Abstract
Objectives
Recent Zika virus (ZIKV) outbreaks challenged existing laboratory diagnostic standards, especially for serology‐based methods. Because of the genetic and structural similarity of ZIKV with other flaviviruses, this results in cross‐reactive antibodies, which confounds serological interpretations.
Methods
Plasma from Singapore ZIKV patients was screened longitudinally for antibody responses and neutralising capacities against ZIKV. Samples from healthy controls, ZIKV patients and DENV patients were further assessed using ZIKV and DENV peptides of precursor membrane (prM), envelope (E) or non‐structural 1 (NS1) viral proteins in a peptide‐based ELISA for epitope identification. Identified epitopes were re‐validated and diagnostically evaluated using sera of patients with DENV, bacteria or unknown infections from Thailand.
Results
Long‐lasting ZIKV‐neutralising antibodies were elicited during ZIKV infection. Thirteen potential linear B‐cell epitopes were identified, and of these, four common flavivirus, three ZIKV‐specific and one DENV‐specific differential epitopes had more than 50% sensitivity and specificity. Notably, ZIKV‐specific peptide 26 on domain I/II of E protein (amino acid residues 271–288) presented 80% sensitivity and 85.7% specificity. Importantly, the differential epitopes also showed significance in differentiating non‐flavivirus patient samples.
Conclusion
Linear B‐cell epitope candidates to differentiate between ZIKV and DENV infections were identified, providing the first step towards the design of a much‐needed serology‐based assay.
Keywords: flavivirus, epitopes, patients, diagnostic
Linear B‐cell epitope candidates to differentiate between Zika virus (ZIKV) and dengue virus (DENV) infections were identified, which includes four common flavivirus, three ZIKV‐specific and one DENV‐specific differential epitopes that showed more than 50% sensitivity and specificity. Notably, ZIKV‐specific peptide 26 on domain I/II of envelope (E) glycoprotein (amino acid residues 271–288) presented 80% sensitivity and 85.7% specificity. Importantly, the differential epitopes also showed significance in discriminating non‐flavivirus patient samples.
Introduction
Zika virus (ZIKV) outbreaks in French Polynesia and Brazil in 2013 and 2015 resulted in unexpected severe neurological and congenital complications,1, 2, 3, 4 leading to a race to develop diagnostic and treatment strategies against the infection. Current ZIKV diagnosis, which relies heavily on molecular methods, poses several limitations because ZIKV patients display a short viraemic phase with low viraemia levels, and thus may escape detection, even in symptomatic patients.5, 6 Hence, serology, as an alternative diagnostic approach, is very much needed to address these shortcomings. Unfortunately, this approach has been hampered because of the cross‐reactive nature of the antibodies in ZIKV patients with other flaviviruses, such as dengue virus (DENV),7, 8, 9, 10, 11 in which ZIKV shares high amino acid identity (55%) and structural homology with DENV.12, 13, 14, 15, 16 Moreover, as both viruses are transmitted by the same mosquito vectors,17 they are often found in overlapping geographical areas.18, 19 Thus, there is a demand for a proper serology diagnostic tool that accurately differentiates the two infections.
Previous studies have shown the possibility of using ZIKV antigens to distinguish ZIKV infections from other flavivirus infections.11, 20, 21, 22 Although computational studies have predicted multiple differential epitopes, validation on patient samples, however, remains a challenge.23 In this report, antibody and neutralising responses by ZIKV patients from Singapore were characterised longitudinally. Common and differential linear B‐cell epitopes recognised by antibodies from Singapore ZIKV and DENV patients were then identified. Importantly, the potential value of these identified epitopes in a diagnostic setting was further assessed using sera from patients from Thailand previously diagnosed with DENV, bacteria and including those of unknown infections. This study aims to further the development of a serology‐driven differential flavivirus diagnosis, particularly between ZIKV and DENV, allowing for accurate diagnosis that will improve patient management. The application can also be further expanded to study sero‐prevalence and vaccine strategies.
Results
ZIKV patients produce a robust and protective humoral response
Forty‐five healthy donors were first screened for the presence of IgM and IgG against ZIKV, DENV and chikungunya virus (CHIKV), the three main arboviruses co‐circulating in Singapore and several parts of Asia18 using virion‐based ELISA.18, 24, 25, 26, 27 Twenty‐two donors who had antibody levels lower than the assigned cut‐off (mean + SD) in all three viruses (Supplementary figure 1a, b) were used as the healthy control pool and set as a baseline reference.
Anti‐ZIKV IgM and IgG levels of ZIKV patients from the Singapore outbreak in 201628, 29, 30 were longitudinally assessed using virion‐based ELISA.18, 24, 25, 26, 27 The majority of the patients showed a robust ZIKV‐specific humoral response (Figure 1a–c and Supplementary figure 1c). Anti‐ZIKV IgM was detected as early as in the acute phase [2–7 days post‐illness onset (pio)] and peaked at early convalescent phase (10–14 days pio), before decreasing during the recovery phases (3 months to 1 year pio, Figure 1a, c and Supplementary figure 1c). ZIKV‐specific IgG titres peaked at early convalescent, persisted at high levels during late recovery and were still detectable a year after infection (Figure 1b, c and Supplementary figure 1c). These patients were also screened for the presence of DENV‐specific antibodies, and 80% of the patients were negative for anti‐DENV IgM in samples taken at the acute phase (Supplementary figure 1d, f). However, 75% of the patients were found to have anti‐DENV IgG (Supplementary figure 1e, f), suggesting that ZIKV IgG, but not IgM, cross‐reacts with DENV.
Figure 1.
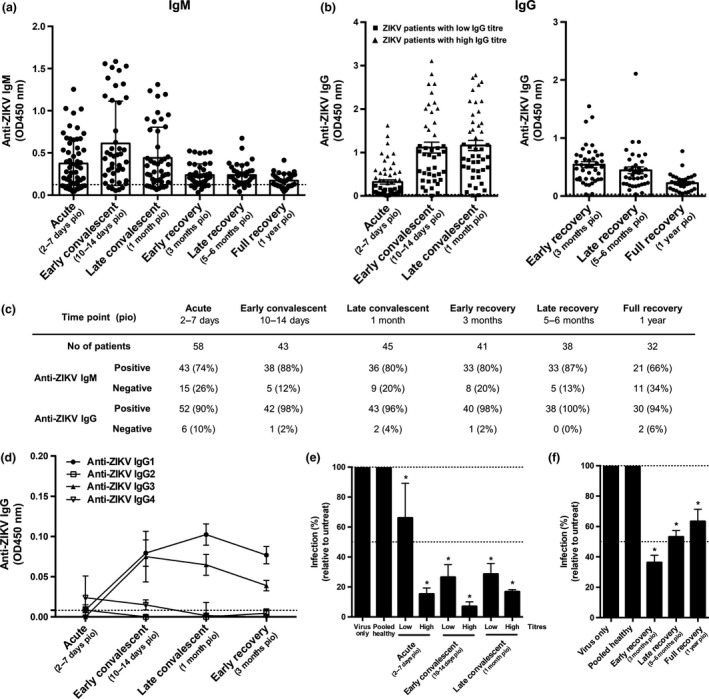
Antibody profiles of ZIKV patients of Singapore cohort in 2016 over time. (a‐c) Total anti‐ZIKV (a) IgM and (b) IgG antibody titres in patients’ plasma samples at dilutions 1:200 and 1:2000, respectively, were determined by virion‐based ELISA using purified ZIKV virions. Pooled plasma of healthy donors was used as negative control. Data are presented as mean ± SEM, with dotted line indicating mean of pooled healthy control. (c) Number and percentage of patients who are positive or negative for anti‐ZIKV IgM and IgG at the respective time points. (d) IgG isotype titres in patients’ plasma samples were determined at 1:200 dilution in a ZIKV virion‐based ELISA. Data are presented as mean ± SEM, with dotted line indicating mean of pooled healthy control. All ELISA readings were conducted in duplicates or triplicates [acute (n = 58), early convalescent (n = 43), late convalescent (n = 45), early recovery (n = 41), late recovery (n = 38) and full recovery (n = 32)]. (e‐f) In vitro neutralising capacity of pooled ZIKV patients and pooled healthy control was tested at 1:1000 plasma dilution via flow cytometry. (e) Plasma samples were pooled according to levels of anti‐ZIKV IgG titre [group of low‐titre patients are denoted as square symbol, while group of high‐titre patients are denoted as triangle symbol as shown in (b)] for acute [low (n = 37), high (n = 21)], early convalescent [low (n = 29), high (n = 14)] and late convalescent [low (n = 28), high (n = 17)] time points. (f) Plasma samples collected at the recovery phases were pooled together at the respective time points [early recovery (n = 41), late recovery (n = 38) and full recovery (n = 32)]. Results are expressed as percentage of control infection. Data are presented as mean ± SD and representative of two independent experiments. Statistical analysis between virus‐only and pooled healthy control or patient samples was carried out using the Mann–Whitney U‐test, two‐tailed, with the Bonferroni correction for multiple testing (*P < 0.05).
IgG isotypes produced by ZIKV patients were then determined, and highest titres of anti‐ZIKV IgG1 and IgG3 subtypes were produced at early convalescent for IgG3 and late convalescent for IgG1 (Figure 1d). To determine whether antibodies produced in these patients were protective against ZIKV, neutralisation assays were carried out via flow cytometry (Figure 1e, f and Supplementary figure 2a). Efficient neutralisation (71–93%) was observed in early and late convalescent stages (Figure 1e), while weak neutralisation (37–47%) was seen in late and full recovery stages (Figure 1f). Neutralisation capacity of ZIKV patients correlated with levels of anti‐ZIKV IgG (Supplementary figures 1c, 5a). Plasma from these patients only minimally neutralised DENV (Supplementary figure 2b), indicating ZIKV specificity.
Identification of specific B‐cell linear epitopes recognised by antibodies from ZIKV and DENV patients
Preliminary mapping of specific ZIKV and DENV epitopes was first performed in a peptide‐based ELISA on the most antigenic flavivirus antigens: prM, E and NS1,24, 25, 31 using pooled linear ZIKV and consensus DENV peptides. Plasma/serum samples of ZIKV and DENV patients32 taken at the late convalescent phase were used as IgG levels were highest at this time point (Supplementary figure 1c). Results specifically showed two common flavivirus (pools 1 and 21), six potential ZIKV‐specific (pools 6, 10, 11, 16, 17 and 24) and one potential DENV‐specific (pool 19) pools were identified within the ZIKV and DENV proteome (Supplementary table 1 and Supplementary figure 3). Thereafter, new peptides selectively designed based on the exposed residues and computational predictions were re‐synthesised for subsequent experiments (Supplementary table 2).23
Interestingly, results showed differences between pooled and individual peptides (Table 1 and Figure 2). These differences could be due to the interferences of the pooled peptides, while single peptides allowed for more enhanced specific binding. Nevertheless, six potential common flavivirus peptides were identified, which displayed less than 0.05 relative difference in the binding capacity between ZIKV and DENV patients (peptides 7, 36, 38, 39, 46 and 49) (Table 1, Figure 2 and Supplementary figure 4). These peptides were also selected based on the close similarity between the ZIKV and DENV peptide sequences (Supplementary table 2). Additionally, three potential ZIKV‐specific (peptides 3, 26 and 32) and four potential DENV‐specific (peptides 9, 17, 43 and 45) peptides with a binding capacity difference of more than 0.1 were identified (Table 1, Figure 2 and Supplementary figure 4).
Table 1.
Singapore ZIKV and DENV patients’ response to ZIKV and DENV peptides
| Protein | Peptide No. | Recognition (%)a | Mean binding capacityb | Relative differencec | Epitope classificationd | ||||
|---|---|---|---|---|---|---|---|---|---|
| ZIKV patients (n = 30–44) | DENV patients (n = 20) | ||||||||
| ZIKV peptide | DENV peptide | ZIKV peptide | DENV peptide | ZIKV patients | DENV patients | ||||
| prM | 1 | 55 | 39 | 55 | 40 | 0.244 | 0.226 | 0.018 | |
| 2 | 63 | 60 | 95 | 80 | 0.634 | 0.612 | 0.022 | ||
| 3 | 70 | 66 | 80 | 75 | 0.132 | −0.033 | 0.165 | ZIKV‐specific | |
| 4 | 30 | 50 | 0 | 15 | −0.356 | −0.346 | 0.010 | ||
| 5 | 59 | 45 | 55 | 50 | 0.306 | 0.272 | 0.034 | ||
| 6 | 59 | 61 | 40 | 50 | −0.120 | −0.079 | 0.041 | ||
| 7 | 86 | 86 | 90 | 85 | 0.056 | 0.014 | 0.042 | Common | |
| 8 | 59 | 64 | 30 | 0 | −0.088 | −0.293 | 0.205 | ||
| 9 | 52 | 55 | 25 | 60 | −0.210 | −0.455 | 0.246 | DENV‐specific | |
| 10 | 62 | 86 | 40 | 70 | −0.263 | −0.355 | 0.092 | ||
| E | 11 | 64 | 61 | 55 | 35 | 0.066 | 0.271 | 0.205 | |
| 12 | 84 | 89 | 65 | 65 | 0.065 | 0.076 | 0.011 | ||
| 13 | 59 | 57 | 30 | 20 | −0.030 | 0.088 | 0.118 | ||
| 14 | 68 | 66 | 60 | 65 | −0.015 | 0.043 | 0.059 | ||
| 15 | 61 | 64 | 30 | 50 | −0.138 | −0.157 | 0.020 | ||
| 16 | 53 | 90 | 55 | 85 | −0.415 | −0.379 | 0.036 | ||
| 17 | 70 | 75 | 55 | 85 | 0.084 | −0.227 | 0.311 | DENV‐specific | |
| 18 | 70 | 100 | 85 | 100 | −0.218 | −0.260 | 0.042 | ||
| 19 | 57 | 57 | 50 | 55 | 0.084 | 0.006 | 0.078 | ||
| 20 | 60 | 50 | 30 | 20 | 0.186 | 0.314 | 0.129 | ||
| 21 | 54 | 78 | 25 | 45 | −0.314 | −0.264 | 0.050 | ||
| 22 | 47 | 37 | 25 | 0 | 0.610 | 0.620 | 0.010 | ||
| 23 | 34 | 30 | 0 | 0 | 0.152 | N.A. | N.A. | ||
| 24 | 86 | 98 | 90 | 100 | −0.235 | −0.041 | 0.194 | ||
| 25 | 77 | 75 | 55 | 40 | 0.177 | 0.209 | 0.032 | ||
| 26 | 64 | 59 | 25 | 25 | 0.340 | 0.173 | 0.167 | ZIKV‐specific | |
| 27 | 68 | 78 | 40 | 50 | −0.081 | −0.114 | 0.033 | ||
| 28 | 87 | 90 | 95 | 95 | 0.095 | −0.001 | 0.097 | ||
| 29 | 76 | 70 | 40 | 45 | −0.109 | −0.018 | 0.090 | ||
| 30 | 64 | 80 | 45 | 55 | −0.182 | −0.157 | 0.025 | ||
| 31 | 93 | 95 | 90 | 75 | 0.319 | 0.681 | 0.362 | ||
| 32 | 100 | 100 | 100 | 100 | 0.189 | 0.039 | 0.150 | ZIKV‐specific | |
| NS1 | 33 | 82 | 86 | 65 | 60 | 0.013 | 0.033 | 0.020 | |
| 34 | 86 | 82 | 90 | 50 | 0.431 | 0.853 | 0.422 | ||
| 35 | 84 | 89 | 65 | 70 | −0.223 | −0.134 | 0.089 | ||
| 36 | 79 | 83 | 80 | 90 | 0.012 | −0.031 | 0.042 | Common | |
| 37 | 84 | 82 | 60 | 60 | −0.012 | −0.023 | 0.011 | ||
| 38 | 82 | 89 | 60 | 70 | 0.019 | 0.000 | 0.019 | Common | |
| 39 | 89 | 91 | 75 | 75 | −0.041 | −0.069 | 0.027 | Common | |
| 40 | 89 | 91 | 80 | 75 | −0.082 | −0.079 | 0.002 | ||
| 41 | 68 | 66 | 35 | 35 | 0.154 | 0.103 | 0.051 | ||
| 42 | 68 | 59 | 35 | 35 | 0.177 | 0.110 | 0.068 | ||
| 43 | 84 | 91 | 75 | 95 | −0.137 | −0.244 | 0.107 | DENV‐specific | |
| 44 | 81 | 83 | 65 | 45 | 0.192 | 0.183 | 0.010 | ||
| 45 | 84 | 89 | 55 | 75 | −0.131 | −0.267 | 0.136 | DENV‐specific | |
| 46 | 84 | 82 | 70 | 60 | 0.118 | 0.098 | 0.020 | Common | |
| 47 | 82 | 86 | 50 | 70 | −0.132 | −0.120 | 0.012 | ||
| 48 | 84 | 59 | 65 | 35 | −0.757 | 1.234 | 1.992 | ||
| 49 | 78 | 86 | 50 | 60 | −0.019 | −0.019 | 0.001 | Common | |
| 50 | 80 | 75 | 80 | 40 | 0.334 | 0.720 | 0.386 | ||
| 51 | 86 | 83 | 90 | 80 | 0.019 | 0.160 | 0.141 | ||
Patient samples are positive if their normalised peptide responses (calculated as OD of patient sample/mean OD of pooled healthy control) are more than 1.01.
Binding capacity of a patient positive for a peptide pair was calculated using normalised values of: [(ZIKV peptide response‐DENV peptide response)/DENV peptide response]. Values close to 0 denote equal recognition of sample to ZIKV and DENV peptides. Values more than 0 denote a sample recognising ZIKV peptide more. Values less than 0 denote a sample recognising DENV peptide more.
Relative difference is calculated as the difference in the mean binding capacity of ZIKV patients and DENV patients. Values are rounded up to 3 decimal places.
Common flavivirus epitopes: ≥ 60% of ZIKV and DENV patients recognise both ZIKV and DENV peptides of peptide pair. ZIKV‐specific epitopes: ≥ 60% of ZIKV patients recognise at least ZIKV peptide of peptide pair. DENV‐specific epitopes: ≥ 60% of DENV patients recognise at least DENV peptide of peptide pair.
Figure 2.
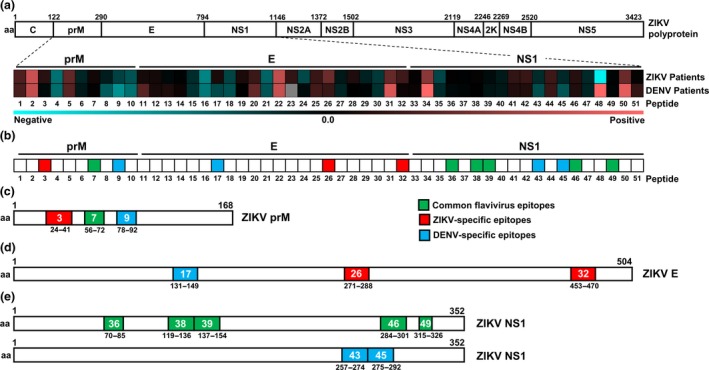
Mapping of common flavivirus, ZIKV‐specific and DENV‐specific linear B‐cell epitopes using ZIKV and DENV patient samples. (a) Polyprotein of ZIKV H/PF/2013 (UniProtKB accession: A0A024B7W1). Plasma samples of ZIKV patients (n = 30–44) and serum samples of DENV patients (n = 20) at late convalescent phase were tested at 1:2000 dilution in a peptide‐based ELISA in duplicates, using peptides that cover the precursor of membrane (prM: peptides 1–10), envelope (E; peptides 11–32) and non‐structural 1 (NS1; peptides 33–51) proteins of ZIKV and DENV proteome. IgG response of patients was normalised to mean of pooled healthy control. Patients’ response to ZIKV and DENV peptide pairs was compared, and the mean binding capacity is presented in a heat‐map. A value of 0 on the scale denotes patients showing equal binding response to a ZIKV and DENV peptide pair, whereas values larger than 0 show preferential of patients to bind to ZIKV peptide. Values smaller than 0 show binding preference of patients to DENV peptide. (b) A schematic representation to denote common flavivirus (green), ZIKV‐specific (red) and DENV‐specific (blue) peptides across prM, E and NS1 based on the heat‐map analysis above. (c–e) Genome organisation of ZIKV prM, E and NS1. Regions of amino acids corresponding to the identified linear B‐cell epitopes in (c) prM, (d) E and (e) NS1 are shown, with green areas denoting common flavivirus, red denoting ZIKV‐specific and blue denoting DENV‐specific epitopes. Numbers in coloured boxes denote the peptide number and the amino acid position in the respective proteome.
Epitope recognition by ZIKV patients over time
In order to characterise the changes in epitope recognition by ZIKV patients over time, the common flavivirus (green) and ZIKV‐specific (red) peptides were screened with plasma of ZIKV patients in acute, late convalescent and full recovery phases. For the common flavivirus hits, more than 60% of the ZIKV patients were able to recognise the six peptide pairs at late convalescent and beyond (Figure 3a). However, at the acute phase, only peptides 7, 36 and 38 were recognised by ZIKV patients (Figure 3a). In terms of binding capacity, there was equal binding between ZIKV and DENV peptide pairs over time for peptides 7, 36, 38 and 49 (Figure 3b).
Figure 3.
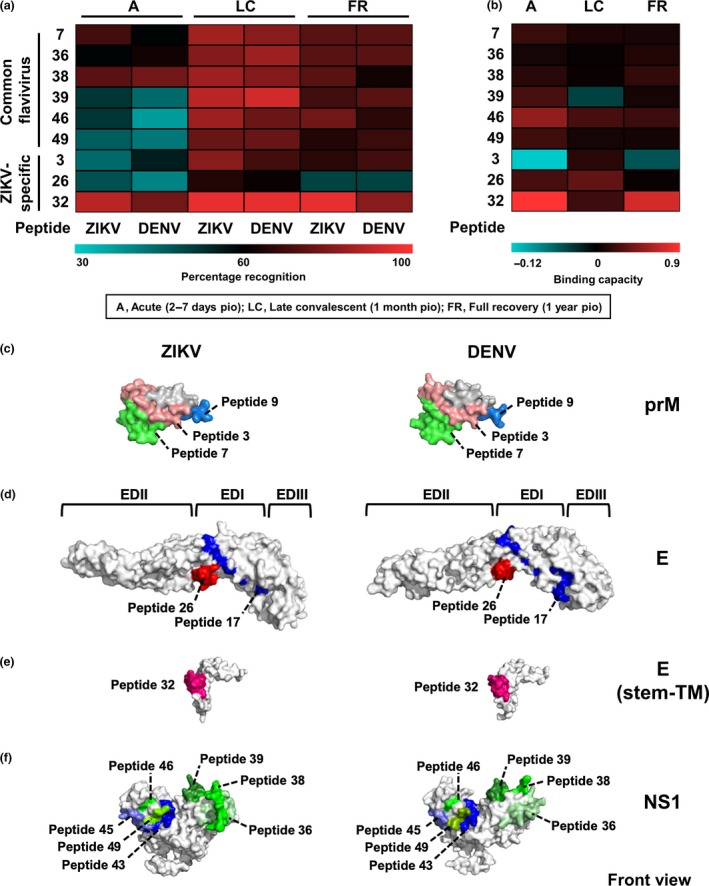
Characterisation of the antibody profile kinetics of ZIKV patients on common flavivirus and ZIKV‐specific linear B‐cell epitopes, and localisation of potential epitopes within the ZIKV and DENV proteome. (a, b) Plasma samples of ZIKV patients (n = 27) at acute, late convalescent and full recovery phases were tested for IgG at 1:2000 dilution in duplicates using ZIKV and DENV peptides in a peptide‐based ELISA. Pooled plasma of healthy donors was used as negative control, and patients’ data were normalised to mean of pooled healthy control. (a) Percentage of ZIKV patients positively binding to ZIKV and DENV peptides, and (b) binding capacity of ZIKV patients positively binding to peptides were calculated and are presented in a heat‐map. (c–e) Schematic diagrams showing the localisation of common flavivirus (denoted as shades of green), ZIKV‐specific (denoted as shades of red) and DENV‐specific (denoted as shades of blue) epitopes on (c) prM protein of ZIKV and DENV (PDB: 3C6E), (d) E glycoprotein of ZIKV (PDB: 5JHM) and DENV (PDB: 1UZG), (e) stem–transmembrane (TM) domain of E glycoprotein of ZIKV (PDB: 5IZ7) and DENV (PDB: 3J2P), and (f) NS1 protein of ZIKV (PDB: 5K6K) and DENV (PDB: 4O6B).
For ZIKV‐specific epitopes, more than 60% of the ZIKV patient samples were able to recognise peptides 3 and 26 (Figure 3a), with positive peptide binding capacity (Figure 3b) at late convalescent phase. However, peptide 32 showed strong recognition by the patient samples (Figure 3a) as well as high binding capacity (Figure 3b) at various time points from acute to full recovery. The localisation of all potential epitopes within the viral proteins is shown in Figure 3c–f.
Evaluation of epitopes with patient cohorts
To assess the diagnostic performance of identified epitopes, the 13 peptides were screened using patient serum samples from a Thailand cohort that had DENV, bacteria or unknown infections. Results of a randomised selection of Singapore ZIKV and DENV patients were also analysed in parallel (Supplementary table 3).
Interestingly, results showed a wide range of specificity and sensitivity for each peptide (Table 2 and Figure 4a). ZIKV‐specific peptide 26 (amino acid residues 271–288) on the E protein of domain I/II (EDI/II) had the best sensitivity and specificity profile (80% and 85.7%, respectively, Table 2 and Figure 4a). Nevertheless, eight peptides (common flavivirus peptides 36, 38, 46 and 49; ZIKV‐specific peptides 3, 26 and 32; and DENV‐specific peptide 9) showed more than 50% sensitivity and specificity (Table 2 and Figure 4a), and were selected for further evaluation. These peptides were used to ‘diagnose’ the patients (Supplementary table 4), and the performance of the peptide combination based on the epitope groupings was determined collectively (Table 2 and Figure 4b). Although the common flavivirus (green) and DENV‐specific (blue) groups demonstrated modest measurements, the ZIKV‐specific (red) peptide mix showed a robust specificity of 96.4% (Table 2 and Figure 4b). Furthermore, when the anti‐peptide IgG response of patients was plotted in a principal component analysis (PCA), it was observed that patients of different diagnoses and cohorts formed separate clusters, and ZIKV patients stood out when compared to the healthy control (Figure 4c). To identify peptides with discriminating power, the binding capacity of positive peptides was calculated. The virus‐specific ZIKV and DENV epitopes were significantly differential (Figure 4d). Peptide 32 (amino acid residues 453–470 on E protein) was the best‐performing ZIKV‐specific epitope and was able to distinguish Singapore ZIKV patients from bacteria and unknown infections from Thailand (Figure 4d, e). DENV‐specific peptide 9 (amino acid residues 78–92 on prM) could be used to differentiate Singapore DENV patients from bacteria‐infected patients from Thailand (Figure 4e). Overall, we have identified the best differential epitopes to differentiate between DENV and ZIKV patients.
Table 2.
Diagnostic evaluation of linear B‐cell epitopes
| Analysis | Epitope classification | Protein | Peptide No. | No. of patientsa | Sensitivity (%)b | Specificity (%)c | |||
|---|---|---|---|---|---|---|---|---|---|
| True positive | True negative | False negative | False positive | ||||||
| Individual peptide | Common flavivirus | prM | 7 | 22 | 4 | 3 | 9 | 88.0 | 30.8 |
| NS1 | 36 | 18 | 9 | 7 | 4 | 72.0 | 69.2 | ||
| 38 | 18 | 7 | 7 | 6 | 72.0 | 53.8 | |||
| 39 | 17 | 6 | 8 | 7 | 68.0 | 46.2 | |||
| 46 | 16 | 8 | 9 | 5 | 64.0 | 61.5 | |||
| 49 | 14 | 11 | 11 | 2 | 56.0 | 84.6 | |||
| ZIKV‐specific | prM | 3 | 6 | 24 | 4 | 4 | 60.0 | 85.7 | |
| E | 26 | 8 | 24 | 2 | 4 | 80.0 | 85.7 | ||
| 32 | 5 | 23 | 5 | 5 | 50.0 | 82.1 | |||
| DENV‐specific | prM | 9 | 8 | 16 | 7 | 7 | 53.3 | 69.6 | |
| E | 17 | 9 | 10 | 6 | 13 | 60.0 | 43.5 | ||
| NS1 | 43 | 11 | 6 | 4 | 17 | 73.3 | 26.1 | ||
| 45 | 4 | 17 | 11 | 6 | 26.7 | 73.9 | |||
| Peptide combination | Common flavivirus | NS1 | 36 | 17 | 8 | 8 | 5 | 68 | 61.5 |
| 38 | |||||||||
| 46 | |||||||||
| 49 | |||||||||
| ZIKV‐specific | prM | 3 | 6 | 27 | 5 | 1 | 54.5 | 96.4 | |
| E | 26 | ||||||||
| 32 | |||||||||
| DENV‐specific | prM | 9 | 8 | 16 | 7 | 7 | 53.3 | 69.6 | |
ZIKV (n = 10) and DENV (n = 10) patients from Singapore, and DENV (n = 5), bacteria (n = 5) and unknown (n = 8) patients from Thailand were used in the diagnostic evaluation.
Sensitivity is calculated as the percentage of [true‐positive patients/(true‐positive patients + false‐negative patients)].
Specificity is calculated as the percentage of [true‐negative patients/(true‐negative patients + false‐positive patients)].
Figure 4.
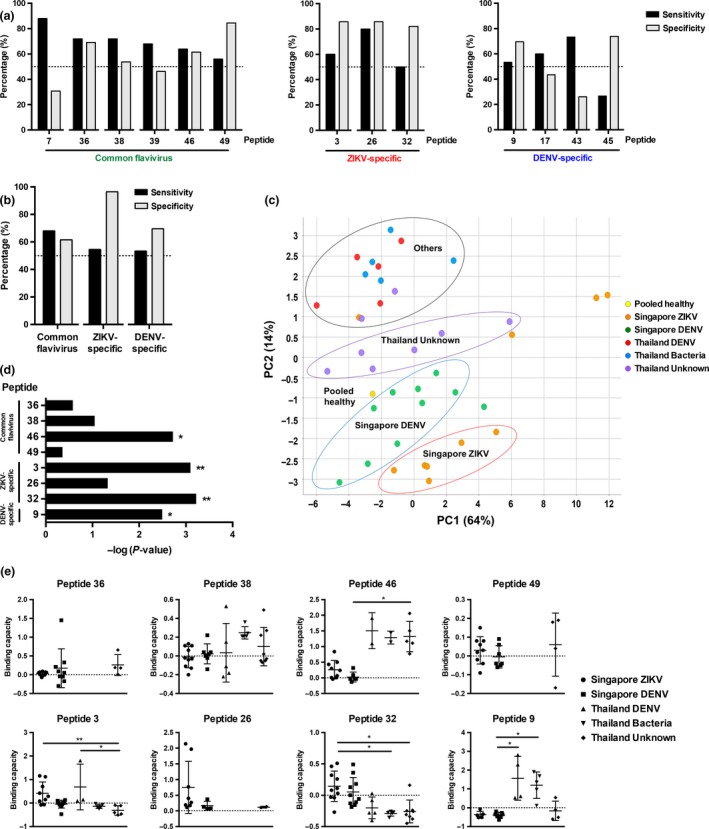
Preliminary diagnostic validation of identified linear B‐cell epitopes with patient cohorts. Convalescent plasma samples of ZIKV (n = 10) and serum samples of DENV (n = 10) patients from Singapore, and DENV (n = 5), bacteria (n = 5) and unknown (n = 8) patients from Thailand were tested in a peptide‐based ELISA in duplicates at 1:2000 dilution. Pooled healthy plasma was used as a negative control. (a) Sensitivity and specificity were determined for individual peptides. (b) Sensitivity and specificity of peptide mix of selected epitopes were determined. (c) Principal component analysis (PCA) of pooled healthy control, and patients’ anti‐IgG peptide response (OD values) were plotted in a graph with the percentage of variance indicated. (d, e) The peptide binding capacity of patients positively binding to peptides was calculated and statistically analysed by using the Kruskal–Wallis tests with the Bonferroni correction for multiple testing. Post hoc tests were done using Dunn's multiple comparison tests to determine (d) peptides with discriminating power and (e) the peptide binding capacity distribution of patients. Data are presented as mean ± SD. (*P < 0.05, **P < 0.01).
Discussion
Zika virus patients were shown to produce high levels of ZIKV‐specific IgG antibodies. Specifically, IgG1 and IgG3 were the subclasses induced following ZIKV infection, closely resembling DENV‐infected patients.33 Although patients from this cohort had detectable DENV IgG levels because of the high level of cross‐reactivity among flaviviruses,7, 8, 9, 10 DENV neutralisation was significantly less efficient compared to ZIKV, indicating that the antibodies were ZIKV‐specific (Figure 1e, f and Supplementary figure 2). This observation is also supported by another study, in which the profiles of ZIKV‐neutralising antibodies of patients from Nicaragua, Sri Lanka and Thailand were not affected by previous DENV infection.34 Nonetheless, it is imperative to consider the possible implications of virus‐infection enhancement.35 Moreover, none of the ZIKV patients in our study displayed severe symptoms to suggest occurrence of antibody‐dependent enhancement (ADE),30 and similar observations were also reported from Brazil.35, 36
Peptides identified from B‐cell epitope mapping have been reported on flavivirus E, prM, NS1 and NS3 antigens from antibodies of patients and animal models.31, 37, 38 Identification of antigenic epitopes and characterisation of cross‐reactive epitopes are crucial in vaccine and immunodiagnostic developments.31, 39, 40 While various reports have shown the specificity of the NS1 antigen to differentiate between ZIKV and DENV,11, 20, 21, 41, 42 the majority of the common flavivirus peptides identified in this study are on the NS1 protein, possibly because of the conserved regions of NS1 among the flaviviruses.8, 43 For example, common flavivirus peptides 36 (amino acid residues 70–85), 38 (amino acid residues 119–136) and 49 (amino acid residues 315–326) were identified as ZIKV‐specific in other patient cohorts from South America.41, 42 However, it remains to be seen whether these peptides could be used to detect all flaviviruses such as yellow fever virus (YFV) and Japanese encephalitis virus (JEV).
Differential ZIKV and DENV epitopes identified were located across prM, E and NS1. Of interest, DENV‐specific peptide 17 (amino acid residues 131–149) and ZIKV‐specific peptide 26 are found on EDI and EDII of E glycoprotein, which share 35% and 51% amino acid identity between ZIKV and DENV, respectively,8 whereas ZIKV‐specific peptide 32 is found in the transmembrane domain of the anchor region (Figure 3e). Interestingly, peptide 32 (amino acid residues 453–470) maps to a region that overlaps with a DENV‐2 epitope (amino acid residues 451–468) described for immune sera of DENV‐2‐infected patients.38 Computational analyses of ZIKV‐specific peptide 32 and DENV‐2‐equivalent epitope showed that they remain moderately accessible on the virus particle.23, 38 Since they share low sequence identity (43.75%), this epitope could be conformationally different and thus differentially recognised by ZIKV‐ and DENV‐specific antibodies. It would also be useful to assess the use of the identified peptides as a ZIKV vaccine target, particularly peptides 26 and 32. Interestingly, despite the similarity between the sequences of these ZIKV and DENV peptide pairs (Supplementary table 2), they were able to distinguish between ZIKV and DENV patients. Moreover, ZIKV patients at different disease stages have different peptide recognition, and the current set‐up could identify ZIKV infection at any point, independent of the patients’ level of ZIKV‐specific antibodies (Supplementary figure 5b, c). However, given that the identified epitopes were screened and validated using adult patient samples, it would be important to assess how these epitope profiles will perform in other patient cohorts, specifically ZIKV‐infected pregnant women from Brazil.27
Identified putative epitopes were preliminary diagnostically evaluated with 38 patient samples.44, 45, 46 Intriguingly, the Singapore DENV and Thailand DENV patients were not clustered together in the PCA (Figure 4c). Most of the Singapore DENV patients selected for validation had moderate‐to‐severe forms of plasma leakage, a clinical feature of severe manifestations of DENV infection,47 whereas DENV patients from Thailand displayed mild symptoms (unpublished data). The latter being ‘negative’ in our assays could thus be due to the differences in epitope recognition in different DENV disease states31 and the different strains of viruses circulating in Singapore and Thailand. Nonetheless, further refinements are required to identify serotype‐specific DENV epitopes.
Furthermore, comparing these results and computationally predicted diagnostic peptide regions23 revealed differences. The majority of the computationally predicted peptide regions were not ZIKV‐specific. NS1 peptide 36, for example, was predicted to be differential,23 but was, in fact, a common flavivirus. Furthermore, peptide 26 on E glycoprotein, predicted to recognise both ZIKV and DENV,23 was shown to be ZIKV‐specific in this study. Despite differences in various approaches, computational prediction remains a useful tool.
Overall, this study offers important valuable information on the human antibody response against ZIKV and insights into epitope cross‐reactivity. Notably, several novel differential ZIKV and DENV epitopes with potential diagnostic efficacies have been identified on prM and E proteins. These results offer useful insights towards the development of diagnostics or vaccines.
Methods
Ethics statement
Written informed consent was obtained from participants in accordance with the tenets of the Declaration of Helsinki. Study protocols of Singapore ZIKV (2016–2018) and DENV (2010–2012) patient cohorts were approved by the SingHealth Centralised Institutional Review Board (CIRB Ref: 2016/2219) and National Healthcare Group (NHG) Domain Specific Review Board (DSRB‐E‐2009/432), respectively. Specimens from Singapore healthy donors (2010–2015) and patients from Thailand (2011–2013) were collected in accordance with the study guidelines of approval numbers: CIRB Ref 2017/2806, MUTM 2011‐008‐01, OXTREC 42‐10 and TCAB‐01‐11, respectively.
Study subjects and sample collection
Singapore ZIKV patients
Collection of specimens from subjects during the ZIKV outbreak in 2016 has been previously described.30 Briefly, 65 patients who were RT‐PCR‐positive for ZIKV in whole blood or urine, and negative for DENV RT‐PCR were enrolled.28 Whole blood specimens were collected in EDTA‐coated vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA) after peripheral venipuncture and were centrifuged at 240 g for 10 min. Plasma was collected and heat‐inactivated for 30 min at 56°C before storage at −80°C. Specimens were obtained over a period of six time points: (1) acute [2–7 days post‐illness onset (pio)], (2) early convalescent (10–14 days pio), (3) late convalescent (1 month pio), (4) early recovery (3 months pio), (5) late recovery (5–6 months pio) and (6) full recovery (1 year pio) phases.
Singapore DENV patients
Twenty DENV patient serum samples (2010–2012) collected before the ZIKV outbreak were used in this study.32 Patients were DENV PCR‐ and/or NS1‐positive upon hospital admission and were a combination of the following: one unknown serotype, six DENV‐1, seven DENV‐2, three DENV‐3 and three DENV‐4 patients. Serum samples used were obtained at late convalescent phase (21–37 days pio).
Thailand patients
Archived serum samples from an undifferentiated fever study conducted at Shoklo Malaria Research Unit (SMRU) were used. Five DENV patients were confirmed by gold standard paired serology, and all but one was DENV PCR‐positive. Five bacteria‐infected patients were diagnosed with leptospirosis, scrub typhus, murine typhus or Streptococcus pneumoniae infections, or a combination of above, and all were DENV PCR‐ and DENV NS1‐, and IgM‐ and IgG RDT‐negative. Eight patients with unknown diagnoses were negative for the above pathogens by serology, blood culture and PCR. Convalescent serum samples used were collected at 14–20 days pio.
Viruses
Zika virus Polynesian isolate (H/PF/2013) was obtained from the European Virus Archive (EVA, Marseille, France). DENV‐3 was used as a reference DENV serotype because it is widespread in South‐East Asia,48, 49, 50, 51 and was kindly provided by the National Public Health Laboratory (NPHL), Singapore. CHIKV SGP011 was isolated from a patient from Singapore.52 Viruses were propagated in VeroE6 cells (ATCC, Manassas, VA, USA) and purified via ultracentrifugation24 before being titred by standard plaque assays in VeroE6 cells.25, 53
Virion‐based ELISA
Antibody titres were determined by a virion‐based ELISA as previously described.18, 24, 25, 26, 27 Briefly, purified virus was immobilised on 96‐well Maxisorp microtitre plates overnight (Thermo Fisher Scientific, Waltham, MA, USA). Wells were blocked with 0.05% PBST [0.05% Tween‐20 (Sigma‐Aldrich, Saint Louis, MO, USA) in PBS] containing 5% skim milk (Nacalai Tesque, Kyoto, Japan) at 37°C for 1.5 h. Heat‐inactivated patient and pooled healthy control plasma samples at 1:200 to 1:8000 dilutions prepared in PBST with 2.5% milk were incubated at 37°C for 1 h. HRP‐conjugated goat anti‐human IgM or IgG (H+L) (Thermo Fisher Scientific) or mouse anti‐human IgG1, IgG2, IgG3 and IgG4 (Thermo Fischer Scientific) antibodies were used for detection. Reactions were developed using TMB (3,3,5,5‐tetramethylbenzidine) substrate (Sigma‐Aldrich) and terminated with Stop reagent (Sigma‐Aldrich), and absorbance was measured at 450 nm in a microplate autoreader (Tecan, Männedorf, Zürich, Switzerland).18, 24, 25, 26, 27 ELISA readings were conducted in duplicates or triplicates.
Sero‐neutralisation
Neutralising capacity of antibodies from ZIKV patients was determined via flow cytometry.54 Briefly, pooled patient and healthy plasma samples at 1:500, 1:1000 and 1:2000 dilutions were incubated with ZIKV or DENV‐3 at MOI 10 for 2 h at 37°C with gentle agitation (350 rpm). Virus–antibody suspensions were then added in duplicates to HEK293T cells (ATCC) at 37°C. After 2 h, media were removed and Dulbecco's modified Eagle's medium (DMEM; GE Healthcare Life Sciences, Pittsburgh, PA, USA) with 10% foetal bovine serum (FBS; GE Healthcare Life Sciences) was added. After 48 h, cells were harvested and stained as described,54 using ZIKV NS3 protein‐specific rabbit polyclonal antibody29 or DENV human monoclonal antibody 1B,25 and counter‐stained with fluorophore‐tagged goat anti‐rabbit or anti‐human IgG (H+L) (Thermo Fisher Scientific). Cells were acquired with MACSQuant Analyser 10 (Miltenyi Biotec, Bergisch Gladbach, Germany). The assay was carried out in duplicates with two independent experiments. Flow cytometry results were analysed with FlowJo (version 10.4.1; Tree Star Inc. Ashland, OR, USA). Data of patient and pooled healthy neutralisation assays were normalised using the respective untreated infections and calculated as a percentage of virus‐only control infection.
Epitope determination
Linear peptide libraries
The sequences used for the design of biotinylated linear peptides of prM, E and NS1 proteins were derived from ZIKV Polynesian isolate (KJ776791) and consensus sequence of DENV‐3 strains (KR296743, KF973487, EU081181, KF041254, JF808120, JF808121, KJ189293, KC762692, KC425219, KJ830751, KF973479 and AY099336).24, 25, 27 Peptides were generated as a ZIKV and DENV peptide pair of corresponding sequences. Preliminary epitope screening was used with a library of peptides (Mimotopes, Mulgrave, VIC, Australia) consisting of 18‐mer overlapping sequences. Five peptides were combined to form one pooled peptide set. Screening and validation of patients were done with higher purity of peptides (≥ 90%; EMC Microcollections GmbH, Tuebingen, Germany) with lengths ranging from 11‐ to 22‐mer (Supplementary table 2). Peptides were dissolved in DMSO (Sigma‐Aldrich) to obtain a stock concentration of 3.75 μg μL−1.
Peptide‐based ELISA
Epitope determination was performed via peptide‐based ELISA as previously described.24, 25, 27 Briefly, streptavidin‐coated plates (Thermo Fisher Scientific) were blocked with 0.1% PBST (0.1% Tween‐20 in PBS) containing 1% sodium caseinate (Sigma‐Aldrich) and 1% bovine serum albumin (BSA; Sigma‐Aldrich) overnight at 4°C, before addition of biotinylated peptides (1:1000 dilution in 0.1% PBST), followed by heat‐inactivated pooled healthy control and patient plasma/serum samples (1:2000 dilution in 0.1% PBST). HRP‐conjugated goat anti‐human IgG (H+L) antibody (Thermo Fisher Scientific) prepared in 0.1% blocking buffer was used for detection of peptide‐bound antibodies. TMB substrate and Stop reagent (Sigma‐Aldrich) were used for development, prior to absorbance measurements at 450 nm (Tecan).24, 25, 27 All incubation steps were at room temperature for 1 h on a rotating shaker, and ELISA readings were conducted in duplicates.
Data analysis
OD values obtained from ZIKV and DENV peptide‐based ELISA experiments were first normalised against mean OD values of pooled healthy donors. Patient samples were considered positive if the normalised response was more than 1.01. Subsequently, peptide binding capacity was calculated using the normalised values as [(ZIKV peptide response − DENV peptide response)/DENV peptide response]. Binding capacities with positive values denote the binding preference of the sample to ZIKV peptide, whereas negative values denote a binding preference to the corresponding DENV peptide. The difference in the mean peptide binding capacity of ZIKV patients and DENV patients of a peptide pair (i.e. ZIKV and DENV peptides with complementary sequence) was calculated. Peptides with a relative difference of 0.1 or more are considered to be differential ZIKV (red) and DENV (blue) epitopes of interest, whereas peptides with a difference of 0.05 or less, and share amino acid similarity between the peptide pairs (Supplementary table 2) are considered to be common flavivirus epitopes (green).
Data visualisation and statistical analysis
Heat‐maps were generated using MultiExperiment Viewer (version 4.8; Microarray Software Suite TM4, Boston, MA, USA). For structural localisation, ZIKV prM was simulated using Phyre (version 2; Structural Bioinformatics Group, London, UK).55 Structures of DENV‐3 prM, ZIKV E glycoprotein, DENV‐3 E glycoprotein, ZIKV stem–transmembrane domain of E glycoprotein, DENV‐3 stem–transmembrane domain of E glycoprotein, ZIKV NS1 and DENV‐3 NS1 were modelled based on PDB 3C6E, 5JHM, 1UZG, 5IZ7, 3J2P, 5K6K and 4O6B, respectively. All structures were visualised using PyMol (Schrödinger, Cambridge, MA, USA). PCA was performed using the OD values of the anti‐peptide IgG response by patients using prcomp function in R (version 3.3.1; R Foundation for Statistical Computing, Vienna, Austria).
Data were analysed using GraphPad Prism (version 7.03; GraphPad Software, San Diego, CA, USA). The Mann–Whitney U‐tests, two‐tailed, with the Bonferroni correction for multiple testing, or the Kruskal–Wallis tests with the Bonferroni correction for multiple testing, and post hoc tests using Dunn's multiple comparison tests were used to derive any statistical significance. Correlation analysis was carried out using Spearman's rank correlation. P‐values less than 0.05 are considered significant.
Conflicts of interest
The authors declare no conflict of interest.
Supporting information
Acknowledgments
We thank Nicholas QR Kng for assisting with the experiments, Kai‐Er Eng for assisting with the manuscript, and Cheryl YP Lee, Yi‐Hao Chan, Guillaume Carissimo, Siew‐Wai Fong and Jonathan Cox for processing of patient samples (SIgN); Vincent Pang, Linda Lee and research assistants from Tan Tock Seng Hospital; SMRU clinic and microbiology laboratory staff for patient recruitment, sample preparation and laboratory diagnosis, Mahidol‐Oxford Tropical Medicine Research Unit (MORU) microbiology staff for supporting Rickettsia reference serology testing, and Armed Forces Research Institute of Medical Sciences (AFRIMS) for dengue reference serology testing; National Public Health Laboratory (NPHL) for providing DENV‐3 virus; Professor Andres Merits for providing the ZIKV NS3 protein‐specific rabbit polyclonal antibody; and study participants for their participation in the study.
This work was supported by core research grants provided to the Singapore Immunology Network (SIgN) by the Biomedical Research Council (BMRC) and also partially supported by the BMRC A*STAR‐led Zika Virus Consortium Fund [project number: 15/1/82/27/001], Agency for Science, Technology and Research (A*STAR), Singapore. SIgN Immunomonitoring and Flow Cytometry platforms are supported by BMRC IAF 311006 grant and BMRC transition funds #H16/99/b0/011.
References
- 1. Teixeira MG, Costa Mda C, de Oliveira WK, Nunes ML, Rodrigues LC. The epidemic of Zika virus‐related microcephaly in Brazil: detection, control, etiology, and future scenarios. Am J Public Health 2016; 106: 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barcellos C, Xavier DR, Pavão AL et al Increased hospitalizations for neuropathies as indicators of Zika virus infection, according to health information system data, Brazil. Emerg Infect Dis 2016; 22: 1894–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He D, Gao D, Lou Y, Zhao S, Ruan S. A comparison study of Zika virus outbreaks in French Polynesia, Colombia and the state of Bahia in Brazil. Sci Rep 2017; 7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cauchemez S, Besnard M, Bompard P et al Association between Zika virus and microcephaly in French Polynesia, 2013–2015: a retrospective study. Lancet 2016; 387: 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson HL, Tran T, Druce J, Dupont‐Rouzeyrol M, Catton M. Neutralization assay for Zika and dengue viruses by use of real‐time‐PCR‐based endpoint assessment. J Clin Microbiol 2017; 55: 3104–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goncalves A, Peeling RW, Chu MC et al Innovative and new approaches to laboratory diagnosis of Zika and dengue: a meeting report. J Infect Dis 2018; 217: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Priyamvada L, Quicke KM, Hudson WH et al Human antibody responses after dengue virus infection are highly cross‐reactive to Zika virus. Proc Natl Acad Sci USA 2016; 113: 7852–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stettler K, Beltramello M, Espinosa DA et al Specificity, cross‐reactivity and function of antibodies elicited by Zika virus infection. Science 2016; 353: 823–826. [DOI] [PubMed] [Google Scholar]
- 9. Kawiecki AB, Christofferson RC. Zika virus‐induced antibody response enhances dengue virus serotype 2 replication in vitro . J Infect Dis 2016; 214: 1357–1360. [DOI] [PubMed] [Google Scholar]
- 10. Barba‐Spaeth G, Dejnirattisai W, Rouvinski A et al Structural basis of potent Zika–dengue virus antibody cross‐neutralization. Nature 2016; 536: 48–53. [DOI] [PubMed] [Google Scholar]
- 11. Wong SJ, Furuya A, Zou J et al A multiplex microsphere immunoassay for Zika virus diagnosis. EBioMedicine 2017; 16: 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang H‐H, Huber RG, Bond PJ et al Systematic analysis of protein identity between Zika virus and other arthropod‐borne viruses. Bull World Health Organ 2017; 95: 517–525I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu X, Vaughan K, Weiskopf D et al Identifying candidate targets of immune responses in Zika virus based on homology to epitopes in other flavivirus species. PLoS Curr 2016; 8 10.1371/currents.outbreaks.9aa2e1fb61b0f632f58a098773008c4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu X, Song H, Qi J et al Contribution of intertwined loop to membrane association revealed by Zika virus full‐length NS1 structure. EMBO J 2016; 35: 2170–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrison SC. Immunogenic cross‐talk between dengue and Zika viruses. Nat Immunol 2016; 17: 1010–1012. [DOI] [PubMed] [Google Scholar]
- 16. Mohns MS, Bailey A, Breitbach ME et al Antibody responses to Zika virus proteins in pregnant and non‐pregnant macaques. PLoS Negl Trop Dis 2018; 12: e0006903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Funk S, Kucharski AJ, Camacho A et al Comparative analysis of dengue and Zika outbreaks reveals differences by setting and virus. PLoS Negl Trop Dis 2016; 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chia PY, Yew HS, Ho H et al Clinical features of patients with Zika and dengue virus co‐infection in Singapore. J Infect 2017; 74: 611–615. [DOI] [PubMed] [Google Scholar]
- 19. Campos G, Bandeira A, Sardi S. Zika virus outbreak, Bahia. Brazil. Emerg Infect Dis 2015; 21: 1885–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steinhagen K, Probst C, Radzimski C et al Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1‐based ELISA devoid of cross‐reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Eurosurveillance 2016; 21: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balmaseda A, Stettler K, Medialdea‐Carrera R et al Antibody‐based assay discriminates Zika virus infection from other flaviviruses. Proc Natl Acad Sci USA 2017; 114: 8384–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mishra N, Caciula A, Price A et al Diagnosis of Zika virus infection by peptide array and ELISA. MBio 2018; 9: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee AJ, Bhattacharya R, Scheuermann RH, Pickett BE. Identification of diagnostic peptide regions that distinguish Zika virus from related mosquito‐borne Flaviviruses. PLoS One 2017; 12: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kam YW, Lum FM, Teo TH et al Early neutralizing IgG response to chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol Med 2012; 4: 330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kam Y‐W, Lee CY‐P, Teo T‐H et al Cross‐reactive dengue human monoclonal antibody prevents severe pathologies and death from Zika virus infections. JCI Insight 2017; 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kam YW, Simarmata D, Chow A et al Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long‐term clinical protection. J Infect Dis 2012; 205: 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kam Y‐W, Leite JA, Amrun SN et al ZIKV‐specific NS1 epitopes as serological markers of acute Zika virus infection. J Infect Dis 2019; 220: 203–212. [DOI] [PubMed] [Google Scholar]
- 28. Ho ZJM, Hapuarachchi HC, Barkham T et al Outbreak of Zika virus infection in Singapore: an epidemiological, entomological, virological, and clinical analysis. Lancet Infect Dis 2017; 17: 813–821. [DOI] [PubMed] [Google Scholar]
- 29. Lum F‐M, Lin C, Susova OY et al A sensitive method for detecting Zika virus antigen in patients’ whole‐blood specimens as an alternative diagnostic approach. J Infect Dis 2017; 216: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lum F‐M, Lye DCB, Tan JJL et al Longitudinal study of cellular and systemic cytokine signatures to define the dynamics of a balanced immune environment during disease manifestation in Zika virus–infected patients. J Infect Dis 2018; 218: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vaughan K, Greenbaum J, Blythe M, Peters B, Sette A. Meta‐analysis of all immune epitope data in the Flavivirus genus: inventory of current immune epitope data status in the context of virus immunity and immunopathology. Viral Immunol 2010; 23: 259–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Low JGH, Ooi E‐E, Tolfvenstam T et al Early dengue infection and outcome study (EDEN) ‐ study design and preliminary findings. Ann Acad Med Singapore 2006; 35: 783–789. [PubMed] [Google Scholar]
- 33. Koraka P, Suharti C, Setiati TE et al Kinetics of dengue virus‐specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. J Clin Microbiol 2001; 39: 4332–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montoya M, Collins M, Dejnirattisai W et al Longitudinal analysis of antibody cross‐neutralization following Zika virus and dengue virus infection in Asia and the Americas. J Infect Dis 2018; 218: 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martín‐Acebes MA, Saiz J‐C, Jiménez de Oya N. Antibody‐dependent enhancement and Zika: real threat or phantom menace? Front Cell Infect Microbiol 2018; 8: 2014–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Terzian ACB, Schanoski AS, Mota MTO et al Viral load and cytokine response profile does not support antibody‐dependent enhancement in dengue‐primed Zika virus‐infected patients. Clin Infect Dis 2017; 65: 1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuhn RJ, Dowd KA, Beth C, Pierson TC. Shake, rattle, and roll: impact of the dynamics of flavivirus particles on their interactions with the host. Virology 2015; 480: 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramanathan B, Poh CL, Kirk K, McBride WJH, Aaskov J, Grollo L. Synthetic B‐cell epitopes eliciting cross‐neutralizing antibodies: strategies for future dengue vaccine. PLoS One 2016; 11: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crill WD, Chang GJ. Localization and characterization of Flavivirus envelope glycoprotein. Cell 2004; 78: 13975–13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun EC, Zhao J, Liu NH et al Comprehensive mapping of west nile virus (WNV)‐and japanese encephalitis virus serocomplex‐specific linear B‐cell epitopes from WNV non‐structural protein 1. J Gen Virol 2012; 93: 50–60. [DOI] [PubMed] [Google Scholar]
- 41. Lee H‐J, Cho Y, Kang HJ et al Identification of peptide based B‐cell epitopes in Zika virus NS1. Biochem Biophys Res Commun 2018; 505: 1010–1014. [DOI] [PubMed] [Google Scholar]
- 42. Freire MCLC, Pol‐Fachin L, Coêlho DF et al Mapping putative B‐cell Zika virus NS1 epitopes provides molecular basis for anti‐NS1 antibody discrimination between Zika and dengue viruses. ACS Omega 2017; 2: 3913–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song H, Qi J, Haywood J, Shi Y, Gao GF. Zika virus NS1 structure reveals diversity of electrostatic surfaces among flaviviruses. Nat Struct Mol Biol 2016; 23: 456–458. [DOI] [PubMed] [Google Scholar]
- 44. Bujang MA, Adnan TH. Requirements for minimum sample size for sensitivity and specificity analysis. J Clin Diagnostic Res 2016; 10: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu HC, Huang YL, Chao TT et al Identification of B‐cell epitope of dengue virus type 1 and its application in diagnosis of patients. J Clin Microbiol 2001; 39: 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Magalhaes T, Yen C‐W, Marín K et al Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci Transl Med 2017; 9: eaan1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Her Z, Kam YW, Gan VC et al Severity of plasma leakage is associated with high levels of interferon γ‐inducible protein 10, hepatocyte growth factor, matrix metalloproteinase 2 (MMP‐2), and MMP‐9 during dengue virus infection. J Infect Dis 2017; 215: 42–51. [DOI] [PubMed] [Google Scholar]
- 48. Messina JP, Brady OJ, Scott TW et al Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 2014; 22: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suwandono A, Kosasih H, Nurhayati et al Four dengue virus serotypes found circulating during an outbreak of dengue fever and dengue haemorrhagic fever in Jakarta, Indonesia, during 2004. Trans R Soc Trop Med Hyg 2006; 100: 855–862. [DOI] [PubMed] [Google Scholar]
- 50. Ng L‐C, Chem Y‐K, Koo C et al 2013 dengue outbreaks in Singapore and Malaysia caused by different viral strains. Am J Trop Med Hyg 2015; 92: 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rajarethinam J, Ang L, Ong J et al Dengue in Singapore from 2004 to 2016: cyclical epidemic patterns dominated by serotypes 1 and 2. Am J Trop Med Hyg 2018; 99: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Her Z, Malleret B, Chan M et al Active infection of human blood monocytes by chikungunya virus triggers an innate immune response. J Immunol 2010; 184: 5903–5913. [DOI] [PubMed] [Google Scholar]
- 53. Hamel R, Dejarnac O, Wichit S et al Biology of Zika virus infection in human skin cells. J Virol 2015; 89: 8880–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ang LW, Kam YW, Lin C et al Seroprevalence of antibodies against chikungunya virus in Singapore resident adult population. PLoS Negl Trop Dis 2017; 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 2015; 10: 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


