Abstract
Objectives.
To recognize the utility of the surgical Apgar score (SAS) in a noncutaneous head and neck squamous cell carcinoma (HNSCC) population.
Study Design.
Retrospective case series with chart review.
Setting.
Academic tertiary medical center.
Subjects and Methods.
Patients (n = 563) undergoing noncutaneous HNSCC resection between April 2012 and March 2015 were included. Demographics, medical history, intraoperative data, and postoperative hospital summaries were collected. SASs were calculated following the published schema. The primary outcome was 30-day postoperative morbidity. A 2-sample t test, analysis of variance, and χ2 (or Fisher exact) test were used for statistical comparisons. A multivariable logistic regression analysis was conducted to identify independent predictors of 30-day morbidity.
Results.
Mean SAS was 6.2 ± 1.5. SAS groups did not differ in age, sex, or race. Sixty-five patients (11.6%) had a SAS between 0 and 4, with 40 incidences of morbidity (61.5%), while 31 (5.5%) patients with SAS from 9 to 10 had 3 morbidity occurrences (9.7%). Results show that 30-day postoperative morbidity is inversely related to increasing SAS (P < .0001). Furthermore, lower SAS was associated with significantly increased operative time (SAS 0-4: 9.3 ± 2.6 hours vs SAS 9-10: 3.0 ± 1.1 hours) and lengths of stay (SAS 0-4: 10.0 ± 7.3 days vs SAS 9-10: 1.6 ± 1.0 days), P < .0001. SAS remained highly significant after adjusting for potential confounding variables in the multivariable analysis (P < .0001).
Conclusions.
An increasing SAS is associated with significantly lower rates of 30-day postoperative morbidities in a noncutaneous HNSCC patient population.
Keywords: surgical Apgar score, postoperative morbidity, surgical outcomes, head and neck cancer, squamous cell carcinoma
The surgical Apgar score (SAS), designed by Gawande et al1 in 2007, was the first attempt to develop an intraoperative assessment score to help predict postoperative outcomes. The score by Gawande et al reflects the structure of the Apgar score developed by Virginia Apgar in the 1950s to assess newborn status.2 As the Apgar score revolutionized neonatology, Gawande’s goal to predict patient outcomes on 3 readily attainable values has the potential to transform perioperative medicine.3
Originally developed in a broad general and vascular surgery population, lower SASs were strongly associated with increased rates of 30-day morbidity and mortality (M&M).1 The SAS (Table 1) is a 10-point score comprising lowest heart rate (HR) in beats per minutes, lowest mean arterial pressure (MAP) in millimeters of mercury, and estimated blood loss (EBL) in milliliters. Remarkably, since its advent, it has been validated in numerous surgical niches.4-10 At present, the closest otolaryngology-related report was based in a small subset of head and neck oncology patients undergoing fibular free flap reconstruction, which was incongruent with the SAS.11 However, a larger, more general head and neck oncology population has not yet been examined.
Table 1.
The 10-Point Surgical Apgar Score.
| Surgical Apgar Score, Points |
|||||
|---|---|---|---|---|---|
| Characteristic | 0 | 1 | 2 | 3 | 4 |
| Lowest heart rate/mina | >85 | 76-85 | 66-75 | 56-65 | ≤55 |
| Lowest mean arterial pressure, mm Hg | <40 | 40-54 | 55-69 | ≥70 | |
| Estimated blood loss, mL | >1000 | 601-1000 | 101-600 | ≤100 | |
Occurrence of pathologic bradydysrhythmia, including sinus arrest, atrioventricular block or dissociation, junctional or ventricular escape rhythms, and asystole, also receives 0 points for lowest heart rate.
A postoperative prediction score has the potential to affect the otolaryngology oncologic population significantly. Head and neck squamous cell carcinoma (HNSCC) is the sixth leading cause of cancer worldwide with an associated 7890 annual US deaths.12,13 Moreover, only about one-third of HNSCC cases present early stage.14 Surgical management of advanced-stage disease is often associated with significant morbidity. Thus, earlier identification and intervention for patients at increased risk of postoperative 30-day M&M may improve surgical outcomes and help with surgical decision making in the immediate postoperative time period. This study aims to evaluate the SASs’ applicability to a tertiary HNSCC surgical population.
Methods
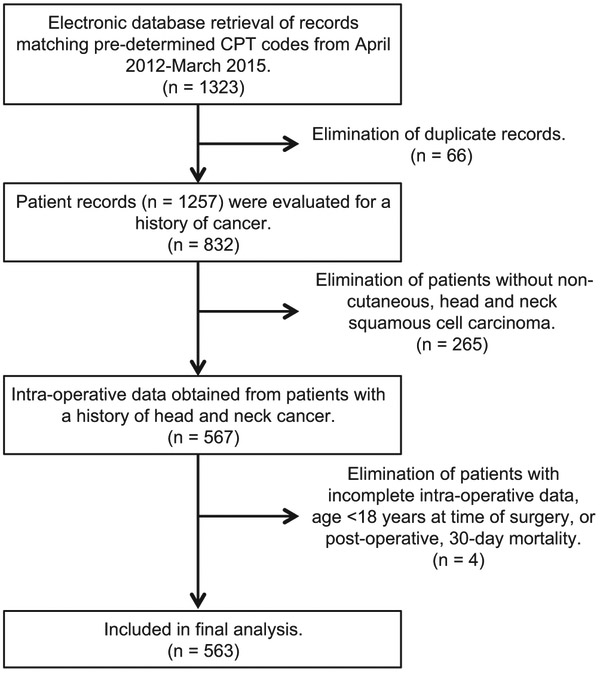
Patients were identified by predetermined Current Procedural Terminology (CPT) codes (Supplemental Table S1, available at in the online version of the article), matching those maintained in the University of Alabama at Birmingham (UAB) Medical Center’s electronic medical record (EMR) from April 1, 2012, to March 31, 2015. Total returned records (n = 1323) were reduced to the studied population (n = 563) as detailed in the flowchart (Figure 1). The UAB Institutional Review Board approved patient data collection, analysis, and publication.
Figure 1.
Flowchart demonstrating selection of included patients. CPT, Current Procedural Terminology.
Patient charts were queried for basic demographics (age, sex, race, self-reported smoking status, body mass index [BMI], and American Society of Anesthesiologists [ASA] status), intraoperative variables (lowest HR, lowest MAP, EBL, and operation start and end times), disease specifics (primary location and American Joint Committee on Cancer [AJCC] stage), and dates of surgery and discharge. Thirty-day postoperative morbidity events were recorded for each patient and subsequently grouped by diagnosis and individual patient’s SAS values. The morbidity incidence percentage (MIP) was calculated by evaluating the frequency of events in an SAS category (ie, seizure, SAS = 2) to the total frequency (ie, seizure, n = 1) of that diagnosis. MIP values are represented by color; higher values (ie, seizure SAS = 1-4, MIP = 100%) are dark red, and lower values (ie, seizure SAS = 5-6, MIP = 0%) are white. Intraoperative vital signs were recorded every 15 seconds with either a Baytech model M4 or DS3 (Baytech, Long Beach Industrial Park, Mississippi). Data were then transmitted and stored in a Microsoft SQL Server (Microsoft, Redmond, Washington) database, which was accessed with SQL Server Management Studio (Microsoft, Redmond, Washington).
The 10-point SAS was calculated and based on the patient’s intraoperative lowest HR, lowest MAP, and EBL (Table 1) as originally described by Gawande et al.1 Subsequently, patients’ EMRs were individually assessed for 30-day postoperative events that reflect incidences of M&M. Included morbidities were defined as previously established by Ettinger et al,11 which reflects that established by the American College of Surgery. Furthermore, disease primary sites were reduced into the following anatomical categories: oropharynx, larynx, hypopharynx, oral cavity, or other for remaining and unknown head and neck tumor primary sites.
Operative time (OT) was calculated based on the elapsed time in hours from documented incision and closure times. Length of stay (LOS) represents the time in days from date of surgery to date of discharge.
Patient characteristics were summarized using descriptive statistics (mean and standard deviation for continuous variables, frequency and percentage for categorical variables, and 95% confidence interval [CI] for all variables). The primary outcome was 30-day postoperative morbidity. A χ2 (or Fisher exact) test was used to assess the relationships between SAS and patient characteristics. A 2-sample t test, analysis of variance (ANOVA), and χ2 (or Fisher exact) test were used to identify specific demographics and clinical variables that were predictive of 30-day morbidity. To quantify the predictive ability of SAS for 30-day morbidity, a receiver operating characteristic (ROC) curve was constructed with calculated area under the curve (AUC). A multivariable logistic regression was used to identify variables that were predictive of 30-day morbidity while adjusting for potential confounders. To avoid overfitting the model, only variables with P < .20 in the bivariate analysis were selected for inclusion into the final model.15 Multicollinearity was assessed using the Spearman correlation matrix and variance inflation factor (VIF).16 The inclusion criteria for the multivariable model were based on correlation ρ < 0.40 and VIF less than 5.17-19 The value P < .05 was considered statistically significant in 2-tailed statistical tests. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, North Carolina). The ROC plot was created using R 3.3.0.20
Results
Table 2 characterizes the enrolled population (n = 563). Mean age was 60.9 ± 11.3 years, and most were male (74.5%). Whites represented 81.8%, African Americans were 16.2%, and 2% were listed as “other.” Average BMI was 25.6 ± 5.9 kg/m2. Few patients self-identified as current smokers (20.8%), and most (88.8%) had poor preoperative health as characterized by ASA scores of 3 or 4. Average OT was 6.6 ± 3.2 hours while LOS was 6.1 ± 5.8 days. Postoperative 30-day morbidity occurred in 37.1% of patients. SAS distribution was as follows: SAS 1 to 4 (11.6%), 5 to 6 (49.7%), 7 to 8 (33.2%), and 9 to 10 (5.5%). Primary disease sites were diverse: oral cavity (35.4%), oropharynx (27.5%), larynx (22.2%), hypopharynx (3.4%), or “other’/unknown primary site (11.6%).
Table 2.
Demographics and Clinical Variables (N = 563).
| Patient Characteristics | Value | 95% CI |
|---|---|---|
| Age, mean ± SD, y | 60.9 ± 11.3 | 59.9-61.8 |
| Sex, No. (%) | ||
| Male | 405 (74.5) | 70.8-78.1 |
| Female | 139 (25.6) | 21.9-29.2 |
| Race, No. (%) | ||
| White | 444 (81.8) | 78.5-85 |
| African American | 88 (16.2) | 13.1-19.3 |
| Others | 11 (2) | 0.8-3.2 |
| BMI, mean ± SD, kg/m2 | 25.6 ± 5.9 | 25.1-26.1 |
| Smoking (current), No. (%) | 93 (20.8) | 17-24.5 |
| ASA status, No. (%) | ||
| 1 | 4 (0.7) | 0.02-1.4 |
| 2 | 59 (10.5) | 8-13 |
| 3 | 456 (81) | 77.8-84.2 |
| 4 | 44 (7.8) | 5.6-10 |
| 30-Day morbidity, No. (%) | ||
| None | 354 (62.9) | 58.9-66.9 |
| Present | 209 (37.1) | 33.1-41.1 |
| Length of stay, mean ± SD, d | 6.1 ± 5.8 | 5.6-6.6 |
| Operation time, mean ± SD, h | 6.6 ± 3.2 | 6.3-6.9 |
| Surgical Apgar score, No. (%) | ||
| 1-4 | 65 (11.6) | 8.9-14.2 |
| 5-6 | 280 (49.7) | 45.6-53.9 |
| 7-8 | 187 (33.2) | 29.3-37.1 |
| 9-10 | 31 (5.5) | 3.6-7.4 |
| Primary site, No. (%) | ||
| Oropharynx | 155 (27.5) | 23.8-31.2 |
| Larynx | 125 (22.2) | 18.8-25.6 |
| Hypopharynx | 19 (3.4) | 1.9-4.9 |
| Oral cavity | 199 (35.4) | 31.4-39.3 |
| Others | 65 (11.6) | 8.9-14.2 |
| AJCC stage, No. (%) | ||
| 1 | 106 (18.9) | 15.6-22.1 |
| 2 | 81 (14.4) | 11.5-17.3 |
| 3 | 100 (17.8) | 14.6-21 |
| 4A | 200 (35.6) | 31.6-39.6 |
| 4B | 35 (6.2) | 4.2-8.2 |
| Unknown | 41 (7.3) | 5-9.2 |
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval.
While no association was detected for age, sex, BMI, or smoking status, SAS was significantly (P < .0001) associated with race, ASA status, LOS, OT, tumor site, and AJCC stage (Table 3). More specifically, African American patients with higher ASA scores and increased LOS and OT were more likely to have a lower SAS. Primary disease site and AJCC stage were significantly associated with SAS (P < .0001 and P = .04, respectively). Patients with SAS 1 to 4 more likely had oral cavity (49.2%) or laryngeal (23.1%) primaries with an AJCC stage greater than 3 (61.5%) while those with SAS 9 to 10 mostly manifested oropharyngeal primaries (54.8%) with a less advanced AJCC stage, 1 and 2 (38.7%).
Table 3.
Result of Associations between SAS and Patient Characteristics.a
| Patient Characteristics | SAS |
P Value | |||
|---|---|---|---|---|---|
| 1-4 (n = 65) | 5-6 (n = 280) | 7-8 (n = 187) | 9-10 (n = 31) | ||
| Age, mean ± SD, y | 60.3 ± 9.1 | 61.1 ± 11.4 | 60.5 ± 11.8 | 61.8 ± 11.3 | .86 |
| Sex | .43 | ||||
| Male | 49 (80.3) | 202 (75.4) | 130 (70.7) | 24 (77.4) | |
| Female | 12 (19.7) | 66 (24.6) | 54 (29.4) | 7 (22.6) | |
| Race | <.0001b | ||||
| White | 44 (72.1) | 320 (78.7) | 162 (88.0) | 28 (90.3) | |
| African American | 16 (26.2) | 52 (19.5) | 17 (9.2) | 3 (9.7) | |
| Others | 1 (1.6) | 5 (1.9) | 5 (2.7) | 0 (0) | |
| BMI, mean ± SD, kg/m2 | 25.2 ± 5.7 | 25.1 ± 6.2 | 26.2 ± 5.8 | 27.3 ± 4.3 | .15 |
| Smoking (current) | 14 (28.0) | 41 (19.2) | 31 (19.8) | 7 (25.9) | .48 |
| ASA status, mean ± SD | 3.1 ± 0.4 | 3.1 ± 0.4 | 2.8 ± 0.5 | 2.6 ± 0.6 | <.0001b |
| Length of stay, mean ± SD, d | 10 ± 7.3 | 7.8 ± 5.5 | 2.9 ± 3.7 | 1.6 ± 1 | <.0001b |
| Operation time, mean ± SD, h | 9.3 ± 2.6 | 7.9 ± 2.7 | 4.3 ± 2.2 | 3 ± 1.1 | <.0001b |
| Primary site | <.0001b | ||||
| Oropharynx | 6 (9.2) | 64 (22.9) | 68 (36.4) | 17 (54.8) | |
| Larynx | 15 (23.1) | 84 (30.0) | 24 (12.8) | 2 (6.5) | |
| Hypopharynx | 2 (3.1) | 13 (4.6) | 4 (2.1) | 0 (0) | |
| Oral cavity | 32 (49.2) | 101 (36.1) | 57 (30.5) | 9 (29.0) | |
| Others | 10 (15.4) | 18 (6.4) | 34 (18.2) | 3 (9.7) | |
| AJCC stage | .04b | ||||
| 1 | 9 (13.9) | 45 (16.1) | 45 (24.1) | 7 (22.6) | |
| 2 | 14 (21.5) | 38 (13.6) | 24 (12.8) | 5 (16.1) | |
| 3 | 11 (16.9) | 55 (19.6) | 30 (16) | 4 (12.9) | |
| 4A | 22 (33.8) | 114 (40.7) | 54 (28.9) | 10 (32.3) | |
| 4B | 7 (10.8) | 14 (5) | 13 (7) | 1 (3.2) | |
| Unknown | 2 (3.1) | 14 (5) | 21 (11.2) | 4 (12.9) | |
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; BMI, body mass index; SAS, surgical Apgar score.
Values are presented as number (%) unless otherwise indicated.
Denotes statistical significance at P < .05.
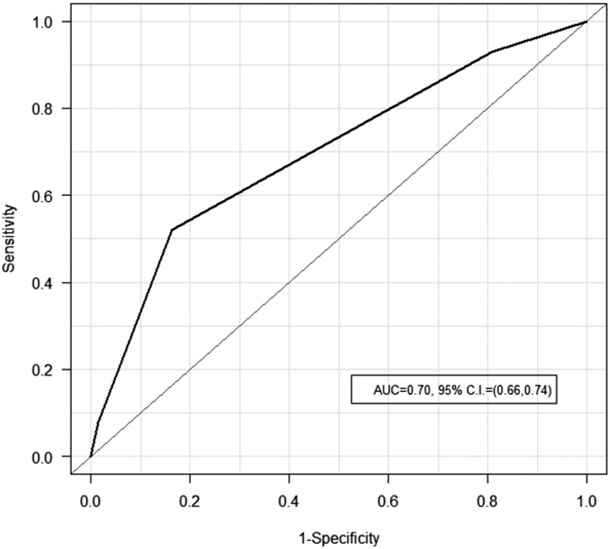
A lower SAS was significantly (P < .0001) associated with increased morbidity (Table 4). In fact, 30-day morbidity peaked at SAS 5 to 6 (64.6%) while most patients with an SAS from 7 to 10 (52.0%) did not experience postoperative morbidities. Evaluation of the AUC (0.70; 95% CI, 0.66-0.74; P < .0001) further highlights that the SAS has predictive ability to discriminate 30-day morbidity from those without (Figure 2). Patient disease location and stage were associated with increased 30-day morbidity. Those with laryngeal, hypopharyngeal, and oral cavity primaries with an AJCC stage 2 or greater were significantly more likely to experience 30-day morbidity (tumor site: P < .0001 and AJCC stage: P = .0008, Table 4). Furthermore, associations between patient characteristics and morbidity occurrence mirror those when compared to SAS. In fact, longer operations (8.8 ± 2.8 hours vs 5.3 ± 2.7 hours), increased LOS (10.4 ± 6.8 days vs 3.5 ± 2.9 days), and increasing ASA status were significantly (P < .0001) associated with an increased incidence of postoperative 30-day morbidity. Patients with a lower BMI (24.1 ± 5.7 kg/m2 vs 26.5 ± 5.9 kg/m2) had a significantly (P < .0001) greater incidence of 30-day morbidity.
Table 4.
Result of Associations between Morbidity and Patient Characteristics.a
| Patient Characteristics | 30-Day Morbidity |
||
|---|---|---|---|
| None (n = 354) | Present (n = 209) | P Value | |
| Age, mean ± SD, y | 60.8 ± 11.2 | 61 ± 11.4 | .87 |
| Sex | .16 | ||
| Male | 263 (76.5) | 142 (71) | |
| Female | 81 (23.6) | 58 (29.0) | |
| Race | .009b | ||
| White | 292 (85.1) | 152 (76.0) | |
| African American | 43 (12.5) | 45 (22.5) | |
| Others | 8 (2.3) | 3 (1.5) | |
| BMI, mean ± SD, kg/m2 | 26.5 ± 5.9 | 24.1 ± 5.7 | <.0001b |
| Smoking (current) | 60 (21.2) | 33 (20) | .76 |
| ASA status, mean ± SD | 2.9 ± 0.5 | 3.1 ± 0.4 | <.0001b |
| Length of stay, mean ± SD, d | 3.5 ± 2.9 | 10.4 ± 6.8 | <.0001b |
| Operation time, mean ± SD, h | 5.3 ± 2.7 | 8.8 ± 2.8 | <.0001b |
| Primary site | <.0001 | ||
| Oropharynx | 109 (30.8) | 46 (22) | |
| Larynx | 64 (18.1) | 61 (29.2) | |
| Hypopharynx | 11 (3.1) | 8 (3.8) | |
| Oral cavity | 113 (31.9) | 86 (41.2) | |
| Others | 57 (16.1) | 8 (3.8) | |
| AJCC stage | .0008b | ||
| 1 | 73 (20.6) | 33 (15.9) | |
| 2 | 46 (13.0) | 35 (16.8) | |
| 3 | 60 (17.0) | 40 (19.2) | |
| 4A | 116 (32.8) | 84 (40.4) | |
| 4B | 21 (5.9) | 14 (6.7) | |
| Unknown | 38 (10.7) | 3 (1.4) | |
| SAS | <.0001b | ||
| 1-4 | 25 (7.1) | 40 (19.1) | |
| 5-6 | 145 (41.0) | 135 (64.6) | |
| 7-8 | 156 (44.1) | 31 (14.8) | |
| 9-10 | 28 (7.9) | 3 (1.4) | |
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; BMI, body mass index; SAS, surgical Apgar score.
Values are presented as number (%) unless otherwise indicated.
Denotes statistical significance at P < .05.
Figure 2.
Receiver operating characteristic curve for surgical Apgar score predicting postoperative 30-day morbidity. AUC, area under the curve; CI, confidence interval.
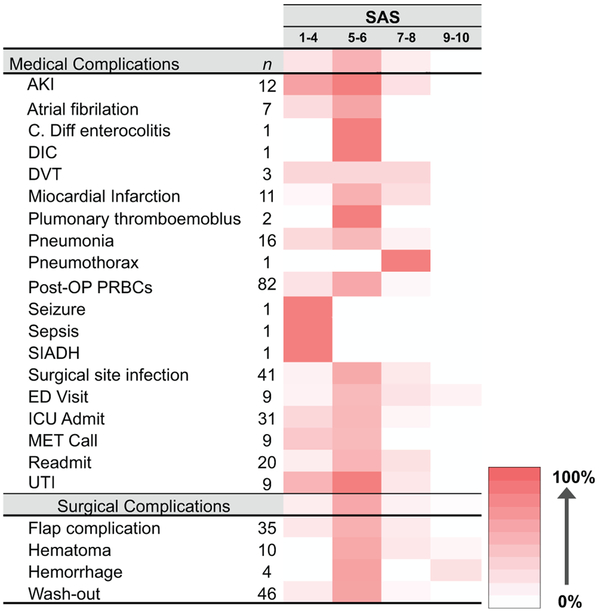
Individual MIP values reveal increased morbidity occurrence in patients with lower SAS (Figure 3). Higher percent values are represented by a darker red color vs white for 0% values. This trend is conserved regardless of morbidity type (medical vs surgical) and severity (minor complications like postoperative wound infections vs major flap complications requiring further surgical intervention). While limited by morbidity diagnosis incidence, even less common morbidities, including seizure, sepsis, and syndrome of inappropriate antidiuretic hormone (SIADH), occurred in SAS 1 to 4.
Figure 3.
Heat map of surgical Apgar score values’ frequency percentage for medical and surgical complications. Higher percentage values are represented with darker red colors vs lower percentages represented by whiter colors. AKI, acute kidney injury; DIC, disseminated intravascular coagulation; DVT, deep venous thrombus; ED, emergency department; ICU, intensive care unit; PRBC, packed red blood cells; SIADH, syndrome of inappropriate antidiuretic hormone; UTI, urinary tract infection.
The multivariable logistic regression analysis identified BMI, ASA status, and SAS as significant (P < .05) independent predictors of 30-day morbidity (Table 5). Given all other variables in the model are held constant, the likelihood of 30-day morbidity occurrence is 89% less for patients with a SAS score of 9 to 10 relative to those with SAS 1 to 4. In addition, patients with SAS scores of 5 to 6 and 7 to 8 are 56% and 85% less likely to have 30-day morbidity than patients with SAS 1 to 4, respectively. For every 5-unit increase in BMI, the likelihood of experiencing 30-day morbidity is 26% less. Last, for every ASA unit increase, odds of a 30-day morbidity event are increased by over 2-fold.
Table 5.
Result of Multivariable Logistic Regression.
| 30-Day Morbidity |
||
|---|---|---|
| Patient Characteristics | Adjusted OR (95% CI) | P Value |
| Sex | .09 | |
| Male | Reference | |
| Female | 1.58 (0.97-2.59) | |
| Race | .54 | |
| White | Reference | |
| African American | 1.43 (0.83-2.49) | |
| Others | 1.18 (0.26-5.45) | |
| BMI | 0.94 (0.91-0.98) | .003a |
| ASA status | 2.05 (1.14-3.68) | .0l5a |
| Primary site | .16 | |
| Oropharynx | Reference | |
| Larynx | 1.18 (0.63-2.21) | |
| Hypopharynx | 0.67 (0.22-2.11) | |
| Oral cavity | 1.32 (0.74-2.34) | |
| Others | 0.39 (0.15-1.07) | |
| AJCC stage | .l7 | |
| 1 | Reference | |
| 2 | 1.51 (0.71-3.24) | |
| 3 | 1.44 (0.71-2.92) | |
| 4A | 1.68 (0.9-3.11) | |
| 4B | 1.35 (0.51-3.6) | |
| Unknown | 0.34 (0.08-1.42) | |
| SAS | <.0001a | |
| 1-4 | Reference | |
| 5-6 | 0.44 (0.22-0.88) | |
| 7-8 | 0.15 (0.07-0.33) | |
| 9-10 | 0.11 (0.03-0.47) | |
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; BMI, body mass index; SAS, surgical Apgar score.
Denotes statistical significance at P < .05.
Discussion
The SAS has successfully shown strong postoperative 30-day M&M predictive abilities among multiple surgical populations. Our study shows the SAS also has utility in a noncutaneous HNSCC population. When SAS decreased, patients were significantly more likely to experience postoperative 30-day morbidity. This association continues even when morbidity diagnoses are considered individually, thereby exhibiting utility regardless of complication severity or type, medical vs surgical. These findings suggest perioperative SAS calculation may serve as a powerful quantitative tool to assist the surgical team in identifying patients at risk for postoperative morbidity events, particularly in the immediate postoperative period. Thus, more aggressive management of low-SAS/“high-risk” patients may improve postoperative outcomes. Decreased SAS also significantly (P < .0001) correlated to increased LOS. This further supports the SASs’ prognostic value, implicating the potential effects of improved outcomes and reduction of medical costs if earlier intervention occurred for SAS-determined at-risk patients. However, future prospective studies are needed to appreciate the true clinical value.
The nature of head and neck cancer and its functional impact on the aerodigestive tract in addition to the increased metabolic rate secondary to neoplastic growth often leads to a poor nutritional status and cachexia.21,22 Interestingly, the results revealed a strong, inverse association between BMI and 30-day morbidity. This follows BMI as a known, effective surrogate measure of presurgical nutritional status.23,24 Clinically, improved presurgical nutritional status translates to decreased postoperative morbidity and mortality.24 Therefore, BMI inclusion into the predicative model may further improve the SASs’ prognostic value in this population. Even more, both primary disease location and AJCC stage were associated with SAS values. That is, patients presenting with a more advanced AJCC stage (≥3) or a primary oral cavity or laryngeal disease correlated to lower SAS values. While a mechanistic explanation of this finding is beyond this study’s scope, it may be derived from increased metabolic activity in more advanced disease and increased dysphagia rates secondary to primary location both resulting in poorer patient nutritional status. On the other hand, considering disease stage as a surrogate measure for ablative extensiveness, AJCC stage >3 individuals may be predisposed to increased complication rates as a result of more invasive intervention. These represent hypotheses for future investigation as the nuances of HNSCC are further elucidated.
Interestingly, smoking did not have an impact on morbidity prediction. However, smoking is a known strong and independent risk factor for the development of head and neck cancer.25 This may be secondary to the limitation of self-reported smoking status. In fact, a recent 67-study review found that smokers are often not forthcoming with the truth.26 Thus, in actuality, with improved variable collection methods, tobacco use may become associated with postoperative 30-day morbidity.
Of note, the studied population had 2 incidences of 30-day mortality after the initial operation. Therefore, the impact of these cases was eliminated from the assessment due to statistical assessment requirements. As such, increased study duration has the potential to collect more 30-day postoperative deaths and thereby allow for analysis with the SAS. Last, the retrospective nature of our study limits the conclusions’ strengths, as data analysis is susceptible to clerical errors with both initial documentation and data retrieval.
Conclusion
The SAS is an efficient and robust method for predicting postoperative 30-day morbidity in the head and neck cancer patient population. As its prognostic value continues to increase with positive results in a variety of surgical populations, the SAS has the potential to improve postoperative care.
Supplementary Material
Acknowledgments
The authors thank Lisa Clemons and Deborah Lowman for their efforts with institutional review board–associated paperwork. In addition, this study would not have been possible without the assistance of David Benz and the UAB Department of Anesthesia, who kindly provided the intraoperative data.
Funding source: Statistical analysis was partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001417.
Footnotes
Competing interests: None.
Sponsorships: None.
Supplemental Material
Additional supporting information is available in the online version of the article.
This article was presented at the 2017 AAO-HNSF Annual Meeting and OTO Experience; September 10-13, 2017; Chicago, Illinois.
References
- 1.Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007; 204:201–208. [DOI] [PubMed] [Google Scholar]
- 2.Apgar V A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg. 1953;32:260–267. [PubMed] [Google Scholar]
- 3.Li F, Wu T, Lei X, Zhang H, Mao M, Zhang J. The Apgar score and infant mortality. PLoS One. 2013;8:e69072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regenbogen SE, Ehrenfeld JM, Lipsitz SR, Greenberg CC, Hutter MM, Gawande AA. Utility of the surgical Apgar score: validation in 4119 patients. Arch Surg. 2009;144:30–37. [DOI] [PubMed] [Google Scholar]
- 5.Janowak CF, Blasberg JD, Taylor L, Maloney JD, Macke RA. The surgical Apgar score in esophagectomy. J Thorac Cardiovasc Surg. 2015;150:806–812. [DOI] [PubMed] [Google Scholar]
- 6.Assifi MM, Lindenmeyer J, Leiby BE, et al. Surgical Apgar score predicts perioperative morbidity in patients undergoing pancreaticoduodenectomy at a high-volume center. J Gastrointest Surg. 2012;16:275–281. [DOI] [PubMed] [Google Scholar]
- 7.Miki Y, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Perioperative risk assessment for gastrectomy by surgical Apgar score. Ann Surg Oncol. 2014;21:2601–2607. [DOI] [PubMed] [Google Scholar]
- 8.Ziewacz JE, Davis MC, Lau D, et al. Validation of the surgical Apgar score in a neurosurgical patient population. J Neurosurg. 2013;118:270–279. [DOI] [PubMed] [Google Scholar]
- 9.Zighelboim I, Kizer N, Taylor NP, et al. “Surgical Apgar score” predicts postoperative complications after cytoreduction for advanced ovarian cancer. Gynecol Oncol. 2010;116: 370–373. [DOI] [PubMed] [Google Scholar]
- 10.Prasad SM, Ferreria M, Berry AM, et al. Surgical Apgar outcome score: perioperative risk assessment for radical cystectomy. J Urol. 2009;181:1046–1052. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger KS, Moore EJ, Lohse CM, Reiland MD, Yetzer JG, Arce K. Application of the surgical Apgar score to microvascular head and neck reconstruction. J Oral Maxillofac Surg. 2016;74:1668–1677. [DOI] [PubMed] [Google Scholar]
- 12.Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91:386–396. [DOI] [PubMed] [Google Scholar]
- 13.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braakhuis BJ, Brakenhoff RH, Leemans CR. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: biological risk factors. Ann Oncol. 2012;23(suppl 10):173–177. [DOI] [PubMed] [Google Scholar]
- 15.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. [DOI] [PubMed] [Google Scholar]
- 16.Allison PD. Logistic Regression Using SAS®: Theory and Application. 2nd ed. Cary, NC: SAS Institute; 2012. [Google Scholar]
- 17.O’Brien R. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–690. [Google Scholar]
- 18.Farrahi J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Psychometric properties of the Persian version of the Pittsburgh Sleep Quality Index addendum for PTSD (PSQI-A). Sleep Breath. 2009;13:259–262. [DOI] [PubMed] [Google Scholar]
- 19.Kutner MH, Nachtsheim C, Neter J. Applied Linear Regression Models. 4th ed. New York, NY: McGraw-Hill/Irwin; 2004. [Google Scholar]
- 20.Carstensen B, Plummer M, Laara E, Hills M. Epi: A Package for Statistical Analysis in Epidemiology. R Package Version 2.26 [computer program]. Vienna, Austria; 2017. https://cran.r-project.org/web/packages/Epi/index.html. [Google Scholar]
- 21.Couch ME, Dittus K, Toth MJ, et al. Cancer cachexia update in head and neck cancer: definitions and diagnostic features. Head Neck. 2015;37:594–604. [DOI] [PubMed] [Google Scholar]
- 22.Snyderman CH. Nutrition and head and neck cancer. Curr Oncol Rep. 2003;5:158–163. [DOI] [PubMed] [Google Scholar]
- 23.Bailey KV, Ferro-Luzzi A. Use of body mass index of adults in assessing individual and community nutritional status. Bull World Health Organ. 1995;73:673–680. [PMC free article] [PubMed] [Google Scholar]
- 24.Evans DC, Martindale RG, Kiraly LN, Jones CM. Nutrition optimization prior to surgery. Nutr Clin Pract. 2014;29:10–21. [DOI] [PubMed] [Google Scholar]
- 25.Gupta PC, Murti PR, Bhonsle RB, Mehta FS, Pindborg JJ. Effect of cessation of tobacco use on the incidence of oral mucosal lesions in a 10-yr follow-up study of 12,212 users. Oral Dis. 1995;1:54–58. [DOI] [PubMed] [Google Scholar]
- 26.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11:12–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.