Abstract
Background
Short and long sleep duration have been linked with poorer cognitive outcomes, but it remains unclear whether these associations are causal.
Methods
We conducted the first Mendelian randomization (MR) study with 77 single-nucleotide polymorphisms (SNPs) for sleep duration using individual-participant data from the UK Biobank cohort (N = 395 803) and summary statistics from the International Genomics of Alzheimer’s Project (N cases/controls = 17 008/37 154) to investigate the potential impact of sleep duration on cognitive outcomes.
Results
Linear MR suggested that each additional hour/day of sleep was associated with 1% [95% confidence interval (CI) = 0–2%; P = 0.008] slower reaction time and 3% more errors in visual-memory test (95% CI = 0–6%; P = 0.05). There was little evidence to support associations of increased sleep duration with decline in visual memory [odds ratio (OR) per additional hour/day of sleep = 1.10 (95% CI = 0.76–1.57); P = 0.62], decline in reaction time [OR = 1.28 (95% CI = 0.49–3.35); P = 0.61], all-cause dementia [OR = 1.19 (95% CI = 0.65–2.19); P = 0.57] or Alzheimer’s disease risk [OR = 0.89 (95% CI = 0.67–1.18); P = 0.41]. Non-linear MR suggested that both short and long sleep duration were associated with poorer visual memory (P for non-linearity = 3.44e–9) and reaction time (P for non-linearity = 6.66e–16).
Conclusions
Linear increase in sleep duration has a small negative effect on reaction time and visual memory, but the true association might be non-linear, with evidence of associations for both short and long sleep duration. These findings suggest that sleep duration may represent a potential causal pathway for cognition.
Keywords: Sleep duration, Mendelian randomization, cognition, dementia
Key Messages
Both short and long sleep duration have been linked with poorer cognitive outcomes, but it remains unclear whether these associations are causal.
We conducted a large linear and non-linear Mendelian randomization (MR) study to investigate the potential causal role of sleep duration on multiple cognitive outcomes.
Our findings suggest that a linear increase in sleep duration is associated with poorer reaction time and visual memory with small effect size, but there is not enough evidence to support associations with cognitive decline, dementia or Alzheimer’s disease.
Non-linear MR analysis suggests that the true association might be J-shaped, which could explain the small linear-effect size.
Sleep duration may represent a potential causal pathway for cognition and thus improving sleep habits within the general population might be useful as a potential therapeutic target to improve cognition.
Introduction
With population ageing, cognitive decline and dementia have become issues of global importance.1 Given that there is currently no effective cure for dementia, identification of modifiable risk factors remains a priority.
In recent decades, numerous observational studies have investigated the association between sleep duration and cognitive performance, but results are conflicting and might be subject to limitations such as residual confounding and over-adjustment of potential mediators.2,3 Reverse causation is also possible, since change in sleep duration might be caused by underlying ill-health,4 with growing evidence that accumulation of biomarkers for cognitive impairment could affect sleep quality.5
Given the difficulties in implementing large-scale randomized trials involving sleep modification, alternative study design such as Mendelian randomization (MR),6 where genetic information is used in an instrumental variable framework, can be used to address some of the limitations of observational studies and estimate causality. Due to the random assortment of genes at conception, MR is less prone to conventional confounding issues with respect to confounders being balanced across genotypes in the population. Reverse causation is also minimized, since cognitive impairment cannot affect individuals’ genotypes.6
In this study, we performed large-scale, linear and non-linear MR analyses using individual-level data from 395 803 participants of UK Biobank and summary statistics from the International Genomics of Alzheimer’s Project (IGAP) stage I, which includes 17 008 Alzheimer’s disease (AD) cases and 37 154 controls. We sought to investigate the potential causal role of sleep duration on baseline assessments of visual memory and reaction time, prospective decline in visual memory and reaction time, hospital-diagnosed all-cause dementia and AD.
Methods
Study participants
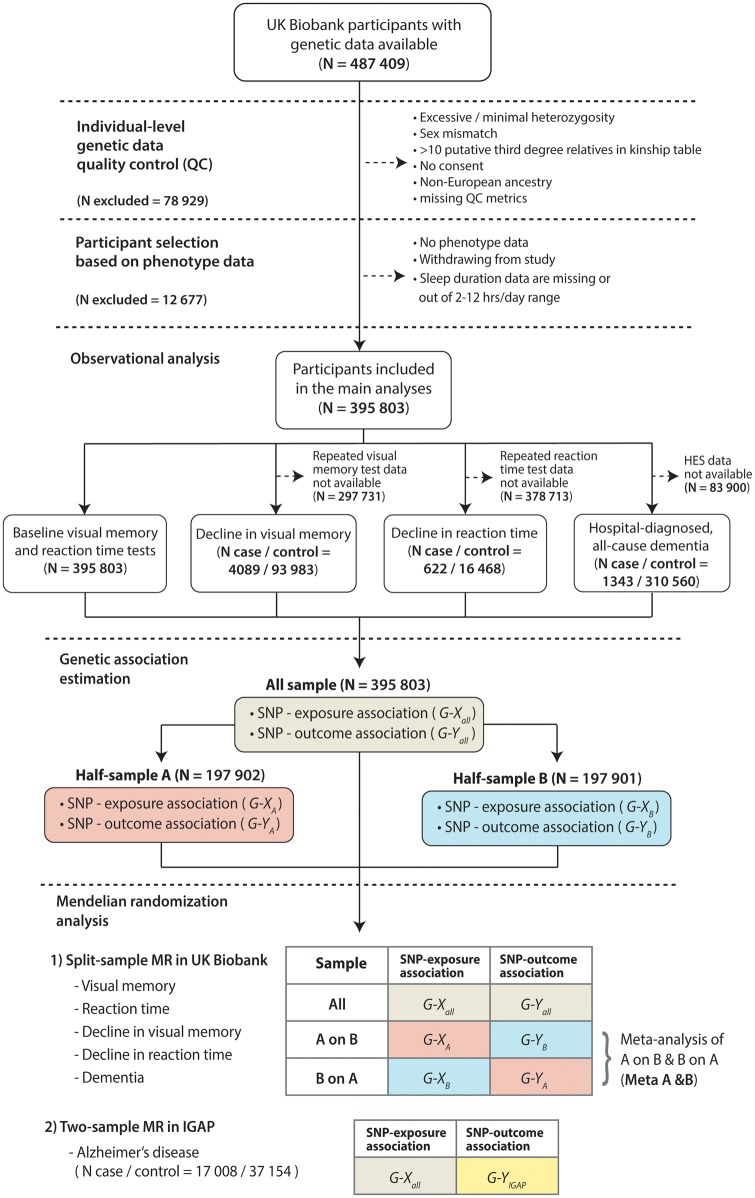
UK Biobank is a large, population-based prospective cohort comprising linked health, hospital-record and genetic data of individuals aged 40–69 years recruited from across the UK between 2006 and 2010.7 Our main analyses included 395 803 UK Biobank participants. In the analyses for decline in visual memory (N case/non-case = 4089/93 983), decline in reaction time (622/16 468) and hospital-diagnosed all-cause dementia (N = 1343/310 560), we included only participants with repeated cognitive assessments and/or hospital-record data available. In the analyses for AD, we used summary statistics from a meta-analysis based upon genome-wide association studies (GWAS) (N case/control = 17 008/37 154) included in the IGAP stage I study (data were available at http://web.pasteur-lille.fr/en/recherche/u744/igap/igap_download.php).8 Details of participant selection are provided in Figure 1 and Supplementary Methods, available as Supplementary data at IJE online.
Figure 1.
Study design.
N, number of observations; HES, Hospital Episode Statistics; SNP, single-nucleotide polymorphism; MR, Mendelian randomization; G-X, genetic association of instrument (SNP) with exposure; G-Y, genetic association of instrument (SNP) with outcome; IGAP, International Genomics of Alzheimer’s Project.
Variable ascertainment
We used self-reported average sleep duration (hours/day) recorded at baseline as our exposure. We used results from baseline assessments of visual memory (number of errors made in pairs-matching test, natural log-transformed) and reaction time (milliseconds, natural log-transformed) as our continuous outcome variables. We used data from repeated assessments of visual memory and reaction time to derive binary cognitive decline variables (case or non-case) based on the standardized regression-based (SRB) method.9 We identified all-cause dementia cases based on previously validated primary and secondary ICD-10 diagnosis codes10 (Supplementary Table 1, available as Supplementary data at IJE online) from linked Hospital Episode Statistics (HES) data. We selected potential confounders based on previous literature,2,3 including sex, age, Townsend deprivation index, qualification, employment status, smoking status, alcohol-intake frequency, body mass index (BMI), systolic blood pressure, diastolic blood pressure, co-morbidities (Supplementary Table 2, available as Supplementary data at IJE online) and use of sleep-inducing medication (Supplementary Table 3, available as Supplementary data at IJE online).
Genetic instrument selection
We took 78 near-independent SNPs for sleep duration with P for association <5 × 10–8 from a recent GWAS11 as our genetic instruments. Of these, one SNP (rs17761776) was excluded following SNP quality control (QC). Cumulatively, the remaining 77 SNPs in our genetic instruments explained 0.65% of the variability in sleep duration (R2 = 0.65%, F-statistic = 33.86). In this study, we used genotype dosage information to estimate allele count under an additive genetic model. More details on the instruments are provided in Supplementary Table 5, available as Supplementary data at IJE online. Information on SNP genotyping, imputation and QC are provided in Supplementary Methods, available as Supplementary data at IJE online.
Statistical analyses
Figure 1 illustrates the design of this study.
Observational analyses
We explored the observational association between sleep duration and each cognitive outcome using linear or logistic regression, with and without adjustment for potential confounders. Sleep duration was modelled as a discrete variable (ranging from 2 to 12 hours/day) and as a categorical variable (≤5, 6, 7, 8, 9, ≥10 hours/day). We performed analysis of variance (ANOVA) and chi-squared tests to compare means and proportions across sleep categories, and paired t-tests to assess within-individual differences for participants who completed both baseline and repeated cognitive assessments.
Genetic-association analyses
Since the GWAS from which we identified our genetic instruments was conducted in UK Biobank,11 we used a split-sample strategy to mitigate the over-estimation of genetic effect sizes in one-sample setting (winner’s curse bias).12,13 We split the data randomly into two sets: A and B, with NA = 197 902 and NB = 197 901. We calculated individual SNP’s genetic association with exposure (G-X) and with outcome (G-Y) by running simple linear or logistic regressions in each set. For MR analyses, we used G-X from set A and G-Y from set B (A on B) and vice versa (B on A). Finally, we meta-analysed the MR estimates from the two (Meta A & B) and compared these to the estimate from the single-sample summary data (All). For AD, we used G-X estimated in our full UK Biobank sample and G-Y from IGAP stage I. Due to data unavailability, we used proxies for nine SNPs (linkage disequilibrium R2 > 0.9) and removed two SNPs without suitable proxy (rs34556183 and rs2139261). The remaining 75 SNPs had R2 = 0.64% and F-statistic = 33.91 in our UK Biobank sample.
MR analyses
We applied the inverse-variance weighted (IVW) method as our main linear MR model. This method estimates the (linear) causal effect of the exposure on the outcome by averaging the genetic instruments’ ratio of instrument–outcome to instrument–exposure association estimates under a fixed-effect meta-analysis model.14 As sensitivity analyses, we ran MR-Egger regression15 and weighted median estimator (WME).16 The former produces an intercept term indicative for horizontal pleiotropy (where the genetic instruments are associated with the outcome through pathways other than the exposure)15 and the latter yields more robust estimates in the presence of some invalid genetic instruments.16
Sensitivity analyses
We further explored the validity of our instruments by testing associations of potential confounders with the genetic score (constructed from summing genotype dosages across instruments), plotting genetic associations of each instrument with the exposure and the outcomes, and repeating our MR analyses with exclusion of potentially invalid instruments. In addition to the split-sample strategy, we also calculated the potential bias due to overlapping samples using a formula described elsewhere.12
Non-linear MR
We investigated the non-linear associations of sleep duration with visual memory and reaction time using the piecewise linear MR method.17 Briefly, we stratified our sample into three strata based on the residual variation of the sleep duration after regressing on the genetic instruments. We then fitted a piecewise linear function in each stratum, which was constrained to be continuous, and took the gradient of each line segment as a localized average causal effect (LACE) in the stratum. Non-linearity was assessed using Cochran’s Q statistic for heterogeneity of the LACE estimates and test for quadratic exposure–outcome model.17 As sensitivity analysis, we re-ran the model with 10 strata using a de-discretized sleep-duration variable by adding small random variability through a series of Monte Carlo simulations.
We used R 3.4.3 and Stata 14 for data processing and statistical analyses. MR analyses and non-linear MR were performed using the mrrobust package in Stata18 and nlmr package in R,17 respectively. Further details of our methods are presented in Supplementary Methods, available as Supplementary data at IJE online.
Results
Baseline characteristics
Table 1 summarizes the baseline characteristics of study participants. The average sleep duration was 7.17 (1.07 SD) hours/day. We observed U-shaped/inverted U-shaped patterns across sleep-duration categories for most variables. Compared with participants who reported sleeping for 7 hours/day, both <7 and >7 hours/day sleep categories had lower scores in the baseline visual-memory and reaction-time tests, with those sleeping 10–12 hours/day scoring the worst [average number of incorrect matches = 4.6 (3.7 SD); average reaction time = 591 (134 SD) milliseconds].
Table 1.
Characteristics of study participants
| Variables | All participants | Sleep duration (hours / day) |
N | P-valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| ≤5 | 6 | 7 | 8 | 9 | ≥10 | ||||
| N = 19 926 | N = 73 813 | N = 155 333 | N = 116 573 | N = 23 536 | N = 6622 | ||||
| (5.0%) | (18.7%) | (39.3%) | (29.5%) | (6.0%) | (1.7%) | ||||
| Baseline characteristics | |||||||||
| Age (years), mean ± SD | 56.9 ± 8 | 57.2 ± 7.7 | 56.6 ± 7.8 | 56.1 ± 8 | 57.3 ± 8.1 | 59 ± 7.8 | 58.7 ± 7.9 | 395 803 | <0.001 |
| Sex, % | 395 803 | <0.001 | |||||||
| Female | 54 | 56.5 | 52.3 | 52.5 | 56.3 | 56.5 | 55.2 | ||
| Male | 46 | 43.5 | 47.7 | 47.5 | 43.7 | 43.5 | 44.8 | ||
| Townsend Deprivation Index, mean ± SD | −1.6 ± 2.9 | −0.7 ± 3.3 | −1.4 ± 3 | −1.7 ± 2.8 | −1.7 ± 2.8 | −1.6 ± 2.9 | −0.7 ± 3.3 | 395 803 | <0.001 |
| College/university/professional qualification, % | 36.4 | 24.6 | 34 | 40.5 | 36.5 | 30.5 | 23.6 | 395 803 | <0.001 |
| Employment status, % | 395 803 | <0.001 | |||||||
| Employed | 57.1 | 50.8 | 62.2 | 64.5 | 51.3 | 35.5 | 24 | ||
| Retired | 35.1 | 34.2 | 30.1 | 29.6 | 41 | 53.6 | 51.7 | ||
| Others | 7.8 | 15 | 7.7 | 5.9 | 7.7 | 10.8 | 24.3 | ||
| Smoking status, % | 395 803 | <0.001 | |||||||
| Never | 54.7 | 49.9 | 52.7 | 56.4 | 55.5 | 52 | 46.6 | ||
| Previous | 35.3 | 34.9 | 35.8 | 34.5 | 35.5 | 37.7 | 38.4 | ||
| Current | 10 | 15.2 | 11.5 | 9.2 | 9 | 10.3 | 14.9 | ||
| Alcohol consumption, % | 395 803 | <0.001 | |||||||
| Rarely | 27.9 | 38.5 | 29.4 | 25.3 | 27.3 | 31.2 | 41.5 | ||
| 1–2 a week | 26.4 | 25.4 | 26.6 | 26.8 | 26.5 | 25.1 | 23 | ||
| 3–4 a week | 24.3 | 18.6 | 23.2 | 26.2 | 24.4 | 21.6 | 15.9 | ||
| Almost daily | 21.3 | 17.5 | 20.8 | 21.7 | 21.8 | 22.1 | 19.6 | ||
| BMI (kg/m2), mean ± SD | 27.4 ± 4.7 | 28.5 ± 5.4 | 27.8 ± 4.9 | 27.1 ± 4.5 | 27.2 ± 4.6 | 27.8 ± 4.9 | 29.1 ± 5.7 | 395 803 | <0.001 |
| SBP (mmHg), mean ± SD | 138 ± 19 | 139 ± 19 | 138 ± 18 | 138 ± 18 | 139 ± 19 | 140 ± 19 | 139 ± 19 | 373 248 | <0.001 |
| DBP (mmHg), mean ± SD | 82 ± 10 | 83 ± 10 | 82 ± 10 | 82 ± 10 | 82 ± 10 | 83 ± 10 | 83 ± 10 | 373 251 | <0.001 |
| Co-morbidities present, % | 38.7 | 49.3 | 39.8 | 35.2 | 37.8 | 47.3 | 63.6 | 395 803 | <0.001 |
| Use of sleep-inducing medication, % | 1.1 | 3.4 | 1.2 | 0.7 | 0.9 | 1.5 | 4.2 | 395 803 | <0.001 |
| Cognitive outcomes | |||||||||
| Baseline cognitive outcomes (all participants) | |||||||||
| VM, mean ± SD | 4.1 ± 3.2 | 4.2 ± 3.3 | 4 ± 3.2 | 4 ± 3.1 | 4.1 ± 3.3 | 4.3 ± 3.4 | 4.6 ± 3.7 | 395 803 | <0.001 |
| RT, mean ± SD | 555 ± 113 | 566 ± 122 | 554 ± 113 | 549 ± 109 | 558 ± 113 | 569 ± 116 | 591 ± 134 | 395 803 | <0.001 |
| Repeated VM assessment | 98 072 | ||||||||
| VM (baseline), mean ± SD | 3.7 ± 2.9 | 3.9 ± 3 | 3.8 ± 2.9 | 3.7 ± 2.9 | 3.8 ± 2.9 | 3.9 ± 3 | 3.9 ± 2.9 | <0.001 | |
| VM (repeated), mean ± SD | 4.2 ± 3.1 | 4.3 ± 3.3 | 4.2 ± 3.1 | 4.1 ± 3 | 4.2 ± 3.1 | 4.3 ± 3.1 | 4.3 ± 3.2 | <0.001 | |
| Decline in VM case, % | 4.2 | 4.8 | 4.3 | 4 | 4.2 | 4.4 | 4.3 | 0.24 | |
| Repeated RT assessment | 17 090 | ||||||||
| RT (baseline), mean ± SD | 548 ± 103 | 552 ± 114 | 546 ± 99 | 544 ± 101 | 552 ± 105 | 555 ± 97 | 582 ± 121 | <0.001 | |
| RT (repeated), mean ± SD | 556 ± 109 | 561 ± 110 | 554 ± 109 | 552 ± 108 | 558 ± 112 | 569 ± 103 | 580 ± 114 | <0.001 | |
| Decline in RT case, % | 3.6 | 3.7 | 3.7 | 3.6 | 3.5 | 5 | 1.9 | 0.16 | |
| Dementia, % | 0.43 | 0.67 | 0.39 | 0.31 | 0.43 | 0.71 | 1.5 | 311 903 | <0.001 |
P-value from ANOVA/chi-squared tests comparing mean/proportion across sleep categories.
VM, visual memory (score reflects number of errors made in pairs-matching test); RT, reaction time (score reflects time to react in millisecond); Decline in VM / RT, decline in visual memory / reaction time derived from standardized regression-based method; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; N, total number of observations (for binary outcomes; N includes both cases and non-cases).
We identified 4089 (4.2%, from a total of Ntotal = 98 072) participants with decline in visual memory, 622 (3.6%, Ntotal = 17 090) with decline in reaction time and 1343 (0.43%, Ntotal = 311 903) diagnosed with dementia. On average, performance in repeated assessments was poorer than baseline for both visual-memory [baseline mean = 3.7 (2.9 SD); repeated mean = 4.2 (3.1 SD); P < 0.001] and reaction-time tests [baseline mean = 548 (103 SD) milliseconds; repeated mean = 556 (109 SD) milliseconds; P < 0.001]. Participants diagnosed with dementia performed worse than those without the disease in baseline cognitive tests [average number of incorrect matches = 5.1 (4.2 SD), P < 0.001; average reaction time = 635 (157 SD) milliseconds, P < 0.001].
Observational analyses
Table 2 outlines the results from observational analyses with categorical sleep duration. For the log-transformed cognitive assessment results, we report exponentiated betas [Exp()] to ease interpretation. The Exp() represent a multiplicative effect size, e.g. Exp() = 1.03, in reaction-time test, which represents an estimated Exp() – 1 = 0.03 = 3% slower reaction time. On average, individuals who reported sleep for less or more than 7 hours/day had more incorrect matches in baseline visual-memory test, slower baseline reaction time and increased risk of dementia, but had little to no difference in the risk of cognitive decline. These associations were attenuated upon adjustment for potential confounders.
Table 2.
Observational associations with categorical sleep duration
| Outcomes | N observation or N case/non-case | Sleep duration in categories (hours/day) |
|||||
|---|---|---|---|---|---|---|---|
| ≤5 | 6 | 7 | 8 | 9 | ≥10 | ||
| Unadjusted model | |||||||
| Baseline cognitive assessment, exponentiated beta (95% CI) | |||||||
| Visual memory | 395 803 | 1.04 (1.03, 1.05)* | 1.01 (1.00, 1.02)** | Ref | 1.03 (1.02, 1.03)* | 1.05 (1.05, 1.06)* | 1.11 (1.09, 1.12)* |
| Reaction time | 395 803 | 1.03 (1.02, 1.03)* | 1.01 (1.01, 1.01)* | Ref | 1.02 (1.12, 1.02)* | 1.03 (1.03, 1.04)* | 1.07 (1.07, 1.08)* |
| Binary cognitive outcomes, OR (95% CI) | |||||||
| Decline in visual memory | 4089/93 983 | 1.19 (1.01, 1.41)** | 1.07 (0.98, 1.17) | Ref | 1.04 (0.96, 1.12) | 1.09 (0.95, 1.26) | 1.06 (0.78, 1.44) |
| Decline in reaction time | 622/16 468 | 1.03 (0.67, 1.59) | 1.04 (0.83, 1.31) | Ref | 0.97 (0.80, 1.18) | 1.43 (1.05, 1.94)** | 0.53 (0.20, 1.44) |
| Dementia | 1343/310 560 | 2.14 (1.73, 2.64)* | 1.26 (1.07, 1.49)** | Ref | 1.39 (1.20, 1.60)* | 2.28 (1.88, 2.78)* | 4.85 (3.84, 6.12)* |
| Adjusted model a | |||||||
| Baseline cognitive assessment, exponentiated beta (95% CI) | |||||||
| Visual memory | 395 803 | 1.02 (1.01, 1.03)* | 1.00 (1.00, 1.01) | Ref | 1.01 (1.00, 1.01)* | 1.02 (1.01, 1.02)* | 1.06 (1.05, 1.08)* |
| Reaction time | 395 803 | 1.00 (1.00, 1.01)* | 1.00 (1.00, 1.00) | Ref | 1.00 (1.00, 1.00)* | 1.00 (1.00, 1.01)* | 1.03 (1.02 1.03)* |
| Binary cognitive outcomes, OR (95% CI) | |||||||
| Decline in visual memory | 4089/93 983 | 1.19 (1.01, 1.41)** | 1.08 (0.99, 1.18) | Ref | 1.01 (0.94, 1.09) | 1.04 (0.90, 1.20) | 1.03 (0.76, 1.40) |
| Decline in reaction time | 622/16 468 | 0.93 (0.60, 1.45) | 1.02 (0.81, 1.28) | Ref | 0.92 (0.76, 1.12) | 1.26 (0.93, 1.72) | 0.43 (0.16, 1.18) |
| Dementia | 1343/310 560 | 1.54 (1.24, 1.91)* | 1.15 (0.97, 1.36) | Ref | 1.12 (0.97, 1.29) | 1.39 (1.14, 1.69)** | 2.28 (1.79, 2.90)* |
P < 0.001; **P < 0.05.
Adjusted for age, sex, socio-economic status, qualification, employment, smoking status, alcohol-intake frequency, body mass index, hypertension, co-morbidities and use of sleep-inducing medication.
OR, odds ratio; 95% CI, 95% confidence interval; numbers represent effect size per additional hour/day in sleep duration; visual memory was measured as natural log of (numbers of errors in pairs-matching test + 1); reaction time was measured as natural log of milliseconds reaction time; exponentiated beta represents a multiplicative effect size (as the outcomes were log-transformed), e.g. an exponentiated beta of 1.03 in reaction time represents an estimated 3% increase in reaction-time test (3% slower).
MR analyses
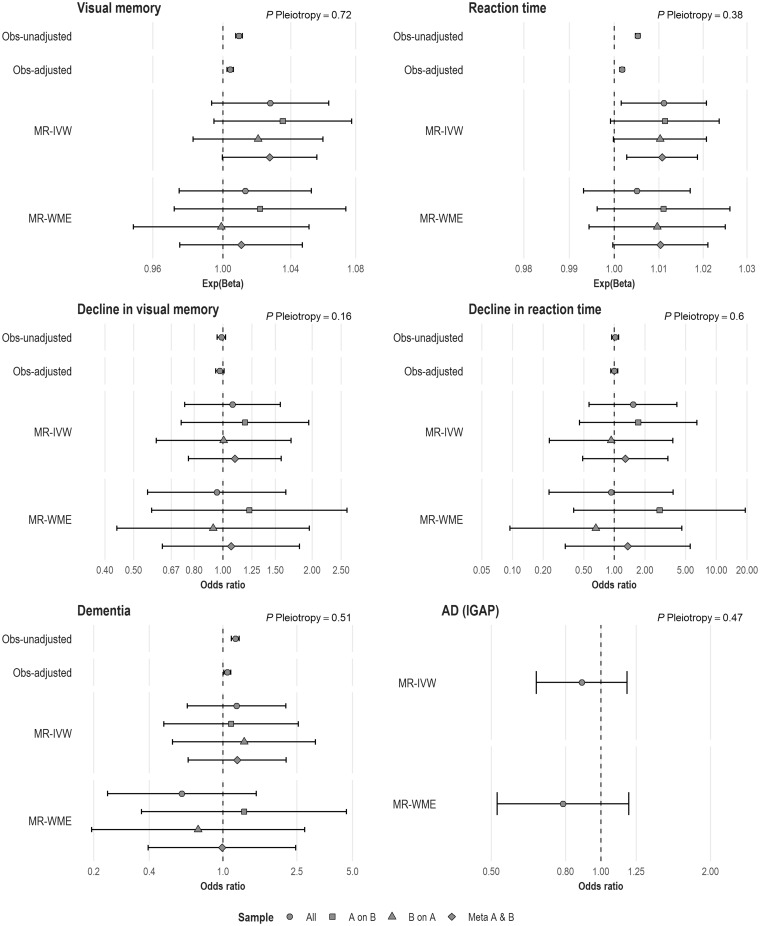
Comparisons between the observational and the MR analyses for linear sleep duration are summarized in Figure 2. Full estimates are provided in Supplementary Table 6, available as Supplementary data at IJE online.
Figure 2.
Results from observational and Mendelian randomization analyses.
Numbers represent effect size per additional hour/day of sleep duration; Exp(Beta), exponentiated beta (represents multiplicative effect size, e.g. an exponentiated beta of 1.03 in reaction time represents an estimated 3% increased/slower reaction time); P Pleiotropy, P-value for overall horizontal pleiotropic effect as indicated by the intercept from MR-Egger regression; Obs-unadjusted, unadjusted observational analysis; Obs-adjusted, observational analysis adjusted for age, sex, socio-economic status, qualification, employment, smoking status, alcohol-intake frequency, body mass index, hypertension, co-morbidities and use of sleep-inducing medication; MR-IVW, Mendelian randomization, inverse-variance-weighted; MR-WME, Mendelian randomization, weighted median estimator.
Linear MR analyses revealed that each additional hour/day in sleep duration was associated with an estimated 1% slower reaction time {exponentiated beta from IVW method in meta-analysis sample – Exp()IVW-meta = 1.01 [95% confidence interval (CI) = 1.00 – 1.02]; P = 0.008}. The evidence for an association with visual memory was directionally consistent [Exp()IVW-meta = 1.03 (95% CI = 1.00–1.06); P = 0.05]. These estimates were similar to observational analysis results.
In both observational and linear MR analyses, we found no evidence of an association with the risk of prospective cognitive decline in visual memory [odds ratio per additional hour/day in sleep duration for the IVW method in our meta-analysis sample– ORIVW-meta = 1.10 (95% CI = 0.76–1.57); P = 0.62] or reaction time [ORIVW-meta = 1.28 (95% CI = 0.49–6.49)].
Observational data suggested some evidence of an association with dementia [OR in adjusted model = 1.05 (95% CI = 1.01–1.10); P = 0.02]. Findings from linear MR-IVW analysis were directionally consistent, but imprecise [ORIVW-meta = 1.19 (95% CI = 0.65–2.19); P = 0.57]. Similarly, we found no evidence of an association between sleep duration and the risk of AD in IGAP [ORIVW = 0.89 (95% CI = 0.67–1.18); P = 0.41].
Sensitivity analyses
In our linear MR analyses, both IVW and WME methods produced broadly consistent results, with MR-Egger intercept P-values ranging from 0.16 to 0.72, suggesting no horizontal pleiotropy effect (Supplementary Figure 1, available as Supplementary data at IJE online).
We found several associations of our genetic score with other variables, including BMI, co-morbidities and some lifestyle factors (P < 0.003, accounting for multiple testing), which we hypothesized might be partly driven by rs9940646, a marker in the FTO gene (widely recognized to be associated with BMI and obesity19). Exclusion of this variant from our genetic score did not completely diminish these associations (Supplementary Table 7, available as Supplementary data at IJE online), but produced consistent MR estimates (Supplementary Table 6, available as Supplementary data at IJE online).
We estimated that the biases due to sample overlap were small (absolute value of bias <0.005 for all outcomes) with type-1 error rate = 0.05 (Supplementary Table 8, available as Supplementary data at IJE online).
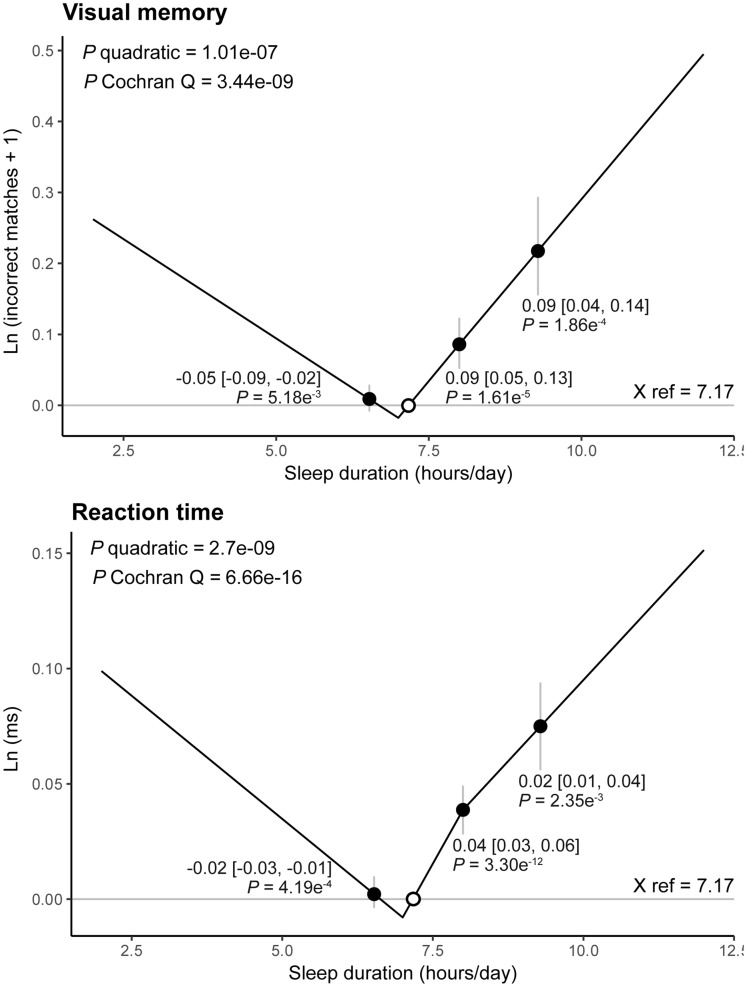
Non-linear MR analyses
The piecewise linear MR with three strata (Figure 3) suggested evidence of non-linear associations of sleep duration with both visual memory (quadratic test P = 1.01e–7, Cochran Q test P = 3.44e–9) and reaction time (quadratic test P = 2.7e–9, Cochran Q test P = 6.66e–16). In both outcomes, the absolute value for LACE estimates in the long-sleep-duration strata were higher (steeper slope in Figure 3) than in the short-sleep-duration strata, suggesting a J-shaped association. This was supported by findings from experimental simulations with 10 strata (Supplementary Figure 2A and B, available as Supplementary data at IJE online).
Figure 3.
Non-linear Mendelian randomization results with piecewise linear method using three strata of sleep duration conditioned on the genetic instruments.
Annotated numbers [black dots (grey vertical lines)] represent localized average causal effect (95% confidence interval) in each stratum; white dots, mean sleep duration used as reference point (X ref); P quadratic/Cochran Q, P-value for non-linearity from quadratic/Cochran Q test; Ln (incorrect matches + 1), natural log of [number of incorrect matches (errors made) in visual-memory test + 1]; Ln (ms), natural log milliseconds of reaction time.
Discussion
Using MR, we found that a linear increase in sleep duration was associated with a small reduced performance in reaction-time and visual-memory tests. This small linear-effect size may indicate that the true association is non-linear, as demonstrated in our non-linear MR model. Whilst the underlying pathways accounting for these associations remain to be elucidated, our findings suggest that sleep duration may represent a potential modifiable risk factor for cognition in mid-life, for which effective pharmacological interventions are currently lacking.
Both short and long sleep duration have been associated with worse cognitive outcomes in previous observational reviews.2,3 These associations were confirmed in our observational analyses and supported by the findings from our non-linear MR analyses. Results from linear and non-linear MR suggest that the causal effect in the long-sleeper group was larger than the short-sleeper group (J-shaped association), consistently with that of a recent meta-analysis20 and a cross-sectional study using objectively measured sleep duration.21
Sleep duration is inextricably linked with sleep quality22 and poor sleep quality could disrupt the circadian rhythm, which regulates gene expression in the frontal, thalamic and hypothalamic regions and the brainstem locus coeruleus.23 This might impair neurogenesis24 and hippocampal function25—region that shows early alteration in several neurodegenerative process leading to cognitive dysfunction. Disordered sleep may have different effects on brain functions linked with specific cognitive domains, e.g. synchronization function of the prefrontal cortex and neuromodulatory system in visual memory26 or the prefrontal cortex and cerebellar functions in reaction time.27
Similarly, short and long sleep duration28–30 and poor sleep quality31 have also been linked with an increased risk of dementia. Although a similar J-shaped association was observed in our observational analysis, we were limited to performing only the linear MR analysis, as the non-linear MR method requires a large number of cases and individual-level data. In our linear MR analysis, we found no clear evidence that an increased sleep duration was associated with a higher risk of all-cause dementia in UK Biobank or with AD in IGAP. This is unsurprising, as the true association might be non-linear and we were limited with only 1343 dementia cases in UK Biobank. Also, IGAP does not capture non-AD dementia types and comprises an older and more heterogeneous population.8
The main strength of our study lies in the MR analysis, which minimizes residual confounding and reverse causation.2 The use of genetic instruments allowed us to estimate a life-long effect of sleep duration on the outcomes and the inclusion of multiple genetic instruments enabled increased power for MR analysis, mitigating weak instrument bias.32 Pleiotropic effects were carefully explored and minimized through MR-Egger analysis, WME and investigation of the effect of individual SNPs. In order to mitigate the potential inflated type-I error rate due to overlapping samples,12 we used a split-sample strategy and found that meta-analysed estimates for both visual memory and reaction time were similar to the single-sample estimate. Moreover, we attempted to quantify the bias12 assuming 100% sample overlap and found it to be small.
Another important strength is that we are one of the first studies to implement non-linear MR analyses and, importantly, these results were consistent with findings from both observational and linear MR analyses, helping to provide better insight into the nature of the association. However, these findings should be interpreted carefully, as sleep duration was only available as a discrete variable in our dataset, which resulted in sub-optimal stratification in our non-linear MR model. Whilst we attempted to improve this by de-discretizing our exposure and found consistent J-shaped associations through simulations, ideally our analysis should be replicated with a more precise continuous measurement of sleep duration (e.g. with actigraphy).
Other limitations include potential reliability issues with the partly novel cognitive assessments and self-reported sleep duration in UK Biobank. However, the cognitive assessments have been validated33 and we also found that lower scores were more frequent in people with dementia. As for sleep duration, self-reported assessment might be more relevant especially in primary health-care settings for practical reasons.34 The MR estimates for prospective cognitive decline were imprecise due to the limited number of cases and practice effects33 may have influenced the reliability of the repeated assessments. Whilst the SRB method can mitigate this issue,9 another method to define cognitive decline could be applied, e.g. by calculating a smallest real-difference cut-off point.33 In addition, the time between assessments in our sample [mean = 5.8 (0.8 SD) years for visual memory; 4.3 (0.9 SD) years for reaction time] might be not long enough for cognitive decline to manifest. Additionally, there may be selection bias in UK Biobank due to low response rates.33
Each of the associations of our genetic score with potential confounders warrants further investigation, but is beyond the scope of this paper. As many of these traits have been widely recognized to be polygenic in nature, they may share some common genetic architecture with sleep duration. Alternatively, these associations may represent downstream effects from sleep duration (i.e. vertical pleiotropy) that do not violate MR assumptions.
In summary, this study provides novel evidence that increased sleep duration may be causally related to poorer reaction time and poorer visual memory, albeit with relatively small linear-effect sizes. The true associations might be J-shaped for both outcomes, but this remains to be confirmed with a more precise sleep-duration measurement. Results for risks of dementia and AD are still too imprecise to draw any definitive conclusions. Our findings suggest that, in clinical care, attention should be paid to sleep-duration patterns and improved sleep habits could represent a potential therapeutic target for cognition. This seems important, as, currently, no single-measure treatment has been shown to decelerate cognitive decline or the risk of dementia. Lastly, we would recommend that most healthy adults should aim to follow the recommendation of 7–9 hours of sleep per day35 and also pay attention to long-term changes in sleep patterns.36
Funding
This work was supported by various funders. A.H. was supported by the Indonesian Endowment Fund For Education (awardee ID 20160412045979); M.K. was supported by the British Heart Foundation (FS/18/5/33319). S.D. was supported by the National Institute for Health Research (RP-PG-0407–10314), Wellcome Trust (086091/Z/08/Z) and the Farr Institute of Health Informatics Research, funded by the Medical Research Council (MR/K006584/1), in partnership with Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Sciences Research Council, the National Institute of Health Research, the National Institute for Social Care and Health Research (Welsh Assembly Government), the Chief Scientist Office (Scottish Government Health Directorates) and the Wellcome Trust. V.G. was supported by a multidisciplinary grant from the Economic and Social Research Council and Medical Research Council (grant number ES/J500185/1). C.D. was supported by a UCL Springboard Population Science fellowship (grant number 105604/Z/14/Z) funded by the Wellcome Trust. This study used the UK Biobank resources that has been funded by the Wellcome Trust Medical Charity, Medical Research Council, Department of Health of Scottish Government, the Northwest Regional Development Agency, the Welsh Assembly Government and the British Heart Foundation.
Supplementary Material
Acknowledgements
This study has been conducted using the UK Biobank Resource (Application Number 13017). We express our gratitude to the participants and researchers involved in UK Biobank and IGAP. We thank James Staley (MRC Integrative Epidemiology Unit, Bristol Medical School, University of Bristol, Bristol, BS8 2BN, UK) and Stephen Burgess (MRC Biostatistics Unit, Cambridge Institute of Public Health, Robinson Way, Cambridge, CB2 0SR, UK) for helpful discussions regarding the non-linear Mendelian randomization methods.
Conflict of interest: None declared.
References
- 1. Brayne C. The elephant in the room—healthy brains in later life, epidemiology and public health. Nat Rev Neurosci 2007;8:233–9. [DOI] [PubMed] [Google Scholar]
- 2. Devore EE, Grodstein F, Schernhammer ES.. Sleep duration in relation to cognitive function among older adults: a systematic review of observational studies. Neuroepidemiology 2016;46:57–78. [DOI] [PubMed] [Google Scholar]
- 3. Lo JC, Groeger JA, Cheng GH, Dijk D-J, Chee M.. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med [Internet]. 2016;17:87–98. http://linkinghub.elsevier.com/retrieve/pii/S1389945715019796 (31 May 2017, date last accessed). [DOI] [PubMed] [Google Scholar]
- 4. Smagula SF, Koh WP, Wang R, Yuan JM.. Chronic disease and lifestyle factors associated with change in sleep duration among older adults in the Singapore Chinese Health Study. J Sleep Res 2016;25:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mander BA, Marks SM, Vogel JW.. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci [Internet] 2015;18:1051–7. http://www.nature.com/doifinder/10.1038/nn.4035 (28 July 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith GD, Ebrahim S. ‘ Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 7. Sudlow C, Gallacher J, Allen N. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lambert J-C, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet [Internet] 2013;45:1452–8. http://web.pasteur-lille.fr/en/recherche/u744/igap/igap_download.php (10 April 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frerichs RJ, Tuokko HA.. A comparison of methods for measuring cognitive change in older adults. Arch Clin Neuropsychol 2005;20:321–33. [DOI] [PubMed] [Google Scholar]
- 10. Pujades-Rodriguez M, Assi V, Gonzalez-Izquierdo A. et al. The diagnosis, burden and prognosis of dementia: A record-linkage cohort study in England. PLoS One 2018;13:e0199026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dashti H, Jones SE, Wood AR, et al. GWAS in 446,118 European adults identifies 78 genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. bioRxiv [Internet]. Cold Spring Harbor Laboratory, 2018. https://www.biorxiv.org/content/early/2018/04/19/274977 (5 December 2018, date last accessed). [Google Scholar]
- 12. Burgess S, Davies NM, Thompson SG.. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol 2016;40:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davey Smith G, Hemani G.. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23:R89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burgess S, Bowden J. Integrating summarized data from multiple genetic variants in Mendelian randomization: bias and coverage properties of inverse-variance weighted methods. 2015. http://arxiv.org/abs/1512.04486 (16 July 2017, date last accessed).
- 15. Bowden J, Smith GD, Burgess S.. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowden J, Davey Smith G, Haycock PC, Burgess S.. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Staley JR, Burgess S.. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol [Internet] 2017;41:341–52. http://doi.wiley.com/10.1002/gepi.22041 (6 July 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spiller W, Davies NM, Palmer TM.. Software application profile: mrrobust—a tool for performing two-sample summary Mendelian randomization analyses. bioRxiv [Internet]. 2017. http://biorxiv.org/content/early/2017/07/05/142125.abstract (6 August 2017, date last accessed). [Google Scholar]
- 19. Frayling TM, Timpson NJ, Weedon MN. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science [Internet] 2007;316:889–94. http://www.ncbi.nlm.nih.gov/pubmed/17434869 (13 December 2018, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim HB, Myung SK, Lee SM, Park YC.. Longer duration of sleep and risk of cognitive decline: a meta-analysis of observational studies. Neuroepidemiology 2016;47:171–80. [DOI] [PubMed] [Google Scholar]
- 21. Spira AP, Stone KL, Redline S. et al. Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep [Internet] 2017;40:zsx073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bin YS. Is sleep quality more important than sleep duration for public health? Sleep [Internet] 2016;39:1629–30. http://www.ncbi.nlm.nih.gov/pubmed/27568809 (3 February 2019, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt C, Peigneux P, Cajochen C.. Age-related changes in sleep and circadian rhythms: impact on cognitive performance and underlying neuroanatomical networks. Front Neurol [Internet] 2012;118 https://www.frontiersin.org/article/10.3389/fneur.2012.00118 (19 November 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kimiwada T, Sakurai M, Ohashi H, Aoki S, Tominaga T, Wada K.. Clock genes regulate neurogenic transcription factors, including NeuroD1, and the neuronal differentiation of adult neural stem/progenitor cells. Neurochem Int [Internet] 2009;54:277–85. 10.1016/j.neuint.2008.12.005 (19 November 2017, date last accessed). [DOI] [PubMed] [Google Scholar]
- 25. Stranahan AM. Chronobiological approaches to Alzheimers disease. Curr Alzheimer Res [Internet] 2012;93–98. http://www.eurekaselect.com/node/88929/article (19 November 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D’Esposito M, Postle BR.. The cognitive neuroscience of working memory. Annu Rev Psychol [Internet] 2015;66:115–42. http://www.annualreviews.org/doi/10.1146/annurev-psych-010814-015031 (3 August 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eckert MA, Keren NI, Roberts DR, Calhoun VD, Harris KC.. Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Front Hum Neurosci 2010;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohara T, Honda T, Hata J. et al. Association between daily sleep duration and risk of dementia and mortality in a Japanese community. J Am Geriatr Soc [Internet] 2018;66:1911–18. http://doi.wiley.com/10.1111/jgs.15446 (3 February 2019, date last accessed). [DOI] [PubMed] [Google Scholar]
- 29. Benito-León J, Bermejo-Pareja F, Vega S, Louis ED.. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol 2009;16:990–97. [DOI] [PubMed] [Google Scholar]
- 30. Westwood AJ, Beiser A, Jain N. et al. Prolonged sleep duration as a marker of early neurodegeneration predicting incident dementia. Neurology 2017;88:1172–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pase MP, Himali JJ, Grima NA. et al. Sleep architecture and the risk of incident dementia in the community. Neurol [Internet] 2017;89:1244–50. http://www.neurology.org/content/89/12/1244.abstract (2 October 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burgess S, Dudbridge F, Thompson SG.. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Statist Med 2016;35:1880–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lyall DM, Cullen B, Allerhand M. et al. Cognitive test scores in UK Biobank: data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLoS One 2016;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA.. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–92. [DOI] [PubMed] [Google Scholar]
- 35. Nathaniel W, Badr MS, Belenky G, Bliwise DL, Buxton OM.. SLEEP—recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015;38:843–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, Singh-Manoux A.. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep 2011;34:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.