Abstract
The pyrethrum plant, Tanacetum cineramfolium (Asteraceae) synthesizes a class of compounds called pyrethrins that have strong insecticidal properties but are safe to humans. Class I pyrethrins are esters of the monoterpenoid trans-chrysanthemic acid with one of three jasmonic-acid derived alcohols. We reconstructed the trans-chry-santhemic acid biosynthetic pathway in tomato fruits, which naturally produce high levels of the tetraterpene pigment lycopene, an isoprenoid which shares a common precursor, dimethylallyl diphosphate (DMAPP), with trans-chrysanthemic acid. trans-Chrysanthemic acid biosynthesis in tomato fruit was achieved by expressing the chrysanthemyl diphosphate synthase gene from T. cinerariifolium, encoding the enzyme that uses DMAPP to make trans-chrysanthemol, under the control of the fruit specific promoter PG, as well as an alcohol dehy-drogenease (ADH) gene and aldehyde dehydrogenase (ALDH) gene from a wild tomato species, also under the control of the PG promoter. Tomato fruits expressing all three genes had a concentration of trans-chrysanthemic acid that was about 1.7-fold higher (by weight) than the levels of lycopene present in non-transgenic fruit, while the level of lycopene in the transgenic plants was reduced by 68%. Ninety seven percent of the diverted DMAPP was converted to trans-chrysanthemic acid, but 62% of this acid was further glycosylated. We conclude that the tomato fruit is an alternative platform for the biosynthesis of trans-chrysanthemic acid by metabolic engineering.
Keywords: Natural pesticides, Plant biochemistry, Specialized metabolism, Secondary metabolites, Fruit-specific metabolic engineering
One sentence summary
Mature fruits of transgenic tomato lines expressing a set of genes for the complete biosynthetic pathway of trans-chrysanthemic acid and controlled by a fruit-ripening specific promoter contained high levels of this acid, a moiety of natural pyrethrin insecticides.
1. Introduction
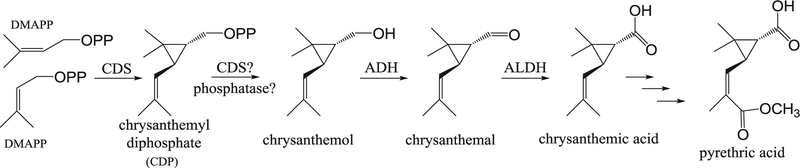
Flowers of the pyrethrum plant (Tanacetum cinerariifolium, Asteraceae) make a set of compounds called pyrethrins, which are toxic to flying insects but are safe to most mammals, including humans. All six of the pyrethrin compounds commonly found in T. cinerariifolium are esters; in the three pyrethrins called Type I, the jasmonic acid-derived alcohols pyrethrolone, cinerolone and jasmolone are esterified with trans-chrysanthemic acid, and in the three Type II pyrethrins, these three alcohols are esterified with pyrethric acid, a derivative of trans-chrysanthemic acid. trans-Chrysanthemic acid is derived from dimethylallyl diphosphate (DMAPP) in several steps (Fig. 1). In the first biosynthetic step in the pathway, the enzyme chrysanthemyl diphosphate synthase (CDS) catalyzes the conversion of DMAPP to trans-chrysanthemyl diphosphate (CDP) (Rivera et al., 2001). In the second step, catalyzed by CDS or one or more phosphatases, CDP is converted to trans-chrysanthemol (Xu et al., 2018; Yang et al., 2014). Two subsequent oxidation reactions, catalyzed sequentially by an alcohol dehydrogenase (ADH) and an aldehyde dehydrogenase (ALDH), convert trans-chrysanthemol to trans-chrysanthemic acid (Xu et al., 2018). The reactions and enzymes involved in the conversion of trans-chrysanthemic acid to pyrethric acid have not yet been elucidated, although hydroxylation, oxidation, and methylation reactions are likely involved (Fig. 1; Xu et al., 2018).
Fig. 1.
The biosynthetic pathway to trans-chrysanthemic and pyrethric acids.
The plant-produced pyrethrins are remarkable natural insecticides. Their target is the sodium transporter in the nerve cells of insects (Du et al., 2015). Binding of pyrethrins to this protein causes constant Na+ efflux, leading to continual firing of the nerve cells and thus to general exhaustion and eventual death of the organism. Their lack of toxicity against most mammals is due mostly to efficient detoxification by hydrolysis in the digestive system (Katsuda, 2012). An important feature of these natural pesticides is that they do not persist in the environment (Demoute, 1989), because they are easily degraded by sunlight and oxidation, making them one of a small number of insecticides available to organic growers. This lability is important in preventing the evolution of resistance in insects, but it has also limits the use of pyrethrins to controlling insects by spraying indoors or on clothing, and they have been particularly important in controlling mosquito-borne diseases such as malaria (Duchon et al., 2009).
Pyrethrins are obtained by oil-based extraction of ground T. cinerariifolium flowers and are typically applied by spraying. Currently, most of the production of natural pyrethrins is carried out in Kenya, Tanzania, Rwanada, Tasmania and China. While current production levels are not publicly available, the most recent study reported that production did not meet demand and that the overall market for natural pyrethrins in the USA was approaching $500 million (USD) (Hitmi et al., 2000). The ability to produce pyrethrins in large amounts and at higher concentrations could address the current limitations on their availability.
The tomato fruit potentially is an excellent plant system for making natural pyrethrins. Breeders have developed tomato varieties with very high biosynthetic flux to C40 tetraterpenes biosynthesis in the fruits, in particular to the red carotenoid pigment lycopene, and this crop is grown efficiently in many parts of the world. frans-Chrysanthemic acid and pyrethric acids, constituents of pyrethrins, share DMAPP, one of the two final products of the plastidic methylerythritol phosphate (MEP) pathway, as a common precursor with carotenoids, and both classes of compounds are synthesized in the plastids (Hirschberg, 2001; Rivera et al., 2001). Given the high levels of DMAPP available in the fruit, tomato fruits would be an ideal system for making at least the monoterpene acid moiety of pyrethrins. Furthermore, the fruit is a terminal structure that develops after the entire plant has grown, and it serves as a “sink” for nutrients. Therefore, redirecting DMAPP and IPP toward trans-chrysanthemic acid biosynthesis (using fruit-ripening promoters) instead of lycopene biosynthesis should not have any adverse consequences on the robust growth and photosynthetic activity of the ve getative parts of the plant. In contrast, diverting IPP and DMAPP from the biosynthesis of carotenes, which are essential for the proper function of photosynthesis, in leaves may have a negative effect on growth of the plant, as has previously been observed (e.g., Rossi et al., 2017).
Various microbial systems have been proposed as alternatives to making plant terpenes (Peralta-Yahya et al., 2011). However, the plant terpenes most successfully produced in these systems are sesquiterpenes but not monoterpenes (Peralta-Yahya et. al, 2011). The exact reasons are not yet clear but may be due in part to the fact that microbes (both bacteria and fungi) already have the enzymes to make farnesyl diphosphate (FDP), the precursor of sesquiterpenes (and sterols) but not geranyl diphosphate, the common precursor of monoterpenes. An additional and serious hurdle is that many monoterpenes are toxic to microorganisms (Zhang et al., 2017). The production of specialized plant chemicals in microbes also requires a carbon source and energy, and usually both are provided by the addition of a sugar, which is obtained from crop plants (usually sugarcane or sugar beet), countering the argument that microbial bioengineering systems do not compete with food production (although for specialty compounds the effect is in either case quite small). Thus, the use of a plant system that is already naturally optimized for the production of substrates for terpenoid biosynthesis could make the process less complex and therefore more likely to succeed, as well as less costly since energy from sunlight and carbon (in the form of CO2) will be provided by the environment. Furthermore, the tomato fruits can then be harvested by existing, well developed machinery and chemicals extracted for use (see Zhang et al., 2014 for a similar argument).
We previously showed that when TcCDS was transiently expressed in Nicotiana benthamiana leaves, trans-chrysanthemic acid and some derivatives were observed in addition to the expected chrysanthemyl diphosphate and trans-chrysanthemol products (Xu et al., 2018). This result is consistent with the hypothesis that the host plant contains one or more endogenous alcohol dehydrogenases (ADH) and aldehyde dehydrogeneases (ALDH) that could convert trans-chrysanthemol to trans-chrysanthemic acid. Here we report the extension of our efforts to produce high levels of trans-chrysanthemic acid in a heterologous plant system by obtaining stable transgenic lines of the cultivated tomato (Solanum lycopersicum) that express the gene for CDS and two genes from a wild tomato species, Solanum habrochaites, encoding an ADH and an ALDH that are capable of carrying out successive oxidation from trans-chrysanthemol to trans-chrysanthemic acid, each under the control of a fruit-specific promoter. The production of specific terpenoid intermediates in the pyrethrin pathway as well as sideproducts was monitored in the ripened fruit. The results indicate that engineering high levels of trans-chrysanthemic acid biosynthesis in tomato fruit is feasible.
2. Materials and methods
2.1. Vector construction and generation of tomato transgenic plants
The complete open reading frame (ORF) of TcCDS was synthesized using the sequence provided by Rivera et al. (2001) and spliced into the binary vector pBIN19 carrying the tomato fruit specific polygalacturonase (PG) promoter and PG terminator as well as kanamycin-resistance marker gene NPTII driven by CaMV 35S promoter (Nicholass et al., 1995). The TcCDS ORF was spliced between the PG promoter and the PG terminator using the BamHI and SpeI restriction sites. The TcCDS sequence in Rivera et al. (2001) is missing the first 4 amino acids of the transit peptide, as determined by Yang et al. (2014). Nevertheless, both versions of TcCDS ORFs direct the synthesis of identical mature proteins, each containing a transit peptide that causes the proteins to be imported into the plastids (see Supplemental Fig. S1; subcellular localization was determined by GFP tagging and confocal microscopy according to Falara et al. (2011)).
The coding region of ShADH (ortholog of Solyc03g044200 from Solanum habrochaites LA1777; See Supplemental Table S1) was obtained by PCR on cDNA from trichomes of S. habrochaites LA1777 using primers SBE634_ADH_F and SBE635_ADH_R (Supplemental Table S1). The product was cloned in the level 0 vector pICH41308 of the Golden Gate modular cloning system (Engler et al., 2014). The resulting plasmid pAGT1217 was then used to construct recombinant binary vectors. Similarly, the coding region of ShALDH (ortholog of So-lyc06g060250 from S. habrochaites LA1777) was amplified from trichome cDNA of S. habrochaites LA1777 using primers SBE537_ALDH_F (Supplemental Table S1), the underlined A replaces a G to remove a Bpil restriction site in the original sequence) and SBE538_ALDH_R and cloned into pICH41308. The resulting plasmid, pAGT918, was then used to construct binary vectors for tomato transformation. The coding regions of ShADH and ShALDH were amplified from plasmids pAGT1217 and pAGT918 respectively and introduced into the binary vector pBIN19 carrying PG promoter and PG terminator at AgeI and SpeI restriction sites.
A binary vector carrying a single gene (TcCDS, ShADH or ShALDH) was introduced into S. lycopersicum cultivar MP1 plants by the University of Nebraska Plant Transformation Facility (http://biotech.unl.edu/plant-transformation). Transgenic plants rooted on kanamycin selection were transferred to soil and grown in a greenhouse with 14/10hday/night photoperiod at 22 °C. Positive transgenic tomato plants were further verified by genomic DNA PCR for the presence of corresponding genes.
Crossing tomato plants was performed as previously described (Kimura and Sinha, 2008). The seeds from crossed flowers germinated on kanamycin selection were transferred to soil and grown in the same conditions as described above. Positive transgenic tomato plants were further verified by genomic DNA PCR for the presence of corresponding genes.
2.2. RNA isolation and qRT-PCR analysis
Total RNA was isolated from ripening pericarps of tomato fruits using the Total RNA Isolation Kit from Omega Biotek containing a DNA digestion step using the manufacturer’s protocol. cDNAs were prepared using the High Capacity cDNA Reverse Transcription Kit from Thermo Fisher Scientific following the manufacturer’s instructions. Primer design was performed using the PrimerExpress software (Supplemental Table S1), and Real-time PCR was performed using the Stepone Realtime PCR system (Applied Biosystems). Assays were done in six independent biological replicates (separate plants growing in the same growth facility). The relative transcript levels for different genes were normalized to that of elongation factor 2 using LinRegPCR software (http://www.hartfaalcentrum.nl/index.php?main=files&fileName=LinRegPCR.zip&description=LinRegPCR:%20qPCR%20dat%20analysis&sub=LinRegPCR).
2.3. GC-MS analysis and LC-MS analysis
For GC-MS analysis, a 1 μl aliquot of sample was injected into a Shimadzu QP-2010 GC-MS system equipped with the Rxi-5Sil column (30 m × 0.25 mm × 0.25 μm film thickness, RESTEK, USA). Helium (1.4 ml/min) was used as a carrier gas with split mode at a ratio of 1:2. The injection temperature was set at 240 °C, and the interface temperature was 280 °C. The oven temperature program was as follows: initial temperature, 50 °C for 3 min, followed by a ramp from 50° to 110°C at a rate of 10 °C min−1 and then from 110 °C to 150 °C at a rate of 5 °C min−1, held for 3 min, and finally increased to 300 °C at a rate of 10 °C min−1, held for 3 min the identification of the volatiles was assigned by comparison of their retention times and mass fragmentations with those of literature and NIST library and by comparison of spectral data with standards.
For LC-MS analysis, samples (10 μl injection volume) were applied to an Ascentis Express C18 column (100 × 2.1 mm, 2.7 μm particle size; Supelco) at 40 °C. Liquid chromatography was carried out using an Acquity UPLC system (Waters). Solvents used for liquid chromatography were water + 0.1% formic acid (solvent A) and acetonitrile (solvent B). A 0.3 ml/min flow rate was used. The LC gradient started at 99% solvent A, 1% solvent B and increased to 99% solvent B over 16 min following a linear gradient. Solvent B was held at 99% for 2 min followed by a return to 99% solvent A for 2 min. Mass spectrometric analysis was carried out with a Xevo G2-XS Q-ToF instrument (Waters). Electrospray ionization was employed in negative-ion mode using 2.0 kV capillary voltage, 40 V sample cone voltage, and 100 °C source temperature. Desolvation gas flow of 600 L/h and temperature of 350 °C were used.
2.4. Extraction and analysis of trans-chrysanthemic acid and related compounds from tomato fruits
The tomato fruit achieves full size while still green, and the point in which the full-size green fruit shows the first sign of red color is called “breaker” (Gillaspy et al., 1993). Pericarps of tomato fruits beyond the breaker stage (as specified) were collected and ground into fine power in liquid nitrogen. For measurement of free trans-chrysanthemic acid, 6g of fruit homogenate was extracted at room temperature with shaking at 50 rpm overnight with 6.0 ml MTBE containing 0.002 ng/μl tetradecane as internal standard. The MTBE layer was transferred to a new tube, followed with dehydration by anhydrous Na2SO4, and then concentrated by evaporating the solvent under vacuum for 10–15 min to a final volume of about 0.5 ml. The sample was analyzed with a Rxi-5Sil column on a Shimadzu QP-2010 GC-MS system as previously described (Xu et al., 2018). The concentration of trans-chrysanthemic acid was calculated based on a standard curve with compound obtained from Sigma Inc. (catalog number 18509). Total trans-chrysanthemic acid including free and conjugated trans-chrysanthemic acid was measured by first treating the homogenate with NaOH, as previously described (Xu et al., 2018), and continuing with the procedure described above. For measurement of free trans-chrysanthemol and trans-chrysanthemal, fruit homogenates (1 g per sample) were extracted with 0.5 ml hexane containing 0.01 ng/μl tetradecane as internal standard at room temperature with shaking at 50 rpm for 3 h. The hexane extract was analyzed by GC-MS. The concentration of free trans-chrysanthemol and trans-chrysanthemal was calculated based on the standard curve of commercial mix of trans and cis-chrysanthemol. For measurement of total trans-chrysanthemol, 0.5 g of fruit homogenates were treated with almond β-glucosidase (Sigma Inc., catalog number G0395) at 37 °C for 2 h, followed by extraction at room temperature with shaking at 50 rpm for 3 h with 0.5 ml hexane containing 0.01 ng/μl tetradecane as internal standard. The hexane extract was analyzed by GC-MS and total trans-chrysanthemol was calculated as above. For analysis of chrysanthemyl diphosphate, 1 g of fruit homogenates were treated by 0.2 M HCl at room temperature for 30 min, and then extracted and analyzed by the same way as described above for analysis of trans-chrysanthemol and trans-chrysanthemal. To measure the concentration of CDP, we treated the samples with 0.2 M HCl, which under these conditions causes hydrolysis of CDP and rearrangement of the resulting trans-chrysanthemol into Yomogi alcohol and Artemisia alcohol, but not hydrolysis of trans-chrysanthemyl glycosides (Supplemental Fig. S2 and S3). Following this treatment, CDP concentrations were calculated from measuring the concentrations of the Yomogi alcohol and Artemisia alcohol.
Previously, we reported the detection of chrysanthemol malonylglycoside and chrysanthemic acid malonylglycoside in Nícotiana benthamiana leaves transiently expressing single TcCDS and co-infiltration with TcCDS, TcADH2 and TcALDH1 (Xu et al., 2018), and such Nícotiana benthamiana material was used here as a source of these compounds as markers. N. benthamiana leaf tissue or S. lycopersicum fruit tissue was powdered in liquid nitrogen. To 0.1 g of powdered tissue was added 0.5 ml of 80% acetonitrile, 20% water with 0.1% formic acid and 10 μM propyl-4-hydroxybenzoate (internal standard). Extracts were centrifuged for 10 min at 10,000g and supernatant transferred to LC vials. Extract supernatant was analyzed by LC-MS using a Xevo G2-XS Q-ToF mass spectrometer (Waters, Inc.) as previously described (Xu et al., 2018).
The measurement of lycopene contents of ripened fruits from different transgenic tomato plants and MP1 was performed as previously described (Gutensohn et al., 2014).
2.5. Enzymatic assays of recombinant TcCDS, ShADH and ShALDH
The truncated open reading frame of TcCDS missing the first 50 codons was synthesized and cloned into the pEXP5-CT/TOPO vector, generating a fusion gene that encodes a “tag” of HIS6 residues at the C- terminus. The complete ORF of ShADH and ShALDH (Supplemental Table S1) were amplified from plasmids pAGT1217 and pAGT918 respectively and introduced into the expression vector pET28a+ or pHIS8, in each case generating a fusion gene construct encoding a “tag” of HIS6 residues at the N-terminus. The expression and purification of the recombinant proteins were performed as described previously (Xu et al., 2013).
For enzymatic assay of TcCDS, 30 μg affinity-purified His-tagged enzyme was incubated at 30 °C for 3 h with 0.4 mM DMAPP, in a final volume of 50 μl of assay buffer containing 50 mM Tris-HCl (PH 7.5), 2 mM DTT, 5 mM MgCl2. The produced CDP was hydrolyzed by 5 units of Roche rAPid alkaline phosphatase (Sigma) at 37 °C for 1 h following the manufacturer’s instructions or 0.2 N HCl at room temperature for 30 min. The reaction products were extracted by 100 μl MTBE and analyzed by GC-MS.
For enzymatic assay of ShADH, 15 μl eluted protein from corresponding recombinant vector was incubated at 30 °C for overnight with 1 mM mix of trans-and cis-chrysanthemol, in a final volume of 50 μl of assay buffer containing 50 mM Tris-HCl (PH 7.5), 2 mM DTT, 1 mM NAD+. For enzymatic assay of ShALDH, a coupled assay was performed by 30 °C overnight incubation of reaction mix composed of 15 μl eluted protein of ShADH and 15 μl eluted protein of ShALDH from corresponding recombinant vectors with 1 mM mix of trans-and cis-chry-santhemol, in a final volume of 50 μl containing 50 mM Tris-HCl (PH 7.5), 2mM DTT, 1 mM NAD+. Reaction products were extracted with 100 μl MTBE and analyzed by GC-MS.
3. Results
3.1. Identification of TcCDS transgenic tomato lines producing trans-chrysanthemic acid
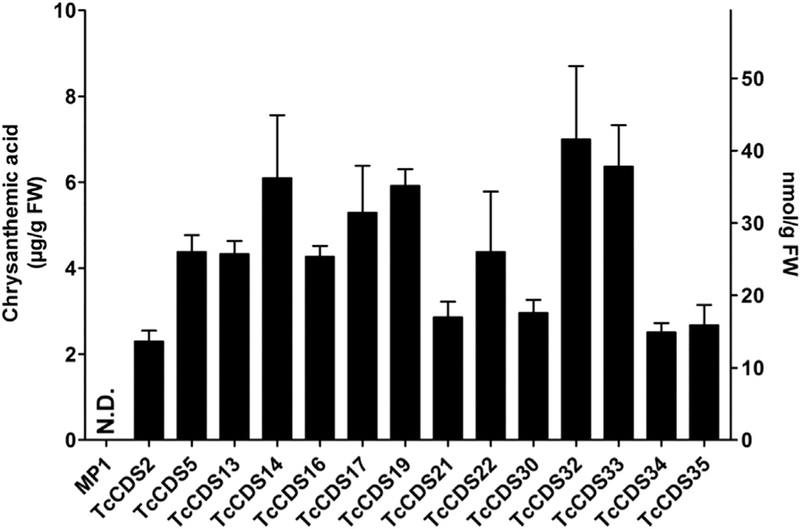
In contrast to the non-transgenic parent tomato line, 2.3–7.0 μgg−1 fresh weight of trans-chrysanthemic acid was detected in ripened fruits of 14 independent TcCDS transgenic lines (Fig. 2). The transgenic line designated as TcCDS32 had the highest concentration of trans-chrysanthemic acid (7.0 μgg−1 fresh weight) and this line was used for analysis of precursors of trans-chrysanthemic acid. We observed that mature fruits of TcCDS32 contained 1.6 μg g−1 fresh weight of trans-chrysanthemol and 0.35 μgg−1 fresh weight of trans-chrysanthemal. The concentration of CDP, analyzed by acid hydrolysis and measuring the resulting monoterpenes yomogi alcohol and artemisia alcohol (Supplemental Fig. S2), was determined to be 3.7 μgg−1 fresh weight.
Fig. 2.
Concentration of trans-chrysanthemic acid in ripened fruits (at the 15th day after breaker, Br + 15) of 14 transgenic tomato lines expressing TcCDS under the control of the PG promoter. Y-axis on the left shows concentrations in μg, and the y-axis on right shows concentration in nmoles. N.D., Not detected.
3.2. Reconstruction of the complete pathway to trans-chrysanthemic acid in tomato fruit
It has been noted that when TcCDS is expressed in a heterologous plant system, appreciable trans-chrysanthemol production is also observed (Xu et al., 2018; Yang et al., 2014). trans-Chrysanthemol production could be the direct outcome of CDS catalysis (Yang et al., 2014), or the action of endogenous non-specific phosphatases might be responsible (Xu et al., 2018). In T. cinerariifolium, trans-chrysanthemol is enzymatically oxidized to trans-chrysanthemal and then to trans-chrysanthemic acid by specific dehydrogenases (Xu et al., 2018), but such oxidation reactions also occur in the leaves of N. benthamiana expressing CDS (Xu et al., 2018) and in tomato fruits, as shown above (Fig. 2). However, the presence of CDP, trans-chrysanthemol, and trans-chrysanthemal in addition to trans-chrysanthemic acid in TcCDS transgenic tomato fruits suggested the hypothesis that the yield of trans-chrysanthemic acid could be increased if the rate of such oxidation reactions were enhanced.
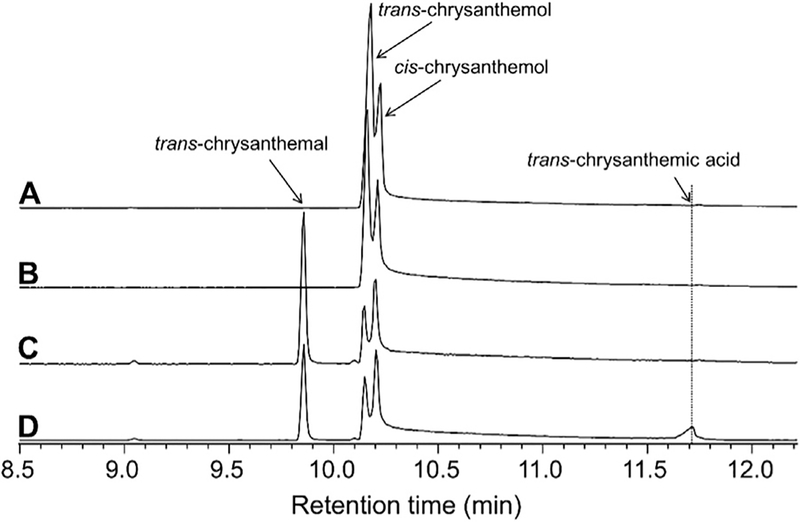
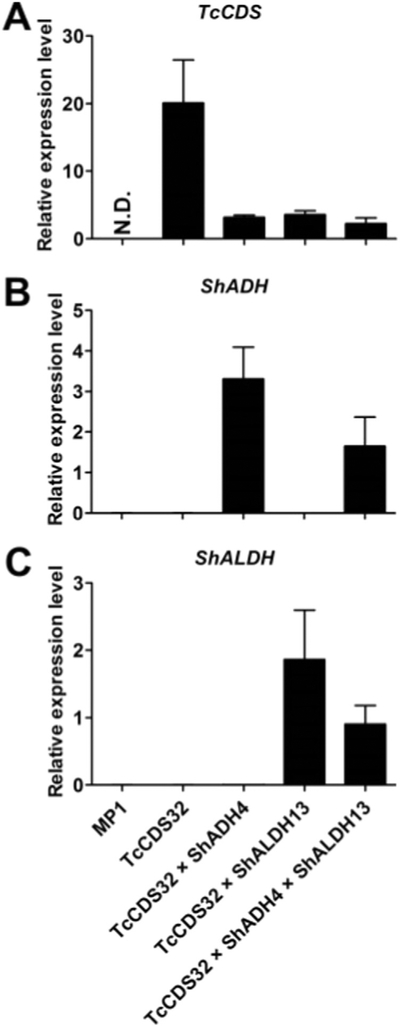
To test this theory, we obtained transgenic tomato plants that express a Solanum habrochaites gene encoding a sesquiterpene alcohol dehydrogenase (ShADH, the ortholog of Solyc03g044200 from S. habrochaites LA1777) under the control of the PG promoter. We also generated transgenic tomato plants that express a Solanum habrochaites gene encoding a sesquiterpene aldehyde dehydrogenase (ShALDH, ortholog of Solyc06g060250 from S. habrochaites LA1777) also under the control of the PG promoter. In S. habrochaites LA1777, ShADH and ShALDH catalyze two successive steps in the oxidation of santalenol and bergamotenol to the respective carboxylic acids, santalenoic and bergamotenoic acids, which are the major products of type VI glandular trichomes in this accession (Bennewitz et al., in prep). In vitro enzymatic assays of ShADH and ShALDH showed that these two enzymes can sequentially oxidize trans-chrysanthemol to trans-chrysanthemic acid in the presence of NAD+ (Fig. 3). Transcript levels of four independent ShADH transgenic tomato lines and six positive ShALDH transgenic tomato lines were examined in ripening fruits (Supplemental Fig. S4). ShADH line 4 and ShALDH line 13 had the highest expression level among ShADH and ShALDH lines transgenic lines, respectively. Therefore these lines were used in sequential crosses with the TcCDS32 transgenic to yield progeny plants that harbor TcCDS and ShADH, TcCDS and ShALDH, as well as plants that contain all three genes. qRT-PCR analysis of transcript levels in the ripened fruits of these lines showed that in the line expressing all three genes, each of these genes was expressed, although at somewhat lower levels than observed in lines containing each gene alone or lines containing only two heterologous genes (Fig. 4). The reduction in transcript level was particularly noticeable for TcCDS, with a transcript level in the line harboring all three genes that was only 11% of that in line TcCDS32. This reduction may have been due in part to the fact that all three heterologous genes contained the same promoter, PG, and thus the three promoters may compete for the same transcription factors.
Fig. 3.
GC-MS analysis of MTBE extracts of in vitro enzymatic assay of ShADH and ShALDH. All reactions were overnight. A, Products obtained after incubating 15μl eluted protein from empty vector with 1 mM mix of trans and cischrysanthemol in the presence of 1 mM NAD+. B, Products obtained after incubating 15 μl eluted ShALDH protein with 1 mM mix of trans and cis-chrysanthemol in the presence of 1 mM NAD+. C, Products obtained after incubating 15 μl eluted ShADH protein with 1 mM mix of trans and cischrysanthemol in the presence of 1 mM NAD+. D, Products obtained after incubating 15 μl eluted ShADH protein and 15 μl eluted ShALDH protein with 1 mM mix of trans and cis-chrysanthemol in the presence of 1 mM NAD+.
Fig. 4.
qRT-PCR analysis of transcript levels in ripening fruits (at the 3rd day after breaker, Br + 3) of non-transgenic tomato and different transgenic lines. A, Transcript levels of TcCDS. B, Transcript levels of ShADH. C, Transcript levels of ShALDH. Results are expressed relative to that of elongation factor 2 (means ± SD, n = 6). N.D., Not detected.
3.3. Production of trans-chrysanthemic acid and related compounds in transgenic tomato fruits expressing CDS, ADH and ALDH
While tomato fruits expressing TcCDS alone produced some trans-chrysanthemal and trans-chrysanthemic acid, the concentration of the desired product, trans-chrysanthemic acid, was expected to increase in transgenic tomato fruit expressing ShADH and ShALDH in addition to TcCDS. We therefore measured the concentration of CDP, trans-chrysanthemic acid and related compounds in ripened fruits of different transgenic tomato plants expressing TcCDS, ShADH and ShALDH and combinations thereof.
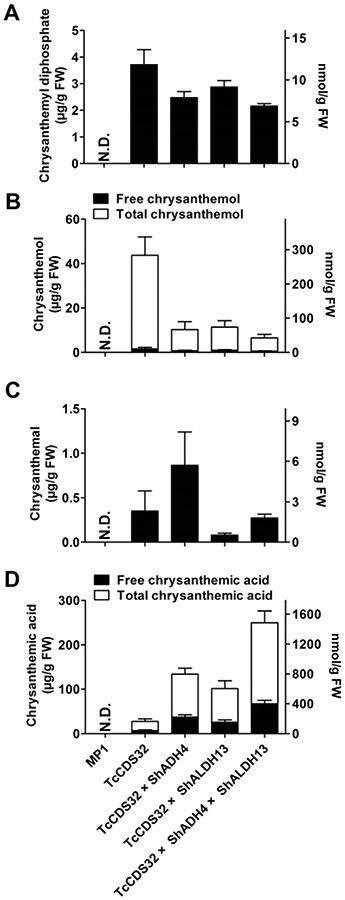
The concentration of CDP, which was 3.7 μgg−1 fresh weight in the TcCDS-expressing line TcCDS32, decreased to 2.5, 2.9, and 2.2 μgg−1 fresh weight respectively in lines TcCDS32 × ShADH4, TcCDS32 × ShALDH13 and TcCDS32 × ShADH4 × ShALDH13 (Fig. 5A). Similarly, the concentration of free trans-chrysanthemol, present at 1.6 μgg−1 fresh weight in TcCDS32, decreased to 0.72, 0.98 and 0.58 μg g−1 fresh weight respectively in lines TcCDS32 × ShADH4, TcCDS32 × ShALDH13 and TcCDS32 × ShADH4 × ShALDH13 (Fig. 5B). Since previous reports indicated that heterologously produced trans-chrysanthemol may be glycosylated (Xu et al., 2018; Yang et al., 2014), we treated fruit extracts with β-glucosidase and measured the levels of total free trans-chrysanthemol, calculating the amount of glycosylated trans-chrysanthemol as the difference between the total and free trans-chrysanthemol levels. Our results indicate that most of the trans-chrysanthemol produced in all transgenic lines was found to be glycosylated, with the concentration of total trans-chrysanthemol measured at 42.2, 9.6, 10.4 and 6.0 μg g−1 fresh weight respectively in lines TcCDS32, TcCDS32 × ShADH4, TcCDS32 × ShALDH13 and TcCDS32 × ShADH4 × ShALDH13 (Fig. 5B). LC/MS analysis indicated that the glycosides of trans-chrysanthemol in tomato fruit were malonylated glucose or related hexoses (Supplemental Fig. S5E,G), similar to those observed in plants of two tobacco species, N. tabacum and N. benthamiana, expressing TcCDS (Supplemental Fig. S5B; Xu et al., 2018; Yang et al., 2014).
Fig. 5.
Production of trans-chrysanthemic acid and its precursors in ripened transgenic tomato fruits (Br + 15) expressing TcCDS, ShADH and ShALDH under the control of PG promoter. A, Concentration of CDP. B, Concentration of trans- chrysanthemol. C, Concentration of trans-chrysanthemal. D, Concentration of trans-chrysanthemic acid. All the data are means ± SD (n ≥ 6). Y-axis on the left shows concentrations in μg, and the y-axis on right shows concentration in nmoles. N.D., Not detected.
As expected, the trans-chrysanthemal concentration, 0.35 μg g−1 fresh weight in TcCDS32, increased to 0.86 μg g−1 fresh weight in the TcCDS32 × ShADH4 line but was only 0.079 and 0.27 μgg−1 f resh weight in TcCDS32 × ShALDH13 and TcCDS32 × ShADH4 × ShALDH13 (Fig. 5C).
Measurements of trans-chrysanthemic acid in fruits of the triple transgenic line TcCDS32 × ShADH4 × ShALDH13 showed a large increase in the concentration of this acid. The 67.1 μg g−1 fresh weight of free trans-chrysanthemic acid in this line represented an 8.6-fold increase over the concentrations found in the TcCDS32 line (7.00 μg g−1 fresh weight). Lines TcCDS32 × ShADH4 and TcCDS32 × ShALDH13 had concentrations of free trans-chrysanthemic acid representing 4.3-fold and 2.7-fold increases, respectively, compared with those in TcCDS32 fruits. Heterologously produced trans-chrysanthemic acid in N. benthamiana leaves was also reported to be mostly in the form of malonylglycosides (Xu et al., 2018), and analysis of trans-chrysanthemic acid in ripened tomato fruits of transgenic plants showed that a large portion of it is indeed present as trans-chrysanthemic acid malonylglycosides (Supplemental Fig. S5F,H), similar to those found in transgenic tobacco plants that produce trans-chrysanthemic acid (Supplemental Fig. S5C; Xu et al., 2018). The concentrations of conjugated trans-chrysanthemic acid, calculated by subtracting the total trans-chrysanthemic acid detected after base hydrolysis from the measured levels of free trans-chrysanthemic acid, were determined to be 13.6, 60.0, 50.3 and 115.8 μg g−1 fresh weight in ripe tomato fruits of TcCDS32, TcCDS32 × ShADH4, TcCDS32 × ShALDH13 and TcCDS32 × ShADH4 × ShALDH13 lines respectively. These levels represent 1.9-, 1.6-, 2.0-and 1.7-fold higher levels compared with the concentrations of free trans-chrysanthemic acid in the corresponding transgenic tomato fruits respectively (Fig. 5D). Overall, co-expression of ShADH and ShALDH in fruit of TcCDS32 line enhanced the accumulation of total trans-chrysanthemic acid, defined as free plus glycosylated forms, up to 183.0 μg g−1 fresh weight – with 62% of it in the glycosylated form – from 20.6 μg g−1 fresh weight in tomato fruits expressing TcCDS only, representing an 8-fold increase (Fig. 5D).
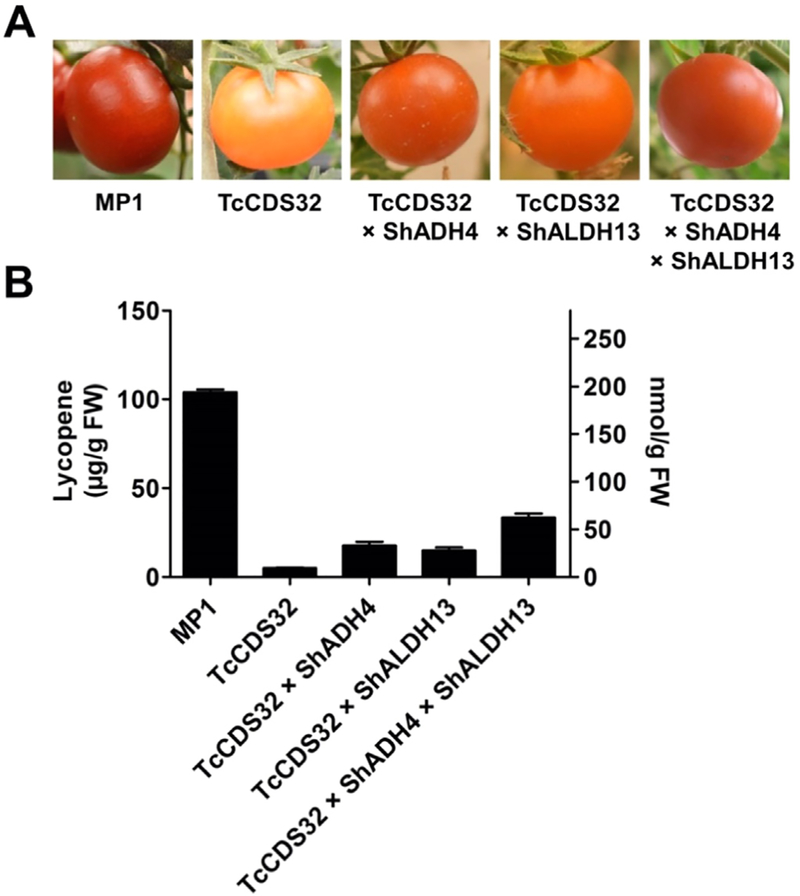
The use of DMAPP by TcCDS in ripening tomato fruits was predicted to compete with the synthesis of the red pigment lycopene, the most abundant terpenoid synthesized during fruit maturation in the tomato cultivar MPI used in this study and in other red fruited varieties. Indeed, ripe tomato fruit expressing TcCDS alone were substantially less red than corresponding non-transgenic fruits (Fig. 6A), with lycopene accumulation decreasing by 95%, at 5.0 μg g−1 fresh weight compared with 104.0 μg g−1 fresh weight in non-transgenic fruits (Fig. 6B). Surprisingly, lycopene in ripened fruits of TcCDS32 × ShADH4, TcCDS32 × ShALDH13 and TcCDS32 × ShADH4 × ShALDH13 plants was higher than in TcCDS plants, with 17.6, 15.0, and 33.6 μg g−1 fresh weight, respectively.
Fig. 6.
Coloration and lycopene contents of ripened fruits (Br + 15) of control and transgenic tomato plants. A, Phenotype of S. lycoperiscum non-transgenic control, TcCDS32, TcCDS32 × ShADH4, TcCDS32 × ShAlDH13 and TcCDS32 × ShADH4 × ShALDH13 plants. B, Lycopene concentrations of ripened fruits of non-transgenic control, TcCDS32, TcCDS32 × ShADH4, TcCDS32 × ShAlDH13 and TcCDS32 × ShADH4 × ShALDH13 plants. All data points are means ± SD (n ≥ 6). Y-axis on the left shows concentrations in μg, and the y-axis on right shows concentration in nmoles.
4. Discussion
4.1. Tomato fruits are a good platform to produce trans-chrysanthemic acid
Ripening tomato fruits synthesize high levels of terpenoids in the carotenoid sublcass (> 90% in the form of lycopene), and the plastidic MEP pathway that produces DMAPP and its isomer IPP, the 5-carbon isoprene building blocks of all terpenes, is highly active. For this reason, tomato fruits were previously utilized as a platform to produce monoterpene flavor compounds such as linalool, geraniol, nerol, and α-phellandrene. Linalool production was achieved by introducing the linalool synthase gene from Clarkia breweri under the control of fruitripening promoter E8 (Lewinsohn et al., 2001). Geraniol production was achieved by introducing basil (Ocimum basilicum) geraniol synthase gene (GES) under the control of the PG promoter (Davidovich-Rikanati et al., 2007), the snapdragon gene encoding the small subunit gene of geranyl diphosphate synthase (GPP-SSU) under the PG promoter (Gutensohn et al., 2013), or by combining both (Gutensohn et al., 2013). Nerol production was achieved by expressing tomato neryl diphosphate synthase gene (NDPS1) under the control of the PG promoter, and the production of α-phellandrene was achieved by creating a transgenic tomato coexpressing both NDPS1 and the tomato phellandrene synthase gene (PHS1), both under the control of the PG promoter (Gutensohn et al., 2014). The general conclusions that could be derived from these experiments were that combining two genes in the pathway, such GPPS-SSU and GES, increased yield of the desired product.
However, in none of these experiments did the total yield of new monoterpenes (by mass) exceeded 5% of the original mass of lycopene in the non-transgenic plants, which ranged from 80 to 180 μg g−1 fresh weight (possibly due to differences in the varieties used and the growing conditions). However, the amounts of lycopene in the transgenic fruits decreased by > 50%. In the case of the production of neryl diphosphate by NDPS1, this was shown to be due in part to the inhibitory effect of neryl diphosphate on geranylgeranyl diphosphate synthase (GGPPS), an enzyme that catalyzes the formation of a lycopene precursor. Finally, these experiments provided evidence that to mato fruits possess enzymes that can hydrolyze monoterpene diphosphates to the corresponding alcohols as well as further oxidize these alcohols to the respective acids. Glycosylation of the heterologously produced terpenes was not, however, investigated in these reports.
Our efforts to produce trans-chrysanthemic acid in tomato fruit began by constructing transgenic lines expressing TcCDS under the control of the PG promoter. Consistent with competition for the common pathway intermediate DMAPP, fruits of such transgenic lines exhibited 95% decrease in lycopene content, from 104 μg g−1 fresh weight in the non-transformed tomato parent line to as little as 5 μg g−1 fresh weight (Fig. 6). As predicted, these transgenic plants contained enzymes with broad specificity that were able to convert chrysanthemyl diphosphate (CDP) to trans-chrysanthemol and further oxidize it to trans-chrysanthemic acid. The latter two compounds were found mostly in glycosylated forms (Fig. 5). But the total production of these compounds, estimated at 63 μg g−1 fresh weight, was far lower than could be expected when 95% of the flux of precursor to lycopene biosynthesis is blocked.
To try to increase the yield of trans-chrysanthemic acid, we made transgenic tomato lines that express, in addition to TcCDS, two dehydrogenases, the first capable of catalyzing the oxidation of trans-chry-santhemol to trans-chrysanthemal, the second catalyzing the oxidation of trans-chrysanthemal to trans-chrysanhemic acid. Although the T. cinerariifolium enzymes and genes involved in these two steps were recently identified (Xu et al., 2018), these genes were not available when the current project was initiated, and we therefore used two S, hab-rochaites (wild tomato) genes, ShADH and ShALDH, which were demonstrated to sequentially oxidize trans-chrysanthemol to trans-chrysanthemic acid. In transgenic tomato fruit expressing TcCDS, ShADH, and ShALDH, the total yield of trans-chrysanthemic acid (63% of it found as a glycosylated compound) increased to 183 μg g−1 fresh weight, representing 8.9-fold increase over total levels of trans-chrysanthemic acid produced in fruits of transgenic plants expressing TcCDS alone.
While the fruits of TcCDS, ShADH, and ShALDH plants accumulated roughly a third of the lycopene that non-transgenic control plants produce (33.6 μg g−1 fresh weight vs. 104.0 μg g−1 fresh weight, see Fig. 6), the total yield of trans-chrysanthemic acid was almost twice the amount of lycopene made in the control plants. Comparing the yield in molar equivalents of C5 isoprene units is even more instructive. In non-transgenic plants, the concentration of the tetraterpene lycopene (i.e., a C40 compound containing eight isoprene units) is 193.8 nmol g−1 fresh weight, or 1550.4 nmol g−1 fresh weight equivalent of isoprene units. In transgenic plants expressing all three heterologous genes, the yield of trans-chrysanthemic acid (free and bound) was 1087.6 nmol g−1 fresh weight, or 2175.2 nmol g−1 fresh weight of isoprene units, and lycopene concentration was 62.5nmol g−1 fresh weight, for a total of 500.1 nmol g−1 fresh weight of isoprene units. Thus the total yield of isoprene units in trans-chrysanthemic acid and lycopene combined in the transgenic plants was 2675.3 nmol g−1 fresh weight, or 1.7-fold the concentration of lycopene in the non-transgenic plants. Because the concentration of lycopene in the transgenic plants was reduced to approximately one-third of its concentration in non-transgenic plants, these results indicate that the yield of the MEP pathway must have increased by about 2-fold in the fruit of the TcCDS, ShADH, and ShALDH plants, perhaps due to a feed-back mechanism that is sensitive to the levels of lycopene or some of its intermediates. A similar situation in tomato leaves was reported by Tieman et al. (2010), in which transgenic plants overexpressing a gene that methylates salicylic acid (SA) made high levels of methylsalicylates and actually synthesized more SA than control plants did, presumably because of the increased flux to SA.
Surprisingly, the level of lycopene produced in the TcCDS, ShADH, and ShALDH transgenics - while only a third of the level found in control plants - was actually 1.7-fold higher than the level found in plants expressing TcCDS alone, even though the triple transgenic plants produce higher levels of total CDS-depended products. This outcome is correlated with the decrease in the steady-state levels of some of the early intermediates produced in TcCDS transgenic plants, such as CDP or trans-chrysanthemol, presumably due to increase in flux toward trans-chrysanthemic acid. This correlation may indicate that at least one of these early intermediates may have inhibitory effects on enzymes involved in lycopene biosynthesis or on enzymes in the pathway leading to DMAPP and IPP.
4.2. The production of diversion products and possible countermeasures
It is commonly observed that a large portion of hydroxyl- and carboxyl-containing terpenoid compounds produced heterologously in plants are glycosylated, reducing the yield of the desired product. For example, in transgenic maize (Zea mays) expressing a geraniol synthase gene from Lippia dulcis, geranoyl-6-O-malonyl-β-D-glucopyranoside was the most abundant geraniol-derived compound (Yang et al., 2011). Likewise, Nicotiana benthamiana plants transiently expressing Artemesia annua genes specifying the biosynthesis of steps in the artemisinin biosynthetic pathway accumulated glycosylated versions of pathway intermediates (Ting et al., 2013; van Herpen et al., 2010).
By expressing all three genes necessary to produce trans-chrysanthemic acid in tomato fruit, we obtained levels of trans-chrysanthemic acid that were more than twice that predicted based on the amount of lycopene found in the fruits. Furthermore, 97% of the DMAPP used by TcCDS ended up as trans-chrysanthemic acid or its glycoside, and only 3% of the new products were intermediates short of trans-chrysanthemic acid, mostly in the form of the malonylglycoside of trans-chrysanthemol (Fig. 5). However, 62% of trans-chrysanthemic acid itself was converted into malonylglycosides. This, however, may not represent a serious problem. Converting the malonylglycoside of trans-chrysanthemic acid back to the free chrysanthemic acid is a relatively easy procedure involving simple alkaline hydrolysis (the malonylglycosides of trans-chrysanthemol, on the other hand, are not hydrolysable in base), so easy protocols for the purification of trans-chrysanthemic acid from tomato fruit could be developed. Furthermore, once the entire pathway for pyrethrin biosynthesis is constructed in tomato fruit, the enzymes that act to catalyze the formation of the ester between trans-chrysanthemic acid and the alcohol will be competing with the non-specific glycosyltransferases that act on trans-chrysanthemic acid, and with proper calibration of gene expression formation of the desired ester could be favored. Alternatively, genetic engineering methods to inactivate the specific glycosyltransferases involved could be applied.
A complete synthesis of pyrethrins will also require the synthesis of the alcohol moiety of these esters. This moiety is derived from jasmonic acid (JA) (Feussner and Wasternack, 2002; Ramirez et al., 2013), and since this compound is found universally in all flowering plants, engineering this part of the pathway in tomato fruit should be possible as well. However, the complete engineering of the production of pyrethrins in tomato or another heterologous host must await the detailed elucidations of the complete biochemical pathway leading to the three pyrethrin alcohols. Overall, our present results indicate that tomato fruits, which can be obtained in high yields by growing plants under normal agricultural practices and can be harvested mechanically, could be used as an efficient platform for producing the monoterpene moiety of pyrethrins.
Supplementary Material
Acknowledgements
We thank Prof. Daniel Jones of Michigan State University for his help with the LC-MS analysis. This publication was made possible by a predoctoral training award to D.L. from Grant Number T32-GM110523 from the National Institute of General Medical Sciences of the National Institutes of Health.
This work was supported by the National Science Foundation collaborative research grants 1565355 to EP and 1565232 to RLL.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ymben.2018.04.004.
References
- Davidovich-Rikanati R, Sitrit Y, Tadmor Y, Iijima Y, Bilenko N, Bar E, Carmona B, Fallik E, Dudai N, Simon JE, Pichersky E, Lewinsohn E, 2007. Enrichment of tomato flavor by diversion of the early plastidial terpenoid pathway. Nat. Biotechnol 25, 899–901. [DOI] [PubMed] [Google Scholar]
- Demoute J-P, 1989. A brief review of the environmental fate and metabolism of pyr-ethroids. Pest. Manag. Sci 27, 375–385. [Google Scholar]
- Du Y, Nomura Y, Zhorov BS, Dong K, 2015. Rotational symmetry of two pyrethroid receptor sites in the mosquito sodium channel. Mol. Pharmacol 88, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchon S, Bonnet J, Marcombe S, Zaim M, Corbel V, 2009. Pyrethrum: amixture of natural pyrethrins has potential for malaria vector control. J. Med. Entomol 46, 516–522. [DOI] [PubMed] [Google Scholar]
- Engler C, Youles M, Gruetzner R, Ehnert TM, Werner S, Jones JD, Patron NJ, Marillonnet S, 2014. A golden gate modular cloning toolbox for plants. ACS Synth. Biol 3, 839–843. [DOI] [PubMed] [Google Scholar]
- Falara V, Akhtar TA, Nguyen TT, Spyropoulou EA, Bleeker PM, Schauvinhold I, Matsuba Y, Bonini ME, Schilmiller AL, Last RL, Schuurink RC, Pichersky E, 2011. The tomato terpene synthase gene family. Plant Physiol. 157, 770–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feussner I, Wasternack C, 2002. The lipoxygenase pathway. Annu. Rev. Plant Biol. 53, 275–297. [DOI] [PubMed] [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W, 1993. Fruits: a developmental perspective. Plant Cell. 5, 1439–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutensohn M, Nguyen TT, McMahon RD 3rd, Kaplan I, Pichersky E, Dudareva N, 2014. Metabolic engineering of monoterpene biosynthesis in tomato fruits via introduction of the non-canonical substrate neryl diphosphate. Metab. Eng 24, 107–116. [DOI] [PubMed] [Google Scholar]
- Gutensohn M, Orlova I, Nguyen TTH, Davidovich-Rikanati R, Ferruzzi MG, Sitrit Y, Lewinsohn E, Pichersky E, Dudareva N, 2013. Cytosolic monoterpene bio-synthesis is supported by plastid-generated geranyl diphosphate substrate in transgenic tomato fruits. Plant J. 75, 351–363. [DOI] [PubMed] [Google Scholar]
- Hirschberg J, 2001. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 4, 210–218. [DOI] [PubMed] [Google Scholar]
- Hitmi A, Coudret A, Barthomeuf C, 2000. The production of pyrethrins by plant cell and tissue cultures of Chrysanthemum cinerariaefolium and Tagetes species. Crit. Rev. Biochem. Mol. Biol 35, 317–337. [DOI] [PubMed] [Google Scholar]
- Katsuda Y, 2012. Progress and future of pyrethroids. Top. Curr. Chem 314, 1–30. [DOI] [PubMed] [Google Scholar]
- Kimura S, Sinha N, 2008. Crossing tomato plants. CSH Protoc. 2008 (pdb prot5082). [DOI] [PubMed] [Google Scholar]
- Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam KH, Amar O, Lastochkin E, Larkov O, Ravid U, Hiatt W, Gepstein S, Pichersky E, 2001. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol. 127, 1256–1265. [PMC free article] [PubMed] [Google Scholar]
- Nicholass FJ, Smith CJ, Schuch W, Bird CR, Grierson D, 1995. High levels of ripening-specific reporter gene expression directed by tomato fruit polygalacturonase gene-flanking regions. Plant Mol. Biol 28, 423–435. [DOI] [PubMed] [Google Scholar]
- Peralta-Yahya PP, Ouellet M, Chan R, Mukhopadhyay A, Keasling JD, Lee TS, 2011. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AM, Yang T, Bouwmeester HJ, Jongsma MA, 2013. A trichome-specific linoleate lipoxygenase expressed during pyrethrin biosynthesis in pyrethrum. Lipids 48, 1005–1015. [DOI] [PubMed] [Google Scholar]
- Rivera SB, Swedlund BD, King GJ, Bell RN, Hussey CE Jr., Shattuck-Eidens DM, Wrobel WM, Peiser GD, Poulter CD, 2001. Chrysanthemyl diphosphate synthase: isolation of the gene and characterization of the recombinant non-head-to-tail monoterpene synthase from Chrysanthemum cinerariaefolium. Proc. Natl. Acad. Sci. USA 98, 4373–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L, Borghi M, Yang JF, Xie DY, 2017. Overexpression of Populus x canescens isoprene synthase gene in Camelina sativa leads to alterations in its growth and metabolism. J. Plant Physiol. 215, 122–131. [DOI] [PubMed] [Google Scholar]
- Tieman D, Zeigler M, Schmelz E, Taylor MG, Rushing S, Jones JB, Klee HJ, 2010. Functional analysis of a tomato salicylic acid methyl transferase and its role in synthesis of the flavor volatile methyl salicylate. Plant J. 62, 113–123. [DOI] [PubMed] [Google Scholar]
- Ting HM, Wang B, Ryden AM, Woittiez L, van Herpen T, Verstappen FW, Ruyter-Spira C, Beekwilder J, Bouwmeester HJ, van der Krol A, 2013. The metabolite chemotype of Nicotiana benthamiana transiently expressing artemisinin biosynthetic pathway genes is a function of CYP71AV1 type and relative gene dosage. New Phytol. 199, 352–366. [DOI] [PubMed] [Google Scholar]
- van Herpen TW, Cankar K, Nogueira M, Bosch D, Bouwmeester HJ, Beekwilder J, 2010. Nicotiana benthamiana as a production platform for artemisinin precursors. PloS One 5, e14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhang F, Liu B, Huhman DV, Sumner LW, Dixon RA, Wang G, 2013. Characterization of the formation of branched short-chain fatty acid:CoAs for bitter acid biosynthesis in hop glandular trichomes. Mol. Plant 6, 1301–1317. [DOI] [PubMed] [Google Scholar]
- Xu HY, Moghe GD, Wiegert-Rininger K, Schilmiller AL, Barry CS, Last RL, Pichersky E, 2018. Coexpression analysis identifies two oxidoreductases involved in the biosynthesis of the monoterpene acid moiety of natural pyrethrin insecticides in Tanacetum cinerariifolium. Plant Physiol. 176, 524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Stoopen G, Yalpani N, Vervoort J, de Vos R, Voster A, Verstappen FW, Bouwmeester HJ, Jongsma MA, 2011. Metabolic engineering of gereanic acid in maize to achieve fungal resistance is compromised by novel glycosylation patterns. Metab. Eng 13, 414–425. [DOI] [PubMed] [Google Scholar]
- Yang T, Gao L, Hu H, Stoopen G, Wang C, Jongsma MA, 2014. Chrysanthemyl diphosphate synthase operates in planta as a bifunctional enzyme with chrysanthemol synthase activity. J. Biol. Chem 289, 36325–36335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu Q, Cao Y, Feng X, Zheng Y, Zou H, Liu H, Yang J, Xian M, 2014. Microbial production of sabinene—a new terpene-based precursor of advanced biofuel. Microb. Cell Factor. 13, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xiao WH, Wang Y, Yao MD, Jiang GZ, Zeng BX, Zhang RS, Yuan YJ, 2017. Chassis and key enzymes engineering for monoterpenes production. Biotechnol. Adv 35, 1022–1031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.