Abstract
Background
This study aimed to evaluate superb microvascular imaging (SMI) as an adjunctive imaging method to evaluate mesenteric lymph nodes in children with mesenteric lymphadenitis compared with healthy children.
Material/Methods
A retrospective study compared children with mesenteric lymphadenitis (n=27) and healthy children (n=30). Lymph node size was determined using grayscale ultrasonography and parameters of lymph node vascularity were compared using color Doppler flow imaging (CDFI) and SMI. The diagnostic performance of ultrasound (US), US combined with SMI, and US combined with CDFI were compared.
Results
Lymph nodes from children with mesenteric lymphadenitis (n=77) and normal lymph nodes (n=84) were evaluated by SMI, which showed that the least diameter of lymph nodes in cases of mesenteric lymphadenitis was 0.58±0.15 mm and of normal mesenteric lymph nodes was 0.47±0.08 mm (p<0.001). SMI identified 92.6% of abnormal mesenteric lymph nodes while CDFI detected 85.2%. US combined with SMI had the highest sensitivity (81.5%), and specificity (78.9%) compared with US alone (sensitivity, 63.0%; specificity, 64.9%), and compared with US combined with CDFI (sensitivity, 74.1%; specificity, 75.4%). US combined with SMI and US combined with CDFI achieved the same specificity (76.7%), which was higher than that of US alone (66.7%).
Conclusions
SMI was superior to color Doppler flow imaging in evaluating the microvasculature in lymphadenopathy in mesenteric lymphadenitis. SMI may be used as an adjunct to grayscale ultrasonography to assist in identifying mesenteric lymphadenopathy in pediatric patients.
MeSH Keywords: Mesenteric Lymphadenitis; Pediatrics; Ultrasonography, Doppler, Color
Background
Mesenteric lymphadenitis is a common clinical finding in the pediatric population. Mesenteric lymphadenitis in childhood is known to have varied clinical presentations that can include fever and abdominal pain. However, physical examination alone is often limited in the young child and requires imaging studies to make the diagnosis. Because abdominal ultrasonography (US) does not expose the child to radiation, it is often the primary imaging choice in clinical practice [1]. Color Doppler flow imaging (CDFI) is a non-invasive technique to investigate the cause of abdominal pain in pediatric patients [2].
With the routine use of high-frequency transducers, CDFI has been frequently used to detect enlarged abdominal lymph nodes, especially in the right lower abdominal quadrant. Recently, an advanced and sensitive Doppler ultrasonography technique, known as superb microvascular imaging (SMI), has shown superior ability to demonstrate very low-speed blood flows with higher resolution and lower artifacts, compared with CDFI and power Doppler flow imaging [3–5]. Mesenteric lymphadenitis is an inflammatory process of the mesenteric lymph nodes that can be associated with inflammatory disorders or neoplasia, and previous studies have shown that lymphadenitis is associated with the development of new lymphoid vasculature [6].
Therefore, this retrospective study aimed to evaluate SMI as an adjunctive imaging method to evaluate mesenteric lymph nodes in children with mesenteric lymphadenitis compared with healthy children.
Material and Methods
Patients and lymph nodes
This retrospective clinical study was approved by the Ethics Committee of Shanghai Pudong New Area Peoples’ Hospital. All patient parental or legal guardian provided informed consent to participate in the present research.
Between January 2018 and June 2018, 32 consecutive pediatric patients diagnosed with mesenteric lymphadenitis were enrolled in the study and included 12 female children and 20 male children. The median age was 5.00±1.31 years (range, 2–8 years). The inclusion criteria for the children in the study group included a clinical diagnosis of mesenteric lymphadenitis, complete imaging data, and agreement to participate in the study. The control group (n=30) included 15 female children and 15 male children with a median age of 4.63±1.19 years (range, 2–9 years) who were symptom-free at the time of ultrasound examination. Abdominal lymph nodes with the least diameter of 4 mm were considered to be enlarged [7]. Laboratory tests were recorded for analysis and included the white blood cell (WBC) count and the C-reactive protein (CRP) levels.
Ultrasound protocols and imaging interpretation
All lymph nodes in the study group and the control group underwent grayscale ultrasound (US), color Doppler flow imaging (CDFI), and superb microvascular imaging (SMI) imaging. All the ultrasound examinations were conducted using a Toshiba Aplio 500 (Toshiba Medical Systems Co., Tokyo, Japan) with a 14 MHz line array transducer. All patients in both groups first underwent US examination with transverse and longitudinal scans of the lower abdomen. Conventional ultrasonic characteristics including size, shape, and echogenicity were recorded for further analysis. Size referred to the longest (L) axis and shortest (S) axis, while shape referred to the ratio of L to S axis (L/S). CDFI (frame rate, 10–15 Hz) and SMI (frame rate, >50 Hz) were used to assess vascular imaging parameters. The velocity scope of SMI was adapted to <2.5 cm/sec. Gentle pressure was applied through the transducer to prevent collapse of the vessels.
US assessments were performed by the same radiologist, who had more than three years of experience in abdominal US and one year of experience in SMI. Two radiologists with more than five and ten years of experience in abdominal imaging interpreted the vascularity index assessed by both CDFI and SMI. If any disagreement occurred, a third senior radiologist, with an experience of >15 years in abdominal US and two years of experience in SMI, was consulted until consensus was achieved. Vascular quantity was classified, as absent (G0), minimal (G1), moderate (G2), or marked (G3), depending on the amount of blood flow in the region of interest (ROI). G0 referred to the absence of blood flow; minimal or G1 vascularity referred to one or two pixels containing flow <0.1 cm in diameter; moderate or G2 flow referred to a certain number of small vessels and/or a main vessel; and marked G3 vascularity was defined as the visualization of more than four vessels. Clinical confirmation by pediatrician was considered as the diagnostic gold standard.
Statistical analysis
Data were analyzed using SPSS version 23.0. An independent t-test was used to analyze continuous variables. The chi-squared (χ2) test or Fisher’s exact test was used to compare categorical variables. A Wilcoxon rank-sum test was used to compare the data on vascularity assessed by CDFI and SMI. The potential associated factors for identifying enlarged and normal mesenteric lymph nodes on US, CDFI, and SMI were analyzed using univariate and multivariate logistic regression analysis. A receiver operating characteristic (ROC) curve was formulated to evaluate the diagnostic performance of US, US combined with CDFI, and US combined with SMI. P<0.05 was considered as statistically significant.
Results
Demographic, clinical data, and baseline ultrasound (US) findings
From the total number of 32 patients in the mesenteric lymphadenitis group, five patients were excluded, due to technical problems in blood sampling (n=2) and incomplete ultrasound (US) imaging (n=3). Table 1 shows the demographic and clinical data of the children included in the study. There was no significant difference in age or gender between the two groups (p>0.05). Body temperature of ≥37.5°C (70.4%), nausea (74.1%), and anorexia (55.6%) were the common clinical characteristics in children with mesenteric lymphadenitis.
Table 1.
Demographic and clinical parameters of children with mesenteric lymphadenitis and children in the control group.
| ML group (n=27) | Control group (n=30) | p-Value | |
|---|---|---|---|
| Age | 5.00±1.31 | 4.65±1.24 | 0.242 |
| Gender | 0.333 | ||
| Female | 10 (61.9%) | 15 (52.3%) | |
| Male | 17 (38.1%) | 15 (47.7%) | |
| Temperature (°C) | 37.93±0.84 | 36.72±0.24 | <0.001* |
| WBC (109/L) | 10.73±3.24 | 8.81±1.37 | 0.007* |
| CRP (mg/L) | 8.03±1.72 | 5.56±1.77 | <0.001* |
| Duration of abdominal pain (days) | 2.33±1.59 | N/A | |
| Nausea | 74.1% (20/27) | N/A | |
| Vomiting | 48.1% (13/27) | N/A | |
| Anorexia | 55.6% (15/27) | N/A |
WBC – white blood cell; CRP – C-reactive protein; ML – mesenteric lymphadenitis.
Indicates statistical significance.
The white blood cell (WBC) count (10.73±3.24×109/L versus 8.81±1.37×109/L), and C-reactive protein (CRP) level (8.03±1.72 mg/dL versus 5.56±1.77 mg/dL) of the children with mesenteric lymphadenitis were significantly greater compared with the children in the control group (p<0.05).
In children with mesenteric lymphadenitis, there were a total of 77 mesenteric lymph nodes with a median number of 2.85±1.17. In the healthy control group, a total of 84 mesenteric lymph nodes were identified with a median number of 2.80±1.21. However, there was no significant difference in the number of mesenteric lymph nodes observed between the two groups (p=0.870). The echogenicity of all mesenteric lymph nodes was found to be either isoechoic or hypoechoic.
In the mesenteric lymphadenitis group, there were 76.6% (59/77) enlarged mesenteric lymph nodes. In the control group, there were 72.6% (61/84) enlarged mesenteric lymph nodes. All the enlarged mesenteric lymph nodes in children with mesenteric lymphadenitis were located in the right lower quadrant, whereas two out of 61 enlarged mesenteric lymph nodes were found in the left lower quadrant in one healthy child. Details of the mesenteric lymph nodes, including their size and shape, are shown in Table 2. The size of the mesenteric lymph nodes was greater in children with mesenteric lymphadenitis compared with the healthy control group. Both the greatest diameter and the least diameter of the mesenteric lymph nodes in children with mesenteric lymphadenitis was significantly greater than that of mesenteric lymph nodes in the normal healthy group (p<0.001). The mesenteric lymph nodes were mostly oval in shape in both groups (p=0.361).
Table 2.
Size and shape of enlarged mesenteric lymph nodes (MLNs).
| Longest diameter | Shortest diameter | L/S | |
|---|---|---|---|
| ML group (n=59) | 1.28±0.28 | 0.58±0.15 | 2.24±0.25 |
| Control group (n=61) | 1.04±0.20 | 0.47±0.08 | 2.31±0.23 |
| p-Value | <0.001* | <0.001* | 0.361 |
L/S – longest diameter/shortest diameter; ML – mesenteric lymphadenitis.
Indicates statistical significance.
Vascularity of mesenteric lymph nodes
There was a significant difference in the vascularity index between the two groups. Color Doppler flow imaging (CDFI) identified blood vessels in 85.2% (23/27) of the mesenteric lymph nodes in the mesenteric lymphadenitis group. Superb microvascular imaging (SMI) detected blood flow signals in 92.6% (25/27) of the mesenteric lymph nodes in the mesenteric lymphadenitis group (Table 3). In comparison, the majority of mesenteric lymph nodes in the control group showed no increase in vascularity using CDFI (83.3%) and SMI (80.0%) (Figure 1). CDFI showed that 74.0% of mesenteric lymph nodes were graded as G1 (44.4%) (Figure 2) and G2 (29.6%) in terms of the number of vessels visualized. SMI showed that 77.8% of mesenteric lymph nodes were graded as G2 (55.6%) and G3 (22.2%). These findings support that SMI was a superior imaging method for identifying both high-velocity and low-velocity blood flow.
Table 3.
Vascularity grading assessed by color Doppler flow imaging (CDFI) and superb microvascular imaging (SMI).
| G0 | G1 | G2 | G3 | z | p-Value | ||
|---|---|---|---|---|---|---|---|
| CDFI | ML group (n=27) | 7 (25.9%) | 9 (33.3%) | 8 (29.6%) | 3 (11.1%) | 4.282 | <0.001* |
| Control group (n=30) | 23 (76.7%) | 7 (23.3%) | 0 (0.0%) | 0 (0.0%) | |||
| SMI | ML group (n=27) | 2 (7.4%) | 3 (11.1%) | 16 (59.3%) | 6 (22.2%) | 4.669 | <0.001* |
| Control group (n=30) | 17 (56.7%) | 6 (20.0%) | 7 (23.3%) | 0 (0.0%) |
Indicates statistical significance.
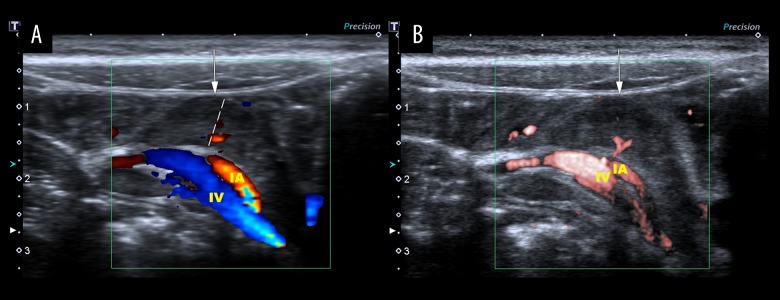
Figure 1.
Ultrasound imaging of one large hypoechoic mesenteric lymph node in a 6-year-old healthy male child. The largest mesenteric lymph node shortest diameter measurement was 0.78 cm (dashed line). (A) Color Doppler flow imaging (CDFI) shows dot-like and linear blood flow signals (G1). (B) Superb microvascular imaging (SMI) shows dot-like blood flow signals with a clearer hilum of the lymph node (G1). IA – ileocolic artery; IV – ileocolic vein.
Figure 2.
Ultrasound imaging of four large hypoechoic mesenteric lymph nodes in a 5-year-old male patient with mesenteric lymphadenitis. The largest mesenteric lymph node shortest diameter measurement was 0.60 cm (dashed line). (A) Color Doppler flow imaging (CDFI) shows dot-like blood flow (G1). (B) Superb microvascular imaging (SMI) shows rich blood flow signals (G2). LCA, left colic artery; LCV, left colic vein.
Diagnostic performance of different imaging methods
The diagnostic utility of vascular parameters assessed by CDFI and SMI for evaluating mesenteric lymphadenitis as an adjunct to conventional US was achieved by using receiver operating characteristic (ROC) curve analysis. In ROC analysis (Figure 3), the highest value for the area under the curve (AUC) was for US combined with SMI, followed by US combined with CDFI, and then for US alone, with the AUC values being 0.841 (95% CI, 0.737–0.945), 0.801 (95% CI, 0.682–0.920), and 0.648 (95% CI, 0.503–0.793), respectively. Sensitivity and specificity rates were highest for US combined with SMI (sensitivity, 81.5%; specificity, 78.9%), followed by US combined with CDFI (sensitivity, 70.4%; specificity, 73.7%), and US alone (sensitivity, 63.0%; specificity, 64.9%). Both US combined with SMI, and US combined with CDFI had a specificity of 76.7%, followed by US alone with a specificity of 66.7% (Table 4).
Figure 3.
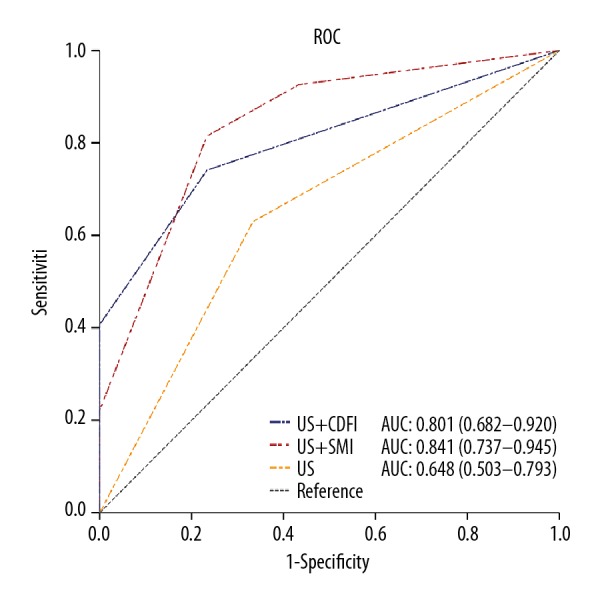
The receiver operating characteristic (ROC) and area under the curve (AUC) for the three diagnostic modes of ultrasound (US), US combined with color Doppler flow imaging (CDFI), and US combined with superb microvascular imaging (SMI).
Table 4.
Diagnostic performance of three ultrasound (US) examination methods.
| Sensitivity | Specificity | Accuracy rate | AUC (95% CI) | P-value | |
|---|---|---|---|---|---|
| US | 63.0% (17/27) | 66.7% (20/30) | 64.9% (37/57) | 0.648 (0.503–0.793) | 0.055 |
| US+CDFI | 74.1% (20/27) | 76.7% (23/30) | 75.4% (44/57) | 0.735 (0.601–0.869) | 0.002 |
| US+SMI | 81.5% (22/27) | 76.7% (23/30) | 78.9% (45/57) | 0.791 (0.668–0.914) | <0.001 |
AUC – area under the curve; CI – confidence interval; US – ultrasound; CDFI – color Doppler flow imaging; SMI – superb microvascular imaging.
Univariate and multivariate logistic analysis
Univariate logistic analysis showed that the US characteristics had significant correlations with mesenteric lymphadenitis, including the least diameter ≥0.58 cm (OR=19.586; P<0.001), the greatest diameter ≥1.28 cm (OR=5.945; P<0.001), and were rated as Grade 2 or lower according to Adler’s classification by SMI (OR=1.619; P<0.001) (Table 5). In the multivariate logistic analysis, measurement of the least diameter ≥0.58 mm (OR=16.650; P=0.002) was the strongest predictor for mesenteric lymphadenitis, followed by vascularity grading of ≥Grade 2 by SMI (OR=1.439; P=0.004) (Table 5).
Table 5.
Univariate and multivariate analysis of mesenteric lymphadenitis evaluated by superb microvascular imaging (SMI).
| Univariate analysis | Odds ratio | p-Value |
|---|---|---|
| Longest diameter ≥1.28 cm | 5.945 | <0.001* |
| Shortest diameter ≥0.58 cm | 19.586 | <0.001* |
| Vascularity grading ≥G2 by SMI | 1.619 | <0.001* |
| Multivariate analysis | Odds ratio | p-Value |
| Shortest axis ≥0.58 cm | 16.650 | 0.002* |
| Vascularity grading ≥G2 by SMI | 1.439 | 0.004* |
Discussion
In children, abdominal pain is a very common clinical presentation in children with mesenteric lymphadenitis [8]. Therefore, children are often referred for ultrasound (US) imaging in primary care. Abdominal ultrasound is a safe and cost-effective method of examination that is not associated with radiation and has a high degree of diagnostic sensitivity. The distinction between normal and abnormal mesenteric lymph nodes by US imaging is largely dependent on the size of the mesenteric lymph nodes. Previous studies have shown that mesenteric lymph nodes with a minimum diameter of ≥4–5 mm can be identified. Vayner et al. [9] conducted a prospective study to evaluate the prevalence of enlarged mesenteric lymph nodes in 189 children with mesenteric lymphadenitis and showed that most of the lymph nodes had minimum diameter of >4 mm. Abdel Gawad et al. [10] showed that mesenteric lymph nodes with a mean least diameter of 19 mm (range, 4.7 mm to 9 cm) were commonly found in children with mesenteric lymphadenitis. Compared with normal mesenteric lymph nodes, with a mean value of 2.96 mm, mesenteric lymph nodes in children with mesenteric lymphadenitis were significantly larger than normal mesenteric lymph nodes (p=0.02) [10].
The findings of the present study, regarding the size of abnormal mesenteric lymph nodes, are supported by those of previously published studies. Both the greatest diameter (1.28±0.28 mm) and least diameter (0.58±0.15 mm) of mesenteric lymph nodes in children with mesenteric lymphadenitis was greater than that of mesenteric lymph nodes in the healthy group (greatest diameter, 1.04±0.20 mm; least diameter, 0.47±0.08 mm) (both, p<0.001). However, Karmazyn et al. [11] examined mesenteric lymph nodes in 61 children with abdominal pain and found enlarged mesenteric lymph nodes in 54% of the study population. Their study suggested that mesenteric lymph nodes with a minimum diameter from 5–10 mm should not be considered as a specific finding in children with mesenteric lymphadenitis [11]. The cutoff value of the least diameter of ≥5 mm for enlarged mesenteric lymph nodes resulted in a high percentage of false-positive results (54%) [11]. Therefore, the findings of the present study that neither the greatest diameter nor the least diameter alone of the mesenteric lymph nodes was sufficient as a sole diagnostic indicator, are supported by previous studies.
Mesenteric lymphadenopathy might be a key indicator of the underlying inflammation that causes abdominal pain [12]. Inflammation is associated with dilation of small vessels and increased permeability of the microvasculature, which lead to increased blood flow. Doppler ultrasonography (US) has been widely used to evaluate the blood flow in healthy organs and inflammatory tissues [13–15]. The Doppler ultrasound technique uses a single-wall filter to remove clutter by suppressing low-velocity flow. With the development of Doppler technology, superb microvascular imaging (SMI) uses a new adaptive algorithm to delineate both high and low-velocity blood flow with higher resolution and fewer motion artifacts [16–18]. The capability of identifying blood flow has been widely shown in several studies [16–18]. In the pediatric population, SMI has been used to evaluate testicular blood flow in children from 2 months to 48 months of age [19]. Karaca et al. applied a four-stage grading system to compare blood flow quantity assessed by color Doppler flow imaging (CDFI) and SMI [19]. The average blood flow was 1.20±0.22 by CDFI and 2.37±0.55 by SMI [19]. Therefore, these authors showed that SMI resulted in more detailed vascular information in the testicles in small children, compared with CDFI or power Doppler imaging [19].
Bayramoglu et al. previously investigated SMI to evaluate vascular blood flow in children and adolescents between 2–18 years of age with lymphadenitis (n=72), lymphoma (n=45), and healthy controls (n=146) [20]. The sensitivity, specificity and accuracy rate of the vascularity index by SMI in differentiating between lymphadenitis and normal lymph nodes was 85%, 84%, and 85%, respectively [20]. The findings from this previous study supported the diagnostic performance of SMI in distinguishing lymphadenitis from normal lymph nodes by using the vascularity index [20]. These previous findings support the findings from the present study that adopted a four-level scoring system to evaluate the microvasculature assessed by two US techniques. SMI detected blood flow signals in 92.6% of the mesenteric lymphadenitis group while CDFI only identified 85.2%. Also, six mesenteric lymph nodes were visualized with increased (G3) blood flow by SMI, whereas only three of them were graded as G3 by CDFI. SMI was superior in identifying both high-velocity and low-velocity blood flow, and this study further demonstrated significant differences between grades of vascularity between normal mesenteric lymph nodes and mesenteric lymphadenitis.
Conventional US imaging parameters for mesenteric lymphadenitis have been limited as the cutoff value of the least diameter for evaluating mesenteric lymphadenitis >0.58 cm has provided a sensitivity, specificity, and diagnostic accuracy of 63.0%, 66.7%, and 64.9%, respectively. Vascularity grading ≥G2 by SMI combining with grayscale findings resulted in sensitivity (81.5%), specificity (76.7%), and accuracy rate (78.9%) in distinguishing between mesenteric lymphadenitis and normal mesenteric lymph nodes.
This study had several limitations. First, this was a preliminary and retrospective study that was performed at a single center with the inclusion of only 27 children with mesenteric lymphadenitis. Therefore, further study prospective studies with a larger study sample size is recommended. Second, in this study, all the imaging studies were conducted by the same radiologist. Ultrasonography examination and interpretation are operator-dependent and so future studies should control for interobserver differences in the evaluation of the imaging findings.
Conclusions
This study evaluated and compared blood flow signals in mesenteric lymph nodes in children with mesenteric lymphadenitis and healthy children using color Doppler flow imaging (CDFI) and superb microvascular imaging (SMI). The findings showed that SMI was superior to CDFI in visualizing the microvasculature in mesenteric lymphadenitis. As an adjunct to grayscale ultrasonography, vascularity assessed by SMI can assist in differentiating mesenteric lymphadenopathy from normal lymph nodes in pediatric patients.
Footnotes
Source of support: This study was supported by the Pu Dong New Area Health and Family Planning Commission Important Vulnerable Course Project (No. PWZbr 2017-25)
References
- 1.Niles LM, Goyal MK, Badolato GM, et al. US Emergency Department trends in imaging for pediatric nontraumatic abdominal pain. Pediatrics. 2017;140:e20170615. doi: 10.1542/peds.2017-0615. [DOI] [PubMed] [Google Scholar]
- 2.Natalia S, Nurith H. Importance of sonographic detection of enlarged abdominal lymph nodes in children. J Ultrasound Med. 2007;26:581–84. doi: 10.7863/jum.2007.26.5.581. [DOI] [PubMed] [Google Scholar]
- 3.Artul S, Nseir W, Armaly Z, et al. Superb microvascular imaging: Added value and novel applications. J Clin Imaging Sci. 2017;7:45. doi: 10.4103/jcis.JCIS_79_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno Y, Fujimoto T, Shibata Y. A new era in diagnostic ultrasound, superb microvascular imaging: Preliminary results in pediatric hepato-gastrointestinal disorders. Eur J Pediatr Surg. 2016;27:20–25. doi: 10.1055/s-0036-1593381. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y-C, Jiang X-Z, Bai Q-K, et al. Evaluating the efficacy of atorvastatin on patients with carotid plaque by an innovative ultrasonography. J Stroke Cerebrovasc Dis. 2019;28(3):830–37. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: Cause or consequence? Angiogenesis. 2007;10:149–66. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 7.Maconi G. Mesenteric lymphadenopathy. In: Maconi G, Bianchi Porro G, editors. Ultrasound of the gastrointestinal tract. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. pp. 29–36. [Google Scholar]
- 8.Chhabra S, Kenny SE. Appendicitis and non-specific abdominal pain in childhood. Paediatr Child Health. 2018;28:231–35. [Google Scholar]
- 9.Vayner N, Coret A, Polliack G, et al. Mesenteric lymphadenopathy in children examined by US for chronic and/or recurrent abdominal pain. Pediatr Radiol. 2003;33:864–67. doi: 10.1007/s00247-003-0985-7. [DOI] [PubMed] [Google Scholar]
- 10.Abdel Gawad EA, Abu Samra MF, Talat AM. The utility of multi-detector CT in detection and characterization of mesenteric lymphadenopathy with histopathological confirmation. The Egyptian Journal of Radiology and Nuclear Medicine. 2016;47:757–64. [Google Scholar]
- 11.Karmazyn B, Werner EA, Rejaie B, et al. Mesenteric lymph nodes in children: What is normal? Pediatr Radiol. 2005;35:774–77. doi: 10.1007/s00247-005-1462-2. [DOI] [PubMed] [Google Scholar]
- 12.Lucey BC, Stuhlfaut JW, Soto JA. Mesenteric lymph nodes seen at imaging: Causes and significance. Radiographics. 2005;25:351–65. doi: 10.1148/rg.252045108. [DOI] [PubMed] [Google Scholar]
- 13.Helbling R, Conficconi E, Wyttenbach M, et al. Acute nonspecific mesenteric lymphadenitis: More than “no need for surgery”. Biomed Res Int. 2017;2017 doi: 10.1155/2017/9784565. 9784565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tourasse C, Coulon A, Dénier J-F. Radio-histological correlations of subtle sonography images. Diagn Interv Imaging. 2014;95:181–95. doi: 10.1016/j.diii.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Jeffrey RB, Shin LK, et al. Color Doppler imaging of the appendix: Criteria to improve specificity for appendicitis in the borderline-size appendix. J Ultrasound Med. 2016;35:2129–38. doi: 10.7863/ultra.15.11064. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel M, Tomczak J, Snoch-Ziółkiewicz M, et al. Comparison of superb micro-vascular ultrasound imaging (SMI) and contrast-enhanced ultrasound (CEUS) for detection of endoleaks after endovascular aneurysm repair (EVAR) Am J Case Rep. 2016;17:43–46. doi: 10.12659/AJCR.895415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y-C, Zhang Y, Deng S-H, et al. A Prospective study to compare superb microvascular imaging with grayscale ultrasound and Color doppler flow imaging of vascular distribution and morphology in thyroid nodules. Med Sci Monit. 2018;24:9223–31. doi: 10.12659/MSM.911695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machado P, Segal S, Lyshchik A, et al. A novel microvascular flow technique: Initial results in thyroids. Ultrasound Q. 2016;32:67–74. doi: 10.1097/RUQ.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 19.Karaca L, Oral A, Kantarci M, et al. Comparison of the superb microvascular imaging technique and the color Doppler techniques for evaluating children’s testicular blood flow. Eur Rev Med Pharmacol Sci. 2016;20:1947–53. [PubMed] [Google Scholar]
- 20.Bayramoglu Z, Caliskan E, Karakas Z, et al. Diagnostic performances of superb microvascular imaging, shear wave elastography and shape index in pediatric lymph nodes categorization: A comparative study. Br J Radiol. 2018;91(1087):20180129. doi: 10.1259/bjr.20180129. [DOI] [PMC free article] [PubMed] [Google Scholar]