Abstract
The aims of this study were to establish the role of MC4Rrs17782313 and ENPP1rs1044498 gene polymorphisms on pre-pregnancy BMI and the newborn’s status. We performed a cross-sectional study on 185 mothers and their offspring. The groups were divided into: control group- underweight or normal mothers with BMIinitial < 25 kg/m2 (n1 = 134) and study group-overweight/obese mothers with BMIinitial ≥ 25 kg/m2 (n2 = 51). All subjects underwent demographic, anthropometric, paraclinical, bioimpedance and genetic parameters. We found association between initial BMI and gestational weight gain (GWG), and a higher frequency of excessive GWG in overweight/obese women (p = 0.037). Higher values of anthropometric and bioimpedance parameters were observed in overweight/obese versus underweight/normal women. The MC4R rs17782313 and ENPP1 rs1044498 variant genotypes had an increased risk of pre-pregnancy overweight (OR = 1.41; 95% CI:[0.72; 2.78]; OR = 1.34; 95% CI:[0.65; 2.75]). The newborns from mothers with excessive GWG had a higher birth weight (BW) (p = 0.001). Higher MUAC values were noticed in newborns with MC4R rs17782313 wild-type genotype. Also, BW was correlated with GWG status smoking in pregnancy, gestational age and neonatal ENPP1rs1044498 variant genotype (p = 0.026). Our study pointed out the role of MC4R rs17782313 and ENPP1 rs1044498 genotypes in obesity determinisms in mothers and their newborns in correlation with BMI, MUAC, TST and bioimpedance parameters.
Subject terms: Paediatric research, Predictive markers
Introduction
Gestational weight gain (GWG) is an essential parameter of birth-related outcomes. Thus, the Institute of Medicine (IOM)1 defined the following recommendations regarding GWG: for underweight women (Body Mass Index - BMI < 18.5 kg/m2), recommended GWG 12.5–18 kg; normal weight women (BMI = 18.5–24.9 kg/m2), recommended GWG 11.5–16 kg; overweight women (BMI = 25–29.9 kg/m2), recommended GWG 7.00–11.5 kg; obese women BMI > 30 kg/m2, recommended GWG 5–9 kg15. Excessive gestational weight gain (GWG) in pregnant women is associated with diabetes, obesity, and dystocia2,3, but also modifications of neonatal birth weight (BW) with afterwards consequences on their evolution, such as macrosomia and obesity, and a wide range of other complications, like cardiovascular diseases, muscular and skeletal impairment, type 2 diabetes mellitus, and even psychological consequences4. World Health Organization data stated that according to the European Childhood Obesity Surveillance Initiative study, the incidence of increased weight among children from Romania is 26.8%, whereas that of obesity is 11.6%, being almost the highest incidence from all countries included in the study5. BW is another important parameter with impact on the newborn’s wellbeing4.
There are multiple genes involved in the determinism of obesity like: Leptin receptor (LEPR)6, Fat mass and obesity-associated gene (FTO)7,8, Single-minded homolog (SIM1) and Propiomelanocortin (POMC)9, Src homology 2B (SH2B) Adaptor Protein 1 gene, Peroxisome proliferator-activated receptor gamma gene (PPAR-γ)10,11, IL-6 572 (C > G, 190 C > T, and 174 G > C gene polymorphisms)12,13, angiotensin converting enzyme (ACE I/D), but also Tumor necrosis factor (TNF) alfa 308 G > A14.
Recently, other genes that are thought to have an impact in the development of obesity were discovered, such as: melanocortin receptor (MCR) MC4R, MC3R, MC2R, and also nucleotide pyrophosphatase/phosphodiesterase (ENPP1). The variant rs17782313 of MC4R is associated with obesity in both children and adults, regulating the control of energetic balance15. The ENPP1 gene inhibits the sensitivity of insulin receptor, being considered a gene with a potential role in determining insulin-resistance and type 2 diabetes mellitus16.
Therefore, the aims of this study were to establish (i) the association between both MC4R rs17782313 and ENPP1 rs1044498 gene polymorphisms and pre-pregnancy BMI and (ii) the impact of these 2 gene polymorphisms on excessive, respectively insufficient gestational weight gain, but also (iii) to establish if the MC4R rs17782313 and ENPP1 rs1044498 single nucleotide polymorphisms (SNPs) were multivariate predictors correlated with birth weight, neonatal mid-upper-arm circumference and tricipital skinfold thickness in newborns.
Results
The demographic description of the studied groups regarding anthropometric, clinical, paraclinical and bioimpedance characteristics
Of all 225 mother-newborn couples that were examined, after applying the exclusion criteria, only 185 remained in the present study.
The mean age of mothers included in the study was 28.10 ± 5.80 years, over 50% of them having superior level education, 22.2% with education level <8 years, 17.8% with educational level between 9 and 12 years, and only 5.9% had never frequented school.
Most of the mothers included in our study were primipara (56.2%), with a GWG mean value of 17.1 ± 6.2 kg. Of all 185 pregnant women 11.4% were smokers, 5.90% presented preeclampsia and only 2.7% were diagnosed with gestational diabetes (Table 1).
Table 1.
Comparison of maternal and neonatal characteristics and initial BMI.
| Study sample n = 185 (%) | Underweight/normal (Initial BMI < 25 kg/m2) n1 = 134 (%) | Overweight/obesity (Initial BMI ≥ 25 kg/m2) n2 = 51 (%) | p-value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age (years) | 28.1 ± 5.8 | 27.5 ± 5.7 | 29.7 ± 5.8 | 0.017* |
| Education | ||||
| 0 years | 11 (5.9) | 7 (5.2) | 4 (7.8) | 0.338 |
| ≤8 years | 41 (22.2) | 33 (24.6) | 8 (15.7) | |
| 9–12 years | 33 (17.8) | 26 (19.4) | 7 (13.7) | |
| >12 years | 100 (54.1) | 68 (50.7) | 32 (2.7) | |
| Parity | ||||
| 0 | 104 (56.2) | 76 (56.7) | 28 (54.9) | 0.824 |
| ≥1 | 81 (43.8) | 58 (43.3) | 23 (45.1) | |
| GWG (kg) | 17.1 ± 6.2 | 17.5 ± 6.2 | 16.0 ± 6.0 | 0.137 |
| Status of GWG [n (%)] | ||||
| Insufficient | 27 (14.6) | 25 (18.7) | 2 (3.9) | 0.037* |
| Normal | 42 (22.7) | 30 (22.4) | 12(23.5) | |
| Excessive | 116 (62.7) | 79 (59.0) | 37 (72.5) | |
| Smoking | 0.067 | |||
| No | 164 (88.6) | 115 (85.8) | 49 (96.1) | |
| Yes | 21 (11.4) | 19 (14.2) | 2 (3.9) | |
| Hypertension in pregnancy | ||||
| No | 174 (94.1) | 127 (94.8) | 47 (92.2) | 0.499 |
| Yes | 11 (5.9) | 11 (5.2) | 11 (7.8) | |
| Diabetes | 0.021* | |||
| No | 180 (97.3) | 133 (99.3) | 47 (92.2) | |
| Yes | 5 (2.7) | 1 (0.7) | 4 (7.8) | |
| Maternal bioimpedance parameters | ||||
| MUAC (cm) | 29.0[27.0; 32.0] | 28.0[26.0; 30.0] | 30.5[33.0; 35.5] | <0.001* |
| TST (mm) | 18.0[13.0; 23.0] | 15.6[11.0; 21.0] | 24.0[18.5; 29.0] | <0.001* |
| FM (kg) | 22.0[18.0; 28.0] | 20.0[16.0; 23.1] | 32.0[26.0; 38.0] | <0.001* |
| MM (kg) | 46.0[42.0; 50.9] | 44.0[41.0; 48.0] | 51.0[48.0; 54.9] | <0.001* |
| BM (kg) | 2.0[2.0; 3.0] | 2.0[2.0; 2.7] | 3.0[2.9; 3.0] | <0.001* |
| TBW (kg) | 34.0[32.0; 38.0] | 33.0[30.5; 36.0] | 39.0[36.0; 41.8] | <0.001* |
| BMR (kcal) | 1476.0[1387.0; 1644.0] | 1430.0[1363.0; 1541.0] | 1658.0[1568.5; 1776.5] | <0.001* |
| Paraclinical parameters | ||||
| ALAT (u/l) | 11.0[8.0; 16.0] | 11.5[8.0; 16.0] | 11.0[8.0; 16.0] | 0.796 |
| ASAT (u/l) | 22.0[18.0; 30.0] | 23.0[19.0; 31.0] | 22.0[17.0; 27.0] | 0.333 |
| Chol total(mg/dl) | 212.0[194.0; 240.0] | 211.0[194.0; 239.0] | 222.0[196.0; 244.0] | 0.381 |
| HDL-chol (mg/dl) | 59.0[50.0; 69.0] | 59.0[51.0; 69.0] | 60.0[48.0; 69.5] | 0.920 |
| LDL-chol (mg/dl) | 134[113.0; 159.0] | 134.0[113.0; 162.0] | 135.0[114.5; 153.0] | 0.942 |
| TG (mg/dl) | 200[169.0; 239.0] | 191.5[158.0; 237.0] | 223.0[187.0; 257.0] | 0.049* |
| Newborn characteristics | ||||
| BW (kg) | 3.4 ± 0.5 | 3.3 ± 0.5 | 3.5 ± 0.5 | 0.094 |
| Apgar Score_1min | 10[2; 10] | 10[2; 10] | 10[3; 10] | 0.048* |
| Height (cm) | 53.8 ± 2.6 | 53.8 ± 2.6 | 53.8 ± 2.5 | 0.939 |
| BMI(kg/m2) | 11.7 ± 1.1 | 11.5 ± 1.0 | 12.0 ± 1.2 | 0.003* |
| MUAC (cm) | 11.0[10.0; 12.0] | 10.3[10.0; 11.0] | 11.0[10.0; 12.0] | 0.059 |
| TST (mm) | 3.0[2.0; 3.0] | 3.0[2.0; 3.0] | 3.0[2.2; 3.0] | 0.988 |
| ALAT(u/l) | 9.4[7.4; 12.0] | 9.5[7.4; 11.8] | 8.9[7.3; 12.4] | 0.773 |
| ASAT(u/l) | 29.1[23.9; 36.4] | 29.3[23.7; 36.9] | 28.4[25.2; 34.9] | 0.713 |
| Chol (mg/dl) | 82.1[58.9; 166.0] | 97.4[61.5; 166.6] | 72.7[53.9; 159.8] | 0.148 |
| HDL- chol (mg/dl) | 38.1[25.3; 52.2] | 39.5[27.3; 53.2] | 32.0[24.5; 47.6] | 0.118 |
| LDL - chol (mg/dl) | 41.1[24.0; 99.9] | 49.2[25.6; 99.7] | 32.2[19.4; 97.7] | 0.100 |
| TG (mg/dl) | 51.6[30.7; 129.6] | 52.5[30.7; 132.5] | 47.1[31.5; 122.5] | 0.849 |
Legend: ALAT: alanine aminotransferase, ASAT: aspartate aminotransferase, BM: Bone mass, BMI: Body mass index, BMR: Basal metabolism rate, BW: birth weight, Chol: cholesterol, FM: Fat mass, GWG: gestational weight gain, HDL-chol: high density lipoprotein – cholesterol; LDL-chol: low density lipoprotein-cholesterol; MM: Muscle mass, MUAC: Middle upper arm circumference, n - absolute number, SD - standard deviation, TBW: Total body water, TG: triglycerides, TST: Tricipital skinfold thickness.
Data expressed as mean ± standard deviation [percentile 25%; percentile 75%]; descriptive statistics for Apgar score were presented as median [minimum; maximum]; p-values obtained from Student-t test for independent samples or Mann-Whitney test or Chi-square test; *statistical significance (p < 0.05).
The comparison between maternal and neonatal characteristics reported to the body mass index at the beginning of the pregnancy is presented in Table 1. Thus, we found a significant association between initial BMI and maternal age (MAge) (p < 0.05), the overweight/obese mothers having a median age higher than those included in the control group.
We encountered a significant association between initial BMI and GWG, the frequency of excessive GWG being higher in overweight or obese women (72.5% versus 59%; p = 0.037) as compared to control group. Moreover, the incidence of diabetes mellitus was higher in obese mothers (7.8% versus 0.7%; p = 0.021).
Regarding anthropometric and bioelectrical impedance analysis (BIA) parameters, we found a significant difference between overweight/obese and underweight/normal weight women, identifying higher values of these parameters in overweight/obese women (Table 1).
Among the newborns’ characteristics, only BMI was significantly different in the newborns of overweight/obese mothers versus underweight/normal weight mothers (12.0 ± 1.0 versus 11.5 ± 1.2, p = 0.003).
Gestational weight gain status and maternal gene polymorphisms
We found no statistically significant associations between MC4R rs17782313 variant genotype and increased weight/obesity before pregnancy (Table 2). After adjusting for maternal covariates as MAge, education level and parity, the presence of MC4R rs17782313 variant genotype (CT + CC) was associated with an increased risk of pre-pregnancy increased weight (adjusted OR = 1.41; 95% CI:[0.72; 2.78]). The same results were obtained for ENPP1 rs1044498 (adjusted OR = 1.34; 95% CI:[0.65; 2.75]).
Table 2.
Associations between MC4R rs17782313 and ENPP1 rs1044498 SNPs and GWG status.
| Study sample n(%) | Underweight/normal n(%) | Overweight/obesity n(%) | p+ | Insufficient GWG n(%) | Normal GWG n(%) | Excessive GWG n (%) | p+ | |
|---|---|---|---|---|---|---|---|---|
| MC4R rs17782313 Maternal SNP | ||||||||
| TT | 106 (57.3) | 81 (60.4) | 25 (49.0) | 0.185 | 17 (63.0) | 25 (59.5) | 64 (55.2) | 0.721 |
| CT + CC | 79 (42.7) | 53 (39.6) | 26 (51.0) | 10 (37.0) | 17 (40.5) | 52 (44.8) | ||
| T-allele | 285 (77.0) | 212 (79.1) | 73 (71.6) | 0.124 | 41 (75.9) | 67 (79.8) | 177 (76.3) | 0.793 |
| C-allele | 85 (23.0) | 56 (20.9) | 29 (28.4) | 13 (24.1) | 17 (20.2) | 55 (23.7) | ||
| p-value for HW(b | 0.118 | 0.136 | 0.439 | 0.131 | 0.100 | 0.071 | ||
| ENPP1 rs1044498 Maternal SNP | ||||||||
| AA | 129 (69.7) | 95 (70.9) | 34 (66.7) | 0.576 | 19 (70.4) | 28 (66.7) | 82 (70.7) | 0.886 |
| AC + CC | 56 (30.3) | 39 (29.1) | 17 (33.3) | 8 (29.6) | 14 (33.3) | 34 (29.3) | ||
| A-allele | 310 (83.8) | 226 (84.3) | 84 (82.4) | 0.645 | 46(85.2) | 68 (80.9) | 196 (84.5) | 0.166 |
| C-allele | 60 (16.2) | 42 (15.7) | 18 (17.6) | 16 (29.6) | 16 (19.1) | 36 (15.5) | ||
| p-value for HW | 0.640 | 0.849 | 0.571 | 0.366 | 0.634 | 0.574 | ||
Legend: GWG = Gestational weight gain; (bHardy-Weinberg equilibrium; +p-values obtained from Chi-square or Fisher’s exact tests.
ENPP1rs1044498 A > C: nucleotide pyrophosphatase/phosphodiesterase gene polymorphisms (AA = reference category); AA – homozygous for A allele; AC – heterozygous; CC - homozygous for C allele;
MC4R rs17782313 T > C: melanocortin- 4 receptor gene polymorphisms (TT = reference category); CC - homozygous for C allele; CT - heterozygous; TT- homozygous for T allele.
We found no significant associations between the variant genotype of MC4R rs17782313 and GWG status (Table 2). After controlling for maternal covariates such as MAge, educational level, parity, smoking during pregnancy, the presence of MC4R rs17782313 variant genotype (CT + CC) was not significantly associated with an increased risk of excessive GWG (adjusted OR = 1.16; 95% CI: [0.54; 2.48]) or insufficient GWG (adjusted OR = 0.96; 95% CI:[0.30; 3.06]). The same results were obtained for ENPP1 rs1044498 SNP (excessive GWG: adjusted OR = 0.77; 95% CI: [0.34; 1.71] and insufficient GWG: adjusted OR = 0.89, 95% CI: [0.28; 2.84]).
The studied genotype frequencies of the two gene polymorphisms were consistent with Hardy-Weinberg equilibrium in the studied sample and GWG status (p > 0.05).
Neonatal gene polymorphisms correlated with birth weight, mid-upper-arm circumference and tricipital skinfold thickness
In the studied sample (n = 185 newborns), the mean BW was 3368 g ± 448, with 9.7% (18 cases) above 4000 grams. We found no significant difference between the means of BW in newborns whose mothers had an initial BMI ≥25 kg/m2 (overweight/obese mothers) in comparison to those from mothers with an initial BMI <25 kg/m2. BW, middle upper arm circumference (MUAC) and tricipital skinfold thickness (TST) were significantly associated with maternal GWG status (one-way ANOVA, F(2; 182) = 8.19, p < 0.001). In the post-test analysis, the mean BW was significantly different in newborns whose mothers had insufficient or normal GWG in comparison to those from mothers with excessive GWG (Tukey test, p = 0.001). Therefore, the mothers with excessive GWG had newborns with higher BW (Table 3).
Table 3.
Relationship between studied gene polymorphisms and neonatal anthropometric bioimpedance characteristics.
| Factors | Birth weight (kg) | MUAC (cm) | TST (cm) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | p+ | Median[Q1; Q3] | p+ | Median[Q1; Q3] | p+ | |
| BMI initial (kg/m 2 ) | ||||||
| <25 | 3.33 ± 0.45 | 0.094 | 10.5[10.0; 11.3] | 0.075 | 2.8[2.3; 3.4] | 0.912 |
| ≥25 | 3.46 ± 0.45 | 11.0[10.5; 11.5] | 2.9[2.4; 3.2] | |||
| GWG status | ||||||
| Insufficient | 3.11 ± 0.49 | <0.001 | 10.3[9.7; 10.8] | 0.005 | 2.3[2.0; 2.8] | <0.001 |
| Normal | 3.28 ± 0.43 | 10.5[10.2; 11.4] | 2.8[2.4; 3.5] | |||
| Excessive | 3.46 ± 0.42 | 11.0[10.5; 11.5] | 2.3[2.0; 2.8] | |||
| MC4R rs17782313 Maternal SNP | ||||||
| TT | 3.37 ± 0.43 | 0.892 | 11.0[10.3; 11.5] | 0.149 | 2.9[2.3; 3.4] | 0.519 |
| CT + CC | 3.36 ± 0.48 | 10.5[10.0; 11.5] | 2.8[2.4; 3.5] | |||
| ENPP1 rs1044498 Maternal SNP | ||||||
| AA | 3.35 ± 0.43 | 0.333 | 10.5[10.0; 11.3] | 0.085 | 2.8[2.3; 3.2] | 0.208 |
| AC + CC | 3.42 ± 0.49 | 11.0[10.3; 11.5] | 3.0 [2.4; 3.4] | |||
| MC4R rs17782313 Neonates SNP | ||||||
| TT | 3.40 ± 0.45 | 0.250 | 11.0[10.3; 11.5] | 0.035 | 2.8[2.3; 3.2] | 0.346 |
| CT + CC | 3.32 ± 0.48 | 10.5[10.0; 11.2] | 3.0 [2.4; 3.4] | |||
| ENPP1 rs1044498 Neonates SNP | ||||||
| AA | 3.35 ± 0.42 | 0.538 | 10.5[10.0; 11.4] | 0.105 | 2.8[2.3; 3.2] | 0.123 |
| AC + CC | 3.40 ± 0.45 | 11.0[10.3; 11.5] | 3.0 [2.3; 3.5] | |||
Legend: SD = standard deviation; Q1 = first quartile; Q3 = third quartile; +p-values obtained from Student-t test for independent samples, ANOVA test or nonparametric test (as Mann-Whitney or Kruskal-Wallis).
BMI: Body mass index, BW: birth weight, MUAC: Middle upper arm circumference, SD: standard deviation, TST: Tricipital skinfold thickness.
ENPP1 rs1044498 A > C: nucleotide pyrophosphatase/phosphodiesterase gene polymorphisms (AA = reference category); AA – homozygous for A allele; AC – heterozygous; CC - homozygous for C allele;
MC4R rs17782313 T > C: melanocortin- 4 receptor gene polymorphisms (TT = reference category); CC - homozygous for C allele; CT - heterozygous; TT- homozygous for T allele.
Our study showed a significant difference regarding the distribution of MUAC values in newborns with MC4R rs17782313 variant genotype in comparison to those with normal genotype, higher values being noticed in the newborns with normal genotype (Table 3).
As a result of a linear multiple regression analysis, BW was significantly positively correlated with GWG status (p = 0.007), gestational age (GA) (p < 0.001) and neonatal ENPP1 rs1044498 variant genotype (p = 0.026), while negatively correlated with smoking during pregnancy (p < 0.001). BW was positively correlated with excessive GWG status. Thus, for each unit increment in GWG status, BW increased by 113 g. The presence of neonatal variant ENPP1 SNP was correlated with an increase in BW by 150 g. Moreover, an increase in BW by 132.1 g was observed per gestational week. The effect of GWG status, neonatal ENPP1 rs1044498 and MC4R rs17782313 variant genotypes, adjusted according to important covariates (as mother’s age, educational level, smoking during pregnancy and gestational age at delivery) was illustrated in Fig. 1. Neonatal MUAC was significantly correlated with GWG status (p = 0.015), smoking in pregnancy (p = 0.024) and gestational age (GA) (p = 0.001). There was a tendency towards statistical association between neonatal ENPP1 rs1044498 variant genotype and MUAC (p = 0.092). Regarding neonatal TST there was a significant correlation with GWG status (p = 0.001), and neonatal ENPP1 rs1044498 variant genotype and MUAC (p = 0.045), the variant genotype being correlated with an increment in TST.
Figure 1.
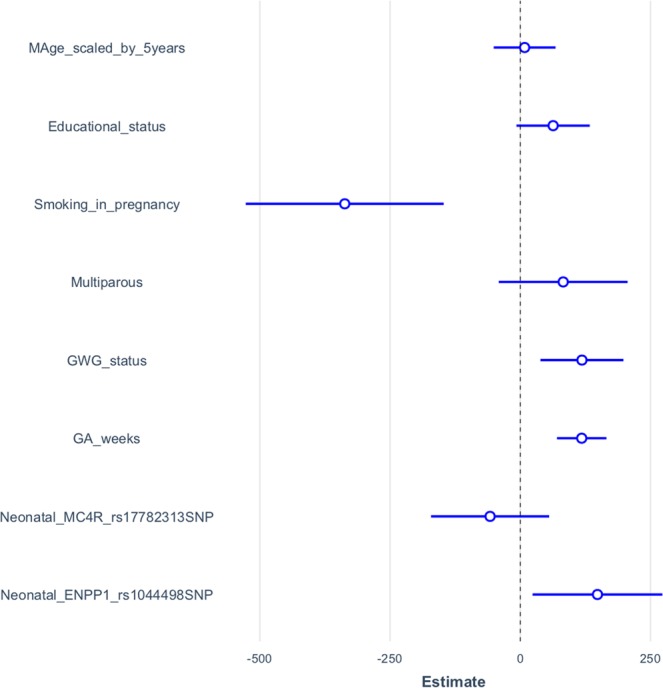
The effects of neonatal MC4Rrs17782313 and ENPP1rs1044498 SNPs on birth weight of full-term newborns adjusted for GWG status and other covariates - unstandardized regression beta coefficients with their confidence intervals (95% CI). Legend: Neonatal MC4Rrs17782313 and ENPP1rs1044498 SNPs were regarded as dichotomous predictors in multiple linear regression and coded as 1 = variant genotype, 0 = wild genotype; also for Multiparous (coded as 1 = yes, 0 = nulliparous) and Smoking in pregnancy (coded as 1 = yes; 0 = no) while Educational status (coded as 0 = 0 years of education; 1 = under 8 years of education; 2 = between 9 and 12 years of education; 3 = more than 12 years of education) and GWG status (0 = insufficient GWG; 1 = normal GWG; 2 = excessive GWG) were regarded as ordinal variables.
Discussions
Predictors for maternal weight gain
The studies reported in literature proved that maternal weight at the beginning of the pregnancy and GWG are important parameters that might result in diabetes mellitus, obesity and poor birth outcomes, with different obstetrical complications2,3. In addition, Farah et al. proved the fact that an excessive GWG leads to an increased neonatal BW with a subsequent risk of faster weight gain and even childhood obesity, but also other complications4. Similarly, in our study, BW was higher in newborns from overweight/obese women (3.5 ± 0.5) compared to underweight/normal weight group (3.3 ± 0.5).
Excessive GWG increases the risk for hypertension17, hypertriglyceridemia, obesity, type 2 diabetes mellitus, insulin resistance18, and is associated with increased BMI, TST, and waist circumference. Similarly, in our study, we noticed that excessive GWG and gestational diabetes incidence were higher in overweight or obese women (p = 0.037/p = 0.021).
Gallagher et al. proved that mothers with increased weight or obesity presented higher GWG and their newborns had a higher BW, but also a greater fat-free mass (FFM) evaluated by BIA19. Farah et al.4 and Butte et al.20 noticed a direct relationship between FFM and fat mass (FM) for GA under 37 weeks, with accurate predictions on BW. In our study, all anthropometric (MUAC, TST) and BIA (FM, MM, bone mass - BM, total body water - TBW and also basal metabolism rate - BMR) measurements were significantly higher in overweight/obese women than in underweight/normal weight women (p < 0.0001), similar to another study of the same authors21. Even though other studies22 proved that BW was correlated with total body water and FFM, but not with FM, in our study we noticed that BW was correlated with both FFM and FM.
There are studies proving that BW is correlated with GA at delivery4,21, but our study failed in proving this correlation. Contradictory data regarding the correlation between BW and smoking were reported by different studies. Thus, Farah et al. found a direct relationship between BW and smoking, and parity4, respectively, while Mărginean et al. in a previous study21 pointed out a reverse relationship between these parameters. The present study also underlined the negative impact of smoking during pregnancy on the newborn’s BW. Even though certain studies11,21 established a correlation between arterial hypertension (AHT) and higher GWG, in our study we did not notice this fact, probably due to the small number of cases with AHT.
Among the multiple MCR genes that proved to be involved in the etiology of obesity, MC4R is associated with monogenic obesity, regardless of age15. Therefore, the MC4R rs17782313 gene polymorphisms was associated in certain studies with obesity in both adults and children15,23. Thus, Bordoni et al.24 observed that the C/C genotype of MC4R rs17782313 gene polymorphism was associated with higher BMI and obesity risk in young Italian population. Similarly, Loos et al. emphasized that the C allele of the same gene was associated with an increased risk for developing obesity15, while Garcia-Solis et al. underlined a positive correlation between FTO rs9939609 homozygotes and MC4R rs17782313 heterozygotes and increased risk for both obesity and high blood pressure values in Mexican school-aged children25. Lazopoulou et al.26 proved that MC4R rs17782313 C allele was associated with a higher BMI and BW, but also that three or more high-risk alleles of the combined FTO and MC4R genotypes result in a 4 folds increased risk for obesity in Greek children and teenagers. Wu et al. noticed on Chinese children an association between MC4R rs17782313 SNP and adiponectin27.
Also, Mejía-Benítez et al.28 found associations between obesity risk, BMI and several SNPs, among which we recall MC4R rs17782313 and ENPP1 rs7754561. Contrariwise, Albuquerque et al.29, Martins et al.30, similar to our study, identified no significant associations between MC4R rs17782313 SNP and GWG, maternal body weight, or other characteristic parameters for obesity.
ENPP1 gene polymorphisms are associated with obesity in children, inhibiting insulin receptors and resulting in a higher incidence of type 2 diabetes mellitus and a lower tolerance for glucose in carriers of ENPP1 K121Q (rs1044498) and A/G_1044TGA haplotypes31,32. Contrariwise, Morandi et al. stated that the Q121 variant allele of ENPP1 K121Q (rs1044498) SNP presents a protective role for obesity in a study performed on 453 Italian children33. Other authors also underlined the relationship between the Q allele of ENPP1 K121Q (rs1044498) SNP and type 2 DM or obesity31,34. In contradiction to the afore-mentioned studies, we found no correlations between ENPP1 rs1044498 variant genotype and GWG status. Our findings are similar to those of Lyon et al.35 who did not notice any correlation between this SNP and obesity or diabetes mellitus.
Predictors for newborn’s birth weight
One of the most important predictors for neonatal BW was proved to be excessive GWG. Therefore, the study of Ferrari et al. proved the fact that newborns coming from mothers with excessive GWG presented a higher BW in comparison to those whose mothers presented normal GWG36. The same study also underlined that the chances for these newborns to be macrosomic were 50% higher. Similarly, in our study, BW, MUAC and TST were correlated with GWG (p < 0.001), meaning that the newborns that came from mothers with excessive GWG had a higher weight than those from mothers with insufficient or normal GWG (p = 0.001). Some studies emphasized that newborns with higher BW are more predisposed to develop obesity and increased weight, but also more prone to cardiovascular and metabolic complications37,38. Previous studies performed by Farah et al.4 and Marginean et al.21 pointed out no correlation between initial BMI of the pregnant women and BW, similarly to the present study in which despite the fact that BW was with 9.7% higher in newborns from mothers with initial BMI ≥25 kg/m2 versus normal BMI, we found no significant difference between the two groups.
The studies of Bordoni et al.24 and Loos et al.15 pointed out the association between the variant allele C of MC4R rs17782313 and both increased BMI and obesity risk in teenagers. Also, the study of Lazopoulou et al.26 proved that BW and BMI were associated with the C allele of MC4R rs17782313, while Garcia-Solis et al.25 established a significant correlation between heterozygous MC4R rs17782313 carriers and both blood pressure and obesity risk in Mexican school-aged children25. Moreover, similar findings were underlined by other studies performed on Chinese people revealing a correlation between the MC4R rs17782313 SNP and adiponectin in Chinese obese children27. On the contrary, in our study, we noticed an association of the wild-type MC4R rs17782313 gene polymorphism and higher MUAC values in newborns.
It is well-documented that ENPP1 gene is associated with obesity in pediatric patients resulting in an increased risk for glucose intolerance and type 2 diabetes mellitus31. Similarly, recent studies32,39 proved that the variant allele of ENPP1 rs104449 increases the risk for obesity and type 2 diabetes mellitus, but no study established the role of this SNP in the newborn’s BW and pregnant woman’s nutritional status. On the other hand, Morandi et al. noticed that variant ENPP1 rs104449 owns a protective role for developing obesity33. In exchange, our study proved a positive association between the same variant gene and GWG status, GA and an increase of 150 g in the mean value of the newborn’s BW. Moreover, a negative association was noticed regarding smoking during pregnancy and BW. Also, neonatal MUAC and TST were correlated with GWG and neonatal variant ENPP1 rs1044498.
Strengths and limitations of our study
The strengths of this study are the uniform population taken into consideration, the assessment of increased weight risk in mother-newborn couples, evaluating obesity risk in newborns and their correlations with other BIA and anthropometric parameters, and also evaluating the role of these genes in the determinism of BW. Some of the limitations of our study consist in the small number of mothers and newborns, the lack of assessment of other cytokines involved in the determinism of obesity, the type of diet, or the lack of BIA measurements in newborns.
There are a few data in literature that are meant to establish the role of these polymorphisms in the determinism of BW, therefore, we consider this study as a pilot one concerning the determinism of MC4R rs17782313 and ENPP1 rs1044498 variant genotypes on GWG status and also on the mother-newborn couple.
Conclusions
In our study we found a significant association between initial BMI and GWG, the frequency of excessive GWG being higher in overweight or obese women. The MC4R rs17782313 and ENPP1 rs1044498 variant genotypes presented an increased risk in pre-pregnancy increased weight. Also, BW, MUAC and TST were significantly associated with GWG status. Higher MUAC values were noticed in newborns with wild-type MC4R rs17782313. The results of multivariate analysis showed that BW was positively correlated with GWG status, GA and neonatal variant ENPP1 rs1044498, while negatively correlated with smoking during pregnancy.
Our study pointed out the role of MC4R rs17782313 and ENPP1 rs1044498 in maternal and neonatal obesity risk in correlation with BMI, MUAC and TST and BIA, which could be useful for diagnosing obesity in mother-newborn couples.
Methods
Ethics approval and informed consent
The approval for this research was granted by the Ethics Committee of the University of Medicine, Pharmacy, Sciences and Technology from Târgu Mureș (No 26/7th of April 2017), and it was performed according to the principles of the Helsinki Declaration. Informed consent was obtained from all individual participants included in the study (the mothers signed informed consent for them and their newborns).
Study sample selection
We developed a cross-sectional study on 185 mothers and their offspring, admitted in a Tertiary Hospital from Romania, in an Obstetrics Gynecology Clinic between May 2017 and October 2017. The groups were divided into: control group - the underweight or normal mothers with Initial BMI <25 kg/m2 (n1 = 134) and study group – the overweight/obese mothers with initial BMI ≥25 kg/m2 (n2 = 51).
The inclusion criteria were: MAge above 18 years and single pregnancies. The exclusion criteria were: intrauterine growth retardation as a result of congenital malformations, chronic disorders, intrauterine infections; the lack of complete clinical, paraclinical, anthropometric and genetic data, but also mothers who refused to sign the informed consent prior to the inclusion in the study.
Variables of interest
Body mass index (BMI), Middle upper arm circumference (MUAC), Tricipital skinfold thickness (TST), Fat mass (FM): We performed all measurements in both mothers and newborns. These measurements included: weight (kg), height (cm), MUAC and TST. Height was measured with a daily calibrated pedometer, being evaluated by SD (0.1-cm error). For MUAC determination, we measured the arm circumference with a tape measure calibrated in centimeters, at the midpoint between shoulder and elbow tips, while TST was assessed in the posterior area of the upper arm with a thickness caliper. BMI was calculated as the ratio between weight (kg) and squared standing height (m2). According to Control Disease Center (CDC), a BMI between 25.0 and 29.9 classified mothers as overweight, while those with a BMI of 30.0 or higher were considered obese.
For the bioelectrical impedance analysis (BIA), we used a Tanita BC-420 MA body composition analyzer (Tanita Corp, Tokyo, Japan). Weight was provided automatically with a 0.5 kg adjustment for the clothes weight, while height, sex, and age were typed manually.
Genetic variables
The DNA was isolated from fresh peripheral blood using the PureLink Genomic DNA kit (ThermoScientific) according to the manufacturer instructions. The concentration and the purity (A260/A280) of the DNA was quantified by spectrophotometry (BioSpectometer basic, Eppendorf).
For genotyping of MC4R rs17782313 and ENPP1 rs1044498 we used TaqMan technology. In this respect we used the following single tub TaqMan SNP Genotyping assay formats: C__32667060_10 for rs17782313, and C__1207994_20 for rs1044498 (from ThermoFisher Scientific). Genotyping was performed according to the standard protocol for genotyping for 7500 Fast Dx Real-Time PCR System from Applied Biosystems. For genotypes interpretation the 7500 Fast Software v2.3 (Applied Biosystems) was used. All gDNA samples isolated from whole blood collected from children included in patient and control groups were successfully genotyped.
Statistical analysis
Qualitative variables were described by relative frequency while description of quantitative variables were released using centrality measures as mean standard deviation or median (interquartile range).
Bivariate associations between the studied gene polymorphisms, maternal GWG and neonatal characteristics (BW, MUAC, TST) were tested by Chi-square test, Student-t test for independent samples, ANOVA test or nonparametric tests (as Mann-Whitney or Kruskal-Wallis).
The effect of the studied gene polymorphisms on GWG and neonates’ characteristics (BW, MUAC, TST) was adjusted for other covariates using logistic regression analysis or linear multiple regression.
Statistical significance was achieved with the estimated significance level of p < 0.05. All statistical analysis was performed using environment for statistical computing R version 3.4.4.
What is known
Excessive gestational weight gain (GWG) is associated with diabetes, obesity, and dystocia, but also modifications of neonatal birth weight (BW) with afterwards consequences on their evolution. One of four Romanian children is obese or overweight, with percentages varying between 19.7% and 35.8%, depending on the geographic area
MC4R and ENPP1 gene polymorphism are associated with increased risk for developing obesity.
What is new
The MC4R rs17782313 and ENPP1 rs1044498 variant genotypes presented an increased risk in pre-pregnancy overweight.
BW was positively correlated with GWG status, gestational age and neonatal variant ENPP1 rs1044498, while negatively correlated with smoking during pregnancy.
Acknowledgements
This research was partially supported by the Collective Research Grants of the University of Medicine and Pharmacy Târgu Mureș, Romania (“The role of mother’s genetic determinism in child’s obesity correlated with bioimpedance and anthropometric parameters” No. 275/4/11.01.2017).
Author Contributions
Dr. Mărginean Claudiu, Dr. Mărginean Cristina Oana and Dr. Bănescu Claudia and conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. Iancu Mihaela performed the statistical analysis and interpreted the data. Dr. Meliț Lorena Elena, Dr. Tripon Florin and Dr. Bănescu Claudia designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cristina Oana Mărginean and Mihaela Iancu contributed equally.
References
- 1.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. (National Academies Press (US), 2009). Available at, http://www.ncbi.nlm.nih.gov/books/NBK32813/. [access date June 28, 2019]. [PubMed]
- 2.O’Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin. Endocrinol. (Oxf.) 2013;78:9–16. doi: 10.1111/cen.12055. [DOI] [PubMed] [Google Scholar]
- 3.Haugen M, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth. 2014;14:201. doi: 10.1186/1471-2393-14-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farah N, Stuart B, Donnelly V, Kennelly MM, Turner MJ. The influence of maternal body composition on birth weight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;157:14–17. doi: 10.1016/j.ejogrb.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 5.Nicolescu R. Evaluarea morbidităţii cronice prin dispensarizare în colectivităţile de copii şi tineri – raport naţional 2013 – COSI, http://insp.gov.ro/sites/cnepss/wp-content/uploads/2014/12/COSI-2013.pdf.
- 6.Mărginean CO, et al. Correlations Between Leptin Gene Polymorphisms 223 A/G, 1019 G/A, 492 G/C, 976 C/A, and Anthropometrical and Biochemical Parameters in Children With Obesity: A Prospective Case-Control Study in a Romanian Population-The Nutrichild Study. Medicine (Baltimore) 2016;95:e3115. doi: 10.1097/MD.0000000000003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duicu C, Mărginean CO, Voidăzan S, Tripon F, Bănescu C. FTO rs 9939609 SNP Is Associated With Adiponectin and Leptin Levels and the Risk of Obesity in a Cohort of Romanian Children Population. Medicine (Baltimore) 2016;95:e3709. doi: 10.1097/MD.0000000000003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mărginean C, et al. The FTO rs9939609 and LEPR rs1137101 mothers-newborns gene polymorphisms and maternal fat mass index effects on anthropometric characteristics in newborns: A cross-sectional study on mothers-newborns gene polymorphisms-The FTO-LEPR Study (STROBE-compliant article) Medicine (Baltimore) 2016;95:e5551. doi: 10.1097/MD.0000000000005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Böttcher, Y., Körner, A., Kovacs, P. & Kiess, W. Obesity Genes: Implication In Childhood Obesity. Paediatr. Child Health 221 31–36 (2012).
- 10.Janani C, Ranjitha Kumari BD. PPAR gamma gene–a review. Diabetes Metab. Syndr. 2015;9:46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Mărginean Claudiu, Mărginean Cristina Oana, Iancu Mihaela, Szabo Bela, Cucerea Manuela, Melit Lorena Elena, Crauciuc Andrei, Bănescu Claudia. The role of TGF-β1 869 T > C and PPAR γ2 34 C > G polymorphisms, fat mass, and anthropometric characteristics in predicting childhood obesity at birth. Medicine. 2016;95(29):e4265. doi: 10.1097/MD.0000000000004265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mărginean CO, et al. The role of IL-6 572 C/G, 190 C/T, and 174 G/C gene polymorphisms in children’s obesity. Eur. J. Pediatr. 2014;173:1285–1296. doi: 10.1007/s00431-014-2315-5. [DOI] [PubMed] [Google Scholar]
- 13.Curti ML, et al. Associations of the TNF-alpha -308 G/A, IL6 -174 G/C and AdipoQ 45 T/G polymorphisms with inflammatory and metabolic responses to lifestyle intervention in Brazilians at high cardiometabolic risk. Diabetol. Metab. Syndr. 2012;4:49. doi: 10.1186/1758-5996-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mărginean CO, Bănescu C, Duicu C, Voidăzan S, Mărginean C. Angiotensin-converting enzyme gene insertion/deletion polymorphism in nutritional disorders in children. Eur. J. Nutr. 2015;54:1245–1254. doi: 10.1007/s00394-014-0802-0. [DOI] [PubMed] [Google Scholar]
- 15.Loos RJF, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maddux BA, Goldfine ID. Membrane glycoprotein PC-1 inhibition of insulin receptor function occurs via direct interaction with the receptor alpha-subunit. Diabetes. 2000;49:13–19. doi: 10.2337/diabetes.49.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Pocobelli G, Dublin S, Enquobahrie DA, Mueller BA. Birth Weight and Birth Weight for Gestational Age in Relation to Risk of Hospitalization with Primary Hypertension in Children and Young Adults. Matern. Child Health J. 2016;20:1415–1423. doi: 10.1007/s10995-016-1939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giapros VI, et al. Vitamin D, parathormone, and insulin resistance in children born large for gestational age. J. Pediatr. Endocrinol. Metab. JPEM. 2014;27:1145–1150. doi: 10.1515/jpem-2013-0327. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher D, et al. Greater Neonatal Fat-Free Mass and Similar Fat Mass Following a Randomized Trial to Control Excess Gestational Weight Gain. Obes. Silver Spring Md. 2018;26:578–587. doi: 10.1002/oby.22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am. J. Obstet. Gynecol. 2003;189:1423–1432. doi: 10.1067/S0002-9378(03)00596-9. [DOI] [PubMed] [Google Scholar]
- 21.Mărginean C, et al. Impact of demographic, genetic, and bioimpedance factors on gestational weight gain and birth weight in a Romanian population: A cross-sectional study in mothers and their newborns: the Monebo study (STROBE-compliant article) Medicine (Baltimore) 2016;95:e4098. doi: 10.1097/MD.0000000000004098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghezzi F, et al. Bioelectrical impedance analysis during pregnancy and neonatal birth weight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001;98:171–176. doi: 10.1016/S0301-2115(01)00330-X. [DOI] [PubMed] [Google Scholar]
- 23.Xi B, Chandak GR, Shen Y, Wang Q, Zhou D. Association between common polymorphism near the MC4R gene and obesity risk: a systematic review and meta-analysis. PloS One. 2012;7:e45731. doi: 10.1371/journal.pone.0045731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bordoni L, Marchegiani F, Piangerelli M, Napolioni V, Gabbianelli R. Obesity-related genetic polymorphisms and adiposity indices in a young Italian population. IUBMB Life. 2017;69:98–105. doi: 10.1002/iub.1596. [DOI] [PubMed] [Google Scholar]
- 25.García-Solís Pablo, Reyes-Bastidas Marissa, Flores Karla, García Olga P., Rosado Jorge L., Méndez-Villa Lorena, Garcia-G Carlota, García-Gutiérrez David, Kuri-García Aarón, Hernández-Montiel Hebert L., Soriano-Leon Ofelia, Villagrán-Herrera Maria Elena, Solis-Sainz Juan C. Fat mass obesity-associated (FTO) (rs9939609) and melanocortin 4 receptor (MC4R) (rs17782313) SNP are positively associated with obesity and blood pressure in Mexican school-aged children. British Journal of Nutrition. 2016;116(10):1834–1840. doi: 10.1017/S0007114516003779. [DOI] [PubMed] [Google Scholar]
- 26.Lazopoulou N, et al. The combined effect of MC4R and FTO risk alleles on childhood obesity in Greece. Horm. Athens Greece. 2015;14:126–133. doi: 10.14310/horm.2002.1524. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, et al. Associations of Two Obesity-Related Single-Nucleotide Polymorphisms with Adiponectin in Chinese Children. Int. J. Endocrinol. 2017;2017:6437542. doi: 10.1155/2017/6437542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mejía-Benítez A, et al. Analysis of the contribution of FTO, NPC1, ENPP1, NEGR1, GNPDA2 and MC4R genes to obesity in Mexican children. BMC Med. Genet. 2013;14:21. doi: 10.1186/1471-2350-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albuquerque D, Nóbrega C, Rodríguez-López R, Manco L. Association study of common polymorphisms in MSRA, TFAP2B, MC4R, NRXN3, PPARGC1A, TMEM18, SEC. 16B, HOXB5 and OLFM4 genes with obesity-related traits among Portuguese children. J. Hum. Genet. 2014;59:307–313. doi: 10.1038/jhg.2014.23. [DOI] [PubMed] [Google Scholar]
- 30.Martins Maisa Cruz, Trujillo Janet, Farias Dayana Rodrigues, Struchiner Claudio Jose, Kac Gilberto. Association of the FTO (rs9939609) and MC4R (rs17782313) gene polymorphisms with maternal body weight during pregnancy. Nutrition. 2016;32(11-12):1223–1230. doi: 10.1016/j.nut.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Meyre D, et al. Variants of ENPP1 are associated with childhood and adult obesity and increase the risk of glucose intolerance and type 2 diabetes. Nat. Genet. 2005;37:863–867. doi: 10.1038/ng1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyre D, Froguel P. [ENPP1, the first example of common genetic link between childhood and adult obesity and type 2 diabetes] Med. Sci. MS. 2006;22:308–312. doi: 10.1051/medsci/2006223308. [DOI] [PubMed] [Google Scholar]
- 33.Morandi A, et al. The Q121 variant of ENPP1 may protect from childhood overweight/obesity in the Italian population. Obes. Silver Spring Md. 2009;17:202–206. doi: 10.1038/oby.2008.470. [DOI] [PubMed] [Google Scholar]
- 34.Grarup N, et al. Studies of the relationship between the ENPP1 K121Q polymorphism and type 2 diabetes, insulin resistance and obesity in 7,333 Danish white subjects. Diabetologia. 2006;49:2097–2104. doi: 10.1007/s00125-006-0353-x. [DOI] [PubMed] [Google Scholar]
- 35.Lyon HN, et al. Common variants in the ENPP1 gene are not reproducibly associated with diabetes or obesity. Diabetes. 2006;55:3180–3184. doi: 10.2337/db06-0407. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari N, Mallmann P, Brockmeier K, Strüder HK, Graf C. Secular trends in pregnancy weight gain in German women and their influences on foetal outcome: a hospital-based study. BMC Pregnancy Childbirth. 2014;14:228. doi: 10.1186/1471-2393-14-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermann GM, Dallas LM, Haskell SE, Roghair RD. Neonatal macrosomia is an independent risk factor for adult metabolic syndrome. Neonatology. 2010;98:238–244. doi: 10.1159/000285629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rooney BL, Mathiason MA, Schauberger CW. Predictors of obesity in childhood, adolescence, and adulthood in a birth cohort. Matern. Child Health J. 2011;15:1166–1175. doi: 10.1007/s10995-010-0689-1. [DOI] [PubMed] [Google Scholar]
- 39.Bacci S, et al. The ENPP1 Q121 variant predicts major cardiovascular events in high-risk individuals: evidence for interaction with obesity in diabetic patients. Diabetes. 2011;60:1000–1007. doi: 10.2337/db10-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]


