Abstract
Routine monitoring of toxic cyanobacteria depends on up-to-date epidemiological information about their distribution. In Australia, anatoxin producing cyanobacteria are not regularly tested for and thought to be rare if not absent from the continent. Our study investigated the presence of anatoxin-a (ATX-a) producing cyanobacteria in surface water samples (n = 226 from 67 sampling locations) collected from 2010 to 2017 across the state of Victoria, Australia. We (1) detected the presence and distribution of anaC (anatoxin-a synthetase C) gene sequences previously associated with various cyanobacteria, including Cuspidothrix issatschenkoi, Aphanizomenon sp., D. circinale, Anabaena sp., and Oscillatoria sp., from 31 sampling locations, and (2) determined the concentration of ATX-a in samples tested using ELISA, in two instances detected at >4 µg · L−1. These data present the first confirmation of ATX-a producers in Australia. Our study indicates that ATX-a should be included in regular testing of cyanobacterial blooms in Australia and highlights the importance of regular investigation of the distributions of toxic cyanobacteria worldwide, particularly amid the known expanding distribution of many cyanobacterial taxa in a period of increased eutrophication and rising surface water temperatures.
Subject terms: Water microbiology, Microbial ecology
Introduction
Cyanobacterial blooms are a major water-quality issue affecting management of surface waters globally1. In addition to impacting water palatability, these blooms have the potential to produce a variety of toxic secondary metabolites or “cyanotoxins”, including hepatotoxins [e.g., Microcystin (MC), Nodularin (NOD)], neurotoxins [e.g., Saxitoxin (SXT), Anatoxin (ATX)] and/or cytotoxins [e.g., Cylindrospermopsin (CYN)] of significant health, veterinary and agricultural importance1,2. Identification and differentiation of toxic cyanobacteria is vital to monitoring and management of various water resources, which in turn is directed by contemporary knowledge of the regional distribution of toxin types.
Many studies in the last decades, have described increasing incidence of cyanobacterial blooms and, more importantly, a rapid global expansion of cyanobacteria species into new environments3,4. Numerous reports indicate increased spread/prevalence of ‘invasive’ cyanobacterial species; including, for example, previously ‘tropical/subtropical’ CYN producing Raphidiopsis raciborskii (previously: Cylindrospermopsis raciborskii) and Chrysosporum ovalisporum (previously: Aphanizomenon ovalisporum) and, ATX producing Raphidiopsis mediterranea and Cuspidothrix issatschenkoi (previously: Aphanizomenon issatschenkoi) emerging in temperate regions of Europe, the USA, Japan and New Zealand (see reviews5–9).
Cyanobacterial blooms are common in many parts of Australia10–13 and toxic cyanobacteria capable of producing MC, NOD, CYN and SXT are widely distributed in tropical, subtropical and temperate regions of Australia14–17. Among various cyanotoxins produced by cyanobacteria globally, anatoxin-a (ATX-a) is a potent neurotoxic alkaloid compound that can cause severe asphyxia and muscular paralysis in humans and animals18. ATX-a was first isolated in the 1970’s from filamentous cyanobacteria, Aphanizomenon flos-aquae19. Since then, several ATX-a producing genera of cyanobacteria, including Anabaena/Dolichospermum20–23, Oscillatoria22,24–26, Aphanizomenon22,27–29, Cu. issatschenkoi22,27–29, Phormidium30,31 and Tychonema32,33 have been identified worldwide. While previous reports have confirmed the expansion of CYN producing R. raciborskii to many parts of Australia (see reviews6–9), and more recently Ch. ovalisporum to south-eastern parts of Australia34, understanding of the prevalence and ecology of ATX-a producers in Australia is limited. Although previous studies10–13 have detected small quantities of Cu. issatschenkoi in the Murray Darling Basin, Australia, an ATX-a producing cyanobacterium is yet to be confirmed35 and therefore, this toxin is not routinely tested for and presumed to be absent in Australian waters.
A range of diagnostic tools are currently used to monitor ATX-a producing cyanobacterial blooms36. These techniques include microscopic identification and enumeration of the phytoplankton community37,38, direct detection/quantification of ATX-a using Enzyme Linked Immunosorbent Assay (ELISA) or liquid chromatography with tandem mass spectrometry (LC-MS/MS)39, and PCR-based assays targeting specific genes in the ATX-a synthetase gene cluster22,25,40,41, mainly anaF28 and anaC22. More recently, a study by Legrand et al.42 has described a nested PCR-based method that is both sensitive and specific for amplifying anaC genes from environmental samples.
Given the availability of diagnostic tools, but lack of prevalence data for ATX-a producers, with the expansion of several cyanobacteria species (see reviews5–9) into new geographical regions and increasing incidence of toxic blooms, we undertook a study to investigate the emergence of ATX-a producing cyanobacteria in 226 cyanobacterial bloom samples collected over a period of seven years from various water bodies across the state of Victoria (VIC), Australia. This study combined the following methods: analyses of cyanobacteria community composition using microscopy, a nested PCR-based amplification and sequencing of environmental anaC genes using two existing general anaC primer pairs22 and direct detection of ATX-a by ELISA. Our study confirms the presence of ATX-a producing cyanobacteria in Australia. Moreover, this study highlights the clear need to better understand and closely monitor the rapidly shifting distributions of toxic cyanobacteria species in global surface waters and to regularly update the testing regimes for these microorganisms.
Results
Sample collection
For the current study, a total of 226 surface water samples (1L surface grabs) were collected from 67 locations across the state of VIC, Australia over summer and autumn months (November to April) from 2010 until 2017 (Fig. 1). All samples were taken during bloom events and exceeded ~1000 cyanobacterial cells · mL−1. In some instances, samples exceeded a biovolume of 10 mm3 L−1.
Figure 1.
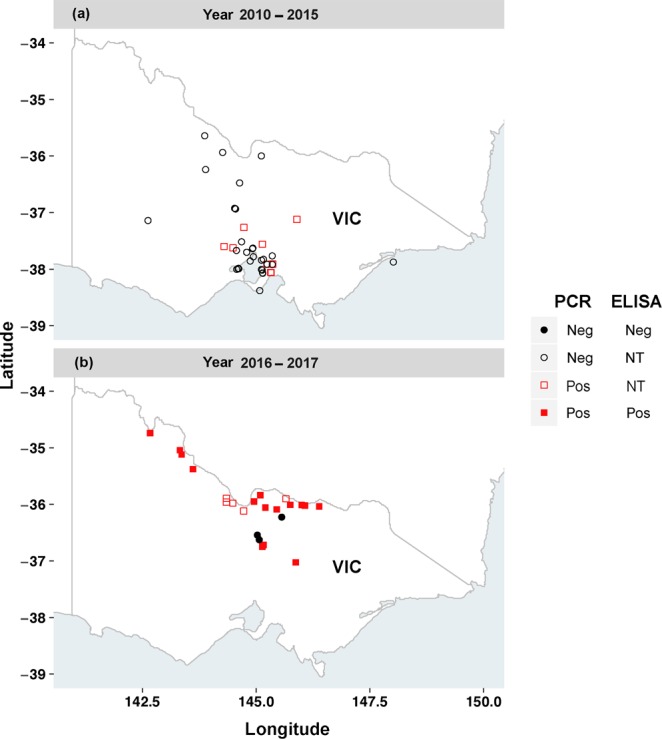
Locations of samples (a) Year 2010–2015, (b) Year 2016–2017 tested in this study for the presence of anatoxin-a (ATX-a) using nested PCR and ELISA. Points in the graph represent summation of nested PCR and ELISA data at each location tested rather than individual samples. Symbols; VIC, Victoria; Pos, Positive; Neg, Negative; NT, Not tested.
anaC gene nested PCR
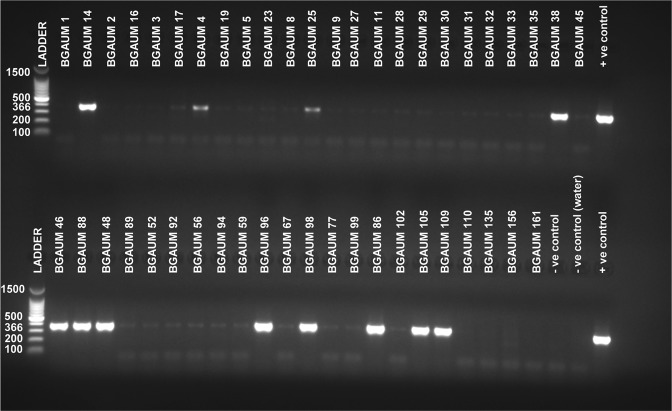
Amplification of the cyanobacterial 16S rRNA and internal spike control assays prior to anaC nested PCR were found to be positive for all samples. For anaC gene screening, DNA extracts from each of the 226 samples were tested by nested PCR using published anaC general primer pairs (Table 1)22 known to specifically target ATX-a producing cyanobacteria. In the primary PCR process, no PCR products except for the positive control were observed on a 1.5% agarose gel electrophoresis, indicating low prevalence of anaC target gene in our samples. Subsequent nested PCR step produced a total of 68 amplicons (from 31 sampling locations across the state of VIC, Australia) from 226 samples with an expected 366 bp size on a 1.5% agarose gel electrophoresis unit (Fig. 2). Out of the total 68 amplicons, 20 amplicons were observed as very strong bands on the agarose gel electrophoresis. The remaining amplicons, i.e., 4/68 and 44/68 were observed as strong and faint bands, respectively (see Supplementary Table S1). The majority of the amplicons (i.e., 59/68, 86.7%) in this study were generated from 82 samples, corresponding to a recent (2016/2017) major cyanobacterial bloom event spreading across 23 different locations along the Murray River and associated waterways (including rivers, creeks, lakes, canals and reservoirs) bordering the states of New South Wales (NSW) and VIC, Australia (Fig. 1b). The remaining nine anaC (i.e., 9/68, 13.2%) positive amplicons produced faint bands on agarose gel electrophoresis, and were represented by non-Murray bloom samples (i.e., n = 144) collected prior to 2016 (Fig. 1a).
Table 1.
List of PCR primers22 used in this study to amplify anatoxin-a synthetase C (anaC) gene.
| Primers | Sequences (5′ - 3′) | Target gene | Product size |
|---|---|---|---|
|
anxgenF anxgenR |
ATGGTCAGAGGTTTTACAAG CGACTCTTAATCATGCGATC |
anaC | 861 bp |
|
anaC-genF anaC-genR |
TCTGGTATTCAGTCCCCTCTAT CCCAATAGCCTGTCATCAA |
anaC | 366 bp |
Figure 2.
Nested PCR results of anatoxin-a synthetase C (anaC) gene of a subset of cyanobacterial bloom samples separated by 1.5% agarose gel electrophoresis. DNA fragments at 366 bp indicate the anaC gene. The positive control represents a known ATX-a producing Cuspidothrix issatschenkoi CY1941. A known negative ATX-a producer, Nostoc punctiforme PCC73102 and non-template control (water), were used as negative controls. The ladder represents DNA fragments with 100 bp. For sample descriptions and sequencing results, see Supplementary Table S1. The present image is cropped and a full-length gel is presented in Supplementary Fig. S1.
To identify potential ATX-a producing cyanobacteria within this recent bloom, cyanobacteria community abundance was determined for each sample by observation under light microscope. The majority of these samples contained Ch. ovalisporum (Forti) E. Zapomelová, O. Skácelová, P. Pumann, R. Kopp & E. Janecek 2012 (previously A. ovalisporum), a known CYN producer, as the dominant cyanobacterium along with Planktolyngbya, Cyanogranis, Chroococcales and Aphanocapsa as the subdominant species. Small quantities of potential ATX-a producers including Cu. issatschenkoi (Usaĉev) Rajaniemi et al. 2005 (previously: Ap. issatschenkoi), species belonging to Aphanizomenonaceae family and Dolichospermum sp., were also present (see Supplementary Table S1).
anaC gene sequencing and phylogenetic analysis
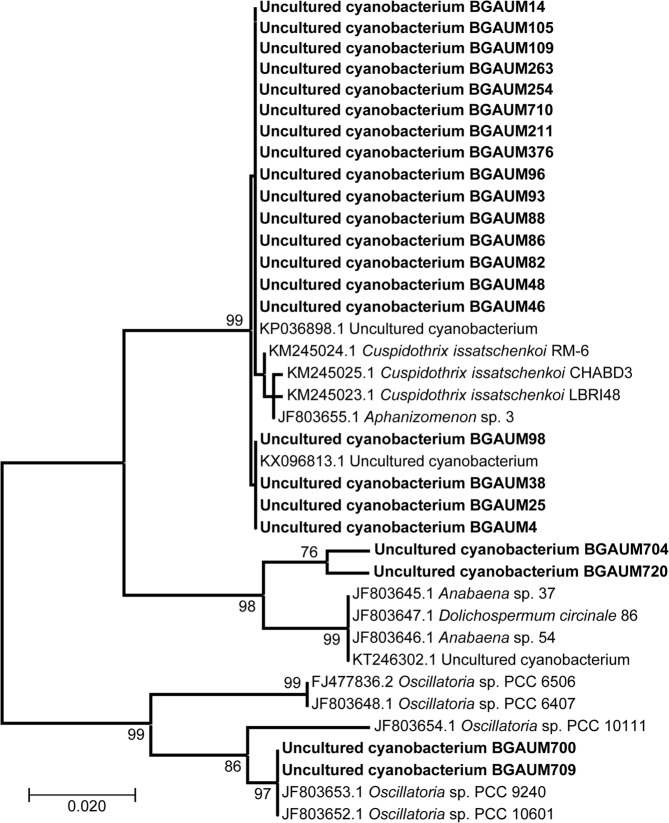
To confirm the identity of anaC nested PCR products, the 24 very strong and strong amplicons were sequenced by bi-directional (capillary) sequencing. Twenty three amplicons produced high quality (>97%) unambiguous sequence data (analysed using Geneious® Version 10) and the remaining amplicon was removed from further analysis. A BLASTn search of the NCBI non-redundant database of each of the 23 sequenced amplicons showed 90–100% nucleotide identity to several previously annotated cyanobacteria anaC genes from uncultured cyanobacteria (Accession Numbers: KX096813, KT246302, KP036898), Oscillatoria sp. (Accession Numbers: JF803653, JF803652), Cu. issatschenkoi (Accession Numbers: KM245023, KM245024, KM245025), Dolichospermum circinale (previously: Anabaena circinalis) (Accession Number: JF803647), Anabaena sp. 37 (Accession Number: JF803645), Anabaena sp. 54 (Accession Number: JF803646) and Aphanizomenon sp. 3 (Accession Number: JF803655) (Table S1).
All 23 anaC sequences obtained from this study along with other peer reviewed anaC sequences from GenBank were used to construct a 340 bp Maximum Likelihood (ML) tree (Fig. 3). A previously described ATX-a producing Phormidium autumnale CAWBG618 (Accession Number: KX016036) with a smaller anaC gene fragment (308 bp) was excluded from the phylogenetic analyses. The overall topology of the anaC gene ML tree recognizes three distinct groups with >98% bootstrap support. The majority of the sequences (19/23) derived from the current study were identical and clustered with a larger group of anaC sequences of many labelled in GenBank as “Uncultured cyanobacterium”, Cu. issatschenkoi and Aphanizomenon sp. The remaining four anaC sequences from this study clustered with two different smaller groups including several anaC GenBank sequences of Anabaena sp., D. circinale and Oscillatoria sp. (see Fig. 3 for Accession Numbers). Small genotypic variations in anaC genes among different ATX producing cyanobacteria have resulted in the formation of subgroups within the three major groups.
Figure 3.
Unrooted phylogenetic tree of partial anatoxin-a synthetase C (anaC) gene sequences (340 bp). 23 sequences generated in this study (marked in bold) along with reference sequences from NCBI GenBank were used to create the tree. Phylogenetic analyses were conducted in MEGA757 using the Maximum Likelihood (ML) based on the Tamura-Nei model56. Bootstrap values from 1000 replicates are shown at the nodes. The scale bar represents 0.02 substitutions/site.
ATX-a concentration in cyanobacterial bloom samples
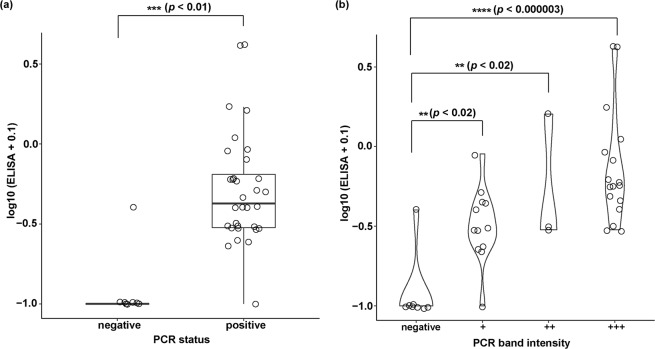
To confirm the presence of anatoxin producers in these water samples, we undertook direct ATX-a detection in 40 (32 anaC nested PCR positive and 8 negative) samples corresponding to a large recent (2016–2017) cyanobacterial bloom extending over 500 kms across the Murray River and associated waterways, Australia. All samples were analysed in duplicate using the Abraxis® ELISA kit. A total of 29 of 32 nested PCR positive samples showed ATX-a concentrations between 0.15–1.6 µg · L−1, with two samples exceeding >4 µg · L−1 and one sample showing <0.15 µg · L−1. Of the eight negative nested PCR samples, seven samples showed zero or <0.15 µg · L−1 (the minimum reading for the test) of ATX-a and one sample had 0.3 µg · L−1 of ATX-a (Table S1). A significant difference (p < 0.01, Welch Two sample t-test) was observed between ATX-a ELISA concentrations of nested PCR-positive and negative samples (Fig. 4a). Additionally, the relationship between observed nested PCR band intensities (i.e., very strong, strong, weak and negative) and ATX-a ELISA values was also found to be highly significant (p < 0.000003 and p < 0.02, Tukey’s honest significant difference (HSD) test and ANOVA) (Fig. 4b). All PCR positive groups showed greater ELISA signal than the PCR negative group, and the highest PCR band intensity (‘very strong’) was greater than the lowest non-zero (‘weak’) intensity (Fig. 4b).
Figure 4.
Box plot (a) and violin plot (b) representing log-transformed anatoxin-a (µg · L−1) measured by ELISA (Y-axis) corresponding to nested PCR results (X-axis) of 40 cyanobacterial bloom samples. The grey line across the box (A) indicates the median anatoxin-a concentration. Asteriks (*) represent statistical significance (p < 0.05). PCR band intentisities (B) were indicated by faint (+), strong (++) and very strong (+++) bands.
Discussion
We examined the presence of ATX-a producing cyanobacteria in 226 samples from 67 locations (Fig. 1) across the state of VIC, Australia by microscopy, nested PCR and ELISA assays. Based on the microscopy data, we detected several potential/known ATX-a producing cyanobacteria in many samples (Table S1). Nested PCR testing of these same samples detected the presence of anaC, a gene required for ATX-a synthesis, at overall sample prevalence of 30.1% (68/226 samples tested) representing 46.2% of sample locations (31/67 sample sites: Figs 1, 2 and Table S1).
Bi-directional sequencing confirmed the identity of the nested PCR amplicons from our samples as the anaC gene. The phylogenetic clustering by unrooted ML analysis clustered these sequences into three distinct groups, of which the majority of anaC sequences most likely represent ATX-a producing C. issatschenkoi along with Aphanizomenon sp., Oscillatoria sp., Anabaena sp. and D. circinale (Table S1 and Fig. 3). Overall, our findings (corroborated by microscopy, nested PCR, sequencing and phylogenetic analysis) agree with previous reports of common ATX-a producing cyanobacteria in many parts of Europe20,28,33,43,44, Asia41,45,46 and New Zealand27,30,31.
Direct toxin detection (using the Abraxis® anatoxin-a ELISA kit) confirmed the presence of ATX-a in all but one of the anaC positive samples (Fig. 4 and Table S1). It is not clear whether these samples represent significant health risks as currently there are no guideline values documented for ATX-a toxin and its congeners in Australia and/or by World Health Organisation (WHO). None of our samples showed toxin levels exceeding the New Zealand provisional maximum value for ATX-a in drinking water (i.e., 6 µg · L−1)47. Two exceeded a provisional guideline value (i.e., 3 µg · L−1) for ATX-a in drinking water outlined by the province of Oregon, USA47, however it should be noted that these samples were taken from raw water (i.e. pre-treatment) and not product water (post-treatment). ATX-a ELISA assay was performed on recently collected (2016–2017) samples (i.e., from the Murray River and associated waterways) only. All samples from 2010–2015 were tested by anaC nested PCR only. Positive anaC detection in these samples is indicative of their potential to produce ATX-a, but actual toxin production has not been confirmed.
Although the ELISA assay quantified ATX-a production in our samples, ELISA values did not strongly correlate with the total number/bio-volume of potential/known ATX-a producing cyanobacteria strains identified in our microscopy analysis (Table S1). The possible reasons for this are, (1) the ATX-a producing cells in our samples declined rapidly prior to our sampling, but the ATX-a did not, (2) despite planktonic cyanobacteria being known as the major ATX-a producers in surface waters, the primary source in some samples may be ATX-a producing benthic cyanobacteria species48,49, or (3) these samples contain other major species of ATX-a producing cyanobacteria that are yet to be identified. Of these possibilities, the latter seems most likely as all samples were transported under cold storage and processed immediately upon receipt and yet none contained large quantities of a known ATX-a producer on microscopic examination. Additionally, while microscopy could not clearly identify a major ATX-a producer at high abundance, associated anaC genes were readily detectable by nested PCR in all ATX-a positive samples, indicating against the primary toxin source being a benthic species not present in the surface water grabs. These possible explanations, however, must be evaluated through further exploration. Additionally, further studies using more accurate quantitative methods for direct toxin detection and quantification (i.e., HPLC-MS / GC-MS)50,51 are needed.
Additional investigation is needed to identify and isolate the specific source of the ATX-a producing cyanobacteria (although phylogenetic clustering implicates a Cuspidothrix/Aphanizomenon/Dolichospermum/Anabaena and Oscillatoria sp.) in our samples, and its current and historical distribution in Australia. Although previous studies in Australia10–13 have reported the presence of potential ATX-a producers [e.g., Cu. issatschenkoi (previously Aphanizomenon issatschenkoi)] by microscopy, their toxigenicity was not confirmed. Phylogenetic analyses using 16S rRNA, rpoC1 or Phycocyanin intergenic spacer (PC-IGS) marker genes on isolated ATX-a producing strains may provide further insights to the biogeography of ATX-a producers in Australia as has been previously studied for CYN producing R. raciborskii (see review by Wilk-Woźniak et al.9).
Materials and Methods
Sample collection and storage
A total of 226 samples representing cyanobacterial blooms were collected between November 2010 to April 2017 from surface water sources across the state of VIC, Australia (Fig. 1). Bloom samples (1 L surface grabs) were collected in polyethylene terephthalate (PETG) bottles. For microscopy, a 50 mL aliquot of the sample was preserved in Lugol’s iodine solution. All samples were dispatched and transported on ice to the laboratory within 24 hours of collection. Upon arrival, 50 mL aliquots of each bloom sample was centrifuged at 3600 × g for 15 min and pellets were stored at −20 °C for genomic DNA extraction and ELISA analysis.
Microscopy and cyanobacteria identification
Microscopic identification and enumeration of cyanobacteria was carried out on a Nikon ECLIPSE Ci-H550S light microscope at 200 to 600 times magnification. All taxa were identified to genus and species level using established cyanobacterial taxonomic keys37,38. Cell counting was performed on a calibrated Sedgewick Rafter Chamber (Pyser-SGI®, United Kingdom) that holds 1 mL of sample over an area of 50 × 20 mm. The biovolume of each taxon was calculated from the cell count data using the “Biovolume calculator”52. In circumstances where a taxon was identified to a genus or family level, the mean cell/filament volume of the largest known toxic species within that genus or family was used to calculate the biovolume.
Genomic DNA extraction and amplification of anaC gene by nested PCR
Sample enrichment and genomic DNA extraction was performed using the PowerBiofilm Kit (MoBio, USA) as described elsewhere53. Each DNA sample was eluted in 100 µL of 10 mM Tris buffer and stored at −20 °C for PCR analysis. All DNA samples were tested for sample degradation and/or for the presence of PCR inhibitors prior to anaC nested PCR assay using cyanobacteria-specific 16S rRNA primers and an internal control as described by Baker et al.53. Presence of the anaC gene (required for ATX-a biosynthesis) was assessed by nested PCR using previously published anaC primer pairs (primary PCR: anxgenF/anxgenR and secondary PCR: anaC-genF/anaC-genR)22 (Table 1) with minor modifications to their published protocols. For each step in the nested PCR assay, a 50 µL mastermix consisted of 2.5 mM MgCl2, 0.2 mM dNTP, 1X Green GoTaq® Flexi Buffer (Promega, USA), 2.5U of GoTaq® DNA Polymerase (Promega, USA) and 5% dimethyl sulfoxide (DMSO). In the primary PCR, 2 µL genomic DNA and 0.3 µM each primer was added to the mastermix and the following thermocycler protocol was used: an initial denaturation at 94 °C for 2 min followed by 30 cycles of 94 °C for 30 sec (denaturation), 52 °C for 30 sec (annealing), 72 °C for 30 sec (extension) and a final extension step at 72 °C for 5 min.
In the secondary (nested) PCR, 2 µL of PCR product generated from the first PCR reaction was added as the template to the mastermix along with 0.3 µM each of internal primer (i.e., anaC-genF and anaC-genR). The thermocycling conditions for the secondary PCR were as follows: an initial denaturation at 94 °C for 2 min followed by 30 cycles of 94 °C for 30 sec (denaturation), 58 °C for 30 sec (annealing), 72 °C for 30 sec (extension) and a final extension step at 72 °C for 5 min. All PCR amplifications were run on a BioRad T100™ thermal cycler. A known ATX-a producing Cu. issatchenkoi CY1941 was used as a positive control and a known negative ATX-a producer (Nostoc punctiforme PCC73102) and non-template control (nuclease free water) were used as negative controls for all PCR reactions [Non-template controls (NTC) from the primary PCR were used as carry-over NTC controls in the secondary PCR]. To determine the size of each amplicon and its specificity, all nested PCR products were subjected to electrophoresis on a 1.5% agarose gel at 90V for 35 min followed by visualisation of bands using a Gel Doc™ XR+ System.
Sequencing and phylogenetic analysis of anaC gene
All nested PCR amplicons were cleaned up prior to sequencing using exoSAP-IT® shrimp alkaline phosphatase. The exoSAP mastermix consisted of Exonuclease I, FastAP™ Thermosensitive Alkaline Phosphatase and 5 µL of nested PCR template. Reactions were incubated in a BioRad T100™ thermal cycler at 37 °C for 30 min followed by 85 °C for 15 min. The purified nested PCR products were then subjected to bi-directional automated sequencing (BigDye® Terminator version 3.1 chemistry, Applied Biosystems, USA) using the internal primers anaC-genF and anaC-genR. The quality of each sequence was assessed using the program Geneious® Version 10 and sequence identity was confirmed by a BLASTn search of the NCBI (National Centre for Biotechnology Information) non-redundant sequence database. Verified sequences were trimmed and aligned along with published anaC sequences from NCBI and adjusted manually using the programs MUSCLE54 and Mesquite, respectively55. An unrooted Maximum Likelihood (ML) tree based on the Tamura-Nei model56 with 1000 bootstrap replicates was constructed using the program MEGA757. The phylogenetic tree was viewed and edited for publication using Adobe Illustrator CC 2017.
Anatoxin-a analysis using ELISA
This work focused on recently collected (2016–2017) anaC-positive (n = 32) and representative negative (n = 8) samples identified by nested PCR to reduce the potential for false negative ELISA results due to the physical degradation of the cyanotoxin during storage58. Direct testing for total ATX-a (i.e., intracellular and extracellular) toxin was performed in duplicate using the ATX-a ELISA kit (Abraxis®, USA) as per the manufacturer’s protocol (available at https://www.abraxiskits.com/wp-content/uploads/2016/06/Anatoxin-a-ELISA-rev053116.pdf). Briefly, samples were freeze/thawed three times to lyse the cells. Prior to testing, the enzyme conjugate and antibody were reconstituted with 3 mL of conjugate and antibody diluent, respectively. Wash buffer (5X) and sample diluent (10X) were diluted at a ratio of 1:5 and 1:10, respectively, using deionised water before use. For each assay, 50 µL of the standard solutions (i.e., standard 0 to standard 5), control and samples were loaded into a 96-well microtitre plate, followed by the addition of 50 µL each of the reconstituted enzyme and antibody solution. After 60 minutes incubation at room temperature, plates were washed four times using 250 µL of 1X diluted wash buffer for each well and each washing step. After drying the plates, 100 µL of substrate colour solution was added to individual wells and contents were incubated for 20–30 minutes at room temperature. Following the addition of stop solution (100 µL), absorbances were read at 450 nm on a Synergy™ hybrid multi-mode microplate reader (BioTek, USA). A standard curve was then constructed by plotting the %B/B0 for each of the standards (where B is the mean absorbance value for each of the standards 1 to 5, B0 is the mean absorbance reading of standard 0) on the y-axis versus the corresponding anatoxin-a concentration on the x-axis. Finally, %B/B0 of each sample (where B is the mean absorbance value for each of the sample, B0 is the mean absorbance reading of standard 0) was interpolated on the standard curve to calculate the quantity of total ATX-a in each of the 40 samples. The measurement range for ATX-a ELISA kit stated by manufacturers is from 0.15–5 µg · L−1. An ATX-a concentration below 0.15 µg · L−1 was considered to be negative or zero µg · L−1.
Statistical analysis
Statistical analyses were computed and displayed using R software59. Welch’s two sample t-test was used to compare log-transformed ATX-a ELISA concentrations between nested PCR-positive and negative samples. Further, the relationship between nested PCR band intensities and log-transformed ELISA concentrations was compared using ANOVA followed with Tukey’s honest significant difference (HSD) test. Significance was accepted for p < 0.05.
Supplementary information
Acknowledgements
N. John’s PhD scholarship was funded by Melbourne Water and Water Research Australia (WaterRA Project 4509-14). We gratefully acknowledge Barwon Water, Southern Rural Water, South Gippsland Water, Goulburn Murray Water, Goulburn Valley Water, Coliban Water, North East Water and Australian Laboratory Services (ALS) for sample provisions. We also acknowledge Dr. Susanna A. Wood (Cawthorn Institute, New Zealand) and Dr. Barbara C. Sendall (Forensic and Scientific Services, Queensland Health, Australia) for providing positive control material. A.R. Jex acknowledges the Australian National Health and Medical Research foundation Career Development Fellowship program (APP1126395). We also acknolwedge funding from the Victorian State Government Operational Infrastructure Support and Australian Government National Health and Medical Research Council Independent Research Institute Infrastructure Support Scheme.
Author Contributions
Manuscript written by N.J. with inputs from N.D.C. and A.R.J.; experimental design by N.J., N.D.C. and A.R.J.; experiments carried out by N.J. and L.B.; data analysis by N.J., B.R.E.A., S.N., N.D.C. and A.R.J. All authors reviewed the manuscript.
Data Availability
A subset of nucleotide sequences representing anaC sequences derived from this study are available from NCBI GenBank (https://www.ncbi.nlm.nih.gov/nucleotide) under Accession Numbers MG836989 - MG836992. Additional Table S1 attached as Supplementary Information.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46945-8.
References
- 1.Chorus, I. & Bartram, J. Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management. (E &FN Spon, London and New York, 1999).
- 2.Codd GA, et al. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999;34:405–415. doi: 10.1080/09670269910001736462. [DOI] [Google Scholar]
- 3.O’Neil J, Davis TW, Burford MA, Gobler C. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae. 2012;14:313–334. doi: 10.1016/j.hal.2011.10.027. [DOI] [Google Scholar]
- 4.Paerl HW, Paul VJ. Climate change: links to global expansion of harmful cyanobacteria. Water Res. 2012;46:1349–1363. doi: 10.1016/j.watres.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Stüken A, et al. Distribution of three alien cyanobacterial species (Nostocales) in northeast Germany: Cylindrospermopsis raciborskii, Anabaena bergii and Aphanizomenon aphanizomenoides. Phycologia. 2006;45:696–703. doi: 10.2216/05-58.1. [DOI] [Google Scholar]
- 6.Kaštovský J, et al. A review of the alien and expansive species of freshwater cyanobacteria and algae in the Czech Republic. Biol. Invasions. 2010;12:3599–3625. doi: 10.1007/s10530-010-9754-3. [DOI] [Google Scholar]
- 7.Sinha R, et al. Increased incidence of Cylindrospermopsis raciborskii in temperate zones–Is climate change responsible? Water Res. 2012;46:1408–1419. doi: 10.1016/j.watres.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Sukenik A, Hadas O, Kaplan A, Quesada A. Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes–physiological, regional, and global driving forces. Front. Microbiol. 2012;3:86. doi: 10.3389/fmicb.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilk-Woźniak E, Solarz W, Najberek K, Pociecha A. Alien cyanobacteria: an unsolved part of the “expansion and evolution” jigsaw puzzle? Hydrobiologia. 2016;764:65–79. doi: 10.1007/s10750-015-2395-x. [DOI] [Google Scholar]
- 10.Baker P, Humpage A. Toxicity associated with commonly occurring cyanobacteria in surface waters of the Murray-Darling Basin, Australia. Mar. Freshwater Res. 1994;45:773–786. doi: 10.1071/MF9940773. [DOI] [Google Scholar]
- 11.Bowling L, Baker P. Major cyanobacterial bloom in the Barwon-Darling River, Australia, in 1991, and underlying limnological conditions. Mar. Freshwater Res. 1996;47:643–657. doi: 10.1071/MF9960643. [DOI] [Google Scholar]
- 12.Al-Tebrineh J, et al. Community composition, toxigenicity, and environmental conditions during a cyanobacterial bloom occurring along 1,100 kilometers of the Murray River. Appl. Environ. Microbiol. 2012;78:263–272. doi: 10.1128/AEM.05587-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths DJ, Saker ML. The Palm Island mystery disease 20 years on: a review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. 2003;18:78–93. doi: 10.1002/tox.10103. [DOI] [PubMed] [Google Scholar]
- 14.Everson S, Fabbro L, Kinnear S, Eaglesham G, Wright P. Distribution of the cyanobacterial toxins cylindrospermopsin and deoxycylindrospermopsin in a stratified lake in north-eastern New South Wales, Australia. Mar. Freshwater Res. 2009;60:25–33. doi: 10.1071/MF08115. [DOI] [Google Scholar]
- 15.Shaw Glen R., Sukenik Assaf, Livne Adi, Chiswell Robyn K., Smith Maree J., Seawright Alan A., Norris Ross L., Eaglesham Geoffrey K., Moore Michael R. Blooms of the cylindrospermopsin containing cyanobacterium, Aphanizomenon ovalisporum (Forti), in newly constructed lakes, Queensland, Australia. Environmental Toxicology. 1999;14(1):167–177. doi: 10.1002/(SICI)1522-7278(199902)14:1<167::AID-TOX22>3.0.CO;2-O. [DOI] [Google Scholar]
- 16.Humpage, A., Gaget, V., Lau, M., Froscio, S. & Laingam, S. CyanoSurvey: a national update on toxic cyanobacteria and their distribution. (Water Research Australia Limited, Adelaide, 2013).
- 17.Falconer IR. Toxic cyanobacterial bloom problems in Australian waters: risks and impacts on human health. Phycologia. 2001;40:228–233. doi: 10.2216/i0031-8884-40-3-228.1. [DOI] [Google Scholar]
- 18.Carmichael WW, Biggs DF, Gorham PR. Toxicology and pharmacological action of Anabaena flos-aquae toxin. Science. 1975;187:542–544. doi: 10.1126/science.803708. [DOI] [PubMed] [Google Scholar]
- 19.Devlin J, et al. Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44h. Can. J. Chem. 1977;55:1367–1371. doi: 10.1139/v77-189. [DOI] [Google Scholar]
- 20.Onodera H, Oshima Y, Henriksen P, Yasumoto T. Confirmation of anatoxin-a (s), in the cyanobacterium Anabaena lemmermannii, as the cause of bird kills in Danish lakes. Toxicon. 1997;35:1645–1648. doi: 10.1016/S0041-0101(97)00038-X. [DOI] [PubMed] [Google Scholar]
- 21.Harada K-I, et al. Liquid chromatography/mass spectrometric detection of anatoxin-a, a neurotoxin from cyanobacteria. Tetrahedron. 1993;49:9251–9260. doi: 10.1016/0040-4020(93)80011-H. [DOI] [Google Scholar]
- 22.Rantala-Ylinen A, et al. Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl. Environ. Microbiol. 2011;77:7271–7278. doi: 10.1128/AEM.06022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James KJ, Sherlock IR, Stack MA. Anatoxin-a in Irish freshwater and cyanobacteria, determined using a new fluorimetric liquid chromatographic method. Toxicon. 1997;35:963–971. doi: 10.1016/S0041-0101(96)00201-2. [DOI] [PubMed] [Google Scholar]
- 24.Edwards C, Beattie KA, Scrimgeour CM, Codd GA. Identification of anatoxin-a in benthic cyanobacteria (blue-green algae) and in associated dog poisonings at Loch Insh, Scotland. Toxicon. 1992;30:1165–1175. doi: 10.1016/0041-0101(92)90432-5. [DOI] [PubMed] [Google Scholar]
- 25.Méjean A, et al. Evidence that biosynthesis of the neurotoxic alkaloids anatoxin-a and homoanatoxin-a in the cyanobacterium Oscillatoria PCC 6506 occurs on a modular polyketide synthase initiated by L-proline. J. Am. Chem. Soc. 2009;131:7512–7513. doi: 10.1021/ja9024353. [DOI] [PubMed] [Google Scholar]
- 26.Cadel-Six S, et al. Different genotypes of anatoxin-producing cyanobacteria coexist in the Tarn River, France. Appl. Environ. Microbiol. 2007;73:7605–7614. doi: 10.1128/AEM.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood SA, Rasmussen JP, Holland PT, Campbell R, Crowe AL. First report of the cyanotoxin anatoxin‐a from Aphanizomenon issatschenkoi (Cyanobacteria) J. Phycol. 2007;43:356–365. doi: 10.1111/j.1529-8817.2007.00318.x. [DOI] [Google Scholar]
- 28.Ballot A, Fastner J, Lentz M, Wiedner C. First report of anatoxin-a-producing cyanobacterium Aphanizomenon issatschenkoi in northeastern Germany. Toxicon. 2010;56:964–971. doi: 10.1016/j.toxicon.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, et al. Distribution and population dynamics of potential anatoxin-a-producing cyanobacteria in Lake Dianchi, China. Harmful Algae. 2015;48:63–68. doi: 10.1016/j.hal.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Heath MW, Wood SA, Ryan KG. Spatial and temporal variability in Phormidium mats and associated anatoxin-a and homoanatoxin-a in two New Zealand rivers. Aquat. Microb. Ecol. 2011;64:69–79. doi: 10.3354/ame01516. [DOI] [Google Scholar]
- 31.Wood SA, et al. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon. 2007;50:292–301. doi: 10.1016/j.toxicon.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Shams S, et al. Anatoxin-a producing Tychonema (cyanobacteria) in European waterbodies. Water Res. 2015;69:68–79. doi: 10.1016/j.watres.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Fastner J, et al. Fatal Neurotoxicosis in Dogs Associated with Tychoplanktic, Anatoxin-a Producing Tychonema sp. in Mesotrophic Lake Tegel, Berlin. Toxins (Basel) 2018;10:60. doi: 10.3390/toxins10020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford A, et al. Use of three monitoring approaches to manage a major Chrysosporum ovalisporum bloom in the Murray River, Australia, 2016. Environ. Monit. Assess. 2017;189:202. doi: 10.1007/s10661-017-5916-4. [DOI] [PubMed] [Google Scholar]
- 35.NHMRC, NRMMC. Australian drinking water guidelines paper 6 national water quality management strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra (2011).
- 36.Méjean A, Paci G, Gautier V, Ploux O. Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon. 2014;91:15–22. doi: 10.1016/j.toxicon.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 37.McGregor, G. B. & Fabbro, L. D. A Guide to the Identification of Australian Freshwater Planktonic Chroococcales (Cyanoprokaryota-Cyanobacteria). (Cooperative Research Centre for Freshwater Ecology, Thrugoona, NSW, 2001).
- 38.Baker, P. D. & Fabbro, L. D. A guide to the identification of common blue-green algae (Cyanoprokaryotes) in Australian freshwaters. (Cooperative Research Centre for Freshwater Ecology, Thrugoona, NSW, 2002).
- 39.Moreira C, Ramos V, Azevedo J, Vasconcelos V. Methods to detect cyanobacteria and their toxins in the environment. Appl. Microbiol. Biotechnol. 2014;98:8073–8082. doi: 10.1007/s00253-014-5951-9. [DOI] [PubMed] [Google Scholar]
- 40.Cadel-Six S, et al. Identification of a polyketide synthase coding sequence specific for anatoxin-a-producing Oscillatoria cyanobacteria. Appl. Environ. Microbiol. 2009;75:4909–4912. doi: 10.1128/AEM.02478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y, Song G, Pan Q, Yang Y, Li R. Identification of genes for anatoxin-a biosynthesis in Cuspidothrix issatschenkoi. Harmful Algae. 2015;46:43–48. doi: 10.1016/j.hal.2015.05.005. [DOI] [Google Scholar]
- 42.Legrand B, Lesobre J, Colombet J, Latour D, Sabart M. Molecular tools to detect anatoxin-a genes in aquatic ecosystems: Toward a new nested PCR-based method. Harmful Algae. 2016;58:16–22. doi: 10.1016/j.hal.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Gugger M, et al. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon. 2005;45:919–928. doi: 10.1016/j.toxicon.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 44.Sabart M, et al. Co-occurrence of microcystin and anatoxin-a in the freshwater lake Aydat (France): Analytical and molecular approaches during a three-year survey. Harmful Algae. 2015;48:12–20. doi: 10.1016/j.hal.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Namikoshi M, et al. Simultaneous production of homoanatoxin-a, anatoxin-a, and a new non-toxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja. Toxicon. 2003;42:533–538. doi: 10.1016/S0041-0101(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 46.Hodoki Y, et al. Anatoxin-a-producing Raphidiopsis mediterranea Skuja var. grandis Hill is one ecotype of non-heterocytous Cuspidothrix issatschenkoi (Usačev) Rajaniemi et al. in Japanese lakes. Harmful Algae. 2013;21:44–53. doi: 10.1016/j.hal.2012.11.007. [DOI] [Google Scholar]
- 47.Chorus, I. Current approaches to cyanotoxin risk assessment, risk management and regulations in different countries. (Fed. Environmental Agency, Germany, 2012).
- 48.Wood SA, Puddick J. The Abundance of Toxic Genotypes Is a Key Contributor to Anatoxin Variability in Phormidium-Dominated Benthic Mats. Mar. Drugs. 2017;15:307. doi: 10.3390/md15100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantoral Uriza EA, Asencio AD, Aboal M. Are We Underestimating Benthic Cyanotoxins? Extensive Sampling Results from Spain. Toxins (Basel) 2017;9:385. doi: 10.3390/toxins9120385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng X, Liang G, Chen J, Qi M, Xie P. Simultaneous determination of eight common odors in natural water body using automatic purge and trap coupled to gas chromatography with mass spectrometry. J. Chromatogr. 2011;1218:3791–3798. doi: 10.1016/j.chroma.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 51.Dahlmann J, Budakowski WR, Luckas B. Liquid chromatography–electrospray ionisation-mass spectrometry based method for the simultaneous determination of algal and cyanobacterial toxins in phytoplankton from marine waters and lakes followed by tentative structural elucidation of microcystins. J. Chromatogr. 2003;994:45–57. doi: 10.1016/S0021-9673(03)00485-0. [DOI] [PubMed] [Google Scholar]
- 52.Victorian Department of Sustainibility and Environment. Bio-volume calculator, https://www.dse.vic.gov.au/dse/index.htm (2007).
- 53.Baker L, et al. Rapid, multiplex-tandem PCR assay for automated detection and differentiation of toxigenic cyanobacterial blooms. Mol. Cell. Probes. 2013;27:208–214. doi: 10.1016/j.mcp.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maddison WP. Mesquite: a modular system for evolutionary analysis. Evolution. 2008;62:1103–1118. doi: 10.1111/j.1558-5646.2008.00349.x. [DOI] [PubMed] [Google Scholar]
- 56.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 57.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamp L, Church JL, Carpino J, Faltin-Mara E, Rubio F. The effects of water sample treatment, preparation, and storage prior to cyanotoxin analysis for cylindrospermopsin, microcystin and saxitoxin. Chem. Biol. Interact. 2016;246:45–51. doi: 10.1016/j.cbi.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 59.R Core Team. R: A language and environment for statistical computing, http://www.R-project.org/ (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A subset of nucleotide sequences representing anaC sequences derived from this study are available from NCBI GenBank (https://www.ncbi.nlm.nih.gov/nucleotide) under Accession Numbers MG836989 - MG836992. Additional Table S1 attached as Supplementary Information.