Abstract
Background:
CDK9 inhibitors are antitumorigenic against solid tumors, including esophageal adenocarcinoma (EAC). However, efficacy of a CDK9 inhibitor combined with 5-fluorouracil (5-FU) and target proteins that are targeted by these agents in EAC are unknown.
Methods:
The anti-EAC efficacy of a new CDK9 inhibitor, BAY1143572, with and without 5-FU was assessed in vitro and in xenograft models in athymic nu/nu mice. Synergy between BAY1143572 and 5-FU in inhibiting cell proliferation was analyzed by calculating the combination index using CompuSyn software. Potential targets of BAY1143572 and 5-FU were identified by reverse-phase protein array. The effects of BAY1143572 and 5-FU on MCL-1 in vitro were analyzed by Western blotting, quantitative real-time polymerase chain reaction, and chromatin immunoprecipitation assay. MCL-1 protein expression in tumors from patients with locoregional EAC treated with chemoradiation and surgery was assessed by immunohistochemistry.
Results:
BAY1143572 had dose-dependent antiproliferative and proapoptotic effects and demonstrated synergy with 5-FU against EAC in vitro. The median volumes of FLO-1 and ESO-26 xenografts treated with 5-FU plus BAY114352 were significantly smaller than those of xenografts treated with either agent alone (p < 0.05). BAY1143572 downregulated MCL-1 by inhibiting HIF-1α binding to the MCL-1 promoter. 5-FU enhanced BAY1143572-induced MCL-1 downregulation and stable MCL-1 overexpression reduced the apoptosis induced by BAY1143572 and 5-FU in vitro. High patients’ tumor MCL-1 expression was correlated with shorter overall and recurrence-free survival.
Conclusions:
BAY1143572 and 5-FU have synergistic antitumorigenic effects against EAC. MCL-1 is a downstream target of CDK9 inhibitors and a predictor of response to neoadjuvant chemoradiation in EAC.
Keywords: adenocarcinoma, CDK9, 5-fluorouracil and MCL-1, esophagus
Introduction
Preoperative chemotherapy or chemoradiation followed by surgery have improved survival outcomes and the likelihood of margin-negative esophagogastrectomy in patients with locoregional esophageal adenocarcinoma (EAC).1 However, these patients’ 5-year survival rates remain low.2,3 Biomarker-driven targeted therapies have had limited success in EAC patients, primarily in less than 20% of those with stage IV human epidermal growth factor receptor 2 (Her2-neu)-overexpressing disease who receive trastuzumab.4–6 To date, no targeted agent has demonstrated benefit as an adjunct to 5-fluorouracil-based chemotherapy or chemoradiation in patients with locally advanced EAC.
EAC, but not normal esophageal squamous epithelium or Barrett esophagus, overexpresses cyclin-dependent kinase 9 (CDK9), an evolutionarily conserved ubiquitous serine threonine kinase.7 CDK9 is a transcriptional CDK and an essential component of positive transcription elongation factor b (p-TEFb). p-TEFb, which phosphorylates the carboxy terminal of RNA polymerase II, prolongs the transcription of proteins with short half-lives, such as myeloid cell leukemia-1 (MCL-1). MCL-1 and other CDK9-regulated proteins play important roles in several cellular processes critical to oncogenesis. Some of these processes, such as apoptosis, are enhanced by CDK9 inhibition and chemotherapy. CDK9 inhibitors and chemotherapeutic agents, owing to their common cellular mechanism, very likely have synergy against tumors.8–10
In phase I/II trials, CDK9 inhibitors elicited limited treatment responses and had high toxicity in patients with solid tumors.11–16 These drugs’ low efficacy is partly due to their lack of specificity against CDK9. Therefore, demonstration of target specificity against CDK 9 is important in improving efficacy of the CDK9 inhibitors. In one recent study, we demonstrated the efficacy of two CDK inhibitors with predominant CDK9 inhibitory effects in in vitro and xenograft models of EAC; we also demonstrated that CDK9 downregulation by shRNA (shCDK9) and treatment with a CDK inhibitor reduces the phosphorylation of RNA polymerase II at serine 2, a CDK9-specific function, and downregulates common CDK9 targets such as MCL-1 and c-Myc in EAC.7 These findings indicate that CDK9 inhibitors have on target effects against CDK9 in EAC.
One novel first-in-class CDK9-specific inhibitor, BAY1143572 (Atuveciclib), potently inhibits CDK9 (p-TEFb) activity; its effect against CDK9 is more than 50-fold greater than that against other CDKs. One recent study showed that BAY1143572 inhibits pSer2 and pSer7 RNA Pol II, as well as MYC and MCL-1, and induces apoptosis in adult T-cell leukemia and lymphoma.17,18 In preclinical models of solid tumors, BAY1143572 at nanomolar doses had antitumor activity without any off-target effects, indicating its high specificity for CDK9.19 Recently, we have demonstrated radiation-sensitizing effects of BAY1143572 in preclinical models of EAC (manuscript under review). However, whether BAY1143572 or any other CDK9 inhibitor have a role as an adjuvant to chemotherapy for EAC is not known.
In this study, we assessed the synergy between BAY1143572 and 5-fluorouracil in suppressing tumor growth and downregulating MCL-1 in in vitro and xenograft models of EAC. In vivo experiments were performed with murine xenografts because of ability to test the efficacy and toxicity of a drug against intact tumor and normal tissue in these models. Experiments with xenograft models generate robust preclinical data, an essential step before proceeding to a human clinical trial. Furthermore, we also studied the prognostic relevance of tumor cell MCL-1 expression in patients with locoregional EAC treated with neoadjuvant chemoradiation (including 5-fluorouracil) and esophagogastrectomy. By these experiments, we have tested a hypothesis that BAY1143572 and 5-fluorouracil have synergistic antitumorigenic properties against EAC and MCL-1 is a shared target of these two agents.
Material and methods
The study was approved by MD Anderson’s Institutional Review Board (PA15-0887 and LAB04-0979, PI: DMM). The requirement for informed consent was waived because all samples were from residual tissue in blocks generated for standard-of-care pathology processing and no additional sampling from patients was required. All experiments involving laboratory animals were approved by MD Anderson’s Institutional Animal Care and Use Committee (IACUC-1155-RN01, PI: DMM) and performed in accordance with the guidelines mandated by the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Cell cultures and reagents
BAY1143572 was purchased from Active Biochemical (Wan Chai, Hong Kong). 5-fluorouracil and the human EAC cell lines OE33, FLO-1, and SKGT4 were purchased from Sigma-Aldrich (St. Louis, MO). OE19, SKGT2, and ESO-26 cells were obtained from Dr. Steven H. Lin (MD Anderson Cancer Center). OE33, OE19, SKGT2, and ESO-26 cells were maintained in RPMI medium containing 2 mM L-glutamine and 10% fetal bovine serum (FBS). FLO-1 and SKGT4 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS. 293FT cells were obtained from Invitrogen (Carlsbad, CA) and maintained in DMEM supplemented with 10% FBS and 500 µg/ml G418. All cell lines were maintained in a 5% CO2 atmosphere at 37°C and passaged at 80% confluence using 0.05% trypsin−ethylenediaminetetraacetic acid for 3−5 min.
Cell proliferation assay
Cell proliferation after treatment with vehicle (control) only, with 5-fluorouracil and/or BAY1143572 was measured using an MTS assay (Cell Titer Aqueous One Solution Cell Proliferation Assay, Promega, Madison, WI). Briefly, cells were incubated with the reaction solution containing MTS reagent for 1 h at 37°C. The absorbance at 490 nm was measured using a microplate reader. The results are presented as values normalized to the control. The experiment was performed in triplicate for each treatment condition. The half maximal inhibitory concentration (IC50) values for BAY1143572 were calculated with an equation derived from a best-fit dose–response curve created in Microsoft Excel.
The synergism between BAY1143572 and 5-fluorouracil in inhibiting cell proliferation was analyzed by calculating combination index (CI) values using CompuSyn software.20,21 The CI is a quantitative measure based on the mass-action law of the degree of drug interaction in terms of synergism and antagonism for a given endpoint of the measured effect. A CI value of less than 0.1 indicates very strong synergism; 0.1−0.3, strong synergism; 0.3−0.7, synergism; 0.7−0.85, moderate synergism; 0.85−0.90, slight synergism; 0.9−1.10, nearly additive; and higher than 1.10, antagonism.22,23
Cell apoptosis
EAC cells were treated with vehicle only, 5-fluorouracil and/or BAY1143572; washed with cold phosphate-buffered saline, resuspended in 100 µl of binding buffer containing 5 µl of recombinant Annexin V−fluorescein isothiocyanate (FITC, BD Biosciences, San Jose, CA) and 10 µl of a 50 µg/ml propidium iodide solution, and then incubated for 15 min at room temperature. The percentage of cells undergoing apoptosis in each treatment cohort was analyzed by flow cytometry at MD Anderson’s Flow Cytometry and Cellular Imaging Facility. The experiments were performed in triplicate for each treatment condition.
Reverse phase protein array
Cell lysates were prepared and serially diluted twofold for five dilutions (from undiluted to a 1:16 dilution). The diluted lysates were then arrayed on nitrocellulose-coated slides in an 11 × 11 format, probed with antibodies by tyramide-based signal amplification, and visualized by 3, 3’-diaminobenzidine colorimetric reaction. The slides were scanned on a flatbed scanner to produce 16-bit TIFF images. Spots on the TIFF images were identified, and the staining density was quantified using the Array-Pro Analyzer software program (Meyer Instruments, Houston, TX). Relative protein levels for each sample were determined by interpolating each dilution curve from the ‘standard curve’ (supercurve) of the antibody. Protein-level data were normalized for protein loading and transformed to linear values.24,25 The linear values were compared across the treatment cohorts, and linear values of treatment group(s) that were 0.1 higher or lower than those of controls were considered to identify upregulated and downregulated proteins, respectively.
Western blotting
For each sample, the total protein was separated by 8% or 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride transfer membranes (GE Healthcare Life Sciences, Pittsburgh, PA). Antibodies against MCL-1 (Santa Cruz Biotechnology, Santa Cruz, CA) and phosphorylated RNA polymerase II CTD (pSer2; Novus Biologicals, Littleton, CO) were used for immunoblotting of MCL-1 and pSer2 proteins. Bands were visualized by enhanced chemiluminescence detection (GE Healthcare Life Sciences). The experiments were performed in triplicate for each treatment condition.
Quantitative real-time polymerase chain reaction
For quantitative real-time polymerase chain reaction (qPCR), 0.5 µg of total RNA isolated from cells treated with vehicle only, with BAY1143572 and/or 5-fluorouracil were reverse-transcribed to cDNA using SuperScript II Reverse Transcriptase (Invitrogen). qPCR was performed with the QuantiFast SYBR Green PCR kit (Qiagen, Valencia, CA) on a Life Technologies instrument. PCR primers were designed using the primer3 program according to the DNA sequence of MCL–1. qPCR was performed in triplicate for each treatment condition. The Ct value of GAPDH was subtracted from that of MCL-1 to obtain a ΔCt value. The ΔCt value of the control was subtracted from the ΔCt value of each treated sample to obtain a ΔΔCt value. The MCL-1 expression levels of the experimental groups relative to those of the controls were expressed as 2−ΔΔCt. All experiments were performed in triplicate.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was performed using the Pierce Agarose ChIP Kit (Thermo Scientific, Rockford, IL). Briefly, cells were treated with 1 µM BAY1143572 or control vehicle for 4 h and then fixed with 1% formaldehyde to cross-link DNA and protein. The chromatin was digested with micrococcal nuclease to obtain chromatin fragments of 200–1000 bp. Ten per cent of the chromatin fragments were used as input DNA. The immunoprecipitation was performed with either an anti-HIF-1α antibody or an immunoglobulin G control (Cell Signaling Technology). The immunoprecipitated DNA was then quantitated using real-time PCR with specific primers for the MCL-1 promoter (forward: 5′-AGGTCACTTGAGGCCATGAG-3′; reverse: 5′-CACGTTCAGACGATTCGGTA-3′). These primers cover the –1051 to –901 bp region of the MCL-1 promoter. The enrichment of targeted genomic regions was normalized with input DNA and presented as a value relative to the immunoglobulin G control.
Lentivirus generation and stable overexpression of MCL-1 in EAC cell lines
Human MCL-1 cDNA was released from the pCMV-SPORT6 (OriGene Technologies Inc., Rockville, MD) vector with the EcoRI enzyme and subcloned into the lentiviral vector pCDH-VMV-MCS-EF1-Puro (Addgene, Cambridge, MA) between EcoRI to create a phMCL-1 vector. The identity and orientation of this construct were confirmed by DNA sequencing. To produce an MCL-1-overexpressing lentivirus, we cotransfected phMCL-1 and control vectors with their packaging and envelope plasmids into 293FT cells using Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer’s instructions. The viral supernatant was collected 48 h after transfection and centrifuged at 3000 rpm for 15 min to remove debris. For transduction with the lentivirus, cells were infected with 2× diluted virus media containing 6 μg/ml polybrene for 16 h. Cells stably overexpressing MCL-1 were selected by incubation in a puromycin-containing medium for at least 2 weeks. Target protein expression was confirmed by Western blotting.
EAC xenograft studies
The animals were provided by Jackson Laboratories (MD, USA) or Experimental Radiation Oncology, MD Anderson Cancer Center. All xenografts experiments were performed with 4- to 6-week-old female athymic nu/nu mice, whose mean weight was 20 g (range, 17–22 g). All mice were treatment naïve and did not undergo any genetic manipulation. We chose athymic nu/nu mice because these immunodeficient rodents cannot reject implanted tumor and the rate of tumor growth in these models is predictable. Female mice were used because of timely availability of the female athymic nu/nu mice as compared with male mice. All laboratory animals were kept in modified barrier housing. All animals had social housing and environmental enrichment in accordance with the current edition of the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act.
BAY1143572 (7.5 mg/ml) and 5-fluorouracil (10 mg/ml) were dissolved in DMSO (vehicle) to generate injectable formulations. To identify the effective dose of BAY1143572, we subcutaneously injected 4.5 × 106 FLO-1 cells into the right flanks of the mice. Once the tumors reached 5 mm in diameter, the mice were randomly divided into three treatment cohorts of seven mice each: (1) a control cohort treated with vehicle only; (2) a cohort treated with 12.5 mg/kg BAY1143572; and (3) a cohort treated with 15 mg/kg BAY1143572. Vehicle alone or BAY1143572 were given daily (in the morning) by intraperitoneal injection for 10 days under isoflurane vaporizer anesthesia. Isoflurane vaporizers are traditional anesthetic systems that allow proper, safe, and effective delivery of inhalant anesthetic agent to rodents.
To assess the synergistic effect of BAY1143572 and 5-fluorouracil in inhibiting the growth of EAC xenografts, we randomly divided FLO-1 xenograft-bearing mice into six cohorts of seven to nine mice each: (1) a control cohort treated with vehicle only; (2) a cohort treated with 12.5 mg/kg BAY1143572; (3) a cohort treated with 15 mg/kg BAY1143572; (4) a cohort treated with 20 mg/kg 5-fluorouracil; (5) a cohort treated with 12.5 mg/kg BAY1143572 plus 20 mg/kg 5-fluorouracil; and (6) a cohort treated with 15 mg/kg BAY1143572 plus 20 mg/kg 5-fluorouracil. ESO-26 xenograft-bearing mice were randomly divided into four cohorts of seven to nine mice each: (1) a control cohort treated with vehicle only; (2) a cohort treated with 15 mg/kg BAY1143572; (3) a cohort treated with 20 mg/kg 5-fluorouracil; and (4) a cohort treated with 15 mg/kg BAY1143572 plus 20 mg/kg 5-fluorouracil. For all xenograft experiments, BAY1143572 was given daily (in the morning) for 10 days and 5-fluorouracil (in the morning) was given every 3 days for 2 weeks; both agents were given by intraperitoneal injection. The number of mice for the cohorts was decided based on our prior studies.
The xenografts were measured with digital calipers every 3 days. The xenograft volume was calculated as (W2 × L)/2, where W is the small diameter of the tumor and L is the large diameter of the tumor. In FLO-1 and ESO-26 bearing xenografts experiments, mice were weighed every other day and monitored daily for toxicity signs such as respiratory distress, gastrointestinal toxicity, intra-abdominal fluid collection, ruffled fur, hunched posture, and reduced food intake and for moribund signs such as impaired ambulation, muscular atrophy, lethargy, bleeding, central nervous system disturbances, and inability to remain upright. All laboratory animals were humanely killed by CO2 asphyxiation when tumors reached the maximum size allowed as per the Institutional Animal Care and Use Committee guidelines or at the end of the experiment. The personnel who humanely killed the mice were adequately trained and used methods that are consistent with American Veterinary Medical Association Guidelines for the Euthanasia of Animals.
MCL-1 immunohistochemistry analysis in tumor samples from EAC patients
MCL-1 immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections of pretreatment tumor samples from 63 patients with locoregional EAC who were treated with neoadjuvant chemoradiation followed by esophagogastrectomy at MD Anderson. The neoadjuvant chemoradiation regiments included fluoropyrimidine-based (plus taxol and/or platinum) chemotherapy and radiation in 59 patients and taxol and/or platinum (without fluoropyrimidine) and radiation in four patients. The clinical and pathologic features of these patients are listed in Supplementary Table 2. For immunohistochemistry, the slides were stained with rabbit monoclonal anti-MCL-1 antibody, clone D5V5L (Cell Signaling Technology, cat. #39224) using a Leica Bond Max automated stainer (Leica Biosystems Nussloch GmbH, Nußloch, Germany). Antigen retrieval was performed with Bond Solution #2 (Leica Biosystems), equivalent to ethylenediaminetetraacetic acid buffer pH 9.0, for 20 min followed by staining with a 1:100 dilution of the primary antibody for 20 min at room temperature. The primary antibody was detected using the Bond Polymer Refine Detection Kit (Leica Biosystems) with diaminobenzidine as the chromogen. Tumor cells with cytoplasmic staining intensity scores of 0, 1, 2, or 3 were manually counted at 200× magnification by a pathologist (AV), who was blinded to the patients’ preclinical data and clinicopathologic features. The H-score for the cytoplasmic immunostaining of MCL-1 was calculated as the percentage of cells with intensity 0 × 0 + the percentage of cells with intensity 1 × 1 + the percentage of cells with intensity 2 × 2 + the percentage of cells with intensity 3 × 3. Tumors with H-scores lower than the median H-score were classified as having low MCL-1 expression, and tumors with H-scores equal to or higher than the median H-score were classified as having high MCL-1 expression.
Statistical analysis
In vitro data are the means ± the standard error (SE) from three independent experiments. For in vitro and xenografts assays, Student’s t test was used to assess differences between groups. Patient demographics, clinical and pathologic information, and survival data were obtained from hospital charts and the hospital tumor registry. The Chi-squared or Fisher exact t test was used to compare categorical data. The prognostic significance of clinical and pathologic characteristics and MCL-1 H-score in relation to overall survival was assessed using univariate Cox regression analysis. Cox proportional hazards models were fitted for the multivariate analysis. After interactions between variables had been examined, a backward stepwise procedure was used to derive the best-fitting model. The statistical analysis was conducted using the SPSS software program (SPSS, Chicago, IL). Kaplan–Meier survival curves were drawn with GraphPad Prism (version 4 for Windows; GraphPad Software, San Diego, CA). p values ⩽0.05 were considered significant.
Results
BAY1143572 is cytotoxic to EAC in vitro and in xenografts
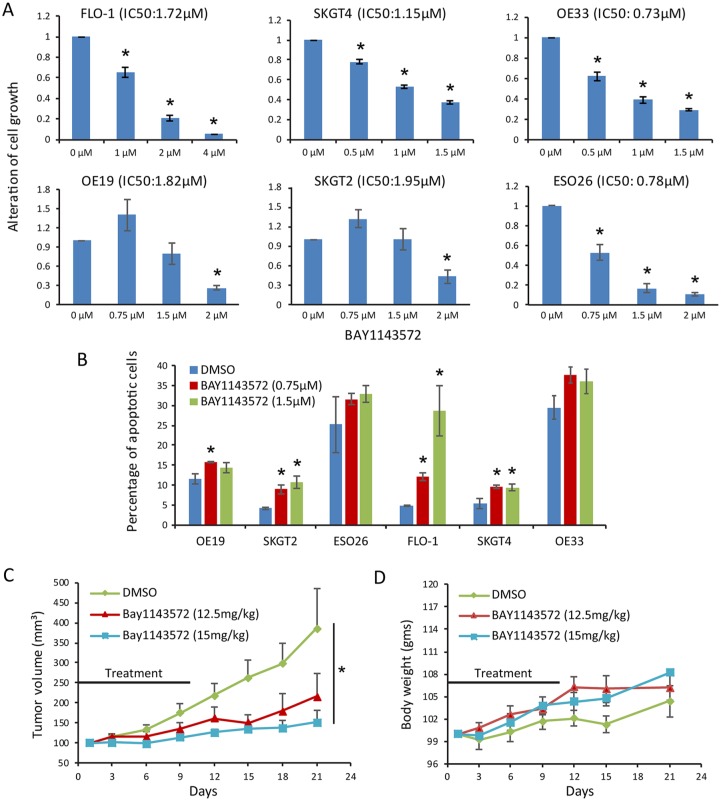
BAY1143572 at doses within a narrow IC50 range (0.73−1.95 µM) had a dose-dependent antiproliferative effect in six EAC cell lines (Figure 1A). Compared with control cells, cells treated with BAY1143572 at a dose within the same narrow IC50 range had higher rates of apoptosis in four of six cell lines; BAY1143572 increased apoptosis by a median of 20% in FLO-1 cells and 5% in SKGT2, SKGT4, and OE19 cells. The increases in apoptosis after treatment with BAY1143572 in OE33 and ESO26 cells were not statistically significant (Figure 1B). Compared with control, 12.5 or 15 mg/kg BAY1143572 reduced FLO-1 xenograft growth by 44% and 61%, respectively (Figure 1C). Mice did not lose weight or show significant signs of toxicity or morbidity throughout the treatment period (Figure 1D).
Figure 1.
BAY1143572 is an effective cytotoxic agent in vitro and in vivo.
Esophageal adenocarcinoma cells were treated with BAY1143572 at the indicated doses for 48 h and then assessed for cell proliferation by MTS assay (A) and for apoptosis by flow cytometry (B). (C) Xenograft-bearing mice were treated with vehicle (DMSO) or with 12.5 or 15 mg/kg BAY1143572 by intraperitoneal injection daily for 10 days. Data are the mean percentages of tumor growth ± SE. *p < 0.05 compared with untreated controls. (D) Body weight chart of the xenograft-bearing mice treated with vehicle or with 12.5 or 15 mg/kg BAY1143572.
Synergy between BAY1143572 and 5-fluorouracil in in vitro models of EAC
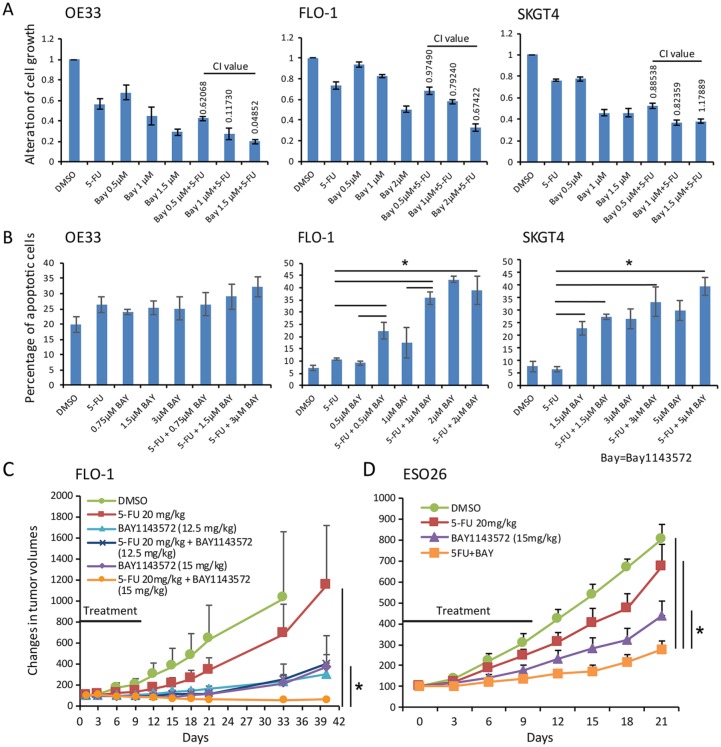
Compared with vehicle, 1.5 µM BAY1143572 plus 10 µM 5-fluorouracil had a very strong synergistic antiproliferative effect in OE33 cells, resulting in an 80% reduction in proliferation (CI, 0.048); 2 µM BAY1143572 plus 5 µM 5-fluorouracil had a strong synergistic antiproliferative effect in FLO-1 cells, resulting in a 67% reduction in proliferation (CI, 0.674); and 1 µM BAY1143572 plus 10 µM 5-fluorouracil had a moderate synergistic antiproliferative effect in SKGT4 cells (CI, 0.83) (Figure 2A). In FLO-1 and SKGT4 cells, BAY1143572 plus 5-fluorouracil induced more apoptosis than that achieved with either agent alone (Figure 2B). In FLO-1 cells, 1 µM BAY1143572 (a dose lower than the IC50) plus 5 µM 5-fluorouracil achieved 20% more apoptosis than BAY1143572 alone did and 25% more apoptosis than 5-fluorouracil alone did. In SKGT4 cells, 5 µM BAY1143572 plus 10 µM 5-fluorouracil achieved 10% more apoptosis than BAY1143572 alone did and 35% more apoptosis than 5-fluorouracil alone did (Figure 2B). In OE33 cells, BAY1143572 plus 5-fluorouracil did not induce significantly more apoptosis than that induced by either agent alone.
Figure 2.
BAY1143572 and 5-fluorouracil synergistically inhibit esophageal adenocarcinoma in vitro and in murine xenografts. (A) Cells pretreated with 5-fluorouracil (5 µM for FLO-1 cells, 10 µM for OE33 and SKGT4 cells) for 24 h were treated with BAY1143572 at the indicated doses for 48 h and then analyzed for cell proliferation by MTS assay. Data are the means ± standard error (SE) of three independent experiments. (B) Cells treated with BAY1143572 with or without 5-fluorouracil were stained with Annexin V–FITC and propidium iodide. Apoptosis was determined by flow cytometry. Data are the means ± SE of 3 independent experiments. *p < 0.05. (C) and (D) The xenograft-bearing mice were treated with BAY1143572 (12.5 or 15 mg/kg for FLO-1 xenografts, 15 mg/kg for ESO-26 xenografts) daily for 10 days and/or 20 mg/kg 5-fluorouracil every 3 days for 2 weeks by intraperitoneal injection. Tumor growth was measured as tumor volume. Data are the percentages of tumor growth.
*p < 0.05.
BAY1143572 enhances the effects of 5-fluorouracil in murine xenografts of EAC
Treatment with 20 mg/kg 5-fluorouracil every 3 days for 2 weeks shrunk FLO-1 xenografts in three mice and slowed xenograft growth in six mice 40 days after treatment initiation. The median xenograft volume of the cohort treated with 5-fluorouracil was 31% smaller than that of the cohort treated with vehicle only. Treatment with 12.5 mg/kg BAY1143572 daily for 10 days shrunk xenografts in three mice and slowed xenograft growth in five mice 40 days after treatment initiation. The median xenograft volume of the cohort treated with 12.5 mg/kg BAY1143572 was 39% smaller than that of the cohort treated with vehicle only. Treatment with 15 mg/kg BAY1143572 daily for 10 days shrunk xenografts in four mice and slowed xenograft growth in four mice 40 days after treatment initiation. The median xenograft volume of the cohort treated with 12.5 mg/kg BAY1143572 was 35% smaller than that of the cohort treated with 5-fluorouracil.
The median xenograft volume of the cohort treated with 15 mg/kg BAY1143572 was 36% smaller than that of the cohort treated with vehicle only. Treatment with 12.5 mg/kg BAY1143572 daily for 10 days plus 20 mg/kg 5-fluorouracil every 3 days for 2 weeks shrunk xenografts in six mice and markedly slowed xenograft growth in one mouse. The median xenograft volume of the cohort treated with 12.5 mg/kg BAY1143572 plus 5-fluorouracil was 65% smaller than that of the cohort treated with 5-fluorouracil alone and 13% smaller than that of the cohort treated with 12.5 mg/kg BAY1143572 alone. Treatment with 15 mg/kg BAY1143572 daily for 10 days plus 20 mg/kg 5-fluorouracil every 3 days for 2 weeks shrunk xenografts in three mice and markedly slowed xenograft growth in five mice. The median xenograft volume of the cohort treated with 15 mg/kg BAY1143572 plus 5-fluorouracil was 94% smaller than that of the cohort treated with 5-fluorouracil alone and 83% smaller than that of the cohort treated with 15 mg/kg BAY1143572 alone (Figure 2C).
The mean ESO-26 xenograft volumes of the cohorts treated with BAY1143572 alone or BAY1143572 plus 5-fluorouracil were 46% and 65% smaller, respectively, than that of the cohort treated with vehicle only. In addition, the mean xenograft volume of the cohort treated with BAY1143572 plus 5-fluorouracil was 55% smaller than that of the cohort treated with 5-fluorouracil alone and 33% smaller than that of the cohort treated with BAY1143572 alone (Figure 2C).
Xenograft-bearing mice treated with 15 mg/kg BAY114372 plus 5-fluorouracil had significant weight loss during the treatment period but were able to gain weight after the treatment was stopped. No other signs of toxicity were observed in any treatment group.
Effects of BAY1143572 with and without 5-fluorouracil on MCL-1 in EAC in vitro
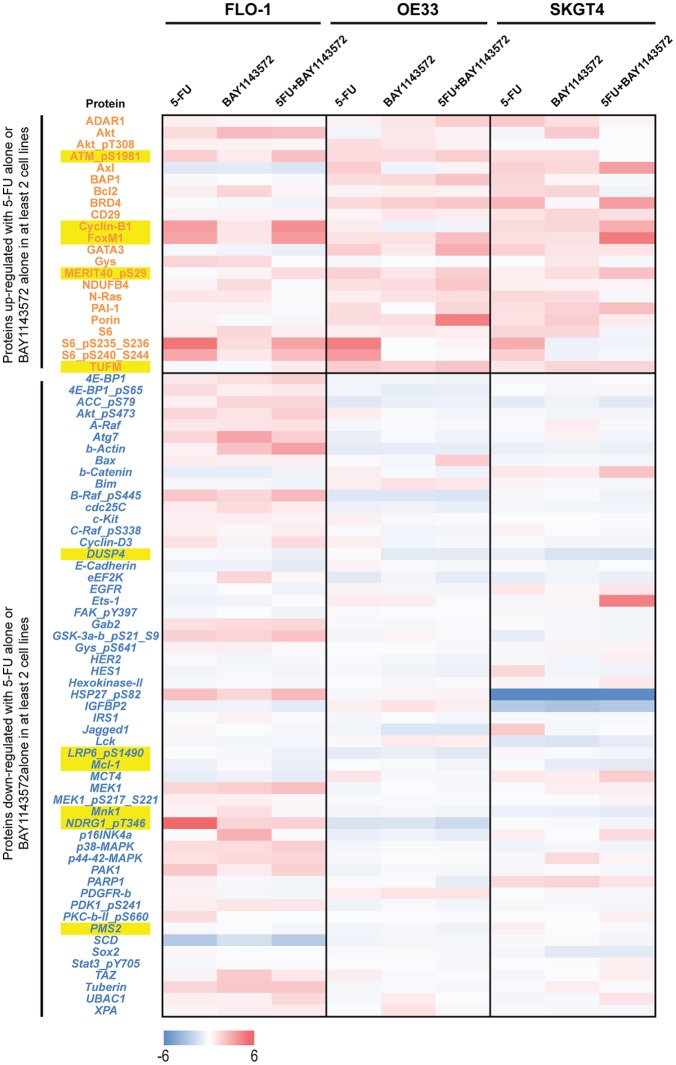
Reverse phase protein array (RPPA) analysis of FLO-1, OE33, and SKGT4 cells revealed that in two of three EAC cell lines, treatment with 5-fluorouracil or BAY1143572 upregulated oncoproteins that included ATMpS1981, a posttranslational form of ATM in response to DNA damage repair; BCL2 and cyclin-B1, regulators of the G2M phase of the cell cycle; FOX-M1, a regulator of DNA damage repair; GATA3, an inducer of epithelial differentiation; MERIT40pS29; and elongation factor Tu, mitochondrial (TUFM; Supplementary Table 1 and Figure 3). Treatment with 5-fluorouracil plus BAY1143572 enhanced the upregulation of ATMpS1981, cyclin-B1, FOX-M1, MERIT40pS29, and TUFM.
Figure 3.
Effects of BAY1143572 with or without 5-fluorouracil on the proteomics profile of esophageal adenocarcinoma. Lysates from cells treated with 1 µM BAY1143572 with or without 5-fluorouracil (10 µM for OE33 and SKGT4 cells, 5 µM for FLO-1 cells) for 30 h were subjected to reverse phase protein array (RPPA) analysis. Protein-level data were normalized for protein loading and transformed to linear values. The heat map indicates the difference in the linear values between control (vehicle treatment only) and the treatment groups. The blue indicating negative (<0) difference between control and the treatment group indicating reduction in the protein and red indicating positive (>0) difference between control and the treatment group indicating increase in the protein expression. Proteins in red font are upregulated oncoproteins after treatment with either 5-fluorouracil or BAY1143572 in at least two cell lines. Proteins in blue font are downregulated oncoproteins after treatment with either 5-fluorouracil or BAY1143572 in at least two cell lines. Yellow highlighted proteins are those with higher upregulation or downregulation after treatment with BAY1143572 plus 5-fluorouracil as compared with single-agent treatment in at least two cell lines.
In two of three EAC cell lines, treatment with 5-fluorouracil or BAY1143572 downregulated several oncoproteins, including ACCpS79, beta-actin, CDC25C (a regulator of transition from G2M to S phase), DUSP4, eF2K, human epidermal growth factor receptor 2, HES1, hexokinase II, IGFBP2, LRP6pS1490 (a Wnt pathway receptor), MCL-1 (a critical protein in apoptotic pathways), MNK1, PMS2, and SCD. Treatment with 5-fluorouracil and BAY1143572 enhanced the downregulation of DUSP4, LRP6pS1490, MCL-1, MNK1, and PMS2. The proteins upregulated or downregulated after treatment with 5-fluorouracil, BAY1143572, or 5-fluorouracil plus BAY1143572 are listed in Supplementary Table 1.
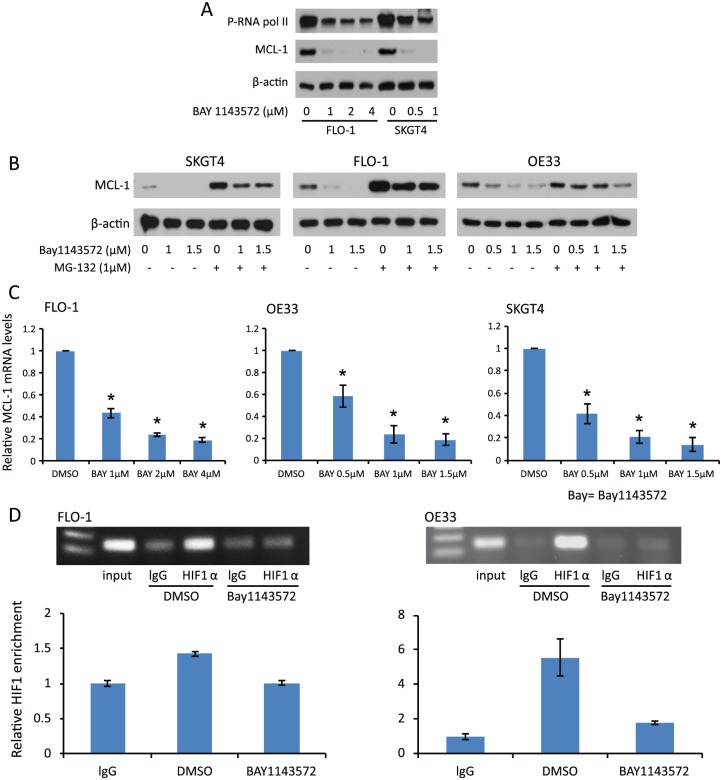
Treatment with different doses of BAY1143572 for 4 h reduced phosphorylated RNA Pol II in two EAC cell lines (eliciting a dose-dependent reduction in one of them), which supports that BAY1143572 has on-target effects against CDK9/p-TEFb (Figure 4A). MCL-1 has not been studied in the context of EAC treated with a CDK9/p-TEFb inhibitor plus a chemotherapeutic agent. Our RPPA analysis demonstrated that BAY1143572 alone and in combination with 5-fluorouracil downregulated MCL-1 in all three EAC cell lines. Therefore, we further assessed the effects of BAY143572 on MCL-1 in vitro.
Figure 4.
Effects of BAY1143572 on MCL-1 protein and RNA levels in in vitro models of esophageal adenocarcinoma. (A) Cells were treated with BAY1143572 at the indicated doses for 4 h. The phosphorylation of RNAPII and the expression of MCL-1 were examined by Western blotting. (B) Cells were treated with 1 µM BAY1143572 for 4 h after pretreatment with or without MG-123 for 1 h. MCL-1 protein levels were assessed by Western blotting. (C) MCL-1 mRNA levels were measured by quantitative real-time polymerase chain reaction (qPCR) after treatment with the indicated doses of BAY1143572 for 4 h. (D) Chromatin immunoprecipitation (ChIP) was used to assess the binding of HIF-1α to the MCL-1 promoter in FLO-1 and OE33 cells treated with 1 µM BAY1143572 or vehicle only for 4 h. qPCR results show the means of experiments performed in triplicate for each treatment condition. Similar results were observed in two independent experiments.
Treatment with 1 µM BAY1143572 reduced MCL-1 protein expression (Figure 4A, B). To determine the role of ubiquitin-dependent MCL-1 degradation in reducing MCL-1 protein after treatment with BAY1143572, we measured the effects of BAY1143572 in cells with and without pretreatment with MG-132, an inhibitor of ubiquitin-dependent proteosomal degradation. The MCL-1 level in cells treated with BAY1143572 and MG132 was significantly higher than that in cells treated with only BAY1143572 (p < 0.05). However, the MCL-1 level in cells treated with MG132 and BAY1143572 was significantly lower than that in cells treated with MG132 alone (Figure 4B). These findings indicate that the inhibition of proteosomal degradation partly rescues MCL-1 and that the rest of the reduction in MCL-1 protein expression is likely due to reduced transcription (RNA level). Indeed, 4 h of treatment with 1 µM BAY1143572 reduced MCL-1 mRNA by 79.2% in SKGT4 cells, 76% in OE33 cells, and 56.4% in FLO-1 cells compared with controls (Figure 4C). ChIP assay revealed that BAY1143572 reduced the binding of HIF-1α to MCL-1 in FLO-1 and OE33 cells. Compared with vehicle only, BAY1143572 significantly reduced the signal of the MCL-1 promoter bound to HIF-1α antibody as compared with control, with a low signal for nonspecific binding by RbIgG (Figure 4D).
Treatment with 5-fluorouracil (5 µM for FLO-1 and 10 µM for SKGT4) had minimal to no effect on MCL-1 protein downregulation in FLO-1 and SKGT4 cells. Compared with either agent alone, 5-fluorouracil plus 1 µM BAY1143572 demonstrated higher reductions of MCL-1 protein expression in FLO-1 and SKGT4 cells (Figure 5A). Treatment with 5-fluorouracil did not significantly reduce the MCL-1 mRNA level in OE33 cells and increased MCL-1 mRNA levels in FLO-1 and SKGT4 cells. Treatment with BAY1143572 and treatment with 5 µM 5-fluorouracil plus BAY1143572 decreased MCL-1 mRNA levels. MCL-1 RNA downregulation in cells treated with BAY1143572 alone and in cells treated with BAY1143572 plus 5-fluorouracil did not differ significantly (Figure 5B).
Figure 5.
Effects of BAY1143572 plus 5-flououracil on MCL-1 protein and RNA levels in in vitro models of esophageal adenocarcinoma. (A) Lysates from cells treated with 1 µM BAY1143572 with or without 5-fluorouracil (10 µM for SKGT4 cells, 5 µM for FLO-1 cells) for 4 h were subjected to Western blotting for MCL-1. (B) Cells were treated with 1 µM BAY1143572 with or without 5-fluorouracil (10 µM for OE33 and SKGT4 cells, 5 µM for FLO-1 cells) for 4 h, and their MCL-1 mRNA levels were measured by quantitative real-time polymerase chain reaction (qPCR). Data are the means ± standard error (SE) of three independent experiments. (C) Western blot of esophageal adenocarcinoma cell lines with stable overexpression of MCL–1. (D) Cells with or without MCL-1 overexpression were treated with 5-fluorouracil and/or BAY1143572 at the indicated doses and then stained with Annexin V–FITC and propidium iodide. Apoptosis was analyzed by flow cytometry. Data are the means ± SE of three independent experiments.
*p < 0.05 compared with control cells.
MCL-1 upregulation decreases BAY1143572’s proapoptotic effects against EAC in vitro
MCL-1 was robustly upregulated in 3 cell lines (Figure 5C). In FLO-1 cells, MCL-1 upregulation reduced apoptosis by 21% after treatment with 0.5 µM BAY1143572, 18% after treatment with 5 µM 5-fluorouracil, and 31% after treatment with BAY1143572 plus 5-fluorouracil as compared with the matching control (p < 0.01). In SKGT4 cells, MCL-1 upregulation reduced apoptosis by 5% after treatment with 0.5 µM BAY1143572, 10% after treatment with 5-fluorouracil, and 8% after treatment with BAY1143572 plus 5-fluorouracil as compared with the matching control (p < 0.01; Figure 5D).
High MCL-1 expression in pretreatment tumor cells predicts shorter overall survival in patients with locoregional EAC
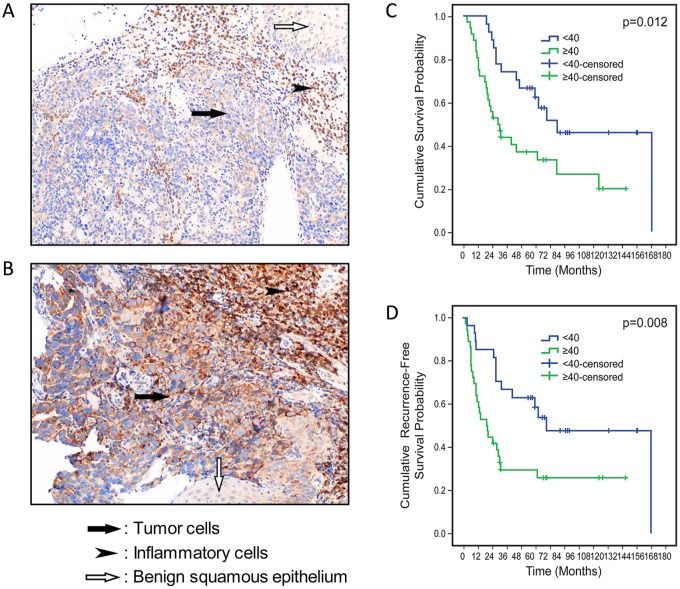
The median H-score of MCL-1 expression in tumor cells in pretreatment samples was 40 (range, 0–250). Higher MCL-1 H-score (H ⩾ 40) correlated with higher pathologic tumor stage (pT3–T4). There was no significant difference in other clinical and pathologic variables between patients whose tumors had low MCL-1 expression (H < 40; Figure 6A) and those whose tumors had high MCL-1 expression (H ⩾ 40; Figure 6B), as shown in Supplementary Table 2. Kaplan–Meier analysis showed that patients with high MCL-1 expression had significantly worse and overall and recurrence (or time to death) survival, than patients with low MCL-1 expression did (Figure 6C and D). The median overall survival duration of patients with tumors with low MCL-1 expression (65 months) was significantly longer than that of patients with tumors with high MCL-1 expression (30 months), p = 0.0.012). In the Cox regression univariate analysis, higher pathologic nodal stage, receipt of therapy for recurrence, and high tumor cell MCL-1 expression were associated with shorter overall survival. In the multivariate analysis, high tumor cell MCL-1 expression and receipt of therapy for recurrence were associated with shorter overall survival, whereas pathologic nodal stage demonstrated a trend towards an association with shorter overall survival (Table 1). The median recurrence-free (or time to death) survival duration of patients with tumors with low MCL-1 expression (60 months) was significantly longer than that of patients with tumors with high MCL-1 expression (18 months, p = 0.008). In the Cox regression univariate and multivariate analyses, higher pathologic nodal stage and high tumor cell MCL-1 expression were associated with shorter recurrence-free (or time to death) survival (Table 2).
Figure 6.
Correlation of MCL-1 protein expression in pretreatment tumor cells with overall survival and recurrence free survival of patients with locoregional esophageal adenocarcinoma treated with neoadjuvant chemoradiation and surgery.
(A) Photomicrograph of MCL-1 immunohistochemical staining of a pretreatment tumor with low MCL-1 expression (200× magnification). (B) Photomicrograph of MCL-1 immunohistochemical staining of a pretreatment tumor with high MCL-1 expression (200× magnification). (C) Kaplan–Meier overall survival curves for patients with high tumor MCL-1 expression and patients with low tumor MCL-1 expression. (D) Kaplan–Meier recurrence free (or time to death) survival curves for patients with high tumor MCL-1 expression and patients with low tumor MCL-1 expression.
Table 1.
Cox regression analysis correlating MCL-1 expression H-score and other clinicopathologic variables with patients’ overall survival.
| Univariate analysis |
Multivariate analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| p | Hazard ratio | 95% confidence interval | p | Hazard ratio | 95% confidence interval | |||
| Age | 0.50 | 0.99 | 0.95 | 1.02 | ||||
| Histology grade (poor or undiff) | 0.63 | 0.85 | 0.44 | 1.63 | ||||
| ypT stage (pT3–T4) | 0.36 | 1.38 | 0.69 | 2.74 | ||||
| ypN stage (pN1–3) | 0.01 | 2.41 | 1.21 | 4.79 | 0.23 | 1.57 | 0.75 | 3.29 |
| Pathologic response (P2) | 0.13 | 1.71 | 0.86 | 3.39 | 0.28 | 1.49 | 0.73 | 3.06 |
| Therapy for recurrence or progression | 0.00 | 3.99 | 1.99 | 8.01 | 0.00 | 3.56 | 1.73 | 7.34 |
| MCL-1 H-score ⩾40 | 0.02 | 2.32 | 1.18 | 4.56 | 0.01 | 2.38 | 1.19 | 4.72 |
Table 2.
Cox regression analysis correlating MCL-1 expression H-score and other clinicopathologic variables with patients’ recurrence-free (or time to death) survival.
| Univariate analysis |
Multivariate analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| p | Hazard ratio | 95% confidence interval | p | Hazard ratio | 95% confidence interval | |||
| Age | 0.16 | 0.97 | 0.94 | 1.01 | 0.28 | 0.98 | 0.95 | 1.02 |
| Histology grade (poor or undiff) | 0.89 | 1.05 | 0.55 | 1.99 | ||||
| ypT stage (ypT3–T4) | 0.18 | 1.59 | 0.80 | 3.14 | 0.60 | 0.80 | 0.35 | 1.84 |
| ypN stage (pN1–3) | 0.03 | 2.07 | 1.06 | 4.04 | 0.04 | 2.16 | 1.02 | 4.57 |
| Pathologic response (P2) | 0.33 | 1.40 | 0.71 | 2.77 | ||||
| MCL-1 H-score ⩾40 | 0.01 | 2.42 | 1.23 | 4.75 | 0.01 | 2.42 | 1.23 | 4.75 |
Discussion
The results of our in vitro and xenograft experiments demonstrate that the inhibition of CDK9/p-TEFb by BAY1143572 alone or in combination with 5-fluorouracil is effective against EAC. In this study, BAY1143572’s dose-dependent effects on cell proliferation and its narrow range of IC50 in six EAC cell lines suggest that CDK9 inhibitors have efficacy against EAC.
The findings of the present study and a previous study7 demonstrate that three CDK inhibitors with predominant CDK9 inhibitory effects, BAY1143572, flavopiridol, and CAN508, have similar antitumorigenic effects against EAC. These drugs also downregulate the phosphorylation of RNA Pol II and transcriptionally downregulate MCL-1 by inhibiting HIF1-α binding to the MCL-1 promoter in EAC. These results indicate that BAY1143572 has on-target effects against CDK9 in EAC. BAY1143572’s higher specificity against CDK9/p-TEFb and strong efficacy in vitro and in murine xenografts of EAC support a study investigating the role of BAY1143572 as an adjunct to chemotherapy or radiotherapy in EAC.
5-fluorouracil is one of the most widely used agents in bimodality and trimodality neoadjuvant therapy as well postoperative chemotherapy in patients with esophageal or gastroesophageal junction adenocarcinoma.1,26–28 However, few studies have assessed the role of targeted agents in combination with 5-fluorouracil-based chemotherapy or chemoradiotherapy in EAC. Most of these studies are limited to targeted agents whose efficacy has already been established in other solid tumors.29–34 Ours is the first study to demonstrate synergy between a CDK9 inhibitor and 5-fluorouracil in vitro and in inhibiting the growth of murine xenografts of EAC. The BAY1143572 dose required to achieve synergy varied across cell lines; a dose lower than the IC50 was required for OE33 and FLO-1 cells, whereas a dose close to the IC50 was required for SKGT4 cells, indicating a heterogeneity of response to BAY1143572 in combination with 5-fluorouracil. Unlike synergy in proliferation in all three EAC cell lines, BAY1143572 significantly enhanced the effects of 5-fluorouracil-induced apoptosis in FLO1 and SKGT4 cells but not in OE33 cells. These findings suggest heterogeneity in the synergistic effects of these agents across different EAC cells. It is likely that effects of BAY1143572 with and without 5-fluorouracil in OE33 and ESO26 are by different mechanism such as cell cycle arrest in G1 or G2-M phase.
In xenograft experiments, synergy between 15 mg/kg BAY1143572 and 5-fluorouracil was evident in most ESO-26 xenografts on day 21 and in most FLO-1 xenografts on day 40. BAY1143572 alone could inhibit the growth of most FLO-1 xenografts until 21 days after treatment initiation. That the inhibitory effects of BAY1143572 plus 5-fluorouracil were longer than those of BAY1143572 alone in FLO-1 xenografts supports the synergy between BAY1143572 and 5-fluorouracil.
Although 5-fluorouracil did not alter MCL-1 RNA and protein expression, the combination of 5-fluorouracil and BAY1143572 enhanced the downregulation of the MCL-1 protein in vitro, indicating that these two agents have synergy in downregulating MCL-1. In addition, 5-fluorouracil enhanced the BAY1143572-induced downregulation of MCL-1 protein but not that of MCL-1 mRNA, indicating that MCL-1 modification is at the post-transcription or post-translational stage by the combination of these agents. BAY1143572 alone and the combination of BAY1143572 and 5-fluorouracil likely have different mechanisms of MCL-1 downregulation, which provides additional support of the relevance of MCL-1 as a likely target of the combination treatment. Compared with control (nonamplified MCL-1), MCL-1 upregulation induced less apoptosis after treatment with 5-fluorouracil and/or BAY1143572 in two EAC cell lines, indicating that the apoptosis mediated by BAY1143572 and 5-fluorouracil depends on MCL–1.
One limitation of the present study was the lack of validation of the synergistic effects of BAY1143572 and 5-fluorouracil on MCL-1 in xenografts. MCL-1, a protein with a short half-life, is regulated by multiple mechanisms. At the end of the experiments, xenografts were unlikely to have reduced MCL-1 levels owing to the transient and reversible effects of CDK9 inhibition on MCL-1 and the normalization of MCL-1 levels by other compensatory mechanisms.35–37 MCL-1 levels in xenografts should be measured immediately after CDK9 inhibitor treatment to demonstrate the effects of CDK9 inhibitors on MCL-1 in xenografts.
MCL-1 is a ubiquitous protein whose expression pattern in EAC is not known. For this reason and to obtain an MCL-1 H-score cut-off that can be used to classify patients as those with good versus those with poor response to neoadjuvant chemoradiation, we used the H-score method and median level of the H-score as the cut-off. We found that MCL-1 expression in patients’ tumor cells was correlated with survival outcomes, further supporting the substantial role MCL-1 has in EAC biology and behavior. Our results showing that MCL-1 expression is at least as significant as established prognostic factors such as nodal stage. This finding indicates that further studies investigating MCL-1 as predictor of neoadjuvant therapy response in patients with localized EAC are warranted.
Our RPPA data showing the upregulation of ATMpS198138 and FOX-M139,40 indicate that the DNA damage repair mechanism is a likely mechanism of synergy between BAY1143572 and 5-fluorouracil. The downregulation of LGRP6pS1490 and MNK1 suggest a synergistic role of BAY1143572 and 5-fluorouracil in the Wnt pathway and/or MAP kinase pathway41 in EAC.
In conclusion, the present study provides ample preclinical data supporting a clinical trial of BAY1143572 alone or in combination with 5-fluorouracil in patients with EAC, using MCL-1 as a potential predictor of response to these therapies.
Supplemental Material
Supplemental material, Supplemental_Table_1 for Targeting CDK9 and MCL-1 by a new CDK9/p-TEFb inhibitor with and without 5-fluorouracil in esophageal adenocarcinoma by Zhimin Tong, Alicia Mejia, Omkara Veeranki, Anuj Verma, Arlene M. Correa, Rashmi Dokey, Viren Patel, Luisa Maren Solis, Barbara Mino, Riham Kathkuda, Jaime Rodriguez-Canales, Steven H. Lin, Sunil Krishnan, Scott Kopetz, Mariela Blum, Jaffer A. Ajani, Wayne L. Hofstetter and Dipen M. Maru in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, Supplemental_Table_2 for Targeting CDK9 and MCL-1 by a new CDK9/p-TEFb inhibitor with and without 5-fluorouracil in esophageal adenocarcinoma by Zhimin Tong, Alicia Mejia, Omkara Veeranki, Anuj Verma, Arlene M. Correa, Rashmi Dokey, Viren Patel, Luisa Maren Solis, Barbara Mino, Riham Kathkuda, Jaime Rodriguez-Canales, Steven H. Lin, Sunil Krishnan, Scott Kopetz, Mariela Blum, Jaffer A. Ajani, Wayne L. Hofstetter and Dipen M. Maru in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Joe Munch in MD Anderson’s Department of Scientific Publications for editing the manuscript and Kim-Anh Vu in MD Anderson’s Department of Anatomic Pathology for helping with the figures.
Footnotes
Author contributions: DM, ZT, AMC, and OV conceived and designed the study. ZT, AM, OV, RD, AV, VP, RK, BM, LMS, JRC, and WLH collected and analyzed the data. DM, ZT, AM, OV, SHL, JAA, MB, SK, and SK wrote and edited the manuscript. All authors approved the final manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded in part by an Independent Investigator Award from the Cancer Prevention and Research Institute of Texas (award number RP140515, to Dr. Dipen Maru). Part of this research was performed in MD Anderson’s Flow Cytometry and Cellular Imaging Facility, which is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support (grant number CA016672).
Availability of data and material: All datasets and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ORCID iD: Dipen M. Maru  https://orcid.org/0000-0002-9134-7709
https://orcid.org/0000-0002-9134-7709
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Zhimin Tong, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Alicia Mejia, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Omkara Veeranki, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Anuj Verma, Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Arlene M. Correa, Department of Thoracic and Cardiovascular Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Rashmi Dokey, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Viren Patel, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Luisa Maren Solis, Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Barbara Mino, Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Riham Kathkuda, Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Jaime Rodriguez-Canales, Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Steven H. Lin, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Sunil Krishnan, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Scott Kopetz, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Mariela Blum, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Jaffer A. Ajani, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Wayne L. Hofstetter, Department of Thoracic and Cardiovascular Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Dipen M. Maru, Division of Pathology and Laboratory Medicine, Unit 085, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX, 77030, USA.
References
- 1. Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011; 29: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 2. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; 16: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 4. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–697. [DOI] [PubMed] [Google Scholar]
- 5. Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crosby T, Hurt CN, Falk S, et al. Long-term results and recurrence patterns from SCOPE-1: a phase II/III randomised trial of definitive chemoradiotherapy +/- cetuximab in oesophageal cancer. Br J Cancer 2017; 116: 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong Z, Chatterjee D, Deng D, et al. Antitumor effects of cyclin dependent kinase 9 inhibition in esophageal adenocarcinoma. Oncotarget 2017; 8: 28696–28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen XX, Xie FF, Zhu XJ, et al. Cyclin-dependent kinase inhibitor dinaciclib potently synergizes with cisplatin in preclinical models of ovarian cancer. Oncotarget 2015; 6: 14926–14939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rathkopf D, Dickson MA, Feldman DR, et al. Phase I study of flavopiridol with oxaliplatin and fluorouracil/leucovorin in advanced solid tumors. Clin Cancer Res 2009; 15: 7405–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walsby E, Pratt G, Shao H, et al. A novel Cdk9 inhibitor preferentially targets tumor cells and synergizes with fludarabine. Oncotarget 2014; 5: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morales F, Giordano A. Overview of CDK9 as a target in cancer research. Cell Cycle 2016; 15: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar SK, LaPlant B, Chng WJ, et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015; 125: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stephenson JJ, Nemunaitis J, Joy AA, et al. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer 2014; 83: 219–223. [DOI] [PubMed] [Google Scholar]
- 14. Dickson MA, Shah MA, Rathkopf D, et al. A phase I clinical trial of FOLFIRI in combination with the pan-cyclin-dependent kinase (CDK) inhibitor flavopiridol. Cancer Chemother Pharmacol 2010; 66: 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heath EI, Bible K, Martell RE, et al. A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Invest New Drugs 2008; 26: 59–65. [DOI] [PubMed] [Google Scholar]
- 16. Morris DG, Bramwell VH, Turcotte R, et al. A phase II study of flavopiridol in patients with previously untreated advanced soft tissue sarcoma. Sarcoma 2006; 2006: 64374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Narita T, Ishida T, Ito A, et al. Cyclin-dependent kinase 9 is a novel specific molecular target in adult T-cell leukemia/lymphoma. Blood 2017; 130: 1114. [DOI] [PubMed] [Google Scholar]
- 18. Lucking U, Scholz A, Lienau P, et al. Identification of atuveciclib (BAY 1143572), the first highly selective, clinical PTEFb/CDK9 inhibitor for the treatment of cancer. ChemMedChem 2017; 12: 1776–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scholz A, Oellerich T, Hussain A, et al. BAY 1143572: a first-in-class, highly selective, potent and orally available inhibitor of PTEFb/CDK9 currently in phase I, inhibits MYC and shows convincing anti-tumor activity in multiple xenograft models by the induction of apoptosis. AACR 106th Annual Meeting, 2015, New Orleans, LA. [Google Scholar]
- 20. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 2010; 70: 440–446. [DOI] [PubMed] [Google Scholar]
- 21. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58: 621–681. [DOI] [PubMed] [Google Scholar]
- 22. D’Alessandro R, Refolo MG, Lippolis C, et al. Strong enhancement by IGF1-R antagonists of hepatocellular carcinoma cell migration inhibition by Sorafenib and/or vitamin K1. Cell Oncol (Dordr) 2018; 41: 283–296. [DOI] [PubMed] [Google Scholar]
- 23. Rozati S, Cheng PF, Widmer DS, et al. Romidepsin and azacitidine synergize in their epigenetic modulatory effects to induce apoptosis in CTCL. Clin Cancer Res 2016; 22: 2020–2031. [DOI] [PubMed] [Google Scholar]
- 24. Tibes R, Qiu Y, Lu Y, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther 2006; 5: 2512–2521. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Liu H, Diao L, et al. Hsp90 inhibitor ganetespib sensitizes non-small cell lung cancer to radiation but has variable effects with chemoradiation. Clin Cancer Res 2016; 22: 5876–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conroy T, Galais MP, Raoul JL, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 2014; 15: 305–314. [DOI] [PubMed] [Google Scholar]
- 27. Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014; 32: 2416–2422. [DOI] [PubMed] [Google Scholar]
- 28. Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008; 26: 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Enzinger PC, Burtness BA, Niedzwiecki D, et al. CALGB 80403 (Alliance)/E1206: a randomized phase II study of three chemotherapy regimens plus cetuximab in metastatic esophageal and gastroesophageal junction cancers. J Clin Oncol 2016; 34: 2736–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J Clin Oncol 2016; 34: 443–451. [DOI] [PubMed] [Google Scholar]
- 32. Moehler M, Gepfner-Tuma I, Maderer A, et al. Sunitinib added to FOLFIRI versus FOLFIRI in patients with chemorefractory advanced adenocarcinoma of the stomach or lower esophagus: a randomized, placebo-controlled phase II AIO trial with serum biomarker program. BMC Cancer 2016; 16: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pant S, Patel M, Kurkjian C, et al. A phase II study of the c-met inhibitor tivantinib in combination with folfox for the treatment of patients with previously untreated metastatic adenocarcinoma of the distal esophagus, gastroesophageal junction, or stomach. Cancer Invest 2017; 35: 463–472. [DOI] [PubMed] [Google Scholar]
- 34. Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter phase II trial. Ann Oncol 2016; 27: 2196–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu H, Yang J, Yuan Y, et al. Regulation of Mcl-1 by constitutive activation of NF-kappaB contributes to cell viability in human esophageal squamous cell carcinoma cells. BMC Cancer 2014; 14: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsieh MJ, Hsieh YH, Lin CW, et al. Transcriptional regulation of Mcl-1 plays an important role of cellular protective effector of vincristine-triggered autophagy in oral cancer cells. Expert Opin Ther Targets 2015; 19: 455–470. [DOI] [PubMed] [Google Scholar]
- 37. Hu J, Dang N, Menu E, et al. Activation of ATF4 mediates unwanted Mcl-1 accumulation by proteasome inhibition. Blood 2012; 119: 826–837. [DOI] [PubMed] [Google Scholar]
- 38. Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003; 421: 499–506. [DOI] [PubMed] [Google Scholar]
- 39. Tan Y, Raychaudhuri P, Costa RH. Chk2 mediates stabilization of the FoxM1 transcription factor to stimulate expression of DNA repair genes. Mol Cell Biol 2007; 27: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khongkow P, Karunarathna U, Khongkow M, et al. FOXM1 targets NBS1 to regulate DNA damage-induced senescence and epirubicin resistance. Oncogene 2014; 33: 4144–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hou S, Du P, Wang P, et al. Significance of MNK1 in prognostic prediction and chemotherapy development of epithelial ovarian cancer. Clin Transl Oncol 2017; 19: 1107–1116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Table_1 for Targeting CDK9 and MCL-1 by a new CDK9/p-TEFb inhibitor with and without 5-fluorouracil in esophageal adenocarcinoma by Zhimin Tong, Alicia Mejia, Omkara Veeranki, Anuj Verma, Arlene M. Correa, Rashmi Dokey, Viren Patel, Luisa Maren Solis, Barbara Mino, Riham Kathkuda, Jaime Rodriguez-Canales, Steven H. Lin, Sunil Krishnan, Scott Kopetz, Mariela Blum, Jaffer A. Ajani, Wayne L. Hofstetter and Dipen M. Maru in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_Table_2 for Targeting CDK9 and MCL-1 by a new CDK9/p-TEFb inhibitor with and without 5-fluorouracil in esophageal adenocarcinoma by Zhimin Tong, Alicia Mejia, Omkara Veeranki, Anuj Verma, Arlene M. Correa, Rashmi Dokey, Viren Patel, Luisa Maren Solis, Barbara Mino, Riham Kathkuda, Jaime Rodriguez-Canales, Steven H. Lin, Sunil Krishnan, Scott Kopetz, Mariela Blum, Jaffer A. Ajani, Wayne L. Hofstetter and Dipen M. Maru in Therapeutic Advances in Medical Oncology