So runs my dream, but what am I?
An infant crying in the night
An infant crying for the light
And with no language but a cry.
–Alfred, Lord Tennyson, 1849
DEFINITION AND BACKGROUND
Infant colic is a characteristic group of behaviors seen in young infants. The most prominent feature is prolonged crying. Additional characteristics, including clenching of the fists and flexion of the hips, have led to the suggestion that these behaviors are related to abdominal discomfort; thus the term “colic,”derived from kolikos, the Greek term for colon. A commonly used set of diagnostic criteria was proposed by Morris Wessel and colleagues,1 based on observations of 98 infants in the newborn nursery at Yale, 25 of whom had inconsolable crying. These criteria are summarized by the frequently quoted “rule of 3s”: crying by an otherwise healthy infant that lasts more than 3 hours per day on more than 3 days a week for more than 3 weeks.
In a most informative article in the New Yorker, Groopman2 quotes the British social anthropologist Sheila Kitzinger as stating the “sound of a crying baby…is just about the most disturbing, demanding, shattering noise we can hear.” He goes on to point out that the US military has used the sound of wailing infants as an instrument of psychological stress, piping recordings of their cries into the cells of detainees at Guantanamo Bay.
Infant fussing and crying can be quantified by a “Barr diary” (Fig. 1),3 which often shows increased crying after feedings. Crying occurs throughout the day but peaks in the hours between 6 am to 12 pm and 6 pm to 12 am (Fig. 2).1 Colic may be a factor in child abuse and infanticide.4,5 In one investigation of more than 100 cases of abusive head trauma to infants, forensic interrogation revealed that shaking was violent and repetitive in most cases. The parent, usually a father, reported that he shook the infant to stop the infant from crying in the most cases, without the intention of actually hurting the infant.6 Recently, a systematic review by Wolke and colleagues1 extracted Barr diary data from more than 8690 infants. They found that up to 25% of normal infants have colic at 6 weeks of age, compared to only 0.6% at 10–12 weeks of age.7
Fig. 1.
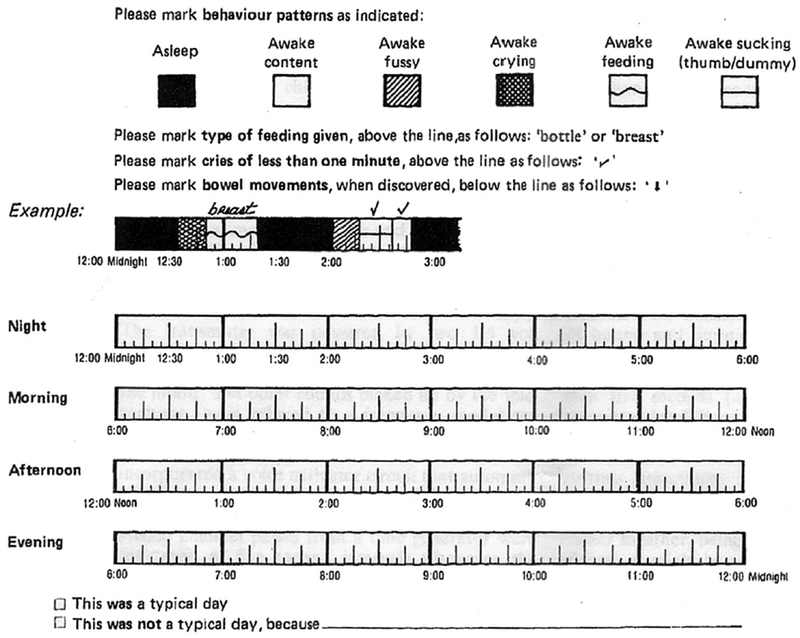
Barr diary. (From Barr RG, Kramer MS, Boisjoly C, et al. Parental diary of infant cry and fuss behavior. Arch Dis Child 1988;63:384; with permission.)
Fig. 2.
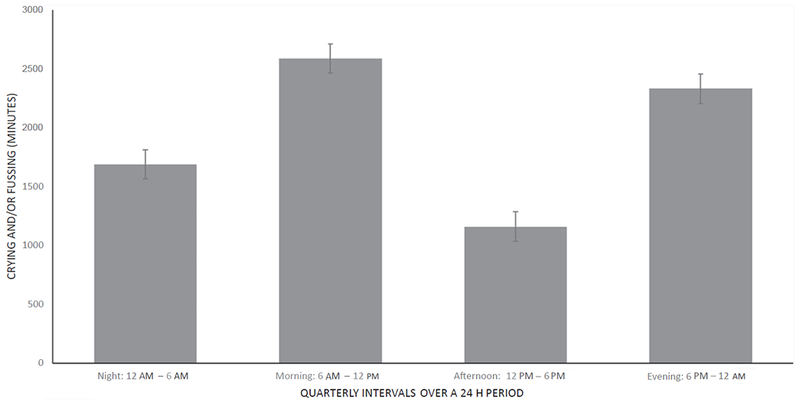
Daily breakdown of crying time in infants with colic: crying and fussing in minutes (n = 27). Means +/− SEM. (Adapted from Rhoads JM, Collins J, Fatheree NY, et al. Infant colic represents gut inflammation and dysbiosis. J Pediatr, in press; with permission.)
ETIOLOGY
The etiology of infantile colic is unknown, but is likely to be multifactorial. Proposed etiologies include gastrointestinal, hormonal, neurodevelopmental, and psychosocial factors.
Gastrointestinal
Several gastrointestinal disorders have been suggested to cause colic, as the infants often lift their legs and pass gas during the crying episodes.8 These factors, although controversial, include cow’s milk protein allergy or allergy to other substances in the maternal diet, excessive gas production, lactose intolerance, poor feeding technique, and dysbiosis.9
Cow’s milk protein allergy
The association between colic and cow’s milk protein allergy is equivocal.10 A previous study indicated that infantile colic is not associated with cow’s milk protein intolerance, based on similar prevalence of colic in formula-fed versus breast-fed infants, as well as a lack of intestinal damage (as determined by fecal alpha-1-antitrypsin and fecal hemoglobin) in colicky infants.11 In contrast, another study presented evidence of intestinal inflammation, with increased fecal calprotectin and less diverse fecal microflora in infants with colic; however, the difference could not be attributed to formula versus breast milk feeding.12 Several systematic reviews of clinical trials or randomized controlled trials in infants with colic have shown that the use of protein hydrolysate formulas decrease crying time in these infants.9,13,14 The limitations of most of these studies were an unclear method of randomization and/or inadequate blinding.9
Intolerance to other substances in the maternal diet
A maternal diet that consists of cruciferous vegetables (such as cauliflower, cabbage, garden cress, bok choy, broccoli, and brussels sprouts), cow’s milk, onion, and chocolate have also been suggested as a cause for colic, in theory related to colonic gas production.15 Many pediatricians recommend that mothers reduce cruciferous vegetables in their diet, although there is little proof that this is beneficial.
Gas production
It has been suggested that colicky infants have more intestinal gas produced as a result of colonic bacterial fermentation. Thirty years ago, an Australian study by Moore and colleagues16 suggested excessive H2 gas production was a problem in many infants with colic, with an initially high level of breath hydrogen decreasing as the colic resolves. We, too, found that baseline breath H2 was high in 25% to 50% of infants with colic,12,17 supporting bacterial overgrowth or simply the inability to excrete colonic gas effectively at this age. Of note, breath H2 level was also high in 25% of control infants without colic. Hyams and colleagues18 showed that most infants with and without colic had a positive lactulose breath H2 test, which is often used to diagnose bacterial overgrowth (defined by adult criteria of a rise of 10 parts per million after ingesting lactulose). They pointed out that many infants with massive rises in H2 gas production following lactulose did not cry or show any signs of discomfort. Thus, the literature is not convincing that excessive gas causes colic, although it may be a contributing factor.12,16,18–21
Lactose intolerance
In the previously mentioned study by Moore and colleagues,16 zero-time (baseline) breath H2 values were significantly higher, by twofold to fourfold, in colicky compared with noncolicky infants at both 6 weeks and 3 months. After lactose-containing milk, there were significantly more positive breath H2 tests (as defined by a breath hydrogen level that increased > 10 parts per million after milk ingestion) in colicky compared with noncolicky infants at 6 weeks (78% vs 36%) and 3 months (89% vs 45%).15 The findings suggested that lactose malabsorption could be important in this condition. Our group, however, found no significant differences between the postprandial levels of breath hydrogen when we compared healthy and colicky infants using a similar protocol.12 Randomized clinical trials of oral lactase administration to facilitate lactose hydrolysis have shown conflicting results in the treatment of infantile colic.22,23 Hence, the association between lactose malabsorption and colic is unclear. Our interpretation is that there is a high level of colonic bacterial fermentation in most infants at this age, but lactose intolerance by itself is unlikely to cause colic.
Poor feeding technique
Improper feeding technique, such as underfeeding or overfeeding, or infrequent burping, has been suggested to be a cause for colic.10 First-born infants have been reported to have an increased risk of colic in 2 studies.24,25 However, in both these studies and in our experience, later birth-order infants are frequently seen with colic. Overreporting or hypervigilance by primiparous parents (rather than parental ineptitude) may be a factor.
Hormonal
A higher level of serotonin has also been suggested to be a cause of infantile colic. Serotonin made in the gut affects mood and social behavior.26 According to one study, colicky infants had a higher level of random urinary 5-OH IAA, a metabolite of serotonin, as compared with the infants in the control group.27
Neurodevelopmental
Neurodevelopmental factors have also been proposed as one of the contributing etiologies.
Normal emotional development
Previous studies have shown that the crying pattern in colicky infants is similar to that of healthy infants (late afternoon and evening onset, peak crying at 2 months of age), although infants with colic have crying of longer duration and are harder to console. In one study, colic was described as a stage of normal emotional development, during which the infant has diminished capacity to regulate crying episodes.28
Migraine and colic
An association between maternal migraine and infantile colic has been shown in several case-control studies. It was shown that mothers with migraine were more than twice as likely to have infants with colic.8
“Missing fourth trimester”theory
According to this theory by Dr. Harvey Karp,29 a developmental specialist, infants are born too “early” by approximately 3 months (or a trimester), which results in inconsolable episodes of crying. According to this theory, they miss out on pleasant womb sensations (which can be mimicked by keeping them warm and quiet, swaddling them tightly, carrying them prone, and shaking them with a jiggling motion). Newborns and horses are compared in this book, with the newborn human being helpless and unable to take care of himself or herself, whereas a newborn horse starts running the same day. The inconsolable crying episodes resolve in most infants at approximately 3 months of age, at which time the infants start to “wake up,”accepting their environment, smiling, rolling over, and cooing.30
Psychosocial
Inadequate parent-infant interaction, parental anxiety, maternal smoking, and advanced maternal age have also been suggested as a potential contributors to or causes of colic.9 Maternal depression and paternal depression have also been shown to be associated with colic.31–33
Inadequate parent-infant interaction
Father-infant and mother-infant interactions were less optimal in one colic group as compared with a control group. Also, there were more dysfunctional interactions between parents in the severe colic group.34 These findings have not been noted in most other studies.
Parental anxiety
Infants born to mothers with high trait anxiety and mothers with a “spoil-fearing” attitude have higher risk of developing colic. In this study, trait anxiety was scored by the “trait” scale of the State-Trait Anxiety Inventory (STAI), and classified into the low, middle, and high score groups. “Spoil-fearing” participants answered “completely true” or “fairly true” to the statement,“If the infant is taken up into the arms (of the caregiver) too much, he/she can become spoiled or too dependent.”35
Maternal smoking
Daily maternal smoking during pregnancy has been related to subsequent colic in infants, but the association with maternal smoking was reported when infants were approximately 5 weeks of age, ruling against infant dependency; and the association with colic did not reach statistical significance.36
Advanced maternal age
Advanced maternal age was shown to be the most important risk factor for infantile colic in one study, whereas another study did not confirm the association.31,37
Maternal and paternal depression
Maternal depression during early pregnancy has been clearly linked to subsequent infantile colic.31,32 In one study, there was a threefold increased risk of infantile colic in mothers who reported distress during pregnancy.32 Interestingly, after adjustment for maternal depressive symptoms, paternal depression has also been associated with colic, as suggested by a prospective population-based study in 4426 2-month-old infants by van den Berg.33 This could be a direct association, or an indirect one through marital, familial, or economic distress.
Inflammation/Dysbiosis
Recently, we found that stools of infants with colic had increased fecal calprotectin, a marker for gut inflammation (Fig. 3A). Levels are known to be higher in breast-fed babies, but when breast-fed babies were separately analyzed from those on formula, fecal calprotectin was consistently higher in those with colic than in those without (Fig. 3B). We also found that the fecal microbial population was different in babies with colic, with fewer Actinobacteria (95% of which are Bifidobacteria) (Fig. 3C). Principal components analysis revealed that microbial β-diversity differed significantly (Fig. 3D). This current hypothesis describing a relationship between the microbial composition of the colon, inflammation, and colic is described later in this article, in the section “Probiotic treatment.”
Fig. 3.
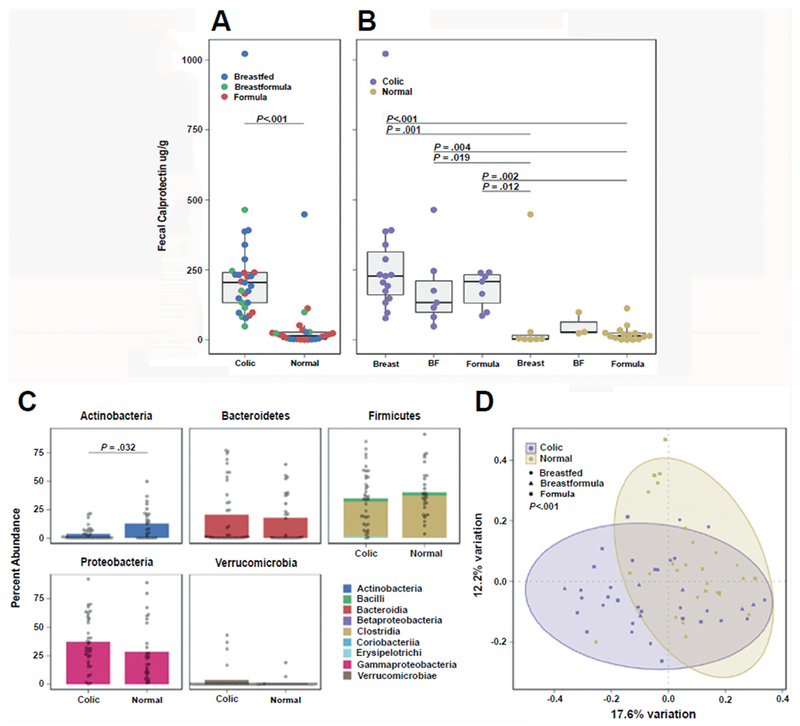
([ISP]A, [ISP][ISP]B): Relationship between fecal calprotectin, colic, and feeding modality in infants. (C, D): Fecal microbial community composition in infants with and without colic. (A) Infants with colic (n = 29) have significantly higher levels of fecal calprotectin than normal controls (n = 25) (P<.0001, Wilcoxon rank sum test). (B) Breastfed infants significantly higher levels of fecal calprotectin than Formula fed infants (P = .019, Kruskal-Wallis with post hoc Dunn’s test and correction for multiple comparisons). (C) Phylum/Classlevel composition of colic (n = 37) vs control (n = 28) samples. Normal control samples have a significantly higher abundance of Actinobacteria, (P = .032, Wilcoxon rank sum tests withcorrection for multiple comparisons). (D) Microbial β-diversity composition of infants with and without colic is significantly different (P = .003, permutational multivariate analysis of variance). (Adapted from Rhoads JM, Collins J, Fatheree NY, et al. Infant colic represents gut inflammation and dysbiosis. J Pediatr, in press; with permission.)
Diagnosis
Most studies use the parental graphing on a Barr diary to quantify crying and fussing, but this is not convenient for most patients seen in the clinic. The diagnosis of colic is traditionally made on the basis of an infant 3 weeks to 3 months of age who has intermittent periods on most days of inconsolable crying and/or fussing. Most often, the periods of worst crying are in the evening and/or after feedings. Other entities to be considered in the differential diagnosis include cow milk protein allergy, urinary tract infection or nephrolithiasis, severe thrush, anal fissure, constipation, occult fracture, and neurologic problems (including seizure activity or maternal drug abuse).
TREATMENT
Conventional Approaches
Many therapies/techniques have arisen over the years, from acupuncture to changing feeding techniques (for example, nipple adjustment), swaddling while prone,29 removing or giving environmental stimulation (white noise, soft sounds, and motion), switching formula, giving gripe water, and skin-to-skin bonding (closeness). These methods have not been scientifically studied, but are rational and anecdotally beneficial.
Acupuncture
Acupuncture is a method of systemic stimulation of neurotransmitters and hormones throughout the central nervous system.38 It is has been shown that acupuncture in animals inhibits somatic and visceral pain and has an effect on the autonomous system.39 Gastric motility and acid secretion can be affected by acupuncture40; jejunal motility can be stimulated41; and functional dyspepsia can be improved in adults.42
Acupuncture features different points of optimal effect; in colic the most commonly used location would be LI4, which is associated with gastrointestinal symptoms.43,44 A randomized controlled trial by Landgren and Hallstrom45 in 80 infants showed a significant decrease of colicky symptoms (P = .03) in the acupuncture group versus controls. A larger multicenter British study showed reduction in crying duration and a shortening of the length of colic symptoms using 2 different types of acupuncture, compared with placebo treatment. Other studies have been completed showing benefit for light (systemic) or targeted (LI4) point stimulation,46 with one study in disagreement these findings.47 Additional randomized controlled trials should be undertaken.
Chiropractic
Chiropractic therapy focuses on the musculoskeletal system and nerves. This therapy makes adjustments to the alignment of the spine and connecting nerves, purportedly pressure areas causing pain. A Cochrane review by Dobson and colleagues48 indicated that the trials have been generally “positive” but inconclusive, because of an inherent risk of performance bias because the assessors (parents) were not blind as to who had received chiropractic treatment. There is a need for further research with this technique.
Complementary Medicine
Alternative or complementary medical treatments are designed to treat the diseased body naturally. Common holistic approaches for colic are herbs, including fennel (Foeniculum vulgare), chamomile (Matricaria recutita), and lemon balm (Melissa officinalis); several of which have been effective in several randomized controlled trials for colic.49 From 5 studies, 491 participants with colic were treated with herbal medicine; results were reviewed. Fennel extract, herbal tea, and fennel oil, as well as a herbal tea called ColiMil were shown to be better than placebo. Of the 5 studies, one reported nonsevere vomiting, sleepiness, and constipation; the other 4 did not report side effects. Multi-herbal treatments (such as Colic Calm) are available over-the-counter and currently not regulated. Fennel preparations were reviewed and found to have no severe adverse events.50 In our opinion, herbal treatments are not recommended as first-line treatment, due to unproven safety and efficacy in infants, as well as unknown biological effects and dosing response.
Medications
Tincture of opium
The medical document Papyrus Ebers (ca. 1500 BCE) was purchased by Georg Ebers in 1873 and resides at the University of Leipzig. It cites a remedy for a colicky baby:
Pods of the poppy plant
Fly dirt which is on the wall
Make into one, strain, and take for four days
It acts at once!
The poppy seed would have provided opium for the cramps of colic, with fly dung added as a thickener. In the United States, paregoric was extensively used for many years for colic. Paregoric consists of 4% opium, benzoic acid, camphor, and anise oil. Before 1970, it was given over-the-counter, but it became a regulated (schedule III) drug thereafter, except as a component of Donnagel-PG (schedule V). Because of addictive properties, it was banned in 2011, only to be resumed in 2012, but is now only to be used for the purpose of weaning opiate-addicted newborns.51
Simethicone
Simethicone is an over-the-counter medication used to relieve gassiness, bloating, and discomfort in adults. Simethicone has been used widely, with outcomes no better than placebo.52 We do not recommend simethicone for in the treatment of infant colic.52
Acid blockers
Most infants referred to subspecialists in pediatric gastroenterology have been treated with acid blockers, often at very high doses. There is evidence that neither omeprazole53 nor lansoprazole54 reduces infant irritability. Acid blocker treatment was successful in reducing acid exposure time but did not reduce crying,53 suggesting that esophageal reflux is not relevant to infant colic. Concerns have been raised that these infants are overmedicated.55
Probiotic treatment
Dysbiosis describes a proven difference in microbial community composition when comparing a clinical group (eg, patients with irritable bowel syndrome56 or inflammatory bowel disease57) with healthy controls. Dysbiosis is a term that is somewhat ambiguous, and it does not prove causality.58 Microbial composition of the dysbiotic stool can be dominated by pathobionts, or commensal organisms that are known to have modestly proinflammatory characteristics. Fecal communities can be programmed by genetic defects, postnatal maturation, medications, dietary choices, antibiotics, life-style choices, and psychological stress.59
In the landmark article describing colic, Wessel and colleagues1 suggested gastrointestinal distress, caused by “excessive proteoids like those of the bean which rapidly undergo gaseous decomposition… [which] causes violent peristalsis, ” and/or “physiologic immaturity of the intestinal tract resulting in overdistension.’They also pointed out that “enemas may decrease putrefactive fermentation” in this condition, thus implicating abnormal fecal composition in the seminal report.
Francesco Savino and his group60,61 in Turin, Italy, were the first to investigate, using stool cultures and subsequently molecular techniques, whether colic is related to gut dysbiosis. His group found reduced lactobacilli and increased Escherichia coli using polymerase chain reaction to measure bacterial ribosomal DNA. Subsequently, our group’s observations from infants in Houston, using denaturing gradient gel electrophoresis and sequencing, substantiated some of these findings, although we found an increase in a different member of genus Proteobacteria, Klebsiella, in half of 18 infants with colic.12 We also suggested that there was reduced fecal microbial diversity, as well as gastrointestinal inflammation in these infants, as measured by an increase in fecal calprotectin, a cytoplasmic protein abundant in neutrophils.
The dysbiosis-inflammation theory proposes that aberrant colonization of the newborn creates a gut environment that affects brain function and the behavior of the infant. Colic represents an ideal model for understanding the effects of individual microbes and their associated microbial-brain interactions, inasmuch as these infants are colonized by only 1 to 3 dominant species, “dominant” referring to the 65% to 97% of the operational taxonomic units (OTUs, or species).62 This limited diversity in infants (especially those with colic [see Fig. 3]) contrasts markedly with studies of the stools of adults, which have a much more balanced composition of approximately 400 OTUs, of which approximately 12% are stable components of a “temporal core.”63 Differential colonization of a relatively sterile gut with proinflammatory commensals, such as Gammaproteobacteria, also known as gram-negative rods, when not balanced by anti-inflammatory commensals (Bifidobacterium, Lactobacillus, Bacteroides) may underlie the gut inflammation that has been reported in our infants with colic.60,64 Fig. 3C from our group shows that there was a significant decrease in the percentage composition of Actinobacteria (95% of which are Bifidobacteria) in infants with colic.
Reduced fecal bifidobacterial species in infants with colic compared with controls were reported by 2 groups,64,65 although low levels were not confirmed by others.66,67 Reduced lactobacilli were also found by Savino’s group,60 although recently a study by the same group using fluorescence in situ hybridization (FISH) techniques failed to find reduced lactobacilli.66 We found that these 2 genera (Lactobacillus and Bifidobacterium) comprise a very small fraction of the gut microbiota in infants 3 weeks to 3 months old,17,62 although bifidobacteria are known to bloom later during infancy. An imbalance between genera of Proteobacteria and Actinobacteria (mainly bifidobacilli) has been found in 2 prospective studies of necrotizing enterocolitis (NEC) in premature infants. Results indicated that those who developed NEC had fecal microbial populations that evolved to become different from those of infants who did not develop NEC.68,69 In both studies, there was a progressive overgrowth of Proteobacteria in those who developed NEC compared with infants with stable gastrointestinal health. The recent study by Savino and colleagues66 also showed an increase in Enterobacteriaceae, as assessed by FISH, in infants with colic compared with those without colic.
To probe gut inflammation in infants with colic, no studies have used colonoscopy to determine if there is a low-grade colitis. Olafsdottir and colleagues70 reported no differences in fecal calprotectin when they compared healthy infants with those with colic. In fact, they noted very high levels of calprotectin in all infants this age. However, subsequently we and others have reported a much higher level of fecal calprotectin in infants with colic compared with age-matched controls.12,67 Furthermore, in longitudinal studies, a dramatic decrease in calprotectin has been consistently seen, in parallel to the resolution of the colic17,71,72 (see Fig. 3A). It should be noted, however, that there is currently no consensus as to whether gut inflammation causes colic.
Simultaneous with these case-control comparisons, Savino and colleagues73 initially, and others subsequently, began to investigate the possibility that a normal fecal commensal, Lactobacillus reuteri, when given as probiotic drops, could modify the course of crying and irritability in these infants. LR ATCC 55730 and its offspring, strain DSM 17938 (the latter cured of an antibiotic-resistance plasmid), originally isolated from a Peruvian mother’s breast milk, have been available commercially for >20 years as “colic drops” in sunflower oil. Each dose of 5 drops contains more than 20 million colony-forming units. To try to quantify outcome, many studies used the Barr diary created by Ronald Barr, a daily timeline that the parents can color in when the infants either cry or fuss (recorded as 5-minute intervals).3,74 We and others have found the Barr diary to be an instrument that is user-friendly, quantitative, and often welcomed by the parents of infants with colic (see Fig. 1).
Subsequent studies of L reuteri DSM 17938 showed efficacy in studies conducted in Turin, Italy,75 Warsaw, Poland,76 Toronto, Canada,77 and Hong Kong, China.78 All (with one exception78) were investigator-masked and parent-masked, and all used Barr diaries. Meta-analyses concluded that a treatment effect was observed at 1, 2, and 3 weeks, with a weighted mean difference of crying + fussing time of 28 to 56 minutes daily, with the best effect at 2 and 3 weeks.79–81 One meta-analysis compared LR 17938 with a variety of other treatments and concluded that only L reuteri DSM 17938 and fennel were effective.79
There were 2 studies that did not find a trend toward improvement in colicky infants treated with L reuteri, authored by Sung and colleagues71 and Fatheree and colleagues.62 The study by Sung and colleagues71 was the largest trial, but it included children who were taking formula, as opposed to all other studies that included only breast-fed infants. There is evidence from rodent studies that stimulation by L reuteri of regulatory T cells is augmented by breast milk.82 The latter trial of breast-fed infants was underpowered and demonstrated an unusually brisk cessation of fussing/crying in the placebo group. It is noted that this was 1 of only 2 trials that reimbursed the parents for multiple clinic visits, potentially augmenting the placebo response. Both of these (nonconfirmatory) trials also included children who were on acid blockers, which may have influenced an impact of the probiotic on crying time and the microbiota.
Nevertheless, the meta-analyses mentioned previously showed very promising results. It should be noted that in more than 350 infants studied in the various trials, no serious adverse events of probiotic, such as bacteremia, were reported. In fact, neutropenia (a condition associated with increased risk of infection) was noted in approximately 50% of children in a recent trial at enrollment, and it resolved in 70% of the infants after 2 months of treatment with the probiotic.62
Several studies investigated other probiotics for infants with colic. One showed that Lactobacillus rhamnosus GG reduced crying time,83 whereas another smaller study (n = 20) looking at the effects of L rhamnosus GG in infants on a casein hydrolysate formula (compared with controls) did not show a difference.17 In other small studies, a symbiotic formula with several species of Lactobacillus and Bifidobacilluş as well as Streptococcus thermophilus and fructooligosaccharides showed benefit,84 as did a symbiotic with heat-killed L reuteri, Brevibacillus brevis, and xyloglucan.85 Neither of these studies used the Barr diary.
One additional comment is that L reuteri DSM 17938 has also been shown to help to prevent the development of colic in 2 studies.86,87
PROGNOSIS
Generally, infants with colic are believed to have an excellent prognosis, as colic is often viewed as a temporary disorder, regardless of etiology. The vast majority of these infants stop crying by 4 to 5 months of age. However, there has always been a concern that “little belly achers” could grow up to become “big belly achers.” A 10-year follow-up study by Savino and colleagues88 recorded (by parental “anamnesis” [history]) gastrointestinal, allergic, and psychological symptoms in 52 children who were hospitalized for severe colic as infants. After age 10, they were compared with 51 who had been hospitalized during their infancy for other reasons. The survey showed that the children who had experienced colic were significantly more likely to develop recurrent abdominal pain, allergic disease (eczema, rhinitis, asthma, and food allergies), and sleeping disorders. Remarkably, 40% to 60% of the children with colic had also gone on to develop aggressiveness, fussiness, or feelings of supremacy, with a relative risk of each calculated to be 10-fold higher than controls.
A fascinating, seemingly outlandish theory in the emerging field of “psychobiotics” (coined by the group in Cork, Ireland) is that neonatal microbiota may regulate the development of the central nervous system.89,90 This theory is mainly supported by observations in rodent models, but may have implications for the development in childhood of autism spectrum disorders and later in life, of schizophrenia. These neurologic disorders might be impacted by microbial manipulation. Examples include (1) development of stress behavior in the germ-free mouse is linked to an enlarged amygdala and hippocampus, rescued by Bifidobacillus infantis (reviewed in Vuong and colleagues91); (2) depression in humans has been linked to increased fecal Actinobacteria and Bacteroides, transmissible to the germ-free mouse92; and (3) functional bowel symptoms linked to high anxiety/depression scores improved symptomatically in response to a probiotic containing Lactobacillus helveticus and Bifidobacterium longum.93
SUMMARY
Parents caring for a miserable infant with colic may be reassured by the consistent finding that “time is on your side.” These infants will recover over the next 2 to 3 months almost 100% of the time. Swaddling, white noise, gentle stimulation (jiggling), and L reuteri drops may be of benefit, in contrast to the traditional acid blockers, which have not been shown to affect infant fussiness and crying.53
In this article, we have shown emerging evidence to support the concept that infant colic could represent a dysbiosis that impacts brain function and even brain development. Recently, Partty and colleagues94 performed a long-term follow-up study of infants treated with a probiotic. These infants (who did not have colic) were treated for the first 6 months of life with 1010 colony-forming units daily of L rhamnosus GG, alongside a control group of similarly normal infants who received a placebo (microcrystalline cellulose). Strikingly, 13 years later, 6 of 35 children who received placebo as infants had gone on to develop attention-deficit disorder or mild autism spectrum disorder (Asperger syndrome), whereas 0 of 40 children who had received the probiotic developed these disorders (P<.01). One might ponder the “unthinkable”: that not only could the symptoms of colic respond favorably to probiotic treatment, but also (in severe cases) successful treatment of the dysbiosis might favorably impact future neurodevelopmental outcome.
KEY POINTS.
Infant colic is a characteristic group of behaviors seen in young infants.
The most prominent feature is prolonged crying.
Additional characteristics, including clenching [ISP][ISP]the fists and flexion of the hips, have led to the suggestion that these behaviors are related to abdominal discomfort.
Infant colic may represent gut inflammation and microbial dysbiosis that impacts brain function and even brain development.
Acknowledgments
Disclosure: Supported by the National Institutes of Health/National Center for Complementary and Integrative Health (R34 AT006727).
Abbreviations
- NEC
Necrotizing enterocolitis
- OTU
Operational taxonomic units
- STAI
State-Trait Anxiety Inventory
REFERENCES
- 1.Wessel MA, Cobb JC, Jackson EB, et al. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics 1954;14:421–35. [PubMed] [Google Scholar]
- 2.Groopman J The colic conundrum: the crying that doctors can’t stop. Ann Med. 2007. Available at: http://www.newyorker.com/magazine/2007-09/17/the-colic-conundrum. Accessed September 17, 2007. [Google Scholar]
- 3.Barr RG, Rotman A, Yaremko J, et al. The crying of infants with colic: a controlled empirical description. Pediatrics 1992;90:14–21. [PubMed] [Google Scholar]
- 4.Barr RG. Crying as a trigger for abusive head trauma: a key to prevention. Pediatr Radiol 2014;44(Suppl 4):S559–64. [DOI] [PubMed] [Google Scholar]
- 5.Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila) 2000;39:395–400. [DOI] [PubMed] [Google Scholar]
- 6.Adamsbaum C, Grabar S, Mejean N, et al. Abusive head trauma: judicial admissions highlight violent and repetitive shaking. Pediatrics 2010;126:546–55. [DOI] [PubMed] [Google Scholar]
- 7.Wolke D, Bilgin A, Samara M. Systematic review and meta-analysis: fussing and crying durations and prevalence of colic in infants. J Pediatr 2017;185:55–61. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand AA. Infant Colic. Semin Pediatr Neurol 2016;23:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall B, Chesters J, Robinson A. Infantile colic: a systematic review of medical and conventional therapies. J Paediatr Child Health 2012;48:128–37. [DOI] [PubMed] [Google Scholar]
- 10.Parker S, Magee T, Colic. The Zuckerman Parker handbook of developmental and behavioral pediatrics for primary care. In: Augustyn M, Zucerkman B, Caronna EB, editors. 3rd edition Philadelphia: Lippincott Williams & Wilkins; 2011. p. 182. [Google Scholar]
- 11.Thomas DW, McGilligan K, Eisenberg LD, et al. Infantile colic and type of milk feeding. Am J Dis Child 1987;141:451–3. [DOI] [PubMed] [Google Scholar]
- 12.Rhoads JM, Fatheree NY, Norori J, et al. Altered fecal microflora and increased fecal calprotectin in infants with colic. J Pediatr 2009;155:823–8. [DOI] [PubMed] [Google Scholar]
- 13.Garrison MM, Christakis DA. A systematic review of treatments for infant colic. Pediatrics 2000;106:184–90. [PubMed] [Google Scholar]
- 14.Lucassen PL, Assendelft WJ, Gubbels JW, et al. Effectiveness of treatments for infantile colic: systematic review. BMJ 1998;316:1563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lust KD, Brown JE, Thomas W. Maternal intake of cruciferous vegetables and other foods and colic symptoms in exclusively breast-fed infants. J Am Diet Assoc 1996;96:46–8. [DOI] [PubMed] [Google Scholar]
- 16.Moore DJ, Robb TA, Davidson GP. Breath hydrogen response to milk containing lactose in colicky and noncolicky infants. J Pediatr 1988;113:979–84. [DOI] [PubMed] [Google Scholar]
- 17.Fatheree NY, Liu Y, Ferris M, et al. Hypoallergenic formula with Lactobacillus rhamnosus GG for babies with colic: a pilot study of recruitment, retention, and fecal biomarkers. World J Gastrointest Pathophysiol 2016;7:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyams JS, Geertsma MA, Etienne NL, et al. Colonic hydrogen production in infants with colic. J Pediatr 1989;115:592–4. [DOI] [PubMed] [Google Scholar]
- 19.Miller JJ, McVeagh P, Fleet GH, et al. Breath hydrogen excretion in infants with colic. Arch Dis Child 1989;64:725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treem WR. Infant colic. A pediatric gastroenterologist’s perspective. Pediatr Clin North Am 1994;41:1121–38. [DOI] [PubMed] [Google Scholar]
- 21.Barr RG, Wooldridge J, Hanley J. Effects of formula change on intestinal hydrogen production and crying and fussing behavior. J Dev Behav Pediatr 1991;12:248–53. [PubMed] [Google Scholar]
- 22.Kanabar D, Randhawa M, Clayton P. Improvement of symptoms in infant colic following reduction of lactose load with lactase. J Hum Nutr Diet 2001;14:359–63. [DOI] [PubMed] [Google Scholar]
- 23.Liebman WM. Infantile colic. Association with lactose and milk intolerance. JAMA 1981;245:732–3. [DOI] [PubMed] [Google Scholar]
- 24.Fazil M. Prevalence and risk factors for infantile colic in District Mansehra. J Ayub Med Coll Abbottabad 2011;23:115–7. [PubMed] [Google Scholar]
- 25.Talachian E, Bidari A, Rezaie MH. Incidence and risk factors for infantile colic in Iranian infants. World J Gastroenterol 2008;14:4662–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav 2002;71: 857–65. [DOI] [PubMed] [Google Scholar]
- 27.Kurtoglu S, Uzum K, Hallac IK, et al. 5-Hydroxy-3-indole acetic acid levels in infantile colic: is serotoninergic tonus responsible for this problem? Acta Paediatr 1997;86:764–5. [DOI] [PubMed] [Google Scholar]
- 28.Vartabedian B Colic solved: the essential guide to infant reflux and the care of your crying, difficult-to-sooth baby. New York (NY): Ballantine Books; 2007. Version 3.1. [Google Scholar]
- 29.Karp HN. Safe swaddling and healthy hips: don’t toss the baby out with the bathwater. Pediatrics 2008;121:1075–6. [DOI] [PubMed] [Google Scholar]
- 30.Karp H The happiest baby on the block: the new way to calm crying and help your newborn baby sleep longer. 2nd edition New York (NY): Bantam Books; 2015. [Google Scholar]
- 31.Paradise JL. Maternal and other factors in the etiology of infantile colic. Report of a prospective study of 146 infants. JAMA 1966;197:191–9. [DOI] [PubMed] [Google Scholar]
- 32.Sondergaard C, Olsen J, Friis-Hasche E, et al. Psychosocial distress during pregnancy and the risk of infantile colic: a follow-up study. Acta Paediatr 2003;92: 811–6. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg MP, van der Ende J, Crijnen AA, et al. Paternal depressive symptoms during pregnancy are related to excessive infant crying. Pediatrics 2009; 124:e96–103. [DOI] [PubMed] [Google Scholar]
- 34.Raiha H, Lehtonen L, Huhtala V, et al. Excessively crying infant in the family: mother-infant, father-infant and mother-father interaction. Child Care Health Dev 2002;28:419–29. [DOI] [PubMed] [Google Scholar]
- 35.Canivet CA, Ostergren PO, Rosen AS, et al. Infantile colic and the role of trait anxiety during pregnancy in relation to psychosocial and socioeconomic factors. Scand J Public Health 2005;33:26–34. [DOI] [PubMed] [Google Scholar]
- 36.Canivet CA, Ostergren PO, Jakobsson IL, et al. Infantile colic, maternal smoking and infant feeding at 5 weeks of age. Scand J Public Health 2008;36:284–91. [DOI] [PubMed] [Google Scholar]
- 37.Crowcroft NS, Strachan DP. The social origins of infantile colic: questionnaire study covering 76,747 infants. BMJ 1997;314:1325–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlsson C Acupuncture mechanisms for clinically relevant long-term effects— reconsideration and a hypothesis. Acupunct Med 2002;20:82–99. [DOI] [PubMed] [Google Scholar]
- 39.Madsen MV, Gotzsche PC, Hrobjartsson A. Acupuncture treatment for pain: systematic review of randomised clinical trials with acupuncture, placebo acupuncture, and no acupuncture groups. BMJ 2009;338:a3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato A, Sato Y, Suzuki A, et al. Neural mechanisms of the reflex inhibition and excitation of gastric motility elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res 1993;18:53–62. [DOI] [PubMed] [Google Scholar]
- 41.Yuan M, Li Y, Wang Y, et al. Electroacupuncture at ST37 enhances jejunal motility via excitation of the parasympathetic system in rats and mice. Evid Based Complement Alternat Med 2016;2016:3840230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng F, Qin W, Ma T, et al. Influence of acupuncture treatment on cerebral activity in functional dyspepsia patients and its relationship with efficacy. Am J Gastroenterol 2012;107:1236–47. [DOI] [PubMed] [Google Scholar]
- 43.Landgren K, Kvorning N, Hallstrom I. Acupuncture reduces crying in infants with infantile colic: a randomised, controlled, blind clinical study. Acupunct Med 2010; 28:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reinthal M, Lund I, Ullman D, et al. Gastrointestinal symptoms of infantile colic and their change after light needling of acupuncture: a case series study of 913 infants. Chin Med 2011;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landgren K, Hallstrom I. Effect of minimal acupuncture for infantile colic: a multicentre, three-armed, single-blind, randomised controlled trial (ACU-COL). Acupunct Med 2017;35:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinthal M, Andersson S, Gustafsson M, et al. Effects of minimal acupuncture in children with infantile colic—a prospective, quasi-randomised single blind controlled trial. Acupunct Med 2008;26:171–82. [DOI] [PubMed] [Google Scholar]
- 47.Skjeie H, Skonnord T, Fetveit A, et al. Acupuncture for infantile colic: a blinding-validated, randomized controlled multicentre trial in general practice. Scand J Prim Health Care 2013;31:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobson D, Lucassen PL, Miller JJ, et al. Manipulative therapies for infantile colic. Cochrane Database Syst Rev 2012;12:CD004796. [DOI] [PubMed] [Google Scholar]
- 49.Savino F, Ceratto S, De MA, et al. Looking for new treatments of infantile colic. Ital J Pediatr 2014;40:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anheyer D, Frawley J, Koch AK, et al. Herbal medicines for gastrointestinal disorders in children and adolescents: a systematic review. Pediatrics 2017; 139 [pii:e20170062]. [DOI] [PubMed] [Google Scholar]
- 51.Paregoric. Available at: http://en.wikipedia.org/wiki/Paregoric. Accessed March 12, 2018. [Google Scholar]
- 52.Savino F, Tarasco V. New treatments for infant colic. Curr Opin Pediatr 2010;22: 791–7. [DOI] [PubMed] [Google Scholar]
- 53.Moore DJ, Tao BS, Lines DR, et al. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. J Pediatr 2003;143: 219–23. [DOI] [PubMed] [Google Scholar]
- 54.Orenstein SR, Hassall E, Furmaga-Jablonska W, et al. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr 2009;154:514–20. [DOI] [PubMed] [Google Scholar]
- 55.Hudson B, Alderton A, Doocey C, et al. Crying and spilling—time to stop the over-medicalisation of normal infant behaviour. N Z Med J 2012;125:119–26. [PubMed] [Google Scholar]
- 56.Ringel Y, Ringel-Kulka T. The intestinal microbiota and irritable bowel syndrome. J Clin Gastroenterol 2015;49(Suppl 1):S56–9. [DOI] [PubMed] [Google Scholar]
- 57.Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olesen SW, Alm EJ. Dysbiosis is not an answer. Nat Microbiol 2016;1:16228. [DOI] [PubMed] [Google Scholar]
- 59.Jandhyala SM, Talukdar R, Subramanyam C, et al. Role of the normal gut microbiota. World J Gastroenterol 2015;21:8787–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savino F, Cresi F, Pautasso S, et al. Intestinal microflora in breastfed colicky and non-colicky infants. Acta Paediatr 2004;93:825–9. [PubMed] [Google Scholar]
- 61.Savino F, Cordisco L, Tarasco V, et al. Molecular identification of coliform bacteria from colicky breastfed infants. Acta Paediatr 2009;98:1582–8. [DOI] [PubMed] [Google Scholar]
- 62.Fatheree NY, Liu Y, Taylor CM, et al. Lactobacillus reuteri for infants with colic: a double-blind, placebo-controlled, randomized clinical trial. J Pediatr 2017;191: 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez I, Muller CE, Walter J. Long-term temporal analysis of the human fecal microbiota revealed a stable core of dominant bacterial species. PLoS One 2013;8:e69621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Weerth C, Fuentes S, Puylaert P, et al. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics 2013;131:e550–8. [DOI] [PubMed] [Google Scholar]
- 65.Partty A, Kalliomaki M, Endo A, et al. Compositional development of Bifidobacterium and Lactobacillus microbiota is linked with crying and fussing in early infancy. PLoS One 2012;7:e32495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Savino F, Quartieri A, De MA, et al. Comparison of formula-fed infants with and without colic revealed significant differences in total bacteria, Enterobacteriaceae and faecal ammonia. Acta Paediatr 2017;106:573–8. [DOI] [PubMed] [Google Scholar]
- 67.Savino F, Garro M, Montanari P, et al. Crying time and RORgamma/FOXP3 expression in Lactobacillus reuteri DSM17938-treated infants with colic: a randomized trial. J Pediatr 2017;192:171–7.e1. [DOI] [PubMed] [Google Scholar]
- 68.Mai V, Young CM, Ukhanova M, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One 2011;6:e20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warner BB, Deych E, Zhou Y, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 2016;387:1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olafsdottir E, Aksnes L, Fluge G, et al. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr 2002;91: 45–50. [DOI] [PubMed] [Google Scholar]
- 71.Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ 2014; 348:g2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Savino F, De MA, Ceratto S, et al. Fecal calprotectin during treatment of severe infantile colic with Lactobacillus reuteri DSM 17938: a randomized, double-blind, placebo-controlled trial. Pediatrics 2015;135(Suppl 1):S5–6. [Google Scholar]
- 73.Savino F, Pelle E, Palumeri E, et al. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 2007;119:e124–30. [DOI] [PubMed] [Google Scholar]
- 74.Barr RG, Kramer MS, Boisjoly C, et al. Parental diary of infant cry and fuss behaviour. Arch Dis Child 1988;63:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savino F, Cordisco L, Tarasco V, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics 2010;126: e526–33. [DOI] [PubMed] [Google Scholar]
- 76.Szajewska H, Gyrczuk E, Horvath A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr 2013;162:257–62. [DOI] [PubMed] [Google Scholar]
- 77.Chau K, Lau E, Greenberg S, et al. Probiotics for infantile colic: a randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. J Pediatr 2015;166:74–8 [DOI] [PubMed] [Google Scholar]
- 78.Mi GL, Zhao L, Qiao DD, et al. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: a prospective single blind randomized trial. Antonie Van Leeuwenhoek 2015;107:1547–53. [DOI] [PubMed] [Google Scholar]
- 79.Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breast-fed infants. J Pediatr Gastroenterol Nutr 2016;62: 668–86. [DOI] [PubMed] [Google Scholar]
- 80.Xu M, Wang J, Wang N, et al. The efficacy and safety of the probiotic bacterium Lactobacillus reuteri DSM 17938 for infantile colic: a meta-analysis of randomized controlled trials. PLoS One 2015;10:e0141445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sung V, Cabana MD, D’Amico F, et al. Lactobacillus reuteri DSM 17938 for managing infant colic: protocol for an individual participant data meta-analysis. BMJ Open 2014;4:e006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y, Fatheree NY, Dingle BM, et al. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS One 2013;8(2):e56547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Partty A, Lehtonen L, Kalliomaki M, et al. Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: a randomized, controlled trial. Pediatr Res 2015;78:470–5. [DOI] [PubMed] [Google Scholar]
- 84.Kianifar H, Ahanchian H, Grover Z, et al. Synbiotic in the management of infantile colic: a randomised controlled trial. J Paediatr Child Health 2014;50:801–5. [DOI] [PubMed] [Google Scholar]
- 85.Vandenplas Y, Bacarea A, Marusteri M, et al. Efficacy and safety of APT198K for the treatment of infantile colic: a pilot study. J Comp Eff Res 2017;6:137–44. [DOI] [PubMed] [Google Scholar]
- 86.Indrio F, Di MA, Riezzo G, et al. Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation: a randomized clinical trial. JAMA Pediatr 2014;168:228–33. [DOI] [PubMed] [Google Scholar]
- 87.Savino F, Ceratto S, Poggi E, et al. Preventive effects of oral probiotic on infantile colic: a prospective, randomised, blinded, controlled trial using Lactobacillus reuteri DSM 17938. Benef Microbes 2015;6:245–51. [DOI] [PubMed] [Google Scholar]
- 88.Savino F, Castagno E, Bretto R, et al. A prospective 10-year study on children who had severe infantile colic. Acta Paediatr Suppl 2005;94:129–32. [DOI] [PubMed] [Google Scholar]
- 89.Kelly JR, Minuto C, Cryan JF, et al. Cross talk: the microbiota and neurodevelopmental disorders. Front Neurosci 2017;11:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rea K, Dinan TG, Cryan JF. The microbiome: a key regulator of stress and neuro-inflammation. Neurobiol Stress 2016;4:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vuong HE, Yano JM, Fung TC, et al. The microbiome and host behavior. Annu Rev Neurosci 2017;40:21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016;21:786–96. [DOI] [PubMed] [Google Scholar]
- 93.Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 2011;105:755–64. [DOI] [PubMed] [Google Scholar]
- 94.Partty A, Kalliomaki M, Wacklin P, et al. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res 2015;77:823–8. [DOI] [PubMed] [Google Scholar]


