Short abstract
Neuropathic pain conditions severely and chronically affect the quality of life in a large human population, but the pain conditions are not adequately managed due to poor understanding of their underlying mechanisms. There is a pressing need for further research into this field to help develop effective and nonaddictive medications to treat neuropathic pain. This article first describes general clinical classification of pain, types and symptoms of neuropathic pain, and current practices of clinical management for neuropathic pain. This is followed by a discussion of various cellular and molecular mechanisms responsible for the development and maintenance of neuropathic pain. In this review, we highlight the loss of function of Kv7 voltage-gated potassium as a mechanism of neuropathic pain and the potential use of Kv7 channel activator as subsequent treatment.
Keywords: Neuropathic pain, voltage-gated K+ channels, hyperalgesia, allodynia, retigabine, flupirtine
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage. It is classically subdivided into acute pain and chronic pain. Acute pain is a physiologic response to an adverse chemical, thermal, or mechanical stimulus.1 Typically, short-lived, acute pain usually resolves within one month. However, certain acute pain pathologies as well as acute pain that is untreated, undertreated, or treated inappropriately can progress to chronic pain. Chronic pain is pain without apparent biologic value that has persisted beyond the normal tissue healing time. Chronic pain is multifaceted and composed of numerous pain syndromes and disorders; some of the more common types being neuropathic pain, radicular pain, and cancer pain.2 Chronic pain is extremely difficult to manage and even harder to cure. Unfortunately, chronic pain is extremely common in the United States with a prevalence of 11.2% or over 25 million adults with an annual cost estimated at $560–$635 billion dollars.3–5 Of the various chronic pain syndromes, neuropathic pain deserves special attention for several reasons: neuropathic pain is generally more severe than other types of chronic pain, it is associated with worse health outcomes compared to nonneuropathic pain, individuals with neuropathic pain have health-related quality of life ratings as low as individuals with clinical depression, and neuropathic pain is pervasive with a reported prevalence of 7%–8%.6–10
Neuropathic pain is typically characterized as a burning or stabbing sensation and is often associated with allodynia or hyperalgesia. Hyperalgesia refers to a heightened response to normally painful stimuli. Allodynia refers to the experience of pain induced by innocuous stimulus. Some important types of allodynia are mechanical allodynia (static or dynamic), thermal allodynia (hot or cold), and movement allodynia. Allodynia is associated with severe pain induced by stimuli that are not normally painful, and it is associated with several disease conditions including complex regional pain syndrome (CRPS), chronic low back pain, and fibromyalgia.11–15
There are several types of neuropathic pain conditions including sympathetic neuropathic pain, peripheral neuropathic pain, and central neuropathic pain based on nervous systems that are involved. Sympathetic neuropathic pain is pain arising from a peripheral nerve lesion and associated with autonomic changes. Complex regional pain syndrome is a common example of sympathetic neuropathic pain. Peripheral neuropathic pain is pain caused by damage to peripheral nerves but without autonomic changes. Examples of peripheral neuropathic pain include postherpetic neuralgia, trigeminal neuropathic pain, chemotherapy-induced peripheral neuropathy, diabetic peripheral neuropathy, and neuroma formation.16 Central neuropathic pain is caused by abnormal central nervous system (CNS) activity. Examples include phantom limb pain, poststroke pain, and pain from spinal cord injuries.16,17 Table 1 lists some examples of neuropathic pain conditions and some treatment modalities. It must be stated that the treatment of neuropathic pain should be multimodal and the most effective treatment will depend on the type of neuropathic pain as well as patient-specific factors.
Table 1.
Types of neuropathic pain and clinical management.
| Types of neuropathic pain | Examples | Management |
|---|---|---|
| Sympathetic neuropathic pain | CRPS types I and II | Physiologic therapiesgroup therapy, individual therapy.MedicationsAnticonvulsants:Gabapentin, carbamazepine, valproate, clonazepam.Antidepressants:Tricyclic antidepressants (nortriptyline and amitriptyline), serotonin selective reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, norepinephrine-dopamine reuptake inhibitors.Opioids:Oxycodone, hydrocodone/acetaminophen, oxycodone/acetaminophen, tramadol, morphine sulfate controlled release, oxycodone hydrochloride controlled release tablets, hydromorphone, methadone.Topical analgesics:Lidocaine patch, capsaicinInterventionsSympathetic blocks, epidural and transformational steroid injections, spinal cord stimulators, peripheral nerve stimulators, intrathecal pumps.Physical and alternative therapiesMassage therapy, acupuncture, nutritional counseling.Mirror therapyPhantom limb pain and complex regional pain syndrome only. |
| Peripheral neuropathic pain | Postherpetic neuralgiaTrigeminal neuralgiaChemotherapy-induced peripheral neuropathyDiabetic peripheral neuropathy | |
| Central neuropathic pain | Phantom limb painPoststroke painPain from spinal cord injuries |
CRPS: complex regional pain syndrome.
Neuropathic pain is overall a more severe form of pain when compared to other nonneuropathic pain conditions, and it can be accompanied by anxiety and depression. Patients with neuropathic pain also reports quality-of-life metrics similar to patients with other serious conditions such as cardiac disease, poorly controlled diabetes mellitus, and clinical depression.8,9,18 The armamentarium for neuropathic pain treatment (Table 1) is vast and includes medication classes such as sodium channel blockers, opioids, local anesthetics, antiepileptic medications, tricyclic antidepressants, and serotonin reuptake inhibitors. The efficacy and specific uses of these various medications have been discussed elsewhere and will not be discussed here.19 In addition, a robust group of invasive procedures joins the list of treatment options. This list of invasive procedures includes facet joint injections, facet joint denervation, epidural steroid injections, transforaminal epidural injections, spinal cord stimulators, and peripheral nerve catheters. Despite the multitude of treatment options available, neuropathic pain is extremely difficult to treat with only 50% of patients reporting relief from the various available treatments. This relief is also often only partial and accompanied by a host of side effects. The numerous conditions resulting in neuropathic pain certainly contribute to its challenging management; despite this, the primary obstacle in neuropathic pain treatment is its complex pathophysiology.
Neuropathic pain pathophysiology
In the past, an etiology-based approach was used to understand the pathophysiology of neuropathic pain. One of the primary limitations of this approach was that the diverse causes of neuropathic pain made selecting the appropriate model difficult. Furthermore, many authors suggested that data acquired from using a specific neuropathic pain syndrome as a model to study the pathophysiology of neuropathic pain had limited generalizability to other neuropathic pain syndromes. More recently, there has been a shift in philosophy away from an etiology-based approach and more toward a mechanism-based approach. This approach is favored both for understanding the pathophysiology of neuropathic pain and treating it. One of the many benefits of the mechanism-based approach is that it somewhat circumvents the conundrum of selecting the most appropriate model or syndrome in which to study neuropathic pain, and it allows for maximum generalizability of research findings. The two widely accepted mechanism-based causes leading to neuropathic pain are central and peripheral sensitization, and each warrants a more detailed discussion. Prior to exploring each further, it must be mentioned that much of our current knowledge on both mechanisms have been derived from animal models. While these models certainly have important implications, they often do not precisely predict human pain processes because neuropathic pain conditions in humans have very complicated causes and are influenced by many factors including genetics and epigenetics.
Central sensitization as a mechanism of neuropathic pain
The dorsal horn of the spinal cord and brainstem is a region of the CNS where sensory information about mechanical, thermal, and painful stimuli carried by peripheral afferent nerves is integrated and then conveyed to the brain.20 Dorsal horn neurons receive millions of inputs per minute; however, the majority of these inputs are subthreshold and the synaptic strength is too weak to produce an action potential.21 However, if the synaptic efficacy in the sensory neurons in the dorsal horn is increased, and then these once subthreshold stimuli are able to elicit action potentials.21 Sustained peripheral noxious stimuli, tissue injury, or nerve damage can cause increased synaptic efficacy in the sensory neurons of the dorsal horn in the spinal cord and brainstem, which can lead to central sensitization in the spinal cord and brainstem nociceptive pathways.20,21 As a results of this increased synaptic efficacy, there is a reduction in pain thresholds, amplification of pain responses, and spread of pain sensitivity to uninjured tissues. Clinically, central sensitization contributes to pain hypersensitivity in the skin, joints, muscles, and viscera. There are several subtypes of central sensitization, some of which include wind-up, activity-dependent classical central sensitization that outlast the initiating stimulus, long-term potentiation, late onset transcription-dependent, and late onset activity-independent sensitization.20,21 Each of these subtypes has distinct mechanisms utilizing various transcription factors, voltage-gated channels, and neurotransmitters. The precise mechanisms governing these subtypes are beyond the scope of this article. Ji et al. provide an excellent summary of the mechanisms underlying each of these subtypes in their previous paper21 and the details will not be restated here. In addition to the enhanced dorsal horn neuron efficacy, other mechanisms that may contribute to central sensitization include the loss of function of dorsal horn inhibitory neurons (disinhibition), changes in the intrinsic electrophysiological properties of dorsal horn neurons, and alterations of Aβ-afferent nerve chemical phenotypes and terminal distributions in the dorsal horn.20 Suffice to say these various mechanisms contribute to the global process of central sensitization. A process which ultimately results in subthreshold stimuli gaining access to the ability to trigger action potentials in nociceptive pathways from the dorsal horns to the brain regions involving the perception of pain.
Peripheral sensitization as a mechanism of neuropathic pain
The key event for peripheral sensitization is nerve damage which ultimately leads to neuroma formation. This is usually followed by abnormal excitability and increased sensitivity to different forms of stimulation including chemical, thermal, and mechanical stimuli. The increased excitability can be developed at multiple sites including the neuroma itself, dorsal root ganglion, peripheral nerve endings, and neighboring intact afferents. Numerous mechanisms contribute to the pathophysiology of peripheral sensitization. The known mechanisms can be grouped into changes in ion channel expression and functions, changes in cytokines, and changes in intracellular signaling pathways.22
A number of studies reported changes in voltage-gated sodium channel expression after nerve injury. Sodium channel Nav1.3, Nav1.7, Nav1.8, and Nav1.9 have been implicated in the primary afferent hyperexcitability resulting in peripheral sensitization. Studies have shown the new formation of clusters of sodium channels at the injured nerve sites and the intact dorsal root ganglion following nerve injury.22 Clinically, sodium channel blockers have been used in treating neuropathic pain with varying degree of success. There are several studies highlighting the importance of cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1β in the pathophysiology of peripheral sensitization. It has been reported that peripheral nerve injuries increase TNF-α and IL-1β immunoreactivity in dorsal root ganglia of both injured and uninjured ipsilateral adjacent afferents.23 Peripheral sensitization can also result from the upregulation of several proteins that have limited expression under physiological conditions.24 Upregulation of transient receptor potential channel expression has been shown to contribute to neuropathic pain resulting from sciatic nerve transection and spinal nerve ligation.25–28 Specifically, upregulation of TRPV1 channels, the thermal receptors that are activated by noxious heat, contributes to neuropathic pain.27,29 Upregulation of TRPM8 channels, the cold-sensing receptors, contributes to cold allodynia and hyperalgesia in animals following sciatic nerve injury.30 A number of signaling pathways such as extracellular-regulated kinases, protein kinase C, and mitogen-activated protein kinase play an important roles in peripheral sensitization.24
Downregulation of Kv7 channel functions and expression as a potential mechanism of peripheral hypersensitization and neuropathic pain
Voltage-gated K+ channels are involved in action potential repolarization and damping membrane depolarization and are fundamentally important in controlling neuronal excitability. Neuronal membrane hyperexcitability due to the loss of function of voltage-gated K+ channels is an important mechanism underlying neuropathic pain. Studies have suggested that changes in potassium channel expression may be important in the pathophysiology of peripheral sensitization and neuropathic pain. Ishikawa et al. showed changes in the expression of voltage-gated potassium channels in the dorsal root ganglion following axotomy.31 Kim et al. showed a downregulation of gene expression of voltage-gated potassium channel alpha subunits in the dorsal root ganglion following chronic constriction injury in rat sciatic nerves.32, Yang et al. showed altered potassium channel mRNA expression following peripheral nerve lesion.33 Considerably less research has been done on medications-targeting potassium channels, especially compared to sodium channel blocking agents. Medications affecting potassium channels may be a powerful addition to the currently available treatments for neuropathic pain, and they warrant a more in depth discussion.
In the large family of voltage-gated K+ channels, the KCNQ gene family encodes a subfamily of voltage-gated potassium channels termed Kv7 channels (Kv7.1–Kv7.5). These channels stabilize membrane excitability via noninactivating K+ currents termed M currents.34,35 Kv7.2–Kv7.5 channels are found in many neuronal tissues, while Kv7.1 are located in cardiac as well as various smooth muscle and epithelial tissues.34 Kv7.2 and Kv7.3 are predominantly expressed in nociceptive dorsal root and trigeminal ganglion neurons,34 suggesting their potential role in regulating nociception. Kv7 channel activity can be modulated by biological factors including intracellular signaling from G-protein-coupled receptor pathways associated with muscarinic acetylcholine receptors, or by covalent modification of cysteine residues by hydrogen peroxide or N-ethylmaleimide.36–42 Phospholipase C-mediated inhibition of Kv7 channels is reported to contribute to peripheral inflammatory pain.43 These signaling pathways lead to functional downregulation of Kv7 channels that contribute to peripheral hypersensitivity of nociceptors. Expression of Kv7.2 channels in somatosensory neurons have been shown to be downregulated in animals following peripheral neuropathy induced by chemotherapy drug oxaliplatin.44 The downregulation of Kv7.2 channel expression in the primary afferent nerves is also thought to be an underlying mechanism of neuropathic pain.44
Kv7 activators and their therapeutic uses for neuropathic pain
Kv7 channels have been explored as therapeutic targets for treating diseases due to neuronal membrane hyperexcitability. Compounds that either potentiate or directly open neuronal Kv7 channels can produce an inhibitory effect on action potential initiation. This inhibitory effect can be beneficial in treating diseases involving neuronal hyperexcitability such as epilepsy and neuropathic pain.45,46 A list of synthetic compounds that can directly bind to Kv7 channels to cause a conformational change leading to channel opening is shown in Table 2.
Table 2.
Kv7 channel activators.
| Compound name | Kv7 channel subtypes targeted |
|---|---|
| Acrylamide (S)-1 | Kv7.2, 7.3, 7.4, 7.5 and Kv7.2–Kv7.3 |
| Acrylamide (S)-2 | Kv7.2 |
| 4,4-diisothiocyanatostilbene-2,20-disulfonic acid | Kv7.1–KCNE1 |
| Maxipost (BMS-204352) | Kv7.2, 7.3, 7.4 and 7.5 |
| Diclofenac | Kv7.2, 7.3 and Kv7.2–Kv7.3 |
| Flufenamic acid | Kv7.1–KCNE1 |
| Meclofenamic acid | Kv7.2, 7.3 and Kv7.2–Kv7.3 |
| Mefenamic acid | Kv7.1–KCNE1 |
| NH6 | Kv7.2-Kv7.3 |
| Niflumic acid | Kv7.1–KCNE1 |
| Retigabine | Kv7.2, 7.3, 7.4, 7.5 and Kv7.2–Kv7.3 |
| (Retigabine analog) flupirtine | Kv7.2 |
| L-364 373 [(3-R)-1, 3-dihydro-5-(2-fluorophenyl)-3-(1H-indol-3-ylmethyl)-1-methyl-2H-1,4-benzodiazepin-2-one (R-L3) | Kv7.1, Kv7.1–KCNE1 |
| Zinc pyrithione | Kv7.1, 7.2, 7.4, 7.5 and Kv7.2–Kv7.3 |
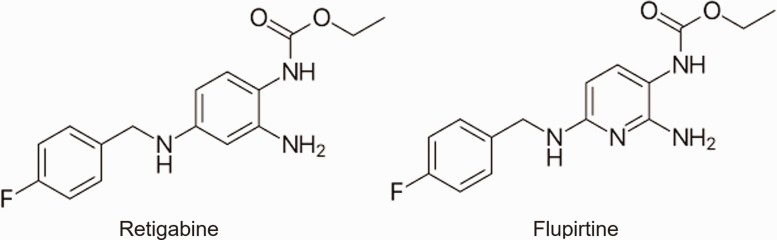
Among them, retigabine (Figure 1) has been most extensively studied for its actions on Kv7 channels in the neurons of the CNS and peripheral nervous system. Retigabine directly opens most Kv7 channels, including heteromeric Kv7.2–Kv7.3 channels, causing enhancements of M currents. The drug exerts a hyperpolarizing effect on neurons, and an action attributed to its ability to activate Kv7 channels expressed in many neurons.47,48 Other studies have shown that retigabine is also involved in the potentiation of other channels, including gamma-aminobutyric acid (GABA) receptors.49 A study conducted in rats found that Kv7.2 channels were expressed on cold-sensing trigeminal ganglion neurons, and that through treatment with retigabine, the excitability of nociceptive cold-sensing neurons was suppressed.50 The study further showed that orofacial cold allodynia and hyperalgesia induced by trigeminal nerve injury in rats could be effectively alleviated by retigabine.50 This study concluded that Kv7.2 channels can be targeted for treatment of trigeminal neuropathic pain. Another study performed in 2003 found similar results, and the study found that injection of retigabine significantly reduced hypersensitivity to injured paws of rats.51
Figure 1.
Chemical structures of retigabine (left) and flupirtine (right).
In humans, retigabine was introduced under the trade name Potiga in the United States and approved in 2011 by Food and Drug Administration for adjuvant treatment of partial-onset seizures in epileptic patients. Its potential uses in treating other disorders such as anxiety and neuropathic pain were also discussed.51–53 However, clinical use of retigabine has been discontinued since June 2017 due to its side effects. Clinical trials with retigabine have identified mostly CNS-related adverse. These include dizziness, somnolence, headache, and fatigue.54,55 Additional unique adverse effects include urinary retention and pigment changes in the skin and retina after prolonged use.56 Clinical use of retigabine is also limited by its short half-life of 8 h, requiring frequent dosing. Flupirtine, a structural analog of retigabine (Figure 1), has been used in Europe for the treatment of acute and chronic pain since the 1980s; it is not clinically available in the United States. Flupirtine has a similar mechanism of action as retigabine at Kv7 channels, and it has also been shown to potentiate GABA-mediated analgesia and muscle relaxation.57,58 The potential role of flupirtine in the treatment of neuropathic pain and fibromyalgia has been previously discussed.59 In addition, bis(1-hydroxy-2(1H)-pyridineselonato-O,S) zinc, commonly known as zinc pyrithione (ZnPy), has recently been shown to be a strong Kv7 activator, potentiating all Kv7 channels except Kv7.3.60 There are no clinical trials of zinc pyrithione for a potential treatment of neuropathic pain.
Concluding remarks
Neuropathic pain is very challenging to manage and despite the numerous treatment options, many patients are still not able to get sustained relief from their neuropathic pain. As a result, researchers and clinicians are constantly exploring additional treatment options. One such option is potassium channel activators which have shown very promising results as a potential therapy for certain types of neuropathic pain in animal models. Neuronal Kv7 channels are of particular interest, as they can be targeted by their activators to suppress neuronal hyperexcitability. Retigabine is one such Kv7 channel activator, and there is a growing body of literature describing its potential use in the treatment of various neuropathic pain conditions. Studies using a rat model of neuropathic pain found that hypersensitivity to injured paws was reduced and orofacial cold hyperalgesia was alleviated through treatment with retigabine.50,51 However, the clinical use of retigabine (for treating seizures) has been discontinued due to its side effects. Future research is necessary to identify the additional activators of Kv7.2 channels as well as other K+ channels with high efficacy and low side effects for the treatment of neurological disorders including neuropathic pain.
Acknowledgments
The authors would like to thank Drs Ferhat Eron, Ryo Ikeda, Zhangfeng Jia, and Ms Jenifer Ling in Dr Jianguo Gu’s laboratory for their previous studies related to the topic of this article.
Authors’ Contributions
All authors participated in the writing of the manuscript. AA-E and JG finished the final version of the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Abd-Elsayed is a consultant for Medtronic, StimWave, Sollis, and Avanos.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research grants DE018661 and DE023090 to JGG.
References
- 1.Carr DB, Goudas LC. Acute pain. Lancet 1999; 353: 2051–2058. [DOI] [PubMed] [Google Scholar]
- 2.Ashburn MA, Staats PS. Management of chronic pain. Lancet 1999; 353: 1865–1869. [DOI] [PubMed] [Google Scholar]
- 3.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization Study in primary care. JAMA 1998; 280: 147–151. [DOI] [PubMed] [Google Scholar]
- 4.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 2015; 16: 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012; 13: 715–724. [DOI] [PubMed] [Google Scholar]
- 6.Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain 2012; 153: 359–365. [DOI] [PubMed] [Google Scholar]
- 7.Johansen A, Romundstad L, Nielsen CS, Schirmer H, Stubhaug A. Persistent postsurgical pain in a general population: prevalence and predictors in the Tromso study. Pain 2012; 153: 1390–1396. [DOI] [PubMed] [Google Scholar]
- 8.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 9.van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014; 155: 654–662. [DOI] [PubMed] [Google Scholar]
- 10.Reda H, Greene K, Rice FL, Rowbotham MC, Petersen KL. Natural history of herpes zoster: late follow-up of 3.9 years (n = 43) and 7.7 years (n = 10). Pain 2013; 154: 2227–2233. [DOI] [PubMed] [Google Scholar]
- 11.Kemler MA, Schouten HJ, Gracely RH. Diagnosing sensory abnormalities with either normal values or values from contralateral skin: comparison of two approaches in complex regional pain syndrome I. Anesthesiology 2000; 93: 718–727. [DOI] [PubMed] [Google Scholar]
- 12.Tahmoush AJ, Schwartzman RJ, Hopp JL, Grothusen JR. Quantitative sensory studies in complex regional pain syndrome type 1/RSD. Clin J Pain 2000; 16: 340–344. [DOI] [PubMed] [Google Scholar]
- 13.Hubscher M, Moloney N, Rebbeck T, Traeger A, Refshauge KM. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain 2014; 30: 886–893. [DOI] [PubMed] [Google Scholar]
- 14.Sethna NF, Meier PM, Zurakowski D, Berde CB. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain 2007; 131: 153–161. [DOI] [PubMed] [Google Scholar]
- 15.Gierthmühlen J, Maier C, Baron R, Tölle T, Treede R-D, Birbaumer N, Huge V, Koroschetz J, Krumova EK, Lauchart M, Maihöfner C, Richter H, Westermann A. Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain 2012; 153: 765–774. [DOI] [PubMed] [Google Scholar]
- 16.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain. Nat Rev Dis Primers 2017; 3: 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Zhao X, Hatch M, Chun S, Chang E. Central neuropathic pain in spinal cord injury. Crit Rev Phys Rehabil Med 2013; 25: 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrance N, Lawson KD, Afolabi E, Bennett MI, Serpell MG, Dunn KM, Smith BH. Estimating the burden of disease in chronic pain with and without neuropathic characteristics: does the choice between the EQ-5D and SF-6D matter? Pain 2014; 155: 1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jay GW, Barkin RL. Neuropathic pain: etiology, pathophysiology, mechanisms, and evaluations. Dis Mon 2014; 60: 6–47. [DOI] [PubMed] [Google Scholar]
- 20.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010; 11: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003; 26: 696–705. [DOI] [PubMed] [Google Scholar]
- 22.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006; 52: 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaible HG, Ebersberger A, Natura G. Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res Ther 2011; 13: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangadharan V, Kuner R. Pain hypersensitivity mechanisms at a glance. Dis Model Mech 2013; 6: 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain 2009; 144: 187–199. [DOI] [PubMed] [Google Scholar]
- 26.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288: 306–313. [DOI] [PubMed] [Google Scholar]
- 27.Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci 2001; 13: 2105–2114. [DOI] [PubMed] [Google Scholar]
- 28.Baron R. Capsaicin and nociception: from basic mechanisms to novel drugs. Lancet 2000; 356: 785–787. [DOI] [PubMed] [Google Scholar]
- 29.Vilceanu D, Honore P, Hogan QH, Stucky CL. Spinal nerve ligation in mouse upregulates TRPV1 heat function in injured IB4-positive nociceptors. J Pain 2010; 11: 588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci 2007; 27: 13680–13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa K, Tanaka M, Black JA, Waxman SG. Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve 1999; 22: 502–507. [DOI] [PubMed] [Google Scholar]
- 32.Kim DS, Choi JO, Rim HD, Cho HJ. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res Mol Brain Res 2002; 105: 146–152. [DOI] [PubMed] [Google Scholar]
- 33.Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N. Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience 2004; 123: 867–874. [DOI] [PubMed] [Google Scholar]
- 34.Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther 2001; 90: 1–19. [DOI] [PubMed] [Google Scholar]
- 35.Wickenden AD, McNaughton-Smith G. Kv7 channels as targets for the treatment of pain. Curr Pharm Des 2009; 15: 1773–1798. [DOI] [PubMed] [Google Scholar]
- 36.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 1980; 283: 673–676. [DOI] [PubMed] [Google Scholar]
- 37.Marrion NV. Control of M-current. Annu Rev Physiol 1997; 59: 483–504. [DOI] [PubMed] [Google Scholar]
- 38.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 2005; 6: 850–862. [DOI] [PubMed] [Google Scholar]
- 39.Higashida H, Hoshi N, Zhang JS, Yokoyama S, Hashii M, Jin D, Noda M, Robbins J. Protein kinase C bound with A-kinase anchoring protein is involved in muscarinic receptor-activated modulation of M-type KCNQ potassium channels. Neurosci Res 2005; 51: 231–234. [DOI] [PubMed] [Google Scholar]
- 40.Roche JP, Westenbroek R, Sorom AJ, Hille B, Mackie K, Shapiro MS. Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels. Br J Pharmacol 2002; 137: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Gamper N, Shapiro MS. Single-channel analysis of KCNQ K+ channels reveals the mechanism of augmentation by a cysteine-modifying reagent. J Neurosci 2004; 24: 5079–5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gamper N, Zaika O, Li Y, Martin P, Hernandez CC, Perez MR, Wang AY, Jaffe DB, Shapiro MS. Oxidative modification of M-type K(+) channels as a mechanism of cytoprotective neuronal silencing. EMBO J 2006; 25: 4996–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linley JE, Pettinger L, Huang D, Gamper N. M channel enhancers and physiological M channel block. J Physiol (Lond) 2012; 590: 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling J, Erol F, Viatchenko-Karpinski V, Kanda H, Gu JG. Orofacial neuropathic pain induced by oxaliplatin: downregulation of KCNQ2 channels in V2 trigeminal ganglion neurons and treatment by the KCNQ2 channel potentiator retigabine. Mol Pain 2017; 13: 1744806917724715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawson K, McKay NG. Modulation of potassium channels as a therapeutic approach. Curr Pharm Des 2006; 12: 459–470. [DOI] [PubMed] [Google Scholar]
- 46.Surti TS, Jan LY. A potassium channel, the M-channel, as a therapeutic target. Curr Opin Investig Drugs 2005; 6: 704–711. [PubMed] [Google Scholar]
- 47.Tatulian L, Brown DA. Effect of the KCNQ potassium channel opener retigabine on single KCNQ2/3 channels expressed in CHO cells. J Physiol (Lond) 2003; 549: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia 2012; 53: 412–424. [DOI] [PubMed] [Google Scholar]
- 49.van Rijn CM, Willems-van Bree E. Synergy between retigabine and GABA in modulating the convulsant site of the GABAA receptor complex. Eur J Pharmacol 2003; 464: 95–100. [DOI] [PubMed] [Google Scholar]
- 50.Abd-Elsayed AA, Ikeda R, Jia Z, Ling J, Zuo X, Li M, Gu JG. KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Mol Pain 2015; 11: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur J Pharmacol 2003; 460: 109–116. [DOI] [PubMed] [Google Scholar]
- 52.Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol 2000; 58: 591–600. [DOI] [PubMed] [Google Scholar]
- 53.Korsgaard MP, Hartz BP, Brown WD, Ahring PK, Strobaek D, Mirza NR. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther 2005; 314: 282–292. [DOI] [PubMed] [Google Scholar]
- 54.Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM, Study G. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology 2007; 68: 1197–1204. [DOI] [PubMed] [Google Scholar]
- 55.Brodie MJ, Lerche H, Gil-Nagel A, Elger C, Hall S, Shin P, Nohria V, Mansbach H, Group RS. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology 2010; 75: 1817–1824. [DOI] [PubMed] [Google Scholar]
- 56.Splinter MY. Efficacy of retigabine in adjunctive treatment of partial onset seizures in adults. J Cent Nerv Syst Dis 2013; 5: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klinger F, Geier P, Dorostkar MM, Chandaka GK, Yousuf A, Salzer I, Kubista H, Boehm S. Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine. Br J Pharmacol 2012; 166: 1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedel HA, Fitton A. Flupirtine. A review of its pharmacological properties, and therapeutic efficacy in pain states. Drugs 1993; 45: 548–569. [DOI] [PubMed] [Google Scholar]
- 59.Szelenyi I. Flupirtine, a re-discovered drug, revisited. Inflamm Res 2013; 62: 251–258. [DOI] [PubMed] [Google Scholar]
- 60.Xiong Q, Sun H, Li M. Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat Chem Biol 2007; 3: 287–296. [DOI] [PubMed] [Google Scholar]