Abstract
Background
Hidradenitis suppurativa (HS) is a chronic, inflammatory, recurrent skin disease of the pilosebaceous unit characterized by protean manifestations. Several studies have found an increased incidence and earlier presentation of this disease in patients carrying trisomy 21. Patients with Down syndrome (DS) have a higher risk of developing a wide range of cutaneous manifestations, including HS and chronic folliculitis. Recently, disseminate recurrent folliculitis (DRF) has been reported as an atypical monosymptomatic feature of HS at its onset.
Objective
To assess the prevalence of HS and DRF by comparing a cohort of patients carrying trisomy 21 vs pediatric controls.
Methods
A retrospective 2-year monocentric clinical study was performed by collecting clinical data of 131 patients with DS, aged 4–36 years, followed at the Dermatology Unit and Down Syndrome Regional Center of Bologna University. Data were matched with those coming from 12,351 pediatric controls.
Results
In DS patients, DRF and HS showed a prevalence of, respectively, 6.8% and 24.4%, while 5.3% of patients presented both diseases. In the control group the prevalence for HS+ and DRF+ was 0.5% and 1.2%, respectively, with a 0.14% of overlap cases. The association between HS and DRF proved to be statistically significant in both groups (P < 0.05). In the DS cohort the mean age of symptoms onset was 15.67 (SD: 2.29) years for HS and 13.11 (SD: 4.93) years for DRF. Buttocks were the most frequently affected body area for DRF followed by the inguinocrural area, while in HS buttocks were less frequently involved than groins and upper thighs.
Conclusions
Because of the later onset of HS, patients with DRF at an early age should be monitored for the possible onset of HS in the apocrine-bearing areas.
Keywords: hidradenitis suppurativa, folliculitis, Down syndrome, pediatric, hair follicle disease
Introduction
Down syndrome (DS) is the most common chromosomal disorder and is characterized by congenital heart defects, endocrinological disorders, neurological abnormalities, immunological disturbances, and a wide range of cutaneous manifestations. The cutaneous diseases associated with DS include alopecia areata, vitiligo, atopic dermatitis, psoriasis, elastosis perforans serpiginosa, syringomas, xerosis with palmoplantar hyperkeratosis, and folliculitis [1]; folliculitis was found to be the most common dermatological manifestation in patients with DS [2].
Folliculitis is an inflammatory reaction of hair follicles with possible involvement of the follicular opening and the perifollicular area. Its classification is based on histopathological or laboratory features, infectious agents, topographic distribution, recognized mechanism, disease duration, and localization within the pilosebaceous compartments [3].
Chronic, relapsing folliculitis with secondary anetoderma has been widely reported in patients affected by DS [4]. The recalcitrant course of the folliculitis, in synergy with the congenital malformation of elastic fibers in patients with DS, can lead to irreversible elastolysis, which is responsible for the anetodermal scarring [2]. The exact etiology is still a matter of discussion. Some authors have postulated the role of infective triggers such as Staphylococcus aureus or Malassezia [5], while others have suggested a link between chronic folliculitis and keratosis pilaris (KP), as the follicular expressions of a generalized xerotic condition [6]. Patients with DS are probably more susceptible to cutaneous infections including bacterial folliculitis, furuncles, abscesses, and secondary impetigo [1].
Long-term disseminate folliculitis of noninfectious etiology is difficult to frame in dermatology [7]. Several skin disorders such as atopic dermatitis, psoriasis, pityriasis rubra pilaris, and hidradenitis suppurativa (HS) often show follicular lesions, variably associated with other typical skin signs.
Disseminate recurrent folliculitis (DRF) has been proposed as one of the presenting features of an atypical monosymptomatic form of HS. A population-based cross-sectional analysis found a 2.1% prevalence of HS in patients with DS vs a 0.3% in nonaffected controls. This association was stronger in the DS population aged between 18 and 29 years, where a more than 5-fold higher risk of developing HS has been reported [8].
The primary endpoint of this study was to investigate the prevalence of HS and DRF of any body area in patients with DS. The second endpoint was to evaluate the clinical features (including anatomical distribution, disease severity) of these 2 conditions in the affected population and to verify whether there was a statistically significant correlation.
Materials and Methods
Medical records were used to perform a 2-year single-center retrospective study on 131 patients with DS followed at the Down Syndrome Regional Center and referred to the Dermatology Unit of the Sant’ Orsola Malpighi University Hospital of Bologna from January 1, 2016, to December 31, 2018.
A second cohort of 12,351 patients served as the control group. This population consisted of all patients aged between 0 and 18 years who were referred to the pediatric dermatology unit during the same 2-year period. This choice was determined by the possibility to retrieve diagnoses and/or clinical images of the patients visited from the database.
Patients lacking written parental consent for the collection of images were excluded.
Iconographic and dermoscopic data were reviewed to detect skin signs of DRF and HS in order to identify 4 different cohorts in the studied population: patients with co-occurrence of hidradenitis and folliculitis (HS+F+), those presenting only DRF (HS−F+), those with HS alone (HS+F−), and those with neither of the 2 conditions (HS−F−).
DRF was defined as a noninfectious inflammatory reaction of hair follicles presenting with multiple papules, pustules, papulopustules, or follicular papules with keratotic plug and perifollicular scarring affecting any body area and characterized by a chronic relapsing course lasting more than 6 months.
The spectrum of KP was excluded by taking into account the following factors: morphological features, anatomical distribution, and dermoscopic pattern [9]. Spiny keratotic papules presenting with variable perifollicular keratosis and degrees of inflammation, mainly scattered on extensor surfaces, favors the diagnosis of KP. Evaluation of dermoscopic images in KP shows short hair shafts or coiled vellus hair within widened follicular openings in most cases.
Moreover, patients presenting recalcitrant folliculitis with positive cutaneous swab for bacteria or fungi were not included.
HS was identified according to the diagnostic criteria provided by the European consensus guidelines for the treatment of HS: history of recurrent suppurating lesions (including inflammatory nodules, sinus tracts, abscess, and subsequent scarring) in the apocrine gland-bearing areas, occurring at least twice in a 6-month period [10].
Past medical and family history of dermatological conditions was recorded in patients affected by HS to establish disease duration and age at HS symptom onset.
For each patient the following data were collected: age, sex, anatomical pictures of affected body segments, elementary lesions and their anatomical distributions, and disease severity of HS according to Hurley’s classification [11].
Statistical Analysis
Associations between HS and DRF, expressed as dichotomous variables, were assessed using chi-square test calculated on contingency tables. Proportions were estimated with 95% exact confidence interval based on the binomial distribution. The risk ratio was estimated by the odds ratio together with 95% approximate confidence interval. Statistical tests were 2-sided and P < 0.05 was considered statistically significant. All analyses were performed using SPSS for Mac v22.0 (IBM Corp, Armonk, NY).
Results
Patients With DS
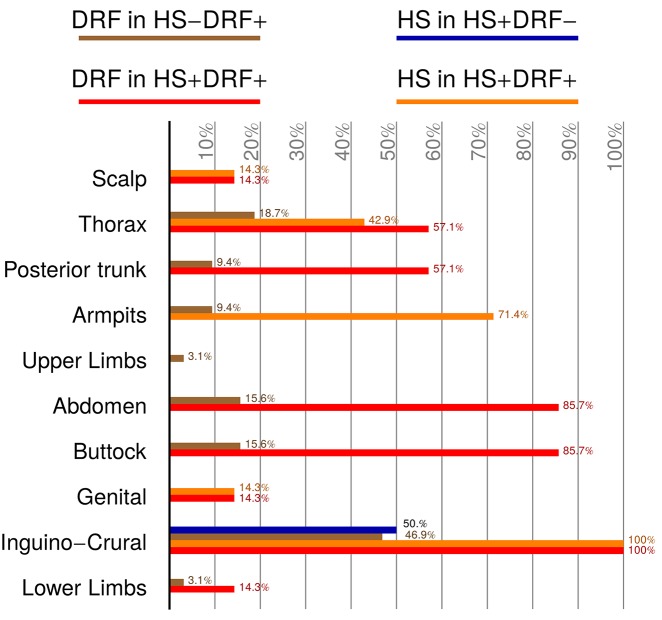
The collection of clinical and iconographic data identified the following 4 cohorts of DS patients: HS+F+, presenting with both HS and DRF (7/131 patients, 5.3%); HS+F−, characterized only by HS (2/131 patients, 1.52%); HS−F+, displaying features of DRF (32/131 patients, 24.42%); and HS−F−, without either disease (90/131, 68.70%). A female-to-male ratio of 1:1 was found in HS+F− patients, while a male prevalence was detected in the other groups. Patient’s age was recorded at the first consultation and at onset of symptoms. The distribution of age at first visit varied considerably in the 4 identified groups. HS−F− presented the lowest age (mean: 9 years; SD: 7.29), while HS+F+ had the highest (mean: 22.98 years; SD: 3.78); intermediate values were found in HS+F− (mean: 16.41 years; SD: 3.67) and HS−F+ (mean: 13.48 years; SD: 6.13). Age range reached the lowest value in HS−F−, where the youngest patient was aged 1 year, while the highest extremity of 36 years belonged to HS−F+. Age at onset of HS symptoms was established in both HS+F+ and HS−F+, being, respectively, 16 (SD: 2.38) and 14.5 (SD: 2.12) years. Age at DRF onset could be established in 18/32 HS−F+ and in 7/7 HS+F+ patients. The mean age at onset of symptoms was 15.67 (SD: 2.29) years for HS and 13.11 (SD: 4.93) years for DRF. Disease severity of HS was assessed using Hurley’s classification score [11]. In the HS+F+ group the disease severity was classified as stage I in 2/7 patients (28.6%) and stage II in 5/7 patients (71.4%). The 2 patients belonging to the HS+F− cohort were staged with Hurley severity score I. Medical history was accurately recorded in all cases: no patient had undergone HS-related surgery or had a positive family history for HS (Table 1). Disease location was assessed for both DRF and HS by identifying the following anatomical body areas: upper limbs, lower limbs, scalp, armpit, inguinocrural, thorax (including inframammary folds), posterior trunk, buttocks, abdomen, and genitals (Figure 1). With regard to DRF localization, the HS+F+ group showed a decreasing involvement of the following areas: inguinocrural (100%), buttocks and abdomen (85.7%), posterior trunk (57.1%), inferior limbs, genitals, and scalp (14.3%). Affected body areas in the HS−F+ group were, in order of decreasing frequency: buttocks (93.7%), inguinocrural (46.9%), thorax (18.7%), abdomen (15.6%), armpit and posterior trunk (9.4%), and lower and upper limbs (3.1%). In the HS+F+ group HS lesions were localized in the following areas: the inguinocrural area was involved in the totality of cases, followed by armpits (71.4%), buttocks and thorax (42.9%), genitals and scalp (14.3%). The HS+F− cohort presented the sole involvement of the inguinocrural area in one case and buttocks in the other (Figure 2).
Table 1.
Demographic Data of the Studied DS and Control Cohorts
| DS Patients | HS+F+ | HS−F+ | HS+F− | HS−F− |
|---|---|---|---|---|
| Sex | n = 7 (5.3%) | n = 32 (24.4%) | n = 2 (1.5%) | n = 90 (68.7%) |
| Male | 4 (57.1%) | 17 (53.1%) | 1 (50%) | 54 (60%) |
| Female | 3 (42.9%) | 15 (46.9%) | 1 (50%) | 36 (40%) |
| Age at first consultation | n = 7 | n = 32 | n = 2 | n = 90 |
| Mean age (SD) | 22.98 (SD: 3.78) | 13.48 (SD: 6.13) | 16.41 (SD: 3.67) | 9 (SD: 7.29) |
| Range | 18–28 | 4–36 | 14–19 | 1–35 |
| Disease severity | n = 7 | — | n = 2 | — |
| Hurley I | 2 (28.6%) | — | 2 (100%) | — |
| Hurley II | 5 (71.4%) | — | 0 (0%) | — |
| Hurley III | 0 (0%) | — | 0(0%) | — |
| Age at DRF onset | n = 7 | n = 18 | n = 2 | — |
| Mean age (SD) | 12.85 (SD: 2.96) | 13.20 (SD: 5.57) | — | |
| Range | 8–16 | 4–23 | — | |
| Age at HS onset | n = 7 | — | n = 2 | — |
| Mean (SD) | 16 (SD: 2.38) | — | 14.5 (SD: 2.12) | — |
| Range | 13–20 | — | 13–16 | — |
| Controls | HS+F+ | HS−F+ | HS+F− | HS−F− |
| Sex | n = 18 (0.14%) | n = 148 (1.2%) | n = 55 (0.44%) | n = 12130 (98.2%) |
| Male | 8 (44%) | 61 (41%) | 19 (35%) | 7,626 (63%) |
| Female | 10 (56%) | 87 (59%) | 36 (65%) | 4,504 (37%) |
| Age at first consultation | n = 18 | n = 148 | n = 55 | n = 12,130 |
| Mean age (SD) | 17.6 (SD: 2.0) | 14.5 (SD: 4.2) | 16.8 (SD: 6.3) | 7.1 (SD: 8.6) |
| Disease severity | n = 18 | — | n = 55 | — |
| Hurley I | 7 (39%) | — | 31 (56%) | — |
| Hurley II | 11 (61%) | — | 24 (44%) | — |
| Hurley III | 0 (0%) | — | 0 (0%) | — |
Figure 1.
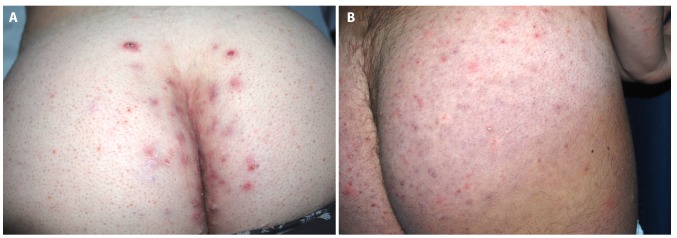
Clinical presentation of HS and DRF in patients carrying trisomy 21. (A) Painful suppurating nodules were detected in close proximity of the intergluteal fold, while the lateral aspects of the buttocks display signs of diffuse folliculitis in an HS+F+ patient. (B) Case of HS−F+ with the gluteal involvement of DRF presenting with active follicular-based papules and pustules, in the absence of inflammatory nodules, abscess, sinus tracts, or scarring. [Copyright: ©2019 Sechi et al.]
Figure 2.
Bar graph illustration of anatomical distribution of DRF and HS lesions compared among the 4 study cohorts. [Copyright: ©2019 Sechi et al.]
Control Group
This group consisted of 12,351 patients and was thus highly heterogeneous. The reported clinical conditions of the control subjects were, in order of decreasing frequency: atopic dermatitis (23.6%), vascular tumors and malformations (16.6%), infective and postinfective etiology (11.8%), melanocytic nevus (10.5%), inherited skin disorders (6%), urticaria and drug adverse event (5.7%), exogenous dermatitis (4.1%), contact irritant or allergic dermatitis (3.9%), psoriasis (3%), epidermal nevus (2.3%), autoimmune disorders (2.3%), lichen planus (2%), appendageal abnormalities (0.8%), and photodermatoses (0.2%). The frequencies of the studied disease were as follows: 1.2% (142/12,351) for DRF and 0.5% (63/12,351) for HS; 0.14% (18 patients) presented both diseases. The remaining 5.5% belonged to conditions (including unknown etiologies) other than those mentioned. Overall data were partially lacking in important details, such as age at onset of symptoms and associated clinical pictures in some cases (Table 1).
The association between HS and DRF, assessed by chi-square test, was statistically significant both in DS and control groups (P value: 0.0039 and 0.00027, respectively). The odds ratio indicates that patients affected by DS and showing DRF have a 9.8 higher risk of developing HS vs patients without DRF.
None of the patients had clinical evidence of any of the occlusion tetrad syndrome signs associated with HS (pilonidal cyst, dissecting cellulitis of the scalp, acne conglobata) [12].
Discussion
In the general population, the prevalence of HS varies from 0.00033% [13] to 4% [14]. Cosmatos et al recently assessed the HS prevalence at 0.053%, with a rate for women almost triple that of men [15].
In our retrospective analysis the prevalence of HS reached 6.8% of the DS population, which is even higher than what is already reported in the literature on patients with DS [8,16]. Compared to the control group the prevalence was 13.6- and 20-fold higher for HS and DRF, respectively. It is also known that HS starts earlier in life in DS, with the highest incidence occurring in the group aged 18–29 compared with the general HS population [8,16]. Many efforts have aimed to better investigate the pediatric onset of HS, and, to date, there are only 14 cases published in the literature [17].
Palmer and Keefe investigated early HS in the general HS population, assessing a 2% prevalence below the age of 11 years [18], whereas different data come from a more recent study, which found a prepubertal onset of HS in 7.7% of the studied HS cohort, by setting the cutoff point at 13.5 years [19].
The prevalence of HS in our control group was 0.5% (63/12,351), which is much higher than the supposed 1% prevalence under 18 years of age in a previously studied HS cohort [20].
The median age at onset of HS symptoms in our DS sample was 16 years (range 13–20) in the HS+F+ group and 14.5 years in the HS+F− group. In all cases, patients with HS had reached the pubertal growth, which is associated with an increased end-organ sensitivity of the pilosebaceous unit to androgens [21].
The mean age at diagnosis of HS in the DS population was quite different in the 2 groups: a mean age of 22.98 years was found in HS+F+ patients and 16.41 years in the HS+F− cohort.
Similar results were obtained in the controls, showing a mean age of 17.6 years in the HS+F+ cohort, 14.5 years in HS+F− group, and 16.8 years in HS−F+ patients; however, several not-DS HS+ patients had a prepubertal onset of HS. A possible bias is the relatively low age of controls, due to the fact that all patients were recruited from the pediatric dermatology outpatient service.
The male-to-female ratio in our DS HS+ groups was 5:4, so a slightly higher male prevalence was detected. The male prevalence of HS in patients with DS is also confirmed by a wide cross-longitudinal study published in the literature [8]; this trend is reversed in the HS population not associated with DS, presenting a female prevalence (female-to-male ratio 2.8:1), with a peak in patients aged 30–39 years [22]. In fact, in the HS+ control group a female-to-male ratio of 1.7:1 was found.
HS severity was assessed using Hurley score: 71.4% of DS patients showing both follicular and HS alterations had Hurley score II, while the remaining DS patients (28.6%) had Hurley I, as well as the totality of HS+F− DS subjects. The severity assessment was not biased by previous HS-related surgery. These findings from the 2 HS+ cohorts differ from the severity distribution among the general HS population, where stage I is detected in 68% of patients, while stage II occurs only in 28% [23]. In the not-DS control subjects the Hurley stage I was identified in 39% of HS+F+ patients and 56% of HS+F− patients. Hurley stage II was detected in 61% and 44% of controls belonging to the HS+F+ and HS+F− subcohorts. It may be speculated that presenting DRF in association with HS is a risk factor of a more severe disease.
In DS a scarcely considered factor is the alteration of the collagen component. Recent in vitro and ex vivo studies have demonstrated an overexpression of the genes encoding the collagen type VI in human skin fibroblast cultures and nuchal skin of DS fetuses [24,25]. Type VI collagen is an extracellular matrix molecule abundantly expressed in the dermis, which plays a key role in the processes of hair follicle cycling and wound healing, tissue repair through the regulation of the dermal matrix assembly, and fibroblastic motility [24,26]. It has a heterotrimeric structure, consisting of 3 distinct polypeptide chains: α1, α2, and α3. The increase in collagen VI is due to an upregulation of the transcription of the COL6A1 and COL6A2 genes located in the chromosome 21, respectively, encoding for the α1 and α2 chains, due to a gene dosage effect [24]. Two diseases associated with impairment of type VI collagen are Ullrich congenital muscular dystrophy and Bethlem myopathy, which are also characterized by abnormal skin findings, including follicular hyperkeratosis, hypertrophic and keloid scarring, and cutaneous xerosis [26]. It is possible to hypothesize that a mechanical stress exerted on compromised follicular units can activate abnormal extracellular remodeling, leading to scarring evolution. This might explain, in the HS+F+ cohort, the progression of DRF into tract formation and cicatrization at intertriginous sites, bypassing the HS severity stage I.
As suggested by Revuz, DRF is a gray area in the field of dermatology, as it cannot be associated univocally with a specific disease or etiology [7]. Even though DRF belongs to the clinical spectrum of HS, it is not mentioned among the diagnostic criteria. As a consequence, a diagnosis of HS cannot be postulated on the basis of DRF presenting as painful papulopustules with a lack of closed comedones or microcysts. Assuming that DRF may represent a mode of presentation of HS [7], in some patients belonging to the HS−F+ group, DRF possibly represents the initial pre-Hurley stage I of HS. This hypothesis could represent the key to explain not only the statistical correlation between HS and DRF, but also the age mismatch at symptom onset between the HS+F+ and HS+F− cohorts.
Disease location of DRF in the HS+F+ DS group favored the inguinocrural area, which was involved in all patients, followed by the buttocks and abdomen detected in 6/7 cases. On the other hand, the buttocks represent the most frequent localization of DRF in HS−F+ DS patients: this frequency remains stable regardless of the age range. Other body areas such as the groin, thigh, armpit, and back are affected mainly in postpubertal patients, whereas prepubertal patients show a constant involvement of the buttocks with minimal involvement of other anatomical sites.
HS lesions in subjects with DS were located, with decreasing prevalence, at the inguinocrural area, armpits, buttocks, thorax (including inframammary folds), genitals, and scalp. As suggested by Giovanardi et al [27] in a recent study, there is no difference in disease distribution of HS in patients with DS vs not-DS. Also, there is no strict overlap between DRF and HS lesion distribution in DS patients showing both affections (HS+F+). As a result, it can be affirmed that the follicular papulopustules of DRF may be the hallmark of a follicular inflammatory disease, which predisposes to develop HS in typical areas. The mechanical friction in the intertriginous area probably remains the main trigger in generating HS lesions in predisposed DS patients.
Another possible predisposing factor in both HS and DRF is obesity, which has also been frequently associated with DS [28]. Weight gain leading to obesity usually occurs after puberty, causing an increase of mechanical friction in the areas of skin-to-skin contact. Moreover, it causes a proinflammatory state through increased levels of resistin and chemerin, which are responsible for insulin resistance and upregulation of the follicular androgen receptor, leading to follicular occlusion [29].
In addition, the intrinsic immunodeficiency of DS [30] facilitates microbial growth and proliferation in the superficial segments of hair follicles and in the perifollicular space. As a consequence, bacterial infections may contribute to HS progression leading to disease extension and scarring evolution [31].
The pathogenesis of HS is multifactorial, with both genetic and environmental factors involved. Considering the genetic factors, an important role is played by loss-of-function mutations of the component of the γ-secretase complex, which results in reduced Notch signaling [32]. Impaired Notch signaling causes alterations of the hair follicle structure and insufficient feedback suppression of TLR-MAPK-activated innate immunity, leading to immune deregulation. The main environmental factors are obesity, smoking, mechanical traumatism, and bacteria. Furthermore, pathogen-associated molecular patterns, microbe-associated molecular patterns, and damage-associated molecular patterns activate the autoinflammatory and inflammasome pathway [33].
The higher prevalence of HS in patients with DS can be explained by the impaired Notch-MKP signaling: in trisomy21, the increased level of amyloid precursor protein (APP) reduces Notch signaling, acting as a competitive substrate of γ-secretase [34], resulting in the persistence of autoinflammation [35]. Furthermore, APP and its cleavage product (secretory N-terminal ectodomain of APP) promote keratinocyte migration, adhesion, and proliferation [36]. This results in the plugging and dilation of hair follicles, which leads to follicular hyperkeratosis, hyperplasia of the follicular epithelium, and rupture of hair follicles with consequent perifolliculitis and cyst formation [36].
This study has several limitations due to the lack of validation criteria for the DRF diagnosis, the lack of a long-term prospective study regarding the HS-DRF association, and the lack of ethnicity variability, since the great majority of the enrolled population consists of Caucasians. A higher incidence of HS has, in fact, been reported in the African American ethnic group in 2 studies [37,38]. Garg et al demonstrated a 2.5-fold higher incidence of HS in this population [37], while Vlassova et al identified African American women as being exposed to the highest risk of developing HS [38].
To sum up, trisomy 21 carries genetic alterations that are responsible for hair follicle imbalances and consequent occlusion. The follicular plugging acts in synergy with other promoting factors such as obesity, mechanical stress, pubertal increase in circulating androgens, and insulin resistance. Added to this is the promoting role of the alteration of the collagen component, which may be responsible for the evolution toward profibrotic phenotype.
Conclusions
Both DRF and HS are commonly detected in patients carrying trisomy 21 and might be considered 2 associated features of the same syndromic condition [39]. On the other hand, there are no studies concerning the prevalence of DRF in the general pediatric population, since the validity of DRF as a separate entity is still debated. DRF is also thought to be associated with a variant of KP, which also occurs under the follicular occlusion disease spectrum [9].
In our clinical practice, we identify pediatric patients at risk of developing HS by taking into account several parameters, which are reported in Table 2; positivity for 2 or more parameters leads us to follow up patients at risk every 6 months, to identify early signs of HS. Although this is not a validated tool, it might be useful to recognize patients eligible for HS screening.
Table 2.
Illustration of the 8 Parameters That Are Assessed to Perform a Risk Stratification of Developing HS in Pediatric Patients
| Recommendation Criteria for HS Screening in Pediatric Patients |
|---|
| Familial background: Family history positive for HS in at least 1 first-degree relative. |
| Genetics: Syndromes with known genetic abnormalities (Bazex-Dupré-Christol syndrome, Dowling-Degos disease, DS, keratitis-ichthyosis-deafness syndrome), Dent disease 2. |
| Ethnicity: Descent from African and Hispanic populations. |
| Clinical findings: History of disseminate recurring folliculitis lasting more than 6 months, involving at least 2 body areas (either mono- or bilaterally) not attributable to known etiological factors. |
| Endocrinological abnormalities: Clinical and laboratory findings of insulin resistance, peripheral hyperandrogenism, polycystic ovarian syndrome. |
| Follicular occlusion signs: Clinical evidence of at least 1 of the occlusion tetrad syndrome signs associated with HS (pilonidal cyst, dissecting cellulitis of the scalp, acne conglobata). |
| Smoking: Smoking habits or smoke exposure in indoor environments (secondhand smoke). |
| Ultrasound: Detection of any of the sonographic features suggestive for subclinical follicular abnormalities, including widening of the hair follicles, thickening or abnormal echogenicity of the dermis, and dermal pseudocystic nodules. |
To determine more accurately whether DRF is a separate entity or an early pre-Hurley stage I of HS, it will be useful to follow DRF lesions longitudinally through photographic and ultrasound evaluation. The latter has become a very promising, widely available imaging modality for HS, able to detect subclinical changes occurring in early stages, as well as the initial hair follicle dilation, increased thinning, or abnormal echogenicity of the dermal layer [40].
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
Authorship: All authors have contributed significantly to this publication.
References
- 1.National Board of Health and Welfare. Cancer incidence in Sweden 2017 [in Swedish] 2018. [Accessed March 5, 2019]. Retrieved from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2018-12-50.pdf.
- 2.Schepis C, Barone C, Siragusa M, Pettinato R, Romano C. An updated survey on skin conditions in Down syndrome. Dermatology. 2002;205(3):234–238. doi: 10.1159/000065859. [DOI] [PubMed] [Google Scholar]
- 3.Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5(5):301–310. doi: 10.2165/00128071-200405050-00003. [DOI] [PubMed] [Google Scholar]
- 4.Schepis C, Siragusa M. Secondary anetoderma in people with Down’s syndrome. Acta Derm Venereol. 1999;79(3):245. doi: 10.1080/000155599750011174. [DOI] [PubMed] [Google Scholar]
- 5.Kavanagh GM, Leeming JP, Marshman GM, Reynolds NJ, Burton JL. Folliculitis in Down’s syndrome. Br J Dermatol. 1993;129(6):696–699. doi: 10.1111/j.1365-2133.1993.tb03334.x. [DOI] [PubMed] [Google Scholar]
- 6.Finn OA, Grant PW, McCallum DI, Raffle EJ. A singular dermatosis of Mongols. Arch Dermatol. 1978;114(10):1493–1494. [PubMed] [Google Scholar]
- 7.Revuz J. Disseminate recurrent folliculitis as the presenting picture of hidradenitis suppurativa. Ann Dermatol Venereol. 2017;144(11):715–718. doi: 10.1016/j.annder.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Garg A, Strunk A, Midura M, Papagermanos V, Pomerantz H. Prevalence of hidradenitis suppurativa among patients with Down syndrome: a population-based cross-sectional analysis. Br J Dermatol. 2018;178(3):697–703. doi: 10.1111/bjd.15770. [DOI] [PubMed] [Google Scholar]
- 9.Wang JF, Orlow SJ. Keratosis pilaris and its subtypes: associations, new molecular and pharmacologic etiologies, and therapeutic options. Am J Clin Dermatol. 2018;19(5):733–757. doi: 10.1007/s40257-018-0368-3. [DOI] [PubMed] [Google Scholar]
- 10.Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 11.Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, editors. Dermatologic Surgery. New York: Marcel Dekker; 1989. pp. 729–739. [Google Scholar]
- 12.Plewig G, Kligman AM. Acne. Berlin, Heidelberg: Springer; 1975. Acne conglobata; pp. 168–203. [Google Scholar]
- 13.Lookingbill DP. Yield from a complete skin examination: findings in 1157 new dermatology patients. J Am Acad Dermatol. 1988;18(1 Pt 1):31–37. doi: 10.1016/s0190-9622(88)70004-3. [DOI] [PubMed] [Google Scholar]
- 14.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35(2 Pt 2):191–194. doi: 10.1016/s0190-9622(96)90321-7. [DOI] [PubMed] [Google Scholar]
- 15.Cosmatos I, Matcho A, Weinstein R, Montgomery MO, Stang P. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68(3):412–419. doi: 10.1016/j.jaad.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Denny G, Anadkat MJ. Hidradenitis suppurativa (HS) and Down syndrome (DS): increased prevalence and a younger age of hidradenitis symptom onset. J Am Acad Dermatol. 2016;75(3):632–634. doi: 10.1016/j.jaad.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 17.Liy-Wong C, Pope E, Lara-Corrales Hidradenitis suppurativa in the pediatric population. J Am Acad Dermatol. 2015;73(5 Suppl 1):S36–S41. doi: 10.1016/j.jaad.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 18.Palmer RA, Keefe M. Early-onset hidradenitis suppurativa. Clin Exp Dermatol. 2001;26(6):501–503. doi: 10.1046/j.1365-2230.2001.00876.x. [DOI] [PubMed] [Google Scholar]
- 19.Deckers IE, van der Zee HH, Boer J, Prens EP. Correlation of early-onset hidradenitis suppurativa with stronger genetic susceptibility and more widespread involvement. J Am Acad Dermatol. 2015;72(3):485–488. doi: 10.1016/j.jaad.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Scheinfeld N. Hidradenitis suppurativa in prepubescent and pubescent children. Clin Dermatol. 2015;33(3):316–319. doi: 10.1016/j.clindermatol.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Ebling FJG. Hidradenitis suppurativa: an androgen-dependent disorder. Br J Dermatol. 1986;115:259–262. doi: 10.1111/j.1365-2133.1986.tb05739.x. [DOI] [PubMed] [Google Scholar]
- 22.Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760–764. doi: 10.1001/jamadermatol.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133(6):1506–1511. doi: 10.1038/jid.2012.472. [DOI] [PubMed] [Google Scholar]
- 24.Karousou E, Stachtea X, Moretto P, et al. New insights into the pathobiology of Down syndrome—hyaluronan synthase-2 overexpression is regulated by collagen VI α2 chain. FEBS J. 2013;280(10):2418–2430. doi: 10.1111/febs.12220. [DOI] [PubMed] [Google Scholar]
- 25.Quarello E, Guimiot F, Moalic JM, Shnoneau M, Ville Y, Delezoide AL. Quantitative evaluation of collagen type VI and SOD gene expression in the nuchal skin of human fetuses with trisomy 21. Prenat Diagn. 2007;27(10):926–931. doi: 10.1002/pd.1803. [DOI] [PubMed] [Google Scholar]
- 26.Theocharidis G, Drymoussi Z, Kao AP, et al. Type VI collagen regulates dermal matrix assembly and fibroblast motility. J Invest Dermatol. 2016;136(1):74–83. doi: 10.1038/JID.2015.352. [DOI] [PubMed] [Google Scholar]
- 27.Giovanardi G, Chiricozzi A, Bianchi L, et al. Hidradenitis suppurativa associated with Down syndrome is characterized by early age at diagnosis. Dermatology. 2018;234(1–2):66–70. doi: 10.1159/000487799. [DOI] [PubMed] [Google Scholar]
- 28.O’Shea M, O’Shea C, Gibson L, Leo J, Carty C. The prevalence of obesity in children and young people with Down syndrome. J Appl Res Intellect Disabil. 2018;31(6):1225–1229. doi: 10.1111/jar.12465. [DOI] [PubMed] [Google Scholar]
- 29.Melnik BC, Plewig G. Impaired Notch signalling: the unifying mechanism explaining the pathogenesis of hidradenitis suppurativa (acne inversa) Br J Dermatol. 2013;168(4):876–878. doi: 10.1111/bjd.12068. [DOI] [PubMed] [Google Scholar]
- 30.Kusters MA, Verstegen RH, Gemen EF, de Vries E. Intrinsic defect of the immune system in children with Down syndrome: a review. Clin Exp Immunol. 2009;156(2):189–193. doi: 10.1111/j.1365-2249.2009.03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Highet AS, Warren RE, Weeks AJ. Bacteriology and antibiotic treatment of perineal suppurative hidradenitis. Arch Dermatol. 1988;124(7):1047–1051. [PubMed] [Google Scholar]
- 32.Melnik BC, Plewig G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: an approach to pathogenesis by evidence from translational biology. Exp Dermatol. 2013;22(3):172–177. doi: 10.1111/exd.12098. [DOI] [PubMed] [Google Scholar]
- 33.Schlapbach C, Hänni T, Yawalkar N, Hunger RE. Expression of the IL-23/TH17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(4):790–798. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Berezovska O, Jack C, Deng A, Gastineau N, Rebeck GW, Hyman BT. NotcH1 and amyloid precursor protein are competitive substrates for presenilin1-dependent gamma-secretase cleavage. J Biol Chem. 2001;276(32):30018–30023. doi: 10.1074/jbc.M008268200. [DOI] [PubMed] [Google Scholar]
- 35.Blok J, Jonkman M, Horváth B. The possible association of hidradenitis suppurativa and Down syndrome: is increased amyloid precursor protein expression resulting in impaired Notch signalling the missing link? Br J Dermatol. 2014;170(6):1375–1377. doi: 10.1111/bjd.12887. [DOI] [PubMed] [Google Scholar]
- 36.Herzog V, Kirfel G, Siemes C, Schmitz A. Biological roles of APP in the epidermis. Eur J Cell Biol. 2004;83(11–12):613–624. doi: 10.1078/0171-9335-00401. [DOI] [PubMed] [Google Scholar]
- 37.Garg A, Lavian J, Lin G, Strunk A, Alloo A. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77(1):118–122. doi: 10.1016/j.jaad.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Vlassova N, Kuhn D, Okoye GA. Hidradenitis suppurativa disproportionately affects African Americans: a single-center retrospective analysis. Acta Derm Venereol. 2015;95(8):990–991. doi: 10.2340/00015555-2176. [DOI] [PubMed] [Google Scholar]
- 39.Gasparic J, Theut Riis P, Jemec GB. Recognizing syndromic hidradenitis suppurativa: a review of the literature. J Eur Acad Dermatol Venereol. 2017;31(11):1809–1816. doi: 10.1111/jdv.14464. [DOI] [PubMed] [Google Scholar]
- 40.Wortsman X, Moreno C, Soto R, Arellano J, Pezo C, Wortsman J. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg. 2013;39(12):1835–1842. doi: 10.1111/dsu.12329. [DOI] [PubMed] [Google Scholar]