Abstract
Dopamine synthesis in the ventral tegmental area (VTA) is necessary for the reinforcement of sexual behavior. The objective of this study determined if sexual stimuli initiates reward, and whether reward is attenuated in sexually inactive rams. Sexually active rams were exposed to urine from estrous (n = 4) or ovariectomized (n = 3) ewes with inactive rams (n = 3) exposed to urine from estrous ewes. Following exposure, rams were exsanguinated and brains perfused. Alternating sections of the VTA were stained for Fos related antigens (FRA), tyrosine hydroxylase, and dopamine beta-hydroxylase activity. Forebrain tissue, mid-sagittal ventral to the anterior corpus callosum, was stained for dopamine D2 receptors. Concentrations of cortisol was determined prior to and following exposure. Exposure to ovariectomized-ewe urine in sexually active rams did not influence (P = 0.6) FRA expression, but fewer (P < 0.05) neurons were positive for tyrosine hydroxylase in the VTA. Sexually inactive rams had fewer (P < 0.05) FRA and tyrosine hydroxylase positive neurons in the VTA than sexually active rams following exposure to estrous ewe urine. VTA neurons staining positive for dopamine beta-hydroxylase did not differ by sexual activity (P = 0.44) or urine exposure (P = 0.07). Exposure to stimulus did not influence (P = 0.46) numbers of forebrain neurons staining positive for dopamine D2 receptors in sexually active rams, but fewer (P = 0.04) neurons stain positive in inactive rams. Serum concentrations of cortisol did not differ (P ≥ 0.52) among rams prior to or following stimulus. In conclusion sexual inactivity is unlikely due to stress, but may be partially a result of decreased tyrosine hydroxylase and/or the response to dopamine.
Keywords: Rams, Sexual behavior, Reward pathway
1. Introduction
Dopamine is a prominent catecholamine neurotransmitter in the brain and is synthesized in the ventral tegmental area (VTA) of the brainstem (Baik, 2013). Dopamine neurons originating in the VTA project to forebrain and basal ganglia structures including the nucleus accumbens. Through this pathway, dopamine influences many physiological functions including the coordination of movement, motivation and sexual behaviors (Tritsch and Sabatini, 2012).
Dopamine plays a vital role in motivated behaviors, such as reinforcement of events that are good or bad and the behaviors associated with good or bad outcomes (Bromberg-Martin et al., 2010). Stimulation or inhibition of dopamine receptors enhances or impairs male sexual behavior (Dominguez and Hull, 2005). Early studies demonstrated that systemic administration of L-DOPA resulted in rats displaying enhanced sexual behavior compared to controls (Da Prada et al., 1973). More recent studies reveal that dopamine agonists have the ability to restore sexual behavior in individuals exhibiting sexual impairment (Niikura et al., 2002) such that a potent D2/D3 dopamine receptor agonist was able to fully restore sexual arousal and ejaculatory ability in sexually sluggish rats (Giuliani et al., 2002). In contrast, treatment with dopamine antagonist impairs male sexual behavior (Dominguez and Hull, 2005).
Rams exhibit a broad range of sexual behavior with nearly 20% of all rams classified as sexually inactive (Alexander et al., 1999; Perkins et al., 1992). Differences in the dopamine pathways between sexually active and inactive rams may play a role in the expression of sexual behavior in the domestic ram. The objective of this study was to determine if sexual disinterest is a result of altered midbrain dopamine synthesis or D2 receptor expression in the forebrain of rams characterized as sexually inactive.
2. Materials and methods
All animal procedures were approved by the University of Wyoming Institutional Animal Care and Use Committee.
2.1. Rams
Sexual activity expressed by the rams was previously characterized (Mirto et al., 2017). Briefly, sexual activity of each ram is determined during a serving capacity test in which the rams are individually exposed to two or three ewes in estrus for 20 min. To eliminate the possibility of sexual inactivity being due to male-oriented behavior, rams were exposed to restrained ewes in estrus and cohort rams in a four-way stanchion giving the subject ram free and equal access to all stimulus animals in a sexual preference test. Rams not exhibiting mounting behavior in either the serving capacity or sexual preference test were fitted with a marking harness and exposed overnight to rams in their home pen or ewes in estrus. Rams were tested each breeding season— as lambs at 6–10 months of age, as yearlings between 18 and 22 months, and at 2+ years of age (Mirto et al., 2017).
Rams mounting ewes within 10 min of the serving capacity test, achieving ≥ 6 ejaculations in 20 min and exclusively mounting females in the preference test were considered high performing “female-oriented” rams. Rams failing to exhibit sexual interest toward female or male stimulus animals, showing a long latency (> 10 min) to mount, or achieving ≤ 3 ejaculations in the serving capacity test were considered to be low performing or sexually-inactive.
2.2. Ewes
Urine collected from non-treated ovariectomized ewes (n = 2) was used as the control stimulus. Estrous ewe urine was collected from ovariectomized ewes exposed to progesterone for 14 days with an Eazi-Breed CIDR® for sheep (Pfizer Animal Health, New York, NY). Following CIDR removal, ewes were treated with estradiol (50 μg) for two consecutive days. Ewes were placed in metabolism crates two days prior to urine collection. Urine was collected fresh on the day of ram exposure and maintained at 37 °C.
2.3. Exposure to olfactory cues
One month prior to exsanguination final behavioral testing was conducted. Rams were 2–3 years of age at the time of exposure to urine stimuli. All animals were exposed to urine within 3 days of each other during the month of November. In order to decrease stress, rams were isolated from ewes and placed in individual pens in an indoor building two days prior to exposure and provided ad libitum access to grass hay and water. Rams had visual and olfactory contact with each other but individual pens prevented physical contact. Ten milliliters of ewe urine from either estrous or ovariectomized ewes was placed on a stack of 3” × 3” cotton pads inside of a facemask and rams were exposed to the olfactory stimulus for 1 h (Mirto et al., 2017).
2.4. Blood and tissue collection
Blood was collected from the jugular vein of each ram prior to and following urine exposure. Blood was allowed to clot overnight at 5° C and serum was separated by centrifugation at 1200g. Serum was stored at −20 °C until analysis. Immediately after exposure rams were overdosed with sodium pentobarbital/sodium phenytoin and exsanguinated. Heads were perfused via the carotid arteries with 500 mL of 0.9% saline with 75,000 units of heparin followed by 3 L of 4% paraformaldehyde in 0.1 M Sorensen’s phosphate buffer. Brains were removed and post-fixed in a 4% paraformaldehyde solution. The fixative solution was replaced at 24 h.
The ventral tegmental area (VTA) was dissected from the midbrain tegmentum at the midline fixed laterally by the cerebral peduncles and the red nucleus. The forebrain dissection was targeted to the nucleus accumbens located at the midline lateral to the septal nuclei and ventral to the head of the caudate nucleus. The VTA tissue block was cryoprotected in a 20% sucrose fixative solution at 4 °C. The forebrain block was paraffin embedded.
2.5. ELISA procedure
Serum concentrations of cortisol were determined using a sheep specific ELISA (Sigma-Aldrich; St. Louis, MO). Frozen plasma samples were thawed and mixed well. Standards and samples (40 μL) were pipetted into pre-coated individual wells. ELISA procedures were per manufacturer specifications with absorbance determined at 400 nm by BioRad plate reader (Richmond, CA) immediately after the color reaction had been stopped.
2.6. Immunohistochemistry
For Tyrosine hydroxylase, dopamine beta-hydroxylase, and Fos related antigen activity, fixed, frozen tissues (in O.C.T; Fisher Scientific, Denver, CO) were cut on a cryostat and stored in cryoprotectant at −20 °C. For each antibody, every third section was stained to ensure a complete survey of the tissue. Tyrosine hydroxylase and dopamine beta-hydroxylase positive cells were determined in discrete but sequential sections. Immunohistochemistry was carried out on free-floating sections washed in phosphate buffered saline (PBS), incubated for 2 h in 4% horse serum diluted in 10 mL 0.4% Triton X-100 PBS. Sections were then incubated with the anti-c-Fos rabbit polyclonal antibody (#PC38, Calbiochem, LaJolla, CA), anti-tyrosine hydroxylase (#T2928, Sigma Chmical, St. Louis, MO) or anti-dopamine beta-hydroxylase antibody (#PA1–18314, Thermo Fisher, Denver, CO), diluted 1:750, overnight on the shaker at 4 °C. Following incubation, sections were washed in PBS and incubated at room temperature for 1 h on the shaker with anti-mouse secondary biotinylated antibody, diluted 1:200 (Vectastain Elite kit, Vector Laboratories, Burlingam, CA). Sections were washed 3 × with PBS and incubated for 1 h in the ABS solution (Vectastain Elite kit). After 2 washes with PBS, slides were incubated with diaminobenzadie (DAB; Vector Laboratories) under cover, for 20 min. Sections were washed with 0.1 M PBS 3 × for 5 min, mounted onto superfrost slides, and placed on a drying rack overnight. Mounted sections were dehydrated in a series of ethanol- 70%, 95% and 100% baths, washed 3 × in xylene and coverslips were applied with permount (Fisher Scientific, Denver, CO).
For D2 receptor staining, cross sections of forebrain were dissected and fixed in 4% buffered paraformaldehyde for 24 h followed by two 70% ethanol washes. Tissues were dehydrated through a series of graded ethanol washes followed by xylene before being infiltrated and embedded in paraffin. The D2 receptor and its conjugates were localized using a rabbit polyclonal antibody (DRD2, #PIPA 5–33480) produced by Thermo Fisher Scientific (Denver, CO) and an anti-rabbit Vectastain Elite ABC kit (Vector laboratories; Burlingame, CA). Forebrain cross sections mounted on superfrost plus slides were deparaffinized and rehydrated in distilled water (i.e. slides were placed in xylene 3 times for 10 min, then absolute ethanol for 5 min twice, then 95% ethanol for 5 min, 70% ethanol for 5 min once, and then into water briefly). All slides were microwaved two times for 5 min in 0.01 M citrate buffer (pH 6.0) and allowed to cool to room temperature. Slides were washed one time in PBS, then endogenous peroxidases were blocked by incubating in 1% hydrogen peroxide for 10 min. Mounted sections were incubated in dilute normal serum (Vectastain ABC kit) for 1 h at room temperature followed by rabbit anti-D2 receptor antibody diluted 1:1000 for 1 h at room temperature. Slides were washed in PBS for 5 min three times then incubated with anti-rabbit biotinylated second antibody (1:200; Vectastain ABC kit) for 30 min. Slides were again washed in PBS 3 x for 30 min. After three 5-min washes, DAB substrate (diaminobenzidine; Vectory Laboratories) was applied and slides were incubated at room temperature, under cover for 20 min. Color development was stopped by placing slides in PBS, and coverslips were applied using Permount (Fisher Scientific; Denver, CO).
2.7. Analysis of immunohistochemistry staining
For all antibodies, nonspecific binding was determined in tissue incubated in absence of primary antibody. Sections were observed on an Olympus Vannox microscope with images captured by a Q-Imaging QIClick digital camera (QImaging, Surrey, BC). Images were not altered other than adjusting the contrast as necessary. ImageJ software (1.49 v; USA National Institute of Health) was used to quantify cells containing Fos activity, tyrosine hydroxylase, dopamine beta-hydroxylase, and D2 receptor positive neurons within the region of interest. Positive cells in contact with the border of the region of interest were not included in the cell count. For quality control, all fields were visually verified and artifacts were removed prior to the final tally. Immune-positive neurons were the average of positive cells counted within the area of interest of the VTA.
2.8. Statistical analysis
All cell counts within the region of interest were totaled per animal for statistical analysis. One-way ANOVA analysis in Minitab (Ver 17.1.0, Minitab Inc.) was used to analyze the data. To determine the effects of stimulus on serum cortisol concentration and neural expression of Fos activity, tyrosine hydroxylase, dopamine beta-hydroxylase, and D2 receptor, preplanned comparisons of stimulus (estrous vs. ovariectomized ewe urine) in high-performing rams were conducted. Influence of ram classification (sexually active vs. sexually inactive) was determined by comparing active to inactive rams exposed to urine from ewes in estrus.
3. Results
3.1. Fos immunostaining
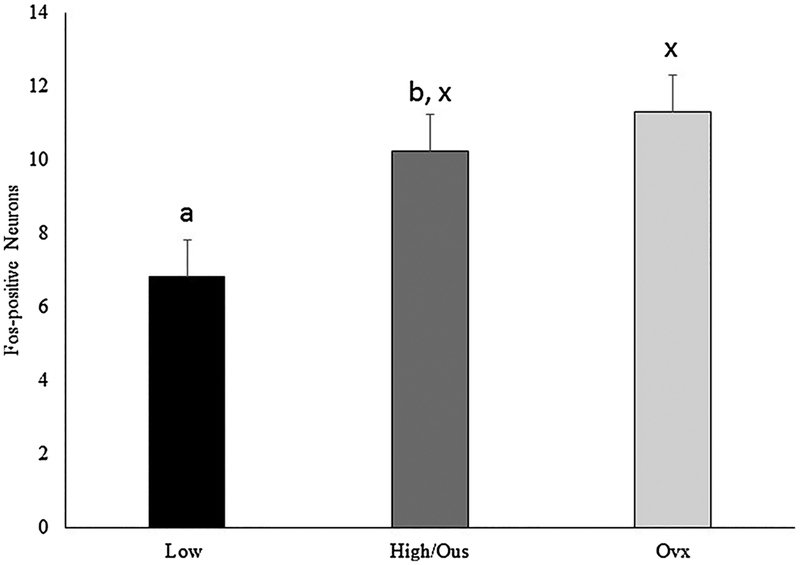
Representative Fos staining is presented in Fig. 1A. Sexually active rams exposed to urine from estrous ewes had a greater number of Fos positive neurons (F (1,5) = 8.23, P = 0.035) in the VTA compared to sexually inactive rams (Fig. 2). The number of Fos positive neurons was similar (F (1,5) = 0.32, P = 0.595) among sexually active rams exposed to urine from estrous or ovariectomized ewes (Fig. 2).
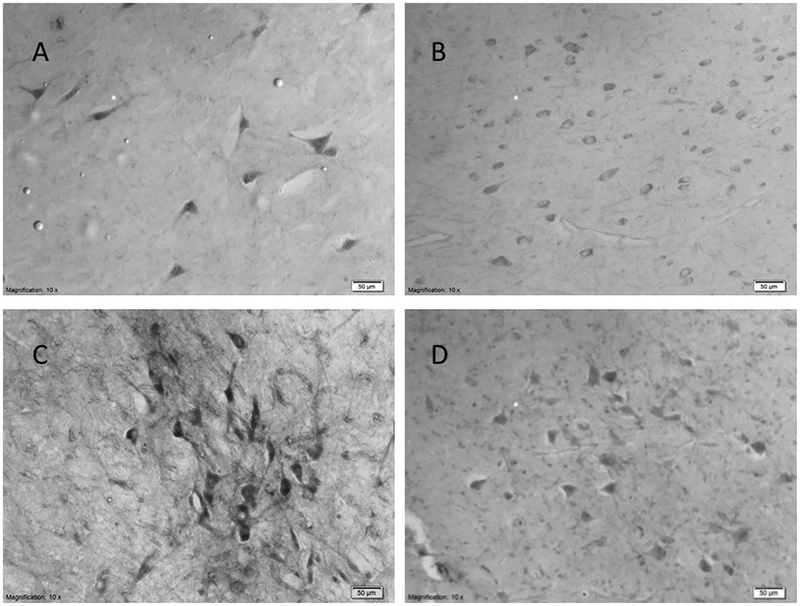
Fig. 1.
Representative staining at 10 × magnification for Fos (A), dopamine D2 receptor (B), tyrosine hydroxylase (C), and dopamine-beta hydroxylase (D). Reference bar scales 50 μm.
Fig. 2.
Number of neurons in the ventral tegmental area staining positive for Fos and Fos related antigens. Preplanned comparisons between sexually active (A) and inactive (I) rams (a,b) and stimulus (x,y) urine from ewes in estrous (EU) or urine from ovariectomized (OU) ewes. Bars with different (a,b or x,y) annotations differ (P < 0.05).
3.2. Tyrosine hydroxylase immunostaining
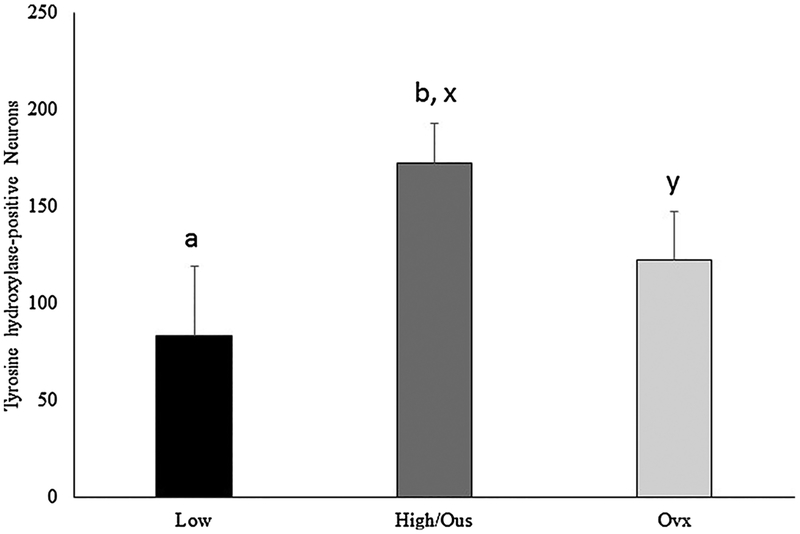
Representative staining for tyrosine hydroxylase is presented in Fig. 1C. Sexually inactive rams had fewer (F (1,5) = 0.1.51, P = 0.01) neurons in the VTA staining positive for tyrosine hydroxylase than sexually active rams. Exposure of sexually active rams to urine from ovariectomized ewe urine resulted in fewer (F (1,5) = 2.93, P = 0.008) tyrosine hydroxylase positive neurons in the VTA compared to rams exposed to urine from ewes in estrus (Fig. 3).
Fig. 3.
Number of neurons in the ventral tegmental area staining positive for tyrosine hydroxylase. Preplanned comparisons between sexually active (A) and inactive (I) rams (a,b) and stimulus (x,y) urine from ewes in estrous (EU) or urine from ovariectomized (OU) ewes. Bars with different (a,b or x,y) annotations differ (P < 0.05).
3.3. Dopamine beta-hydroxylase immunostaining
Representative staining for dopamine beta-hydroxylase is presented in Fig. 1D. The number of neurons staining positive for dopamine beta-hydroxylase was greater (F (1,4) = 7.84, P ≤ 0.049) than number staining positive for tyrosine hydroxylase. Differences in dopamine beta-hydroxylase expression were not evident (F (1,5) = 5.06, P ≥ 0.07) among sexually inactive (2406 ± 484 average count/μm2) or active rams regardless of exposure to estrous (2347 ± 193.7 average count/μm2) or ovariectomized (2555 ± 237 average count/μm2) ewe urine.
3.4. D2 receptor
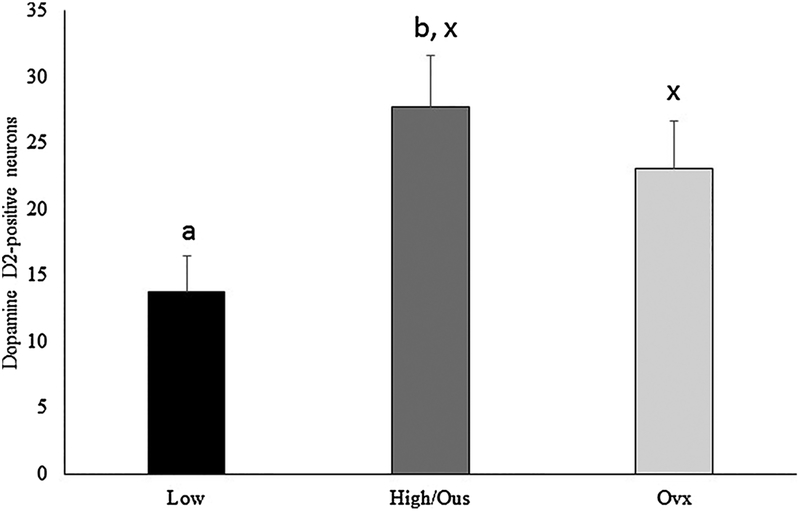
Representative staining for the dopamine D2 receptor is presented in Fig. 1B. Sexually active rams had a greater number of neurons staining positive for the D2 receptor in the forebrain (F (1,5) = 7.66, P = 0.039) when compared to sexually inactive rams. Differences in neurons expressing the D2 receptor were not noted in sexually active rams (F (1,5) = 0.76), P = 0.461) regardless of exposure (Fig. 4).
Fig. 4.
Number of neurons in the forebrain, nucleus accumbens area staining positive for the dopamine D2 receptor. Preplanned comparisons between sexually active (A) and inactive (I) rams (a,b) and stimulus (x,y) urine from ewes in estrous (EU) or urine from ovariectomized (OU) ewes. Bars with different (a,b or x,y) annotations differ (P < 0.05).
3.5. Cortisol
Serum concentrations of cortisol did not differ among treatment groups prior to (F (1,5) = 0.43, P ≥ 0.539) or following (F (1,5) = 0.47, P ≥ 0.524) exposure to urine. Serum concentration of cortisol was 3.60 ± 3.2 ng/ml and 1.47 ± 4.33 ng/ml in sexually active and inactive rams.
4. Discussion
Previous studies determined variation in basal concentrations of testosterone do not account for differences in sexual performance observed in adult rams (Alexander et al., 1999; D’Occhio and Brooks, 1982; Pinckard et al., 2000; Stellflug, 2006) or bulls (Price et al., 1986). This concept is supported in studies using sheep (Perkins et al., 1992), rats (Whalen et al., 1961) and guinea pigs (Harding and Feder, 1976) where differences in circulating concentration of testosterone were not detected among sexually active and inactive males. In our study, basal concentrations of serum testosterone did not differ between sexually active and inactive rams prior to or following exposure to females in estrous (Mirto et al., 2017).
Therefore, differences in ram libido cannot be accounted for by differences in testosterone and require further explanation. Immediate early genes, such as c-Fos, are induced during neuronal activity associated with an extracellular stimulus (Minatohara et al., 2015). These extracellular stimuli evoke two types of responses in target cells: a rapid, transcriptional-independent event producing an immediate reaction to the stimulus and a longer-term, transcriptional-dependent response (Curran and Morgan, 1987). The role of immediate early genes is to mediate long-term responses of neurons to external stimuli (Curran and Morgan, 1986, 1987; Goelet et al., 1986). These are a class of genes whose transcription becomes activated within minutes after stimulation and remains detectable approximately thirty minutes following activation (Sheng and Greenberg, 1990). Previous findings by Alexander et al. (2001) demonstrate that the number of neurons staining positive for Fos and Fos related antigens in rams with fence line exposure to ewes in estrus were similar in sexually active and inactive rams in the medial preoptic area (mPOA), bed nucleus of the stria terminalis (BNST), medial amygdala, and ventral medial hypothalamus. Sexually inactive rams receive sensory cues from estrous ewes and their lack of investigatory behavior does not appear to be due to a deficit in neural response in the presence of stimuli (Alexander et al., 2001). Likewise, Mirto et al. (2017) reported Fos expression in the olfactory bulb and amygdala to be similar in sexually active and inactive rams. However, differences in Fos expression were detected among sexually active and inactive rams in the BNST and mPOA (Mirto et al., 2017). Discrepancies among the studies may be due to the type of exposure the rams received. Rams in the Alexander et al. (2001) study received fence line exposure to estrus ewes while rams in the Mirto et al. (2017) study received isolated olfactory stimulation. In the current study, urine from estrus ewes provoked a greater response of Fos activity in the VTA of sexually active than inactive rams, but a discrimination of the stimulus quality was not detected by Fos activity in sexually active rams exposed to either urine from estrous or ovariectomized ewes. Bell et al. (2013) reported adult hamsters have greater Fos expression independent of exposure to vaginal secretions from estrous females compared to juveniles. These findings suggest experienced adult hamsters may have an enhanced sensitivity to a stimulus (Bell et al., 2013).
Although discrimination of stimulus was not evident with the expression of Fos activity in the VTA of sexually active rams, expression of tyrosine hydroxylase clearly discriminated among stimulus with tyrosine hydroxylase activity greater in sexually active than inactive rams. The VTA is the site of dopamine synthesis and is important for normal motivated behavior such as drinking, feeding, aggression, and sexual behavior. The mesolimbic dopamine system consists of synthesizing neurons located in the VTA projecting to the nucleus accumbens and forebrain (Balfour et al., 2004; Pfaus, 2009). Rewarding stimuli up-regulates dopamine synthesis (Danjo et al., 2014). The first direct anatomical evidence of dopaminergic neurons being activated in the VTA during sexual behavior was observed in mice (Balfour et al., 2004), demonstrating that the mesolimbic pathway is activated during male sexual behavior. Reduced dopamine synthesis in the VTA of sexually inactive rams would damper input to the nucleus accumbens and decrease rewarding aspects of mating behavior. Increased expression of tyrosine hydroxylase was apparent following olfactory stimulation alone. The increased expression of tyrosine hydroxylase following isolated olfactory exposure may indicate recognition and anticipation of sexual experience in sexually active rams. A previous study in rodents also observed an increase in dopamine positive neurons throughout the VTA when exposed to sex related environmental cues and sexual behavior (Balfour et al., 2004). Reduced numbers of neurons staining positive for tyrosine hydroxylase but similar Fos positive neurons in sexually active rams exposed to a sexually deterring (ie. ovariectomized ewe urine) stimulus indicates tyrosine hydroxylase activity is sensitive to the quality of the stimulus.
A temporary decrease in the firing of dopamine neurons predominantly promotes the D2 receptors (Bromberg-Martin et al., 2010). The silencing of dopamine neurons could potentially trigger aversive reactions and learning responses (Danjo et al., 2014). The number of D2 receptors did not differ between sexually active rams exposed to urine from estrus and ovariectomized ewes implying an equal response to an olfactory stimulus. However, sexually inactive rams had fewer neurons staining positive for the D2 receptor compared to sexually active rams. The decreased number of D2 receptors in combination with decreased tyrosine hydroxylase expression within the VTA of sexually inactive rams could be a result of aversive or avoidance responses to the stimulus (Danjo et al., 2014). During the sexual preference or performance tests sexually inactive rams exhibit limited mounting behavior (Mirto et al., 2017) which may be a result of behavioral avoidance towards ewes (Hikida et al., 2010). The behavioral avoidance of sexually inactive rams may be accounted for by differences of D2 receptor availability and tyrosine hydroxylase expression observed in sexually active and inactive rams. The more robust tyrosine hydroxylase expression and increased D2 receptor availability in sexually active rams exposed to estrous ewe urine may contribute to the high level of sexual motivation and performance observed (Sanna et al., 2015).
The neurons quantified for tyrosine hydroxylase appear to be distinct to those synthesizing norepinephrine. Dopamine beta-hydroxylase is the enzyme responsible for the conversion of dopamine to norepinephrine. Neurons expressing dopamine beta-hydroxylase were larger in area, and did not differ by treatment or ram classification in contrast to neurons expressing tyrosine hydroxylase. This is important because the production of norepinephrine would be a confounding factor since dopamine is the precursor to norepinephrine synthesis. Norepinephrine mediates the fight-or-flight stress response, potentially altering behavioral responses to sexual stimuli (Sulzer et al., 2016). Exposure of sexually active rams to a putative sexual stimulus elicited an increase in tyrosine hydroxylase but did not influence dopamine beta-hydroxylase activity suggesting dopamine, but not norepinephrine, was increased following exposure to urine from estrous ewes.
Exposure to stress evokes a series of endocrine responses (Abbott et al., 2003; Creel, 2001). Stress is often defined as a condition prompting a response or alterations of homeostasis in the individual caused by the external environment (Creel, 2001). In some species, such as primates, it has been reported that low sexual activity is a result of males being subordinate (Abbott et al., 2003). However, in sheep this does not appear to be the case. Erhard et al. (1998) reported sexually active and inactive rams compete equally for limited food sources suggesting sexual inactivity is not a result of social subordination. Serum concentrations of cortisol were indistinguishable between sexually active and inactive rams prior to or following exposure, demonstrating that stress was equal among rams during the exposure periods making chronic stress to be an unlikely cause of sexual inactivity.
5. Conclusion
Sexual inactivity may be due to decreased tyrosine hydroxylase in the VTA reducing the dopamine influence to the forebrain. In high sexually active rams, tyrosine hydroxylase activity in the VTA differentiates among putative sexually evocative and non-evocative odors. Reduced numbers of neurons expressing the D2 receptor in the forebrain coupled with reduced expression of tyrosine hydroxylase may limit sexual reward and contribute to sluggish sexual activity in these rams.
Acknowledgments
This work was supported in part by National Institutes for Health 3RO1RR014270–10S1 NIH/DHHS and United States Department of Agriculture USDA-NRI 2007–55618–18176
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, et al. , 2003. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm. Behav 43, 67–82. [DOI] [PubMed] [Google Scholar]
- Alexander BM, Stellflug JN, Rose JD, Fitzgerald JA, Moss GE, 1999. Behavior and endocrine changes in high-performing, low-performing, and male-oriented domestic rams following exposure to rams and ewes in estrus when copulation is precluded. J. Anim. Sci 77, 1869–1874. [DOI] [PubMed] [Google Scholar]
- Alexander BM, Rose JD, Stellflug JN, Fitzgerald JA, Moss GE, 2001. Low-sexually performing rams but not male-oriented rams can be discriminated by cell size in the amygdala and preoptic area: a morphometric study. Behav. Brain Res 119, 15–21. [DOI] [PubMed] [Google Scholar]
- Baik J-H, 2013. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour ME, Yu L, Coolen LM, 2004. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 29, 718–730. [DOI] [PubMed] [Google Scholar]
- Bell MR, De Lorme KC, Figueira RJ, Kashy DA, Sisk CL, 2013. Adolescent gain in positive valence of a socially relevant stimulus: engagement of the mesocorticolimbic reward circuitry. Eur. J. Neurosci 37, 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O, 2010. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel S, 2001. Social dominance and stress hormones. Trends Ecol. Evol 16, 491–497. [Google Scholar]
- Curran T, Morgan JI, 1986. Barium modulates c-fos expression and post-translational modification. Proc. Natl. Acad. Sci. U. S. A 83, 8521–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Morgan JI, 1987. Memories of fos. BioEssays News Rev. Mol. Cell. Dev. Biol 7, 255–258. [DOI] [PubMed] [Google Scholar]
- D’Occhio MJ, Brooks DE, 1982. Threshold of plasma testosterone required for normal mating activity in male sheep. Horm. Behav 16, 383–394. [DOI] [PubMed] [Google Scholar]
- Da Prada M, Carruba M, Saner A, O’Brien A, Pletscher A, 1973. The action L-dopa on sexual behaviour of male rats. Brain Res. 55, 383–389. [DOI] [PubMed] [Google Scholar]
- Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S, 2014. Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A 111, 6455–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM, 2005. Dopamine, the medial preoptic area, and male sexual behavior. Physiol. Behav 86, 356–368. [DOI] [PubMed] [Google Scholar]
- Erhard HW, Price EO, Dally MR, 1998. Competitive ability of rams selected for high and low levels of sexual performance. Anim. Sci 66, 403–408. [Google Scholar]
- Giuliani D, Ottani A, Ferrari F, 2002. Influence of sildenafil on copulatory behaviour in sluggish or normal ejaculator male rats: a central dopamine mediated effect? Neuropharmacology 42, 562–567. [DOI] [PubMed] [Google Scholar]
- Goelet P, Castellucci VF, Schacher S, Kandel ER, 1986. The long and the short of long-term memory–a molecular frameworkm. Nature 322, 419–422. [DOI] [PubMed] [Google Scholar]
- Harding CF, Feder HH, 1976. Relation between individual differences in sexual behavior and plasma testosterone levels in the guinea pig. Endocrinology 98, 1198–1205. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S, 2010. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66, 896–907. [DOI] [PubMed] [Google Scholar]
- Minatohara K, Akiyoshi M, Okuno H, 2015. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front. Mol. Neurosci 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirto AJ, Austin KJ, Uthlaut VA, Roselli CE, Alexander BM, 2017. Fos expression in the olfactory pathway of high- and low-Sexually performing rams exposed to urine from estrous or ovariectomized ewes. Appl. Anim. Behav. Sci 186, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura S, Yokoyama O, Komatsu K, Yotsuyanagi S, Mizuno T, Namiki M, 2002. A causative factor of copulatory disorder in rats following social stress. J. Urol 168, 843–849. [PubMed] [Google Scholar]
- Perkins A, Fitzgerald JA, Price EO, 1992. Luteinizing hormone and testosterone response of sexually active and inactive rams. J. Anim. Sci 70, 2086–2093. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, 2009. REVIEWS: pathways of sexual desire. J. Sex. Med 6, 1506–1533. [DOI] [PubMed] [Google Scholar]
- Pinckard KL, Stellflug J, Stormshak F, 2000. Influence of castration and estrogen replacement on sexual behavior of female-oriented, male-oriented, and asexual rams. J. Anim. Sci 78, 1947–1953. [DOI] [PubMed] [Google Scholar]
- Price EO, Katz LS, Moberg GP, Wallach SJR, 1986. Inability to predict sexual and aggressive behaviors by plasma concentrations of testosterone and luteinizing hormone in Hereford bulls. J. Anim. Sci 62, 613–617. [DOI] [PubMed] [Google Scholar]
- Sanna F, Piludu MA, Corda MG, Melis MR, Giorgi O, Argiolas A, 2015. Involvement of dopamine in the differences in sexual behaviour between Roman high and low avoidance rats: an intracerebral microdialysis study. Behav. Brain Res 281, 177–186. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME, 1990. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4, 477–485. [DOI] [PubMed] [Google Scholar]
- Stellflug JN, 2006. Comparison of cortisol, luteinizing hormone, and testosterone responses to a defined stressor in sexually inactive rams and sexually active female-oriented and male-oriented rams. J. Anim. Sci 84, 1520–1525. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Cragg SJ, Rice ME, 2016. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 6, 123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL, 2012. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen RE, Beach FA, Kuehn RE, 1961. Effects of exogenous androgen on sexually responsive and unresponsive male rats. Endocrinology 69, 373–380. [DOI] [PubMed] [Google Scholar]