic inflammatory conditions (Barr, 2017; Henley and Lightman, 2014). We have previously shown that acute ozone- and acrolein-induced respiratory injury and inflammation are associated with the activation of neuroendocrine stress pathways in both healthy and diabetic rat models (Bass et al., 2013; Miller et al., 2015; Snow et al., 2017) and in healthy humans (Miller et al., 2016a). We have also demonstrated that ozone and acrolein exposure increases circulating epinephrine and corticosterone, and produces metabolic alterations similar to a fight-or-flight response (Bass et al., 2013; Miller et al., 2015, 2016a; Snow et al., 2017). Even though ozone inhalation has been shown to contribute to airway responses through vagal C-fibers in the lung (Taylor-Clark and Undem, 2010; Schelegle and Walby, 2012), activate stress responsive regions in the central nervous system (Gackière et al., 2011), induce autonomic reflex mechanisms (Gordon et al., 2014), and increase the release of adrenocorticotrophic hormone (Thomson et al., 2013), the precise mechanisms by which it activates the SAM and HPA axes are not well understood.
Once released into the circulation, epinephrine and corticosterone exert their tissue effects through adrenergic and glucocorticoid receptors (GR). Adrenergic receptors (AR) are G-protein-coupled receptors involved in a variety of cellular processes induced during a fight-or-flight stress response (Ghanemi and Hu, 2015; Tank and Lee, 2015). Two distinct classes of AR, α and β, are widely distributed in blood vessels and body organs in a tissue-specific manner. β adrenergic receptors (βAR) are widely distributed in the lung. Nearly 70% of the βAR are β2-type (β2AR) with high affinity for epinephrine while the remaining 30% are β1-type (β1AR) with high affinity for both epinephrine and nor-epinephrine (Barnes, 2004). The β2AR when activated by epinephrine induces bronchial relaxation and alters lung vascular permeability (Barnes, 2004).
Circulating corticosterone exerts its cellular effects by binding to GR. GR are distributed in virtually all tissues in the body, including lung, and are involved in regulation of metabolic and immune processes (Oakley and Cidlowski, 2011). Activation of GR by glucocorticoids leads to immunosuppression through transcriptional repression of proinflammatory genes (De Bosscher et al., 2000). GR agonists are the primary therapeutic agents used to reduce chronic lung inflammation present in pulmonary diseases (Barnes, 2016). In many instances, a combination of β2AR and GR agonists are used to reverse and suppress airway bronchoconstriction and inflammation present in asthma and COPD (Cain and Cidlowski, 2015; Waldeck, 2002).
We have recently demonstrated that total bilateral adrenalectomy (AD), which diminishes circulating epinephrine and corticosterone in rats, inhibits ozone-induced metabolic changes as well as lung injury, protein leakage, and inflammation (Miller et al., 2016b; Henriquez et al., 2017). Pharmacological inhibition of AR and GR also led to suppression of ozone-induced vascular leakage and neutrophil inflammation, suggesting a proinflammatory role of endogenously released epinephrine and corticosterone (Henriquez et al., 2018). Although glucocorticoids have been shown to suppress immune response and inhibit lymphocyte function in rats, the proinflammatory effect of newly released epinephrine and corticosterone has also been reported after an acute restraint stress (Dhabhar et al., 2012) and after a treatment with exogenous epinephrine and glucocorticoids (Dhabhar, 2014). However, the potential mechanism by which the activation of βAR and GR regulate acute ozone-induced inflammation is not clear. Herein we hypothesized that combined treatment of rats with clenbuterol (CLEN; β2AR agonist), plus dexamethasone (DEX; GR agonists), would restore ozone-induced pulmonary vascular leakage and inflammation in adrenalectomized (AD) rats, while exacerbating ozone effects in sham control (SH) rats with intact SAM and HPA axes.
MATERIALS AND METHODS
Animals
Male, 11–12-week-old Wistar Kyoto (WKY) rats, purchased from Charles River Laboratory (Raleigh, North Carolina), were pair-housed in polycarbonate cages with beta chip bedding. Animal rooms were maintained at 50–65% relative humidity [RH], 21°C temperature and 12 h light/dark cycle in our animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Water and food (5001 Purina rat chow; Brentwood, Missouri) were provided ad libitum except where indicated. All animal procedures were approved by the U.S. Environmental Protection Agency (U.S. EPA), National Health and Environmental Effects Research Laboratory (NHEERL) Animal Care and Use Committee prior to the start of the experiment.
Animal surgeries and drug treatments
At 12–13 weeks of age, rats underwent either total bilateral AD or control sham (SH) surgeries as described previously (Miller et al., 2016b). Briefly, the rats were anesthetized by i.p. injection of ketamine (25–50 mg/kg in saline). Once under anesthesia, buprenorphine was injected (0.02 mg/kg/ml in saline; s.c.) for analgesia. During the surgery, additional anesthesia was induced by inhalation of vaporized isoflurane (∼3%) using a nose-only cone as needed. Veterinarians from Charles River Laboratories Inc. performed surgeries under aseptic conditions while animals were placed in sternal recumbency. Surgeries were performed using protocols established at Charles River Laboratories Inc. Except for the removal of adrenal glands, SH surgeries involved similar anesthesia and surgical approaches as AD. The recovery was assured by the observation of the animal’s movement while on heating pads. Once awake, animals were treated with meloxicam (0.2 mg/kg; s.c.) for analgesia. Additional analgesia was provided by administering buprenorphine (0.02 mg/ml/kg, s.c.) every 8–12 h for 2 times. Half of the rats (D + 2 groups as indicated below) were injected with temperature-sensitive transponders s.c. over the caudal dorsal surface to allow measurement of body temperature (BDMS, Seaford, Delaware). SH rats received normal tap water for drinking while AD rats were provided saline (0.9% sodium chloride) after the surgery to maintain adequate fluid and electrolyte balance. Following surgery, the animals were pair housed with Enviro Dry enrichment/nesting material, provided with powdered as well as pelleted food, and allowed to recover for 4–6 days prior to any drug treatment related to this study. This approach was based on a number of AD studies that used/recommended a 4–6-day recovery period to avoid secondary changes in the body occurring as a result of AD (Miller et al. 2016b; Nicolaides et al., 2013; Sakakibara et al., 2014).
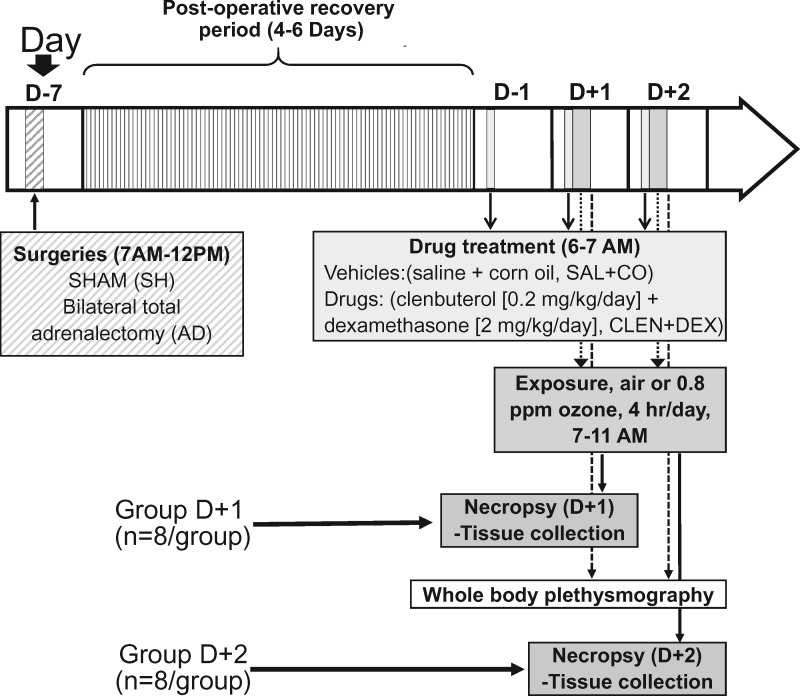
The general experimental design is shown in Figure 1. SH and AD rats were randomized by body weight into four treatment groups (vehicle: air, vehicle: ozone, CLEN+DEX: air, and CLEN+DEX: ozone) over 2 time periods (1-day [D + 1] or 2-day [D + 2] exposures). This resulted in 8 total groups with 8 rats/group. Vehicle or drug treatments began 1 day prior to the first air or 0.8 ppm ozone exposure (4 h/day for 1 or 2 consecutive days). Vehicle and drug treatments were administered each day prior to the beginning of inhalation exposures. Vehicle injections were comprised of saline (1 ml/kg, i.p.) followed by pharmaceutical grade corn oil (1 ml/kg, s.c.). Drug treatments included clenbuterol hydrochloride, a long acting β2AR agonist (CLEN; 0.2 mg/kg in saline, i.p.) followed by a GR agonist, dexamethasone (DEX; 2 mg/ml corn oil/kg, s.c.). Since we were attempting to restore the activities of epinephrine and corticosterone at the basal levels that were completely depleted by AD procedure and provide the additional replenishment for increased levels observed during ozone exposure in SH rats, these relatively high doses were selected. Although these doses are several fold higher than those used therapeutically, these CLEN and DEX concentrations are within the range used in other laboratory rodent studies for the purpose of inducing bronchodilation and immunosuppression, respectively (Griffin et al., 2018; Jonasson et al., 2013).
Figure 1.
Experimental design and timeline. The timing for surgery, drug treatments, exposures, whole body plethysmography (WBP), and necropsies are indicated by arrows. Animals assigned to 1-day or 2-day air or ozone exposure (4 h/day) are referred to as groups D + 1 and D + 2, respectively. Animals belonging to D + 2 groups were subjected to WBP immediately after the first and second day of exposure. Necropsy and tissue collection were performed immediately after exposure on D + 1 groups (within 2 h) or immediately after exposure and WBP for D + 2 groups (within 2.5 h).
Ozone exposure
Ozone was generated using a silent arc discharge generator (OREC, Phoenix, Arizona) from oxygen and transported to Rochester style “Hinners” chambers using mass flow controllers (Coastal Instruments Inc., Burgaw, North Carolina). Ozone concentration was controlled and recorded by photometric analyzers (API Model 400, Teledyne, San Diego, California). Mean chamber temperature, relative humidity, and air flow were recorded hourly (control chamber: 71.05 ± 0.32°F, 54.08 ± 0.67 and 256.1 ± 0.57 l/min; ozone chamber: 73.10 ± 0.14°F, 50.40 ± 0.44 and 261.2 ± 0.43 l/min, respectively). Rats were exposed to air or 0.8 ppm ozone, 4 h/day for either 1 day (D + 1) or 2 consecutive days (D + 2). The actual daily mean chamber concentration of ozone was 0.800 ± 0.04 ppm (mean ± SD).
In-life assessment
Body weights were measured once prior to and then daily after the surgery for all rats, including each day post-exposure. In the D + 2 groups, subcutaneous temperature was assessed (n = 4/group), immediately before and after each ozone exposure. Similarly, whole body plethysmography (WBP) was performed in the D + 2 groups (n = 8/group) following each day of exposure to detect potential alterations in breathing patterns. These rats were first acclimated to the WBP chambers for 2 days prior to the first plethysmography assessment. After each exposure, rats were removed from the exposure chambers and transported to a separate quiet room where, on a rotational basis, rats representing each exposure/treatment group were placed in rat-sized WBP chambers (n = 8 rats/run). After a 2 min acclimation period, ventilatory parameters were recorded for an additional 5 min. Inclusion criteria for acceptable breaths included inspiratory volume and expiratory volume within 40%, and breathing frequency of ≤ 375 bpm to minimize the influence of high frequency breathing related to odor discrimination (i.e., overt sniffing activity). Furthermore, all traces were visually inspected to ensure that valid breath patterns were not excluded. During calibration and data acquisition, a band pass filter (low of 0.25 Hz and high of 35 Hz) and AC filter designed for rats/guinea pigs were used. Measurements were averaged every 10 s for the 5-min period using EMKA iox 2 software (SCIREQ, Montreal, Canada). Ventilatory parameters obtained included: breathing frequency, peak inspiratory flow (PIF), peak expiratory flow (PEF), inspiratory time (Ti), expiratory time (Te), relaxation time (RT), and calculated parameters such as pause, and enhanced pause (PenH).
Blood collection at necropsy, complete blood counts, and assessment of circulating stress hormones
An overdose of fatal plus (sodium pentobarbital, Virbac AH, Inc., Fort Worth, Texas; >200 mg/kg, i.p.) was used to euthanize rats. For D + 1 groups, rats were necropsied within 2 h of termination of the first exposure, whereas for D + 2 groups, rats were necropsied after their second exposure and WBP data acquisition (within 2.5 h). Blood samples from the abdominal aorta were collected in vacutainer tubes. Complete blood counts were performed using EDTA blood samples on a Beckman-Coulter AcT blood analyzer (Beckman-Coulter Inc., Fullerton, California), which provided a relative measure of total white blood cells (WBC) and lymphocytes. Blood smears were also obtained and then stained with Diff-quick to enumerate relative neutrophil and monocyte numbers. The remaining EDTA blood samples were centrifuged at 3500 × g for 10 min and resulting plasma samples were stored at −80°C until analysis. Serum was collected after centrifugation of blood samples collected in serum separator tubes at 3500 × g for 10 min and stored at −80°C until use. Epinephrine levels in plasma samples were quantified using a kit obtained from Rocky Mountain Diagnostics following the manufacturer’s protocol (Colorado Springs, Colorado). Corticosterone levels were quantified in serum employing an immunoassay kit and following manufacturer’s directions (Arbor Assays, Ann Arbor, Michigan).
Bronchoalveolar lavage and cell counts
Bronchoalveolar lavage fluid (BALF) was collected by cannulating the trachea. The left lung was tied and the right lung was lavaged 3 times using the same aliquot of Ca2+- and Mg2+-free PBS, pH 7.4, 37°C, at 28 ml/kg body weight for total lung capacity with the right lung weight being 60% of the total lung weight. Whole BALF (0.5 ml) was diluted to 10 ml using isotone and spiked with 0.2 ml saponin to lyse cells. Nuclei were counted using a Z1 Coulter Counter (Coulter Inc., Miami, Florida). Whole BALF was also used to prepare cytospin slides, which were stained with Diff-quick and cell differentials were performed under light microscopy (300 cells/slide, 1 slide/animal). The remaining BALF samples were centrifuged (1500 × g for 5 min) and cell-free BALF aliquots were analyzed for lung injury markers. The lavaged caudal lobe from the right lung was removed, blotted, frozen in liquid nitrogen, and stored at −80°C for RNA extraction.
Assessment of lung protein leakage markers and inflammatory cytokines
BALF total protein was assessed using Coomassie Plus Protein Reagent from Thermo Fisher Diagnostics (Rockford, Illinois) and albumin standards from Sigma-Aldrich (St. Louis, Missouri). BALF albumin levels were determined using a kit from Sekisui Diagnostics (Lexington, Massachusetts) while N-acetylglucosaminidase (NAG) activity was assessed using reagents and controls from Sigma-Aldrich Diagnostics (St. Louis, Missouri). These assays were modified for use on the Konelab Arena 30 clinical analyzer (Thermo Chemical Lab Systems, Espoo, Finland). Cell-free BALF samples were used to quantify cytokine proteins using the V-PLEX proinflammatory panel 2 (rat) kit following the manufacturer’s protocol (Mesoscale Discovery, Gaithersburg, Maryland). The electrochemiluminescence signals for each protein were detected using Meso Scale Discovery® platform (Mesoscale Discovery Inc., Rockville, Maryland). For some cytokines, the BALF levels in control animals were below the limit of detection. Those values were imputed with the lowest quantified value in that group for a given cytokine.
Flow cytometry of circulating WBC
For flow cytometry analyses of blood samples, the general procedures were performed following previously published methods (DeWitt et al., 2016). Briefly, fresh aortic blood samples collected in EDTA blood tubes (500 μl) from the D + 1 animals were treated with RBC lysis buffer (Affymetrix eBioscience, Santa Clara, California) and then washed twice with HBSS without Ca2+ and Mg2+ (Thermo Fisher, Waltham, Massachusetts). The cells were suspended in staining buffer containing HBSS with 1% bovine serum albumin (BSA) and 0.1% sodium azide (Sigma-Aldrich, St. Louis, Missouri). WBC concentrations were determined using a Z1 Coulter Counter (Coulter, Inc., Miami, Florida). Cells were washed once with PBS and incubated for 30 min at room temperature with LIVE/DEADTM fixable violet dead cell stain (Thermo Fisher, Waltham, Massachusetts) to determine viable cells. After incubation, cells were washed 3 times with staining buffer and then incubated with mouse anti-rat CD32 (BD Pharmingen, San Jose, California) to block FC receptor-mediated nonspecific antibody binding as per manufacturer’s instructions. Cells were then washed three times with staining buffer and labeled for 30 min with the following monoclonal antibodies: APC-Cy7 mouse anti-rat CD45, PE mouse anti-rat RP1, PE-Cy7 mouse anti-rat CD4, and FITC mouse anti-rat CD8a (BD Pharmingen, San Jose, California). Cells labeled with fluorochrome-conjugated isotype control antibodies (APC-Cy7 mouse anti-rat IgG, ĸ; PE mouse anti-rat IgG2a, ĸ; PE-Cy7 mouse anti-rat IgG2a, ĸ; FITC mouse anti-rat IgG1 [BD Pharmingen, San Jose, California]) were used as negative controls. Additional cell samples, including unstained cells and fluorescence minus one (FMO) controls were utilized to aid in identifying and ensuring accurate gating of negative and positive cell populations. After staining, cells were washed three times with staining buffer, fixed with 0.05% formaldehyde in PBS and kept in the dark at 4°C (no longer than 1 day) until FACS analysis. Data were collected on a LSR II flow cytometer (BD Biosciences, Mississauga, Canada) using FACS Diva software (BD Biosciences, Mississauga, Canada). The quantification of the data was performed using FlowJo software (TreeStar, Inc., Ashland, Oregon). Live cells were gated based on CD45 expression for the analysis of subpopulations CD4−/CD8a−, CD4−/CD8a+, CD4+/CD8a−, CD4+/CD8a+, and RP1+. Due to the low number of leukocytes available, a minimum of 1000 events per sample were counted.
Lung RNA isolation and real time-quantitative PCR
Anatomically uniform portions of frozen caudal lung lobes were separated and weighed (20–30 mg) for RNA extraction using the RNeasy mini kit (Qiagen, Valencia, California). RNA was quantified using Qubit 2.0 fluorimeter (Thermo Fisher, Waltham, Massachusetts). Qscript cDNA Supermix (Quanta Biosciences, Beverly, Massachusetts) was used to synthesize cDNA by reverse transcription. Primers were designed using Rattus Norvegicus sequences annotated using NCBI and obtained from Integrated DNA Technologies, Inc. (Coralville, Iowa; Table 1). SYBR® Green PCR Master Mix (Thermo Fisher, Waltham, Massachusetts) was used to perform quantitative PCR using the Applied Biosystems 7900HT Sequence Detection System (Foster City, California). Relative mRNA expression was calculated employing the ΔΔCt method using β-actin as the housekeeping gene and the vehicle-air treated SH group as control. The determination of relative lung mRNA expression was restricted to D + 1 groups since ozone-induced changes in RNA expression peak rapidly after first ozone exposure (Hollingsworth et al., 2007; Kodavanti et al., 2015). Il6, Tnf, Cxcl2, and Il4 were selected as proinflammatory endpoints, which have shown consistent up-regulation after ozone exposure in previous publications (Henriquez et al., 2017). Furthermore, expression of Tsc22d3, also known as Gilz, a validated glucocorticoid responsive gene (Pepin et al., 2015), and Adrb2, the gene coding for β2AR, were measured to determine the influence of AD, ozone and CLEN+DEX on activation of AR and GR in the lungs.
Table 1.
Forward and Reverse Primer Sequences Designed for Each Gene Used in PCR
| Gene | Forward | Reverse |
|---|---|---|
| β-Actin (Actb) | 5′-CAACTGGGACGATATGGAGAAG-3′ | 5′-GTTGGCCTTAGGGTTCAGAG-3′ |
| Tumor necrosis factor (Tnf) | 5′-ACCTTATCTACTCCCAGGTTCT-3′ | 5′-GGCTGACTTTCTCCTGGTATG-3′ |
| Interleukin 6 (Il6) | 5′-CTTCACAAGTCGGAGGCTTAAT-3′ | 5′-GCATCATCGCTGTTCATACAATC-3′ |
| Interleukin 4 (Il4) | 5′-GTCACCCTGTTCTGCTTTCT-3′ | 5′-GACCTGGTTCAAAGTGTTGATG-3′ |
| Chemokine (C-X-C motif)-ligand 2 (Cxcl2 or Mip2) | 5′-GCCTCGCTGTCTGAGTTTAT-3′ | 5′-GAGCTGGCCAATGCATATCT-3′ |
| TSC22 domain family protein 3 (Tsc22d3 or Gilz) | 5′-CCGAATCATGAACACCGAAATG-3′ | 5′-GCAGAGAAGAGAAGAAGGAGATG-3′ |
| Beta-2 adrenergic receptor (Adrb2) | 5′-CTCCTTAACTGGTTGGGCTATG-3′ | 5′-CCTGGAAGGCAATCCTGAAA-3′ |
Statistics
Approximately 10% of male WKY rats develop spontaneous cardiac hypertrophy, which has been shown to be associated with secondary pulmonary complications causing high baseline levels of lung injury markers and inflammation (Shannahan et al., 2010). While this hypertrophy does not appear to be associated with systemic hypertension, we have noted that it is often associated with both left- and right-sided chamber enlargement, and increased BALF protein, and inflammatory cells (Shannahan et al., 2010). Therefore, we have developed an exclusion criterion to remove these animals from further analysis whenever heart/body weight ratio is above 20% of the average for the group such that the data interpretation is not affected. In this study too, we excluded data from animals exhibiting cardiac hypertrophy and hence the number of observations ranged from n = 6–8. For each endpoint, data were log transformed when normal distribution and homoscedasticity were not satisfied using Shapiro–Wilk and Levene’s tests, respectively. Data were analyzed using a two-way analysis of variance (ANOVA). The effects of AD were determined by analyzing respective vehicle- and CLEN+DEX-treated groups separately using independent two-way ANOVAs; while drug treatment effects were determined by analyzing SH and AD groups separately as independent factors using two-way ANOVA. This strategy was used separately for D + 1 and D + 2 experiments. Tukey’s test was used to correct for multiple comparisons. Significant differences were considered when p ≤ 0.05 was achieved. For all bar graphs, data (n = 6–8 animals/group) are expressed as mean ± SEM. GraphPad prism 7.03 and Statext 2.7 software were used for statistical analysis. For gene expression analysis, outliers were identified and discarded using the ROUT method (robust regression and outlier removal, Motulsky and Brown, 2006) prior to statistical analysis.
RESULTS
Changes in Body Weight and Subcutaneous Temperature Induced by AD, CLEN+DEX Treatment and Ozone Exposure
To examine the general physiological effects of ozone exposure, AD and CLEN+DEX treatment, body weights, and subcutaneous temperatures were monitored prior to and after each day of exposure in the D + 2 groups (Figure 2). AD rats had significantly reduced body weight gain after surgery relative to SH rats (up to 12% on D + 2; significant for air-exposed vehicle-treated rats at D + 1 and D + 2; ozone-exposed vehicle-treated rats at D + 1; CLEN+DEX-treated, ozone-exposed rats D + 1 and D + 2). Significant reductions were also noted in air-exposed CLEN+DEX-treated SH rats and ozone-exposed vehicle-treated SH rats after each day of exposure (up to 10% on D + 2) (Figure 2A).
Figure 2.
Body weight and temperature changes induced by ozone exposure in vehicle- and CLEN+DEX-treated SH and AD rats. A, Body weights of rats were recorded before surgery (D-7) and immediately after each day of exposure to air or ozone (0.8 ppm, 4 h/day) in the D + 2 animals (n = 8/group). B, Subcutaneous temperatures were measured using a receiver, which acquired signals through subcutaneously injected transponders, immediately before and after each day of exposure to air or ozone (0.8 ppm, 4 h/day) for the D + 2 groups (n = 4/group). Bar graphs show group mean ± SEM. Significant differences between groups (p-value ≤ .05) are indicated by * for ozone effect when compared with corresponding air-exposed rats, # for AD effect when compared with corresponding SH rats, and † for CLEN+DEX effect when compared with corresponding vehicle-treated rats.
Acute ozone exposure has been shown to cause hypothermia in animals as determined by reduction in core body temperature (Gordon et al., 2014). We noted that subcutaneous temperature was reduced by approximately 3°C in all ozone-exposed SH and AD rats when determined immediately following exposure on both days. Neither AD nor CLEN+DEX treatment changed subcutaneous temperature in air-exposed rats or modified the ozone-related reductions in body temperature (Figure 2B).
Circulating Stress Hormones Are Changed After Ozone Exposure in SH and AD Rats Treated With Vehicle and CLEN+DEX
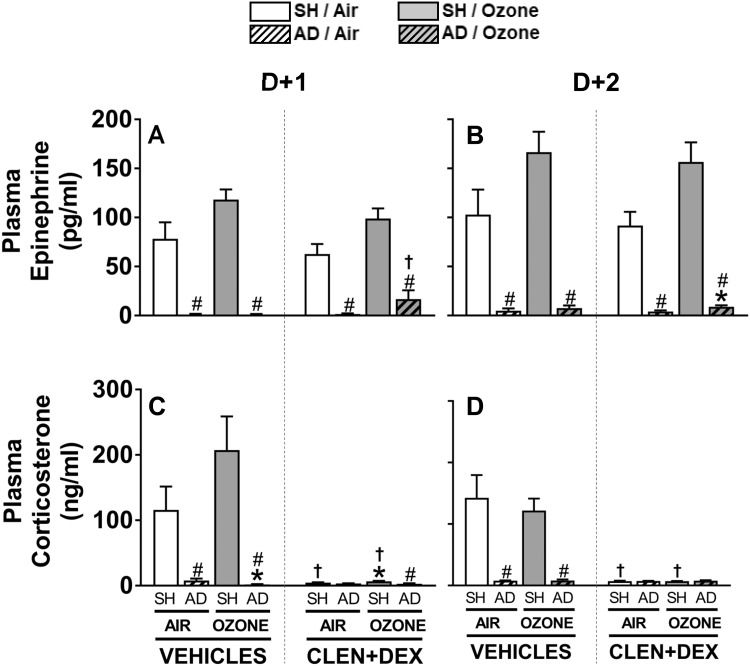
Based on the expected variability in levels of stress hormones due to several influencing factors (ie, precise time of blood collection, stress levels in animals during euthanasia injection), we have seen variable degrees of response to ozone in our past studies in male WKY rats (Miller et al., 2015, 2016b). In this study, although not significant, ozone exposure tended to increase circulating epinephrine levels at D + 1 and D + 2 (p = .13, each day using single group comparison) in vehicle-treated SH rats (Figs. 3A and 3B). The trend for ozone-induced increase in circulating corticosterone on D + 1 in vehicle-treated SH rats was not significant either (p = .24 using single group comparison) (Figure 3C). However, as expected, AD essentially eliminated detectable plasma epinephrine and corticosterone levels at D + 1 and D + 2 regardless of exposure and drug treatment (Figure 3). Moreover, CLEN+DEX treatment markedly reduced circulating corticosterone in all air- and ozone-exposed SH rats (Figs. 3C and 3D) but had no influence on epinephrine levels in air and ozone-exposed SH rats (Figs. 3A and 3B).
Figure 3.
Ozone-induced changes in circulating stress hormones in vehicle- and CLEN+DEX-treated SH and AD rats. Circulating stress hormones, epinephrine (A-B) and corticosterone (C-D), were measured in samples collected within 2.5 h after air or ozone (0.8 ppm) exposure (4 h/day) in D + 1 and D + 2 groups. Bar graphs show mean ± SEM of n = 6–8/group. Significant differences between groups (p-value ≤ .05) are indicated by * for ozone effect when compared with corresponding air-exposed rats, # for AD effect when compared with corresponding SH rats, and † for CLEN+DEX effect when compared with corresponding vehicle-treated rats.
Ozone-Induced Changes in Ventilatory Parameters in SH and AD Rats With and Without CLEN+DEX Treatment
After the filtered air or ozone exposures, rats were transported to a quiet room and were placed in WBP chambers. In order to detect maximal ventilatory distress responses in the ozone-sensitive WKY rats, WBP assessments were performed within ≅30 min of termination of the exposure. Examining first the ventilatory changes related to AD alone (i.e., AD, air-exposed, vehicle-treated rats), we observed trends of minor reductions (∼20%) in breathing frequency, (Figs. 4A and 4B) on both days, relative to the analogous group of SH rats. Data are consistent with the observed generally sedate behavior of the AD rats. No other ventilatory differences were observed for the AD vehicle-treated air-exposed rats when compared with SH vehicle-treated air-exposed rats. However, these air-exposed, vehicle-treated rats exhibited higher breathing frequencies than previous reports in which air control rats were left undisturbed much longer periods prior to obtaining basal breathing frequencies (Tepper et al., 1989, 1990; Schelegle et al., 2001).
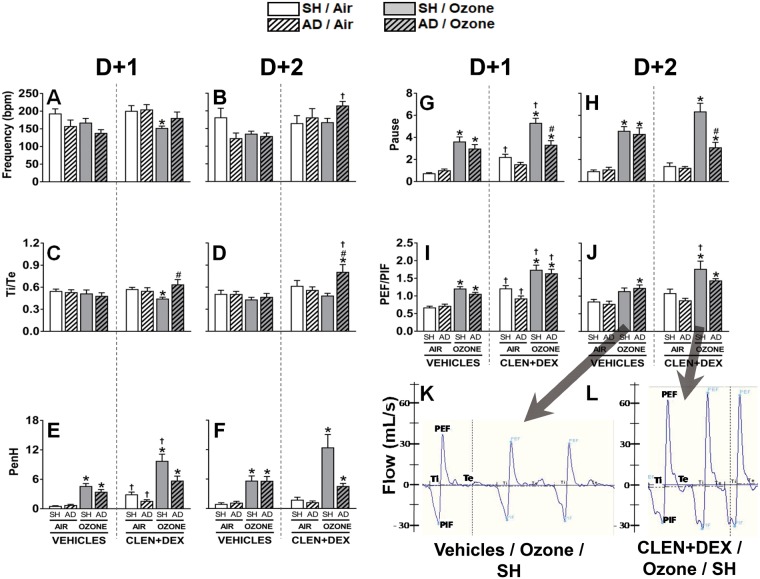
Figure 4.
Ventilatory parameters in rats are modulated by ozone, AD, and CLEN+DEX. Breathing frequency in breaths per minute (bpm; A, B), Ti/Te ratio (C, D), PenH [(Te/RT) − 1 × (PEF/PIF)] (E, F), Pause [(Te/RT) − 1] (G, H), PEF/PIF (I, J), and representative traces of respiration from vehicle-treated and CLEN+DEX-treated rats exposed to ozone (K, L) were assessed within 30 min following air or ozone (0.8 ppm) exposure (4 h/day) on both days for the D + 2 groups. Bar graphs show mean ± SEM of n = 6–8/group. Significant differences between groups (p-value ≤ .05) are indicated by * for ozone effect when compared with corresponding air-exposed rats, # for AD effect when compared with corresponding SH rats, and † for CLEN+DEX effect when compared with corresponding vehicle-treated rats.
Examining next differences in ventilatory patterns of ozone-exposed, vehicle-treated SH and AD rats, the trends of characteristic changes in breathing patterns occurring in ozone-sensitive WKY rats were observed at both time points, including: minor slowing of the respiratory rate (Figs. 4A and 4B); increases in PEF relative to PIF (PEF/PIF; Figs. 4I and 4J); increases in Pause [(Te/RT) – 1] (Figs. 4G and 4H), and thus, enhanced pause (PenH), the product of the PEF/PIF ratio and Pause (Figs. 4E and 4F). No significant differences were observed between vehicle-treated SH and AD rats in terms of these ozone-related ventilatory effects.
When examining the effects of CLEN+DEX treatment in air-exposed SH and AD rats, drug treatment did not change relative breathing frequency or Ti/Te ratios, but it did induce minor increases in PenH at D + 1 but not D + 2, largely due to increased PEF/PIF ratios.
With regards to the ozone-related ventilatory changes on D + 1, the CLEN+DEX-treated SH rats showed significant reductions in breathing frequency and Ti/Te ratios (Figs. 4A and 4C) in combination with the highest PenH values recorded (reflecting significant increases in both PEF/PIF and Pause components) (Figs. 4E–J). Similar trends were observed on D + 2, including still greater increases in PenH for this group. In carefully examining the shape of the waveform of these breaths (ie, WPB chamber flow rate fluctuations), we observed that in these rats, the PEF/PIF ratios were increased in part due to higher PEF values (Figs. 4I and 4J), and in part due to truncated PIF values as these rats often exhibited a biphasic inspiration pattern, with blunting their PIF values (Figs. 4K and 4L).
Lastly, we compared the superimposed effects of ozone exposure and CLEN+DEX treatment in the AD rats. These rats exhibited relatively higher breathing frequencies (D + 2) and Ti/Te ratios (at both time points), and had increased PEF/PIF ratios (at D + 1) like that of their analogous SH group, again in part due to biphasic inspiration (data not shown). In contrast, these AD rats exhibited lesser increases in Penh, primarily because the pause component was not as elevated) (Figs. 4E–H). Results suggest a mixed ventilatory pattern of worsening inspiratory effects (possibly due to underlying edema, hypoxia, or dehydration) in combination with reduced expiratory airflow obstruction.
Ozone-Induced Protein Leakage and Macrophage Activation Are Reduced by AD and Exacerbated by CLEN+DEX Treatment
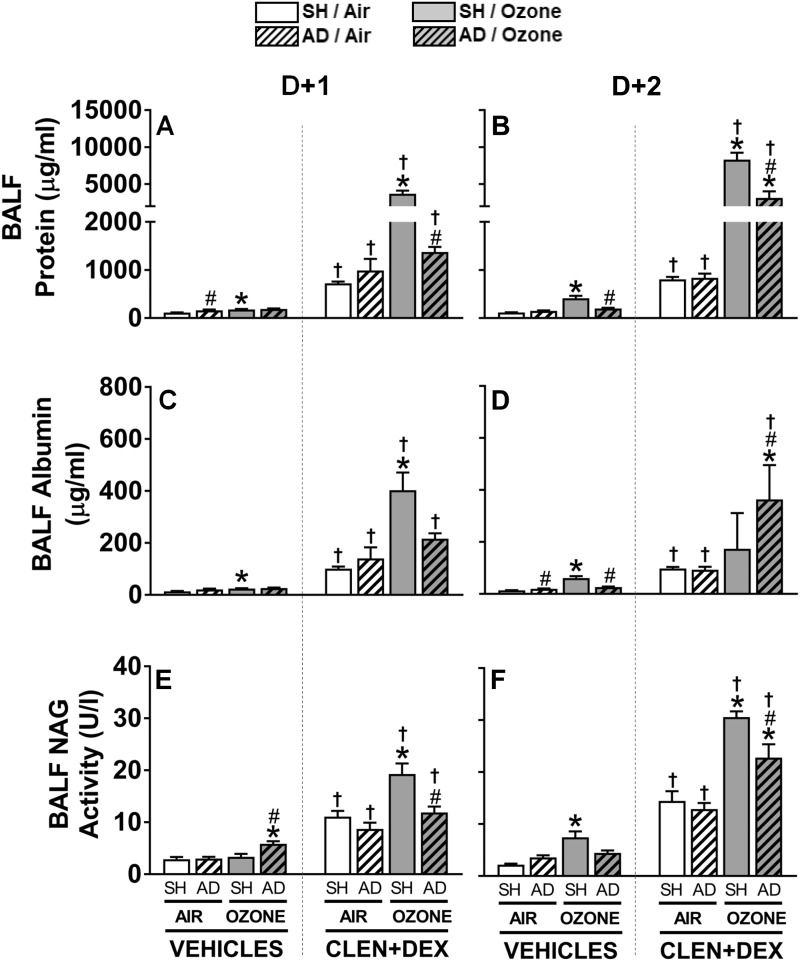
BALF protein and albumin were assessed to determine microvascular leakage in the lung. An increase in BALF protein was noted in air-exposed vehicle-treated AD rats at D + 1. In vehicle-treated SH rats, ozone exposure was also associated with significant increases in BALF protein, which was progressive over 2 days. However, BALF protein levels did not increase in vehicle-treated AD rats after ozone exposure (Figs. 5A and 5B). Regardless of the exposure or surgery status, CLEN+DEX treatment was associated with marked protein leakage at both time points in air-exposed SH and AD rats. Ozone exposure of CLEN+DEX-treated SH rats greatly exacerbated increases in BALF protein at both D + 1 and D + 2. AD moderately reduced the ozone-induced increase of BALF protein in CLEN+DEX-treated rats at D + 2 (Figs. 5A and 5B). Changes in BALF albumin generally followed a similar pattern of changes as BALF protein; except that the increase in albumin was greatest in ozone-exposed AD rats treated with CLEN+DEX at D + 2 (Figs. 5C and 5D).
Figure 5.
Ozone-induced pulmonary vascular leakage and macrophage activation are modulated by AD and CLEN+DEX. BALF protein (A, B), albumin (C, D), and NAG activity (E, F) were assessed in rats within 2.5 h following air or ozone (0.8 ppm) exposure (4 h/day) for D + 1 and D + 2 groups. Bar graphs show mean ± SEM of n = 6–8/group. Significant differences between groups (p-value ≤ .05) are indicated by * for ozone effect when compared with corresponding air-exposed rats, # for AD effect when compared with corresponding SH rats, and † for CLEN+DEX effect when compared with corresponding vehicle-treated rats.
As we have noted in previous publications (Henriquez et al., 2018), ozone exposure was also associated with BALF NAG activity (a marker of macrophage activation) increases in vehicle-treated SH rats at D + 2. AD diminished this ozone effect at D + 2 (Figure 5F). CLEN+DEX significantly increased BALF NAG activity at both time points in all SH and AD rats exposed to air. Ozone exposure further exacerbated NAG activity increases in CLEN+DEX-treated rats at D + 1 and D + 2 while AD blunted this ozone-induced response (Figs. 5E and 5F).
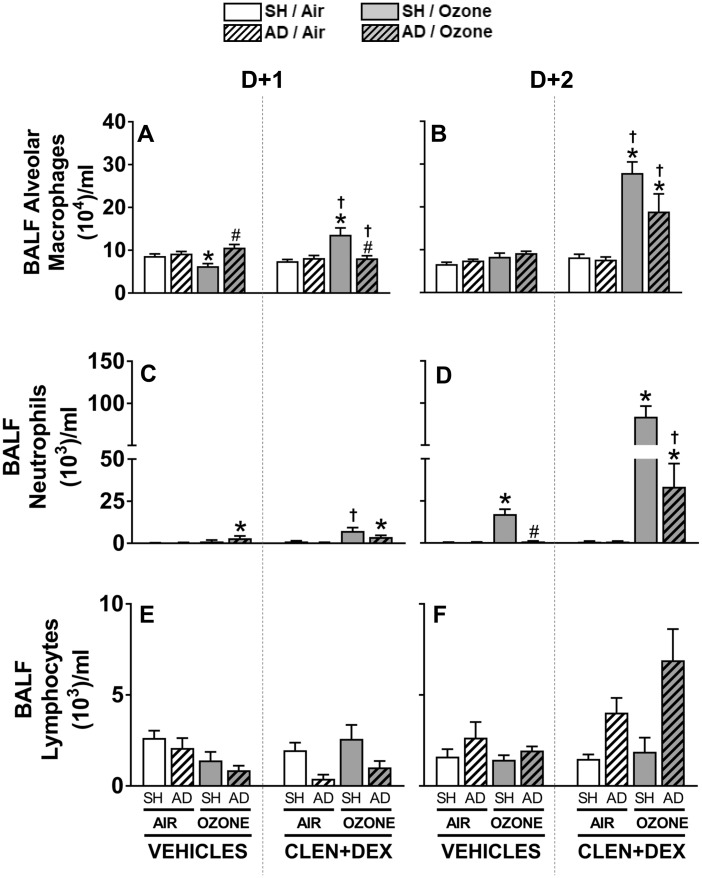
Ozone-Induced Pulmonary Inflammation Is Reduced by AD and Restored by CLEN+DEX
Ozone exposure decreased BALF alveolar macrophages in vehicle-treated SH rats but AD reversed this effect at D + 1 (Figure 6A). CLEN+DEX pretreatment increased alveolar macrophages in ozone—but not air-exposed SH rats at both time points. This CLEN+DEX effect was significantly smaller in AD rats exposed to ozone relative to SH rats (Figs. 6A and 6B). Ozone exposure increased BALF neutrophils at D + 2 in vehicle-treated SH rats. This ozone effect was diminished in vehicle-treated AD rats (Figure 6D). Treatment with CLEN+DEX in air-exposed SH and AD rats did not increase BALF neutrophils, however it markedly exacerbated ozone-induced neutrophilia in both SH and AD rats at D + 2 (SH > AD) (Figure 6D). BALF lymphocytes were not impacted significantly by ozone or CLEN+DEX at any time point, but, in AD rats treated with CLEN+DEX, the number tended to be higher than in SH rats at D + 2 (Figs. 6E and 6F).
Figure 6.
Ozone-induced lung inflammation is modulated by AD and CLEN+DEX. BALF macrophages (A, B), neutrophils (C, D), and lymphocytes (E, F) were calculated based on cell differentials and total cell counts in rats within 2.5 h following air or ozone (0.8 ppm) exposure (4 h/day) for D + 1 and D + 2 groups. Bar graphs show mean ± SEM of n = 6–8/group. Significant differences between groups (p-value ≤ .05) are indicated by * for ozone effect when compared with corresponding air-exposed rats, # for AD effect when compared with corresponding SH rats, and † for CLEN+DEX effect when compared with corresponding vehicle-treated rats.
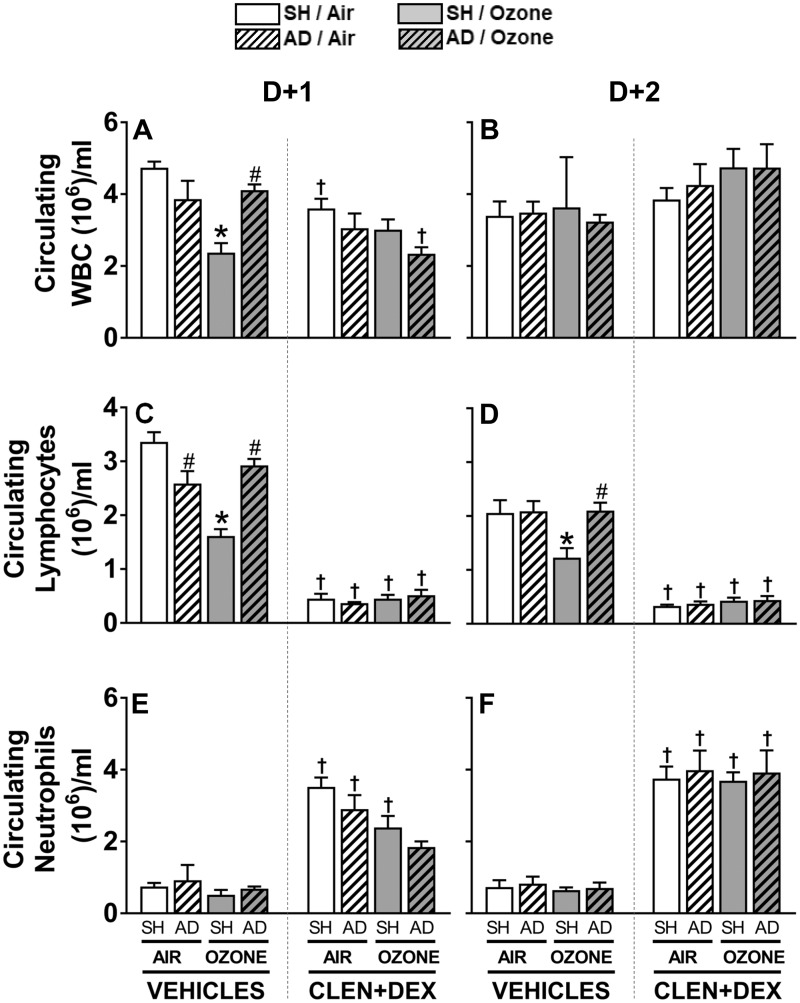
Ozone-Induced Reduction of Circulating WBC and Lymphocytes Is Modulated by AD and CLEN+DEX Treatment
In vehicle-treated SH rats, ozone exposure decreased circulating total WBC at D + 1; however, this ozone effect was not apparent in vehicle-treated AD rats. CLEN+DEX treatment decreased or tended to decrease WBC in all rats at D + 1 with a maximum drop occurring in ozone-exposed AD rats (Figure 7A). This CLEN+DEX effect was not apparent on D + 2 (Figure 7B). A small drop in circulating lymphocytes was noted in air-exposed vehicle-treated AD rats at D + 1. Ozone exposure in vehicle-treated SH rats markedly decreased circulating lymphocytes at D + 1 and D + 2. This ozone effect was not observed in vehicle-treated AD rats. CLEN+DEX treatment was associated with remarkable reduction of circulating lymphocytes in all animals at both time points regardless of surgery or exposure condition (Figs. 7C and 7D). Circulating neutrophils were not affected by AD or ozone in vehicle-treated rats, however, CLEN+DEX treatment caused significant increases in circulating neutrophils in all rats regardless of surgery or exposure (except for ozone-exposed AD rats at D + 1) (Figs. 7E and 7F).
Figure 7.
Ozone-induced changes in circulating WBC and lymphocytes are modulated by AD and CLEN+DEX. Circulating WBC (A, B), lymphocytes (C, D), and neutrophils (E, F) were assessed following air or ozone (0.8 ppm) exposure (4 h/day) for D + 1 and D + 2 groups. Bar graphs show mean ± SEM of n = 6–8/group. Significant differences between groups (p-value ≤ .05) are indicated by * for ozone effect when compared with corresponding air-exposed rats, # for AD effect when compared with corresponding SH rats, and † for CLEN+DEX effect when compared with corresponding vehicle-treated rats.
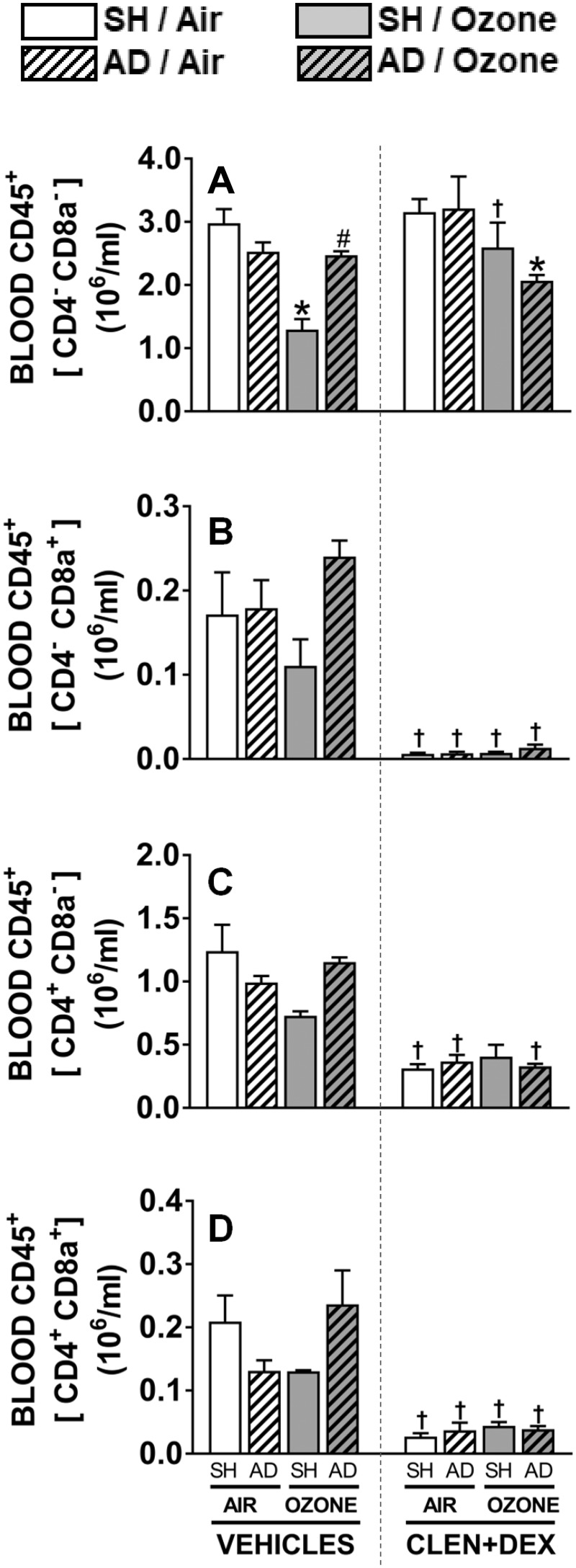
In order to further examine the types of lymphocytes impacted by ozone and CLEN+DEX at the D + 1 time point, subpopulations of lymphocytes were quantified as a percentage of total leukocytes (CD45+) and then normalized using total CD45+ leukocyte count. Generally, CD4−CD8a− and CD4+CD8a− made up the substantial portion of WBC (60–95%, Figs. 8A and 8C). Ozone exposure, as noted above, decreased overall leukocyte counts, and tended to decrease all cells positive for lymphocyte markers such as CD4−CD8a+ (Figure 8B), CD4+CD8a− (Figure 8C), and CD4+CD8a+ (Figure 8D) in vehicle-treated SH rats; however, this trend was not observed in AD rats. CLEN+DEX treatment significantly decreased all T-lymphocyte subpopulations regardless of surgery or exposure status except for CD4−CD8− leukocytes (Figure 8). CD4−CD8− leukocytes were decreased in ozone-exposed AD rats treated with CLEN+DEX when compared with AD rats exposed to air and treated with CLEN+DEX.
Figure 8.
Flow cytometry assessment of circulating leukocyte subpopulations after ozone exposure in SH and AD rats treated with vehicle or CLEN+DEX. Percentage of circulating leukocyte subpopulations CD4-CD8a− (A), CD4−CD8a+ (B), CD4+CD8a− (C), and CD4+CD8a+ (D) were determined following air or ozone (0.8 ppm) exposure (4 h/day) for D + 1 groups. Cells positive for CD4−CD8a+, CD4+CD8a−, and CD4+CD8a+ were considered T-lymphocyte subpopulations. Relative numbers were determined based on CD45+ cells (total leukocytes). Bar graphs show mean ± SEM of n = 6–8/group. Significant differences between groups (p-value ≤ .05) are indicated by * for ozone effect when compared with corresponding air-exposed rats, # for AD effect when compared with corresponding SH rats, and † for CLEN+DEX effect when compared with corresponding vehicle-treated rats.
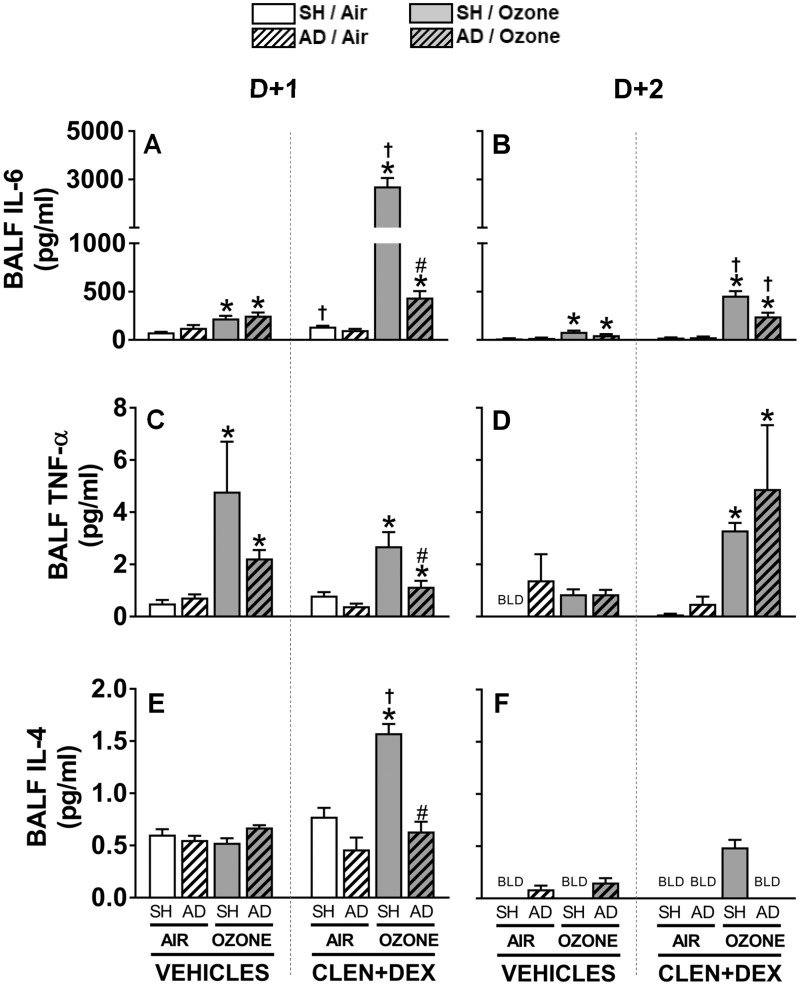
Ozone-Induced Effects on BALF Cytokines in SH and AD Rats Treated With Vehicle or CLEN+DEX
Proinflammatory cytokine proteins were assessed in BALF to determine the role of AD and CLEN+DEX on ozone-mediated lung inflammation. BALF IL-6 levels were increased in all vehicle-treated SH and AD rats exposed to ozone relative to air at both time points. CLEN+DEX in air-exposed rats had little effect on IL-6 levels; however, CLEN+DEX treatment resulted in highly exacerbated IL-6 increases in SH rats exposed to ozone at D + 1 and D + 2. This interactive effect of ozone and CLEN+DEX was less remarkable in AD rats (Figs. 9A and 9B). Ozone exposure increased BALF levels of TNF-α at D + 1 regardless of surgery or treatment. CLEN+DEX-treatment did not influence ozone-induced increases in BALF TNF-α at D + 1. However, on D + 2, CLEN+DEX treatment increased BALF TNF-α in ozone-exposed SH and AD rats (Figs. 9C and 9D). BALF IL-4 levels were generally low in D + 1 samples and below the detection limit in most D + 2 samples with no apparent ozone effect in vehicle-treated rats. CLEN+DEX treatment increased IL-4 levels in ozone-exposed SH, but not AD rats, at D + 1 (Figs. 9E and 9F).
Figure 9.
Ozone-induced changes in BALF cytokine levels are influenced by AD and/or CLEN+DEX treatment. BALF IL-6 (A, B), TNF-α (C, D), and IL-4 (E, F) proteins were quantified in samples collected within 2.5 h following air or ozone (0.8 ppm) exposure (4 h/day) for D + 1 and D + 2 groups. Bar graphs show mean ± SEM of n = 6–8/group. Significant differences between groups (p-value ≤ .05) are indicated by * for ozone effect when compared with corresponding air-exposed rats, # for AD effect when compared with corresponding SH rats, and † for CLEN+DEX effect when compared with corresponding vehicle-treated rats. BLD: below the limit of detection.
Ozone-Induced Pulmonary Cytokine mRNA Changes in SH and AD Rats Treated WIth Vehicle or CLEN+DEX
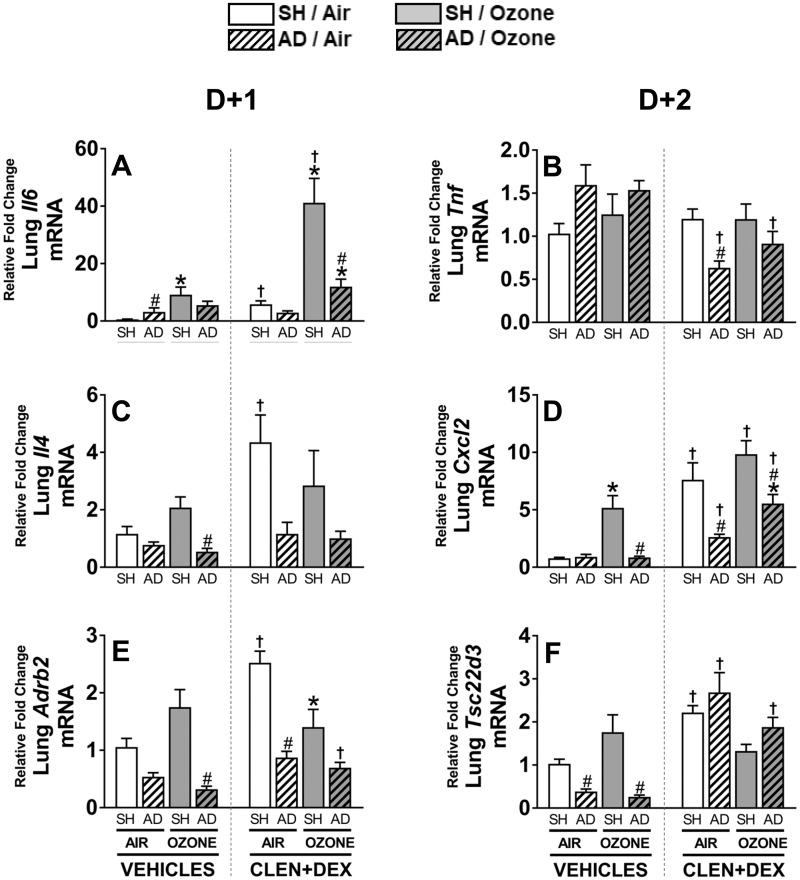
Lung expression of genes involved in inflammatory processes and those responsive to AR and GR signaling was assessed in all samples from D + 1 groups to determine if stress hormone receptors are involved in transcriptional regulation of genes known to be induced by ozone (Henriquez et al., 2017). Overall changes in Il6 mRNA expression corroborated those observed for BALF IL-6 protein at D + 1 and D + 2. Ozone exposure up-regulated Il6 expression only in vehicle-treated SH but not AD rats. CLEN+DEX treatment exacerbated ozone-induced Il6 expression in SH and tended to exacerbate the effect in the AD rats (SH > AD) but had little effect in air-exposed rats (Figure 10A). Even though BALF TNF-α protein was increased, Tnf gene expression was not changed by ozone exposure or CLEN+DEX treatment in SH rats. However, CLEN+DEX treatment down-regulated Tnf expression in both air- and ozone-exposed AD rats (Figure 10B). Il4 expression was not affected by ozone in vehicle-treated SH or AD rats exposed to ozone. CLEN+DEX treatment in SH but not AD rats increased Il4, especially in air-exposed SH rats (Figure 10C). Cxcl2 expression was up-regulated after ozone exposure in vehicle-treated SH rats, and AD effectively inhibited this effect. CLEN+DEX treatment increased Cxcl2 expression in all rats exposed to air or ozone; however, these increases were smaller in AD rats relative to SH rats (Figure 10D). Ozone tended to increase Ardb2, the gene expressing the β2AR protein, in vehicle-treated SH rats (p = 0.18); however, its expression was decreased in AD rats treated with vehicle. CLEN+DEX significantly induced Ardb2 expression in the air-exposed SH rats while this effect was less pronounced in ozone-exposed SH rats and air- or ozone-exposed AD rats (Figure 10E). Expression of Tsc22d3, a GR responsive gene, tended to increase after ozone exposure in the lungs of vehicle-treated SH rats; however, AD effectively down-regulated Tsc22d3 expression in air- and ozone-exposed vehicle-treated rats. CLEN+DEX treatment significantly increased the expression of Tsc22d3 for all groups except for ozone-exposed SH rats (Figure 10F).
Figure 10.
Ozone-induced changes in lung gene expression, and the effects of AD and CLEN+DEX. Relative lung gene expressions for Il6 (A), Tnf (B), Il4 (C), Cxcl2 (D), Adrb2 (E), and Tsc22d3 (F) were determined in tissues collected within 2.5 h following air or ozone (0.8 ppm) exposure (4 h/day) for D + 1 groups (n = 6–8/group). Significant differences between groups (p-value ≤ .05) are indicated by * for ozone effect when compared with corresponding air-exposed rats, # for AD effect when compared with corresponding SH rats, and † for CLEN+DEX effect when compared with corresponding vehicle-treated rats.
DISCUSSION
We have previously shown that neuroendocrine activation leading to increased circulating stress hormones was necessary for mediating ozone-induced lung injury and inflammation since AD rats were protected from these ozone effects (Henriquez et al., 2017; Miller et al., 2016b). Because AD is invasive and also eliminates circulating mineralocorticoids along with stress hormones, one cannot rule out their contribution in diminution of ozone-induced lung effects. The goal of this study was to evaluate if agonists of stress hormone receptors β2AR and GR were able to restore ozone-induced lung injury, inflammation and immune cell trafficking in AD rats, and exacerbate these effects in SH rats. Here, we reconfirm that the pulmonary and systemic effects of ozone inhalation, characterized by vascular leakage, neutrophilic inflammation, cytokine release in the lungs and peripheral vascular lymphopenia were significantly diminished by AD (Miller et al., 2016b). The treatment with a combination of β2AR and GR agonists (CLEN+DEX) was able to restore the majority of these ozone effects in AD rats, and further exacerbate ozone-induced lung protein leakage, inflammation and lymphopenia in SH rats. It was also noted that CLEN+DEX itself, at the dose level used, caused lung injury, increases in cytokines and in genes responsive to activation of β2AR and GR. β2AR and GR agonists are widely used for the treatment of chronic lung diseases (Barnes, 2011; Fireman, 1995), and their use has been shown to exacerbate lung inflammation in asthmatics during increased air pollution episodes (Qian et al., 2009). Our data provide mechanistic support for a potential interaction between air pollution-induced increases in endogenous epinephrine and cortisol/corticosterone, and the use of long-acting bronchodilators plus steroidal therapeutic agents that might explain exacerbation of lung injury/inflammation.
β2AR and GR agonists are widely used for the treatment of chronic lung diseases such as asthma and COPD by promoting bronchodilation and reducing inflammation, respectively. Since ozone-induced lung protein leakage and inflammation are also associated with increased levels of endogenous β2AR and GR agonists, it is likely that air pollution effects are exacerbated in those receiving this therapy. A variety of agonists and antagonists of different formulations are given orally or via the inhalation route to patients as a singular therapy or combination (Brusselle and Bracke, 2014; Cazzola et al. 2012; Yayan and Rasche, 2016). In some cases, intramuscular or intravenous routes of drug delivery are also used (Rowe et al., 2004). In research applications, however, the intraperitoneal route is most commonly used. We used this route for administration of drugs, as it provides a consistent means of administering the drugs as well as providing adequate absorption and distribution systemically. CLEN, a specific β2AR agonist, although not prescribed in the United States, is used in other counties with a recommended dose of 0.02–0.06 mg/day for bronchodilation, and much higher doses of up to 0.12 mg/day for inducing weight loss (Drug Enforcement Administration, 2013). Likewise, DEX is a widely used steroid in humans, in veterinary practice and employed extensively in research. At the recommended repeated adult dose levels of 0.75–9 mg every 6–12 h for an average 70 kg person it is anti-inflammatory, whereas for patients with adrenal insufficiency, doses of up to 0.15 mg/kg/day every 6–12 h are given (http://reference.medscape.com/drug/decadron-dexamethasone-intensol-dexamethasone-342741-, accessed September 20, 2017). In this study, we used higher levels than what is used therapeutically in humans to assure a sufficient coverage of expected change due to AD-mediated depletion and ozone-induced increases in circulating stress hormones.
As we demonstrated previously (Miller et al., 2016b), AD nearly completely eliminated stress hormones, corticosterone and epinephrine, from the circulation. Importantly, corticosterone, but not epinephrine, was significantly diminished by CLEN+DEX treatment. Since the HPA axis is regulated by a negative feedback inhibition controlled by glucocorticoids themselves (Keller-Wood, 2015), it is likely that this is due to the known DEX-dependent inhibition of corticosterone synthesis and release (Kolebinov et al., 1975). The increased expression of the glucocorticoid responsive gene Tsc22d3, which has been shown to increase following ozone exposure in multiple organs (Henriquez et al., 2017; Thomson et al., 2013, 2016), and the up-regulation of adrenergic receptor β2 gene (Adrb2), together with increased trend of circulating epinephrine and corticosterone in SH rats following ozone exposure, supports the conclusion that β2AR and GR are involved in mediating ozone-induced lung injury and inflammation.
In the current report, we wanted to determine if the ozone-induced changes discussed above were further influenced by AD in combination with systemic CLEN+DEX administration. We therefore, included in-life screening assessments such as body weight (to assess dehydration), changes in SQ temperature (to assess the degree of resultant hypothermia), and WBP (to detect acute ventilatory responses). We infer that any noted group differences relate to one or more of our study factors (ie, SH vs AD surgeries, vehicle vs CLEN+DEX treatment, or air vs ozone exposures).
While total bilateral AD and ozone exposure each decreased body weight gain as observed in our previous study (Miller et al., 2016b), the treatment with selected high concentrations of CLEN+DEX resulted in further decreases, especially in AD rats. Although water intake did not appear to be impacted in AD rats receiving 0.9% saline for drinking (Miller et al., 2016b), herein when the AD rats were exposed to air or ozone, they did not have access to these oral electrolytes. Thus, the AD rats concurrently receiving high doses of the adrenergic and glucocorticoid agonists incurred significant weight loss, presumably due largely to body fluid loss. Such changes in fluid and electrolyte balance could contribute to some of the observed effects (eg, hypothermia, ventilatory changes, and alveolar fluid accumulation) discussed below.
Hypothermia following air pollution exposure has been postulated to be a protective autonomic mechanism in rodents (Gordon et al., 2014), likely reducing the pollutant dosimetry and/or impact on the lung (Gorr, 2017; Terrien et al., 2011). In this study, we too observed a hypothermic response to ozone in SH rats via assessment of subcutaneous temperature, a measurement known to reflect ozone-induced effects on core body temperature (Gordon et al., 2014). Interestingly, although AD reversed most ozone effects on the lung and periphery, hypothermia was not reversed, suggesting this autonomic response is likely regulated upstream and independent of the effects of stress hormones. It is also likely that this response might be regulated by the contribution of parasympathetic autonomic output not associated with HPA activation. Moreover, it is possible that the sympathetic outflow regulating post-ganglionic nor-epinephrine action, especially producing local effects at sympathetic nerve endings might contribute to hypothermia, since AD did not influence the levels of circulating nor-epinephrine (Miller et al., 2016b).
We likewise used WBP parameters to assist in characterizing ozone-induced ventilatory changes related to AD, with and without systemic CLEN+DEX treatments. Acutely following exposure, all ozone-exposed WKY rats exhibited some degree of dyspnea. In healthy humans and certain rat strains, including the F344 (Tepper et al., 1989, 1990) and Wistar strains (Schelegle et al., 2001), a common respiratory response to acute ozone inhalation is the development of a short, shallow breathing pattern (ie, tachypnea). However, compared with the F344, Wistar, and many other rat strains, WKY rats are one of the most ozone responsive in terms of developing increased BALF protein concentrations (Kodavanti et al., 2015) and disproportionate increases in PenH (Dye et al., 2015). Therefore, to obtain data on the obstructive effects occurring in these ozone-exposed WKY rats, we performed WBP within ≅30 min of the end of exposure. This timing is optimal to detect maximal increases in Penh, an index of airflow limitation related to small airway injury, obstruction, or disease. In so doing, our air-exposed rats underwent similar WBP assessments, in a rotating manner, with the ozone-exposed rats. This approach necessitated handling and placement of rats into the WBP chambers, and while we did allow a brief period for acclimation to the chamber, the rats remained alert (unrestrained, conscious) when ventilatory data were collected. Hence, a limitation of our approach is that breathing frequencies of our air-exposed controls rats are higher than that of previous reports (eg, Schelegle et al. [2001] in which rats were undisturbed and exposed to ozone for 8 h in glass WBP chambers, or Tepper et al. [1989, 1990] in which rats remained in restraint tubes for head-out plethysmography for 30 min prior to recording breathing frequencies).
Recognizing this limitation, we focused on group differences in breathing patterns between the air- or the ozone-exposed rats with and without corresponding interventions (ie, prior surgery or drug treatment). Data revealed that in air-exposed, SH rats treated with CLEN+DEX, PenH was mildly increased (Figure 4); however, in ozone-exposed SH rats, Penh increases were more significantly exacerbated by the combined drug treatment (Figure 4). It is important to note that these rats (ie, SH, ozone-exposed, CLEN+DEX-treated) often exhibited biphasic inspiration waveforms, which likely contributed to their relatively higher PEF/PIF ratios. In comparison, the analogous AD group (ie, AD, ozone-exposed, CLEN+DEX-treated) had lower Penh values, but higher Ti/Te ratios, especially by D + 2. These complex ventilatory changes may reflect superimposed changes in ventilatory drive and/or airway obstruction secondary to airway injury that likely developed as a result of the combined drug treatment and repeated ozone exposures. In case of ozone which causes small airway injury, Penh increases correlate with pulmonary protein leakage (Miller et al., 2018).
As reported earlier (Miller et al., 2016b), AD prevented the ozone-induced vascular protein leakage in the lungs. Conversely, CLEN+DEX treatment in air-exposed animals was enough to induce marked vascular leakage, NAG activity increases, and exacerbated ozone effect indicating a crucial role for β2AR and GR in this response. It should be noted that the doses used for CLEN and DEX in this study are several fold higher than therapeutic levels, however, CLEN treatment in horses (Dodam et al., 1993) and salbutamol treatment in humans have also been shown to decrease pulmonary vascular resistance (Spiekerkoetter et al., 2002). Epinephrine has been reported to induce pulmonary edema in rats (Hao et al., 2001). In addition, lung epithelial permeability is increased by β2AR agonists (Unwalla et al., 2012, 2015). Thus, the endogenous and administered catecholamines have been associated with increased edema (Rassler, 2013). However, in some studies, β2AR agonists administration have been shown to increase alveolar fluid clearance (Downs et al., 2012). Also, increased glucocorticoid activity has been implicated in decreased permeability of lung epithelium (Kielgast et al., 2016; Matheson et al., 2004) and alleviation of pulmonary edema (Matthay, 2014). Collectively, it is likely that circulating endogenous or administered AR and GR agonists might influence vascular integrity locally and cause pulmonary edema. It is also possible that the lung protein leakage is mediated by hemodynamic changes induced by drug treatment and ozone exposure. CLEN-induced increases in heart rate was attributed to β2AR-mediated hypotension (Hoey et al., 1995). Although we did not assess cardiac physiological changes in this study with CLEN+DEX, ozone exposure has been shown to induce bradycardia and reduce blood pressure causing cardiac depression (Gordon et al., 2014; Wagner et al., 2014). These hemodynamic changes could lead to increased pulmonary vascular hydrostatic pressure, congestion of pulmonary venules, and edema. These changes likely further contributed to the dyspnea observed in the CLEN+DEX-treated SH rats exposed to ozone.
The changes in lavageable macrophages after ozone exposure are likely influenced by the temporality of the assessment (Kumarathasan et al., 2015; Laskin et al., 1998). Increased adhesion of activated alveolar macrophages might reduce the efficiency of recovery during lavage procedure (Bhalla et al., 1996; Gordon et al., 2016). While the decrease in alveolar macrophages after ozone exposure on D + 1 was prevented by AD, it is noteworthy that CLEN+DEX treatment increased alveolar macrophages but only in ozone-exposed rats (SH > AD). How these increases in BALF macrophage number might relate to their activity status is unclear, however, it has been shown that ozone and β adrenergic inhibition cooperatively inhibit macrophage activity (McGovern et al., 1996).
Lung neutrophilia is one of the hallmarks of ozone exposure (Alexis et al., 2010; Hollingsworth et al., 2007; Kodavanti et al., 2015) and acute stress scenarios directly modulate neutrophil recruitment, migration, and mobilization through the action of stress hormones (Dhabhar et al., 2012). Consistent with our previous publications (Henriquez et al., 2017; Miller et al., 2016b), ozone exposure increased the accumulation of neutrophils in BALF while an absence of stress hormone (by AD) was associated with decreased BALF neutrophils in ozone-exposed rats. Interestingly, ozone-induced increases in BALF neutrophil numbers was 2–3-fold higher in SH treated with CLEN+DEX. This is in line with the pulmonary vascular permeability changes induced by ozone, and the subsequent modulation by AD and CLEN+DEX. Surprisingly, CLEN+DEX treatment by itself did not induce neutrophilic influx despite increasing protein leakage in air-exposed rats, suggesting that vascular leakage is likely a direct effect of β2AR stimulation in the lung, while neutrophil increase is a systemic response mediated through ozone-induced activation of neuroendocrine axis.
Effects of ozone inhalation are not limited to the respiratory system. Our results support the previous observation that ozone exposure reduces circulating WBC, especially lymphocytes, while increasing circulating epinephrine and corticosterone in rats (Henriquez et al., 2018; Miller et al., 2016b). Since CLEN+DEX also produces a substantial reduction in circulating lymphocytes (likely due to the immunosuppressive dose of DEX) while increasing BALF neutrophils, it is likely that these effects of ozone are modulated by stress hormones in a dynamic and cell-specific manner (Dhabhar et al., 2012). Although the separation between circulating T-lymphocytes and B-lymphocytes could not be assessed since CD3, a pan marker of T-lymphocytes, was not included in our analysis, it was apparent that T-lymphocyte subpopulations CD4−CD8a+ (cytotoxic), CD4+CD8a− (Helper), and CD4+CD8a+ were decreased by CLEN+DEX and to some extent by ozone. This demonstrates that T-lymphocytes are sensitive to stress hormone levels and their receptor activation. It is well-known that glucocorticoids induce thymic atrophy (Pazirandeh et al., 2002; Roggero et al., 2006) and apoptosis in circulating T-cells (Cidlowski et al., 1996), which is consistently observed in different models of stress (Szabo et al., 2017). The pattern of ozone-induced decrease and AD-mediated recovery of circulating leukocyte cell subpopulations suggests that endogenous levels of glucocorticoid hormones are sufficient to dynamically regulate the relative size of cytotoxic and helper T-cell pools.
We have previously reported that global lung gene expression changes observed after ozone exposure are diminished in AD rats (Henriquez et al., 2017), suggesting that diminution of circulating stress hormones reduce ozone effects at the transcriptome level. In this study, we sought to determine the specific influence of activating β2AR and GR in AD rats on inflammatory markers. We noted that ozone exposure was associated with increases in proteins and mRNA for inflammatory markers, and as observed earlier (Henriquez et al., 2017), this response was inhibited in AD rats. Moreover, we noted that CLEN+DEX itself was sufficient to induce inflammatory genes, such that no further increases were discernible by ozone. Air pollution-induced IL-6 increases have been shown to be augmented by treatment with β2AR agonists (Chiarella et al., 2014). These increases confirm that activation of β2AR and GR modulates lung transcriptional changes, which likely influence the inflammatory response to ozone.
The concentration of ozone used in this study (0.8 ppm) is much higher than what is likely encountered environmentally. However, this level in resting rats can be comparable to exercising humans exposed to 0.2 ppm ozone (Hatch et al., 2013), and it has been recently shown that ambient ozone concentrations can rise up to 0.2 ppm under certain circumstances as observed recently in the United States (EPA, 2007).
There are a number of limitations to our study. The dynamic nature of ozone-induced changes was not examined to determine how the recovery or progression of injury might occur after ozone exposure, especially in the case of AD. Furthermore, only male WKY rats were used since males of a strain derived from WKY, the spontaneously hypertensive rats, have shown greater sensitivity to tobacco smoke-induced lung injury and inflammation than females (Shen et al., 2016). However, ozone-induced effects have not been examined in female WKY rats. Lastly, the CLEN+DEX doses used in this study, although within the range of those used by many research publications (Huang et al., 2000; Jonasson et al., 2013; Ryan et al., 2011; Sadarani and Majumdar, 2015; Sato et al., 2008; Sun et al., 2009), were much higher than human therapeutic doses, and the interaction of CLEN and DEX with air pollutants at therapeutic levels needs to be carefully examined.
In conclusion, our results suggest that both endogenous and exogenous levels of β2AR agonists and glucocorticoids dynamically regulate ozone-induced pulmonary and extra-pulmonary responses. Ozone-induced lung vascular protein leakage, inflammation, and peripheral lymphopenia were diminished in AD rats, and agonists of β2AR and GR restored this phenotype. Moreover, these agonists by themselves were sufficient to induce lung vascular leakage and cytokine increases producing ozone-like lung injury and lymphopenia. These data provide a potential mechanistic link for the epidemiological evidence that air pollution effects are exacerbated in those socioeconomically disadvantaged communities with high levels of psychosocial stresses and high levels of circulating stress hormones (Douwes et al., 2011). Furthermore, this study may suggest that the pulmonary effects of air pollutants might be exacerbated in asthmatics or COPD patients receiving chronic bronchodilator treatment with or without immunosuppressant steroids, since air pollutants mediate their effects through circulating stress hormones.
ACKNOWLEDGMENTS
The authors thank Drs Barbara Buckley, Andrew Ghio, Wayne Cascio, and Ian Gilmour of the U.S. EPA for their critical review of the manuscript, and Ms Cynthia Fisher for her help in the mRNA PCR experiment. We acknowledge the help of Dr Mark Higuchi for performing inhalation exposures and Mr Abdul Malek Khan in finalizing ozone exposure parameters. We also thank Ms Judy Schmid of the U.S. EPA for her expert advice on statistical analysis of the data. This work was supported by the US EPA Intramural Research Program, the EPA UNC Center for Environmental Medicine, Asthma and Lung Biology Cooperative Agreement (CR83515201), as well as EPA UNC Cooperative Training Agreement (CR 83578501). A.R.H. was supported in part by Fulbright (Becas Chile, CONICYT; IIE 15120279.)
REFERENCES
- Alexis N. E., Lay J. C., Hazucha M., Harris B., Hernandez M. L., Bromberg P. A., Kehrl H., Diaz-Sanchez D., Kim C., Devlin R. B., et al. (2010). Low-level ozone exposure induces airways inflammation and modifies cell surface phenotypes in healthy humans. Inhal. Toxicol. 22, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An K., Salyer J., Brown R. E., Kao H. F., Starkweather A., Shim I. (2016). Salivary biomarkers of chronic psychosocial stress and CVD risks: A systematic review. Biol. Res. Nurs. 18, 241–263. [DOI] [PubMed] [Google Scholar]
- Awotidebe T. O., Awopeju O. F., Bisiriyu L. A., Ativie R. N., Oke K. I., Adedoyin R. A., Olusola O. D., Erhabor G. E. (2017). Relationships between respiratory parameters, exercise capacity and psychosocial factors in people with chronic obstructive pulmonary disease. Ann. Phys. Rehabil. Med. 60, 387–392. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. (2004). Distribution of receptor targets in the lung. Proc. Am. Thorac. Soc. 1, 345–351. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. (2011). Glucocorticosteroids: Current and future directions. Br. J. Pharmacol. 163, 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. (2016) Glucocorticosteroids In: Pharmacology and Therapeutics of Asthma and COPD. Handbook of Experimental Pharmacology, Vol 237 (Page C., Barnes P., Eds.). Springer, Cham. [Google Scholar]
- Barr D. A. (2017). The childhood roots of cardiovascular disease disparities. Mayo Clin. Proc. 92, 1415–1421. [DOI] [PubMed] [Google Scholar]
- Bass V., Gordon C. J., Jarema K. A., MacPhail R. C., Cascio W. E., Phillips P. M., Ledbetter A. D., Schladweiler M. C., Andrews D., Miller D., et al. (2013). Ozone induces glucose intolerance and systemic metabolic effects in young and aged Brown Norway rats. Toxicol. Appl. Pharmacol. 273, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla D. K., Hoffman L. A., Pearson A. C. (1996). Modification of macrophage adhesion by ozone: Role of cytokines and cell adhesion molecules. Ann. N.Y. Acad. Sci. 796, 38–46. [DOI] [PubMed] [Google Scholar]
- Brusselle G., Bracke K. (2014). Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 11, S322–S328. [DOI] [PubMed] [Google Scholar]
- Cain D. W., Cidlowski J. A. (2015). Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 29, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M., Page C. P., Calzetta L., Matera M. G. (2012). Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 64, 450–504. [DOI] [PubMed] [Google Scholar]
- Chiarella S. E., Soberanes S., Urich D., Morales-Nebreda L., Nigdelioglu R., Green D., Young J. B., Gonzalez A., Rosario C., Misharin A. V., et al. (2014). β2-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis. J. Clin. Invest. 124, 2935–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cidlowski J. A., King K. L., Evans-Storms R. B., Montague J. W., Bortner C. D., Hughes F. M. Jr (1996). The biochemistry and molecular biology of glucocorticoid-induced apoptosis in the immune system. Recent Prog. Horm. Res. 51, 457–490. [PubMed] [Google Scholar]
- Clark M. L., Heiderscheidt J. M., Peel J. L. (2015). Integrating behavior change theory and measures into health-based cookstove interventions: A proposed epidemiologic research agenda. J. Health Commun. 20, 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty J. E., Kubzansky L. D. (2008). Traffic-related air pollution and stress: Effects on asthma. Environ. Health Perspect. 116, A376–A377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K., Vanden Berghe W., Haegeman G. (2000). Mechanisms of anti-inflammatory action and of immunosuppression by glucocorticoids: Negative interference of activated glucocorticoid receptor with transcription factors. J. Neuroimmunol. 109, 16–22. [DOI] [PubMed] [Google Scholar]
- DeWitt J. C., Williams W. C., Creech N. J., Luebke R. W. (2016). Suppression of antigen-specific antibody responses in mice exposed to perfluorooctanoic acid: Role of PPARα and T- and B-cell targeting. J. Immunotoxicol. 13, 38–45. [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S. (2014). Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 58, 193–210. [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S., Malarkey W. B., Neri E., McEwen B. S. (2012). Stress-induced redistribution of immune cells–from barracks to boulevards to battlefields: A tale of three hormones–Curt Richter Award winner. Psychoneuroendocrinology 37, 1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodam J. R., Moon R. E., Olson N. C., Exposito A. J., Fawcett T. A., Huang Y. C., Theil D. R., Camporesi E., Swanson C. R. (1993). Effects of clenbuterol hydrochloride on pulmonary gas exchange and hemodynamics in anesthetized horses. Am. J. Vet. Res. 54, 776–782. [PubMed] [Google Scholar]
- Douwes J., Brooks C., Pearce N. (2011). Asthma nervosa: Old concept, new insights. Eur. Respir. J. 37, 986–990. [DOI] [PubMed] [Google Scholar]
- Downs C. A., Kriener L. H., Yu L., Eaton D. C., Jain L., Helms M. N. (2012). β-Adrenergic agonists differentially regulate highly selective and nonselective epithelial sodium channels to promote alveolar fluid clearance in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L1167–L1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration. (2013). ODE@usdoj.gov. Available at: https://www.deadiversion.usdoj.gov/drug_chem_info/clenbuterol.pdf. Accessed September 20, 2017.
- Dye J. A., Ledbetter A. D., Schladweiler M. C., Costa D. L., Kodavanti U. P. (2015). Whole body plethysmography reveals differential ventilatory responses to ozone in rat models of cardiovascular disease. Inhal. Toxicol. 27, 14–25. [DOI] [PubMed] [Google Scholar]
- EPA, 2007. Airnow.gov. Retrieved from https://airnow.gov/index.cfm? action=airnow.mapsarchivedetail&domainid=29&mapdate=20170831&tab=2. Accessed September 7, 2017.
- Fireman P. (1995). B2 agonists and their safety in the treatment of asthma. Allergy Proc. 16, 235–239. [DOI] [PubMed] [Google Scholar]
- Gackière F., Saliba L., Baude A., Bosler O., Strube C. (2011). Ozone inhalation activates stress-responsive regions of the CNS. J. Neurochem. 117, 961–972. [DOI] [PubMed] [Google Scholar]
- Ghanemi A., Hu X. (2015). Elements toward novel therapeutic targeting of the adrenergic system. Neuropeptides 49, 25–35. [DOI] [PubMed] [Google Scholar]
- Gordon C. J., Johnstone A. F., Aydin C., Phillips P. M., MacPhail R. C., Kodavanti U. P., Ledbetter A. D., Jarema K. A. (2014). Episodic ozone exposure in adult and senescent Brown Norway rats: Acute and delayed effect on heart rate, core temperature and motor activity. Inhal. Toxicol. 26, 380–390. [DOI] [PubMed] [Google Scholar]
- Gordon C. J., Phillips P. M., Johnstone A. F., Beasley T. E., Ledbetter A. D., Schladweiler M. C., Snow S. J., Kodavanti U. P. (2016). Effect of high-fructose and high-fat diets on pulmonary sensitivity, motor activity, and body composition of brown Norway rats exposed to ozone. Inhal. Toxicol. 28, 203–215. [DOI] [PubMed] [Google Scholar]
- Gorman L. S. (2013). The adrenal gland: Common disease states and suspected new applications. Clin. Lab. Sci. 26, 118–125. [PubMed] [Google Scholar]
- Gorr T. A. (2017). Hypometabolism as the ultimate defence in stress response: How the comparative approach helps understanding of medically relevant questions. Acta Physiol. 219, 409–440. [DOI] [PubMed] [Google Scholar]
- Griffin É. W., Yssel J. D., O’Neill E., Ryan K. J., Boyle N., Harper P., Harkin A., Connor T. (2018). The β2-adrenoceptor agonist clenbuterol reduces the neuroinflammatory response, neutrophil infiltration and apoptosis following intra-striatal IL-1β administration to rats. Immunopharmacol. Immunotoxicol. 40, 99–106. [DOI] [PubMed] [Google Scholar]
- Hao Y., Okamura S., Wang L. M., Mineshita S. (2001). The involvement of bradykinin in adrenaline-induced pulmonary edema in rats. J. Med. Dent. Sci. 48, 79–85. [PubMed] [Google Scholar]
- Hatch G. E., McKee J., Brown J., McDonnell W., Seal E., Soukup J., Slade R., Crissman K., Devlin R. (2013). Biomarkers of dose and effect of inhaled ozone in resting versus exercising human subjects: Comparison with resting rats. Biomark. Insights 8, BMI.S11102–B67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley D. E., Lightman S. L. (2014). Cardio-metabolic consequences of glucocorticoid replacement: Relevance of ultradian signalling. Clin. Endocrinol. 80, 621–628. [DOI] [PubMed] [Google Scholar]
- Henriquez A. R., Snow S. J., Schladweiler M. C., Miller C. N., Dye J. A., Ledbetter A. D., Richards J. E., Mauge-Lewis K., McGee M. A., Kodavanti U. P. (2018). Adrenergic and glucocorticoid receptor antagonists reduce ozone-induced lung injury and inflammation. Toxicol. Appl. Pharmacol. 339, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez A., House J., Miller D. B., Snow S. J., Fisher A., Ren H., Schladweiler M. C., Ledbetter A. D., Wright F., Kodavanti U. P. (2017). Adrenal-derived stress hormones modulate ozone-induced lung injury and inflammation. Toxicol. Appl. Pharmacol. 329, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewagalamulage S. D., Lee T. K., Clarke I. J., Henry B. A. (2016). Stress, cortisol, and obesity: A role for cortisol responsiveness in identifying individuals prone to obesity. Domest. Anim. Endocrinol. 56, S112–S120. [DOI] [PubMed] [Google Scholar]
- Hirotsu C., Tufik S., Andersen M. L. (2015). Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 8, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey A. J., Matthews M. L., Badran T. W., Pegg G. G., Sillence M. N. (1995). Cardiovascular effects of clenbuterol are beta 2-adrenoceptor-mediated in steers. J. Anim. Sci. 73, 1754–1765. [DOI] [PubMed] [Google Scholar]
- Hollingsworth J. W., Kleeberger S. R., Foster W. M. (2007). Ozone and pulmonary innate immunity. Proc. Am. Thorac. Soc. 4, 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Gazzola C., Pegg G. G., Sillence M. N. (2000). Differential effects of dexamethasone and clenbuterol on rat growth and on beta2-adrenoceptors in lung and skeletal muscle. J. Anim. Sci. 78, 604–608. [DOI] [PubMed] [Google Scholar]
- Jonasson S., Wigenstam E., Koch B., Bucht A. (2013). Early treatment of chlorine-induced airway hyperresponsiveness and inflammation with corticosteroids. Toxicol. Appl. Pharmacol. 271, 168–174. [DOI] [PubMed] [Google Scholar]
- Joseph J. J., Golden S. H. (2017). Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N.Y. Acad. Sci. 1391, 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood M. (2015). Hypothalamic-pituitary–adrenal axis-feedback control. Compr. Physiol. 5, 1161–1182. [DOI] [PubMed] [Google Scholar]
- Kielgast F., Schmidt H., Braubach P., Winkelmann V. E., Thompson K. E., Frick M., Dietl P., Wittekindt O. H. (2016). Glucocorticoids regulate tight junction permeability of lung epithelia by modulating claudin 8. Am. J. Respir. Cell Mol. Biol. 54, 707–717. [DOI] [PubMed] [Google Scholar]
- Kodavanti U. P., Ledbetter A. D., Thomas R. F., Richards J. E., Ward W. O., Schladweiler M. C., Costa D. L. (2015). Variability in ozone-induced pulmonary injury and inflammation in healthy and cardiovascular-compromised rat models. Inhal. Toxicol. 27, 39–53. [DOI] [PubMed] [Google Scholar]
- Kolebinov NKh., Ankov V. K., Belovezhdov N. I. (1975). Depression of cortisol secretion with dexamethasone in healthy persons. Vutr. Boles 14, 89–93. [PubMed] [Google Scholar]
- Kumarathasan P., Blais E., Saravanamuthu A., Bielecki A., Mukherjee B., Bjarnason S., Guénette J., Goegan P., Vincent R. (2015). Nitrative stress, oxidative stress and plasma endothelin levels after inhalation of particulate matter and ozone. Part. Fibre Toxicol. 12, 28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin D. L., Heck D. E., Laskin J. D. (1998). Role of inflammatory cytokines and nitric oxide in hepatic and pulmonary toxicity. Toxicol. Lett. 102–103, 289–293. [DOI] [PubMed] [Google Scholar]
- Matheson M., McClean M., Rynell A. C., Berend N. (2004). Methylprednisolone reduces airway microvascular permeability but not airway resistance induced by N-formylmethionine leucyl-phenylalanine in the rabbit. Respirology 9, 211–214. [DOI] [PubMed] [Google Scholar]
- Matthay M. A. (2014). Resolution of pulmonary edema. Thirty years of progress. Am. J. Respir. Crit. Care Med. 189, 1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern T. J., el-Fawal H. A., Chen L. C., Schlesinger R. B. (1996). Ozone-induced alteration in beta-adrenergic pharmacological modulation of pulmonary macrophages. Toxicol. Appl. Pharmacol. 137, 51–56. [DOI] [PubMed] [Google Scholar]
- Miller C. N., Dye J. A., Schladweiler M. C., Richards J. H., Ledbetter A. D., Stewart E. J., Kodavanti U. P. (2018). Acute inhalation of ozone induces DNA methylation of apelin in lungs of Long-Evans rats. Inhal. Toxicol. Jun 27: 1–9. doi: 10.1080/08958378.2018.1483984. [Epub ahead of print] PMID: 29947284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. B., Ghio A. J., Karoly E. D., Bell L. N., Snow S. J., Madden M. C., Soukup J., Cascio W. E., Gilmour M. I., Kodavanti U. P. (2016a). Ozone Exposure Increases Circulating Stress Hormones and Lipid Metabolites in Humans. Am. J. Respir. Crit. Care Med. 193, 1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. B., Karoly E. D., Jones J. C., Ward W. O., Vallanat B. D., Andrews D. L., Schladweiler M. C., Snow S. J., Bass V. L., Richards J. E., et al. (2015). Inhaled ozone (O3)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicol. Appl. Pharmacol. 286, 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. B., Snow S. J., Schladweiler M. C., Richards J. E., Ghio A. J., Ledbetter A. D., Kodavanti U. P. (2016). Acute Ozone-Induced Pulmonary and Systemic Metabolic Effects Are Diminished in Adrenalectomized Rats. Toxicol. Sci. 150, 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J., Brown R. E. (2006). Detecting outliers when fitting data with nonlinear regression - A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7, 123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides N. C., Chrousos G. P., Charmandari E. (2013). Adrenal Insufficiency. Endotext [Internet]. South Dartmouth (MA: ): MDText.com, Inc., 2000–2013 Nov 18. [Google Scholar]
- Oakley R. H., Cidlowski J. A. (2011). Cellular processing of the glucocorticoid receptor gene and protein: New mechanisms for generating tissue-specific actions of glucocorticoids. J. Biol. Chem. 286, 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazirandeh A., Xue Y., Prestegaard T., Jondal M., Okret S. (2002). Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis. Faseb J. 16, 727–729. [DOI] [PubMed] [Google Scholar]
- Pepin A., Biola-Vidamment A., Latré de Laté P., Espinasse M. A., Godot V., Pallardy M. (2015). TSC-22D proteins: New regulators of cell homeostasis? Med. Sci. 31, 75–83. [DOI] [PubMed] [Google Scholar]
- Qian Z., Lin H. M., Chinchilli V. M., Lehman E. B., Duan Y., Craig T. J., Wilson W. E., Liao D., Lazarus S. C., Bascom R. (2009). Interaction of ambient air pollution with asthma medication on exhaled nitric oxide among asthmatics. Arch. Environ. Occup. Health 64, 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassler B. (2013). Role of α- and β-adrenergic mechanisms in the pathogenesis of pulmonary injuries characterized by edema, inflammation and fibrosis. Cardiovasc. Hematol. Disord. Drug Targets 13, 197–207. [DOI] [PubMed] [Google Scholar]
- Roggero E., Pérez A. R., Tamae-Kakazu M., Piazzon I., Nepomnaschy I., Besedovsky H. O., Bottasso O. A., del Rey A. (2006). Endogenous glucocorticoids cause thymus atrophy but are protective during acute Trypanosoma cruzi infection. J. Endocrinol. 190, 495–503. [DOI] [PubMed] [Google Scholar]
- Rowe B. H., Edmonds M. L., Spooner C. H., Diner B., Camargo C. A. Jr (2004). Corticosteroid therapy for acute asthma. Respir. Med. 98, 275–284. [DOI] [PubMed] [Google Scholar]
- Ryan K. J., Griffin E. W., Connor T. J. (2011). Complementary anti-inflammatory actions of the β2-adrenoceptor agonist clenbuterol and the glucocorticoid dexamethasone in rat brain. J. Neuroimmunol. 232, 209–216. [DOI] [PubMed] [Google Scholar]
- Sadarani B. N., Majumdar A. S. (2015). Resveratrol potentiates the effect of dexamethasone in rat model of acute lung inflammation. Int. Immunopharmacol. 28, 773–779. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Katoh M., Suzuki M., Kawabe R., Iwase K., Nadai M. (2014). Effect of adrenalectomy on expression and induction of UDP-glucuronosyltransferase 1A6 and 1A7 in rats. Biol. Pharm. Bull. 37, 618–624. [DOI] [PubMed] [Google Scholar]
- Sato S., Nomura S., Kawano F., Tanihata J., Tachiyashiki K., Imaizumi K. (2008). Effects of the beta2-agonist clenbuterol on beta1- and beta2-adrenoceptor mRNA expressions of rat skeletal and left ventricle muscles. J. Pharmacol. Sci. 107, 393–400. [DOI] [PubMed] [Google Scholar]
- Schelegle E. S., Alfaro M. F., Putney L., Stovall M., Tyler N., Hyde D. M. (2001). Effect of C-fiber-mediated, ozone-induced rapid shallow breathing on airway epithelial injury in rats. J. Appl. Physiol. 91, 1611–1618. [DOI] [PubMed] [Google Scholar]
- Schelegle E. S., Walby W. F. (2012). Vagal afferents contribute to exacerbated airway responses following ozone and allergen challenge. Respir. Physiol. Neurobiol. 181, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan J. H., Schladweiler M. C., Richards J. H., Ledbetter A. D., Ghio A. J., Kodavanti U. P. (2010). Pulmonary oxidative stress, inflammation, and dysregulated iron homeostasis in rat models of cardiovascular disease. J. Toxicol. Environ. Health A 73, 641–656. [DOI] [PubMed] [Google Scholar]
- Shen Y. H., Pham A. K., Davis B., Smiley-Jewell S., Wang L., Kodavanti U. P., Takeuchi M., Tancredi D. J., Pinkerton K. E. (2016). Sex and strain-based inflammatory response to repeated tobacco smoke exposure in spontaneously hypertensive and Wistar Kyoto rats. Inhal. Toxicol. 28, 677–685. [DOI] [PubMed] [Google Scholar]
- Shmool J. L., Kubzansky L. D., Newman O. D., Spengler J., Shepard P., Clougherty J. E. (2014). Social stressors and air pollution across New York City communities: A spatial approach for assessing correlations among multiple exposures. Environ. Health 13, 91.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow S. J., McGee M. A., Henriquez A., Richards J. E., Schladweiler M. C., Ledbetter A. D., Kodavanti U. P. (2017). Respiratory effects and systemic stress response following acute acrolein inhalation in rats. Toxicol. Sci. 158, 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekerkoetter E., Fabel H., Hoeper M. M. (2002). Effects of inhaled salbutamol in primary pulmonary hypertension. Eur. Respir. J. 20, 524–528. [DOI] [PubMed] [Google Scholar]
- Sun J., Yang D., Li S., Xu Z., Wang X., Bai C. (2009). Effects of curcumin or dexamethasone on lung ischaemia-reperfusion injury in rats. Eur. Respir. J. 33, 398–404. [DOI] [PubMed] [Google Scholar]
- Szabo S., Yoshida M., Filakovszky J., Juhasz G. (2017). “Stress” is 80 years old: From Hans Selye original paper in 1936 to recent advances in GI ulceration. Curr. Pharm. Des. 6, 170. doi: 10.2174/1381612823666170622110046. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Tank A. W., Lee W. D. (2015). Peripheral and central effects of circulating catecholamines. Compr. Physiol. 5, 1–15. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark T. E., Undem B. J. (2010). Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J. Physiol. 588, 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper J. S., Costa D. L., Lehmann J. R., Weber M. F., Hatch G. E. (1989). Unattenuated structural and biochemical alterations in the rat lung during functional adaptation to ozone. Am. Rev. Respir. Dis. 140, 493–501. [DOI] [PubMed] [Google Scholar]
- Tepper J. S., Wiester M. J., Weber M. F., Ménache M. G. (1990). Measurements of cardiopulmonary response in awake rats during acute exposure to near-ambient concentrations of ozone. J. Appl. Toxicol. 10, 7–15. [DOI] [PubMed] [Google Scholar]
- Terrien J., Perret M., Aujard F. (2011). Behavioral thermoregulation in mammals: A review. Front. Biosci. (Landmark Ed.) 16, 1428–1444. [DOI] [PubMed] [Google Scholar]
- Thomson E. M., Pal S., Guénette J., Wade M. G., Atlas E., Holloway A. C., Williams A., Vincent R. (2016). Ozone inhalation provokes glucocorticoid-dependent and -independent effects on inflammatory and metabolic pathways. Toxicol. Sci. 152, 17–28. [DOI] [PubMed] [Google Scholar]
- Thomson E. M., Vladisavljevic D., Mohottalage S., Kumarathasan P., Vincent R. (2013). Mapping acute systemic effects of inhaled particulate matter and ozone: Multiorgan gene expression and glucocorticoid activity. Toxicol. Sci. 135, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwalla H. J., Horvath G., Roth F. D., Conner G. E., Salathe M. (2012). Albuterol modulates its own transepithelial flux via changes in paracellular permeability. Am. J. Respir. Cell Mol. Biol. 46, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwalla H. J., Ivonnet P., Dennis J. S., Conner G. E., Salathe M. (2015). Transforming growth factor-β1 and cigarette smoke inhibit the ability of β2-agonists to enhance epithelial permeability. Am. J. Respir. Cell Mol. Biol. 52, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. G., Allen K., Yang H. Y., Nan B., Morishita M., Mukherjee B., Dvonch J. T., Spino C., Fink G. D., Rajagopalan S., et al. (2014). Cardiovascular depression in rats exposed to inhaled particulate matter and ozone: Effects of diet-induced metabolic syndrome. Environ. Health Perspect. 122, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck B. (2002). Beta-adrenoceptor agonists and asthma–100 years of development. Eur. J. Pharmacol. 445, 1–12. [DOI] [PubMed] [Google Scholar]
- Wright R. J. (2011). Psychological stress: A social pollutant that may enhance environmental risk. Am. J. Respir. Crit. Care Med. 184, 752–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayan J., Rasche K. (2016). Asthma and COPD: Similarities and differences in the pathophysiology, diagnosis and therapy. Adv. Exp. Med. Biol. 910, 31–38. [DOI] [PubMed] [Google Scholar]