Abstract
The development of a subnormal level of testosterone (T) is not universal in ageing men, with 75% of men retaining normal levels. However, a substantial number of men do develop T deficiency (TD), with many of them carrying a portfolio of cardiovascular (CV) risk factors, including type 2 diabetes (T2D) and the metabolic syndrome. TD increases the risk of CV disease (CVD) and the risk of developing T2D and the metabolic syndrome. The key symptoms suggesting low T are sexual in nature, including erectile dysfunction (ED), loss of night-time erections and reduced libido. Many men with heart disease, if asked, admit to ED being present; a problem that is often compounded by drugs used to treat CVD. A large number of studies and meta-analyses have provided evidence of the link between TD and an increase in CVD and total mortality. Patients with chronic heart failure (CHF) who have TD have a poor prognosis and this is associated with more frequent admissions and increased mortality compared with those who do not have TD. Conversely, in men with symptoms and documented TD, T therapy has been shown to have beneficial effects, namely improvement in exercise capacity in patients with CHF, improvement of myocardial ischaemia and coronary artery disease. Reductions in BMI and waist circumference, and improvements in glycaemic control and lipid profiles, are observed in T-deficient men receiving T therapy. These effects might be expected to translate into benefits and there are more than 100 studies showing CV benefit or improved CV risk factors with T therapy. There are flawed retrospective and prescribing data studies that have suggested increased mortality in treated men, which has led to regulatory warnings, and one placebo-controlled study demonstrating an increase in coronary artery non-calcified and total plaque volumes in men treated with T, which is open for debate. Men with ED and TD who fail to respond to phosphodiesterase type 5 (PDE5) inhibitors can be salvaged by treating the TD. There are data to suggest that T and PDE5 inhibitors may act synergistically to reduce CV risk.
Keywords: Testosterone deficiency, erectile dysfunction, cardiovascular risk, chronic heart failure, myocardial ischaemia, coronary artery disease, reduced libido, night time erections, cardiovascular risk factors, PDE5 inhibitors
Testosterone deficiency (TD) is a well-established and significant medical condition.[1,2] It has been defined as a clinical and biochemical syndrome, associated with older age and comorbidities.[3] It is characterised by a deficiency in serum androgen levels, with or without reduced genomic sensitivity to androgens.[2] The latter relates to the functionality of androgen receptors. For example, if there is CAG repeat polymorphism on exon 1 of the androgen receptor gene, the response to any given serum testosterone (T) concentration is reduced as the number of CAG repeats increases.[4–8]
Biochemical TD must be associated with relevant signs and symptoms for a diagnosis to be made. The hormone has important physiological functions and deficiency can adversely affect the brain, peripheral nerves, muscle, fat, bone, the cardiovascular (CV) system and especially the male genital and reproductive systems. T is important for the regulation of carbohydrate metabolism, lipids and proteins, and positively affects glucose control, liver fat, cardiac biomarkers, muscle growth and adipogenesis.[1,9–15]
Epidemiology
Epidemiological studies vary regarding the prevalence of TD. Perhaps the most useful study is the European Male Ageing Study, which evaluated more than 3,000 men between the ages of 40 and 79 years, recording biochemistry results and symptoms. An overall prevalence of 2.5% was reported and rates varied from 0.1% in men aged 40–49 years to 5.1% in those aged 70–79. In this study, TD was defined as three or more sexual symptoms associated with a total T (TT) level <11 nmol/l and a free T (FT) level less than 0.22 nmol/l.[16] Three-quarters of men maintained normal T levels into old age. Based on biochemical levels, the prevalence of secondary TD was 11.8%, primary TD 2.0% and compensated (subclinical) TD 9.5% (worthy of observation but not treatment).[17]
Aetiology
Normal T levels depend on a healthy hypothalamic–pituitary–gonadotropin axis. Primary TD results from disruption at the level of the testes, secondary TD from disruption at the level of the hypothalamus and pituitary and combined TD from disruption at both these levels.[3] Secondary TD is the most common form.[17,18]
Pituitary tumours can cause centrally mediated TD. Primary testicular failure may result from orchitis, Klinefelter’s syndrome, chemotherapy or radiation. Additional causes of TD include obesity, diabetes, metabolic syndrome, HIV infection, chronic renal failure, and chronic glucocorticoid and opioid use.[19,20]
Making the Diagnosis
The signs and symptoms of TD are shown in Table 1. Sexual symptoms are the most common, but there are many less specific symptoms such as fatigue, sleep disturbance, visceral obesity, loss of physical strength, decreased muscle mass, decreased energy and motivation, hot flushes, changes in cognition and memory, depression and decreases in bone mineral density.[1–3,21–23]
Table 1: Clinical Signs and Symptoms Suggestive of Testosterone Deficiency[1–3,21–23].
| Sexual | Physical |
| Delayed puberty | Decreased body hair |
| Small testes | Gynaecomastia |
| Infertility | Decreased muscle mass and strength |
| Decreased sexual desire and activity | Hot flushes or sweats |
| Erectile dysfunction | Sleep disturbances |
| Delayed ejaculation | Fatigue |
| Decreased volume of ejaculate | Osteoporosis/height loss/low-trauma fractures |
| Decreased or absent morning/night-time erections | |
| Cardiometabolic | Psychological |
| Increased BMI/obesity | Changes in mood, e.g. anger, irritability, sadness, depression |
| Visceral obesity | Decreased wellbeing or poor self-rated health |
| Metabolic syndrome | Decreased cognitive function (including impaired concentration, verbal memory and spatial performance) |
Source: Hackett et al. 2017.[1] Reproduced with permission from Elsevier.
The patient should be asked if they are taking any medications – prescription or otherwise – that can lower T levels, e.g. corticosteroids or opiates, or drugs that can cause erectile dysfunction (ED), and if they have previously taken T therapy.[1,21]
Physical examination should include height, weight, BMI and waist circumference, together with an assessment of body hair, any significant breast enlargement and the appearance of the penis and testicles.[1,21,22,24] A prostate examination is also recommended.[1,21]
The British Society for Sexual Medicine (BSSM) recommendations for case finding are to test for TD in adult men with persistent and multiple signs of TD and to screen for TD in all men presenting with ED, loss of spontaneous erections or low sexual desire, type 2 diabetes (T2D), BMI >30 kg/m2 or waist circumference >102 cm and those on long-term opiate, antipsychotic or anticonvulsant medications.[1]
T has a diurnal rhythm in younger men, which may become less marked with age but not invariably so.[22] Thus, the recommendation is to measure T between 7 am and 11 am on at least two occasions, with a reliable method, preferably 4 weeks apart and, if possible, not during an acute illness.[1] A fasting sample is generally advised because T levels are influenced by insulin and glucose, and this is particularly important for the second test.[25] If the first result was close to the lower normal range (8–12 nmol/l), it is advised to include levels of sex hormone binding globulin (SHBG).[1]
The bioavailable forms of T are those not bound to SHBG, known as FT, and this may provide the most reliable clinical androgen status in some patients.[16]
FT may be calculated using the equation of Vermeulen et al.[26] Online FT calculators are provided by the International Society for the Study of the Aging Male (ISSAM), available at: http://issam.ch/freetesto.htm and the Primary Care Testosterone Advisory Group (PCTAG), available at: http://www.pctag.uk/testosterone-calculator/. Reference ranges quoted by laboratories represent the normal population and these are often much lower than the advised action levels recommended by the various guideline groups.
The BSSM and International Society for Sexual Medicine (ISSM) recommend the following action levels:[1,27]
TT less than 8.00 nmol/l, or FT less than 0.18 nmol/l (based on two separate levels taken between 8 am and 11 am) usually requires T therapy.
TT higher than 12.00 nmol/l, or FT higher than 0.225 nmol/l does not require T therapy.
TT levels between 8.00 nmol/l and 12.00 nmol/l may require a trial of T therapy, for a minimum of 6 months, according to symptoms.
The BSSM and ISSAM also recommend the following:[1,22]
Increased luteinising hormone (LH) levels and T levels below normal or in the lower quartile range indicates testicular failure, so T therapy should be considered.[28]
Men with increased LH levels, normal T levels but TD symptoms should be considered to have TD.
European Male Ageing Study data demonstrated that clinical symptoms were more closely related to calculated FT than TT.[29]
Testosterone Levels in Men
Ruige et al. demonstrated that higher T levels were associated with a decreased risk for CV events in men aged >70 years (HR 0.84; 95% CI [0.76–0.92]) but this was not the case in younger men (HR 1.01; 95% CI [0.95–1.08]).[30]
Araujo et al. performed a meta-analysis that included 18 studies and more than 22,000 subjects and concluded that both overall and CV mortality were related to T levels.[31]
Corona et al. comprehensively reviewed 1,178 articles and included 70 in their meta-analysis. They demonstrated a clear association between low T/high oestradiol levels and CV disease (CVD). They made the point that longitudinal studies demonstrated that overall mortality and CV mortality were highest in those with lowest T levels.[32]
Coronary Artery Disease and Testosterone Deficiency
Clinical studies show favourable effects of short- or long-term exposure to T therapy on coronary and peripheral vasomotion and peripheral arterial stiffness.[33–35] Physiological concentrations of intracoronary T cause epicardial coronary artery dilatation and increases in volume blood flow in men with coronary artery disease (CAD).[33]
T therapy in hypogonadal men delays time to ischaemia, improves mood and is associated with potentially beneficial reductions in biomarkers, total cholesterol and serum tumour necrosis factor-alpha.[13,36]
Three early randomised, placebo-controlled trials demonstrated that administration of T improves myocardial ischaemia in men with CAD. All three trials found that in men with CAD, T prolongs the time to exercise-induced ST-segment depression as measured on treadmill stress testing.[37–39]
Numerous studies have demonstrated a negative correlation between endogenous T levels and intima-media thickness of the carotid arteries, abdominal aorta and thoracic aorta, which suggests that men with lower levels of endogenous T may be at a higher risk of developing more generalised atherosclerosis.[40–47] Evidence also suggests that men with lower T levels are more likely to develop CAD during their lifetime and CAD severity has been shown to correlate with the degree of TD.[48–55]
Budoff et al. studied 170 T deficient men over the age of 65 years in a double-blinded, placebo-controlled trial setting in the US.[56] Men with symptoms suggestive of hypogonadism were enrolled in the study between June 2010 and June 2014. Treatment was with T gel, with the dose adjusted to maintain the T level in the normal range for young men, or placebo gel for 12 months. Plaque volume was determined by coronary CT angiography. For the primary outcome, T therapy compared with placebo was associated with a significantly greater increase in non-calcified plaque volume from baseline to 12 months. There were no major adverse CV events in either group.
The authors point out that this trial had several strengths, including a placebo-controlled design, selection of men with unequivocally low T and a relatively high retention rate. However, they also point out that the study had some limitations. The assumptions about the composition of plaque components as detected by coronary CT angiography were not confirmed by direct radiological and pathological studies. Furthermore, the volume and radiological characteristics of coronary artery plaques are only surrogate outcomes and do not account for other factors that can influence the frequency and extent of plaque rupture and thrombosis. In fact, T therapy was associated with a significant increase in the volume of fibrous plaque, which may be more stable than other types of plaque. The major limitation is that the trial was not large enough or long enough to draw conclusions about the risk of T therapy on major adverse CV events. Larger studies are needed to understand the clinical implications of this finding.
All-cause Mortality, Cardiovascular Mortality and Testosterone Deficiency
Long-term studies, reviews and meta-analyses have supported the association between TD and increased all-cause and CV mortality.[2,30,31,57–60]
A meta-analysis by Araujo et al. included 12 studies involving more than 17,000 participants. Although there was considerable heterogeneity in these studies resulting from study and subject characteristics, low endogenous T levels were associated with both overall and CV mortality.[31]
Corona et al. included 70 papers in their meta-analysis and showed a clear association between low T/high oestradiol levels and increased risk of CVD and CV mortality.[32]
Muraleedharan et al. studied 581 men with T2D who were followed up for a mean of 5.8 years. Low T was defined as less than 10.4 nmol/l. A total of 51 men received T therapy for at least 2.0 years. Mortality rates were 20.0% in the low T group versus 9.1% in the normal T group, independent of comorbidities and other therapies, and T therapy reduced mortality similar to the controls.[58]
Daka et al. demonstrated that low concentrations of T predicted acute MI in men with T2D.[60]
Yeap et al. studied 3,690 older men over 10 years, TT and FT levels in the normal range were associated with reduced all-cause and CV mortality. Interestingly, both low and high levels of T were associated with all-cause mortality, and higher levels of dihydrotestosterone reduced ischaemic heart disease mortality.[59]
A review by Muraldeedharan et al. raised the issue that these studies do not prove a pathogenic link, but low T may simply be a marker of illness.[61]
Testosterone Therapy and Cardiovascular Risk
T therapy has been controversial, with worries that treatment will increase CV risk. An extensive review of the literature published between 1940 and 2014 found only four studies that reported increased CV risk.[62] The authors concluded that two of these were retrospective analyses with serious methodological limitations; one was a prospective trial with only four major adverse CV events and the other a meta-analysis that was criticised for using few studies and CV endpoints of questionable clinical importance, e.g. non-specific ECG findings and palpitations.[63–66]
The study by Vigen et al. was indeed retrospective, involving 8,709 men with a baseline TT level no higher than 10.4 nmol/l.[63] These men were undergoing angiography and were followed up for a mean of 840 days. Results showed 681 of the 7,486 patients who did not receive T therapy died, 420 had a MI and 486 had a stroke. Of the remaining 1,233 patients receiving T therapy, only 67 died, 23 had a MI and 33 had a stroke. The authors then performed a complex statistical analysis using more than 50 covariates and concluded that there was a greater risk in the T therapy group. Some 1,132 patients were excluded because they were prescribed T therapy after the event when they should have been included in the untreated group, which falsely increased the events by 70%. When challenged, the investigators revised the number to 132 but admitted that 104 women had been mistakenly included in the results. Worryingly, there were no data confirming the correct diagnosis of TD syndrome or T therapy and duration of therapy.[62]
Figure 2: Men Stratified by Phosphodiesterase Type 5 Inhibitor Treatment.
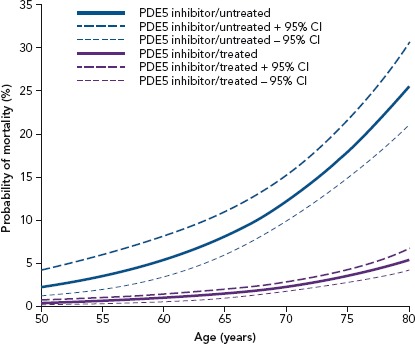
The estimated mortality probability and 95% CI from the fitted logistic regression: men stratified by testosterone replacement therapy. PDE5 = phosphodiesterase type 5. Source: Hackett et al. 2017.[71] Reproduced with permission from Baishideng Publishing Group.
The Finkle et al. study provided prescribing data in men treated with T, but there were no records of T blood results or the patients’ symptoms.[64] The researchers defined non-fatal coronary events as the major endpoint assessed in the 12 months before and 3 months after therapy. This is clearly a major weakness because the benefits of T therapy would take longer than this to appear and many other studies have excluded the first 3 months of treatment from analysis because of the likelihood of events relating to the pre-existing condition. In addition to this, data on fatal CV events and all-cause mortality were not collected and 12-month post-treatment data were collected but not presented. The lack of mortality data fails to recognise that any treatment that decreases mortality is, of course, likely to increase non-fatal events. The design was not prospective and although used as evidence against T therapy, it has been discredited by several design flaws and statistical analyses.[62]
Following these publications, in January 2014 the US Food and Drug Administration (FDA) convened an advisory committee meeting to review CV risks of T therapy. Subsequently, the FDA expanded the stated purpose of this committee to include a review of the suitable populations for T therapy. On 17 September 2014, the advisory committee voted to restrict therapeutic indications for T therapy and requested that the pharmaceutical industry perform a CV safety study. In March 2015, all US commercial T products underwent a mandatory label change that restricted the indicated population and warned against the possible risk of MI and stroke.[67]
The EU and Health Canada also issued warnings regarding T therapy and potential CV risk. A review by the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee recommended updating the product information warning about the potential increased CV risk in hypogonadal men using T therapy, but did not confirm an increase in heart problems with T medicines.[68] These label changes have led to significant media coverage, which is ongoing.
Testosterone Therapy and Decreased Cardiovascular Risk
There are more than 100 studies showing CV benefits of higher endogenous T levels or improved CV risk factors with T therapy.[69] This may not be surprising because long-term T therapy reduces fat mass, increases lean mass, improves glycaemic control, reduces insulin resistance and waist circumference and improves the ability to exercise.
In a retrospective observational study involving 1,031 hypogonadal men aged over 40 years, 398 of whom took T therapy, the cumulative mortality was 21% in the untreated group versus 10% in the treated group (p<0.0001). The mortality rates were 3.4 deaths per 100 person-years for T-treated men and 5.7 deaths per 100 person-years in men not treated with T. The greatest effect was observed in younger men and those with T2D.[70]
In a prospective study, 581 men with T2D and low T (defined as TT <10.4 nmol/l) were followed up for a mean of 5.8 years. Fifty-one men were treated for at least 2.0 years. Mortality rates were 20.0% in the low T group versus 9.1% in the normal T group, independent of comorbidities and therapies, and 9.4% in those with TD in the treated group.[58]
Both studies demonstrated that mortality was reduced by approximately half in those who received T therapy compared with those who did not. A retrospective study followed up 857 men with T2D for 4 years after baseline T measurements. Patients were randomised to long-acting T undecanoate or placebo. Low baseline TT and FT levels were associated with increased all-cause mortality. T therapy and phosphodiesterase type 5 (PDE5) inhibitor use were independently associated with lower all-cause mortality (Figures 1–4).[71] Interestingly, the greatest benefits from the two treatments were seen in older men (Figure 5).[72]
Figure 1: Men Stratified by Testosterone Replacement Therapy.
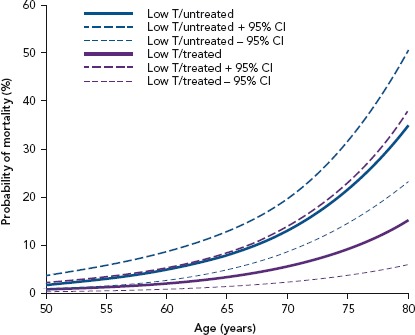
The estimated mortality probability and 95% CI from the fitted logistic regression: men stratified by testosterone replacement therapy. T = testosterone. Source: Hackett et al. 2017.[71] Reproduced with permission from Baishideng Publishing Group.
Figure 4: Men on All or No Treatments.
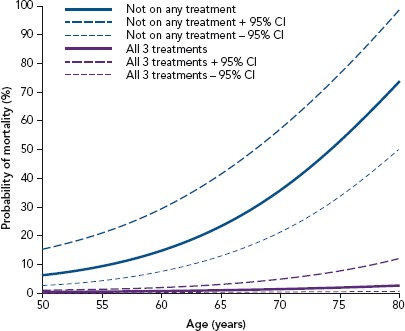
The estimated mortality probability and 95% CI from the fitted logistic regression comparing men on all three treatments (testosterone, phosphodiesterase type 5 inhibitor and statin) and on none of the treatments. Source: Hackett et al. 2017.[71] Reproduced with permission from Baishideng Publishing Group.
Figure 5: Mortality of Men with Type 2 Diabetes Not Receiving Phosphodiesterase Type 5 Inhibitors.
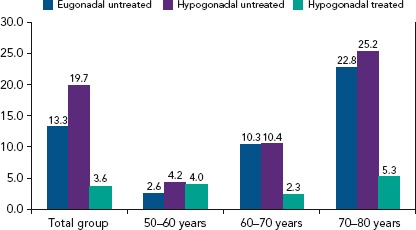
Mortality data of men with type 2 diabetes who were not receiving phosphodiesterase type 5 inhibitors followed for approximately 4 years (n=682). Source: Hackett et al. 2016.[72] Reproduced with permission from Wiley.
This finding regarding PDE5 inhibitor treatment was supported by two database studies demonstrating CV and mortality benefits.[73,74]
Registry data provide useful evidence where parenteral T undecanoate was used for up to 10 years in 656 men with a mean age of 60.7 years. Long-term treatment was well tolerated, with excellent adherence. Importantly, mortality related to CVD was significantly decreased in the group taking T versus the untreated group.[75]
A large retrospective study examined 83,010 male veterans with low TT levels who were categorised into three groups:[76]
group 1: T therapy resulting in normalisation of T levels;
group 2: T therapy without normalisation of T levels; and
group 3: did not receive T therapy.
All-cause mortality (HR 0.44; 95% CI [0.42–0.46]), risk of MI (HR 0.76; 95% CI [0.63–0.93]) and stroke (HR 0.64; 95% CI [0.43–0.96]) were significantly lower in group 1 (n=43,931, median age 66.0 years, mean follow-up 6.2 years) than group 3 (n=13,378, median age 66.0 years, mean follow-up 4.7 years). Similarly, all-cause mortality (HR 0.53; 95% CI [0.50–0.55]), risk of MI (HR 0.82; 95% CI [0.71–0.95]) and stroke (HR 0.70; 95% CI [0.51–0.96]) were significantly lower in group 1 than group 2 (n=25,701, median age 66.0 years, mean follow-up 4.6 years). There was no difference in the risk of MI or stroke between groups 2 and 3.[76]
A further large study compared acute MI rates in 6,355 men aged 66 years and older who received at least one T injection, compared with a matched placebo group over an 8 year period. It found no overall increase in events, those at greatest risk experienced a significant reduction in events and mortality and there was no increased risk from thromboembolism.[77]
A virtual controlled study examined electronic medical records from 1996 to 2011 to identify 5,695 men who had a low initial TT level, a subsequent T level and up to 3 years of follow-up. It demonstrated a positive impact of T therapy – particularly on mortality – and there was no suggestion of increased risk with sustained higher serum levels.[78] In a more recent study, the same group reported a significant reduction in CV events in a cohort of hypogonadal men with angiographically confirmed CAD.[79]
Wallis et al. compared 10,311 T-treated men with 28,029 controls. They found a reduction in all-cause and CV mortality with T therapy. They also found an increase in mortality in the first 6 months compared with normal, which was attributed to the impact of underlying undertreated TD. Reassuringly, this study also reported a 40% reduction in new diagnoses of prostate cancer in the treated group versus the control group.[80]
Lastly, a study comparing 8,808 T-treated men with 35,527 untreated men with low T reported a 33% reduction in cardiac events associated with T therapy.[81]
These results corroborate those of registry studies, which have published data collected over 6 years of follow-up, with no suggestion of increased mortality.[82]
Heart Failure
TD is common during the course of chronic heart failure (CHF). Reduction in circulating T level predicts a deterioration of functional capacity and suggests a role for managing TD in CHF. T is a determinant of exercise capacity, muscle mass and strength. TD is involved in the pathophysiology of CHF, contributing to some features of this syndrome such as reduced muscle mass, abnormal energy handling, fatigue, dyspnoea and, ultimately, cachexia.[83–85]
Figure 3: Long-term Mortality Data in Diabetes.
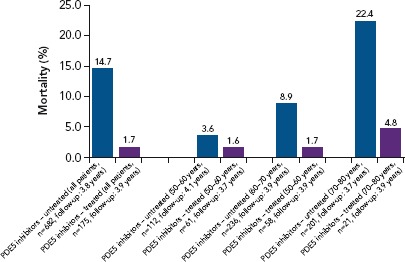
Long-term mortality data in diabetes: the BLAST long-term study (n=857). Source: Hackett et al. 2017.[71] Reproduced with permission from Baishideng Publishing Group.
Approximately 25% of patients affected by CHF have T levels below normal ranges and this is related to disease progression. In addition, reduction of circulating T levels may contribute to some specific features of CHF, such as abnormal energy handling, weakness, dyspnoea and cachexia in particular. T therapy may improve muscle strength and functional pulmonary capacity in (CHF) in men with TD.[86]
Jankowska et al. studied 208 men with CHF and 366 healthy male controls.[87] Low T levels were found in all New York Heart Association (NYHA) classes of heart failure. It has also been shown that reduced T levels in men with CHF indicates a poor prognosis and is associated with increased hospital admissions and mortality.[88,89]
A meta-analysis of a small number of randomised controlled trials studied the effect of T therapy on exercise capacity in HF patients.[90] The four studies (n=198; men, 84%; mean age 67 years) tested either transdermal or intramuscular T, given between 12 weeks and 12 months. The endpoints were 6-minute walk test (6MWT), incremental shuttle walk test, or peak VO2 by cardiopulmonary exercise test. The 6MWT increased by 54.0 metres, incremental shuttle walk test increased by 46.7 metres, and peak VO2 increased by 2.7 ml/kg/min in the T-treated patients versus placebo. The increase in peak VO2 and distance walked in the T-treated group correlated with the increase in FT.
This degree of improvement in the 6MWT is similar to that seen with other therapies in patients with HF. The NYHA functional class improved by ≥1 grade in 9.8% of patients in the placebo group versus 35.0% of patients in the T therapy group. There were no significant differences in major adverse cardiac events between the T therapy and placebo groups.[90]
Concern About Prostate Cancer
There is no compelling evidence that T therapy is associated with an increased risk of prostate cancer. This statement is supported by guidelines from the European Association of Urology, the BSSM, the International Consultation on Sexual Medicine (ICSM) and the ISSM.[1,3,21,23] The ISSAM states that there is no evidence that T therapy converts subclinical prostatic lesions into clinically detectable prostate cancer, and the ICSM states that there is no compelling evidence that T therapy is associated with prostate cancer progression.[3,22]
T therapy may make occult prostate cancer cases detectable within an early phase of treatment and present a beneficial effect in relation to early detection. Future longitudinal studies are needed to confirm these findings.[91]
Testosterone Therapy
The choice of therapy lies between the transdermal route and long-acting T undecanoate injection, depending on patient choice. Adverse events related to T therapy are relatively rare, but follow-up is important because T therapy – especially shorter-acting injections – can increase the haematocrit.[92]
In patients with TD, ischaemia should be addressed wherever possible and underlying risk factors corrected. Mathur et al. tested the effect of T therapy on ischaemia during 12 months of treatment.[93] Long-term treatment with T increased time to develop ischaemia on a treadmill (129 seconds in T patients versus 12 seconds in placebo patients, p=0.020).
Nevertheless, it would seem sensible to be cautious in patients with CVD in an unstable state and approach replacement therapy in the same way as the correction of hypothyroidism, by slowly titrating the dose up to normal over 3 months.
Contraindications to Testosterone Therapy
The main contraindications to T therapy are a haematocrit >54%, male breast cancer, locally advanced or metastatic prostate cancer, an active desire to have children (T therapy reduces spermatogenesis) and severe CHF (NYHA class IV).[1,21]
Additional contraindications include an unevaluated prostate nodule or induration, prostate specific antigen >4 ng/ml (or >3 ng/ml in those at high risk of prostate cancer), severe untreated sleep apnoea and severe lower urinary tract symptoms associated with benign prostatic hyperplasia.[1,3,22,24]
Discussion
Both ED and TD are now regarded as independent CV risk factors.[94–96] ED is common in cardiac patients and virtually all international guidelines recommend testing for TD in men with ED.[97]
PDE5 inhibitors are first-line therapy for men with ED.[98] Patients unresponsive to PDE5 inhibitors may be rescued with T therapy, especially if their T level is less than 8 nmol/l.[99]
The Birmingham, Lichfield, Atherstone, Sutton and Tamworth (BLAST) study found that in patients with T2D, T therapy, PDE5 inhibitors and statin therapy appeared to be synergistic and independent in preventing morbidity and mortality. T therapy and PDE5 inhibitors were independently associated with reduced all-cause mortality, with the greatest benefit from both treatments being seen in older men.[71,72]
Evidence also suggests that PDE5 inhibitors improve insulin sensitivity.[100,101] These findings of reduced mortality in men taking PED5 inhibitors are in close agreement with Anderson et al., who followed up 5,956 UK men with T2D over 6.9 years. A 31.0% reduction in all-cause mortality and a 26.0% reduction in MI were reported with a low rate of PDE5 inhibitor prescribing at 22.8%.[73]
PDE5 inhibitors protect against endothelial reperfusion injury, improve endothelial function and reduce systemic and pulmonary blood pressure.[101] Andersson et al. followed up 43,415 Swedish men after first MI for 5 years and found a significant reduction in all-cause and CV mortality and a 30% reduction in new diagnoses of heart failure and related admissions in men prescribed PDE5 inhibitors.[74] T levels were not recorded and the benefits of PDE5 inhibitors appeared dose related and were not seen with other treatments for ED.
An assessment of CV risk is inadequate and inaccurate if a question regarding ED is not included in the risk calculation, as in QRISK®3.[95]
Conclusion
The balance of evidence is that T therapy does not increase CV risk. Many studies have demonstrated that a low serum T concentration is associated with increased CV risk and mortality and that T therapy may have clinically relevant CV benefits.
Evidence demonstrates reduced CV risk with a higher endogenous T concentration, evidence of improvement of known CV risk factors with T therapy and reduced mortality in T deficient men who received T therapy versus untreated men, evidence of improvement of myocardial ischaemia in men with CAD, improved exercise capacity in men with CHF and improvement in serum glucose levels, HbA1c and insulin resistance in men with diabetes and prediabetes.
Bearing these facts in mind, when dealing with cardiac patients clinicians need to be alert to the possibility of undisclosed sexual problems and underlying TD, which are amenable to treatment.
The following statements are the conclusions of the BSSM:[1]
Other benefits of replacing T in deficient men are that beyond 6 months there is evidence of benefit of T therapy in terms of body composition, features of the metabolic syndrome and bone mineralisation.
T therapy also improves sexual desire, erectile function and sexual satisfaction. Reductions in BMI and waist circumference, and improvements in glycaemic control and lipid profiles, are observed in hypogonadal men receiving T therapy.
Trials of T therapy should extend beyond 6 months and maximal benefit is often seen after 12 months.
Patients should be informed about the benefits and side-effects of therapy to allow a joint decision regarding the appropriateness of treatment.
The adverse effects of T therapy should be fully discussed and, where appropriate its potential effect on future fertility for each patient and his partner.
When T therapy is prescribed, it should be accompanied by weight loss and lifestyle advice as standard management.
For patients who are severely symptomatic, with T levels <8 nmol/l, dietary and lifestyle advice alone is unlikely to produce meaningful improvement within a relevant clinical period.
References
- 1.Hackett G, Kirby M, Edwards D et al. British Society for Sexual Medicine guidelines on adult testosterone deficiency, with statements for UK practice. J Sex Med. 2017;14:1504–23. doi: 10.1016/j.jsxm.2017.10.067. [DOI] [PubMed] [Google Scholar]
- 2.Hackett G, Kirby M, Edwards D UK policy statements on testosterone deficiency. Int J Clin Pract. 2017. p. 71. [DOI] [PMC free article] [PubMed]
- 3.Khera M, Adaikan G, Buvat J et al. Diagnosis and treatment of testosterone deficiency: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015) J Sex Med. 2016;13:1787–804. doi: 10.1016/j.jsxm.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Zitzmann M. Mechanisms of disease: pharmacogenetics of testosterone therapy in hypogonadal men. Nat Clin Pract Urol. 2007;4:161–6. doi: 10.1038/ncpuro0706. [DOI] [PubMed] [Google Scholar]
- 5.Schneider G, Nienhaus K, Gromoll J et al. Aging males’ symptoms in relation to the genetically determined androgen receptor CAG polymorphism, sex hormone levels and sample membership. Psychoneuroendocrinology. 2010;35:578–87. doi: 10.1016/j.psyneuen.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Francomano D, Greco EA, Lenzi A et al. CAG repeat testing of androgen receptor polymorphism: is this necessary for the best clinical management of hypogonadism? J Sex Med. 2013;10:2373–81. doi: 10.1111/jsm.12268. [DOI] [PubMed] [Google Scholar]
- 7.Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92:3844–53. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- 8.Stanworth RD, Akhtar S, Channer KS et al. The role of androgen receptor CAG repeat polymorphism and other factors which affect the clinical response to testosterone replacement in metabolic syndrome and type 2 diabetes: TIMES2 sub-study. Eur J Endocrinol. 2013;170:193–200. doi: 10.1530/EJE-13-0703. [DOI] [PubMed] [Google Scholar]
- 9.Ng Tang, Fui M, Hoermann R, Prendergast LA et al. Symptomatic response to testosterone treatment in dieting obese men with low testosterone levels in a randomized, placebo-controlled clinical trial. Int J Obes. 2017;41:420–6. doi: 10.1038/ijo.2016.242. [DOI] [PubMed] [Google Scholar]
- 10.Borst SE, Mulligan T. Testosterone replacement therapy for older men. Clin Interv Aging. 2007;2:561–6. doi: 10.2147/CIA.S1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng Tang Fui M, Prendergast LA, Dupuis P et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Medicine. 2016;14:153. doi: 10.1186/s12916-016-0700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackett G, Cole N, Bhartia M et al. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study) Int J Clin Pract. 2014;68:203–15. doi: 10.1111/ijcp.12235. [DOI] [PubMed] [Google Scholar]
- 13.Mohler E, Ellenberg SS, Lewis CE et al. The effect of testosterone on cardiovascular biomarkers in the testosterone trials. J Clin Endocrinol Metab. 2018;103:681–8. doi: 10.1210/jc.2017-02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyos CM, Yee BJ, Phillips CL et al. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnea: a randomized placebo-controlled trial. Eur J Endocrinol. 2012;167:531–41. doi: 10.1530/EJE-12-0525. [DOI] [PubMed] [Google Scholar]
- 15.Huang G, Bhasin S, Tang ER et al. Effect of testosterone administration on liver fat in older men with mobility limitation: results from a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68:954–9. doi: 10.1093/gerona/gls259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu FC, Tajar A, Beynon JM et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 17.Tajar A, Forti G, O’Neill TW et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–8. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 18.Corona G, Maseroli E, Rastrelli G et al. Characteristics of compensated hypogonadism in patients with sexual dysfunction. J Sex Med. 2014;11:1823–34. doi: 10.1111/jsm.12549. [DOI] [PubMed] [Google Scholar]
- 19.Wu FC, Tajar A, Pye SR et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 20.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–53. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 21.Dohle GH, Arver S, Bettocchi C Guidelines on male hypogonadism. The Netherlands: European Association of Urology, 2017. http://uroweb.org/guideline/male-hypogonadism/ Available at. (accessed January 2019)
- 22.Lunenfeld B, Mskhalaya G, Zitzmann M et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15. doi: 10.3109/13685538.2015.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean JD, McMahon CG, Guay AT et al. The International Society for Sexual Medicine’s process of care for the assessment and management of testosterone deficiency in adult men. J Sex Med. 2015;12:1660–86. doi: 10.1111/jsm.12952. [DOI] [PubMed] [Google Scholar]
- 24.Bhasin S, Cunningham GR, Hayes FJ et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 25.Caronia LM, Dwyer AA, Hayden D et al. Abrupt decrease in serum testosterone levels after an oral glucose load in men: implications for screening for hypogonadism. Clin Endocrinol (Oxf) 2013;78:291–6. doi: 10.1111/j.1365-2265.2012.04486.x. [DOI] [PubMed] [Google Scholar]
- 26.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jc.84.10.3666. [DOI] [PubMed] [Google Scholar]
- 27.International Society for Sexual Medicine ISSM quick reference guide on testosterone deficiency for men. Wormerveer, the Netherlands: ISSM, 2015. https://professionals.issm.info/wp-content/uploads/sites/2/2018/05/ISSM-Quick-Reference-Guide-on-TD.pdf Available at. (accessed 14 June 2019)
- 28.Tajar A, McBeth J, Lee DM et al. Elevated levels of gonadotrophins but not sex steroids are associated with musculoskeletal pain in middle-aged and older European men. Pain. 2011;152:1495–501. doi: 10.1016/j.pain.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonio L, Wu FC, O’Neill TW et al. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal testosterone. J Clin Endocrinol Metab. 2016;101:2647–57. doi: 10.1210/jc.2015-4106. [DOI] [PubMed] [Google Scholar]
- 30.Ruige JB, Mahmoud AM, De Bacquer D et al. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;11:870–5. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 31.Araujo AB, Dixon JM, Suarez EA et al. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–19. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corona G, Rastrelli G, Monami M et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 33.Webb CM, McNeill JG, Hayward CS et al. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–6. doi: 10.1161/01.CIR.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 34.Webb CM, Elkington AG, Kraidly MM et al. Effects of oral testosterone treatment on myocardial perfusion and vascular function in men with low plasma testosterone and coronary heart disease. Am J Cardiol. 2008;101:618–24. doi: 10.1016/j.amjcard.2007.09.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong PJ, Patrizi G, Chong WC et al. Testosterone enhances flow-mediated brachial artery reactivity in men with coronary artery disease. Am J Cardiol. 2000;85:269–72. doi: 10.1016/S0002-9149(99)00630-X. [DOI] [PubMed] [Google Scholar]
- 36.Malkin CJ, Pugh PJ, Morris PD. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90:871–6. doi: 10.1136/hrt.2003.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.English KM, Steeds RP, Jones TH et al. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–11. doi: 10.1161/01.CIR.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 38.Rosano GM, Leonardo F, Pagnotta P et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–70. doi: 10.1161/01.CIR.99.13.1666. [DOI] [PubMed] [Google Scholar]
- 39.Webb CM, Adamson DL, de Zeigler D, Collins P. Effect of acute testosterone on myocardial ischemia in men with coronary artery disease. Am J Cardiol. 1999;83:437–9. doi: 10.1016/S0002-9149(98)00880-7. [DOI] [PubMed] [Google Scholar]
- 40.Makinen J, Jarvisalo M, Pollanen P et al. Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol. 2005;45:1603–8. doi: 10.1016/j.jacc.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 41.van den Beld AW, Bots ML, Janssen JA et al. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol. 2003;157:25–31. doi: 10.1093/aje/kwf160. [DOI] [PubMed] [Google Scholar]
- 42.Fukui M, Kitagawa Y, Nakamura N et al. Association between serum testosterone concentration and carotid atherosclerosis in men with type 2 diabetes. Diabetes Care. 2003;26:1869–73. doi: 10.2337/diacare.26.6.1869. [DOI] [PubMed] [Google Scholar]
- 43.De Pergola G, Pannacciulli N, Ciccone M et al. Free testosterone plasma levels are negatively associated with the intima-media thickness of the common carotid artery in overweight and obese glucose-tolerant young adult men. Int J Obes Relat Metab Disord. 2003;27:803–7. doi: 10.1038/sj.ijo.0802292. [DOI] [PubMed] [Google Scholar]
- 44.Svartberg J, Von Muhlen D, Mathiesen E et al. Low testosterone levels are associated with carotid atherosclerosis in men. J Intern Med. 2006;259:576–82. doi: 10.1111/j.1365-2796.2006.01637.x. [DOI] [PubMed] [Google Scholar]
- 45.Fu L, Gao QP, Shen JX. Relationship between testosterone and indexes indicating endothelial function in male coronary heart disease patients. Asian J Androl. 2008;10:214–8. doi: 10.1111/j.1745-7262.2008.00335.x. [DOI] [PubMed] [Google Scholar]
- 46.Oskui PM, French WJ, Herring MJ et al. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013;6:E000272. doi: 10.1161/JAHA.113.000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb Vasc Biol. 1991;11:1245–9. doi: 10.1161/01.ATV.11.5.1245. [DOI] [PubMed] [Google Scholar]
- 48.Zhao SP, Li XP. The association of low plasma testosterone level with coronary artery disease in Chinese men. Int J Cardiol. 1998;63:161–4. doi: 10.1016/S0167-5273(97)00295-7. [DOI] [PubMed] [Google Scholar]
- 49.Akishita M, Hashimoto M, Ohike Y et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210:232–6. doi: 10.1016/j.atherosclerosis.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 50.Rosano G, Sheiban I, Massaro R et al. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res. 2007;19:176–82. doi: 10.1038/sj.ijir.3901504. [DOI] [PubMed] [Google Scholar]
- 51.Hu X, Rui L, Zhu T et al. Low testosterone level in middle-aged male patients with coronary artery disease. Eur J Intern Med. 2011;22:133–6. doi: 10.1016/j.ejim.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 52.English KM, Mandour O, Steeds RP et al. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000;21:890–4. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 53.Dobrzycki S, Serwatka W, Nadlewski S et al. An assessment of correlations between endogenous sex hormone levels and the extensiveness of coronary heart disease and the ejection fraction of the left ventricle in males. J Med Invest. 2003;50:162–9. [PubMed] [Google Scholar]
- 54.Li L, Guo CY, Jia EZ et al. Testosterone is negatively associated with the severity of coronary atherosclerosis in men. Asian J Androl. 2012;14:875–8. doi: 10.1038/aja.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 1994;14:701–6. doi: 10.1161/01.ATV.14.5.701. [DOI] [PubMed] [Google Scholar]
- 56.Budoff M, Ellenberg S, Lewis C et al. Testosterone treatment and coronary artery plaque volume in men with low testosterone. JAMA. 2017;317:708–16. doi: 10.1001/jama.2017.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haring R, Volzke HV, Steveling A et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J. 2010;31:1494–501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 58.Muraleedharan V, Marsh H, Kapoor D et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–33. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 59.Yeap B, Alfonso H, Chubb S et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99:E9–E18. doi: 10.1210/jc.2013-3272. [DOI] [PubMed] [Google Scholar]
- 60.Daka P, Langer RD, Larsson CA. Low concentrations of serum testosterone predicts acute myocardial infarction in men with type 2 diabetes mellitus. BMC Endocr Disord. 2015;15: 1–12. doi: 10.1186/s12902-015-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muraleedharan V, Jones TH. Testosterone and mortality. Clin Endocrinol. 2014;81:477–87. doi: 10.1111/cen.12503. [DOI] [PubMed] [Google Scholar]
- 62.Morgentaler A, Miner MM, Caliber M et al. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90:224–51. doi: 10.1016/j.mayocp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Vigen R, O’Donnell CI, Barón AE et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–36. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 64.Finkle WD, Greenland S, Ridgeway GK et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basaria S, Coviello AD, Travison TG et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.US Food and Drug Administration. Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use 2015. https://www.fda.gov/Drugs/DrugSafety/ucm436259.htm Washington DC: FDA, 3 March 2015 Available at. (accessed 14 June 2019) [DOI] [PubMed]
- 68.European Medicines Agency PRAC review does not confirm increase in heart problems with testosterone medicines. London: European Medicines Agency, 10 Octoberz 2014. https://www.ema.europa.eu/documents/referral/testosterone-article-31-referral-prac-review-does-not-confirm-increase-heart-problems-testosterone_en.pdf Available at. (accessed 14 June 2019)
- 69.Morgentaler A, Zitzman M, Abdulmaged M et al. Fundamental concepts regarding testosterone deficiency and treatment: International Expert consensus resolutions. Mayo Clin Proc. 2016;91:881–96. doi: 10.1016/j.mayocp.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Shores MM, Smith NL, Forsberg CW et al. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–8. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 71.Hackett GI, Ramachandran S, Strange R et al. Statin, testosterone and PDE5Is and age-related mortality in type 2 diabetes. World J Diabetes. 2017;8:104–11. doi: 10.4239/wjd.v8.i3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hackett G, Heald AH, Sinclair A et al. Serum testosterone, testosterone replacement therapy and all-cause mortality in men with type 2 diabetes: retrospective consideration of the impact of PDE5 inhibitors and statins. Int J Clin Pract. 2016;70:244–53. doi: 10.1111/ijcp.12779. [DOI] [PubMed] [Google Scholar]
- 73.Anderson S, Hutchings DC, Woodward M et al. Phosphodiesterase type-5 inhibitor use in type 2 diabetes is associated with a reduction in all-cause mortality. Heart. 2016;102:1750–6. doi: 10.1136/heartjnl-2015-309223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersson DP, Lagerros YT, Grotta A et al. Association between treatment for erectile dysfunction and death or cardiovascular outcomes after myocardial infarction. Heart. 2017;103:1264–70. doi: 10.1136/heartjnl-2016-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Traish AM, Haider A, Haider KS et al. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22:414–33. doi: 10.1177/1074248417691136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma R, Oni OA, Gupta K et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–15. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 77.Baillargeon J, Urban RJ, Kuo YF et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48:1138–44. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anderson J, May H, Lappe D. Cardiovascular impact of testosterone therapy in men with low testosterone levels. Circulation. 2014;130((Suppl 2)):A13220. [Google Scholar]
- 79.Anderson JL, May HT, Lappé DL et al. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated health care system. Am J Cardiol. 2016;117:794–9. doi: 10.1016/j.amjcard.2015.11.063. [DOI] [PubMed] [Google Scholar]
- 80.Wallis CJ, Lo K, Lee Y et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4:498–506. doi: 10.1016/S2213-8587(16)00112-1. [DOI] [PubMed] [Google Scholar]
- 81.Cheetham CT, An JJ, Jacobsen SJ. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med. 2017;177:491–9. doi: 10.1001/jamainternmed.2016.9546. [DOI] [PubMed] [Google Scholar]
- 82.Haider A, Yassin A, Dorros G et al. Effects of long-term testosterone therapy on patients with ‘diabesity’: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515. doi: 10.1155/2014/683515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Volterrani M, Rosano G, Iellamo F. Testosterone and heart failure. Endocrine. 2012;42:272–7. doi: 10.1007/s12020-012-9725-9. [DOI] [PubMed] [Google Scholar]
- 84.Malkin CJ, Jones TH, Channer KS. Testosterone in chronic heart failure. Front Horm Res. 2009;37:183–96. doi: 10.1159/000176053. [DOI] [PubMed] [Google Scholar]
- 85.Pugh PJ, Jones RD, West JN et al. Testosterone treatment for men with chronic heart failure. Heart. 2004;90:446–7. doi: 10.1136/hrt.2003.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giagulli VA, Guastamacchia E, De Pergola G et al. Testosterone deficiency in male: a risk factor for heart failure. Endocr Metab Immune Disord Drug Targets. 2013;13:92–9. doi: 10.2174/1871530311313010011. [DOI] [PubMed] [Google Scholar]
- 87.Jankowska EA, Biel B, Majda J et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–37. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- 88.Wehr E, Pilz S, Boehm BO et al. Low free testosterone is associated with heart failure mortality in older men referred for coronary angiography. Eur J Heart Fail. 2011;13:482–8. doi: 10.1093/eurjhf/hfr007. [DOI] [PubMed] [Google Scholar]
- 89.Dos Santos MR, Seyegh AL, Groehrs RV et al. Testosterone deficiency increases hospital readmission and mortality rates in male patients with heart failure. Arq Bras Cardiol. 2015;105:256–64. doi: 10.5935/abc.20150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toma M, McAlister FA, Coglianese EE et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012;5:315–21. doi: 10.1161/CIRCHEARTFAILURE.111.965632. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X, Zhong Y, Saad F Clinically occult prostate cancer cases may distort the effect of testosterone replacement therapy on risk of PCa. World J Urol. 2019. epub ahead of press. [DOI] [PubMed]
- 92.Jones SD, Dukovac T, Sangkum P et al. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev. 2015;3:101–12. doi: 10.1002/smrj.43. [DOI] [PubMed] [Google Scholar]
- 93.Mathur A, Malkin C, Saeed B et al. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161:443–9. doi: 10.1530/EJE-09-0092. [DOI] [PubMed] [Google Scholar]
- 94.Hackett G, Kirby M. Erectile dysfunction and testosterone deficiency as cardiovascular risk factors? Int J Clin Pract. 2017;72:e13054. doi: 10.1111/ijcp.13054. [DOI] [PubMed] [Google Scholar]
- 95.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi: 10.1136/bmj.j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mulhall JP, Trost LW, Brannigan RE et al. Evaluation and management of testosterone deficiency: AUA Guideline. J Urol. 2018;200:423–32. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 97.Hodges LD, Kirby M, Solanki J et al. The temporal relationship between erectile dysfunction and cardiovascular disease. Int J Clin Pract. 2007;61:2019–25. doi: 10.1111/j.1742-1241.2007.01629.x. [DOI] [PubMed] [Google Scholar]
- 98.Hackett G, Kirby M, Wylie K et al. British Society for Sexual Medicine Guidelines on the Management of Erectile Dysfunction in Men – 2017. J Sex Med. 2018;15:430–57. doi: 10.1016/j.jsxm.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 99.Hackett G, Cole N, Saghir A, et al. Testosterone undecanoate improves sexual function in men with type 2 diabetes and severe hypogonadism: results from a 30-week randomized placebo-controlled study. BJU International. 2016;118:804–13. doi: 10.1111/bju.13516. [DOI] [PubMed] [Google Scholar]
- 100.Ramirez CE, Nian H, Yu C et al. Treatment with sildenafil improves insulin sensitivity in prediabetes: a randomized, controlled trial. J Clin Endocrinol Metab. 2015;100:4533–40. doi: 10.1210/jc.2015-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pofi R, Gianfrilli D, Badagliacca R et al. Everything you ever wanted to know about phosphodiesterase 5 inhibitors and the heart (but never dared ask): How do they work? J Endocrinol Invest. 2016;39:131–42. doi: 10.1007/s40618-015-0339-y. [DOI] [PubMed] [Google Scholar]


