Abstract
Objective
GAANTRY (Gene Assembly in Agrobacterium by Nucleic acid Transfer using Recombinase technologY) is a flexible and effective system for stably stacking multiple genes within an Agrobacterium virulence plasmid Transfer-DNA (T-DNA). We examined the ability of the GAANTRY Agrobacterium rhizogenes ArPORT1 ‘10-stack’ strain to generate transgenic potato plants.
Results
The 28.5 kilobase 10-stack T-DNA, was introduced into Lenape potato plants with a 32% transformation efficiency. Molecular and phenotypic characterization confirmed that six of the seven tested independent transgenic lines carried the entire desired construct, demonstrating that the GAANTRY 10-stack strain can be used can be used in a tissue culture-based callus transformation method to efficiently generate transgenic potato plants. Analysis using droplet digital PCR showed that most of the characterized events carry one or two copies of the 10-stack transgenes and that ‘backbone’ DNA from outside of the T-DNA was absent in the transgenic plants. These results demonstrate that the GAANTRY system efficiently generates high quality transgenic potato plants with a large construct of stacked transgenes.
Electronic supplementary material
The online version of this article (10.1186/s13104-019-4493-8) contains supplementary material, which is available to authorized users.
Keywords: GAANTRY, Gene-stacking, Site-specific recombinase, Solanum tuberosum
Introduction
Agrobacterium are soil microbes that have been harnessed for their ability to transfer DNA into plant cells [1, 2]. This technique has revolutionized agriculture, providing a way to identify and test gene functions and to transfer superior trait genes into crops without the added and often unwanted genes that come along during breeding schemes. An important aspect associated with the transfer of DNA into plants is the stability of the insertion event. Agrobacterium T-DNAs can sometimes be incomplete or concatenated repeats [3], exhibit genetic instability or gene silencing [4]. Agrobacterium-mediated transformation of plants with one or a few genes is relatively routine, but the assembly and transformation of large constructs carrying multiple genes and their efficient use to generate high-quality transgenic plants has been a challenge.
Previous research developed the GAANTRY (Gene Assembly in Agrobacterium by Nucleic acid Transfer using Recombinase technologY) system for transgene stacking [5]. The system is based on the combined use of unidirectional integration and excision controlled by three site-specific serine recombinases and has been shown to be an effective and stable system for stacking multiple genes within an Agrobacterium virulence plasmid T-DNA [5, 6]. The gene stacking system utilizes easy-to-handle ‘P Donor’ and ‘B Donor’ cloning vectors for the insertion of sequences of interest. The P and B Donor vectors contain either attP or attB recognition sites enabling precise integration into the GAANTRY ArPORT1 strain. The resulting Agrobacterium strain can then be directly used for plant transformation. The gene stacking strategy is efficient, precise, modular, and allows control over of the orientation and order in which genes are stacked within the T-DNA. The stacking process was previously demonstrated to successfully assemble a 28.5 kb 10-stack T-DNA construct containing ten cargo sequences, including eight transgenes that confer functional phenotypes in transgenic Arabidopsis plants [5]. The 10 stack T-DNA contains eight transcriptional units that confer functional phenotypes and one cargo (inserted twice) was the TBS [7] insulator sequence that blocks interactions between promoters and nearby enhancers. The transcriptional units included the sul1 [8], firefly luciferase [9], eGFP [10], bar [11], uidA [12], CsMybA [13], tdTomato [14], and nptII [15] genes. The 10-stack construct was shown to produce high quality events that contain low copies of a complete T-DNA with rare incorporation of vector ‘backbone’ sequence in model plant Arabidopsis [5].
We wanted to examine the ability of the GAANTRY ArPORT1 10-stack strain to produce stable clean single copy transgenic events in the important crop species of potato. Potato is a member of the Solanaceae family, which contains multiple other crop species and utilizes a tissue culture-based callus transformation method, which is distinctly different than the floral dip transformation method used for Arabidopsis [5].
We present the utilization of the GAANTRY transgene stacking system to produce genetically engineered Lenape potato plants carrying the 28.5 kb ‘10-stack’ T-DNA. Multiple independent transgenic events were produced, phenotypically and molecularly characterized. Our analysis evaluated both the fidelity and completeness of T-DNA integration, and the copy number of the inserted sequences within the potato genome.
Main text
Results and discussion
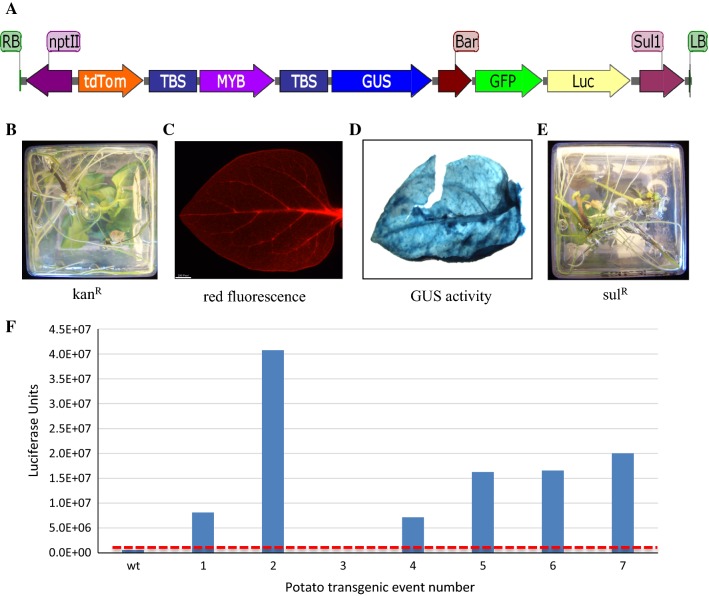
The GAANTRY ArPORT1 10-stack strain was previously assembled and validated [5]. Details and the GenBank accessions for the donor plasmids carrying the promoter and gene sequences assembled within the 10-stack assembly are described in Additional file 2: Table S1. A simplified diagram of the 28.5 kb 10-stack T-DNA is shown in Fig. 1a.
Fig. 1.
Diagram of the GAANTRY 10-stack construct and phenotypes in transgenic potato plants. a Schematic representation of the GAANTRY 10 stack T-DNA. b Transgenic potato shoots rooting in media containing 100 mg/L kanamycin (observed from the bottom of the magenta box). c Red fluorescence observed in a transgenic potato leaf. d Leaf GUS activity. e Transgenic potato shoots rooting in media containing 25 mg/L sulfadiazine. f Firefly luciferase activity measured in wildtype and seven 10-stack transgenic potato plants. The red dashed line marks the level of background luminescence detectable in wildtype potato leaf samples. Wildtype potato plants do not root on kanamycin or sulfadiazine media and do not exhibit detectable red fluorescence or GUS activity under these assay conditions
The 10-stack GAANTRY strain was used to transform potato B5141-6 (cv. Lenape) in two independent transformation experiments. A total of 86 internodes were co-cultivated with the ArPORT1 10-stack strain. A total of 28 shoots regenerated and rooted under kanamycin selection. Resistance to kanamycin is conferred by the nptII transgene, which is located adjacent to the right border (RB) of the T-DNA (Fig. 1a). Nodal cuttings from the rooted kanamycin resistant plants were excised and rooted on media containing the sulfadiazine selection agent (resistance is conferred by sul1, located adjacent to the Left Border (LB) of the T-DNA). Twenty-five plants survived and rooted in the presence of sulfadiazine, indicating the presence and expression of the sul1 resistance gene in 89% of the transgenic plants that were recovered. The overall transformation rate based on kanamycin selection was 32.6% and it was 29.1% if both kanamycin and sulfadiazine resistance was required. Ten randomly selected kanamycin-resistant independent T0 transgenic events were transferred to soil and grown in the greenhouse. Seven of the plants successfully grew to maturity and were further characterized.
The selection marker and reporter gene phenotypes of these seven transgenic potato plants were further examined. All seven plants exhibited resistance to kanamycin in tissue culture media (Fig. 1b) and produced leaves with detectable red fluorescence (Fig. 1c). Six of the events (85%) were positive for β-glucuronidase histochemical staining of leaves (Fig. 1d) and these same six events exhibited resistance to sulfadiazine by successfully rooting on sulfadiazine containing tissue culture media (Fig. 1e), but line 3 was sensitive to sulfadiazine and did not root. Luciferase enzyme activity was detected in in leaf protein extracts from six of the events, but line 3 lacked detectable activity (Fig. 1f).
The presence of other portions of the 10-stack T-DNA was examined using PCR amplification of the transgene sequences using genomic DNA isolated from each transgenic event as template. The analysis illustrates that the mybA and uidA transgenes were detected in all of the seven tested lines, while the bar and eGPF transgenes were detected in six of the tested events, but not within line 3 (Fig. 2).
Fig. 2.
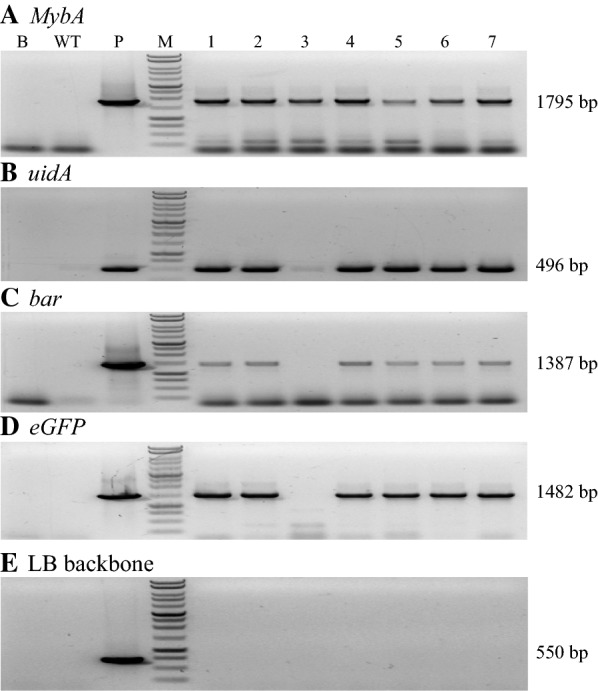
Genomic PCR screening of the 10-stack transgenic potato plants. Seven 10-stack potato transgenic events along with water (blank; B) and wildtype (WT) negative controls, and A. rhizogenes ArPORT1 10-stack genomic DNA (positive control; P) were analyzed for the presence of the mybA (a), uidA (b), bar (c), and eGFP genes (d). e The presence of LB ‘backbone’ sequence was also examined using primers that detect sequences outside of the T-DNA left border. The expected sizes each of the PCR amplicons are shown on the right of each panel
The nptII transgene copy number was measured for the seven selected transgenic events using droplet digital PCR [16]. Five of these events (71%) carried a single copy of the nptII transgene, while two events (29%) carried two copies (Additional file 1: Fig. S1). The transgene copy number for the sul1 gene from these seven events was also determined, and the results show that four of the plants (57%) were single copy, two carried two copies (29%) and one event (line #3; 14%) lacked the sul1 transgene (Additional file 1: Fig. S1).
The presence of sequences from outside of the T-DNA construct was also examined using genomic PCR screening. The analysis did not detect the presence of ‘backbone’ sequence from outside of the left border region in any of the seven transgenic lines, despite the fact that two of these lines contained two copies of sul1 gene located adjacent to the Left Border of the T-DNA (Fig. 2e and Additional file 1: Fig. S1).
Taken together, these results suggest that four of the seven lines likely carried a single complete copy of the 28.5 kb 10-stack T-DNA construct. Two of the independent events appeared to carry two complete T-DNA copies, and a single event (line #3) contains only a partial T-DNA integration which lacks the sul1, luciferase, eGFP, and bar transgenes.
Together these results indicate that 4 out of 7 (57%) of the transgenic lines likely carry an intact, backbone free, single copy T-DNA integration demonstrating that the GAANTRY system can be used in tissue culture-based transformation method for the production of transgenic potato with stacked gene constructs.
Rates of potato transformation for this study appeared to be somewhat lower than what we typically observe for potato transformation. This may be due to the larger size of the 10-stack T-DNA and/or characteristics of the ArPORT1 strain in potato transformation. Both the high rate of single copy transgenic production (57% of the tested lines) and the lower rates of transformation may be because the GAANTRY T-DNA is launched directly from the low copy virulence plasmid, rather than a higher copy binary vector. The study by Oltmanns et al. [17] launched the T-DNA from the picA locus of the Agrobacterium tumefaciens chromosome and also recovered a lower transformation rate than observed for a binary vector. The lower transformation rate of the GAANTRY system is not a significant problem, since multiple high-quality transgenic events were recovered within the small population of seven transgenic events that were analyzed.
Genetic engineering offers a powerful way to introduce or modify complex traits within crop plants. The GAANTRY system enables the assembly and maintenance of multi-gene stacked constructs and can produce high quality transgenic events with low copy number transgene integrations that are free of sequences outside of the T-DNA in both Arabidopsis [5], and potato. Thus, the ArPORT1 10-stack GAANTRY strain provides an effective technique to generate and efficiently introduce multiple genes into crop plants like potato and suggests that the GAANTRY system will likely be useful in other Solanaceous plants as well.
Methodology
Potato (Solanum tuberosum) B5141-6, the variety formerly known as Lenape [18] were maintained in a tissue culture chamber at 23 °C, 16 h light and grown in a greenhouse in Sunshine Mix #1 (Sungro Horticulture). Plant material was micropropagated in tissue culture on Shoot Media (Table 1) from excised 1 or 2 node segments (1 cm in size) when plantlets reached approximately 10 cm in height, or every 6–8 weeks.
Table 1.
Potato culture media
| Mediaa | SH | CC | Stage | ||
|---|---|---|---|---|---|
| I | II | III | |||
| MSb with vitamins (g/L) | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 |
| Sucrose (g/L) | 30 | 20 | 20 | 20 | 30 |
| pHc | 5.6 | 5.4 | 5.6 | 5.6 | 5.6 |
| GA3 (mg/L) | – | – | – | 10 | – |
| ZRd (mg/L) | – | 2 | 4 | 4 | – |
| NAAe (mg/L) | – | 0.1 | 0.1 | – | – |
| Kanamycinf (mg/L) | – | – | 100 | 100 | 100 |
| Sulfadiazineg (mg/L) | – | – | – | – | 25 |
| Carbenicillinh (mg/L) | – | – | 500 | 500 | 500 |
aSH: shoot maintenance; CC: co-cultivation; Stage I: callus induction; II: shoot induction; III: root induction
bMS: Murashige and Skoog [20] with Vitamins (M404, PhytoTechnology Laboratories). GA3: Gibberellic Acid 10 mg/ml stock in ethanol [0.29 mM]; (G745, Sigma-Aldrich) 0.29 μM final concentration
cThe pH is adjusted to 5.4 or 5.6 with 0.1 N potassium hydroxide before addition of 0.2% Gelzan CM (G3251, PhytoTechnology Laboratories) for solidification
dZR: Zeatin Riboside 1 mg/ml stock [4.56 mM] (Z875, PhytoTechnology Laboratories); final concentrations 9.1–18.2 μM
eNAA: 1-napthalene acetic acid (1 mg/ml stock, [5.4 mM] (N605, PhytoTechnology Laboratories); 0.54 μM final concentration
fKanamycin 50 mg/ml stock; (K378, PhytoTechnology Laboratories) 100 μg/ml final concentration
gSulfadiazine 25 mg/ml stock; 25 μg/ml final concentration
hCarbenicillin (C346, PhytoTechnology Laboratories) 500 μg/ml final concentration. Cefotaxime, timentin or vancomycin can be added to Stage I and II in addition to Carbenicillin as required for aggressive Agrobacterium strain
Agrobacterium rhizogenes 10-stack strain
The Agrobacterium rhizogenes strain ArPORT1, harboring the 10-stack T-DNA contains the following 8 genes which confer functional phenotypes in plants: sul1 [8] (sulfadiazine resistance), firefly luciferase [9] (luminescence), eGFP [10] (green fluorescence), bar [11] (herbicide resistance), uidA [12] (β-glucuronidase ‘GUS’ activity), CsMybA [13] (anthocyanin accumulation), tdTomato [14] (red fluorescence), and nptII [15] (kanamycin resistance). The promoters and terminators controlling transgene expression and the GenBank accessions for the donor vectors carrying these cargo sequences are shown in Additional file 2: Table S1 as previously described [5].
Transformation and regeneration of transgenic potato plants
Potato transformation, selection and regeneration was conducted using a modified version of a previously described method [19]. Overnight Agrobacterium cultures were grown in a shaking incubator at 28 °C in Luria–Bertani (LB) medium containing 100 mg/l kanamycin. Cultures are washed twice and re-suspended in co-cultivation (CC) media (without Gelzan or hormones) to an OD600 of 0.2 AU. 1 cm long internode segments from 6 to 8 weeks old plantlets were excised and submerged in the Agrobacterium suspension and gently shaken for 20 min at 28 °C. Segments were rinsed with liquid CC media, blotted dry on sterile filter paper and placed on CC media for 2 days. Segments were transferred to Stage I media with weekly transfers. After 4 weeks segments were transferred to Stage II media with biweekly transfers. Shoots were transferred to Stage III media when they appeared, usually during weeks 4–6. Shoots that rooted in stage III were re-rooted in Stage III media to minimize non-transgenic escape plants. Rooted shoots were then transferred to soil, hardened off for 1–2 weeks and then planted in the greenhouse for the production of mini-tubers. See Table 1 for media recipes.
Genomic DNA isolation and PCR
Genomic potato DNA was isolated using the Gentra PureGene DNA isolation kit (Qiagen). Droplet digital PCR was performed as previously described [16]. End-point PCR and droplet digital PCR amplification reactions were performed with the primers and probes shown in Additional file 3: Table S2.
Detecting β-glucuronidase (GUS) activity
Lines were sampled and histochemically assayed for β-glucuronidase activity as previously described [12].
Luciferase activity
Luminescence was analyzed using the Dual Luciferase Assay Kit (Promega). The manufacture’s protocol was followed, except a Luciferase Lysis Buffer (100 mM potassium phosphate; 1 mM EDTA; 10% glycerol; 1% Triton X; pH 7.8) was used for plant tissue extraction.
Detection of red fluorescence
Red fluorescence was examined using a Leica MZ16FA dissecting microscope with a 590–650 nm bandpass filter. The excitation light provided was from 510 to 560 nm.
Limitations
Transformation efficiency appeared lower than is typical for potato transformation with a binary vector containing a small (~ 8 kb) T-DNA. Whether this reduced transformation efficiency was due to the larger size of the 10-stack T-DNA or an attribute of the Agrobacterium rhizogenes ArPORT1 strain was not determined.
Additional files
Additional file 1: Fig. S1. Transgene copy number measurements in seven potato 10-stack events.
Additional file 2: Table S1. 10-stack cargo sequences in Donor plasmids.
Additional file 3: Table S2. Primers and probes used for transgene detection.
Acknowledgements
The authors would like to thank all study participants for their contribution in success of this work.
Abbreviations
- GAANTRY
Gene Assembly in Agrobacterium by Nucleic acid Transfer using Recombinase technologY
- ArPORT1
Agrobacterium rhizogenes strain that contains the genetic components required for GAANTRY gene stacking within the virulence plasmid. Can be used directly for plant transformation
Authors’ contributions
JT and RT conceived the project, analyzed and interpreted the data and drafted the manuscript. KM, RC and EG performed the research, collected and analyzed project data. All authors read and approved the final manuscript.
Funding
This work was supported by the USDA Agricultural Research Service CRIS project 2030-21220-002-00-D. This funding agency had no involvement in the study design, collection, analysis, interpretation of data nor in the writing the manuscript.
Availability of data and materials
All data and material are available upon request. Mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Ethics approval and consent to participate
Not applicable. The current research did not involve human subjects, human material, or human data or animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev. 2003;67:16–37. doi: 10.1128/MMBR.67.1.16-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vain P. Thirty years of plant transformation technology development. Plant Biotechnol J. 2007;5:221–229. doi: 10.1111/j.1467-7652.2006.00225.x. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen R, Snyder C, Jones JDG. T-DNA is organized predominantly in inverted repeat structures in plants transformed with Agrobacterium tumefaciens C58 derivatives. Mol Gen Genet. 1987;207:471–477. doi: 10.1007/BF00331617. [DOI] [Google Scholar]
- 4.Jude F, Rivkin AC, Michael TP, Zander M, Motley ST, Sandoval JP, Slotkin SK, Chen H, Castanon R, Nery JR, Ecker JR. The complex architecture and epigenomic impact of plant T-DNA insertions. PLoS Genet. 2018;15(1):e1007819. doi: 10.1371/journal.pgen.1007819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier R, Thomson JG, Thilmony R. A versatile and robust Agrobacterium-based gene stacking system generates high quality transgenic plants. Plant J. 2018 doi: 10.1111/tpj.13992. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Yau Y-Y, Perkins-Balding D, Thomson JG. Recombinase technology: applications and possibilities. Plant Cell Rep. 2011;30:267–285. doi: 10.1007/s00299-010-0938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hily JM, Singer SD, Yang Y, Liu Z. A transformation booster sequence (TBS) from Petunia hybrida functions as an enhancer-blocking insulator in Arabidopsis thaliana. Plant Cell Rep. 2009;28(7):1095–1104. doi: 10.1007/s00299-009-0700-8. [DOI] [PubMed] [Google Scholar]
- 8.Thomson JG, Cook M, Guttman M, Smith J, Thilmony R. Novel sulI binary vectors enable an inexpensive foliar selection method in Arabidopsis. BMC Res Notes. 2011;4:44. doi: 10.1186/1756-0500-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ow DW, de Wet JR, Helinski DR, Howell SH, Wood KV, Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- 10.Leffel SM, Mabon SA, Stewart CN., Jr Applications of green fluorescent protein in plants. Biotechniques. 1997;23:912–918. doi: 10.2144/97235bi01. [DOI] [PubMed] [Google Scholar]
- 11.Rathore KS, Chowdhury VK, Hodges TK. Use of bar as a selectable marker gene and for the production of herbicide-resistant rice plants from protoplasts. Plant Mol Biol. 1993;21:871–884. doi: 10.1007/BF00027118. [DOI] [PubMed] [Google Scholar]
- 12.Jefferson R. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. doi: 10.1007/BF02667740. [DOI] [Google Scholar]
- 13.Dasgupta K, Thilmony R, Stover E, Oliveria ML, Thomson JG. Novel R2R3-MYB transcription factors from Prunus americana regulate differential patterns of anthocyanin accumulation in tobacco and citrus. GM Crops Food. 2017;8(2):85–105. doi: 10.1080/21645698.2016.1267897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann DG, Abercrombie LL, Rudis MR, Millwood RJ, Dunlap JR, Stewart CN., Jr Very bright orange fluorescent plants: endoplasmic reticulum targeting of orange fluorescent proteins as visual reporters in transgenic plants. BMC Biotechnol. 2012;12:17. doi: 10.1186/1472-6750-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trieu-Cuot P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5′-aminoglycoside phosphotransferase type III. Gene. 1983;23:331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- 16.Collier R, et al. Accurate measurement of transgene copy number in crop plants using droplet digital PCR. Plant J. 2017;90:1014–1025. doi: 10.1111/tpj.13517. [DOI] [PubMed] [Google Scholar]
- 17.Oltmanns H, Frame B, Lee LY, Johnson S, Li B, Wang K, Gelvin SB. Generation of backbone-free, low transgene copy plants by launching T-DNA from the Agrobacterium chromosome. Plant Physiol. 2010;152:1158–1166. doi: 10.1104/pp.109.148585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zitnack A, Johnston GR. Glycoalkaloid content of B5141-6 potatoes. Am Potato J. 1970;47:256–260. doi: 10.1007/BF02864825. [DOI] [Google Scholar]
- 19.Synder GW, Belknap WR. A modified method for routine Agrobacterium-mediated transformation of in vitro grown potato microtubers. Plant Cell Rep. 1993;12(6):324–327. doi: 10.1007/BF00237428. [DOI] [PubMed] [Google Scholar]
- 20.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1963;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Transgene copy number measurements in seven potato 10-stack events.
Additional file 2: Table S1. 10-stack cargo sequences in Donor plasmids.
Additional file 3: Table S2. Primers and probes used for transgene detection.
Data Availability Statement
All data and material are available upon request. Mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.