Abstract
Aim:
To assess a program combining virtual reality (VR) games and proprioceptive neuromuscular facilitation (PNF) and to compare it with the standalone techniques in stroke survivors.
Methods:
A randomized controlled clinical trial. A total of 48 participants were recruited in the outpatient clinic of a University Hospital in Salvador, Brazil. They were randomly assigned to 3 groups (n = 16 each): PNF, VR, and PNF/VR. Participants attended twice-weekly 50-minute sessions over a 2-month period. The PNF/VR group performed both PNF and VR exercises employing Nintendo Wii electronic games. Motor performance was assessed before and immediately after the treatment using the Fugl-Meyer Assessment scale.
Results:
An improvement in the mean scores was observed after treatment independent of the allocation group with significant intragroup changes: 14.5, 10.5, and 10.4 for PNF, VR, and PNF/VR, respectively. Score changes were also observed in the analyses of specific sections as follows: (1) a significant improvement in the passive movement and pain score was observed in the PNF and PNF/VR groups; (2) the same was observed for the motor function of the upper limb in all groups, for the motor function of the lower limb in the VR group and for balance in the PNF and PNF/VR groups.
Conclusions:
The use of a program combining virtual rehabilitation and PNF presented results that were comparable with those obtained with the isolated techniques.
Keywords: physical therapy, stroke, rehabilitation, virtual rehabilitation, hemiplegia, stroke rehabilitation, rehabilitation research
Introduction
Stroke is the second-most cause of death in the world and one of the main causes of death and disability.1 Both ischemic and hemorrhagic forms of stroke can lead to significant neurological deficits.2 Hemiparesis, hemiplegia, sensory, and cognitive impairment are among the most common clinical manifestations. Consequently, there is frequently a reduction in functional capacity and quality of life.3,4 Physical therapy enhances the recovery of motor function and functionality, promoting cortical reorganization and motor relearning. A number of methods exist, such as neurodevelopmental therapy, proprioceptive neuromuscular facilitation (PNF), task-oriented training,5 and virtual reality (VR) protocols.
Proprioceptive neuromuscular facilitation was originally developed by Kabat in the 1950s for the treatment of patients with motor deficits.6,7 In this therapeutic philosophy, every individual has a potential to progress, even with significant disabilities.8 PNF techniques promote functional movements through facilitation, inhibition, strengthening, and relaxation of muscle groups using concentric, eccentric, and isometric contractions.9 The exercise patterns for each segment are based on functional and 3-dimensional movements performed in routine daily activities.10
It has been suggested that part of the limitations of the traditional therapy are related to the fact that repetition of the same movements can result in patient being less engaged in treatment and, as a consequence, in loss of effectiveness. Combining traditional techniques with interactive technologies may impact positively by allowing the individual to participate and become immersed in the rehabilitation environment.11 In this context, VR is a relatively new strategy that has shown favorable results in the recovery of motor function and improvement of functional capacity,12 as the Nintendo Wii (Nintendo Co Ltd, Kyoto, Japan) electronic game has been progressively recognized as a useful therapeutic instrument.1 Therapeutic strategies including this tool seem to have greater potential to maintain patient motivation, which may improve their adherence to physiotherapy.12,13 This device has become the focus of much attention due to its low cost and possibility for the patient to use it independently, even for in-home training.14
A growing number of studies have examined the usefulness of the Wii platform for rehabilitation and compared it with conventional physical therapy. Nevertheless, practical issues make it necessary to investigate not only the superiority of one technique over the other but also the possibility of associating them. Exercises with games are highly dependent on the patient involvement and their physical capacity to play. In addition, game-exclusive strategies may leave training gaps that are difficult to fill, and do not foresee passive work for the situations in which a motor deficit or postural difficulties hamper the use of games. When a more conventional strategy (such as PNF) is part of the treatment, it can be used to address conditions that may be difficult to treat with VR only. Wii games are not originally conceived for therapy and, as a consequence, may lack completeness or may not optimally explore movement amplitude. It is a topic still under discussion, but relevant for clinical practice because knowledge of the therapeutic potential of these techniques together may influence the planning for a given stroke survivor. The aim of this study was to compare the efficacy of a training program combining VR and PNF with that of standalone VR or PNF for the promotion of sensorimotor rehabilitation.
Methods
This study was a randomized controlled clinical trial, conducted from June 2015 to December 2017. All participants were recruited at the outpatient clinic of the Prof Edgard Santos University Hospital in Salvador, Brazil, from June 2015 to July 2017 and provided written consent to participate. Prior to the inclusion of the first volunteer, the research protocol received full approval from our institutional review board (ICS/UFBA Nb. 943738) and was registered in the ClinicalTrials.gov database (CT Identifier NCT03171077). The study was performed in accordance with the Declaration of Helsinki and current national regulations for human research.
Demographic data were collected to characterize the sample, namely, sex, age, laterality, affected body parts, type, and time of stroke. An improvement in the sensorimotor function was the primary endpoint, assessed by computing the score change in the Fugl-Meyer Assessment (FMA), which is a stroke-specific performance-based 155-item quantitative scale.15 A trained examiner (a physical therapist that was blinded to the participant allocation groups) performed all pre-treatment and post-treatment evaluations. The initial score was recorded and the same examiner performed a second assessment after 2 months of treatment. Secondary endpoints were improvements in passive motion and pain, sensory function, upper limb motor function, lower limb motor function, and balance, which were assessed through specific FMA domains.
Sample
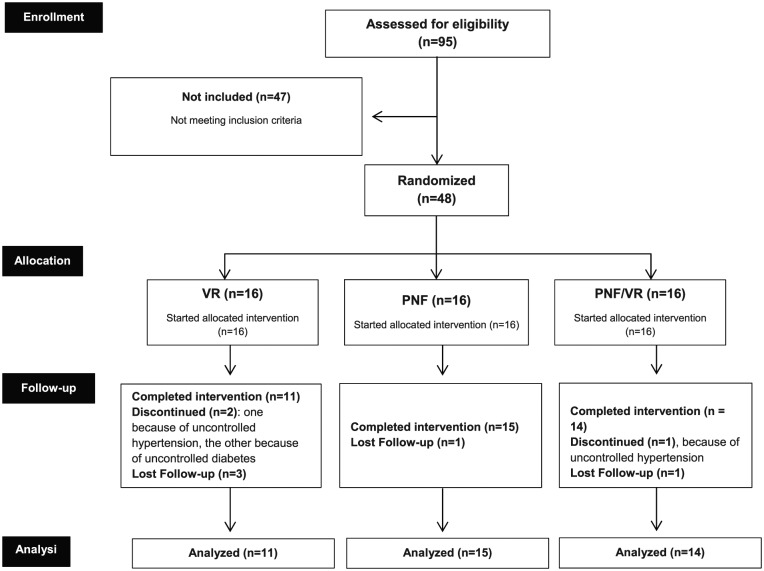
Participants were selected by members of the study staff (V.A.d.S.J., M.d.S.S., N.M.d.S.R.) according to the following inclusion criteria: age between 18 and 80 years; of any sex; diagnosis of stroke confirmed clinically and by neuroimaging; presence of hemiparesis for at least 6 months; ability to perform independent walking (with or without support); and absence of known or self-reported visual or auditory deficits. The study did not include individuals already participating in other rehabilitation programs, using functional electrical stimulation devices, botulinum toxin, or those meeting any of the following non-inclusion criteria: orthopedic conditions that could render the proposed activities harmful or impossible; chronic progressive disabling conditions; a score below 24 in the Mini-Mental State Exam16; or cognitive sequelae that could hamper the understanding of the electronic games. Subjects presenting with uncontrolled systemic arterial hypertension were excluded, as were those who were absent from 3 or more meetings. The sample distribution and size are detailed in the CONSORT (consolidated standards for reporting of trials) flow diagram (Figure 1).
Figure 1.
Flow diagram detailing the distribution of the 48 study participants. PNF indicates proprioceptive neuromuscular facilitation; VR, virtual reality.
Participants were allocated into 3 parallel groups according to the result of a simple random sampling procedure: PNF, VR, and PNF/VR. Someone who was not connected with the study performed randomization using a computer-based random number generator. Information on the allocation of each participant was stored in opaque, sealed envelopes and placed in the folders of the respective participants to ensure confidentiality.
Intervention
The 50-minute sessions took place twice per week. Participants from all groups started the therapy session with 10 minutes of upper and lower limb stretching. The post-interventional assessment was performed at the next scheduled session, after completion of the therapy.
Only fully qualified physiotherapists, who completed a standardized training program on the study protocol, applied the therapy. The PNF sequence included scapula, upper limb, pelvis, lower limb, and gait training and was distributed between 2 weekly sessions. Training was performed in different positions (decubitus, sitting, standing) according to the specific exercise. In the first session, the participants performed 10 minutes of diagonal scapula exercises (anterior and posterior elevation) and 30 minutes of upper limb diagonals (Flexion-Abduction-External Rotation and Extension-Abduction-Internal Rotation). In the second session, participants performed 10 minutes of pelvic diagonals with anterior elevation and posterior depression followed by 20 minutes of lower limb diagonals (Flexion-Conduction-External Rotation and Flexion-Abduction-Internal Rotation) and 10 minutes of gait cycle.
In the VR group, the tasks were performed with the aid of a Nintendo Wii device. The site of the intervention was a 20-square-meter room equipped with a multimedia projector, which projected the image on the wall at a height of 1 m and 20 cm. A professional physical therapist supervised the treatment continuously, and every participant received prior instructions on the conduct of the games. The therapeutic protocol included 4 electronic games: Balance Bubble Plus, Rhythm Parade, Tennis, and Box. The games included exercises for multidirectional displacement, stationary gait, and upper limb.
The duration of each therapeutic session with the Nintendo Wii was the same as that of the other groups, including the 10 initial minutes of stretching. In the first session of the week, the participants performed exercises with the Balance Bubble Plus and Tennis games (20 minutes each). In the second session, Rhythm Parade and Boxing were used (20 minutes each).
The PNF/VR group performed both PNF and VR exercises. The duration of the exercises was modified so that half of the time was devoted to PNF and the other half to VR. The total duration of the sessions remained consistent. The participants performed 5 minutes of scapula diagonals and 15 minutes of lower limb diagonals in the first weekly session. The rest of the session was devoted to VR exercises. In the second session, participants performed 5 minutes of pelvic diagonals, 10 minutes of lower limb diagonals, 5 minutes of gait cycle, and then the VR exercises. The games for the PNF/VR group were the same as those for the VR group, except for their duration. Whereas they were performed for 20 minutes each in the VR group, they were executed for 10 minutes in the PNF/VR group.
Statistical analysis
The statistical analysis was performed using R software version 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria). Absolute and relative frequencies are presented for qualitative variables, and means and standard deviations for quantitative variables, with maximum and minimum values when pertinent. The normality of the data distribution was verified using the Shapiro-Wilk test and the assessment of the symmetry and flattening of the distribution. Analysis of variance (ANOVA) or the Kruskal-Wallis tests were used to assess the existence of significant differences in the measurements between groups. For comparisons between the pre-treatment and post-treatment assessments, Student t-test or the Wilcoxon test was used for paired samples. In all evaluations, the 95% confidence interval and a statistical significance threshold of P < .05 were adopted.
Results
A total of 95 patients were evaluated for eligibility. Of these, 40 were included, 47 met at least 1 non-inclusion criterion and 8 were excluded (Figure 1). The participants were divided equally into the 3 groups. The demographic characteristics of the participants are detailed in Table 1. No significant differences were found between groups regarding age, sex, time since vascular insult, side of hemiparesis, handiness, or stroke type. The study was ended after the 3 groups finished the interventions and post-assessments.
Table 1.
Demographic characteristics of a sample of 40 stroke patients submitted to 3 different therapeutic interventions: proprioceptive neuromuscular facilitation, virtual reality with electronic games (Nintendo Wii), or a hybrid strategy.
| Characteristics | PNF (n = 15) | VR (n = 11) | PNF/VR (n = 14) | Total (n = 40) | P |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 8 (53.4%) | 6 (54.6) | 9 (64.3) | 23 (57.5) | .810 |
| Female | 7 (46.6) | 5 (45.4) | 5 (35.7) | 17 (42.5) | |
| Age (mean ± SD), years | 58.2 ± 7.7 | 55.5 ± 9.6 | 52.7 ± 13.3 | 55.6 ± 10.5 | .380 |
| Time from stroke | |||||
| Mean ± SD (months) | 95.8 ± 99.4 | 87.9 ± 64.7 | 46.7 ± 58.6 | 76.1 ± 79.5 | .210 |
| ∆t ⩽ 12 months | 3 (20%) | 2 (18.2%) | 6 (42.9%) | 11 (27.5%) | |
| 12 < ∆t ⩽ 24 months | 0 (0%) | 1 (9.1%) | 3 (21.4%) | 4 (10%) | |
| ∆t > 24 months | 12 (80%) | 8 (72.7%) | 5 (35.7%) | 25 (62.5%) | |
| Hemiparesis, n (%) | |||||
| Right | 9 (60) | 8 (72.7) | 5 (35.7) | 22 (55) | .16 |
| Left | 6 (40) | 3 (27.3) | 9 (64.3) | 18 (45) | |
| Handiness, n (%) | |||||
| Right handed | 14 (93.3) | 11 (100) | 14 (100) | 39 (97.5) | .999 |
| Left handed | 1 (6.7) | 0 (0) | 0 (0) | 01 (2.5) | |
| Stroke type, n (%) | |||||
| Ischemic | 13 (86.7) | 9 (81.8) | 13 (92.9) | 35 (89.6) | .999 |
| Hemorrhagic | 2 (13.3) | 1 (9.1) | 1 (7.1) | 4 (10) | |
| Unknown | 0 (0) | 1 (9.1) | 0 (0) | 1 (0.4) | |
Abbreviations: PNF, proprioceptive neuromuscular facilitation; VR, virtual rehabilitation.
Table 2 shows the results of the comparison of the effects of the 3 different therapeutic strategies. Post-treatment improvement in the mean FMA scores was observed independent of the allocation groups with significant intragroup differences in the total scores of 14.5, 10.5, and 10.4 for PNF, VR, and PNF/VR, respectively.
Table 2.
Pre-treatment and post-treatment scores (Fugl-Meyer Scale) of 40 stroke patients submitted to 3 different therapeutic interventions: proprioceptive neuromuscular facilitation, virtual reality with electronic games (Nintendo Wii), or a hybrid strategy.
| Sections (Fugl-Meyer Scale) | Pre-treatment score | Post-treatment score | P |
|---|---|---|---|
| PNF (n = 15) | |||
| Passive motion and pain | 77.8 ± 9.9 | 83.9 ± 4.4 | .011* |
| Sensory assessment | 20.9 ± 3.9 | 21.5 ± 3.9 | .400 |
| Upper limb (motor) | 30.8 ± 23.5 | 33.8 ± 24.7 | .018* |
| Lower limb (motor) | 24.3 ± 5.3 | 26.7 ± 3.6 | .052 |
| Balance | 10.5 ± 1.3 | 11.3 ± 1.4 | .041* |
| Total | 164.4 ± 34.4 | 177.3 ± 30.7 | .001* |
| VR (n = 11) | |||
| Passive motion and pain | 80.45 ± 8.7 | 83.7 ± 4.7 | .082 |
| Sensory assessment | 20.27 ± 2.2 | 21.3 ± 4.0 | .221 |
| Upper limb (motor) | 38.91 ± 23.2 | 43.8 ± 23.7 | .010* |
| Lower limb (motor) | 24.82 ± 4.7 | 27.2 ± 3.9 | .018* |
| Balance | 10.64 ± 1.4 | 11.5 ± 2.0 | .146 |
| Total | 175.09 ± 34.3 | 187.5 ± 30.9 | .006* |
| PNF/VR (n = 14) | |||
| Passive motion and pain | 83.21 ± 4.3 | 85.5 ± 3.5 | .033* |
| Sensory assessment | 20.57 ± 6.3 | 22.3 ± 2.4 | .492 |
| Upper limb (motor) | 40.43 ± 20.7 | 45.1 ± 18.2 | .003* |
| Lower limb (motor) | 26.36 ± 4.5 | 28.4 ± 3.0 | .132 |
| Balance | 11.14 ± 1.5 | 11.1 ± 1.7 | .015* |
| Total | 181.71 ± 28.8 | 192.1 ± 25.3 | .001* |
Abbreviations: PNF, proprioceptive neuromuscular facilitation; VR, virtual rehabilitation.
p <0.05.
Modifications were also observed in the analyses of FMA sections. A statistically significant improvement in the passive movement and pain score was observed in the PNF group, as well for motor function of upper limb and balance. The same was observed for motor function of the upper and lower limbs in the VR group and for passive movement and pain, upper limb, and balance in the PNF/VR group.
No significant differences were observed in the intergroup analysis comparing the final scores and the score changes between groups (Table 3).
Table 3.
Comparison of post-treatment scores and score changes (Fugl-Meyer Scale) after 3 different therapeutic interventions for motor impairment following stroke: proprioceptive neuromuscular facilitation, virtual reality with electronic games (Nintendo Wii), and a hybrid strategy.
| Post-treatment scores |
P | Score changes |
P | |||||
|---|---|---|---|---|---|---|---|---|
| PNF | VR | PNF/VR | PNF | VR | PNF/VR | |||
| PMP | 83.9 ± 4.4 | 83.7 ± 4.7 | 85.5 ± 3.5 | .470 | 6.1 ± 5.6 | 3.3 ± 8.7 | 2.3 ± 3.4 | .608 |
| SA | 21.5 ± 3.9 | 21.3 ± 4.0 | 22.3 ± 2.4 | .939 | 0.7 ± 3.8 | 1.0 ± 4.9 | 1.7 ± 5.9 | .779 |
| ULMF | 33.8 ± 24.7 | 43.8 ± 23.7 | 45.1 ± 18.2 | .337 | 3.0 ± 5.2 | 4.9 ± 4.2 | 4.7 ± 6.4 | .727 |
| LLMF | 26.7 ± 3.6 | 27.2 ± 3.9 | 28.4 ± 3.0 | .552 | 2.3 ± 2.8 | 2.4 ± 4.2 | 1.8 ± 3.9 | .718 |
| Balance | 11.3 ± 1.4 | 11.5 ± 2.0 | 11.1 ± 1.7 | .844 | 0.8 ± 1.7 | 0.8 ± 1.4 | –0.1 ± 1.4 | .296 |
| Total | 177.3 ± 30.7 | 187.5 ± 30.9 | 192.1 ± 25.3 | .482 | 12.9 ± 10.4 | 12.4 ± 15 | 10.4 ± 11.1 | .596 |
Abbreviations: LLMF, lower limb motor function; PMP, passive motion and pain; PNF, proprioceptive neuromuscular facilitation; SA, sensory assessment; ULMF, upper limb motor function; VR, virtual rehabilitation.
Discussion
This study compared different treatment strategies (PNF, VR, or PNF/VR) for improving sensorimotor function recovery after stroke. As the exposure time influences functional outcomes, the time dedicated to each technique was reduced in the combined group to maintain a consistent treatment exposure. A significant difference was found in the FMA scores in the intragroup analysis. The study of specific FMA domains also showed significant changes from the pre-therapeutic scores. This was the case for the passive movement and pain section in the PNF and PNF/VR groups, for the motor function of the upper limb section in all groups and the motor function of the lower limb in the VR group. No significant difference among strategies was observed.
Few studies have assessed the effectiveness of Nintendo Wii in the context of stroke rehabilitation.12,17-20 Although the approaches and aims varied, their results agreed in affirming the use of Nintendo Wii games as a feasible and safe therapeutic strategy. In 2010, Saposnik et al18 reported the results of a pilot randomized trial assessing the feasibility, safety, and efficacy of VR using Nintendo Wii for arm rehabilitation. In comparison with a recreational group, participants improved in motor function, as assessed by the Wolf Motor Function Test (WMFT). In 2015, da Silva Ribeiro et al17 compared the impact of conventional physical therapy and virtual rehabilitation with Nintendo Wii on FMA scores in a randomized clinical trial with 30 patients. The results were similar to those of this study, with an improvement in the total scores within each group, score changes in passive movement and pain, upper limb motor function, and balance, but no significant difference between treatment modalities. In 2016, the final results of the EVREST (Efficacy and safety of non-immersive virtual reality exercising in stroke rehabilitation) study were reported with a total of 141 participants.12 In patients who had a stroke within 3 months or less, non-immersive VR using Nintendo Wii as an add-on therapy and recreational activities were associated to a similar incidence of adverse events. Again, there was no significant difference for the upper extremity motor performance assessed using the total time to complete the WMFT.
To the best of our knowledge, this was the first study that assessed the combination of VR and PNF. Although significant differences were not observed among groups, it is interesting to note that the improvement in the specific domains of the FMA was not homogeneous. Score improvement in passive movement and pain was observed in the groups exposed to PNF activities (PNF and PNF/VR) but not standalone VR. A possible factor that may have contributed for this phenomenon is the fact that training in PNF explores movements with maximum tolerated amplitude, which is often not the case with Nintendo Wii electronic games.
We noticed a significant improvement in lower limb motor function with VR. It may be hypothesized that this additional finding was related to the choice of games. The present protocol includes a game for stationary gait exercise (Rhythm Parade), which was not the case in the study of da Silva Ribeiro et al.17 A significant improvement in upper limb function was, however, observed for all groups. A score increase of 4 or more points was observed in 17 participants: 7 from the PNF group, 5 from the VR group, and 5 from the PNF group.
More recently, the use of a Nintendo Wii-based balance-training platform was proposed for balance rehabilitation. A feasibility study with 7 patients in a laboratory setting was reported in 2017.19 The system was proposed to be a step toward an effective balance-training platform. In the present trial, an improvement in balance was observed in the PNF and PNF/VR groups. We believe that this phenomenon may be related to the strengthening of the proximal musculature promoted by PNF. Pelvic and scapula diagonal exercises help stabilize the trunk. In parallel, gait training stimulates weight transfer to the paretic limb. In a study by Sharma and Kaur10 with chronic patients, pelvic PNF combined with core stabilization exercises produced a significant improvement in balance and gait scales. Barcala et al21 assessed balance after stroke using the Berg scale and found a significant improvement with kinesiotherapy, with or without Nintendo Wii virtual rehabilitation.
This study has limitations. The small sample may have reduced its capacity of detecting minor differences between intervention types. These results may also be specific for the present protocols and should not be extrapolated to every type of VR or conventional physical therapy. Furthermore, studies with a longer follow-up are needed to assess the sustainability of the effects.
The fact that no significant difference was observed between the results of the treatment protocols does not mean that their adaptability and indications are the same. In clinical practice, treatment options must be evaluated on a case-by-case basis. The level of acceptance of the objectives by the patient has to be taken into consideration, bearing in mind that, to date, the superiority of PNF or VR cannot be affirmed, nor the combination of both.
In summary, according to the results of this study, the impact of a training program combining VR and PNF on the sensorimotor performance assessed through the FMA scale is similar to that obtained with standalone techniques. Thus, there seems to be no significant loss in associating them, and so far it is not possible to affirm the superiority of one therapeutic strategy over another.
Acknowledgments
The authors thank FAPESB for the financial support.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially funded by Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), Brazil.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: VASJ and ILM performed the essence of the text. ILM and NMSR participated in all phases of the manuscript development and guided the discussion meetings. All authors participated in the literature review, project design, results, and revisions of the manuscript.
ORCID iDs: Vitor Antônio dos Santos Junior  https://orcid.org/0000-0002-9132-7476
https://orcid.org/0000-0002-9132-7476
Igor Lima Maldonado  https://orcid.org/0000-0001-6037-0407
https://orcid.org/0000-0001-6037-0407
References
- 1. de Souza LB, Paim CDRP, Imamura M, Alfieri F. Use of interactive video game for stroke rehabilitation. Acta Fisiátrica. 2011;18:217-221. [Google Scholar]
- 2. Botelho TS, Neto CDM, de Araújo FLC, de Assis SC. Epidemiologia do acidente vascular cerebral no Brasil. Temas Saúde. 2016;16:361-377. [Google Scholar]
- 3. Iano Y, Lima N, Arthur R. Tratamentos fisioterapêuticos em pacientes pós-AVC: uma revisão do papel da neuroimagem no estudo da plasticidade neural. Ensaios Ciência. 2010;14:187-208. [Google Scholar]
- 4. Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2532-2553. [DOI] [PubMed] [Google Scholar]
- 5. Teasell R, Meyer MJ, McClure A, et al. Stroke rehabilitation: an international perspective. Top Stroke Rehabil. 2009;16:44-56. [DOI] [PubMed] [Google Scholar]
- 6. Westwater-Wood S, Adams N, Kerry R. The use of proprioceptive neuromuscular facilitation in physiotherapy practice. Phys Ther Rev. 2010;15:23-28. [Google Scholar]
- 7. Adler SS, Beckers D, Buck M. PNF in Practice: An Illustrated Guide. 2008. 3rd ed (December 3, 2007). Springer; 2008. [Google Scholar]
- 8. Chen J-C. Progress in sensorimotor rehabilitative physical therapy programs for stroke patients. World J Clin Cases. 2014;2:316-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guiu-Tula FX, Cabanas-Valdés R, Sitjà-Rabert M, Urrútia G, Gómara-Toldrà N. The efficacy of the proprioceptive neuromuscular facilitation (PNF) approach in stroke rehabilitation to improve basic activities of daily living and quality of life: a systematic review and meta-analysis protocol. BMJ Open. 2017;7:e016739. doi: 10.1136/bmjopen-2017-016739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma V, Kaur J. Effect of core strengthening with pelvic proprioceptive neuromuscular facilitation on trunk, balance, gait, and function in chronic stroke. J Exerc Rehabil. 2017;13:200-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massetti T, da Silva TD, Crocetta TB, et al. The clinical utility of virtual reality in neurorehabilitation: a systematic review. J Cent Nerv Syst Dis. 2018;10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saposnik G, Cohen LG, Mamdani M, et al. Efficacy and safety of non-immersive virtual reality exercising in stroke rehabilitation (EVREST): a randomised, multicentre, single-blind, controlled trial. Lancet Neurol. 2016;15:1019-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mouawad MR, Doust CG, Max MD, McNulty PA. Wii-based movement therapy to promote improved upper extremity function post-stroke: a pilot study. J Rehabil Med. 2011;43:527-533. [DOI] [PubMed] [Google Scholar]
- 14. Bateni H. Changes in balance in older adults based on use of physical therapy vs the Wii Fit gaming system: a preliminary study. Physiotherapy. 2012;98:211-216. [DOI] [PubMed] [Google Scholar]
- 15. Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13-31. [PubMed] [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198. [DOI] [PubMed] [Google Scholar]
- 17. da Silva Ribeiro NM, Ferraz DD, Pedreira É, et al. Virtual rehabilitation via Nintendo Wii® and conventional physical therapy effectively treat post-stroke hemiparetic patients. Top Stroke Rehabil. 2015;22:299-305. [DOI] [PubMed] [Google Scholar]
- 18. Saposnik G, Teasell R, Mamdani M, et al. Effectiveness of virtual reality using Wii gaming technology in stroke rehabilitation: a pilot randomized clinical trial and proof of principle. Stroke. 2010;41:1477-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verma S, Kumar D, Kumawat A, et al. A low-cost adaptive balance training platform for stroke patients: a usability study. IEEE Trans Neural Syst Rehabil Eng. 2017;25:935-944. doi: 10.1109/TNSRE.2017.2667406. [DOI] [PubMed] [Google Scholar]
- 20. Pacheco TBF, Oliveira Rego IA, Campos TF, Cavalcanti FADC. Brain activity during a lower limb functional task in a real and virtual environment: a comparative study. NeuroRehabilitation. 2017;40:391-400. [DOI] [PubMed] [Google Scholar]
- 21. Barcala L, Grecco LAC, Colella F, Lucareli PR, Salgado AS, Oliveira CS. Visual biofeedback balance training using Wii Fit after stroke: a randomized controlled trial. J Phys Ther Sci. 2013;25:1027-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]