Abstract
Background
It is well known that the genotype of ALDH2 is associated with coronary artery disease (CAD), and in-stent restenosis (ISR) is a primary complication of percutaneous coronary intervention (PCI), a primary recommended treatment for CAD. The aim of this study was to identify the relationship between aldehyde dehydrogenase 2 (ALDH2) genotype and in-stent restenosis (ISR).
Methods
This study recruited 531 patients who were undergoing PCI at two Chinese hospitals from 2015 to 2017 and 183 were diagnosed with ISR after PCI during the one-year follow-up period. We used polymerase chain restriction fragment length polymorphism (PCR-RFLP) and sequencing to determine ALDH2 polymorphisms.
Results
Among all 531 patients (mean age = 59.4 ± 9.8; 65.9% male), 68.7% carried the wild-type genotype, 28.4% were heterozygous for the mutation, and 2.8% were homozygous for the mutation. Multiple logistical regression analyses indicated no correlation between ALDH2 genotype and the occurrence of restenosis after PCI (OR = 1.448, 95% CI: 0.965–2.168, p = 0.073), though a significant association was observed for patients with diabetes (OR = 4.053, 95% CI: 1.668–10.449, p = 0.003).
Conclusion
In this study, we found that carrying an ALDH2*2 allele had no notable relationship with ISR one year after PCI but that it did have a significant association with complications in diabetic patients. Further studies with larger sample sizes will be necessary to reveal a consensus.
Electronic supplementary material
The online version of this article (10.1186/s12872-019-1161-9) contains supplementary material, which is available to authorized users.
Keywords: ALDH2, SNP, In-stent restenosis, Percutaneous coronary intervention, Genetic polymorphisms
Background
Coronary artery disease (CAD) is one of the most common causes of death worldwide, with over 7 million deaths each year [1]. According to the WHO, in 2011, China has the second-largest number of CAD deaths in the world. CAD is a complex disease with both genetic and environmental components, with genetic factors accounting for 40–50% of the etiology of CAD [2]. Treatment for CAD depends on drug therapy and percutaneous coronary intervention (PCI). As the recommended therapy when symptoms persist despite drug treatment, PCI is important not only in acute coronary syndrome (ACS) but also in stable CAD. Although application of PCI has greatly improved ACS patient prognosis, prolonged life and enhanced quality of life, some patients suffer from in-stent restenosis (ISR), which can lead to relapse and a poor prognosis [3]. Thus, ISR has become a common problem for PCI.
Understanding the mechanisms involved in the development of ISR is crucial for its prevention. The direct cause of ISR is stent-induced endothelial injury. Delayed reendothelialization in the stented segment can result in activation of cytokines and growth factors, which in turn stimulate vascular smooth muscle cell (VSMC) differentiation, migration and proliferation [4], followed by narrowing of the coronary lumen [5]. Moreover, oxidative stress promotes the reactivity of aldehydes, such as acetaldehyde, malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), further aggravating endothelial dysfunction. Human mitochondrial aldehyde dehydrogenase 2 (encoded by the ALDH2 gene) is a key enzyme that metabolizes and eliminates 4-HNE [6]. Previous studies have indicated that mice with ALDH2 functional defects exhibit increased sensitivity to externally induced oxidative stress [7] and that inhibition of ALDH2 by oxidative stress is significantly associated with cardiac dysfunction in diabetic rats [8]. Therefore, ALDH2 is considered to be a marker and protector against oxidative stress [9], and deletion of the gene can increase oxidative stress.
There is evidence that genetic factors such as single-nucleotide polymorphism in the genes encoding human vascular endothelial cell growth factor (VEGF) and endothelial nitric oxide synthase (eNOS) are related to a risk of ISR [10], though it remains unknown whether polymorphisms in genes involved in ROS metabolism are also associated with this risk. The human ALDH2 gene is located on chromosome 12 (12q24.2), and the loss-of-function polymorphism rs671 (Glu504Lys or ALDH2*2) in ALDH2 decreases ALDH2 activity by approximately 90% [11]. Due to the important role of ALDH2 in 4-HNE metabolism and in the 4-HNE-mediated oxidative stress in endothelial dysfunction, we speculate the ALDH2*2 polymorphism might affect the risk of ISR after PCI by influencing vascular endothelial function.
Methods
Study population
This study was conducted at Xiangya Hospital of Central South University and First Affiliated Hospital of Zhengzhou University. All patients were diagnosed with ACS and underwent PCI within 4 weeks after their diagnosis. All patients returned to the hospital for reexamination by coronary angiography about one year after discharge according to individual clinical practices. The inclusion criterion was a diagnosis of in-stent restenosis via coronary angiography after PCI treatment during the one-year follow-up period. The exclusion criteria were as follows: (1) presence of severe heart valve disease; (2) history of thrombosis; (3) cerebrovascular accident in the 4 months before the procedure; (4) bleeding susceptibility; (5) malignant disease such as tumor; (6) platelet dysfunction; (7) acute or chronic inflammatory disease; (8) incomplete information. A 5-ml sample of peripheral blood was drawn from all patients into ethylenediaminetetraacetic acid (EDTA) tubes and at centrifuged at 3000 rpm for 10 min; the plasma and blood cells were separated and stored at − 20 °C. Genomic DNA was extracted from the blood cells. A questionnaire and case registration form were used to record in detail the reasons for readmission, basic information, clinical presentation, coronary angiograph results, the event of IRS, and the degree of restenosis. The time of all patients to reexamination the angiography was recorded and the time from PCI to reexamination were calculated. Smoker vs non-smoker was distinguished by no cigarette smoking for half a year; drinker vs non-drinker was distinguished by no alcohol drinking for half a year. Hypertension, hyperlipidemia and diabetes mellitus were defined according to European Society of Hypertension (ESH)/European Society of Cardiology (ESC) guidelines for the management of arterial hypertension [12], the third Report of The National Cholesterol Education Program (NCEP) [13] and American Diabetes Association criteria [14], respectively.
Definition of in-stent restenosis
The definition of ISR was greater than 50% reduction in the luminal diameter within the stent, distal segments adjacent to the stent or the 5-mm proximal, as based on follow-up angiography [12]. In patients with multiple restenosis, the number of lesions were recorded. The results of the luminal narrowing and in-stent restenosis were confirmed by two experienced interventional cardiologists according to ESC guidelines for cardiovascular diagnoses.
ALDH2*2 (Glu504Lys) polymorphism assay
DNA was extracted by phenol-chloroform extraction performed according to previously described protocols [15]. The polymerase chain restriction fragment length polymorphism (PCR-RFLP) method was employed for genotyping using the following primers: upstream, 5′- GATGTGGAGGTTGCAACGAG − 3′; downstream, 5′- CCTACAGGCCTTGGCGTATA − 3′. The PCR amplification system used 15 ng of genomic template and 10 μM primers with the following reaction conditions: 36 cycles of 94 °C for 45 s, 60 °C for 45 s, and 72 °C for 1 min, followed by extension at 72 °C for 10 min and storage at 4 °C. The PCR product (3 μL) was digested using the restriction endonuclease Eco57 I at 37 °C overnight and electrophoresed through a 1.5% agarose gel with ethidium bromide staining. The PCR products were sent to Sangon Biotech Company for sequencing. Data were analyzed with the use of Chromas version 2.33. Genomic sequences of ALDH2 obtained from GenBank (NC_000012.12:111766887–111809985) were used as references.
Statistical analysis
The t test and chi-square test were performed with SPSS 20.0 software, and logistic regression analysis was performed using R statistical software version 3.3.3. Measurement data are expressed as the mean ± standard deviation and median (interquartile range). Analysis of continuous variables such as biochemical tests was performed using the t test and non-normally distributed data was performed using Mann-Whitney test or Kruskal-Wallis H test. Analysis of categorical variables such as sex, risk factors, coronary artery lesions, medications and ALDH2 genotype were analyzed by the chi-square test. Logistic regression analysis was applied to assess the relationship between genotype and ISR risk. The independent variables examined were based on previous studies [16, 17]. A p value less than 0.05 was considered to indicate statistical significance.
Results
Eligible patients and clinical characteristics between ISR and non-ISR patients
According to our inclusion and exclusion criteria, 531 subjects were enrolled in this study from 2015 to 2018 (Fig. 1). All of the patients visited the hospital and were subjected to repeated coronary angiographies after PCI. ISR was observed in 183 (34.5%) patients. The clinical characteristics of the patients enrolled in this study are listed in Table 1. Baseline demographics were balanced between the ISR group and the non-ISR group. Compared with non-ISR patients, ISR patients had higher HBA1C levels but lower thrombocytocrit levels. The incidence of type 2 diabetes and the use of clopidogrel and statins were notably overrepresented in patients with ISR. However, no differences in age, sex, follow-up period, risk factors, biochemical tests, or other medical treatments were found between the patients with and without ISR. Despite similarities with regard to the site of coronary lesion and degree of stenosis before PCI, patients with complicated coronary artery disease and multivessel disease were found to be more likely to develop ISR (Table 1).
Fig. 1.
Patients selection flowchart
Table 1.
Baseline characteristics of patients who undergoing PCI
| Variable | Total (n = 531) | ISR (n = 183) | no-ISR (n = 348) | p-value |
|---|---|---|---|---|
| Age, years | 59.4 ± 9.8 | 58.8 ± 9.8 | 60.5 ± 9.8 | 0.069 |
| Male, n (%) | 350 (65.9) | 114 (62.3) | 236 (67.4) | 0.202 |
| Follow-up period, days | 363 (289–386) | 364 (294–391) | 363 (285–385) | 0.471 |
| Risk factors, n (%) | ||||
| Cigarette smoking | 143 (26.9) | 42 (23) | 101 (29) | 0.134 |
| Alcohol drinking | 96 (18.1) | 29 (15.8) | 67 (19.3) | 0.332 |
| Hypertension | 247 (46.5) | 92 (50.3) | 155 (44.5) | 0.208 |
| Diabetes mellitus | 115 (21.7) | 49 (26.8) | 66 (19.0) | 0.038 |
| Hyperlipemia | 31 (5.8) | 6 (3.3) | 25 (7.2) | 0.068 |
| Biochemical tests | ||||
| Thrombocytocrit, mmol/L | 0.19 ± 0.07 | 0.18 ± 0.04 | 0.19 ± 0.08 | 0.041 |
| MPV, fl | 9.03 ± 3.0 | 8.85 ± 1.7 | 9.12 ± 3.5 | 0.338 |
| HBA1C, % | 6.23 ± 1.9 | 6.53 ± 1.9 | 6.07 ± 1.9 | 0.007 |
| Glucose, mmol/L | 5.43 ± 1.9 | 5.6 ± 1.9 | 5.35 ± 1.9 | 0.16 |
| Serum creatinine, μmol/L | 75.7 ± 35.2 | 71.8 ± 22.4 | 77.8 ± 40.2 | 0.06 |
| TC, mmol/L | 3.82 ± 1.1 | 3.79 ± 1.1 | 3.83 ± 1.2 | 0.673 |
| TG, mmol/L | 1.62 ± 1.1 | 1.59 ± 0.8 | 1.64 ± 1.2 | 0.646 |
| HDL, mmol/L | 1.07 ± 0.3 | 1.07 ± 0.3 | 1.07 ± 0.3 | 0.997 |
| LDL, mmol/L | 2.3 ± 0.9 | 2.3 ± 0.9 | 2.29 ± 0.9 | 0.895 |
| Coronary artery lesions, n (%) | < 0.001 | |||
| Single-vessel disease | 225 (42.4) | 64 (35) | 161 (46.3) | |
| Double-vessel disease | 168 (31.6) | 49 (26.8) | 119 (34.2) | |
| Triple-vessel disease | 138 (26) | 70 (38.3) | 68 (49.3) | |
| Medications, n (%) | ||||
| Clopidogrel | 420 (79.1) | 156 (85.2) | 264 (75.9) | 0.011 |
| Ticagrelor | 119 (22.4) | 37 (20.2) | 82 (23.6) | 0.38 |
| Statins | 505 (95.1) | 179 (97.8) | 326 (93.7) | 0.036 |
| ACEI/ARB | 196 (37) | 75 (41) | 121 (34.9) | 0.166 |
| β-blocker | 383 (72.1) | 133 (72.7) | 250 (71.8) | 0.838 |
| CCB | 158 (29.9) | 58 (32) | 100 (28.8) | 0.442 |
| Antidiabetic therapy | 264 (49.7) | 99 (54.1) | 165 (47.4) | 0.143 |
| Nitrate | 520 (97.9) | 181 (98.9) | 339 (97.4) | 0.251 |
| ALDH2 genotype, n (%) | 0.028 | |||
| ALDH2 *1/*1 | 365 (68.7) | 115 (62.8) | 250 (71.8) | |
| ALDH2 *1/*2 | 151 (28.4) | 59 (32.2) | 92 (26.4) | |
| ALDH2 *2/*2 | 15 (2.8) | 9 (4.9) | 6 (1.7) | |
Note: This statistical analysis was performed by SPSS 20.0 software. MPV Mean Platelet Volume, HBA1C Hemoglobin A1c, TC Total Cholesterol, TG Triglyceride, HDL High Density Lipoprotein, LDL Low Density Lipoprotein, ACEI Angiotensin-Converting Enzyme Inhibitors, ARB Angiotensin Receptor Blocker, CCB Calcium channel blocker
Genotype distribution of the ALDH2*2 polymorphism in ISR and non-ISR patients
The genotype distribution of the ALDH2*2 polymorphism is shown in Fig. 2. Among the 531 patients, 365 (68.7%) were the wild-type allele (ALDH2*1/*1), 151 (28.4%) carried the heterozygous (ALDH2*1/*2) allele and 15(2.8%)carried the mutant homozygote allele (ALDH2*2/*2). As the ALDH2*2 polymorphism is reportedly dominant, carriers of ALDH2*2 were combined for the ensuing association analysis [2]. As shown in Table 1, carriers of the ALDH2*2 allele were notably overrepresented among the ISR patients(p = 0.028).
Fig. 2.
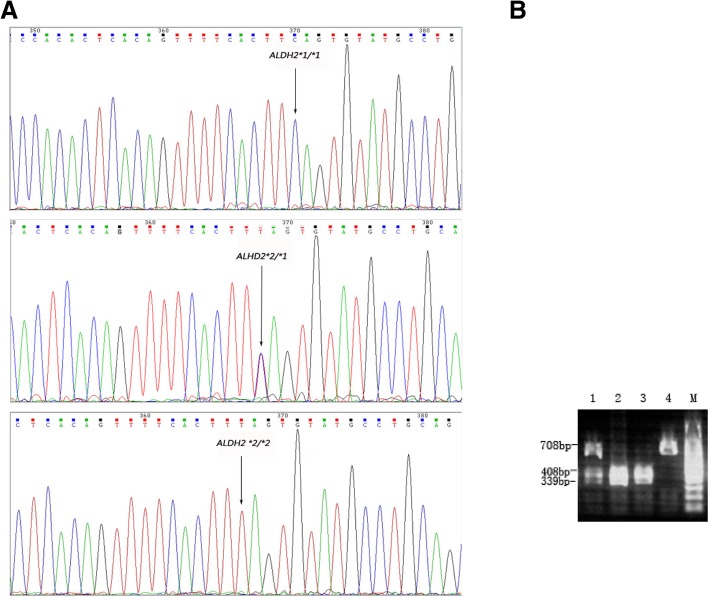
Genotyping Results of the ALDH2. Panel a was the results of the measure sequence: wild genotype of ALDH2 *1/*1, heterozygote genotype of ALDH2 *1/*2, homozygous mutant genotype of ALDH2 *2/*2. Panel b was the results of the ALDH2 by PCR-RFLP: Sample 1 represents the heterozygote genotype of ALDH2 *1/*2, sample 2 and sample 3 represents the wild genotype of ALDH2 *1/*1, sample 4 represents the homozygous mutant genotype of ALDH2 *2/*2, sample M represents marker
Association of the ALDH2*2 polymorphism with ISR risk
The results of the chi-square test revealed obvious difference in the prevalence of diabetes mellitus (p = 0.038), coronary artery lesions (p < 0.01) and the use of clopidogrel (p = 0.011) and statins (p = 0.036) between the ISR and non-ISR patients. Due to the low frequency of the mutant allele, heterozygotes with minor allele homozygotes were combined for comparison with wild-type allele homozygotes. Ten variables, including age, alcohol drinking, cigarette smoking, diabetes mellitus, coronary artery lesions, ALDH2 genotype and medications (clopidogrel, statins, ACEI/ARB, and nitrite) were selected for univariate regression, and the results showed that patients with type 2 diabetes (OR = 1.647, 95% CI: 1.029–2.611, p = 0.035), carriers of the ALDH2*2 allele (OR = 1.537, 95% CI: 1.005–2.339, p = 0.046) and those who use of the antiplatelet medication clopidogrel (OR = 1.697, 95% CI: 1.018–2.934, p = 0.049) were at an increased risk for ISR (Table 2). In multivariate analysis, a significant association between coronary artery lesions (OR = 1.508, 95% CI: 1.193–1.911, p = 0.006, Table 2) and ISR was observed, and the use of clopidogrel for antiplatelet therapy was also a risk factor for ISR (OR = 1.680, 95% CI: 1.035–2.794, p = 0.039); in contrast, the association of ALDH2 genotype with the risk of IRS was only marginal (OR = 1.50, 95% CI: 0.96–2.32, p = 0.073). Among the 183 patients diagnosed with ISR, the median (interquartile range) of ALDH2 *1/*1 was 70 (50–90) %, ALDH2 *1/*2 was 85 (70–90) % and ALDH2 *2/*2 was 80 (60–92.5) %. Analysis of correlation was performed using the Kruskal-Wallis H test and the result suggested that there was a statistically significant difference in the percentage diameter stenosis between the three groups (p = 0.034, Fig. 3).
Table 2.
Risk Factors of in-sent retenosis by univariate and multivariate regression in all patients
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95%) | p-value | OR (95%) | p-value | |
| Age | 1.01 (0.989–1.030) | 0.378 | 1.009 (0.990–1.030) | 0.32 |
| Alcohol drinking (vs non) | 0.877 (0.512–1.460) | 0.622 | 1.230 (0.651–2.319) | 0.521 |
| Cigarette smoking (vs non) | 0.807 (0.506–1.264) | 0.357 | 0.654 (0.377–1.113) | 0.123 |
| Diabetes mellitus (vs non) | 1.647 (1.029–2.611) | 0.035 | 1.424 (0.909–2.221) | 0.12 |
| Coronary artery lesions (vs single-vessel disease) | 1.278 (0.994–1.642) | 0.057 | 1.508 (1.193–1.911) | 0.006 |
| ALDH2 *2 carriers vs ALDH2 *1 homozygotes | 1.537 (1.005–2.339) | 0.046 | 1.448 (0.965–2.168) | 0.073 |
| Clopidogrel | 1.697 (1.018–2.934) | 0.049 | 1.680 (1.035–2.794) | 0.039 |
| Statins | 2.924 (0.992–12.503) | 0.085 | 2.493 (0.867–9.054) | 0.118 |
| ACEI/ARB | 1.317 (0.872–1.982) | 0.188 | 1.052 (0.709–1.556) | 0.799 |
| Nitrite | 1.739 (0.441–11.505) | 0.483 | 1.367 (0.277–9.903) | 0.718 |
Note: This statistical analysis was performed by R statistical software version 3.3.3. ACEI Angiotensin-Converting Enzyme Inhibitors, ARB Angiotensin Receptor Blocker
Fig. 3.
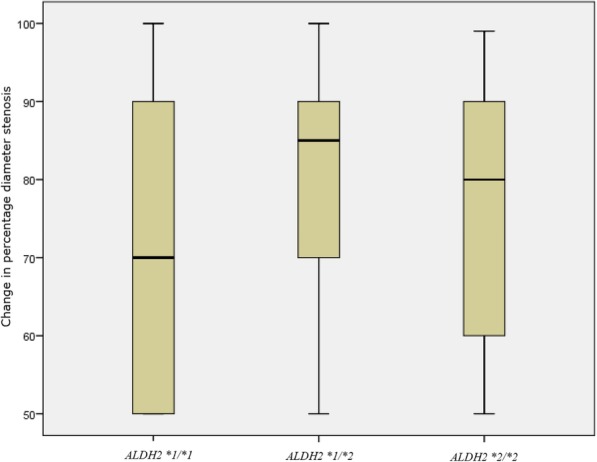
Correlation of genotype distribution and changes in percentage diameter stenosis. Correlation of genotype distribution and changes in percentage diameter stenosis was analyzed in 183 patients diagnosed with ISR. Among them, the median (interquartile range) of ALDH2 *1/*1 was 70 (50–90) %, ALDH2 *1/*2 was 85 (70–90) % and ALDH2 *2/*2 was 80 (60–92.5) %. Kruskal-Wallis H test was used to analyze the correlation, the result suggested that there was a statistically significant difference in the percentage diameter stenosis between the three groups (p = 0.034)
Association of the ALDH2*2 polymorphism with ISR risk stratified by diabetes
Among ISR patients with diabetes, 27 (55.1%) carried the ALDH2*1/*1, 20 (40.8%) carried the ALDH2*1/*2 allele, and 2 (4.1%) carried the ALDH2*2/*2 allele (Table 3). Of the non-ISR patients with diabetes, 54 (81.8%) carried the ALDH2*1/*1 allele, 11 (16.7%) carried the ALDH2*1/*2 allele, and 1 (1.5%) carried the ALDH2*2/*2 allele (Table 3). The results of the chi-square test showed significant overrepresentation of ALDH2*2 carriers among ISR patients with diabetes (p = 0.008). Of the ISR patients who did not have diabetes, equal proportions of ALDH2*1/*1, ALDH2*1/*2 and ALDH2*2/*2 were 88 (65.7%), 39 (29.1%), and 7 (5.2%), respectively. No difference in ALDH2 genotype distribution between the ISR and non-ISR patients was observed among those without diabetes (p = 0.139, Table 3).
Table 3.
The genotype of ALDH2 distribution in patients with or without diabetes mellitus
| Genotype, n (%) | Total | ISR | non-ISR | p-value |
|---|---|---|---|---|
| Patients with diabetes | 0.008 | |||
| ALDH2 *1/*1 | 81 (70.4) | 27 (55.1) | 54 (81.8) | |
| ALDH2 *1/*2 | 31 (27) | 20 (40.8) | 11 (16.7) | |
| ALDH2 *2/*2 | 3 (2.6) | 2 (4.1) | 1 (1.5) | |
| Patients without diabetes | 0.139 | |||
| ALDH2 *1/*1 | 284 (68.3) | 88 (65.7) | 196 (69.5) | |
| ALDH2 *1/*2 | 120 (28.8) | 39 (29.1) | 81 (28.7) | |
| ALDH2 *2/*2 | 12 (2.9) | 7 (5.2) | 5 (1.8) |
Note: This statistical analysis was performed by SPSS 20.0 software
To investigate whether the occurrence of diabetes affects the relationship between the ALDH2*2 polymorphism and ISR risk, an additional association analysis was performed by stratification of patients with or without diabetes mellitus. In Table 4, 8 variables including age, alcohol consumption, cigarette smoking, coronary artery lesions, ALDH2 genotype, and clopidogrel, statin and ACEI/ARB use are shown with their p values from univariate regression; among these, only ALDH2*2 (OR = 3.667, 95% CI: 1.606–8.727, p = 0.002) genotype was associated with a significantly increased risk for IRS among patients with diabetes. After entering these 8 variables into the multivariate model, the results were the same as those of univariate regression, with only ALDH2*2 (OR = 4.053, 95% CI: 1.668–10.449, p = 0.003) carriers showing significantly increased risk. Similarly, univariate regression and multivariate regression were employed to assess risk factors in non-diabetic patients, with no association found between ALDH2 genotype and ISR (Table 4). Interestingly, both univariate and multivariate regression analyses indicated that coronary artery lesions were not associated with the risk of ISR among diabetic patients (OR = 1.095, 95% CI: 0.68–1.767, p = 0.709; OR = 1.068, 95% CI: 0.626–1.818, p = 0.808); however, in non-diabetic patients, the number of coronary artery lesions was significantly associated with ISR in both univariate and multivariate regression analyses (OR = 1.746, 95% CI: 1.355–2.261, p < 0.01; OR = 1.677, 95% CI: 1.284–2.2, p < 0.01). Additionally, the use of clopidogrel was associated with ISR in patients without diabetes (OR = 1.918, 95% CI: 1.129–3.382, p = 0.019; OR = 1.857, 95% CI: 1.073–3.329, p = 0.031) but not in those with diabetes (OR = 1.472, 95% CI: 0.551–4.221, p = 0.451; OR = 0.899, 95% CI: 0.297–2.829, p = 0.851).
Table 4.
Risk Factors of in-sent retenosis by univariate and multivariate regression in patients with or without diabetes mellitus
| Variable | univariate analysis | multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95%) | p-value | OR (95%) | p-value | ||
| Diabetes | Age | 1.018 (0.974–1.065) | 0.431 | 1.014 (0.966–0.966) | 0.574 |
| Alcohol drinking (vs non) | 0.644 (0.164–2.183) | 0.495 | 0.944 (0.178–4.66) | 0.944 | |
| Cigarette smoking (vs non) | 0.636 (0.187–1.929) | 0.439 | 0.436 (0.093–1.113) | 0.267 | |
| Coronary artery lesions (vs single-vessel disease) | 1.095 (0.680–1.767) | 0.709 | 1.068 (0.626–1.818) | 0.808 | |
| ALDH2 *2 carriers vs ALDH2 *1 homozygotes | 3.667 (1.606–8.727) | 0.002 | 4.053 (1.668–10.449) | 0.003 | |
| Clopidogrel | 1.472 (0.551–4.221) | 0.451 | 0.899 (0.297–2.829) | 0.851 | |
| Statins | 1.500 (0.140–32.83) | 0.743 | 1.817 (0.145–2.794) | 0.651 | |
| ACEI/ARB | 1.376 (0.654–2.914) | 0.400 | 1.205 (0.528–2.748) | 0.656 | |
| Non-diabetes | Age | 1.015 (0.995–1.037) | 0.145 | 1.009 (0.987–1.031) | 0.424 |
| Alcohol drinking (vs non) | 0.868 (0.508–1.445) | 0.591 | 1.177 (0.581–2.374) | 0.649 | |
| Cigarette smoking (vs non) | 0.801 (0.505–1.253) | 0.337 | 0.694 (0.379–1.241) | 0.225 | |
| Coronary artery lesions (vs single-vessel disease) | 1.746 (1.355–2.261) | < 0.001 | 1.677 (1.284–2.200) | < 0.001 | |
| ALDH2 *2 carriers vs ALDH2 *1 homozygotes | 1.191 (0.766–1.841) | 0.433 | 1.112 (0.693–1.774) | 0.657 | |
| Clopidogrel | 1.918 (1.129–3.382) | 0.019 | 1.857 (1.073–3.329) | 0.031 | |
| Statins | 3.333 (1.117–14.329) | 0.055 | 2.594 (0.839–11.376) | 0.138 | |
| ACEI/ARB | 1.210 (0.785–1.855) | 0.385 | 1.008 (0.635–1.586) | 0.974 | |
Note: This statistical analysis was performed by R statistical software version 3.3.3. ACEI Angiotensin-Converting Enzyme Inhibitors, ARB Angiotensin Receptor Blocker
Discussion
This study analyzed the association between the ALDH2*2 polymorphism and risk for ISR one year after PCI. Previous studies have demonstrated that the rate of the ALDH2*2 polymorphism is approximately 30–50% in Asians, but the frequency worldwide is only approximately 8% [18]. Therefore, analysis of the clinical relevance of this polymorphism is important for Asian populations. Several previous studies have reported that the ALDH2*2 polymorphism is a risk factor for the course of CAD, especially in Asians [18]. For example, Yavari et al. found the ALDH2*2 polymorphism to be an independent genetic risk factor for CAD in the Han Chinese population [19]. In addition, ALDH2-deficient mice exhibit significantly increased production of mitochondrial ROS and endothelial dysfunction after prolonged treatment with acetaldehyde [20]. Indeed, oxidative stress due to the production of reactive oxygen species (ROS) and inflammation are key pathophysiological processes for the development of ISR and for in-stent atherosclerosis [21]. ROS can cause endothelial dysfunction and directly promote VSMC proliferation and migration by inducing inflammation. ROS can also promote the formation of lipid free radicals and lipid peroxides, which result in the formation of reactive aldehydes, such as acetaldehyde, MDA and 4-HNE, further aggravating endothelial dysfunction. PC12 cells deficient in ALDH2 are highly sensitive to 4-HNE-induced oxidative damage and accumulate proteins that are modified by 4-HNE. Ethanol can reduce 4-HNE levels by activating ALDH2 and thus shows cardioprotective effects during ischemia-reperfusion injury [22, 23]. Therefore, ALDH2 is considered to be a marker of and protector against oxidative stress, and defects in ALDH2 can increase oxidative stress [24]. Asymmetric dimethylarginine (ADMA) is a newly discovered risk factor for cardiovascular disease that acts via inhibition of NO synthase and decreased NO production, which leads to vascular endothelial dysfunction. Evidence has shown that 4-HNE can inhibit expression of miR-21, a microRNA that suppresses expression of the ADMA-inactivating enzyme dimethylarginine dimethylaminohydrolase 1 (DDAH1). In addition 4-HNE can directly form an adduct with DDAH1, inhibiting DDAH1 activity and promoting endothelial dysfunction [25]. The ALDH2*2 polymorphism is associated with CAD susceptibility [15], and the mechanism of this effect is partially explained by decreased DDAH1 expression and increased ADMA levels in endothelial cells. The stent-induced endothelial injury and delayed reendothelialization by DES is critical for the development of ISR, and interindividual differences in reendothelialization capacity might influence the risk of ISR and the prognosis of CAD after PCI [26, 27].
In this study, we analyzed 531 CAD patients who had received PCI and were diagnosed with in-stent restenosis about one year after the procedure. Patients were grouped according to the occurrence of ISR. Frequency matching was also performed for all variables potentially associated with a risk of ISR to evaluate the pure impact of the ALDH2 polymorphism. The overall frequency of heterozygosity for the polymorphism was 31.2% in this study, and this frequency was comparable with that of other studies [18]. Our results showed that there was a statistical difference in the changes in percentage diameter stenosis among the three genotypes of 183 patients diagnosed with ISR. But it was different from our expectation that the percentage diameter stenosis of ALDH2 *1/*2 was the highest, which may be caused by low frequency of ALDH2 *2/*2. We acknowledged this study’s limitation that there is no specific value degree of stenosis for those patients who had reduction in in-stent luminal diameter of less than 50%, and we could not acquire their angiographic images for technical reason. Furthermore, our results indicated that ALDH2*2 carriers were at an increased risk of ISR, especially among those with diabetes. Wang et al. observed that myocardial MDA contents and ROS levels were significantly increased in diabetic rats, which also showed reduced left ventricular ejection fraction and fractional shortening accompanied by decreased ALDH2 expression and activity. We hypothesized that a diabetic status may result in increased oxidative stress, which may include an increase in endogenous aldehydes levels. Furthermore, the level of oxidative stress in ALDH2*2 patients may be increased, thus accounting for the delayed reendothelialization of target vessels after DES-induced endothelial injury and the increased risk for ISR after PCI. In addition, the risk of ISR is higher in the Asian population than in European and American populations, perhaps because ALDH2*2 is more commonly found in Asians than in other populations [28].
Study limitation
There are several limitations of this study. First, the sample size was small. Moreover, for analyzing the changes in percentage diameter stenosis as a continuous variable, we had to exclude those patients with a reduction of less than 50% in in-stent luminal diameter, because the angiographic images were not re-accessible for a technical reason. A prospective study with a larger sample size is required to validate our findings. Second, given that the distribution of different gene mutations can vary depending on ethnicity, our results might not be repeatable in other ethnic populations. Third, data regarding BMI, stent type, and angiographic and echocardiographic information were not collected at baseline, though these data might have prognostic significance. Lastly, the major weakness of this study is the lack of systematic follow-up of all patients, which may have resulted in selection bias. Although we showed no association between ALDH2 genotype and ISR, we cannot ensure that our eligible cohort is representative of the population due to the loss patients because of poor compliance and incomplete follow-up.
Conclusion
Our findings indicate that ALDH2*2 (heterozygous genotype) was not associated with ISR one year after PCI. Although our study demonstrated that ALDH2*2/1/ALDH2*2/2 were not associated with ISR after PCI, the well-documented cardiovascular protection provided by the ALDH2*1/1 genotype is unquestionable. Furthermore, the prevalence of nonfunctional alleles in different ethnic populations should be considered to achieve a consensus regarding the characteristics of these genotypes [2].
Additional file
Table S1. Clinical information of all patients in this study. (XLSX 90 kb)
Acknowledgements
Not applicable.
Abbreviations
- 4-HNE
4-Hydroxynonenal
- ACEI
Angiotensin-converting enzyme inhibitor
- ACS
Acute coronary syndrome
- ADMA
Asymmetric Dimethylarginine
- ALDH2
Aldehyde dehydrogenase 2
- ARB
Angiotensin receptor blocker
- BMS
Bare metal stent
- CAD
Coronary artery disease
- CCB
Calcium channel blocker
- DDAH1
Dimethylarginine Dimethylaminohydrolase 1
- DES
Drug-eluting stent
- EDTA
Ethylenediaminetetraacetic acid
- eNOS
Endothelial nitric oxide synthase
- HBA1C
Hemoglobin A1c
- HDL
High-density lipoprotein
- ISR
In-stent restenosis
- LDL
Low-density lipoprotein
- MDA
Malondialdehyde
- MPV
Mean platelet volume
- PCI
Percutaneous coronary intervention
- ROS
Reactive oxygen species
- SNP
Single-nucleotide polymorphism
- TC
Total cholesterol
- TG
Triglyceride
- VEGF
Vascular endothelial cell growth factor
- VSMC
Vascular smooth muscle cell
Author’s contributions
LZL designed the study and analyzed the data; WJY wrote the paper; PYS, YBC, JY and CMZ collected the clinical data; XPC contribute the study design and FYL approved the final manuscript. All authors have read and approved the MS.
Funding
The study was supported by the fundamental research funds for the Central Universities of Central South University (Program: 2018zzts081), National Natural Science Foundation of China (81873494 to Fanyan Luo), and Hunan Natural Science Foundation (2018JJ2665 to Fanyan Luo), all funding bodies have played a role in designing research and collection, analyzing and interpreting data, and writing the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article Additional file 1.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Xiangya Hospital, Central South University, Changsha (201803732). We obtained individual verbally permission of participants before the study.
Consent for publication
Not applicable.
Competing interests
None of the authors declares competing interests regarding this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lizhi Lv and Weijie Ye contributed equally to this work.
Contributor Information
Lizhi Lv, Email: prolvlizhi@126.com.
Weijie Ye, Email: 463111153@qq.com.
Peiyuan Song, Email: song_ng_peiyuan@126.com.
Yubin Chen, Email: 470643668@qq.com.
Jing Yang, Email: fccyangj3@zzu.edu.cn.
Congmin Zhang, Email: fcczhangcm@zzu.edu.cn.
Xiaoping Chen, Email: chenxp74@hotmail.com.
Fanyan Luo, Phone: +86 139-7312-9106, Email: drlfy1998@csu.edu.cn.
References
- 1.Members ATF, Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Eur Heart J. 2012;33(20):2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 2.Pang J, Wang J, Zhang Y, Xu F, Chen Y. Targeting acetaldehyde dehydrogenase 2 (ALDH2) in heart failure—recent insights and perspectives. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2017;1863(8):1933–1941. doi: 10.1016/j.bbadis.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub WS, Grau-Sepulveda MV, Weiss JM, DeLong ER, Peterson ED, O'brien SM, Kolm P, Klein LW, Shaw RE, McKay C. Prediction of long-term mortality after percutaneous coronary intervention in older adults: results from the National Cardiovascular Data Registry. Circulation. 2012;125(12):1501–1510. doi: 10.1161/CIRCULATIONAHA.111.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Salu K, Wang L, Liu X, Li S, Lorenz G, Wnendt S, Verbeken E, Bosmans J, dWF V. Use of a tacrolimus-eluting stent to inhibit neointimal hyperplasia in a porcine coronary model. J Invasive Cardiol. 2005;17(3):142. [PubMed] [Google Scholar]
- 5.Pleva L, Kusnierova P, Plevova P, Zapletalova J, Karpisek M, Faldynova L, Kovarova P, Kukla P. Increased levels of MMP-3, MMP-9 and MPO represent predictors of in-stent restenosis, while increased levels of ADMA, LCAT, ApoE and ApoD predict bare metal stent patency. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(4):586–594. doi: 10.5507/bp.2015.037. [DOI] [PubMed] [Google Scholar]
- 6.Wang R-S, Nakajima T, Kawamoto T, Honma T. Effects of aldehyde dehydrogenase-2 genetic polymorphisms on metabolism of structurally different aldehydes in human liver. Drug Metab Dispos. 2002;30(1):69–73. doi: 10.1124/dmd.30.1.69. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel P, Schuhmacher S, Kienhöfer J, Müller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res. 2008;80(2):280–289. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Wang H, Hao P, Xue L, Wei S, Zhang Y, Chen Y. Inhibition of aldehyde dehydrogenase 2 by oxidative stress is associated with cardiac dysfunction in diabetic rats. Mol Med. 2011;17(3–4):172–179. doi: 10.2119/molmed.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng L-M, Chen X-P, Sun J, Guo Y-J, Li L, Mo L, Xie W, Li Y-J, Yang T-L, Li C-C. Influence of ALDH2 Glu504Lys polymorphism on nitroglycerin response in chronic heart failure and involvement of calcitonin gene related peptide (CGRP) Int J Clin Pharmacol Ther. 2012;50(10):701–711. doi: 10.5414/CP201635. [DOI] [PubMed] [Google Scholar]
- 10.Bagyura Z, Kiss L, Hirschberg K, Berta B, Széplaki G, Lux Á, Szelid Z, Soós P, Merkely B. Association between VEGF gene polymorphisms and in-stent restenosis after coronary intervention treated with bare metal stent. Dis Markers. 2017;2017:9548612. doi: 10.1155/2017/9548612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100(18):1872–1878. doi: 10.1161/01.CIR.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 12.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Blood Press. 2013;22(4):193–278. doi: 10.3109/08037051.2013.812549. [DOI] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection E Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Jama. 2001;285(19):2486. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Idiopathic B, Endocrinopathies D. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–S20. doi: 10.2337/diacare.26.2007.S5. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y-J, Chen L, Bai Y-P, Li L, Sun J, Zhang G-G, Yang T-L, Xia J, Li Y-J, Chen X-P. The ALDH2 Glu504Lys polymorphism is associated with coronary artery disease in Han Chinese: relation with endothelial ADMA levels. Atherosclerosis. 2010;211(2):545–550. doi: 10.1016/j.atherosclerosis.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Shen Y, Li C, Zhang RY, Zhang Q, Shen WF, Ding FH, Lu L. Association of increased serum CTRP5 levels with in-stent restenosis after coronary drug-eluting stent implantation: CTRP5 promoting inflammation, migration and proliferation in vascular smooth muscle cells. Int J Cardiol. 2017;228:129–136. doi: 10.1016/j.ijcard.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Ma W, Liang Y, Zhu J, Chen T, Feng G, Yang Y, Liu X, Wang X. Relationship of paraoxonase-1 Q192R genotypes and in-stent restenosis and re-stenting in Chinese patients after coronary stenting. Atherosclerosis. 2016;251:305–310. doi: 10.1016/j.atherosclerosis.2016.07.901. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, Wang J, Xue M, Xu F, Chen Y. ALDH2---the genetic polymorphism and enzymatic activity regulation: their epidemiologic and clinical implications. Curr Drug Targets. 2015;18(15):1810–1816. doi: 10.2174/1389450116666150727115118. [DOI] [PubMed] [Google Scholar]
- 19.Yavari A, Ashrafian H. Potentiating mitochondrial aldehyde dehydrogenase 2 to treat post-infarction heart failure. Cardiovasc Res. 2014;103(4):429. doi: 10.1093/cvr/cvu175. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel P, Müller J, Zurmeyer S, Schuhmacher S, Schulz E, Oelze M, Pautz A, Kawamoto T, Wojnowski L, Kleinert H. ALDH-2 deficiency increases cardiovascular oxidative stress---evidence for indirect antioxidative properties. Biochem Biophys Res Commun. 2008;367(1):137–143. doi: 10.1016/j.bbrc.2007.12.089. [DOI] [PubMed] [Google Scholar]
- 21.Nebeker JR, Virmani R, Bennett CL, Hoffman JM, Samore MH, Alvarez J, Davidson CJ, McKoy JM, Raisch DW, Whisenant BK. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the research on adverse drug events and reports (RADAR) project. J Am Coll Cardiol. 2006;47(1):175–181. doi: 10.1016/j.jacc.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 22.Churchill EN, Disatnik M-H, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of ɛPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46(2):278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, Song Z, Yang G-P, Zhang B-K, Chen M, Wu T, Guo R. The ALDH2 rs671 polymorphism affects post-stroke epilepsy susceptibility and plasma 4-HNE levels. PLoS One. 2014;9(10):e109634. doi: 10.1371/journal.pone.0109634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X-J, Liu B, Ma Q-L, Peng J. Mitochondrial aldehyde dehydrogenase, a potential drug target for protection of heart and brain from ischemia/reperfusion injury. Curr Drug Targets. 2014;15(10):948–955. [PubMed] [Google Scholar]
- 25.Chen L, Zhou J-P, Kuang D-B, Tang J, Li Y-J, Chen X-P. 4-HNE increases intracellular ADMA levels in cultured HUVECs: evidence for miR-21-dependent mechanisms. PLoS One. 2013;8(5):e64148. doi: 10.1371/journal.pone.0064148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino J, Siegel N, Leimgruber R, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84(5):1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18(14):3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virmani R, Kolodgie FD, Yahagi K. Epidemiology and pathophysiology of coronary artery disease. In: Practical Interventional Cardiology. US: CRC Press; 2017. p. 3–15.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical information of all patients in this study. (XLSX 90 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article Additional file 1.