Abstract
Background:
Helicobacter pylori (H. pylori) infection is known to be associated with peptic ulcer and gastric cancer. Detection of H. pylori infection is a significant part of peptic ulcer and gastric cancer prevention and management. 13C-urea breath test (UBT) provides a good option for the pathogen detection due to its accuracy and safety.
Objective:
This review aims to evaluate the 13C-UBT diagnostic accuracy studies conducted among Asian population and validate its use for the Asian population.
Methods:
Original articles were systematically searched in PubMed, Scopus, and Google Scholar using the PICOS strategy by applying relevant keywords. Only studies published in English and conducted in Asia were included. Our search returned 276 articles. After assessment, 11 articles which answered our research question and met the criteria set for systematic review and meta-analysis were accepted. A total of 15 study protocols were extracted from the 11 accepted articles.
Findings:
Majority of the studies were conducted in Hong Kong (six), followed by Taiwan (five), Japan (two), and one each in Singapore and Israel. All studies had used histology as part of its gold standard of reference. All but one study was performed on adult populations. The summary estimate for sensitivity was 97% (95% CI: 96, 98%), and specificity was 96% (95% CI: 95, 97%), with significant heterogeneity between studies. Adjusting for the dose (50 mg) and breath sample collection time (20 minutes) had improved both accuracy estimates and significantly reduced heterogeneity.
Conclusion:
This review supports the test-and-treat strategy for H. pylori infection management. Prevalence and cost-effectiveness studies are mandatory for health authorities to adopt this strategy into national policy.
Introduction
Gastric cancer remains a major public health concern, especially in Asia. It is one of the top five most prevalent cancers in the world, and a leading cause of mortality whereby it is one of the top three cancers with the highest number of annual mortality globally [1]. Gastric cancer incidence is the highest in East Asia, particularly Japan, South Korea and China [1]. Globally, Helicobacter pylori (H. pylori) is the major environmental cause of gastric cancer [1]. H. pylori infection has long been known to be associated with peptic ulcer and gastric cancer [2]. Detection of H. pylori infection is a significant part of gastric cancer prevention and management. 13C-UBT provides a good option for the pathogen detection due to its accuracy and safety [3]. However, differences between test kits may render the 13C-UBT tools from different manufacturers not suitable for the Asian population. The difference between test kits may be with regards to the dose of isotope, requirement to fast, timing of breath sample collection, usage or non-usage of a test drink to alter gastric emptying, and equipment for analysis [4]. There is also a major difference in terms of the carbon isotope used; either 13C or 14C. The 13C-UBT is preferred due to its non-radioactive nature and excellent sensitivity and specificity [5]. It is thus recommended by the Second Asia-Pacific Consensus Guidelines for H. pylori Infection that 13C-UBT tests are validated locally [4]. There have been a few meta-analyses to pool the sensitivity and specificity of 13C-UBT in diagnosing H. pylori infection, but no recent study had focussed on the Asian population. Those reviews had emphasized on different research questions; a review had assessed the test’s accuracy among children [6], another looked into population with partial gastrectomy done [7], and there was also a review in 2006 that concentrated on multiple H. pylori diagnostic tests (including 13C-UBT) in bleeding peptic ulcer patients [8]. Hence, this review aims to evaluate the 13C-UBT diagnostic accuracy studies conducted among Asian population and justify its use as a safe and accurate H. pylori detection tool validated for the Asian population.
Materials and Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. The study protocol was not registered.
Search Protocol
A systematic search for articles published since inception until 2018 was conducted on PubMed, Scopus, and Google Scholar databases. Study titles, abstracts and keywords were searched by applying the PICOS strategy. Original articles were systematically searched using keywords of “Asia” for “P” (Population), “13C-Urea Breath Test” and its MeSH terms for “I” (Intervention), and “diagnostic accuracy”, “sensitivity”, “specificity”, and their MeSH terms for “O” (Outcome). There was no C (Comparison) or S (Study design) terms in the search protocol. Boolean operations namely “AND”, “OR”, or “NOT” were used to narrow and widen the search as according to the outlined objective. The initial studies obtained for first screening were retrieved from the final search algorithm of “P” AND “I” AND “O”. Only English language literatures and human studies were searched and included for this review. Other sources like unpublished reports, indirect article finding from bibliography of accepted articles, and grey literatures were not searched.
Study Selection
Studies were first screened by the title. Studies with titles that do not conform to the objective of this review were immediately discarded. The remaining studies which were judged to be addressing the relevant research question were then randomly allocated to two reviewers for the screening of abstracts. As with the first stage of screening, studies deemed as not addressing the intended research question were excluded. Further assessment by two other reviewers followed after retrieval of the full text. Accepted studies were then subjected to systematic data extraction into a summary table with standardized headings. Studies were only included if: 1) it was conducted among Asian population; 2) it was an original article designed to measure the diagnostic accuracy of 13C-UBT for detection of H. pylori infection; and 3) the diagnostic accuracy metrics and measurements, namely true positive, true negative, false positive, and false negative numbers, were reported or were able to be indirectly extracted from the full-text. The exclusion criteria were: 1) the study had used 13C-UBT as the gold standard tool (either alone or in combination with other tools); 2) no English full text was available; and 3) inability to obtain the full text.
Assessment of Study Quality
All accepted studies were assessed for quality using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. This tool was validated for the purpose of assessing study quality of studies included in systematic reviews measuring diagnostic accuracy, and is an improvement of the original tool (QUADAS) published in 2003 [10,11]. The QUADAS-2 tool comprises of four key domains, namely patient selection, index test, reference standard, and flow and timing (of index test and reference standard). Although all domains were relevant for risk of bias assessment, only the former three domains were designed to assess applicability of each study to this review’s research question. There were no summary quality scores generated as it was deemed invalid for diagnostic accuracy systematic reviews [12]. QUADAS-2 was instead used to aid in selection of studies. Only studies which scored low risk of bias for reference standard and low risk of applicability concerns (all domains) were accepted. Studies were independently assessed by two reviewers and any discrepancy was resolved by consensus between the reviewers.
Data Extraction
A standardized table with relevant headings was used to extract data. Extraction of studies included the identification of study locality, population characteristics (age group or other specific identifiers), name of the manufacturer-specific 13C-UBT used, characteristics of the 13C-UBT protocol used (dose of 13C-urea, presence/absence of test meal, duration from ingestion of carbon isotope to collection of post-ingestion exhaled air and others), the gold standard for H. pylori detection, total number of patients/respondents, and relevant diagnostic accuracy values. The extracted data regarding numbers of true positive, true negative, false positive, and false negative cases were also stated as to whether they were taken directly from the referenced text, or indirectly calculated or extrapolated from the available data. Studies were arranged in descending order according to year of publication, with the most recent study being placed in the first row.
Statistical Analysis
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for each study. Heterogeneity was assumed at significance level of p < 0.10 and was tested by chi squared. When heterogeneity was present, the degree was quantified using the I2 statistic. Values of less than 25% are considered as homogenous and 25% to <50% are considered as having low heterogeneity. For values of 50% or more, significant heterogeneity is assumed. Studies were then subjected to sub-group analyses to account for the heterogeneity between studies. Groups were not prespecified and were decided during analysis based on the criteria of having at least three studies in all sub-groups. All analyses were performed using the software Meta-Disc (version 1.4) [13].
Results
Study Selection
The result of systematic search yielded 53 articles from PubMed, 64 articles from Scopus and 159 articles from Google Scholar, which totalled up to 276. After removal of duplicates (32), 165 articles were excluded after the screening of title and abstract, while a further 68 were omitted after full text review. The reasons for exclusion were due to full-text was in language other than English (2), study was not done in Asia (33), 13C-UBT was used as the gold standard or part of the gold standard (32), and insufficient data for accuracy analysis (1). The screening process resulted in 11 articles being accepted for systematic review and meta-analysis [14,15,16,17,18,19,20,21,22,23,24]. The flowchart of study selection is illustrated in Figure 1.
Figure 1.
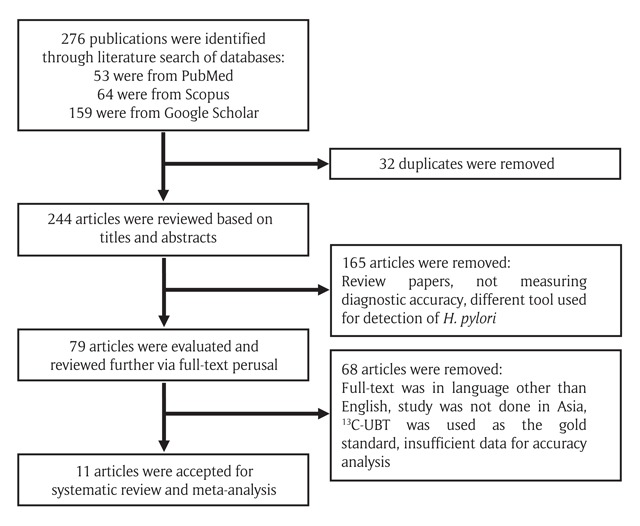
Flowchart of Study Selection for Meta-analysis.
Characteristics of Included Studies
Table 1 summarizes the quality assessment of the 11 accepted articles, along with the questions answered from QUADAS-2, while Tables 2 and 3 summarize the study characteristics of all included studies. Out of the 11 articles, there were considerable bias noted for six articles in the domain of index test [15,17,19,20,23,24]. This was due to the mentioned articles having index test protocol which had not prespecified the cut-off value for 13C-UBT used, which may result in an overestimation of the test performance. Two articles had unclear risk of bias for study selection [18,22]. In both articles, patient selection was based on an established condition or disease (partial gastrectomy patients and non-ulcer dyspepsia patients). This may lead to an exaggeration of diagnostic accuracy if the same test protocol was to be used on a different population [11]. However, all articles had low risk of bias for other domains, and there was no concern for applicability to the research question of this review.
Table 1.
Summary of Quality Assessment of Diagnostic Accuracy Study (QUADAS-2) for Accepted Studies.
| No. | First Author (Year) | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | ||
| 1. | Wardi (2012) | U | L | L | L | L | L | L |
| 2. | Peng (2005) | L | H | L | L | L | L | L |
| 3. | Urita (2004) | L | H | L | L | L | L | L |
| 4. | Wong (2003) | L | L | L | L | L | L | L |
| 5. | Chua (2002) | L | L | L | L | L | L | L |
| 6. | Kato (2002) | L | H | L | L | L | L | L |
| 7. | Wong WM (2001) | L | H | L | L | L | L | L |
| 8. | Wong BCY (2001) | L | L | L | L | L | L | L |
| 9. | Peng (2000) | U | L | L | L | L | L | L |
| 10. | Wong (2000) | L | H | L | L | L | L | L |
| 11. | Wang (1998) | L | H | L | L | L | L | L |
L = Low risk.
H = High risk.
U = Unclear risk.
Table 2.
Summary Table of Accepted Studies.
| No. | First Author (Year) | Population & Country | Name of Test | Cut-off Threshold | Other Specific Features of Test | Gold Standard |
|---|---|---|---|---|---|---|
| 1. | Wardi (2012) | Adults, partial gastrectomy, Israel | 13C-BreathID | 5.0 δ over baseline | 75 mg, 4.5 g citric acid-based powder, breath sample at 10 to 15 minutes (delta time) | Histology |
| 2. | Peng (2005a) | Adults, routine upper scope, Taiwan | 13C-UBT (INER, Taiwan) | 2.0 δ over baseline | 50 mg, 6-hour fast, no test meal, breath sample at 15 minutes | Culture or histology + CLO |
| Peng (2005b) | Adults, routine upper scope, Taiwan | 13C-UBT (INER, Taiwan) | 5.0 δ over baseline | 100 mg, 6-hour fast, no test meal, breath sample at 15 minutes | Culture or histology + CLO | |
| 3. | Urita (2004) | Adults, diagnostic upper scope, Japan | Modified 13C-UBT (sample via nostril) | 2.5 δ over baseline | 100 mg, overnight fast, breath sample at 20 minutes | Histology + serology |
| 4. | Wong (2003) | Adults, referred to upper scope unit, Hong Kong | Tablet 13C-UBT (Diabact UBT) | 3.0 δ over baseline | 50 mg, overnight fast, 456 mg anhydrous citric acid, breath sample at 20 minutes | RUT + histology |
| 5. | Chua (2002) | Adults, referred to upper scope unit, Singapore | 13C-UBT (Hp-Plus, Sweden) | 3.5 δ over baseline | Amount of 13C-urea not stated, test meal with solution containing citric acid, breath sample at 30 minutes | Histology + CLO + serology (2/3) |
| 6. | Kato (2002) | Children, referred to upper scope unit, Japan | 13C-Urea Breath Test | 3.5 δ over baseline | 75 and 100 mg, 4-hour fast, no test meal, breath sample at 20 minutes | Culture or histology + RUT |
| 7. | Wong WM (2001a) | Adults, referred to upper scope unit, Hong Kong | 13C-Urea Breath Test | 7.5 δ over baseline | 50 mg, overnight fast, no test meal, breath sample at 20 minutes | Histology + CLO |
| Wong WM (2001b) | Adults, referred to upper scope unit, Hong Kong | 13C-Urea Breath Test | 3.0 δ over baseline | 50 mg, overnight fast, 2.4 g citrate solution as test meal, breath sample at 20 minutes | Histology + CLO | |
| 8. | Wong BCY (2001) | Adults, referred to upper scope unit, Hong Kong | 13C-Urea Breath Test | 5.0 δ over baseline | 75 mg, overnight fasting, 2.4 g citric acid 200 mL test meal solution, breath sample at 30 minutes | Histology + RUT + CLO + culture + PCR |
| 9. | Peng (2000) | Adults, non-ulcer dyspepsia, Taiwan | 13C-UBT (INER, Taiwan) | 3.0 δ over baseline | 100 mg, 6-hour fast, 100 mL fresh milk as test meal, breath sample at 15 minutes | Culture or histology + CLO |
| 10. | Wong (2000a) | Adults, referred to upper scope unit, Hong Kong | 13C-Urea Breath Test | 5.0 δ over baseline | 75 mg, overnight fast, 2.4 g citric acid solution as test meal, breath sample at 30 minutes | Histology + CLO |
| Wong (2000b) | Adults, referred to upper scope unit, Hong Kong | 13C-Urea Breath Test | 5.0 δ over baseline | 75 mg, overnight fast, no test meal, breath sample at 30 minutes | Histology + CLO | |
| 11. | Wang (1998a) | Adults, routine upper scope, Taiwan | 13C-UBT (INER, Taiwan) | 3.0 δ over baseline | 100 mg, 6-hour fast, 100 mL fresh milk as test meal, breath sample at 15 minutes | Histology or culture or urease test |
| Wang (1998b) | Adults, routine upper scope, Taiwan | 13C-UBT (INER, Taiwan) | 4.0 δ over baseline | 100 mg, 6-hour fast, 100 mL fresh milk as test meal, breath sample at 30 minutes | Histology or culture or urease test | |
PCR Polymerase chain reaction.
CLO Campylobacter-like organism test (rapid urease test).
RUT Rapid urease test.
Table 3.
Summary Table of Accepted Studies with Indicators of Diagnostic Accuracy.
| No. | Author (Year) | Total, n | TP | TN | FP | FN | Sensitivity | Specificity | Overall Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Wardi (2012) | 76 | i9 | i57 | i5 | i5 | 64.2 | 91.9 | 86.8 | 64.2 | 91.9 |
| 2. | Peng (2005a) | 50 | 27 | 22 | 0 | 1 | 96.4 | 100.0 | 98.0 | 100.0 | 95.6 |
| Peng (2005b) | 50 | 18 | 32 | 0 | 0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
| 3. | Urita (2004) | 127 | i42 | i85 | i0 | i0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 4. | Wong (2003) | 200 | i99 | i101 | i0 | i0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 5. | Chua (2002) | 100 | 65 | 31 | 0 | 4 | 94.2 | 100.0 | 96.0 | 100.0 | 88.6 |
| 6. | Kato (2002) | 220 | i87 | i129 | i2 | i2 | 97.8 | 98.5 | 98.2 | 97.8 | 98.5 |
| 7. | Wong WM (2001a) | 101 | i49 | i50 | i2 | i0 | 100.0 | 96.2 | 98.0 | 96.1 | 100.0 |
| Wong WM (2001b) | 105 | i50 | i54 | i1 | i0 | 100.0 | 98.2 | 99.1 | 98.0 | 100.0 | |
| 8. | Wong BCY (2001) | 294 | i151 | i127 | i4 | i12 | 92.6 | 96.9 | 94.5 | 97.4 | 91.2 |
| 9. | Peng (2000) | 136 | i76 | i49 | i6 | i5 | 93.8 | 89.1 | 91.9 | 92.7 | 90.7 |
| 10. | Wong (2000a) | 202 | i110 | i86 | i2 | i4 | 96.5 | 97.7 | 97.0 | 98.2 | 95.6 |
| Wong (2000b) | 202 | i108 | i86 | i2 | i6 | 94.7 | 97.7 | 96.0 | 98.2 | 93.5 | |
| 11. | Wang (1998a) | 352 | 197 | 143 | 10 | 2 | 99.0 | 93.4 | 96.6 | 95.2 | 98.6 |
| Wang (1998b) | 352 | 196 | 142 | 11 | 3 | 98.5 | 92.8 | 96.0 | 94.7 | 97.9 | |
i Figures indirectly derived from the original articles via deduction of other available figures.
TP True Positive.
TN True Negative.
FP False Positive.
FN False Negative.
PPV Positive Predictive Value.
NPV Negative Predictive Value.
Out of the included articles, four articles reported two protocol variations in the 13C-UBT accuracy evaluation. In these articles, each test protocol was counted as an individual study which is separate from the other. Thus, a total of 15 protocol comparisons (referred to as studies hereinafter) were included in the meta-analysis. Majority of the studies were conducted in Hong Kong (six), followed by Taiwan (five), Japan (two), and one each in Singapore and Israel. All studies had histology as its gold standard of reference or at least as part of the tools forming the gold standard. All but one study was performed on adult populations. The only study conducted among children was also the solitary study that had used two variations of the 13C-urea in the UBT (75 mg and 100 mg) for its subjects and reported the results jointly, owing to the different age groups [15]. Since results were pooled in that study, the different dosages of 13C-urea used were not reported separately in our meta-analysis.
Overall Accuracy and Exploration of Heterogeneity
When studies were pooled together, the summary estimate for sensitivity was 97% (95% CI: 96, 98%), and specificity was 96% (95% CI: 95, 97%) (Figures 2 and 3). While these were respectable numbers, suggesting a highly accurate test tool, the analysis was performed on studies which were largely heterogenous. Chi-squared p-values for both sensitivity and specificity pooled accuracy analyses were less than 0.1, indicating significant heterogeneity between the studies.
Figure 2.
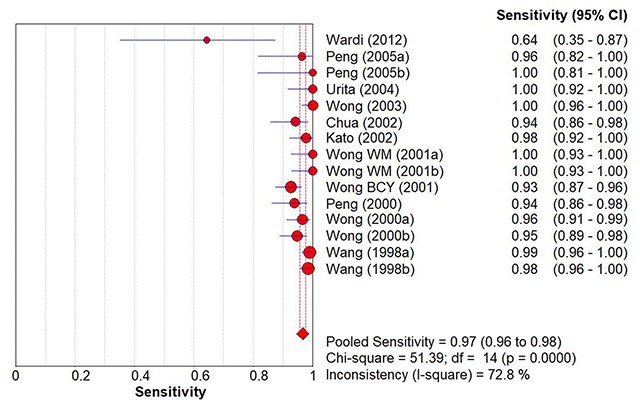
Overall Pooled Sensitivity of 13C-UBT to Detect H. pylori Infection.
Figure 3.
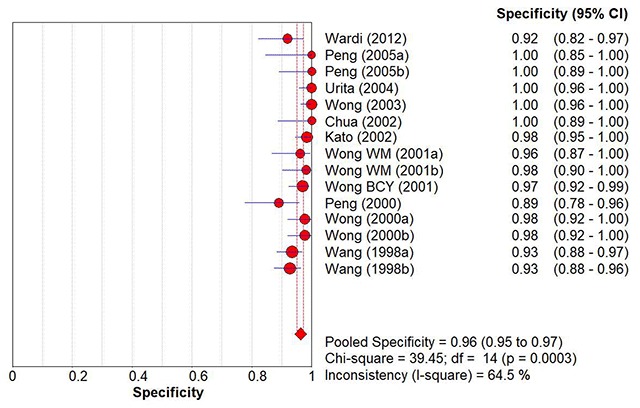
Overall Pooled Specificity of 13C-UBT to Detect H. pylori Infection.
We conducted multiple sub-group analyses to stratify the studies into groups which were more homogenous. The summary statistics for the diagnostic accuracy of 13C-UBT to detect H. pylori infection and the findings of sub-group analysis were presented in Table 4. Dose of 13C-urea and breath sample collection time appeared to account for the largest variations in terms of heterogeneity of the outcome (pooled accuracy values). When adjusted according to the doses of 13C-containing urea used, studies with 50 mg in their 13C-UBT protocol had the best sensitivity (100%; 95% CI: 98, 100%) and specificity (99%; 95% CI: 96, 100%) compared to other protocols. The studies in this group were also homogenous (chi-squared p-values = 0.24 [sensitivity] & 0.17 [specificity]). Breath collection time showed similar accuracy improvement for protocols using 20 minutes as the time of breath sample collection following ingestion of 13C-urea. For these studies, the summary estimate for sensitivity was 99% (95% CI: 98, 100%) while for specificity it was similarly improved at 99% (95% CI: 97, 100%). These studies were also statistically homogenous. For other sub-groups, there was no apparent improvement of the diagnostic accuracy of the test after stratification, and heterogeneity persisted in at least one accuracy domain (sensitivity or specificity) analysis.
Table 4.
Summary Statistics for the Diagnostic Accuracy of 13C-Urea Breath Test.
| Sub-groups | Number of Studies | P-value (I2)a | P-value (I2)b | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|
| All studies* | 15 | <0.01 (72.8%) | <0.01 (64.5%) | 0.97 (0.96, 0.98) | 0.96 (0.95, 0.97) |
| By dose of 13C-urea** | |||||
| 50 mg | 4 | 0.24# (28.7%) | 0.17# (40.6%) | 1.00 (0.98, 1.00) | 0.99 (0.96, 1.00) |
| 75 mg | 4 | 0.01 (76.5%) | 0.28# (21.0%) | 0.93 (0.90, 0.96) | 0.96 (0.94, 0.98) |
| 100 mg | 5 | 0.07 (53.0%) | <0.01 (76.1%) | 0.98 (0.97, 0.99) | 0.94 (0.92, 0.96) |
| By cut-off threshold | |||||
| <5.0 δ over baseline | 9 | 0.02 (54.4%) | <0.01 (75.9%) | 0.98 (0.97, 0.99) | 0.96 (0.95, 0.97) |
| ≥5.0 δ over baseline | 6 | <0.01 (76.5%) | 0.30# (17.3%) | 0.94 (0.92, 0.96) | 0.97 (0.95, 0.98) |
| By breath sample collection time | |||||
| 10 to 15 minutes | 5 | <0.01 (83.0%) | 0.06 (55.4%) | 0.96 (0.94, 0.98) | 0.94 (0.90, 0.96) |
| 20 minutes | 5 | 0.26# (24.0%) | 0.15# (40.3%) | 0.99 (0.98, 1.00) | 0.99 (0.97, 1.00) |
| 30 minutes | 5 | 0.07 (54.6%) | 0.10# (48.4%) | 0.96 (0.94, 0.97) | 0.96 (0.94, 0.98) |
| By locality*** | |||||
| Hong Kong | 6 | <0.01 (75.5%) | 0.36# (8.4%) | 0.96 (0.94, 0.98) | 0.98 (0.96, 0.99) |
| Taiwan | 5 | 0.13# (44.0%) | 0.06 (54.8%) | 0.98 (0.96, 0.99) | 0.93 (0.91, 0.96) |
| By manufacturer | |||||
| 13C-UBT (INER, Taiwan) | 5 | 0.13# (44.0%) | 0.06 (54.8%) | 0.98 (0.96, 0.99) | 0.93 (0.91, 0.96) |
| Others | 10 | <0.01 (77.5%) | 0.05 (46.7%) | 0.96 (0.94, 0.97) | 0.98 (0.97, 0.99) |
* Four articles had data for two sets of samples, tools and findings; a total of 15 studies were obtained.
** One study was not included in this sub-group due to absence of reported 13C-urea amount used, and another study was excluded due to use of multiple amounts in a single study (for different age groups in children).
*** Studies done in Japan, Israel and Singapore were excluded due to having only two or less studies per locality.
a P-value for heterogeneity (chi-squared) and I2 test for heterogeneity quantification for sensitivity analysis.
b P-value for heterogeneity (chi-squared) and I2 test for heterogeneity quantification for specificity analysis.
# P-value is not significant (≥0.1).
Discussion
Principal Findings
This systematic review had identified 15 study protocols from 11 articles that addressed the diagnostic accuracy of 13C-UBT to detect H. pylori infection in the Asian population. Our meta-analysis shows that: 1) 13C-UBT had outstanding diagnostic accuracy with sensitivity of 97% and specificity of 96%; 2) there were significant heterogeneity which persisted in most sub-group analyses performed; and 3) adjusting for the dose of 13C-urea (50 mg) and breath sample collection time (20 minutes) had improved accuracy estimates and significantly reduced heterogeneity, as to render the studies homogenous.
Analysis of Heterogeneity
13C-UBT protocols differ between manufacturers, regions, and populations tested. There were multiple variables in a protocol for 13C-UBT procedure. These differences were likely to cause significant heterogeneity across the studies. Potential sources of heterogenous characteristics include the dose of 13C-urea used in test, cut-off threshold values, and breath sample collection time following ingestion of 13C-urea. The dose of 13C is usually determined by the manufacturer. For example, all studies that had used 13C-UBT manufactured by Institute of Nuclear Energy Research (INER), Taiwan, had 100 mg of 13C-urea in their 13C-UBT protocol, except for one study which had used both 50 mg and 100 mg variations of the test kit [18]. Cut-off threshold value represents a pivotal factor for diagnostic accuracy. The authors in six studies had not prespecified the cut-off values in their 13C-UBT protocols; instead using the ROC curve or manually calculating the best cut-off value from results to determine the best accuracy estimates via trade-offs between sensitivity and specificity [15,17,19,20,23,24]. A low cut-off threshold value may improve sensitivity but conversely reduce specificity, and vice versa. Regarding the breath sample collection time, it was also a matter of quid pro quo; too long and accuracy improves but may be inconvenient to patients, while too short and test is more convenient to both patient and operator, but may impair accuracy.
We attempted to diminish the effects of test protocol variations on heterogeneity by stratifying the studies according to the variations. For dose of 13C-urea used and breath sample collection time, our results showed that there was not only improvement in homogeneity of the studies, but also increased sensitivity and specificity (Table 4). However, it was only for 13C-urea dose of 50 mg and breath sample collection time of 20 minutes. For other sub-groups, significant heterogeneity persisted in analysis of at least one of the accuracy parameters (sensitivity or specificity). This was observed even for the five studies which had used the test from the same manufacturer (INER, Taiwan) [17,18,20]. This suggests that there were other possible sources of heterogeneity. Prior studies have argued that the test protocol itself is not solely responsible for the differences in test performance. Individual or patient characteristics also play a crucial role. Heterogeneity persisted in part because the 13CO2 exhaled (and collected in the sample collection bag) depends not only on the 13C-urea dose given and the amount hydrolysed by the urease from H. pylori, but also on individual attributes like the individual’s CO2 production, the degree of 13CO2 diluted within the body’s CO2 and bicarbonate pool, the anthropometric measurements, and important differentiating factors like age and sex [25,26]. We had stratified the studies into groups of similar localities in order to partly account for the differences between the populations tested, and it was noted that accuracy estimates did not improve, nor did the analysis for heterogeneity. The persistence of heterogenous qualities between the studies in this sub-group was expected, mostly due to our inability to further sub-divide the studies into groups which were more homogenous, in view of the limited number of studies.
Epidemiologic and Policy Implications
Based on the results of our meta-analysis, 13C-UBT is an accurate tool to diagnose H. pylori infection, with the advantage of being non-invasive and safe. The use of 13C-UBT is recommended for the detection of H. pylori prior to eradication therapy, and post therapy to confirm eradication. The Second Asia-Pacific Consensus Guidelines for H. pylori recommended the test-and-treat approach, whereby uninvestigated dyspepsia patients with no alarm symptoms to suggest gastric cancer (such as dysphagia, weight loss, overt gastrointestinal bleeding, iron deficiency anaemia, or abdominal mass) are tested for H. pylori infection and managed accordingly [4]. This recommendation was echoed by the Maastricht V/Florence Consensus Conference [5]. It was argued that the test-and-treat approach, as compared to merely prescribing proton pump inhibitors (PPI) or directly performing oesophago-gastro-duodenoscopy (OGD), may provide better outcome, improved convenience for patients and greater cost-effectiveness in the long run. In an individual patient data meta-analysis conducted in 2005, comparing two arms between the test-and-treat and endoscope-and-treat patients, there was huge cost saving of US$389 per patient in the test-and-treat arm. However, the same meta-analysis also concluded that the endoscope-and-treat patients had a marginal but significant benefit in terms of symptom improvement and patient satisfaction [27].
The test-and-treat strategy must be appropriated with local settings. This approach is not recommended for regions with H. pylori infection prevalence of less than 10%, in which case the false positive rate may increase and causes unnecessary treatments [5]. Cost-effectiveness studies must also be conducted in similar settings prior to policy revamp. This is paramount in order to account for the local status regarding H. pylori prevalence, availability of resources and health services readiness. Further concern related to the test-and-treat strategy is regarding the delay of gastric cancer diagnosis. It was agreed that employing the test-and-treat strategy and possibly delaying referral for endoscopy for a brief time was unlikely to affect prognosis in gastric cancer [4]. A meta-analysis performed in 2009 on six randomised trials found that eradication of H. pylori reduces the risk of gastric cancer (relative risk: 0.65; 95% CI: 0.43, 0.98) [28]. In a more recent randomised placebo-controlled trial, compared to placebo, subjects who received H. pylori treatment had odds of 0.61 (95% CI: 0.38, 0.96) for gastric cancer incidence [29]. These evidences show that the eradication treatment will reduce the risk for gastric cancer and as such, an accurate and acceptable tool is mandatory for H. pylori detection. For the test-and-treat approach, 13C-UBT is an excellent tool to be used among the Asian population, due to its impeccable accuracy, convenience, non-invasiveness, and safety.
Strengths and Weaknesses of Review
A major strength of this review is the study selection process, whereby two independent reviewers were involved in the screening of articles at all stages. There was also no limit placed for timeframe, resulting in reduced bias for study selection. We had also stratified the studies accordingly and attempted to explore the heterogeneity by adjusting for variations between the studies. This in turn had reduced the heterogeneity, most noticeably after stratifying the studies according to dose of 13C-urea in test protocol and the time of sample collection. Further, we had included all test protocols available in each article and analyse them separately during the process of meta-analyses, in order to improve homogeneity.
There were also weaknesses which are duly acknowledged. We had only included studies published in English and this may have introduced a measure of bias. Literatures in Japanese, Korean, and Chinese languages were not able to be included due to the authors’ lingual limitations. We also regret that during the process of exploring heterogeneity, a more comprehensive sub-group analysis was not able to be conducted due to the limited number of studies.
Conclusion
To conclude, 13C-UBT is validated as an accurate tool for the diagnosis of H. pylori infection in the Asian population, with a sensitivity of 97% (95% CI: 96, 98%), and a specificity of 96% (95% CI: 95, 97%). Adjusting the test protocol for dose of 13C-urea and breath sample collection time may further improve accuracy estimates. The findings of this review support the test-and-treat strategy for H. pylori infection management and prevention of related diseases. Prevalence and cost-effectiveness studies are mandatory to aid health authorities to adopt this strategy into national policy. However, results must also be interpreted with considerations for the limitations, especially regarding the heterogenous nature of the test protocols included in this review.
Competing Interests
The authors have no competing interests to declare.
Author Contributions
All authors conform to the Authorship Guidelines of AGH and have made substantial contributions to the study, have access to data used in the manuscript, have approved the content of the manuscript, and have agreed to the authorship.
References
- 1.Stewart BW and Wild CP. World Cancer Report 2014; 2014. http://www.searo.who.int/publications/bookstore/documents/9283204298/en/ ISBN: 9283204298.
- 2.Marshall BJ and Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 323(8390): 1311–1315. DOI: 10.1016/S0140-6736(84)91816-6 [DOI] [PubMed] [Google Scholar]
- 3.Gisbert JP and Pajares JM. Review article: 13C-urea breath test in the diagnosis of helicobacter pylori infection – A critical review. Aliment Pharmacol Ther. 2004; 20(10): 1001–1017. DOI: 10.1111/j.1365-2036.2004.02203.x [DOI] [PubMed] [Google Scholar]
- 4.Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific consensus guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009; 24(10): 1587–1600. DOI: 10.1111/j.1440-1746.2009.05982.x [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017; 66(1): 6–30. DOI: 10.1136/gutjnl-2016-312288 [DOI] [PubMed] [Google Scholar]
- 6.Leal YA, Flores LL, Fuentes-Pananá EM, Cedillo-Rivera R and Torres J. 13C-Urea breath test for the diagnosis of Helicobacter pylori infection in children: A systematic review and meta-analysis. Helicobacter. 2011; 16(4): 327–337. DOI: 10.1111/j.1523-5378.2011.00863.x [DOI] [PubMed] [Google Scholar]
- 7.Tian XY, Zhu H, Zhao J, She Q and Zhang GX. Diagnostic performance of urea breath test, rapid urea test, and histology for helicobacter pylori infection in patients with partial gastrectomy: A meta-analysis. J Clin Gastroenterol. 2012; 46(4): 285–292. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=2012174449 DOI: 10.1097/MCG.0b013e318249c4cd [DOI] [PubMed] [Google Scholar]
- 8.Gisbert JP and Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: A systematic review and meta-analysis. Am J Gastroenterol. 2006; 101(4): 848–863. DOI: 10.1111/j.1572-0241.2006.00528.x [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009; 62 DOI: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 10.Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM and Kleijnen J. The development of QUADAS: A tool for quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003; 3(25): 1–13. DOI: 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155: 529–536. DOI: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 12.Whiting P, Harbord R and Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol. 2005; 5(19). DOI: 10.1186/1471-2288-5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamora J, Abraira V, Muriel A, Khan K and Coomarasamy A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006; 6(31). DOI: 10.1186/1471-2288-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua TS, Fock KM, Teo EK and Ng TM. Validation of 13C-urea breath test for the diagnosis of Helicobacter pylori infection in the Singapore population. Singapore Med J. 2002; 43(8): 408–411. [PubMed] [Google Scholar]
- 15.Kato S, Ozawa K, Konno M, et al. Diagnostic accuracy of the 13C-urea breath test for childhood helicobacter pylori infection: A multicenter Japanese study. Am J Gastroenterol. 2002; 97(7): 1668–1673. DOI: 10.1016/S0002-9270(02)04181-3 [DOI] [PubMed] [Google Scholar]
- 16.Wong WM, Lam SK, Lai KC, et al. A rapid-release 50-mg tablet-based 13 C-urea breath test for the diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther. 2003; 17: 253–257. DOI: 10.1046/j.0269-2813.2003.01417.x [DOI] [PubMed] [Google Scholar]
- 17.Peng NJ, Lai KH, Liu RS, et al. Capsule 13C-urea breath test for the diagnosis of helicobacter pylori infection. World J Gastroenterol. 2005; 11(9): 1361–1364. DOI: 10.3748/wjg.v11.i9.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng NJ, Hsu PI, Lee SC, et al. A 15-minute [13C]-urea breath test for the diagnosis of Helicobacter pylori infection in patients with non-ulcer dyspepsia. J Gastroenterol Hepatol. 2000; 15(3): 284–289. DOI: 10.1046/j.1440-1746.2000.02159.x [DOI] [PubMed] [Google Scholar]
- 19.Urita Y, Hike K, Torii N, et al. Breath sample collection through the nostril reduces false-positive results of 13C-urea breath test for the diagnosis of helicobacter pylori infection. Dig Liver Dis. 2004; 36(10): 661–665. DOI: 10.1016/j.dld.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 20.Wang WM, Lee SC, Ding HJ, et al. Quantification of Helicobacter pylori infection: Simple and rapid 13C-urea breath test in Taiwan. J Gastroenterol. 1998; 33(3): 330–335. DOI: 10.1007/s005350050092 [DOI] [PubMed] [Google Scholar]
- 21.Wong B, Wong WM, Wang WH, et al. An evaluation of invasive and non-invasive tests for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2001: 505–511. DOI: 10.1046/j.1365-2036.2001.00947.x [DOI] [PubMed] [Google Scholar]
- 22.Wardi J, Shalev T, Shevah O, Boaz M, Avni Y and Shirin H. A rapid continuous-real-time 13C-urea breath test for the detection of helicobacter pylori in patients after partial gastrectomy. J Clin Gastroenterol. 2012; 46(4): 293–296. DOI: 10.1097/MCG.0b013e31823eff09 [DOI] [PubMed] [Google Scholar]
- 23.Wong WM, Wong BCY, Li TM, et al. Twenty-minute 50 mg 13C-urea breath test without test meal for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2001; 15(9): 1499–1504. DOI: 10.1046/j.1365-2036.2001.01078.x [DOI] [PubMed] [Google Scholar]
- 24.Wong WM, Wong BCY, Wong KW, et al. 13C-urea breath test without a test meal is highly accurate for the detection of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2000; 14(10): 1353–1358. DOI: 10.1046/j.1365-2036.2000.00843.x [DOI] [PubMed] [Google Scholar]
- 25.Klein PD, Malaty HM, Czinn SJ, Emmons SJ, Martin RF and Graham DY. Normalizing results of 13 C-Urea breath besting for CO2 production rates in children. J Pediatr Gastroenterol Nutr. 1999; 29(3): 297–301. DOI: 10.1097/00005176-199909000-00011 [DOI] [PubMed] [Google Scholar]
- 26.Slater C, Preston T and Weaver LT. Is there an advantage in normalising the results of the Helicobacter pylori [13C] urea breath test for CO2 production rate in children? Isotopes Environ Health Stud. 2004; 40(1): 89–98. DOI: 10.1080/10256010310001621164 [DOI] [PubMed] [Google Scholar]
- 27.Ford AC, Qume M, Moayyedi P, et al. Helicobacter pylori “test and treat” or endoscopy for managing dyspepsia: An individual patient data meta-analysis. Gastroenterology. 2005; 128(7): 1838–1844. DOI: 10.1053/j.gastro.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 28.Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: Can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009; 151(2): 121–128. DOI: 10.7326/0003-4819-151-2-200907210-00009 [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012; 104(6): 488–492. DOI: 10.1093/jnci/djs003 [DOI] [PMC free article] [PubMed] [Google Scholar]


