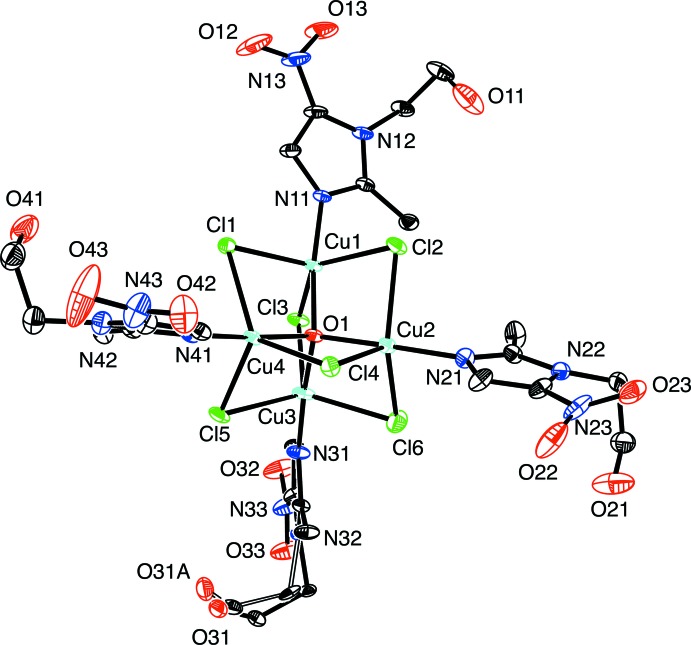
The title tetranuclear cluster contains a tetrahedral arrangement of copper(II) ions bonded to a central oxygen atom. The extended structure shows short O⋯N interactions between the nitro groups of adjacent clusters, which are oriented perpendicular to each other in a manner that has previously been described as an ONO2⋯π(N)NO2 interaction.
Keywords: crystal structure, tetranuclear copper, metronidazole, bridging chloride, NO2 Interactions
Abstract
The title tetranuclear copper complex, [Cu4Cl6O(C6H9N3O3)4] or [Cu4Cl6O(MET)4] [MET is 1-(2-hydroxyethyl)-2-methyl-5-nitro-1H-imidazole or metronidazole], contains a tetrahedral arrangement of copper(II) ions. Each copper atom is also linked to the other three copper atoms in the tetrahedron via bridging chloride ions. A fifth coordination position on each metal atom is occupied by a nitrogen atom of the monodentate MET ligand. The result is a distorted CuCl3NO trigonal–bipyramidal coordination polyhedron with the axial positions occupied by oxygen and nitrogen atoms. The extended structure displays O—H⋯O hydrogen bonding, as well as unusual short O⋯N interactions [2.775 (4) Å] between the nitro groups of adjacent clusters that are oriented perpendicular to each other. The scattering contribution of disordered water and methanol solvent molecules was removed using the SQUEEZE procedure [Spek (2015 ▸). Acta Cryst. C71, 9–16] in PLATON [Spek (2009 ▸). Acta Cryst. D65, 148–155].
Chemical context
Metronidazole (C6H9N3O3; MET) is a medication that was discovered to be effective against both bacteria and parasites more than 50 years ago (Samuelson, 1999 ▸). MET is currently incorporated in the World Health Organization (WHO) list of essential medicines, i.e. medications that are considered to be effective and safe to meet the most important needs in a health system (WHO, 2015 ▸). Despite the widespread use of MET as a drug, relatively little structural data concerning its interactions with metal ions exist, and there are few structurally characterized copper compounds of MET (Galván-Tejada et al., 2002 ▸; Barba-Behrens et al., 1991 ▸; Athar et al., 2005 ▸; Ratajczak-Sitarz et al., 1998 ▸; Bharti et al., 2002 ▸). Our recent work has sought to develop further metal–MET chemistry and we have reported structures containing Cu (Palmer et al., 2015 ▸; Quinlivan & Upmacis, 2016 ▸), as well as Ag (Palmer & Upmacis, 2015 ▸) and Au (Quinlivan et al., 2015 ▸). Tetranuclear copper(II) compounds of the form [Cu4OX
6
L
4] are relatively well known, with the first example described in 1996 (Bertrand & Kelley, 1966 ▸). In this regard, although the structure of a [Cu4OX
6
L
4] structure, where L = imidazole, has been previously described (Atria et al., 1999 ▸), a counterpart containing L = MET has not been reported. Herein, we describe the structure of a tetranuclear Cu–MET complex [Cu4Cl6O(MET)4] that is obtained by the reaction of anhydrous copper(I) chloride with MET in MeOH under aerobic conditions.
Structural commentary
The structure of the [Cu4Cl6O(MET)4] complex is shown in Fig. 1 ▸. Four copper atoms are arranged around an oxygen atom in a tetrahedral fashion, with Cu—O distances ranging from 1.8960 (18) to 1.913 (2) Å. The Cu—O—Cu angles range from 108.36 (10) to 110.80 (9)°, indicating a fairly uniform tetrahedron with little distortion. In fact, the degree of distortion from a tetrahedral arrangement can be readily quantified by the τ4 four-coordinate geometry index that is reported and discussed elsewhere (Yang et al., 2007 ▸; Palmer et al., 2015 ▸, Brescia et al., 2018 ▸). Briefly, τ4 is obtained from the expression, τ4 = [360 − (α + β)]/141, where α and β represent the two largest angles; a τ4 value of 1.00 indicates an idealized tetrahedral geometry, whereas a value of 0.00 indicates an idealized square-planar geometry. In the title complex, α = 110.80 (9)° and β = 109.55 (9)°, such that τ4 is 0.990, which indicates negligible deviation from a tetrahedral geometry for oxygen (Yang et al., 2007 ▸).
Figure 1.
The molecular structure of [Cu4Cl6O(MET)4]. For clarity, hydrogen atoms have been omitted. The ethoxy group of the MET ligand attached to Cu3 (comprising C34, C35 and O31) is disordered over two sets of sites in a 0.515 (19):0.485 (19) ratio.
Each of the four copper atoms is linked to the other three copper atoms via three chloride bridges, with the Cu—Cl bridging distances varying from 2.3579 (10) to 2.4435 (9) Å (for Cu2—Cl6 and Cu1—Cl2, respectively). Each copper atom is also bound to a nitrogen atom of a MET ligand. The Cu—N lengths range from 1.949 (2) to 1.972 (3) Å (for Cu1—N11 and Cu4—N41, respectively). Thus, each copper atom sits within a trigonal–bipyramidal arrangement, with the oxygen and nitrogen atoms forming the axial coordination points, and the bridging chloride ligands occupying the equatorial plane. The trigonal–bipyramidal structure is somewhat distorted, as indicated by the fact that the O—Cu—N angles are less than 180°, ranging from 173.12 (10) to 176.91 (10)° (for O1—Cu1—N11 and O1—Cu2—N21, respectively), and the Cl—Cu—Cl angles differ significantly from 120°, ranging from 109.97 (3) to 134.02 (3)° (for Cl2—Cu2—Cl4 and Cl3—Cu1—Cl2, respectively). Furthermore, the O—Cu—Cl angles are all less than 90°, ranging from 83.33 (6) to 86.13 (6)° (for O1—Cu1—Cl2 and O1—Cu—Cl1, respectively), indicating that the equatorial chloride ligands are displaced slightly more towards the axial oxygen atom in the center of the molecule, than towards the nitrogen-containing ligand in the opposite axial position.
The τ5 geometry index is a general descriptor of five-coordinate molecules and provides a way to determine the extent of distortion of a molecule from trigonal bipyramidal to square pyramidal (Addison et al., 1984 ▸). The τ5 geometry index is calculated by using the equation: τ5 = (β − α)/60, where β − α is the difference between the two largest angles (Addison et al., 1984 ▸; Palmer & Parkin, 2014 ▸). The values for τ5 are calculated to be 0.65 (Cu1), 0.74 (Cu2), 0.84 (Cu3) and 0.73 (Cu4) for the five-coordinate copper centers, giving an average τ5 value of 0.74. The τ5 values obtained indicate that the copper-centered structures are closer to an idealized trigonal–bipyramidal (1.00) than a square-pyramidal geometry (0.00).
Supramolecular features
Fig. 2 ▸ shows the packing in the unit cell. As well as the O—H⋯O hydrogen bonds shown in Table 1 ▸, O11—H11A and O21—H21A probably form links to the disordered solvent molecules removed with SQUEEZE (see Experimental). The most interesting observation is the existence of short O⋯N interactions between the N13/O12/O13 and N33/O32/O33 nitro groups of adjacent clusters that are oriented perpendicular to each other, as illustrated in Fig. 3 ▸ with O12⋯N33 = 2.775 (4) Å. This type of contact has previously been described as an ONO2⋯π(N)NO2 interaction (Daszkiewicz, 2013 ▸); such contacts are typically shorter than 3 Å.
Figure 2.
Unit-cell packing of [Cu4Cl6O(MET)4] viewed down [100].
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O41—H41A⋯O31i | 0.89 (2) | 2.13 (3) | 2.738 (8) | 125 (2) |
Symmetry code: (i)  .
.
Figure 3.
Detail of the O⋯N interaction between the nitro groups of adjacent clusters.
Database survey
The tetranuclear copper motif, L 4Cu4Cl6O, where L is a nitrogen-containing Lewis base ligand, is common. For instance, several structures have been reported in which L contains either an imidazole or substituted imidazole moiety (Clegg et al., 1988 ▸; Norman et al., 1989 ▸ Erdonmez et al., 1990 ▸; Atria et al., 1999 ▸; Cortés et al., 2006 ▸; Chiarella et al., 2009 ▸, 2010 ▸; She et al., 2010 ▸) or a benzimidazole moiety (Tosik et al., 1991 ▸ Zhang et al., 2003 ▸; Jian et al., 2004 ▸; Li et al., 2011 ▸).
The title compound [Cu4Cl6O(MET)4] contains Cu—X distances that are similar to those in [Cu4Cl6O(imidazole)4] (Atria et al., 1999 ▸). For example, the Cu—O distances in [Cu4Cl6O(MET)4] are 1.8960 (18)–1.913 (2) Å, compared to 1.903 (4)–1.924 (4) Å for [Cu4Cl6O(imidazole)4]. Likewise, the Cu—Cl distances in [Cu4Cl6O(MET)4] are 2.3579 (10)–2.4435 (9) Å, compared to 2.374 (2)–2.564 (2) Å for [Cu4Cl6O(imidazole)4]. Moreover, the Cu—N distances in [Cu4Cl6O(MET)4] are 1.949 (2)–1.972 (3) Å, compared to 1.934 (6)–1.961 (6) Å.
Synthesis and crystallization
Anhydrous copper(I) chloride (0.015 g, 0.00015 mol) was mixed with MET (0.05075 g, 0.00030 mol) in methanol (2 ml) in a glass vial, forming a dark olive-colored solution. After allowing the solution to evaporate for eight days, gold-colored plates, suitable for X-ray diffraction, were obtained.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. Hydrogen atoms on carbon were placed in calculated positions (C—H = 0.95–1.00 Å) and included as riding contributions with isotropic displacement parameters U iso(H) = 1.2U eq(Csp 2) or 1.5U eq(Csp 3). Atoms C34, C35 and O31 and their attached H atoms were modeled as disordered over two sets of sites in a 0.515 (19):0.485 (19) ratio. The structure contains two methanol molecules and one water molecule, but they are disordered and were removed by the SQUEEZE procedure in PLATON (Spek, 2015 ▸); the stated crystal data (M r, μ, etc.) only refer to the main molecule.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Cu4Cl6O(C6H9N3O3)4] |
| M r | 1167.51 |
| Crystal system, space group | Monoclinic, C2/c |
| Temperature (K) | 130 |
| a, b, c (Å) | 22.125 (3), 13.361 (2), 32.633 (5) |
| β (°) | 94.752 (2) |
| V (Å3) | 9613 (3) |
| Z | 8 |
| Radiation type | Mo Kα |
| μ (mm−1) | 2.14 |
| Crystal size (mm) | 0.36 × 0.20 × 0.10 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2008 ▸) |
| T min, T max | 0.586, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 78050, 15003, 11100 |
| R int | 0.048 |
| (sin θ/λ)max (Å−1) | 0.720 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.045, 0.118, 1.03 |
| No. of reflections | 15003 |
| No. of parameters | 579 |
| No. of restraints | 120 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 1.55, −1.09 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989019008570/hb7801sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989019008570/hb7801Isup2.hkl
CCDC reference: 1923275
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
RKU thanks Pace University for research support. Gerard Parkin (Columbia University) is thanked for helpful discussions.
supplementary crystallographic information
Crystal data
| [Cu4Cl6O(C6H9N3O3)4] | F(000) = 4688 |
| Mr = 1167.51 | Dx = 1.613 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 22.125 (3) Å | Cell parameters from 9836 reflections |
| b = 13.361 (2) Å | θ = 2.2–29.8° |
| c = 32.633 (5) Å | µ = 2.14 mm−1 |
| β = 94.752 (2)° | T = 130 K |
| V = 9613 (3) Å3 | Plate, gold |
| Z = 8 | 0.36 × 0.20 × 0.10 mm |
Data collection
| Bruker APEXII CCD diffractometer | 11100 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.048 |
| Absorption correction: multi-scan (SADABS; Bruker, 2008) | θmax = 30.8°, θmin = 1.3° |
| Tmin = 0.586, Tmax = 0.746 | h = −31→31 |
| 78050 measured reflections | k = −19→19 |
| 15003 independent reflections | l = −46→46 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.045 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.118 | w = 1/[σ2(Fo2) + (0.0497P)2 + 31.4385P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.002 |
| 15003 reflections | Δρmax = 1.55 e Å−3 |
| 579 parameters | Δρmin = −1.09 e Å−3 |
| 120 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Cu1 | 0.80290 (2) | −0.01300 (3) | 0.39169 (2) | 0.02214 (8) | |
| Cu2 | 0.70660 (2) | −0.01915 (3) | 0.31768 (2) | 0.02614 (8) | |
| Cu3 | 0.66594 (2) | 0.02107 (3) | 0.40449 (2) | 0.03036 (9) | |
| Cu4 | 0.71326 (2) | −0.19124 (3) | 0.38380 (2) | 0.02342 (8) | |
| Cl1 | 0.81567 (3) | −0.18214 (5) | 0.41707 (2) | 0.02840 (14) | |
| Cl2 | 0.81362 (3) | −0.00755 (7) | 0.31780 (2) | 0.03451 (17) | |
| Cl3 | 0.75591 (3) | 0.09683 (6) | 0.43943 (2) | 0.03046 (16) | |
| Cl4 | 0.67511 (3) | −0.19355 (6) | 0.31175 (2) | 0.03082 (15) | |
| Cl5 | 0.63812 (3) | −0.13927 (7) | 0.42920 (2) | 0.03596 (17) | |
| Cl6 | 0.63258 (5) | 0.09252 (9) | 0.33861 (3) | 0.0547 (3) | |
| N11 | 0.88549 (10) | 0.03600 (18) | 0.40341 (7) | 0.0233 (5) | |
| N12 | 0.96498 (10) | 0.13435 (19) | 0.39975 (9) | 0.0302 (5) | |
| N13 | 1.04472 (14) | 0.0232 (3) | 0.43287 (15) | 0.0646 (12) | |
| N21 | 0.68926 (12) | 0.0204 (2) | 0.26024 (8) | 0.0307 (5) | |
| N22 | 0.67466 (12) | 0.1147 (2) | 0.20463 (8) | 0.0312 (6) | |
| N23 | 0.62700 (16) | −0.0128 (3) | 0.15652 (10) | 0.0497 (9) | |
| N31 | 0.60566 (11) | 0.0980 (2) | 0.43173 (8) | 0.0316 (6) | |
| N32 | 0.51725 (10) | 0.15805 (18) | 0.44639 (7) | 0.0243 (5) | |
| N33 | 0.55277 (14) | 0.2806 (3) | 0.50054 (11) | 0.0513 (9) | |
| N41 | 0.70835 (10) | −0.33784 (19) | 0.38980 (7) | 0.0254 (5) | |
| N42 | 0.71449 (13) | −0.4914 (2) | 0.41434 (9) | 0.0355 (6) | |
| N43 | 0.71274 (19) | −0.5839 (3) | 0.34682 (11) | 0.0591 (10) | |
| O1 | 0.72212 (8) | −0.05076 (15) | 0.37460 (6) | 0.0222 (4) | |
| O11 | 0.9926 (2) | 0.1888 (4) | 0.31860 (12) | 0.0965 (15) | |
| H11A | 0.991 (3) | 0.228 (4) | 0.2983 (14) | 0.145* | |
| O12 | 1.05456 (14) | −0.0584 (3) | 0.44694 (19) | 0.125 (2) | |
| O13 | 1.08424 (12) | 0.0857 (3) | 0.43043 (14) | 0.0822 (12) | |
| O21 | 0.55815 (16) | 0.1846 (3) | 0.17440 (17) | 0.0943 (14) | |
| H21A | 0.5252 (8) | 0.206 (4) | 0.1839 (17) | 0.141* | |
| O22 | 0.59231 (17) | −0.0862 (2) | 0.15657 (10) | 0.0714 (11) | |
| O23 | 0.64160 (13) | 0.0312 (3) | 0.12553 (8) | 0.0606 (9) | |
| O32 | 0.59897 (13) | 0.3128 (3) | 0.52030 (10) | 0.0717 (11) | |
| O33 | 0.50093 (13) | 0.3067 (3) | 0.50600 (10) | 0.0683 (10) | |
| O41 | 0.81469 (16) | −0.5364 (3) | 0.47601 (15) | 0.0891 (14) | |
| H41A | 0.8525 (9) | −0.5590 (19) | 0.479 (2) | 0.134* | |
| O42 | 0.70514 (17) | −0.5744 (2) | 0.30998 (9) | 0.0643 (9) | |
| O43 | 0.7238 (3) | −0.6628 (3) | 0.36399 (13) | 0.139 (2) | |
| C11 | 0.90440 (12) | 0.1254 (2) | 0.39148 (9) | 0.0261 (6) | |
| C12 | 0.93477 (13) | −0.0145 (2) | 0.42001 (11) | 0.0333 (7) | |
| H12A | 0.9349 | −0.0801 | 0.4312 | 0.040* | |
| C13 | 0.98391 (13) | 0.0457 (3) | 0.41783 (12) | 0.0380 (8) | |
| C14 | 1.00057 (14) | 0.2241 (3) | 0.39029 (12) | 0.0403 (8) | |
| H14A | 1.0302 | 0.2388 | 0.4139 | 0.048* | |
| H14B | 0.9729 | 0.2822 | 0.3864 | 0.048* | |
| C15 | 1.03387 (19) | 0.2108 (4) | 0.35240 (16) | 0.0627 (13) | |
| H15A | 1.0563 | 0.2729 | 0.3469 | 0.075* | |
| H15B | 1.0636 | 0.1557 | 0.3567 | 0.075* | |
| C16 | 0.86509 (14) | 0.2053 (3) | 0.37248 (12) | 0.0369 (7) | |
| H16A | 0.8229 | 0.1822 | 0.3698 | 0.055* | |
| H16B | 0.8780 | 0.2213 | 0.3452 | 0.055* | |
| H16C | 0.8684 | 0.2652 | 0.3899 | 0.055* | |
| C21 | 0.69669 (14) | 0.1114 (3) | 0.24465 (9) | 0.0311 (6) | |
| C22 | 0.66075 (16) | −0.0364 (3) | 0.22997 (11) | 0.0382 (8) | |
| H22A | 0.6493 | −0.1046 | 0.2323 | 0.046* | |
| C23 | 0.65153 (15) | 0.0209 (3) | 0.19587 (10) | 0.0355 (7) | |
| C24 | 0.66535 (15) | 0.2064 (3) | 0.18002 (11) | 0.0398 (8) | |
| H24A | 0.6692 | 0.1905 | 0.1507 | 0.048* | |
| H24B | 0.6972 | 0.2557 | 0.1889 | 0.048* | |
| C25 | 0.60375 (18) | 0.2517 (3) | 0.18455 (15) | 0.0514 (10) | |
| H25A | 0.6014 | 0.2739 | 0.2133 | 0.062* | |
| H25B | 0.5983 | 0.3113 | 0.1666 | 0.062* | |
| C26 | 0.7245 (2) | 0.1984 (3) | 0.26728 (12) | 0.0515 (10) | |
| H26A | 0.7510 | 0.1746 | 0.2908 | 0.077* | |
| H26B | 0.6925 | 0.2407 | 0.2771 | 0.077* | |
| H26C | 0.7483 | 0.2373 | 0.2489 | 0.077* | |
| C31 | 0.54558 (12) | 0.0912 (2) | 0.42365 (9) | 0.0247 (5) | |
| C32 | 0.61649 (13) | 0.1716 (2) | 0.46056 (9) | 0.0299 (6) | |
| H32A | 0.6552 | 0.1934 | 0.4719 | 0.036* | |
| C33 | 0.56247 (14) | 0.2077 (2) | 0.47002 (10) | 0.0308 (6) | |
| C34 | 0.4518 (4) | 0.1791 (10) | 0.4409 (4) | 0.027 (2) | 0.515 (19) |
| H34A | 0.4346 | 0.1503 | 0.4145 | 0.033* | 0.515 (19) |
| H34B | 0.4451 | 0.2523 | 0.4399 | 0.033* | 0.515 (19) |
| C35 | 0.4205 (4) | 0.1352 (8) | 0.4754 (3) | 0.034 (2) | 0.515 (19) |
| H35A | 0.4351 | 0.1686 | 0.5014 | 0.041* | 0.515 (19) |
| H35B | 0.3763 | 0.1469 | 0.4706 | 0.041* | 0.515 (19) |
| O31 | 0.4317 (4) | 0.0314 (7) | 0.4788 (3) | 0.040 (2) | 0.515 (19) |
| H31A | 0.4316 (19) | 0.012 (3) | 0.5031 (8) | 0.060* | 0.515 (19) |
| C34A | 0.4496 (4) | 0.1552 (12) | 0.4507 (5) | 0.036 (3) | 0.485 (19) |
| H34D | 0.4353 | 0.2234 | 0.4568 | 0.044* | 0.485 (19) |
| H34E | 0.4283 | 0.1335 | 0.4243 | 0.044* | 0.485 (19) |
| C35A | 0.4336 (5) | 0.0858 (14) | 0.4841 (4) | 0.053 (4) | 0.485 (19) |
| H35D | 0.4523 | 0.1103 | 0.5108 | 0.063* | 0.485 (19) |
| H35E | 0.3890 | 0.0857 | 0.4854 | 0.063* | 0.485 (19) |
| O31A | 0.4535 (6) | −0.0129 (10) | 0.4775 (2) | 0.054 (3) | 0.485 (19) |
| H31D | 0.483 (6) | −0.012 (11) | 0.489 (4) | 0.081* | 0.485 (19) |
| C36 | 0.51388 (13) | 0.0216 (3) | 0.39382 (11) | 0.0357 (7) | |
| H36A | 0.5428 | −0.0279 | 0.3850 | 0.053* | |
| H36B | 0.4966 | 0.0594 | 0.3699 | 0.053* | |
| H36C | 0.4813 | −0.0126 | 0.4068 | 0.053* | |
| C41 | 0.71482 (13) | −0.3938 (2) | 0.42409 (9) | 0.0287 (6) | |
| C42 | 0.70446 (13) | −0.4025 (2) | 0.35718 (9) | 0.0281 (6) | |
| H42A | 0.6992 | −0.3840 | 0.3290 | 0.034* | |
| C43 | 0.70926 (16) | −0.4966 (2) | 0.37164 (10) | 0.0352 (7) | |
| C44 | 0.71595 (19) | −0.5751 (3) | 0.44430 (12) | 0.0480 (9) | |
| H44A | 0.7000 | −0.5519 | 0.4701 | 0.058* | |
| H44B | 0.6897 | −0.6302 | 0.4330 | 0.058* | |
| C45 | 0.7785 (2) | −0.6121 (4) | 0.45304 (15) | 0.0602 (11) | |
| H45A | 0.7781 | −0.6746 | 0.4693 | 0.072* | |
| H45B | 0.7964 | −0.6269 | 0.4269 | 0.072* | |
| C46 | 0.71969 (17) | −0.3551 (3) | 0.46674 (10) | 0.0393 (8) | |
| H46A | 0.7583 | −0.3765 | 0.4809 | 0.059* | |
| H46B | 0.6861 | −0.3813 | 0.4813 | 0.059* | |
| H46C | 0.7179 | −0.2818 | 0.4662 | 0.059* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.01369 (14) | 0.02604 (17) | 0.02712 (17) | −0.00543 (12) | 0.00426 (12) | −0.00550 (13) |
| Cu2 | 0.02511 (17) | 0.03088 (19) | 0.02237 (16) | −0.00373 (14) | 0.00162 (13) | −0.00551 (14) |
| Cu3 | 0.01595 (15) | 0.0409 (2) | 0.0351 (2) | −0.00491 (14) | 0.00700 (13) | −0.01941 (16) |
| Cu4 | 0.01913 (15) | 0.02818 (18) | 0.02352 (16) | −0.00774 (13) | 0.00518 (12) | −0.00593 (13) |
| Cl1 | 0.0216 (3) | 0.0303 (4) | 0.0323 (3) | −0.0077 (3) | −0.0032 (3) | 0.0011 (3) |
| Cl2 | 0.0254 (3) | 0.0527 (5) | 0.0265 (3) | −0.0088 (3) | 0.0084 (3) | −0.0048 (3) |
| Cl3 | 0.0178 (3) | 0.0377 (4) | 0.0364 (4) | −0.0069 (3) | 0.0055 (3) | −0.0146 (3) |
| Cl4 | 0.0320 (3) | 0.0325 (4) | 0.0270 (3) | −0.0100 (3) | −0.0035 (3) | −0.0038 (3) |
| Cl5 | 0.0238 (3) | 0.0510 (5) | 0.0351 (4) | −0.0049 (3) | 0.0145 (3) | −0.0015 (3) |
| Cl6 | 0.0554 (6) | 0.0713 (7) | 0.0395 (5) | 0.0348 (5) | 0.0163 (4) | 0.0098 (4) |
| N11 | 0.0136 (9) | 0.0250 (12) | 0.0317 (12) | −0.0037 (8) | 0.0049 (9) | −0.0061 (9) |
| N12 | 0.0161 (10) | 0.0266 (13) | 0.0485 (16) | −0.0053 (9) | 0.0071 (10) | −0.0078 (11) |
| N13 | 0.0175 (13) | 0.049 (2) | 0.127 (4) | 0.0027 (13) | −0.0002 (17) | 0.006 (2) |
| N21 | 0.0349 (13) | 0.0325 (14) | 0.0240 (12) | −0.0034 (11) | −0.0015 (10) | −0.0082 (10) |
| N22 | 0.0273 (12) | 0.0421 (15) | 0.0240 (12) | −0.0001 (11) | 0.0020 (10) | −0.0039 (11) |
| N23 | 0.0530 (19) | 0.051 (2) | 0.0406 (17) | 0.0300 (16) | −0.0228 (15) | −0.0193 (15) |
| N31 | 0.0183 (11) | 0.0410 (15) | 0.0358 (14) | −0.0054 (10) | 0.0040 (10) | −0.0198 (12) |
| N32 | 0.0215 (11) | 0.0276 (12) | 0.0237 (11) | 0.0048 (9) | 0.0007 (9) | −0.0023 (9) |
| N33 | 0.0396 (16) | 0.055 (2) | 0.057 (2) | 0.0174 (15) | −0.0098 (14) | −0.0336 (16) |
| N41 | 0.0189 (10) | 0.0317 (13) | 0.0259 (12) | −0.0082 (9) | 0.0033 (9) | −0.0044 (10) |
| N42 | 0.0404 (15) | 0.0300 (14) | 0.0340 (14) | −0.0092 (11) | −0.0094 (12) | −0.0012 (11) |
| N43 | 0.090 (3) | 0.0332 (17) | 0.050 (2) | 0.0034 (17) | −0.0176 (19) | −0.0105 (15) |
| O1 | 0.0163 (8) | 0.0279 (10) | 0.0227 (9) | −0.0040 (7) | 0.0036 (7) | −0.0080 (8) |
| O11 | 0.085 (3) | 0.148 (4) | 0.061 (2) | −0.048 (3) | 0.031 (2) | −0.016 (2) |
| O12 | 0.0294 (16) | 0.068 (2) | 0.273 (7) | 0.0062 (16) | −0.022 (3) | 0.061 (3) |
| O13 | 0.0176 (12) | 0.066 (2) | 0.161 (4) | −0.0098 (13) | −0.0059 (17) | 0.005 (2) |
| O21 | 0.0401 (18) | 0.073 (3) | 0.168 (4) | −0.0018 (17) | −0.004 (2) | −0.021 (3) |
| O22 | 0.097 (3) | 0.0370 (16) | 0.070 (2) | 0.0127 (16) | −0.0530 (19) | −0.0170 (14) |
| O23 | 0.0467 (16) | 0.104 (3) | 0.0293 (13) | 0.0229 (16) | −0.0096 (11) | −0.0139 (15) |
| O32 | 0.0461 (16) | 0.088 (2) | 0.077 (2) | 0.0221 (16) | −0.0221 (15) | −0.0596 (19) |
| O33 | 0.0407 (15) | 0.080 (2) | 0.082 (2) | 0.0242 (14) | −0.0056 (14) | −0.0527 (18) |
| O41 | 0.055 (2) | 0.070 (2) | 0.134 (4) | −0.0152 (17) | −0.038 (2) | 0.037 (2) |
| O42 | 0.108 (3) | 0.0457 (17) | 0.0396 (15) | −0.0042 (17) | 0.0097 (16) | −0.0170 (13) |
| O43 | 0.290 (7) | 0.045 (2) | 0.071 (3) | 0.046 (3) | −0.061 (4) | −0.0152 (19) |
| C11 | 0.0193 (12) | 0.0275 (14) | 0.0320 (14) | −0.0053 (10) | 0.0064 (10) | −0.0070 (11) |
| C12 | 0.0186 (13) | 0.0289 (15) | 0.053 (2) | 0.0019 (11) | 0.0050 (13) | −0.0024 (14) |
| C13 | 0.0141 (12) | 0.0352 (17) | 0.065 (2) | −0.0015 (11) | 0.0042 (13) | −0.0069 (16) |
| C14 | 0.0221 (14) | 0.0362 (18) | 0.063 (2) | −0.0153 (13) | 0.0074 (14) | −0.0067 (16) |
| C15 | 0.037 (2) | 0.071 (3) | 0.084 (3) | −0.022 (2) | 0.026 (2) | −0.004 (2) |
| C16 | 0.0244 (14) | 0.0344 (17) | 0.052 (2) | −0.0068 (12) | 0.0012 (13) | 0.0044 (15) |
| C21 | 0.0313 (15) | 0.0392 (17) | 0.0229 (14) | −0.0076 (13) | 0.0035 (11) | −0.0058 (12) |
| C22 | 0.0420 (18) | 0.0303 (16) | 0.0394 (18) | 0.0068 (14) | −0.0137 (14) | −0.0113 (14) |
| C23 | 0.0357 (16) | 0.0406 (18) | 0.0284 (15) | 0.0114 (14) | −0.0086 (12) | −0.0134 (13) |
| C24 | 0.0319 (16) | 0.051 (2) | 0.0359 (17) | −0.0074 (15) | −0.0003 (13) | 0.0097 (15) |
| C25 | 0.042 (2) | 0.043 (2) | 0.069 (3) | 0.0006 (17) | 0.0065 (19) | 0.0059 (19) |
| C26 | 0.069 (3) | 0.048 (2) | 0.0355 (19) | −0.025 (2) | −0.0065 (18) | −0.0030 (16) |
| C31 | 0.0176 (12) | 0.0325 (15) | 0.0245 (13) | −0.0023 (10) | 0.0040 (10) | −0.0050 (11) |
| C32 | 0.0244 (13) | 0.0346 (16) | 0.0305 (15) | −0.0031 (12) | 0.0001 (11) | −0.0095 (12) |
| C33 | 0.0273 (14) | 0.0327 (16) | 0.0310 (15) | 0.0078 (12) | −0.0053 (11) | −0.0106 (12) |
| C34 | 0.017 (3) | 0.035 (5) | 0.030 (5) | 0.010 (3) | 0.003 (3) | 0.000 (3) |
| C35 | 0.027 (3) | 0.043 (5) | 0.035 (4) | 0.001 (3) | 0.013 (3) | 0.003 (3) |
| O31 | 0.033 (3) | 0.037 (4) | 0.052 (4) | 0.004 (3) | 0.013 (3) | 0.011 (3) |
| C34A | 0.022 (4) | 0.045 (7) | 0.040 (7) | 0.019 (4) | −0.010 (4) | −0.005 (5) |
| C35A | 0.023 (4) | 0.091 (11) | 0.045 (5) | −0.001 (7) | 0.010 (4) | 0.014 (8) |
| O31A | 0.055 (6) | 0.065 (7) | 0.041 (4) | −0.023 (5) | −0.007 (3) | 0.020 (4) |
| C36 | 0.0197 (13) | 0.0441 (19) | 0.0427 (18) | −0.0038 (12) | −0.0005 (12) | −0.0198 (15) |
| C41 | 0.0229 (13) | 0.0331 (16) | 0.0294 (14) | −0.0129 (11) | −0.0008 (11) | −0.0043 (12) |
| C42 | 0.0250 (13) | 0.0332 (16) | 0.0265 (14) | −0.0074 (11) | 0.0038 (11) | −0.0078 (12) |
| C43 | 0.0394 (17) | 0.0300 (16) | 0.0348 (16) | −0.0058 (13) | −0.0052 (13) | −0.0074 (13) |
| C44 | 0.059 (2) | 0.039 (2) | 0.044 (2) | −0.0141 (17) | −0.0106 (18) | 0.0024 (16) |
| C45 | 0.060 (3) | 0.058 (3) | 0.060 (3) | −0.004 (2) | −0.009 (2) | 0.011 (2) |
| C46 | 0.049 (2) | 0.0420 (19) | 0.0265 (15) | −0.0144 (16) | 0.0005 (14) | −0.0057 (14) |
Geometric parameters (Å, º)
| Cu1—O1 | 1.8960 (18) | N31—C31 | 1.337 (3) |
| Cu1—N11 | 1.949 (2) | N31—C32 | 1.368 (4) |
| Cu1—Cl1 | 2.4152 (9) | N32—C31 | 1.348 (4) |
| Cu1—Cl3 | 2.4351 (8) | N32—C33 | 1.381 (4) |
| Cu1—Cl2 | 2.4435 (9) | N32—C34 | 1.472 (9) |
| Cu2—O1 | 1.908 (2) | N32—C34A | 1.516 (10) |
| Cu2—N21 | 1.955 (3) | N33—O33 | 1.226 (4) |
| Cu2—Cl6 | 2.3579 (10) | N33—O32 | 1.240 (4) |
| Cu2—Cl2 | 2.3726 (9) | N33—C33 | 1.423 (4) |
| Cu2—Cl4 | 2.4351 (9) | N41—C41 | 1.343 (4) |
| Cu3—O1 | 1.9022 (19) | N41—C42 | 1.368 (4) |
| Cu3—N31 | 1.955 (2) | N42—C41 | 1.342 (4) |
| Cu3—Cl5 | 2.3877 (10) | N42—C43 | 1.390 (4) |
| Cu3—Cl6 | 2.4113 (11) | N42—C44 | 1.484 (5) |
| Cu3—Cl3 | 2.4312 (8) | N43—O42 | 1.207 (4) |
| Cu4—O1 | 1.913 (2) | N43—O43 | 1.209 (5) |
| Cu4—N41 | 1.972 (3) | N43—C43 | 1.426 (5) |
| Cu4—Cl5 | 2.4186 (8) | O11—C15 | 1.404 (6) |
| Cu4—Cl4 | 2.4314 (9) | O21—C25 | 1.370 (5) |
| Cu4—Cl1 | 2.4332 (8) | O41—C45 | 1.458 (6) |
| N11—C11 | 1.335 (4) | C11—C16 | 1.480 (4) |
| N11—C12 | 1.356 (4) | C12—C13 | 1.359 (4) |
| N12—C11 | 1.350 (4) | C14—C15 | 1.501 (6) |
| N12—C13 | 1.373 (4) | C21—C26 | 1.482 (5) |
| N12—C14 | 1.481 (4) | C22—C23 | 1.352 (5) |
| N13—O12 | 1.195 (5) | C24—C25 | 1.510 (5) |
| N13—O13 | 1.217 (4) | C31—C36 | 1.480 (4) |
| N13—C13 | 1.426 (4) | C32—C33 | 1.348 (4) |
| N21—C21 | 1.334 (4) | C34—C35 | 1.490 (10) |
| N21—C22 | 1.359 (4) | C35—O31 | 1.411 (9) |
| N22—C21 | 1.356 (4) | C34A—C35A | 1.495 (13) |
| N22—C23 | 1.376 (4) | C35A—O31A | 1.413 (13) |
| N22—C24 | 1.469 (4) | C41—C46 | 1.480 (4) |
| N23—O23 | 1.236 (5) | C42—C43 | 1.343 (5) |
| N23—O22 | 1.246 (5) | C44—C45 | 1.474 (6) |
| N23—C23 | 1.425 (4) | ||
| O1—Cu1—N11 | 173.12 (10) | C31—N31—C32 | 107.4 (2) |
| O1—Cu1—Cl1 | 86.13 (6) | C31—N31—Cu3 | 125.4 (2) |
| N11—Cu1—Cl1 | 99.55 (7) | C32—N31—Cu3 | 127.1 (2) |
| O1—Cu1—Cl3 | 84.63 (6) | C31—N32—C33 | 106.1 (2) |
| N11—Cu1—Cl3 | 96.61 (7) | C31—N32—C34 | 123.8 (6) |
| Cl1—Cu1—Cl3 | 112.86 (3) | C33—N32—C34 | 129.5 (6) |
| O1—Cu1—Cl2 | 83.33 (6) | C31—N32—C34A | 122.8 (7) |
| N11—Cu1—Cl2 | 91.01 (7) | C33—N32—C34A | 129.4 (7) |
| Cl1—Cu1—Cl2 | 110.37 (3) | O33—N33—O32 | 124.6 (3) |
| Cl3—Cu1—Cl2 | 134.02 (3) | O33—N33—C33 | 119.6 (3) |
| O1—Cu2—N21 | 176.91 (10) | O32—N33—C33 | 115.9 (3) |
| O1—Cu2—Cl6 | 86.06 (6) | C41—N41—C42 | 107.0 (3) |
| N21—Cu2—Cl6 | 91.20 (8) | C41—N41—Cu4 | 129.2 (2) |
| O1—Cu2—Cl2 | 85.06 (6) | C42—N41—Cu4 | 123.4 (2) |
| N21—Cu2—Cl2 | 95.77 (8) | C41—N42—C43 | 106.5 (3) |
| Cl6—Cu2—Cl2 | 132.47 (4) | C41—N42—C44 | 125.2 (3) |
| O1—Cu2—Cl4 | 83.85 (6) | C43—N42—C44 | 128.2 (3) |
| N21—Cu2—Cl4 | 98.64 (8) | O42—N43—O43 | 124.0 (4) |
| Cl6—Cu2—Cl4 | 115.31 (4) | O42—N43—C43 | 118.0 (3) |
| Cl2—Cu2—Cl4 | 109.97 (3) | O43—N43—C43 | 117.9 (4) |
| O1—Cu3—N31 | 176.21 (10) | Cu1—O1—Cu3 | 110.80 (9) |
| O1—Cu3—Cl5 | 85.31 (6) | Cu1—O1—Cu2 | 108.46 (9) |
| N31—Cu3—Cl5 | 96.51 (9) | Cu3—O1—Cu2 | 108.36 (10) |
| O1—Cu3—Cl6 | 84.68 (6) | Cu1—O1—Cu4 | 108.74 (10) |
| N31—Cu3—Cl6 | 91.57 (9) | Cu3—O1—Cu4 | 109.55 (9) |
| Cl5—Cu3—Cl6 | 126.01 (4) | Cu2—O1—Cu4 | 110.93 (9) |
| O1—Cu3—Cl3 | 84.61 (6) | N11—C11—N12 | 110.5 (3) |
| N31—Cu3—Cl3 | 97.52 (7) | N11—C11—C16 | 125.5 (3) |
| Cl5—Cu3—Cl3 | 116.05 (3) | N12—C11—C16 | 124.0 (3) |
| Cl6—Cu3—Cl3 | 115.56 (4) | N11—C12—C13 | 107.8 (3) |
| O1—Cu4—N41 | 175.50 (9) | C12—C13—N12 | 108.4 (3) |
| O1—Cu4—Cl5 | 84.21 (6) | C12—C13—N13 | 126.4 (3) |
| N41—Cu4—Cl5 | 100.26 (7) | N12—C13—N13 | 125.2 (3) |
| O1—Cu4—Cl4 | 83.84 (6) | N12—C14—C15 | 112.4 (3) |
| N41—Cu4—Cl4 | 93.78 (7) | O11—C15—C14 | 109.9 (3) |
| Cl5—Cu4—Cl4 | 113.26 (3) | N21—C21—N22 | 110.5 (3) |
| O1—Cu4—Cl1 | 85.25 (6) | N21—C21—C26 | 125.7 (3) |
| N41—Cu4—Cl1 | 93.57 (7) | N22—C21—C26 | 123.7 (3) |
| Cl5—Cu4—Cl1 | 111.98 (3) | C23—C22—N21 | 108.1 (3) |
| Cl4—Cu4—Cl1 | 131.90 (3) | C22—C23—N22 | 108.5 (3) |
| Cu1—Cl1—Cu4 | 79.38 (2) | C22—C23—N23 | 125.7 (3) |
| Cu2—Cl2—Cu1 | 79.70 (2) | N22—C23—N23 | 125.6 (3) |
| Cu3—Cl3—Cu1 | 79.95 (3) | N22—C24—C25 | 111.6 (3) |
| Cu4—Cl4—Cu2 | 80.61 (2) | O21—C25—C24 | 111.5 (4) |
| Cu3—Cl5—Cu4 | 80.86 (3) | N31—C31—N32 | 110.3 (2) |
| Cu2—Cl6—Cu3 | 80.74 (3) | N31—C31—C36 | 125.6 (3) |
| C11—N11—C12 | 107.5 (2) | N32—C31—C36 | 124.2 (2) |
| C11—N11—Cu1 | 123.7 (2) | C33—C32—N31 | 107.8 (3) |
| C12—N11—Cu1 | 128.4 (2) | C32—C33—N32 | 108.4 (3) |
| C11—N12—C13 | 105.8 (2) | C32—C33—N33 | 126.4 (3) |
| C11—N12—C14 | 124.5 (3) | N32—C33—N33 | 125.1 (3) |
| C13—N12—C14 | 129.7 (3) | N32—C34—C35 | 110.2 (7) |
| O12—N13—O13 | 122.9 (3) | O31—C35—C34 | 110.9 (8) |
| O12—N13—C13 | 117.5 (3) | C35A—C34A—N32 | 112.3 (8) |
| O13—N13—C13 | 119.7 (4) | O31A—C35A—C34A | 111.8 (9) |
| C21—N21—C22 | 107.3 (3) | N42—C41—N41 | 110.2 (3) |
| C21—N21—Cu2 | 126.3 (2) | N42—C41—C46 | 124.1 (3) |
| C22—N21—Cu2 | 126.1 (2) | N41—C41—C46 | 125.7 (3) |
| C21—N22—C23 | 105.6 (3) | C43—C42—N41 | 108.6 (3) |
| C21—N22—C24 | 125.2 (3) | C42—C43—N42 | 107.6 (3) |
| C23—N22—C24 | 127.8 (3) | C42—C43—N43 | 124.9 (3) |
| O23—N23—O22 | 125.4 (3) | N42—C43—N43 | 127.3 (3) |
| O23—N23—C23 | 118.8 (4) | C45—C44—N42 | 110.4 (3) |
| O22—N23—C23 | 115.9 (4) | O41—C45—C44 | 109.5 (4) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O41—H41A···O31i | 0.89 (2) | 2.13 (3) | 2.738 (8) | 125 (2) |
Symmetry code: (i) x+1/2, y−1/2, z.
References
- Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J. & Verschoor, G. C. (1984). J. Chem. Soc. Dalton Trans. pp. 1349–1356.
- Athar, F., Husain, K., Abid, M., Agarwal, S. M., Coles, S. J., Hursthouse, M. B., Maurya, M. R. & Azam, A. (2005). Chem. Biodivers. 2, 1320–1330. [DOI] [PubMed]
- Atria, A. M., Vega, A., Contreras, M., Valenzuela, J. & Spodine, E. (1999). Inorg. Chem. 38, 5681–5685.
- Barba-Behrens, N., Mutio-Rico, A. M., Joseph-Nathan, P. & Contreras, R. (1991). Polyhedron, 10, 1333–1341.
- Bertrand, J. A. & Kelley, J. A. (1966). J. Am. Chem. Soc. 88, 4746–4747.
- Bharti, N., Shailendra, Coles, S. J., Hursthouse, M. B., Mayer, T. A., Garza, M. G., Cruz-Vega, D. E., Mata-Cardenas, B. D., Naqvi, F., Maurya, M. R. & Azam, A. (2002). Helv. Chim. Acta, 85, 2704–2712.
- Brescia, T. K., Mulosmani, K., Gulati, S., Athanasopoulos, D. & Upmacis, R. K. (2018). Acta Cryst. E74, 309–312. [DOI] [PMC free article] [PubMed]
- Bruker (2008). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chiarella, G. M., Melgarejo, D. Y. & Fackler Jr, J. P. (2009). Acta Cryst. C65, m228–m230. [DOI] [PubMed]
- Chiarella, G. M., Melgarejo, D. Y., Prosvirin, A. V., Dunbar, K. R. & Fackler, J. P. (2010). J. Clust Sci. 21, 551–565.
- Clegg, W., Nicholson, J. R., Collison, D. & Garner, C. D. (1988). Acta Cryst. C44, 453–461.
- Cortés, P., Atria, A. M., Garland, M. T. & Baggio, R. (2006). Acta Cryst. C62, m311–m314. [DOI] [PubMed]
- Daszkiewicz, M. (2013). CrystEngComm, 15, 10427–10430.
- Erdonmez, A., van Diemen, J. H., de Graaff, R. A. G. & Reedijk, J. (1990). Acta Cryst. C46, 402–404.
- Galván-Tejada, N., Bernès, S., Castillo-Blum, S. E., Nöth, H., Vicente, R. & Barba-Behrens, N. (2002). J. Inorg. Biochem. 91, 339–348. [DOI] [PubMed]
- Jian, F. F., Zhao, P. S., Wang, H. X. & Lu, L. D. (2004). Bull. Kor. Chem. Soc. 25, 673–675.
- Li, H., Jiang, H. & Sun, H. (2011). Acta Cryst. E67, m1372. [DOI] [PMC free article] [PubMed]
- Norman, R. E., Rose, N. J. & Stenkamp, R. E. (1989). Acta Cryst. C45, 1707–1713.
- Palmer, J. H. & Parkin, G. (2014). Dalton Trans. 43, 13874–13882. [DOI] [PMC free article] [PubMed]
- Palmer, J. H. & Upmacis, R. K. (2015). Acta Cryst. E71, 284–287. [DOI] [PMC free article] [PubMed]
- Palmer, J. H., Wu, J. S. & Upmacis, R. K. (2015). J. Mol. Struct. 1091, 177–182.
- Quinlivan, P. J. & Upmacis, R. K. (2016). Acta Cryst. E72, 1633–1636. [DOI] [PMC free article] [PubMed]
- Quinlivan, P. J., Wu, J.-S. & Upmacis, R. K. (2015). Acta Cryst. E71, 810–812. [DOI] [PMC free article] [PubMed]
- Ratajczak-Sitarz, M., Katrusiak, A., Wojakowska, H., Januszczyk, M., Krzyminiewski, R. & Pietrzak, J. (1998). Inorg. Chim. Acta, 269, 326–331.
- Samuelson, J. (1999). Antimicrob. Agents Chemother. 43, 1533–1541. [DOI] [PMC free article] [PubMed]
- She, G., Liu, S. Y., Wu, X. M., Guo, J. H., Wang, X. G. & Liu, Q. X. (2010). Chin. J. Inorg. Chem. 26, 515–520.
- Sheldrick, G. M. (2008). Acta Cryst A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Spek, A. L. (2015). Acta Cryst. C71, 9–18. [DOI] [PubMed]
- Tosik, A., Bukowska-Strzyzewska, M. & Mrozinski, J. (1991). J. Coord. Chem. 24, 113–125.
- WHO (2015). Who. Tech. Rep. Ser. 994, 1-546.
- Yang, L., Powell, D. R. & Houser, R. P. (2007). Dalton Trans. pp. 955–964. [DOI] [PubMed]
- Zhang, Y.-Q., Xu, D.-J. & Su, J.-R. (2003). Acta Cryst. E59, m919–m920.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989019008570/hb7801sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989019008570/hb7801Isup2.hkl
CCDC reference: 1923275
Additional supporting information: crystallographic information; 3D view; checkCIF report