Abstract
Neurobiological models of adolescent decision-making emphasize developmental changes in brain regions involved in affect (e.g., ventral striatum) and cognitive control (e.g., lateral prefrontal cortex). Although social context plays an important role in adolescent decision-making, current models do not discuss brain regions implicated in processing social information (e.g., dorsomedial prefrontal cortex). We conducted a coordinate-based meta-analysis using the Multilevel peak Kernel Density Analysis (MKDA) method to test the hypothesis that brain regions involved in affect, cognitive control, and social information processing support adolescent decision-making in social contexts (N = 21 functional neuroimaging studies; N = 1292 participants). Results indicated that dorsomedial prefrontal cortex, inferior frontal gyrus/insula and ventral striatum are consistently associated with adolescent decision-making in social contexts. Activity within these regions was modulated by the type of social context and social actors involved. Findings suggest including brain regions involved in social information processing into models of adolescent decision-making. We propose a ‘constructionist’ model, which describes psychological processes and corresponding neural networks related to affect, cognitive control, and social information processing.
1. Introduction
Many decisions in a teenagers’ life affect or are influenced by other people. For instance, the decision to speed through a yellow light with a risk-endorsing friend in the car can affect the safety of the driver, the friend, and the adolescents’ relationships with their parents. Yet, speeding through a yellow light may also enhance a teen’s social status with their friend, potentially making it worth the consequences. The work of developmental neuroscientists seeks to understand how these everyday instances of decision-making in social contexts unfold in the developing brain.
Adolescence is a time of heightened risk-taking behaviors and increased social-affective sensitivity. These processes occur in parallel with tremendous changes in the developing brain. Prominent neurobiological models of adolescent behavior emphasize adolescents’ orientation towards rewards and risk-taking and thus focus on the developmental changes that occur within neural networks implicated in affective sensitivity (e.g., ventral striatum; VS) and cognitive control (e.g., lateral prefrontal cortex; Steinberg, 2008; Shulman et al., 2016; Casey et al., 2008; 2015). Yet models of decision-making in adolescence do not take into consideration the important role of neural regions involved in social information processing (e.g., dorsomedial prefrontal cortex, temporo-parietal junction), despite the fact that adolescents show uniquely heightened activation within these regions in response to social information (e.g., Blakemore, 2008; Blakemore & Mills, 2014; Nelson et al., 2005; 2016). In the present work, we underscore the greater need to focus on the social context when examining adolescent decision-making. We perform a coordinate-based quantitative meta-analysis to examine whether brain regions involved in social information processing are involved during adolescent decision-making in social contexts. We close by proposing a ‘constructionist’ model of adolescent decision-making that models psychological processes and corresponding neural networks related to affective salience, social information processing, and cognitive control.
1.1. Heightened risk-taking and social-affective sensitivity in adolescence
To date, important scientific advances in our understanding of adolescent neurocognition have been guided by separate neurobiological models that describe adolescents’ heightened sensation seeking and social-affective sensitivity. The Dual Systems Model (Steinberg, 2008; Shulman et al., 2016) and Imbalance Model (Casey, 2008; 2015) suggest that adolescents demonstrate heightened activation in the affective system (e.g., ventral striatum (VS), insula, amygdala) at a developmental period of vulnerability when the cognitive control system (e.g., lateral PFC) is not yet mature (also see Romer, Reyna, & Satterthwaite, 2017; Li, 2017 for recent adaptions of these models). Heightened affective sensitivity paired with an inability to engage in effective regulation, is thought to result in an orientation towards rewards and greater risk-taking behavior. Early fMRI studies support these models, demonstrating unique VS sensitivity to rewards among adolescents compared to children or adults (for review of this seminal work, see Galvan, 2010) as well as altered activation during regulatory tasks in the prefrontal cortex (for review see Crone & Dahl, 2012). Although recent studies have continued to provide empirical support for differential activation of affective and cognitive control networks during adolescence (e.g., Barkley-Levenson & Galvan, 2014; Braams et al., 2014; Van Duijvenvoorde et al., 2016; Van Leijenhorst et al., 2010; Qu, Galvan, Fuligni, Lieberman, & Telzer, 2015; for a meta-analysis see Silverman, Jedd, & Luciana, 2015), there has also been a call for a more nuanced understanding of interactions across brain regions involved in cognitive, affective, and social processing (Pfeifer & Allen, 2016; Crone & Dahl, 2012).
Much of the existing research on the neurodevelopment of adolescent decision-making has been conducted in a social vacuum. Yet in daily life, adolescent decision-making often occurs in the context of peers, parents, or other important social agents who may impact decisions (Albert et al., 2013; Blakemore & Mills, 2014; Schriber & Guyer, 2016). Compared to children, adolescents spend more time with peers, form more sophisticated and complex social relationships, are more sensitive to peer acceptance, and become more self-conscious (Brown, 2004; see Blakemore & Mills, 2014). Indeed, adolescents show uniquely heightened embarrassment when being watched by their peers (Somerville et al., 2013) and have compromised emotion regulation compared to children or adults in the presence of socially appetitive cues (Somerville et al., 2011). In addition, among adolescents, a greater orientation towards rewards and greater risk-taking behaviors are more likely to occur in a social than non-social context (Albert et al., 2013; Steinberg et al., 2017; Duckworth & Steinberg, 2015). For example, adolescents are more susceptible to risk-taking than adults in the presence of peers (Gardner & Steinberg, 2005) and tend to conform to the attitudes of their peers about risky behaviors more so than adults (Knoll et al., 2015). As such, it has been proposed that adolescence is a uniquely sensitive period for sociocultural information processing (Blakemore & Mills, 2014).
Because adolescent decision-making is most likely to occur in a social context, neurobiological models of adolescent decision-making could benefit by incorporating neural regions that support social information processing. According to models of social cognition, and the Social Brain Model (Blakemore, 2008) in particular, information from the social context is processed by a collection of regions that support the ability to mentalize such as the dorsomedial prefrontal cortex (dmPFC), temporo-parietal junction (TPJ), and posterior superior temporal sulcus (pSTS) (Blakemore, 2008; Blakemore & Mills, 2014; Mitchell, Macrae, & Banaji, 2005; Spunt & Lieberman, 2013; Saxe, 2006; Van Overwalle, 2009). Mentalizing involves recognizing that another person has a mind, thinking about another’s thoughts and feelings, and predicting another’s behavior to guide one’s own decisions (Waytz, Gray, Epley, & Wegner, 2010). The literature consistently shows functional changes in these social brain regions across development. In particular, adolescents show greater mPFC activity during mentalizing tasks than adults (Blakemore et al., 2007; Burnett et al., 2009; Gunther Moor et al., 2012; Pfeifer et al., 2009; Van den Bos et al., 2011; Wang et al., 2006, Somerville et al., 2013). For example, relative to adults, adolescents show greater mPFC activation when thinking about intentions (Blakemore et al., 2007). These findings underscore adolescence as a key period of social sensitivity (Blakemore, 2008; Blakemore & Mills, 2014).
1.2. Social context and adolescent decision-making
Surprisingly, social context has remained an elusive construct throughout the developmental neuroimaging literature. A wide range of social contexts have been studied without the broader concept being explicitly defined. Here, we define decision-making in a social context as decisions in which others are involved. We aim to understand neural activity specifically related to decision-making in a social context. To refine the construct of social context, we distinguish between social processes that affect the input for a decision versus the outcome of a decision. As such, we define two types of decisions in a social context: 1) those in which the decision-maker is affected by others (i.e., social influence decisions) and 2) those in which an individual’s decisions affect others (i.e., social outcome decisions). Note that other social processes such as social emotion processing, face processing or receiving peer evaluation are other crucial processes that develop during adolescence (Blakemore & Mills, 2014), but these processes have not been studied in the context of decision-making per se, and as such are not examined in this meta-analysis. Given the social reorientation that occurs during adolescence, both social influence and social outcome decisions are common and highly salient in adolescents’ daily lives (Blakemore, in press; Nelson et al., 2005; 2016). We expect social influence and social outcome decisions to be moderated by social actors, or who adolescents are interacting with in the moment (Telzer, van Hoorn, Rogers, & Do, 2018).
1.2.1. Social influence decisions.
Social influence decisions occur when adolescents’ behaviors or attitudes are explicitly or implicitly influenced by others, such as friends, the larger peer group or family (Brechwald & Prinstein, 2011; Telzer, Van Hoorn, Rogers, & Do, 2018). This may include very explicit social pressure, such as friends being present and egging on an adolescent to drink or drive fast, or online social media websites that use ‘likes’ as quantifiable social endorsements (e.g., Instagram, Facebook). Through such explicit feedback, the peer group reinforces social norms, which in turn may guide subsequent decisions (Brechwald & Prinstein, 2011). Social influence can also be more implicit and guided by the (mis)perception of social norms, such as thinking one’s peers drink a lot, leading to greater substance use (Prinstein & Wang, 2005; McDonald & Crandall, 2015). As such, an adolescent may adapt their decisions to conform to perceived social norms to gain social approval and connection with others (DeWall & Richman, 2011). This implied “psychological presence” of others can lead to mentalizing about others’ goals, values and expectations, and influences subsequent behavior (Shah, 2003).
1.2.2. Social outcome decisions.
Social outcome decisions denote instances when the outcome of one’s decisions affect other people. In the risk-taking domain, adolescents’ decisions often not only affect themselves but close others as well. For instance, taking their parents’ car for a joy ride can result in being grounded (personal risk), crashing the car (financial risk to the family), or sacrificing their friend’s safety if they were in the car together (Guassi Moreira & Telzer, 2018b). Social outcomes could also be more abstract, such as angering parents, offending friends or hurting one’s social standing. In the prosocial domain, social outcome decisions can include fairness considerations, strategic bargaining, trust, reciprocity, and prosocial behaviors (Crone, Will, Overgaauw, & Guroglu, 2012). For example, an adolescent may offer help to a friend who is sad or struggles with homework, or reciprocate someone else’s trust. Among these different social decisions, each requires the need to draw an inference about the mental state of another person (Lee & Harris, 2013).
1.2.3. Social actors.
Adolescent decision-making in social contexts (i.e., social influence or social outcome decisions) is likely dependent on several factors, including who adolescents are interacting with (i.e., the social actors). Oftentimes experimental paradigms involve anonymous others to carefully control for previous experiences or potential beliefs that may be attributed to known others. However, previous work also suggests that decision-making may change depending on the beneficiary. For example, adolescents are more prosocial towards friends than anonymous others (Guroglu et al., 2014; Padilla-Walker, Carlo, & Memmott-Elison, 2017) and alter their risky decisions when they affect themselves or their family (Guassi-Moreira & Telzer, 2018b). Moreover, while peer rejection has been linked to greater risk-taking behaviors such as substance use (Prinstein & La Greca, 2004), supervision by parents is associated with lower levels of risk-taking (Borawski, Ievers-Landis, Lovegreen, & Trail, 2003). Together, this work highlights that the type of social actor can moderate adolescent decision-making in social contexts, and likely the recruitment of neural regions representing information about the social context.
1.3. Present study
Despite the importance of the social context, neurobiological models of adolescent decision-making have not explicitly incorporated regions representing the social context. We took a data-driven, quantitative approach to test the hypothesis that brain regions involved in affect, cognitive control, and social information processing support adolescent decision-making in social contexts. In order to do so, we performed a coordinate-based quantitative meta-analysis on the existing developmental neuroimaging literature. Meta-analysis is advantageous because it summarizes the set of brain regions that show consistent (i.e., reliable) increases in activation across a range of studies (Kober & Wager, 2010). The set of brain regions that are consistently activated during a certain class of studies are referred to as a “neural reference space” and represent the brain regions that are probabilistically more likely to show increased activation during the process of interest as compared to chance (Barrett et al. 2007; Lindquist et al. 2012; Wager et al., 2007). Meta-analysis can also demonstrate brain regions that are more likely to be involved in one experimental condition versus another, and thus can speak to the relative specificity of neural function. Neuroimaging studies are prone to Type-I error due to small sample sizes and may lack generalizability because single studies can only assess a few conditions (Wager et al. 2007). Meta-analysis is thus ideal to generate data-driven hypotheses (cf. Pfeifer & Allen, 2016), through summarizing data from multiple studies.
Our main goal was to examine the neural reference space associated with decision-making in a social context during adolescence. Based on existing neurobiological models of decision-making and social cognition, we expected that the neural reference space associated with adolescent decision-making in social contexts would encompass brain regions associated with affective (i.e., VS, insula, amygdala), cognitive control (lPFC), and social information processing (dmPFC, TPJ) (Blakemore, 2008; Blakemore & Mills, 2014; Casey, 2008; 2015; Nelson et al., 2016; Smith et al., 2014; Steinberg, 2008; Shulman et al., 2016). These affective, cognitive, and social brain regions are part of complex and dynamically interacting neural networks (Casey, 2015). While taking a functional connectivity or network-approach is certainly insightful (e.g., see McCormick, van Hoorn, Cohen, & Telzer, 2018), most individual studies and meta-analytic techniques to date allow for assessing consistent mean levels of activation only. As such, this is our focus in the current work.
Next, we examined how the neural reference space is modulated by characteristics of the social context. In particular, we disentangled effects of social context type by comparing neural activation consistently associated with social influence decisions versus social outcome decisions. We also built on growing research examining effects of social actors (Guassi-Moreira & Telzer, 2018b; Guroglu et al., 2014; Padilla-Walker et al., 2017; Prinstein et al., 2001) by comparing the neural reference space when social actors were known versus unknown others.
2. Methods
2.1. Database
The database for the meta-analysis included 21 empirical functional neuroimaging studies of adolescent decision-making in a social context (N = 1292 total participants; 61 contrasts and 331 data points (peak coordinates)) published between 2011 and June 2017. As a follow up, we searched websites of research laboratories that conduct fMRI research on relevant topics to ensure that our search encompassed the most recent papers. A final literature search was conducted by independent researchers and yielded no additional papers. As such, the database represents all studies of adolescent decision-making in social contexts that met our inclusion criteria until the stop-point for data collection for this project.
Using scholarly search engines such as PsycInfo, PubMed and Google Scholar, we sampled papers based on specific search criteria: our targeted measurement (e.g., “fMRI”), our target sample type and age (e.g., “human,” “adolescence/adolescents”), overall area of research or phenomenon (e.g., “social influence,” “social evaluation,” “social decision-making,” “social context,” “peer influence,” “parental influence,” “risk-taking,” “risky behavior,” “social (peer) exclusion,” “peer feedback”), and specific tasks that are typically used in this literature (e.g., “family donation task,” “trust game,” “prisoner’s dilemma,” “reward tasks,” and “ultimatum game”). Our initial search was broad, in order to be inclusive and to ensure that our codes represented the full range of tasks used in the literature. We did not restrict our search from a particular start date, but reflecting the novelty of this field, the earliest eligible study in our database was published in 2006. Our initial literature search produced 82 papers, including literature reviews and meta-analyses on separate topics in developmental neuroscience. Following the initial search, 30 papers were excluded that did not have social context or were literature reviews or meta-analyses; 17 additional papers were excluded due to tasks that we considered unrelated to our phenomenon of interest (i.e., Cyberball) and 14 papers were excluded as they used methods or analyses that are not currently compatible with our analysis method (e.g., functional connectivity analyses; longitudinal analyses), resulting in a final sample of 21 papers.
2.1.1. Inclusion and exclusion criteria.
Only fMRI tasks involving decision-making were included. Social feedback processing (receiving social rejection or acceptance from peers; e.g., a Chatroom Task) and outcome processing (e.g., a task analyzing neural responses to reward but not decision-making) were beyond the scope of the current meta-analysis, as they did not explicitly model decision-making. Cyberball and similar tasks (e.g., Chatroom) were excluded because we considered receiving explicit social rejection to be a different phenomenon from the more subtle social decision-making we were interested in. Since our goal was to specifically focus on social influence and social outcome decisions, we did not include non-social decision-making studies as the set of non-social decision-making studies is quite large and diverse in respect to the types of decision-making tasks/procedures included. See Table 1 for an extensive overview of studies including tasks employed, coding for social context categories (social influence decisions, social outcome decisions, other) and social actor type (known other, unknown other), contrasts included, as well as other dimensions of interest such as sample size.
Table 1.
Overview of studies included in the meta-analysis (N = 21 studies, N = 1292 total participants), task description, and social context category. For an overview of excluded studies (N = 13 studies), see Supplementary Table 1.
| First author, year | Task | Description | Contrasts included | N (#F) | Age Range (Mean) | Social Context Category | Social Actor (specific person) |
|---|---|---|---|---|---|---|---|
| Braams & Crone, 2016 | Heads or tails gambling game | Participants guessed heads or tails and won or lost money depending on whether the computer picked the chosen side. Outcomes affected best friend or self.1 | outcome friend > outcome self; outcome self > outcome friend | 249 (132) | 8.01–25.95 (14.5) | social outcome | Known (best friend) |
| Braams, 2017 | Heads or tails gambling game | Participants guessed heads or tails and won or lost money depending on whether the computer picked the chosen side. Outcomes affected best friend, self, or mother.2 | win for friend > lose for friend; win for mom > lose for mom | 233 (117) | 9.9–26.6 (16.1) | social outcome | Known (best friend, parent) |
| Chein, 2011 | Stoplight Task | Computerized driving game in which participants chose to stop (safe decision) or go (risky decision) at yellow lights of intersections. Goal is to reach the end of the track as fast as possible. Played alone and with peers observing.3 | adult decisions > adolescent decisions | 40 (21) | 14–18, 19–22, 24–29 (15.7, 20.6, 25.6) | social influence | Known (same-sex same-age friends) |
| Guassi Moreira, 2018a | Stoplight Task | Computerized driving game in which participants chose to stop (safe decision) or go (risky decision) at yellow lights of intersections. Goal is to reach the end of the track as fast as possible. Played with a parent and an unknown adult observing. | safe decision mother > safe decision adult; risky decision mother > risky decision adult | 23 (9) | 15 (15.22) | social influence | Known (Parent) Unknown (adult “expert in adolescent driving behavior”) |
| Gunther Moor, 2012 | Dictator game (DG) | Played Cyberball before DG to establish social exclusion and inclusion. In DG participants divided coins between themselves and other players (team 1: includers; team 2: excluders). | DG excluders > DG includers; DG excluders > DG includers in 19–21 group vs younger age groups | 53 (31) | 10–12, 14–16, 19–21 (11.8, 15.74, 20.38) | social outcome | Unknown (team 1: includers; team 2: excluders) |
| Guroglu, 2011 | Ultimatum Game (UG) | UG in which participants were responders that accepted or rejected offers from the proposer. | accept > reject; unfair > fair w/fair alternative; unfair > fair w/ hyperfair alternative | 68 (32) | 10.3–20.4 10, 13, 15, 20 (10.4, 13.4, 15.4, 20.4) | social outcome | Unknown (avatars) |
| Op de Macks, 2016 | Jackpot Task | Participants played a gambling task in which they decided to play or pass, with variable risk levels (33% or 67% chance to win) and stakes (1 or 3 points), and received either social rank feedback or monetary feedback. | social play > monetary play | 58 (58) | 11–13 (12.4) | social influence | Unknown (same-sex same-age peers) |
| Peake, 2013 | Stoplight Task; Cyberball | Computerized driving game in which participants chose to stop (safe decision) or go (risky decision) at yellow lights of intersections. Goal is to reach the end of the track as fast as possible. Participants played Stoplight, were excluded in Cyberball, then played Stoplight again, with the excluders watching the participant. | stop decision post exclusion > stop decision pre exclusion; go decision pre exclusion > go decision post exclusion; stop decision pre exclusion > stop decision post exclusion | 20 (10) | 14–16.8 (15.3) | social influence | Unknown (peers) |
| Perino, 2016 | Social Go-NoGo Task | Participants completed a social go-nogo task with the target stimuli (letters) superimposed on socially appetitive and socially aversive scenes. Participants had to withhold button presses on trials with an X letter. | socially appetitive > socially aversive | 35 (20) | 12–17 | other - social distraction | Unknown (pictures of social scenes) |
| Rodrigo, 2014 | Social Context Decision Task | Participants read short passages describing social situations in which they were accompanied by a close friend and had to decide between a dangerous or a safe choice (risk condition) or between 2 neutral choices (ambiguous condition). | risky decision > ambiguous decision; risky > ambiguous adolescent vs adult; dangerous > safe; dangerous > safe young adult vs adolescent | 60 (30) | 17–18, 21–22 (17.5, 21.4) | other - scenarios with peers | Known (close friend) |
| Sherman, 2016 | Instagram Task | Participants were shown a feed of Instagram photos from peers and themselves (some risky, some neutral) with the number of likes displayed (popular or unpopular), and could decide to like photos or move on to the next photo. | neutral image unpopular > neutral image popular; risky image unpopular > risky image popular; own image unpopular > own image popular; risky image > neutral image; risky image < neutral image; own neutral image > other neutral image4 ; own risky image > other risky image8 | 32 (18) | 13–18 | social influence | Unknown (peers) |
| Steinmann, 2014 | Ultimatum Game | Participants were responders and accepted or rejected offers from the proposer. | unfair - fair decision for adult > unfair - fair decisions for children; unfair - fair decision for adult < unfair – fair decisions for children; unfair - fair decision for adolescents > unfair – fair decisions for children | 45 (20) | 8–12, 13–18, 19–28 (10.2, 15.5, 24.8) | social outcome | Unknown |
| Telzer, 2011 | Family Assistance task | Participants accepted or rejected monetary offers that would benefit their families or themselves. Choices were either costly or non- costly donations or costly or non- costly rewards. | Accept costly donation > Accept a non-costly reward | 25 (13) | 19–20 (20.2) | social outcome | Known (family) |
| Telzer, 2013 | Family Assistance task | Participants accepted or rejected monetary offers that would benefit their families or themselves. Choices were either costly or non- costly donations or costly or non- costly rewards. | Accept costly donation > control; Accept non- costly donation > control; Accept costly donation > Accept non-costly donation; Accept non- costly donation > Accept costly donation | 32 (18) | 15–17 (16.3) | social outcome | Known (family) |
| Telzer, 2015 | Stoplight Task | Computerized driving game in which participants chose to stop (safe decision) or go (risky decision) at yellow lights of intersections. Goal is to reach the end of the track as fast as possible. Participants completed the task alone and with their mother watching. | Risky decision (collapsed over mom and alone context); Stop decision > Go decision (collapsed over mom and alone context); Go decision > stop decision (collapsed over mom and alone context); Pass decisions (collapsed over mom and alone context); Stop decision w/ mom > Stop decision alone; Stop decision w/ mom > control; Go decision w/mom > go decision alone | 25 (10) | 14 (14.43) | social influence | Known (mother) |
| Telzer, 2017* | Cyberball; Stoplight Task | Played Cyberball and were excluded by 2 unknown peers; Then completed a computerized driving game in which participants chose to stop (safe decision) or go (risky decision) at yellow lights of intersections. Goal is to reach the end of the track as fast as possible in order to earn points for their team. Compared a chronically victimized (CV) group with a non- victimized group (NV). | Risky decision CV > Risky decision NV; Risky decision NV > Risky decision CV; Safe decision CV > safe decision NV | 46 (46) | 14.8–16.1 (15.3) | social influence | Unknown (peers) |
| van den Bos, 2011 | Trust Game | Participant acts as Player 2, the trustee, in the Trust Game. When Player 1 gives money to Player 2, participant can choose to share increased amount of money with Player 1 or keep the given money. | Receiving trust > No trust (all ages); Receiving trust > no trust receiving5; Choose to defect > choose to reciprocate (all ages); Choose to reciprocate > choose to defect (all ages); Choose to defect > choose to reciprocate6 | 54 (30) | 12–22 (16.2) | social outcome | Unknown (peers) |
| van den Bos, 2014* | mini- Ultimatum Game | Participants decide whether they accept or reject a monetary offer from another player. Offers were either fair, unfair, no alternative, and hyperfair alternative. | Accept unfair offer > Reject unfair offer (entire sample) | 34 (0) | 15.0–22.0 (17.71) | social outcome | Unknown (peers) |
| van Hoorn, 2016 | Public Goods Game | Participant could donate tokens to the group or keep for themselves in one of three conditions: peers observing, peers giving feedback, or no peers present (i.e., alone). | Prosocial decision w/someone watching > prosocial decision when alone; Prosocial decision w/ feedback > prosocial decision when alone | 61 (32) | 12–13, 15–16 (12.93, 16.08) | social influence | Unknown (peer actors) |
| Verdejo-Garcia, 2015 | Ultimatum Game | Participants decide whether they accept or reject a monetary offer from another player. Examining group differences between adolescents in excess weight (EW) vs. normal weight (NW) | unfair decision NW > fair decision NW; unfair decision > fair decision7 ; reject offer NW > accept offer NW; reject offer > accept offer8 | 80 (49) | 12–18 (Normal weight: 15.32, Excess weight: 15.06) | social outcome | Unknown (peers) |
| Welborn, 2015 | Artwork Ratings | Participants rated artwork before the scan session and during the scan were shown actual ratings made by their parents and peers of the same pieces of art. Participants then made their ratings again to examine changes due to parental or peer influence. | Peer influence > no influence; No influence > peer influence; Parental influence > no influence; Parent and peer influence > no influence | 19 (7) | 16.44–18.43 (17.56) | social influence | Known (peers from same school; parents) |
Notes. Social context was coded into 3 categories: social outcome decisions, social influence decisions, and other. Social actor was coded as known or unknown.
Analyses focused on processing outcomes, but there was no jitter between events, so included in meta-analysis to retain power. Only timepoint 1 whole brain analyses included.
Only whole brain analyses included.
Interactions age group x social context could not be included.
ROI analysis.
Trust versus no trust, comparing 2 youngest with oldest age group.
Choice to defect > reciprocate, comparing youngest age group to two older age groups.
Unfair offer > fair offer comparing normal weight vs excess weight.
Reject offer < accept offer comparing normal weight vs excess weight.
All studies were conducted with non-patient populations. ‘Special’ populations included in this meta-analysis were: Telzer 2017 Chronically victimized and non-victimized females; Van den Bos 2014 Antisocial teens (some of which had a diagnosis) and healthy controls (age and IQ matched); Verdejo Garcia 2014 normal weight and excess weight (high BMI).
We focused on studies in the adolescent age range, defined as ages between 10–22 years (Steinberg, 2008). To ensure that we had ample power and were inclusive, we included a slightly wider age range (ages 8–26 years) in our final database. To balance concerns about power with those about validity, papers including a wider age range were only included when the majority of participants fell within the adolescent age range (e.g., Braams & Crone, 2016 with only a few participants on the youngest and oldest ends of the age range (ages <10 years and > 22 years)) and when papers directly compared adolescents to adults or children; papers exclusively assessing adults or children were excluded. Importantly, each of the papers included had a mean age within the traditional age range of adolescence, with a collective mean age (SD) of 15.80(0.62). All studies included healthy, typically developing participants and excluded patient samples. We did not explicitly search for patient populations, but examined all studies that qualified within our criteria, and have been inclusive of all papers we could identify. Prior meta-analyses excluded contrasts that focus on comparing specific groups of participants (e.g., overweight v. healthy weight) but we chose to retain three studies that contrasted specific groups within their sample. We retained these papers to ensure power and err on the side of inclusion because none included an exclusively patient-based sample (Telzer et al., 2017: chronically victimized and non-victimized; Van den Bos et al., 2014: antisocial (some of them diagnosed) and typically developing controls; Verdejo Garcia et al., 2015: excess weight and normal weight). Analyses with and without these studies yielded no substantial differences (for more details, see the neural reference space section below).
We also excluded studies that utilized methods or analyses not compatible with the nature of our meta-analytic technique, the Multilevel Peak Kernel Density Analysis (MKDA; for technical details see section 2.2). Group-level longitudinal findings were not included as they track brain changes over time within the same individuals rather than assessing brain processes within individuals at a specific point in time, like all cross-sectional findings included. If data from each time-point was provided in a longitudinal study, only the first time-point was added to the database as a singular data point (example: Braams & Crone, 2016). If multiple studies reported different analyses on the same sample, we did not include those findings twice, as they would be non-independent. An exception to this rule was Braams & Crone (2017), as the task employed was slightly different between studies. In all other cases, a study with whole-brain analyses was preferred over a study with region of interest analyses (e.g. Telzer et al., 2011 included, Telzer et al., 2010 excluded).
Finally, this meta-analysis was limited to contrast analyses reported in each study and if information was not clearly reported in the paper, we reached out to the first authors for additional information. Studies reporting percent signal change (e.g. Smith et al., 2015), parametric analyses, individual differences analyses (e.g., Pfeifer et al., 2011 correlations with the Resistance to Peer Influence questionnaire), psychophysiological interactions (PPI) analyses (e.g., Somerville et al., 2013 mPFC-striatum connectivity), and network analyses could not be included as the MKDA only summarizes reported peak activations from study-level experimental contrasts. As a result, not all studies with relevant findings are included in this quantitative meta-analysis. In Supplementary Table 1 we describe excluded studies and reasons for exclusion.
2.1.2. Coding.
Each study contrast was coded on a number of dimensions by two researchers (JVH and HS), including sample size, gender ratio, category of social task and social actor type. Any disagreements between the two researchers were resolved through discussion and a third individual (EHT).
Tasks involving social influence decisions, i.e., when decisions are impacted by others, included both tasks with explicit feedback on behavioral choices provided by others (e.g., Van Hoorn et al., 2016), as well as more subtle forms where people were observing decisions (e.g., Chein et al., 2011), ranking how well adolescents do relative to others (e.g., Op de Macks et al., 2016), and social manipulations such as priming social exclusion before partaking in risk-taking behaviors (e.g., Peake et al., 2013).
Tasks involving social outcome decisions, i.e., making decisions in which outcomes affect others, have been studied most often using economic games that involve some kind of distribution of tokens or points. Tasks that fall within this category include the Trust Game (e.g., Van den Bos, 2011), Ultimatum Game (e.g., Steinmann et al., 2014), Dictator Game (e.g., Gunther Moor et al., 2012), and Family Donation Task (e.g., Telzer et al., 2011). Moreover, some previous work has manipulated the social context in risk-taking paradigms by specifying that the outcomes of decisions would affect others; we also included these types of studies (e.g., Braams & Crone, 2016).
Finally, two studies that did not neatly fall within either of these categories (social go-nogo; Perino et al., 2016; risky vs neutral decisions with a peer; Rodrigo et al., 2014) were classified as “other” social tasks. These contributed to the overall neural reference space but were not included in analyses specifically examining social influence decisions or social outcome decisions.
Some prior behavioral research suggests that social relationships may differentially modulate behavior and neural activity, especially when interacting with known others relative to unknown others (Guassi-Moreira & Telzer, 2018a,b; Guroglu et al., 2014; Padilla-Walker et al., 2017; Prinstein et al., 2001). Thus, we also coded studies based on the type of ‘social actor’, which refers to who the other individual is that the participant is either playing for, against, or is aware exists and is observing.
2.2. An Overview of Multilevel Peak Kernel Density Analysis (MKDA)
The meta-analysis examines reported peak coordinates across the brain using Multilevel peak Kernel Density Analysis (MKDA; Wager et al., 2007; Kober & Wager, 2010) in Neuroelf (http://neuroelf.net). MKDA groups peaks within a single contrast and creates contrast maps for each, using study (or independent contrasts in a study if multiple contrasts are reported in a single study) as an overall unit of analysis. In typical neuroimaging meta-analyses coordinates are convolved with spheres ranging between 10mm and 15mm (for data-driven evidence see Salimi-Khorshidi et al. 2009); building off of prior research that has specifically used the MKDA procedure (Kober et al., 2008; Kober & Wager, 2010; Lindquist et al. 2016; Brooks et al., 2016; Wager et al., 2007), coordinates from each contrast were convolved with 12-mm spheres to create binary comparison indicator maps. Since study contrasts are the main units of analysis, to prevent any single study from biasing the results (due to many peaks, more liberal thresholding, or statistical power), the indicator maps were then weighted depending on the type of analyses used (fixed or random) and the sample size of the contrast. This approach allows the MKDA to control for differences in the quality of the data entering the meta-analysis due to the reliability of the statistical analyses used or the sample size.
Specifically, following previous meta-analyses (Brooks et al., 2016; Lindquist et al. 2012; Lindquist et al., 2016), studies were weighted by the square root of sample size and studies with fixed effects were down-weighted by .75, resulting in studies with higher sample sizes having more influence, and fixed effects having less (for in depth explanation, see Kober et al., 2008; Kober & Wager, 2010; Lindquist et al., 2012, Wager et al., 2007). Note that there were no studies in the current database that used fixed effects analyses. The resulting meta-analytic contrast maps are then created based on the proportion (i.e., density) of contrasts activating near any given voxel. This proportion is thresholded by comparing it to a null distribution created through Monte Carlo simulations that compute the likelihood of finding any activation in any voxel within gray matter (excluding white matter).
We first examined the overall neural reference space across all studies of adolescent decision-making in social contexts, i.e., the brain areas that show consistent activation that is greater than would be expected by chance across all studies in our database. Five thousand Monte Carlo simulations were performed, and following our prior work (Lindquist et al. 2012; 2016) only voxels surpassing a stringent height-based threshold of p < .01 were considered significant. Practically, this means that the findings observed to be consistent across all studies in the literature would have been found by chance only 1% of the time. Resulting maps were cluster-level thresholded using a family wise error rate of p < .05.
Following the computation of the neural reference space, we then computed a series of meta-analytic contrasts assessing how characteristics of the social context modulate neural activation (discussed in more detail below). These meta-analytic contrasts created binary comparison indicator maps of the respective study-level contrasts that were then compared to a null distribution created through Monte Carlo simulations. Again, voxels surpassing the height-based threshold of p < .01 were considered significant. In one meta-analytic contrast in which there were no voxels that surpassed this more stringent threshold, we report exploratory findings at a more lenient threshold (p < .02). We opt to do so given the relatively small sample size in this relatively new literature; exploratory results should be interpreted in the context of discovery for future work. Resulting maps were cluster-level thresholded using a family wise error rate of p < .05.
2.3. Analysis plan for testing the neural reference space of adolescent decision-making in social context
2.3.1. Neural reference space.
In our key analysis, we sought to examine the neural reference space for adolescent decision-making in a social context across different tasks and domains. The neural reference space contains consistent increases in brain activation during decision-making in a social context that occurs more frequently than would be expected by chance across the literature. To ensure that specific studies/samples included did not unduly bias our findings, we ran this analysis with both a full and a more conservative version of the database. First, we included all studies from our database (21 studies, 61 contrasts and 331 data points). Second, we ran two more conservative analyses. The first excluded contrasts that contained age comparisons (from Van den Bos et al., 2011; Chein et al., 2011; Rodrigo et al., 2014; Steinmann et al., 2014; Gunter Moor et al., 2012), resulting in 52 contrasts, 19 studies and 298 data points. The second additionally excluded contrasts from three studies that contained comparisons amongst groups (e.g., chronically victimized v. non-victimized adolescents) within their sample (Van den Bos et al., 2014; Telzer et al., 2017; Verdejo-Garcia et al., 2014), resulting in 47 contrasts, 18 studies and 264 points. Findings from both of these more conservative neural spaces were largely identical to the larger neural reference space, thus we included all studies in subsequent meta-analytic contrasts to retain power and more fully characterize the literature. We report the findings from the more conservative reference spaces in the supplementary material1.
2.3.2. Social Context Type.
To examine the influence of the type of social context (social influence v. social outcome decisions), we next conducted a series of targeted meta-analytic contrasts. For contrasts included from each study, please see Table 1.
Our meta-analytic contrasts compared task types: (1) social influence decisions versus all other social tasks, and (2) social outcome decisions versus all other social tasks. This allowed us to disentangle the brain regions associated with these two different categories of social contexts. Here, we also ran a more constrained analysis where we excluded the two ‘other’ social context studies (Perino et al., 2016; Rodrigo et al., 2014), resulting in 56 contrasts, 19 studies, and 261 data points. The findings with the unconstrained meta-analytic contrast were again largely identical, thus we included the other studies to retain power.
2.3.3. Social Actor Type.
Finally, to gain more insight into the effects of different social actors on neural activity, we examined potential differences based on social actor type. In order to do so, we conducted a meta-analytic contrast comparing across all tasks whether they involved known others (e.g., family members, known peers) versus unknown others (e.g., peer confederates, unknown adult), as well as the reverse meta-analytic contrast unknown others versus known others.
3. Results
3.1. Neural reference space of adolescent decision-making in social contexts
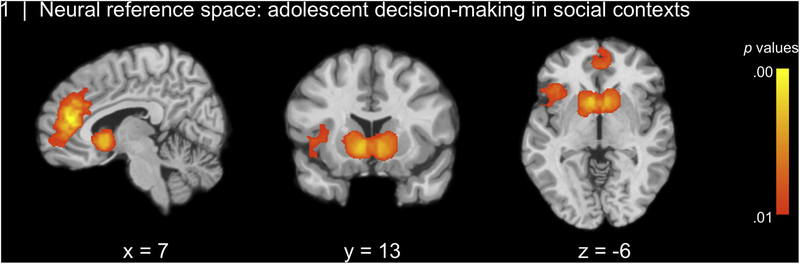
As predicted, the neural reference space for adolescent decision-making in social contexts showed that decision-making in social contexts elicits activation in brain regions implicated in affective sensitivity (bilateral VS, insula), cognitive control (IFG), but also social information processing (dmPFC extending into mPFC) (see Figure 1; Table 2).
Figure 1.
During adolescence, decision-making in social contexts elicits activation in brain regions implicated in social processing (dmPFC), affective sensitivity (insula, ventral striatum), and cognitive control (IFG).
Table 2.
Coordinates for overall neural reference space, thresholded voxel-wise at p < .01, cluster-wise FWE-corrected p < .05.
| Region | k | x | y | z | max | mean |
|---|---|---|---|---|---|---|
| Neural reference space all studies | ||||||
| R Dorsomedial Prefrontal Cortex (dmPFC) | 631 | 6 | 42 | 15 | 0.23 | 0.12 |
| R Dorsal Anterior Cingulate Cortex (dACC) | a | 3 | 27 | 27 | 0.15 | 0.10 |
| L Medial Prefrontal Cortex (mPFC) | a | −3 | 45 | 0 | 0.14 | 0.10 |
| R Ventromedial Prefrontal Cortex (vmPFC) | a | 9 | 51 | −9 | 0.11 | 0.09 |
| L Ventral Striatum (VS) | 470 | −6 | 12 | −6 | 0.18 | 0.12 |
| R Ventral Striatum | b | 9 | 9 | −9 | 0.17 | 0.12 |
| L Insula | 299 | −39 | 18 | 0 | 0.14 | 0.10 |
| L Inferior Frontal Gyrus (IFG) | c | −36 | 27 | −9 | 0.14 | 0.10 |
Abbreviations. k = cluster size in mm3; x, y, z = coordinates in Montreal Neurological Institute (MNI) space; max = maximum value within cluster; mean = average value within cluster. L = left, R = right
dmPFC cluster
= ventral striatum cluster
insula/IFG cluster.
3.1.1. Social Context Type.
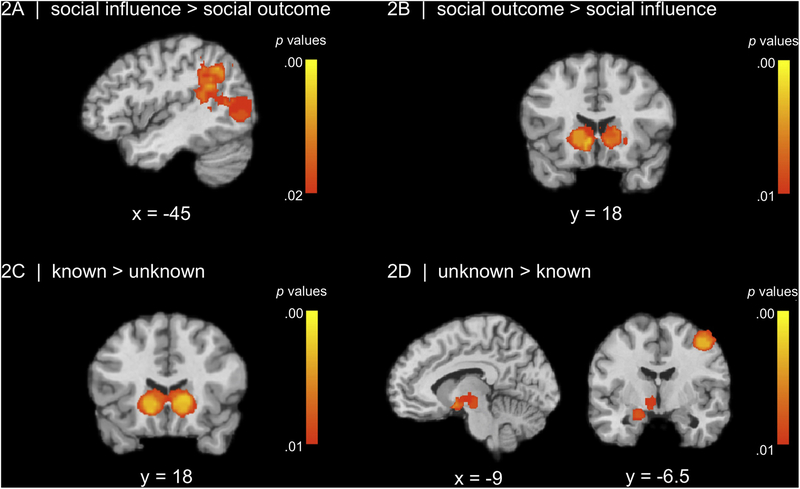
For social influence decisions > all other social tasks, there were no significant effects at our conservative a priori threshold of p<.01. Given the novelty of the database and the relatively small sample of studies included, we thus report exploratory analyses at a less stringent threshold. At the threshold of p < .02, we found a cluster of regions implicated in social information processing that encompassed inferior parietal lobule (IPL), temporo-parietal junction (TPJ), and posterior superior temporal sulcus (pSTS) (see Figure 2A; Table 3). These exploratory findings should be interpreted in the context of discovery for future research, although it is notable that they are consistent with a priori predictions that regions involved in mentalizing would be active when making decisions in the presence of social others. For social outcome decisions > all other social tasks, we observed a large cluster in the VS, which highlights the rewarding/salient nature of making decisions where the outcome affects others (see Figure 2B; Table 3).
Figure 2.
(A) Social influence decisions > Social outcome decisions and other social tasks elicits activity in regions implicated in social processing (IPL, TPJ, pSTS), highlighting the contribution of the social brain, although at a slightly less stringent threshold of p < .02 (B) Social outcome decisions > Social influence decisions and other social tasks yields activation in the VS. (C) Decision-making with known > unknown others yields activity in the VS (D) Decision-making with unknown vs known others yields activation in the subgenual ACC/amygdala.
Table 3.
Coordinates for meta-analytic contrasts, thresholded voxel-wise at p < .01, except where noted by *p < .02, cluster-wise FWE-corrected p < .05.
| Region | k | x | y | z | max | mean |
|---|---|---|---|---|---|---|
| Contrast: social influence decisions > social outcome decisions and other tasks* | ||||||
| L Inferior Parietal Lobule (IPL) | 615 | −45 | −57 | 39 | 0.19 | 0.10 |
| L Superior Temporal Gyrus (STS) | d | −42 | −51 | 24 | 0.17 | 0.12 |
| L Supramarginal Gyrus (TPJ) | d | −45 | −45 | 39 | 0.15 | 0.10 |
| L Angular Gyrus (TPJ) | d | −48 | −69 | 42 | 0.14 | 0.10 |
| L Middle Temporal Gyrus | d | −42 | −63 | 12 | 0.12 | 0.10 |
| L Middle Occipital Gyrus | d | 0.36 | 0.72 | 9 | 0.08 | 0.08 |
| Contrast: social outcome decisions > social influence decisions and other tasks | ||||||
| L Ventral Striatum (VS) | 365 | −6 | 18 | −3 | 0.24 | 0.15 |
| R Ventral Striatum (VS) | e | 9 | 18 | −6 | 0.21 | 0.15 |
| L Putamen | e | −24 | 15 | 9 | 0.15 | 0.13 |
| R Putamen | e | 24 | 21 | −3 | 0.12 | 0.12 |
| Contrast: social actors known > unknown | ||||||
| R Ventral Striatum (VS) | 445 | 18 | 18 | 3 | 0.36 | 0.25 |
| L Ventral Striatum | f | −9 | 18 | 0 | 0.36 | 0.25 |
| Contrast: social actors unknown > known | ||||||
| R Subgenual Anterior Cingulate (sgACC) | 330 | 3 | 18 | −12 | 0.1 | 0.10 |
| L Amygdala | g | −21 | −9 | −18 | 0.14 | 0.11 |
| L Thalamus | g | −6 | −21 | −3 | 0.11 | 0.09 |
| R Lentiform Nucleus | g | 18 | 0 | −6 | 0.1 | 0.09 |
| R Ventral Striatum (VS) | g | 3 | 3 | −3 | 0.2 | 0.11 |
| R Postcentral Gyrus | 291 | 51 | −12 | 51 | 0.19 | 0.13 |
Abbreviations. k = cluster size in mm3; x, y, z = coordinates in Montreal Neurological Institute (MNI) space; max = maximum value within cluster; mean = average value within cluster.
IPL/TPJ/pSTS cluster
ventral striatum cluster
ventral striatum cluster
subgenual ACC/amygdala cluster.
3.1.2. Social Actor Type.
Known others > unknown others elicited activity in bilateral VS (See Figure 2C; Table 3). For the reverse contrast, unknown others > known others, we observed activation in the subgenual ACC extending into the amygdala as well as the right postcentral gyrus (See Figure 2D; Table 3).
4. Discussion
Adolescence is a time when the social world is particularly salient (Blakemore, in press), and decision-making is especially influenced by social information in emotionally-charged ‘hot’ social contexts (Duckworth & Steinberg, 2015). The goal of the current meta-analysis was to investigate the neural bases of adolescent decision-making in social contexts across the emerging developmental neuroimaging literature. Recent adaptions of neurobiological models of adolescent risk-taking acknowledge the important role of the social context (e.g., Shulman et al., 2016), but the discussion of the underlying neural circuitry involved in adolescent decision-making has yet to expand beyond brain networks implicated in affective sensitivity and cognitive control (Pfeifer & Allen, 2016). Our results provide meta-analytic evidence that VS, insula/IFG, and dmPFC are consistently implicated in adolescent decision-making in social contexts. These findings support the notion that it is crucial to move beyond the popular notion of dueling affective and cognitive control systems in order to gain traction on understanding adolescent neurocognition. Our findings underscore the fact that studies of developmental social-affective processes must measure and model psychological and neural processes related to affect, cognitive control, and social information processing, taking into account not only the developmental window during which processes are occurring, but also the momentary context in which adolescents’ behavior is occurring.
4.1. Overall neural reference space.
Our key analysis leveraged 21 fMRI studies and revealed that the neural reference space of adolescent decision-making encompassed regions largely consistent with neurobiological models of both adolescent risk-taking and social cognition, including the VS, IFG/insula and dmPFC.
Across human (Delgado, 2007; Galvan, 2010; Telzer, 2016) and animal (Berridge & Kringelbach, 2008) models, the VS has been recognized as a key node in reward/saliency processing and incentive-driven behaviors. As such, the VS plays a prominent role in neurobiological models of adolescent risk-taking behaviors, and it is proposed that risky decisions in the peer context may be even more rewarding during adolescence, as evidenced by increased VS activity when peers are present during adolescent risk-taking (Chein et al., 2011). The VS serves an adaptive role in positive contexts as well, for example in prosocial decision-making, where activity in the VS is interpreted as part of the “warm glow” of giving (Moll et al., 2006). The current findings confirm the prominent role of the VS in adolescent decision-making in social contexts.
The IFG was also part of the neural reference space. The IFG is related to a wide range of functions, including cognitive control (Aron & Poldrack, 2006; Cascio et al., 2015). Recent work has also associated the left vlPFC (i.e., IFG) with tendencies toward impulsive sensation seeking (Chase et al., 2017), as well as the moderation between behavioral responses to one’s best friend’s positive affect and risky behavior (Ambrosia et al., 2018). In the context of social cognition, the IFG has been implicated in (re)appraisal of social stimuli, emotional judgment, and top-down aspects of emotion recognition such as deciding what action to take based on someone’s emotion (Blakemore, 2008; Nelson & Guyer, 2011; Guyer et al., 2012). In the emotion literature, the IFG is routinely involved in emotional experiences and perceptions, perhaps because it is allowing a person to draw on semantic emotion category knowledge to make meaning of their and others’ affective feelings (Brooks et al., 2017; Lindquist et al., 2012). These more social-emotional functions of the IFG may explain its contribution to adolescent decision-making in social contexts, over the contribution of the dlPFC, for instance, which is more often linked to domain-general cognitive control (MacDonald, Cohen, Stenger, & Carter, 2000; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). Indeed, the IFG cluster we observed spanned both the IFG and anterior insula; it may thus be part of what has been termed the “frontoinsula,” a cluster of brain regions that frequently co-activate as part of an extended brain network that responds to affectively salient stimuli (Seeley et al., 2007; Kleckner et al., 2017; Touroutoglou et al., 2012).
The anterior insula is also part of the so-called “salience network” (Seeley, et al. 2007) insofar as it represents affective states and helps guide attention during goal-directed behavior (Menon & Uddin, 2010). Our other work suggests that it more generally responds to pleasant and unpleasant stimuli (Lindquist et al., 2016) and represents the importance of social stimuli for humans (Aztil et al., in prep). These findings are consistent with the idea that the salience network is part of a broader group of brain regions (including those traditionally part of the so-called ‘default mode network’) that are involved in representing affective states and maintaining homeostasis of the organism (Kleckner et al. 2017). As a highly social species, social others are certainly important to homeostasis for humans. In keeping with these findings, in social-affective contexts, the insula is associated with learning after social feedback (Jones et al., 2014) and the evaluation of others’ mental states and emotional expressions (Blakemore, 2008; Lamm & Singer, 2010). Moreover, adolescents with high familial conflict show disrupted connectivity within aspects of this network (insula-VS connectivity) during risk-taking in the presence of their mother (Guassi Moreira & Telzer, 2018c). Although the insula remains largely overlooked in current neurobiological models of adolescent decision-making, the results of our meta-analysis underscore the key role of the insula in adolescent decision-making in a social context (cf. Smith et al., 2014).
Finally, our neural reference space included the dmPFC extending into the mPFC, a region included in the social brain model (Blakemore, 2008; Blakemore & Mills, 2014). The mPFC is often discussed as a region implicated in understanding others’ mental states, with the dorsal peak found in our study generally attributed to mentalizing, or thinking about oneself and others’ psychological states (Nelson et al., 2005; Blakemore, 2008; Blakemore & Mills, 2014; Jenkins & Mitchell, 2011; Denny et al., 2012). Some current neurobiological models of adolescent decision-making do recognize a role of the mPFC, such as in the dual systems model, which includes the mPFC in the socioemotional system that increases motivation to pursue rewards (Shulman et al., 2016). The role of the dmPFC may also differ in part based on task demands (e.g., dmPFC is also associated with the cognitive component of risk, but this part is cytoarchitecturally closer to the dorsal ACC; Van Duijvenvoorde et al., 2015). Nonetheless, our findings represent a more dorsal part of the mPFC that is not often discussed in the literature. In sum, many of the regions highlighted as part of our neural reference space of adolescent decision-making in social contexts involve regions outside current neurobiological models of adolescent decision-making.
4.2. Modulation of the neural reference space of decision-making in social contexts
The second goal of this meta-analysis was to examine how the neural reference space of decision-making in social contexts was modulated by different types of social context. In other words, we further delineated neural activity that was relatively more likely to occur during social influence decisions (i.e., when one’s decisions are affected by others) and social outcome decisions (i.e., when one’s decisions affect others).
4.2.1. Social influence decisions.
Social influence tasks yielded a cluster of regions associated with social cognition (IPL, TPJ and pSTS), highlighting the role of social brain regions when adolescents’ decisions are affected by others - either in their presence or with actual feedback. This effect was found using a more liberal threshold than our other findings; as such the interpretation of this finding should be seen as more exploratory. However, given both our a priori hypotheses and the nascence of the field we opted to include these more liberal results as they may guide future research. Interestingly, while the dmPFC was part of the overall neural reference space, the TPJ and pSTS seem to be more specific to social influence. The TPJ and pSTS have been implicated in predicting biological movements (Frith & Frith, 2007),in understanding other people’s mental states (Saxe, 2006) and beliefs about stimuli (social or otherwise) more generally (Mitchell et al., 2005). In the context of social influence, adolescents likely recruit these regions to evaluate social norms and the perspectives of others, which in turn affects their behavior (Shaw, 2003; Telzer et al., 2018). While many of the studies included in the social influence category used versions of risk-taking tasks, we surprisingly did not find VS activity for this contrast. This is an interesting finding, given that it is often thought that peers may make risk-taking a more rewarding experience, as evidenced by heightened activation in VS during risk-taking with peers compared to alone during adolescence (Chein et al., 2011). It is possible that decision-making under social influence recruits regions implicated in social processing more consistently than VS when considering a broader range of behaviors beyond just risk-taking, such as in this meta-analysis. Taken together, the current findings implicate that it is crucial to take a broad approach to studying the neural correlates of social influence, as different neural processes may be implicated depending on the task behaviors and context used (cf. Van Hoorn et al., 2016) and modeling of task data (see e.g., Sherman et al., 2017).
4.2.2. Social outcome decisions.
Social outcome decisions elicited activity in the VS, supporting the idea that decisions that affect the outcomes of others are motivationally salient and rewarding to adolescents (Moll et al., 2006; Telzer, 2016; Do, Guassi Moreira, & Telzer, 2018c). VS activity is often associated with positively valenced affect (e.g., Forbes & Dahl, 2005), and so it could be argued that this effect is confounded by a difference in the valence of the behaviors studied in social outcome versus social influence decisions. In other words, social outcome tasks might include positively valenced behaviors, such as prosocial decisions, whereas social influence might include negatively valenced behaviors, such as risk-taking. However, this is highly unlikely given that the social outcome category also encompassed gambling for others, and the social influence category included neutral or positively valenced behaviors such as prosocial behavior. Given that there was a range of behaviors studied within each category, a more parsimonious explanation is that the act of making decisions that affect others is itself motivationally salient and rewarding for adolescents.
4.2.3. Social actor type.
Finally, we investigated how the neural reference space was modulated by social relationships across decision-making tasks in a social context. Given that the social context is so salient in adolescence, it is important to disentangle whether the closeness of social actors (i.e., known versus unknown others) differentially affects neural processing involved in decision-making. The present findings showed that decision-making in a social context in which known others are involved elicited more VS activity than when unknown others are involved, highlighting the motivational relevance of known others for adolescents (Telzer, 2016). This finding is in line with previous behavioral evidence showing that adolescents tend to be more prosocial towards friends than strangers (Guroglu et al., 2014; Padilla-Walker, Carlo, & Memmott-Elison, 2017), and close friendships, as opposed to broader peer groups, are protective for adolescents’ mental health (Narr et al., 2017). Hence, when researchers use unknown social actors in studies to create a more controlled experimental environment (i.e., one in which adolescents do not have pre-existing beliefs about social actors), they may be misrepresenting the extent of VS activity recruited in everyday life when adolescents interact with known social actors.
Social interactions with unknown others elicited more subgenual ACC/amygdala activity. These regions are part of the salience network, and show heightened responding to threat, negativity, and the unknown (Masten et al., 2011; Lindquist et al., 2016). The thoughts, feelings and behaviors of unknown others may be relatively uncertain and hence require more information gathering. Adolescents need to figure out whether unknown others constitute a (social) ‘threat’, which in turn can affect subsequent decision-making. Taken together, our results highlight differences in neural recruitment depending on the social relationship, such that known others consistently elicit VS activity, while subgenual ACC/amygdala is consistently recruited for unknown others.
4.3. A constructionist model of adolescent decision-making
Together, our results suggest that models of adolescent decision-making would be well advised to consider the role of neural systems involved in affect, cognitive control, and social information processing. Our findings are consistent with a constructionist approach to the mind (Barrett, 2017; Lindquist, 2013), which hypothesizes that all mental states can be decomposed into more basic affective, semantic, sensory, and cognitive control elements; brain networks supporting these functions are thought to combine to create the myriad mental states (emotions, cognitions, perceptions) that humans experience on a daily basis (Lindquist & Barrett, 2012; Barrett & Satpute, 2013).
A constructionist approach to adolescent decision-making describes the current findings and offers novel predictions for future research. For instance, it suggests that adolescent behaviors can be described as the combination of more basic processes such as affective salience (whether a person or situation is especially meaningful to the observer), social information processing (understanding the feelings and thoughts of the social actors involved), and cognitive control (whether an adolescent tries to actively regulate or inhibit their behavior). Each of these psychological functions has been associated with specific canonical neural networks (Barrett & Satpute, 2013; Lindquist & Barrett, 2012; McCormick et al., 2018; Smith et al. 2009; Spunt & Lieberman, 2013). Our constructionist approach predicts that adolescent decision-making in a given context will be associated with the relative activity within and between these networks and will vary as a product of development (e.g., age, pubertal status) and the context (e.g., the presence or type of peers).
Although no research to date has explicitly tested the constructionist hypothesis that between-network connectivity predicts different decision-making outcomes, some existing research is consistent with this approach. For instance, studies find that greater connectivity within the salience network (e.g., between the VS and insula) predicts adolescent risky decision-making (Guassi Moreira & Telzer, 2018c). Other studies find that greater connectivity between the salience network and social information processing network (e.g., the VS and mPFC) predicts adolescent risky decision-making (Qu et al. 2015). VS-mPFC connectivity is uniquely heightened during adolescence when adolescents think they are being watched by a peer (Somerville et al., 2013) and VS-mPFC connectivity at rest shows regionally specific linear age-related changes from childhood to late adolescence (Fareri et al., 2015). VS-mPFC connectivity subsequently correlates with age-related increases in testosterone levels (Fareri et al., 2015) as well as reward sensitivity (Van Duijvenvoorde et al., 2016), cognitive control, and substance use (Lee & Telzer, 2016). On the one hand, such connectivity may be specific to reward-related processes, insofar as mPFC is a dopaminoceptive region with dopaminergic projections from the substantia nigra/ventral tegmental area (see Telzer, 2016). On the other hand, especially task-related increases in functional connectivity between VS-mPFC and VS-insula may represent the integration of social signals with motivational and affective processes that govern goal-directed behavior (Somerville et al., 2013). In keeping with this constructionist interpretation, other research finds evidence for increased functional connectivity within regions associated with social information processing and between these regions and regions associated with affective sensitivity, motivation, and cognitive control when adolescents experience social evaluation (McCormick et al., 2018). A limitation of our meta-analytic procedure is that we could not address functional connectivity. However, future research should continue to examine the dynamic coupling of these interacting neural systems as well as networks involved in cognitive control across different social contexts and across development to gain a deeper understanding of how diverse neural systems work together to support adolescent behavior.
4.4. Limitations and future directions
Although the current findings are central to the developmental period of adolescence, it is important to acknowledge that they may or may not be unique to adolescence. To date, the emerging neuroimaging literature on this topic is relatively small (i.e., we could only include 21 papers), which prevents the comparison of the adolescent neural reference space versus the neural reference space in other developmental periods. Moreover, the MKDA does not examine longitudinal changes, and so such studies were excluded in the present meta-analysis, precluding our ability to examine developmental trajectories. To further unpack the developmental trajectory of adolescent decision-making in social contexts, future studies should aim to include diverse age groups, especially children (ages <12; also see Li, 2017), as well as ‘older’ adult groups (age 30+instead of college students) as these are highly underrepresented in current developmental comparisons. As the developmental neuroimaging field is moving from cross-sectional studies to longitudinal designs, that allow within- and between-subject comparisons, this shift will ultimately provide a more comprehensive understanding of how individual differences and environmental processes impact developmental trajectories (Crone & Elzinga, 2015). Nevertheless, the current meta-analysis was the first empirical test of the neural reference space supporting adolescent decision-making in social contexts and can be considered a stepping stone for future research into this important topic.
In conclusion, we underscore the importance of integrating social contexts when studying adolescent neurocognition, and we provide meta-analytic evidence that dmPFC, VS and insula/IFG are consistently activated during adolescent decision-making in social contexts. In addition, we show that the neural reference space is modulated by the type of task (i.e., social influence or social outcome decisions) and the social actor (i.e., known vs unknown social actors). Our findings highlight the need for the field to broaden the lens and study brain regions associated with social information processing to gain traction on the processes supporting adolescent neurocognition in social contexts. While our results do not imply that social brain regions are implicated in a standard ‘cold’ decision-making task, such tasks may not be truly representative of decision-making in real-life, which seldom takes place in a social vacuum. Exploration of broader brain networks implicated in adolescent decision-making in social contexts may lead to a refinement rather than verification of current neurobiological models (Pfeifer & Allen, 2016). These meta-analytic findings represent a first step towards refining current neurobiological models of adolescent decision-making. With further research, especially that increases our understanding of the dynamic interplay between networks supporting affective responding, cognitive control, and social information processing across development, the field can refine existing models to understand how the context shapes adolescent behavior. Ultimately, understanding the neural processes involved in adolescent decision-making will help us to solve the complex puzzle of why adolescents make adaptive decisions in some situations, but maladaptive decisions in other situations.
Supplementary Material
Highlights.
Social context plays an important role in adolescent decision-making
Neurobiological models do not discuss brain regions implicated in social processing
Meta-analysis tested neural coding for adolescent decision-making in social context
dmPFC, IFG/insula and ventral striatum are consistently involved in this process
Neurobiological models should incorporate social information processing regions
Acknowledgements
This work was supported by the National Institutes of Health (R01DA039923 to EHT) and National Science Foundation (SES1459719 to EHT). The authors would like to thank the members of the Developmental Social Neuroscience Lab for their helpful comments on a previous version of this manuscript, and Melissa Burroughs for her help with the study overview table.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We report findings from the more conservative neural reference spaces thresholded at p < .01 and p < .02 in the supplement. The insula/IFG effect does not reach significance at our determined threshold of p < .01, but is visible at p < .02, which suggests this is a power issue rather than the studies adding in qualitatively different data.
References
- Ambrosia M, Eckstrand KL, Morgan JK, Allen NB, Jones NP, Sheeber L, … & Forbes EE (2018). Temptations of friends: adolescents’ neural and behavioral responses to best friends predict risky behavior. Social Cognitive and Affective Neuroscience, 13(5), 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert D, Chein J, & Steinberg L (2013). The teenage brain: Peer influences on adolescent decision-making. Current Directions in Psychological Science, 22(2), 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, & Poldrack RA (2006). Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. Journal of Neuroscience, 26(9), 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aztil S, Parrish MJ, Satpute A, Shablack H, Brooks JA, & Lindquist KA (in preparation). A social dimension of the evaluative brain
- Barrett LF (2017). How emotions are made: The secret life of the brain Houghton Mifflin Harcourt. [Google Scholar]
- Barrett LF, & Satpute AB (2013). Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Current Opinion in Neurobiology, 23(3), 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley-Levenson E, Galván A (2014). Neural representation of expected value in the adolescent brain. Proceedings of the National Academy of Sciences, 111, 1646–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Kringelbach ML (2008). Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology, 199(3), 457–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ (in press). Avoiding social risk in adolescence. Current Directions in Psychological Science
- Blakemore SJ, den Ouden H, Choudhury S, & Frith C (2007). Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience, 2(2), 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, & Mills KL (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. [DOI] [PubMed] [Google Scholar]
- Borawski EA, Ievers-Landis CE, Lovegreen LD, Trail ES (2003). Parental monitoring, negotiated unsupervised time, and parental trust: the role of perceived parenting practices in adolescent health risk behaviors. Journal of Adolescent Health, 33(2), 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, Peters S, Peper JS, Güroğlu B, & Crone EA (2014). Gambling for self, friends, and antagonists: differential contributions of affective and social brain regions on adolescent reward processing. NeuroImage, 100, 281–289. [DOI] [PubMed] [Google Scholar]
- Braams BR, & Crone EA (2016). Longitudinal changes in social brain development: Processing outcomes for friend and self. Child Development [DOI] [PubMed]
- Braams BR, & Crone EA (2017). Peers and parents: a comparison between neural activation when winning for friends and mothers in adolescence. Social Cognitive and Affective Neuroscience, 12(3), 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechwald WA, & Prinstein MJ (2011). Beyond homophily: A decade of advances in understanding peer influence processes. Journal of Research on Adolescence, 21(1), 166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BB (2004). Adolescents’ relationships with peers. Handbook of Adolescent Psychology (2nd ed.). (pp. 363–394). Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- Burnett S, Bird G, Moll J, Frith C, & Blakemore SJ (2009). Development during adolescence of the neural processing of social emotion. Journal of Cognitive Neuroscience, 21(9), 1736–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CN, Carp J, O’Donnell MB, Tinney FJ Jr, Bingham CR, Shope JT, … & Falk EB (2014). Buffering social influence: neural correlates of response inhibition predict driving safety in the presence of a peer. Journal of Cognitive Neuroscience, 27(1), 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66, 295–319. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, & Galvan A (2008). The adolescent brain. Developmental Review, 28(1), 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Fournier JC, Bertocci MA, Greenberg T, Aslam H, Stiffler R, … & Phillips ML (2017). A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Translational Psychiatry, 7(4), e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, & Steinberg L (2011). Peers increase adolescent risk-taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14(2), F1–F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. [DOI] [PubMed] [Google Scholar]
- Crone EA, & Elzinga BM (2015). Changing brains: How longitudinal functional magnetic resonance imaging studies can inform us about cognitive and social‐affective growth trajectories. Wiley Interdisciplinary Reviews: Cognitive Science, 6(1), 53–63. [DOI] [PubMed] [Google Scholar]
- Crone EA, Will G-J, Overgaauw S, & Güroğlu B (2012). Social decision-making in childhood and adolescence. In van Lange PAM, Rockenbach B, and Yamagishi T. Reward and Punishment in Social Dilemmas NY: Oxford University Press. [Google Scholar]
- Delgado MR (2007). Reward‐related responses in the human striatum. Annals of the New York Academy of Sciences, 1104(1), 70–88. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, & Ochsner KN (2012). A meta-analysis of functional neuroimaging studies of self-and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24(8), 1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall CN, & Richman SB (2011). Social exclusion and the desire to reconnect. Social and Personality Psychology Compass, 5(11), 919–932. [Google Scholar]
- Do K, Guassi Moreira J, & Telzer EH (2017). But is helping you worth the risk? Defining prosocial risk-taking in adolescence. Developmental Cognitive Neuroscience, 25, 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, & Steinberg L (2015). Unpacking Self-Control. Child Development Perspectives, 9(1), 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Gabard-Durnam L, Goff B, Flannery J, Gee DG, Lumian DS, Caldera C, Tottenham N (2015). Normative development of ventral striatal resting state connectivity in humans. NeuroImage, 118, 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2005). Neural systems of positive affect: relevance to understanding child and adolescent depression?. Development and Psychopathology, 17(3), 827–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, & Frith U (2007). Social cognition in humans. Current Biology, 17(16), R724–R732. [DOI] [PubMed] [Google Scholar]
- Galvan A (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience, 4(6), 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, & Casey BJ (2008). Risk-taking and the adolescent brain: who is at risk? Developmental Science, 10(2), F8–F14. [DOI] [PubMed] [Google Scholar]
- Gardner M, & Steinberg L (2005). Peer influence on risk-taking, risk preference, and risky decision-making in adolescence and adulthood: an experimental study. Developmental Psychology, 41(4), 625. [DOI] [PubMed] [Google Scholar]
- Guassi Moreira J & Telzer EH (2018a). Mother still knows best: Maternal influence uniquely modulates adolescent reward sensitivity during risk-taking. Developmental Science,21, e12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guassi Moreira J & Telzer EH (2018b). Family conflict influences whether adolescents take greater or fewer risks when their parent is affected. Developmental Science, 21, e12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guassi Moreira J & Telzer EH (2018c). Family conflict is associated with longitudinal changes in insular-striatal functional connectivity during adolescent risk-taking under maternal influence. Developmental Science, 21(5), e12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor B, Op de Macks ZA, Güroğlu B, Rombouts SA, Van der Molen MW, & Crone EA (2012). Neurodevelopmental changes of reading the mind in the eyes. Social Cognitive and Affective Neuroscience, 7(1), 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güroğlu B, van den Bos W, van Dijk E, Rombouts SA, & Crone EA (2011). Dissociable brain networks involved in development of fairness considerations: understanding intentionality behind unfairness. NeuroImage, 57(2), 634–641. [DOI] [PubMed] [Google Scholar]
- Güroğlu B, van den Bos W, & Crone EA (2014). Sharing and giving across adolescence: An experimental study examining the development of prosocial behavior. Frontiers in Psychology, 291(5),1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, & Nelson EE (2012). Neural circuitry underlying affective responses to peer feedback in adolescence. Social Cognitive and Affective Neuroscience, 7, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AC, & Mitchell JP (2011). Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience, 6(3), 211–218. [DOI] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Powers A, Mehta N, … & Casey BJ (2014). Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll LJ, Magis-Weinberg L, Speekenbrink M, & Blakemore SJ (2015). Social influence on risk perception during adolescence. Psychological Science, 26(5), 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, & Wager TD (2010). Meta-analysis of neuroimaging data. Wiley Interdisciplinary Reviews Cognitive Science, 1(2), 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia Chengie, Simmons WK, Quigley KS, Dickerson BC, & Barrett LF (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behavior, 1,0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, & Singer T (2010). The role of anterior insular cortex in social emotions. Brain Structure and Function, 214(5–6), 579–591. [DOI] [PubMed] [Google Scholar]
- Lee T & Telzer EH (2016). Negative coupling between the right fronto-parietal and limbic resting state networks predicts increased self-control and later substance use onset in adolescence. Developmental Cognitive Neuroscience, 20, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R (2017). Flexing dual-systems models: How variable cognitive control in children informs our understanding of risk-taking across development. Developmental Cognitive Neuroscience, 27, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA (2013). Emotions emerge from more basic psychological ingredients: A modern psychological constructionist model. Emotion Review, 5(4), 356–368. [Google Scholar]
- Lindquist KA, & Barrett LF (2012). A functional architecture of the human brain: emerging insights from the science of emotion. Trends in Cognitive Sciences, 16(11), 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, & Barrett LF (2012). The brain basis of emotion: a meta-analytic review. Behavioral and Brain Sciences, 35(3), 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, & Feldmann Barrett L (2016). The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 1–13. [DOI] [PMC free article] [PubMed]
- Masten CL, Morelli SA, & Eisenberger NI (2011). An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. NeuroImage, 55(1), 381–388. [DOI] [PubMed] [Google Scholar]
- McCormick EM, van Hoorn J, Cohen JR, & Telzer EH (2018). Functional architecture of social brain network in children and adolescents. Social Cognitive and Affective Neuroscience, 13, 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RI, & Crandall CS (2015). Social norms and social influence. Current Opinion in Behavioral Sciences, 3, 147–151. [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, & Banaji MR (2005). Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron, 50(4), 655–663. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, & Grafman J (2006). Human fronto–mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences, 103(42), 15623–15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, & Guyer AE (2011). The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience, 1(3), 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, & Pine DS (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35(02), 163–174. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Jarcho JM, & Guyer AE (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks ZA, Bunge SA, Bell ON, Kriegsfeld LJ, Kayser AS, & Dahl RE (2016). The effect of social rank feedback on risk-taking and associated reward processes in adolescent girls. Social Cognitive and Affective Neuroscience, 12(2), 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla‐Walker LM, Carlo G, & Memmott‐Elison MK (2017). Longitudinal Change in Adolescents’ Prosocial Behavior Toward Strangers, Friends, and Family. Journal of Research on Adolescence Epub ahead of print. doi: 10.1111/jora.12362 [DOI] [PubMed]
- Peake SJ, Dishion TJ, Stormshak EA, Moore WE, & Pfeifer JH (2013). Risk-taking and social exclusion in adolescence: Neural mechanisms underlying peer influences on decision-making. NeuroImage, 82, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino MT, Miernicki ME, & Telzer EH (2016). Letting the good times roll: Adolescence as a period of reduced inhibition to appetitive social cues. Social Cognitive and Affective Neuroscience, 11(11), 1762–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, & Allen NB (2016). The audacity of specificity: Moving adolescent developmental neuroscience towards more powerful scientific paradigms and translatable models. Developmental Cognitive Neuroscience, 17, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, & Lieberman MD (2009). Neural correlates of direct and reflected self-appraisals in adolescents and adults: When social perspective-taking informs self-perception. Child Development, 80(4), 1016–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, Oswald TM, Mazziotta JC, Iacoboni M, & Dapretto M (2011). Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron, 69(5), 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein MJ, Boergers J, & Spirito A (2001). Adolescents’ and their friends’ health-risk behavior: Factors that alter or add to peer influence. Journal of Pediatric Psychology, 26(5), 287–298. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, & La Greca AM (2004). Childhood peer rejection and aggression as predictors of adolescent girls’ externalizing and health risk behaviors: a 6-year longitudinal study. Journal of Consulting and Clinical Psychology, 72(1), 103–112. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, & Wang SS (2005). False consensus and adolescent peer contagion: Examining discrepancies between perceptions and actual reported levels of friends’ deviant and health risk behaviors. Journal of Abnormal Child Psychology, 33(3), 293–306. [DOI] [PubMed] [Google Scholar]
- Qu Y, Galvan A, Fuligni AJ, Lieberman MD, & Telzer EH (2015). Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk-taking. Journal of Neuroscience, 35(32), 11308–11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo MJ, Padrón I, De Vega M, & Ferstl EC (2014). Adolescents’ risky decision-making activates neural networks related to social cognition and cognitive control processes. Frontiers in Human Neuroscience, 8, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Reyna VF, & Satterthwaite TD (2017). Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Developmental Cognitive Neuroscience, 27, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, & Nichols TE (2009). Meta-analysis of neuroimaging data: A comparison of image-based and coordinate-based pooling of studies. NeuroImage, 45(3), 810–823. [DOI] [PubMed] [Google Scholar]
- Saxe R (2006). Uniquely human social cognition. Current Opinion in Neurobiology, 16(2), 235–239. [DOI] [PubMed] [Google Scholar]
- Schriber RA, & Guyer AE (2016). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J (2003). Automatic for the people: how representations of significant others implicitly affect goal pursuit. Journal of Personality and Social Psychology, 84(4), 661. [DOI] [PubMed] [Google Scholar]
- Sherman LE, Payton AA, Hernandez LM, Greenfield PM, & Dapretto M (2016). The power of the like in adolescence: Effects of peer influence on neural and behavioral responses to social media. Psychological Science, 27(7), 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Steinberg L, & Chein J (2017). Connecting brain responsivity and real-world risk-taking: Strengths and limitations of current methodological approaches. Developmental Cognitive Neuroscience [DOI] [PMC free article] [PubMed]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, & Steinberg L (2016). The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience, 17, 103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, & Luciana M (2015). Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage, 122, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Chein J, & Steinberg L (2014). Peers increase adolescent risk-taking even when the probabilities of negative outcomes are known. Developmental Psychology, 50(5), 1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, Strang N, & Chein J (2015). Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Developmental Cognitive Neuroscience, 11, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, & Chein J (2014). The role of the anterior insula in adolescent decision-making. Developmental Neuroscience, 36(3–4), 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, … & Beckmann CF (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, & Casey BJ (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, & Casey BJ (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science, 24(8), 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, & Lieberman MD (2013). The busy social brain: Evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychological Science, 24(1), 80–86. [DOI] [PubMed] [Google Scholar]
- Steinberg L (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Icenogle G, Shulman EP, Breiner K, Chein J, Bacchini D, … & Fanti KA (2017). Around the world, adolescence is a time of heightened sensation seeking and immature self‐regulation. Developmental Science [DOI] [PMC free article] [PubMed]
- Steinmann E, Schmalor A, Prehn-Kristensen A, Wolff S, Galka A, Möhring J, … & Siniatchkin M (2014). Developmental changes of neuronal networks associated with strategic social decision-making. Neuropsychologia, 56, 37–46. [DOI] [PubMed] [Google Scholar]
- Telzer EH (2016). Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, 17, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, & Fuligni AJ (2010). Gaining while giving: An fMRI study of the rewards of family assistance among White and Latino youth. Social Neuroscience, 5(5–6), 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, & Fuligni AJ (2011). Neural regions involved in self-control and mentalizing are recruited during prosocial decisions towards the family. NeuroImage, 58, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, & Galván A (2013). Meaningful family relationships: Neurocognitive buffers of adolescent risk-taking. Journal of Cognitive Neuroscience, 25(3), 374–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Miernicki ME, & Galván A (2015). The quality of adolescents’ peer relationships modulates neural sensitivity to risk-taking. Social Cognitive and Affective Neuroscience, 10(3), 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien NT, & Qu Y (2015). Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk-taking. Social Cognitive and Affective Neuroscience, 10(10), 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Miernicki ME, & Rudolph K (2017). Chronic childhood peer victimization heightens neural sensitivity to risk-taking. Development and Psychopathology, 1–14. [DOI] [PMC free article] [PubMed]
- Telzer EH, Van Hoorn J, Rogers CR & Do KT (2018). Social influence on positive youth development: A developmental neuroscience perspective. Advances in Child Development and Behavior, 54, 215–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, & Barrett LF (2012). Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage, 60, 1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bos W, van Dijk E, Westenberg M, Rombouts SA, & Crone EA (2011). Changing brains, changing perspectives: The neurocognitive development of reciprocity. Psychological Science, 22(1), 60–70. [DOI] [PubMed] [Google Scholar]
- Van den Bos W, Vahl P, Güroglu B, Van Nunspeet F, Colins O, Markus M, …Crone EA (2014). Neural correlates of social decision-making in severely antisocial adolescents. Social Cognitive and Affective Neuroscience, 9, 2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijvenvoorde ACK, Huizenga HM, Somerville LH, Delgado MR, Powers A, … Figner B (2015). Neural correlates of expected risks and returns in risky choice across development. Journal of Neuroscience, 35(4), 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, & Crone EA (2016). Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. NeuroImage, 1(124), 409–420. [DOI] [PubMed] [Google Scholar]
- Van Hoorn J, Fuligni AJ, Crone EA, & Galván A (2016). Peer influence effects on risk-taking and prosocial decision-making in adolescence: Insights from neuroimaging studies. Current Opinion in Behavioral Sciences, 10,59–64. [Google Scholar]
- Van Hoorn J, Van Dijk E Güroğlu B, & Crone E (2016). Neural correlates of prosocial peer influence on public goods game donations during adolescence. Social Cognitive and Affective Neuroscience, 11(6), 923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Gunter Moor B, Op de Macks ZA, Rombouts SA, Westenberg PM, & Crone EA (2010). Adolescent risky decision-making: neurocognitive development of reward and control regions. NeuroImage, 51(1), 345–355. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F (2009). Social cognition and the brain: a meta‐analysis. Human Brain Mapping, 30(3), 829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo‐García A, Verdejo‐Román J, Rio‐Valle JS, Lacomba JA, Lagos FM, & Soriano‐Mas C (2015). Dysfunctional involvement of emotion and reward brain regions on social decision-making in excess weight adolescents. Human Brain Mapping, 36(1), 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, & Kaplan L (2007). Meta-analysis of functional neuroimaging data: Current and future directions. Social Cognitive and Affective Neuroscience, 2(2), 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waytz A, Gray K, Epley N, & Wegner DM (2010). Causes and consequences of mind perception. Trends in Cognitive Sciences, 14, 383–388. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, & Dapretto M (2006). Developmental changes in the neural basis of interpreting communicative intent. Social Cognitive and Affective Neuroscience, 1(2), 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welborn BL, Lieberman MD, Goldenberg D, Fuligni AJ, Galván A, & Telzer EH (2015). Neural mechanisms of social influence in adolescence. Social Cognitive and Affective Neuroscience, 11(1), 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.