Abstract
Nonalcoholic fatty liver disease (NAFLD), first described in 1980, is now recognized as one of the most common causes of elevated liver enzymes and chronic liver disease in Western countries. The incidence of NAFLD in both adults and children is rising, in conjunction with the burgeoning epidemics of obesity and type 2 diabetes mellitus. NAFLD often coexists with other sequelae of the metabolic syndrome: central obesity, type 2 diabetes, hypertension, and hyperlipidemia. NAFLD encompasses a spectrum of pathologic liver diseases ranging from simple hepatic steatosis to a predominant lobular necro-inflammation, with or without centrilobular fibrosis (called nonalcoholic steatohepatitis or NASH). NASH can progress to cirrhosis, decompensated liver disease, and hepatocellular carcinoma. Though the natural history of NASH is still not clearly defined, it has been observed to progress to cirrhosis in 15%–20% of those affected. Insulin resistance is nearly universal in NASH and is thought to play an important role in its pathogenesis leading to dysregulated lipid metabolism. The prevalence of insulin resistance is reported in the general population to be approaching 45%, suggesting that NAFLD and NASH will continue to be an important public health concern. To date, NASH has proven to be a difficult disease to treat. Front-line therapy with lifestyle modifications resulting in weight loss through decreased caloric intake and moderate exercise is generally believed to be beneficial in patients with NASH, but is often difficult to maintain long term. Given that insulin resistance plays a dominant role in the pathogenesis, many studies have examined the use of insulin sensitizers: the biguanides (metformin), thiazolidinediones (pioglitazone, troglitazone, and rosiglitazone), glucagon-like peptide-1-receptor agonists, or incretins (exenatide) in NASH. This review will provide an overview of insulin resistance in NAFLD and provide a detailed summary on the clinical data regarding the use of insulin sensitizers in NASH.
Keywords: exenatide, hepatic steatosis, insulin resistance, insulin sensitizers, metformin, nonalcoholic fatty liver disease, pioglitazone, rimonabant, rosiglitazone, troglitazone
BACKGROUND
Nonalcoholic fatty liver disease (NAFLD) is a common liver disease that often coexists with other features of the metabolic syndrome: central obesity, type 2 diabetes mellitus, hypertension, and hyperlipidemia. Histologically, NAFLD resembles alcohol-induced liver disease, but, by definition, NAFLD develops in patients who consume little or no alcohol. The incidence of NAFLD in adults and children is rapidly rising due to the ongoing epidemics of obesity and type 2 diabetes in Western countries and due to the increased recognition of the disease.1 The prevalence of insulin resistance is reported in the general population to be approaching 45%.2 The estimated prevalence of disease depends on the type of screening method used for diagnosis, but it is generally accepted that NAFLD affects approximately 20%–30% of the adult population.3 Higher prevalence rates are noted among patients with the metabolic syndrome or type 2 diabetes mellitus. It is estimated that up to 40% of patients with NAFLD may have histologic findings consistent with nonalcoholic steatohepatitis (NASH), which may progress to cirrhosis and lead to complications from end-stage liver disease in 15%–20% of cases.4,5
There are few published studies that address the long-term follow-up of NAFLD and thus the natural history of the disease is difficult to ascertain. The available data are obtained from single-center series where patients underwent sequential liver biopsies over a defined period of time. Generally, the histologic findings of simple hepatic steatosis and steatosis with nonspecific inflammation tend to have a benign course, whereas histologic NASH can progress to advanced fibrosis and cirrhosis. NASH-related cirrhosis may have a similar prognosis as cirrhosis from other causes, leading to liver failure or hepatocellular carcinoma. Progression with higher stages of fibrosis has been found to occur in 37.8% of patients, and regression of fibrosis has also been noted in 20.8% of the patients in series with paired biopsies.6 Age and the presence of inflammation on the initial biopsy may independently predict the development of advanced fibrosis in NASH.6 In addition, obesity and diabetes have been shown in cross-sectional studies to be independent factors associated with advanced fibrosis in NASH.7 These individuals should be included in treatment trials to have the greatest improvement in the natural history of disease.
Due to the increasing prevalence and association with other metabolic disorders, it is important that clinicians have a deep understanding of NAFLD and its clinical spectrum of disease presentation as well as therapeutic options. NAFLD has become the most common diagnosis for referral to liver specialists for unexplained abnormal liver enzymes in the United States.8 However, patients are not just presenting to gastroenterologists and hepatologists, but also to family practitioners, internists, pediatricians, endocrinologists, cardiologists, surgeons, and allied health practitioners. This amplifies the importance of a multidisciplinary approach to the diagnosis and treatment of patients with NAFLD.
Insulin Resistance in NAFLD
The pathogenesis of NASH is not currently well understood, but the prevailing hypothesis is the so-called “two-hit” model. The “first hit” is the development of hepatic steatosis as a result of insulin and leptin resistance leading to enhanced free fatty acid flux to the liver, upregulated hepatic de novo lipogenesis, increased fatty acid oxidation, and decreased export of hepatic triglycerides (as seen in Figure 1).9 This is followed by a “second hit” that leads to hepatocellular injury, inflammation, and ultimately fibrosis by way of oxidative stress, which promotes lipid peroxidation, cytochrome P450 activation, and the production of pro-inflammatory cytokines.9 One study supporting the “two-hit” hypothesis showed that patients with NAFLD have higher resting plasma insulin levels and reduced sensitivity to insulin compared with control individuals, and that those with NASH had a higher likelihood of having abnormal mitochondrial morphology suggesting a propensity towards increased oxidative damage.10 The “two-hit” hypothesis has been contested recently due to the complexity of the pathogenic processes that lead to steatosis and steatohepatitis. These processes are likely to be multifactorial, influenced by both environmental and genetic factors. Familial associations have also been recognized. A recent study derived from the Framingham Offspring Cohort showed that early-onset paternal obesity was associated with higher odds of elevated serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in offspring.11 Further elucidation of familial risk factors in biopsy-proven patients with NASH is needed to better understand these associations.
Figure 1.
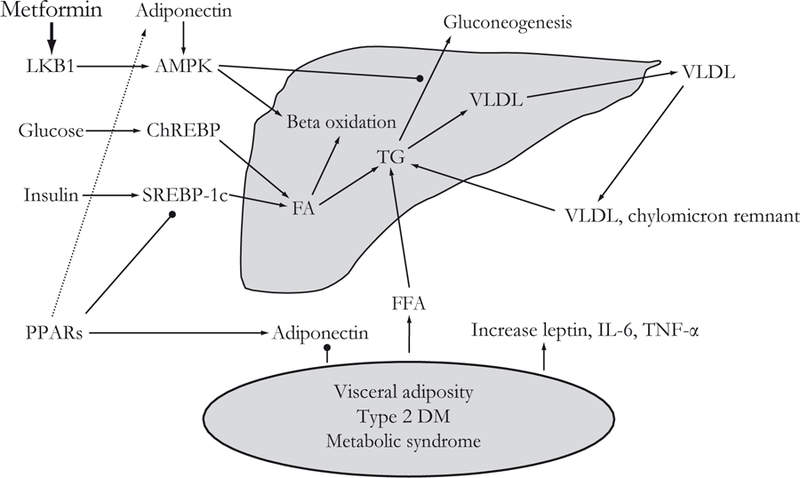
Hepatic steatosis results from excess storage of triglycerides in the liver. Glucose and insulin, via induction of ChREBP and SREBP-1c, respectively, promote fatty acid and triglyceride accumulation. Triglyceride levels are decreased by beta oxidation of fatty acids and conversion of triglycerides to VLDL with subsequent release from the liver. Visceral adiposity, diabetes, and the metabolic syndrome decrease circulating adiponectin levels and increase free fatty acids, leading to their eventual esterification and storage in the liver as triglycerides. Metformin increases the phosphorylation and nuclear export of LKB1. This activates AMPK, leading to phosphorylation of a number of proteins responsible for increasing fatty acid oxidation and inhibiting gluconeogenesis. PPARs promote fatty acid oxidation by increasing adiponectin levels and inhibiting SREBP-1c. AMPK=5’adenosine monophosphate-activated protein kinase; ChREBP=carbohydrate responsive element binding protein; DM=diabetes mellitus; FA=fatty acid; FFA=free fatty acid; IL-6=interleukin-6; LKB1=serine/ threonine-protein kinase 11; PPAR=peroxisome proliferators-activated receptor; SREBP-1c=sterol regulatory element binding protein-1c; TG=triglyceride; TNF-α=tumor necrosis factor-alpha; VLDL=very low density lipoprotein.
It is important to emphasize that insulin resistance is nearly universal in patients with NAFLD, and is thought to play a key role in its pathogenesis by promoting peripheral lipolysis and de novo lipogenesis. Several cross-sectional studies have found strong associations between direct measures of insulin resistance and NAFLD. A long-term prospective study demonstrated that presence of the metabolic syndrome is associated with an increased risk for ultrasound-defined NAFLD (adjusted odds ratio [OR]=4.0 and 11.2 for men and women, respectively).12 Another study revealed that mild insulin resistance is very common at the earliest stages of NAFLD and more severe insulin resistance (ie, clinical presence of the metabolic syndrome or type 2 diabetes) correlates with more advanced stages of NAFLD.13 Finally, a cross-sectional study found that presence of the metabolic syndrome was associated with higher OR of NASH (OR=3.2) and fibrosis (OR=3.5), even adjusting for sex and body mass index (BMI).14
Therapeutic Options in NASH
Recent advances into the pathogenesis of NAFLD have spawned new studies into pharmacologic approaches to treatment. The overarching aim of therapy for NAFLD is to improve peripheral and hepatic insulin resistance, which is thought to be necessary to reverse hepatic steatosis and hepatocellular damage, thus preventing progression to end-stage liver disease and its associated complications. In conjunction with any pharmacologic therapy, it is important to stress lifestyle modifications, which include exercise and weight loss. In addition, treatment of other risk factors for the metabolic syndrome, if present, including hyperlipidemia, diabetes, and hypertension should be addressed. Despite universal recommendations for lifestyle modifications, there is sparse evidence for improvement in meaningful long-term outcomes, such as histology or hepatic fibrosis progression. A recent publication reviewed the literature looking for studies examining weight loss and lifestyle modifications for the treatment of NAFLD.15 It revealed only 14 studies that had study entry assessments satisfying well-defined criteria. Histologic outcomes were reported in only five of these cases. All but one study was a small, pilot, uncontrolled study, or limited case series lacking well-defined treatment details. In the three studies where treatment was carried out to reduce excess nutrition and increase exercise, a remarkable effect on weight loss and an improvement in liver histology were reported.15 This lack of quality data makes it difficult to create clear evidence-based guidelines defining recommendations for dietary modification and exercise in NAFLD. However, the consensus recommendation is that patients lose 7%–10% of their body weight through dietary modifications and exercise over a course of 6–12 months.16 This is based on short-term studies revealing that this degree of weight loss improved insulin resistance and hepatic histology.17 Clearly, weight loss is important to improve insulin resistance in NAFLD. However, the focus of this review will be on pharmacologic agents to improve insulin sensitivity in NASH.
ROLE OF INSULIN SENSITIZERS
Insulin sensitizer therapy has become the main treatment approach for NASH given that insulin resistance is near universal in this disease. However, a large number of the studies investigating the use of these therapies are small, proof-of-concept studies without stringent study design. The largest amount of data in human studies is available for two types of insulin sensitizers: the biguanides (metformin) and the thiazolidinediones (TZDs: pioglitazone, troglitazone, rosiglitazone). The clinical data for each will be detailed below. In addition to a review of the clinical data on these therapies, we will summarize preclinical and early clinical data on novel therapies that may prove to be of clinical benefit in NAFLD.
Metformin
Metformin belongs to a class of insulin-sensitizing drugs called the biguanides, which were initially shown to have benefit in type 2 diabetes. Metformin has been recognized since the 1950s, but it did not receive approval by the US Food and Drug Administration (FDA) for type 2 diabetes until 1994.18 Phenformin, another biguanide, was withdrawn from the market due to the risk of lactic acidosis. Metformin lowers hepatic glucose production and promotes glucose uptake in the muscles. The mechanism of action for metformin was only recently elucidated.18 Metformin exerts its insulin-sensitizing effect via activation of the 5’adenosine monophosphate (AMP)-activated protein kinase (AMPK) pathway, which leads to increased lipid and glucose catabolism (Figure 1).19 In NAFLD, it was first tested in ob/ob mice, a model of hepatic steatosis, and revealed that metformin treatment reversed hepatomegaly, steatosis, and ALT abnormalities via reduced hepatic tumor necrosis factor (TNF)-α expression, which led to decreased lipid accumulation.20
Several trials have examined the role of metformin in adults with NAFLD (Table 1). Marchesini et al.21 treated 20 patients with biopsyproven NASH with open-label metformin for 4 months. They showed significant improvements in insulin resistance, aminotransferase levels, and ultrasound-defined liver volume. However, no follow-up biopsies were performed. In another open-label trial, Nair et al.22 reported 15 patients who completed 12 months of metformin therapy followed by a posttreatment liver biopsy in 10 patients. There was a significant decrease in aminotransferase levels at 3 months that paralleled a significant increase in insulin sensitivity at 3 months. However, after 3 months there was no further improvement in insulin sensitivity and an increase in aminotransferase levels was noted. In the 10 patients who received a posttreatment biopsy, three patients showed improvement in steatosis, two patients had decreased inflammation, and one patient showed decreased fibrosis.22
Table 1.
Studies of metformin in adult patients with nonalcoholic fatty liver disease (NAFLD).
| Reference | Type of study | No. of patients* | Diabetic patients | Drug† | Compared with | Duration of treatment | NASH vs. all NAFLD | ALT improved‡ | Histology improved‡ |
|---|---|---|---|---|---|---|---|---|---|
| Marchesini et al. 200121 | Open label, single arm | 20 | No | Metformin | Baseline | 4 months | NASH | Yes | Not assessed |
| Nair et al. 200422 | Open label, single arm | 15 | Mixed | Metformin | Baseline | 48 weeks | NAFLD | Transient | Modest |
| Uygun et al. 200423 | Open label, randomized | 17 | No | Metformin | LM | 6 months | NASH | Yes | No |
| Bugianesi et al. 200524 | Open label, randomized | 55 | No | Metformin | Vitamin E or LM | 12 months | NAFLD | Yes | Yes |
| Duseja et al. 200725 | Case control | 25 | No | Metformin | LM | 6 months | NAFLD | Yes | Not assessed |
| Akyuz et al. 200726 | Open label, nonrandomized | 12 | Mixed | Metformin | Rosiglitazone or LM | 12 months | NAFLD | No | No |
| de Oliveira et al. 200827 | Open label, single arm | 20 | Mixed | Metformin + NAC |
Baseline | 12 months | NASH | Yes | Yes |
| Idilman et al. 200828 | Open label, randomized | 24 | Mixed | Metformin | Rosiglitazone or LM | 48 weeks | NASH | No | No |
| Loomba et al. 200829 | Open label, single arm | 28 | Mixed | Metformin | Baseline | 48 weeks | NASH | Yes | Yes |
| Nar & Gedik 200930 | Open label, randomized | 19 | Yes | Metformin | LM | 6 months | NAFLD | No | Not assessed |
| Omer et al. 200931 | Open label, randomized | 22 | Yes | Metformin | Rosiglitazone | 12 months | NAFLD | No | No |
Number of patients who received the study drug.
All patients in the study arm were also placed on lifestyle modifications.
Improvements in ALT or histology are reported if significantly better than comparison group.
ALT=alanine aminotransferase; LM=lifestyle modification; NAC=N-acetylcysteine; NAFLD=nonalcoholic fatty liver disease; NASH=non-alcoholic steatohepatitis.
Uygun et al.23 performed a controlled, open-label trial comparing dietary modification (n=17) to dietary modification plus metformin (n=17) over a course of 6 months. The metformin group had a significant decrease in aminotransferases and insulin levels, but both groups showed histologic improvements in inflammation. Bugianesi et al.24 performed a randomized, controlled trial comparing metformin to vitamin E or dietary modifications for 12 months. Fifty-five patients received metformin and were significantly more likely to have aminotransferase normalization. Follow-up biopsies performed in 17 patients receiving metformin showed significant improvements in steatosis, inflammation, and fibrosis compared with baseline.24
Loomba et al. performed an open-label study evaluating the role of metformin on biochemical, anthropometric, and histologic features in 28 patients with biopsy-proven NASH.29 Twentysix patients completed 48 weeks of treatment and underwent a repeat liver biopsy. Approximately 50% of the patients showed enhanced insulin sensitivity and 30% of the patients had strict histologic improvement defined as a three-point improvement in the NASH activity score (0–12) (a sum of three histologic parameters including lobular inflammation [0–4], steatosis [0–4], and ballooning degeneration [0–4]). In addition, 50% of patients had biochemical improvements. Histologic improvement in this study had a significant association with weight loss. Most of the weight loss occurred in the first 24 weeks and then stabilized. Histologic responders achieved greater weight loss compared with non-responders. This suggests that metformin-associated weight loss may lead to biochemical and histologic improvement, in addition to the direct effect of metformin on improving insulin sensitivity.29 In this study, predictors of treatment response to metformin included baseline BMI of <40 and weight loss while receiving therapy. A meta-analysis published in the Cochrane database included published data from the studies of Uygun et al. and Buganiesi et al., and concluded that metformin significantly leads to normalization of aminotransferases and improved steatosis by ultrasound imaging compared with dietary modification, though the total number of patients in these studies was small.32
More recently, Duseja et al.25 published an open-label trial on metformin use in 25 adult Indian patients with NAFLD who did not achieve normalization of ALT levels after 6 months of lifestyle interventions and ursodeoxycholic acid. Results were compared with 25 patients with NAFLD who were treated only with lifestyle interventions. In comparison to untreated controls, all patients treated with metformin had partial biochemical response and 56% of those treated achieved normalization of ALT. Akyuz et al.26 evaluated the effect of metformin in NAFLD in 12 patients compared with rosiglitazone or lifestyle modifications. In the metformin-treated individuals, ALT normalization occurred in 33.3% at 6 months and in 22.2% at 12 months. Steatosis and fibrosis did not change after treatment in the four patients who underwent posttreatment liver biopsies.26 Idilman et al.28 enrolled 74 patients with NASH and randomized them to lifestyle modifications, or lifestyle modifications plus metformin or rosiglitazone. Twenty-four patients received metformin for 48 weeks. In this trial, metformin therapy failed to show a significant biochemical or histologic improvement. Nar and Gedik30 performed an open-label trial comparing metformin to lifestyle modifications in type 2 diabetic patients with ultrasound-proven NAFLD. Nineteen patients received metformin therapy. In this trial, there was no difference between metformin or lifestyle modifications in biochemical or ultrasonographic response.
Omer et al.31 performed an open-label, randomized trial in 64 patients with insulin resistance and elevated ALT with a pretreatment biopsy confirming NAFLD. In the group treated with metformin for 12 months (n=22), there was no significant change in ALT nor improvement in posttreatment histology. De Oliveira et al.27 performed a pilot study evaluating the effect of the combination of metformin with N-acetylcysteine (NAC), which has antioxidant properties. Twenty patients with biopsy-proven NASH were treated for 12 months. ALT and homeostasis model assessment of insulin resistance (HOMA-IR) levels improved significantly after treatment. Posttreatment biopsies revealed that liver steatosis, fibrosis, and NASH activity scores all improved with treatment. These results are difficult to interpret due to the lack of a control group and the possible contribution of NAC to the study results.27
In summary, only a small number of patients with biopsy-proven NASH have been treated with metformin in controlled clinical trials. This makes evidence-based recommendations for its role in NASH difficult, though metformin is generally well tolerated. In addition, its role in inducing weight loss may be of importance in patients with NASH. Metformin should not be used in those with renal insufficiency or with congestive heart failure due to the risk of lactic acidosis. A recently completed trial comparing metformin with vitamin E or placebo in pediatric patients with NASH (TONIC: Treatment of Nonalcoholic Liver Disease in Children; ) should provide better insight into the effect of metformin in NASH. It is unclear whether the results of this trial could be extrapolated into an adult population. These results are expected to be published in late 2009.
Thiazolidinediones
TZDs include troglitazone (which has since removed from the market due to a risk of idiosyncratic hepatotoxic injury), rosiglitazone, and pioglitazone. They are an oral class of antidiabetic drugs, which function as agonists of the peroxisone-proliferator activated receptorgamma (PPAR-γ). PPAR-γ is a transcription factor that regulates gene expression in liver, adipose, vascular endothelium, and muscle tissue. Its main action is to promote differentiation of pre-adipocytes into adipocytes, which leads to the redistribution of triglycerides from liver and muscle into proliferating adipocytes (as seen in Figure 1). Clinically, this leads to improved glycemic control, decreased hepatic fat content, and improved insulin sensitivity.18 Mechanistically, TZDs have also been shown to improve insulin sensitivity by increasing adiponectin levels (Figure 1). Adiponectin is an adipokine that has been shown to improve insulin resistance and decrease hepatic fat content.33 This was shown by Lutchman et al.34 with an open-label study, which revealed that TZD therapy for 48 weeks led to an increase in adiponectin levels, which correlated with a decrease in hepatic steatosis. Finally, the TZDs have been shown to activate the AMPK pathway, which could also explain their insulin-sensitizing effect.35,36 However, there are unwanted side effects and potential risks with this class of drugs, including weight gain, fluid retention, increased fracture rate, and possibly excess cardiovascular events.37,38
There has been significant interest in evaluating TZDs to treat NASH (Table 2). The first open-label pilot trial, published in 2001 by Caldwell et al.,39 examined troglitazone for up to 6 months in 10 women with biopsy-proven NASH. ALT levels normalized in 70% of NASH patients at the end of treatment, but this biochemical response was associated with only mild histologic improvement, and all follow-up biopsies had evidence of NASH. Neuschwander-Tetri et al.40 published an open-label uncontrolled trial using rosiglitazone in 30 overweight patients with biopsy-proven NASH for 48 weeks. There was significant improvement in ALT, insulin sensitivity, liver steatosis, and inflammation, but no change in hepatic fibrosis. Promrat et al.41 conducted a study in nondiabetic patients with biopsy-proven NASH and reported that pioglitazone normalized aminotransferase levels and led to a significant histologic response in the majority of treated patients. This study included 18 nondiabetic NASH patients who received pioglitazone at 30 mg daily for 48 weeks. Sanyal et al.42 also showed promising results in another pilot trial comparing pioglitazone plus vitamin E with vitamin E alone in nondiabetic patients with NASH for 6 months. Both groups had reduction in hepatic inflammation but only a statistically significant reduction was noted in the pioglitazone plus vitamin E group. It was unclear whether the benefits were due to pioglitazone alone or whether vitamin E had an additive effect with pioglitazone. Wang et al.44 examined the role of rosiglitazone in an open-label trial of 68 uncontrolled type 2 diabetics with NAFLD who were previously treated with insulin secretagogues and/or metformin. Patients were treated for a total of 24 weeks. Of the 60 patients who completed the study, 33.3% of patients achieved a normal ALT.
Table 2.
Studies of thiazolidinediones (TZDs) in adult patients with nonalcoholic fatty liver disease (NAFLD).
| Reference | Type of study | Number of patients* | Diabetic patients | Drug† | Compared with | Duration of treatment | NAFLD vs. biopsy-proven NASH | ALT improved‡ | Histology improved‡ |
|---|---|---|---|---|---|---|---|---|---|
| Caldwell et al. 200139 | Open label, single arm | 10 | Mixed | Troglitazone | Baseline | ≤6 months | NASH | Yes | No |
| Neuschwander-Tetri et al. 200340 | Open label, single arm | 30 | Mixed | Rosiglitazone | Baseline | 48 weeks | NASH | Yes | Yes |
| Promrat et al. 200441 | Open label, single arm | 18 | No | Pioglitazone | Baseline | 48 weeks | NASH | Yes | Yes |
| Sanyal et al. 200442 | Open label, randomized | 10 | No | Pioglitazone + vitamin E | Vitamin E | 6 months | NASH | No | Yes |
| Belfort et al. 200643 | Blinded, randomized, placebo controlled | 26 | Yes | Pioglitazone | Placebo | 6 months | NASH | Yes | Yes |
| Wang et al. 200644 | Open label, single arm | 68 | Yes | Rosiglitazone | Baseline | 6 months | NAFLD | Yes | Not assessed |
| Akyuz et al. 200726 | Open label, nonrandomized | 11 | Mixed | Rosiglitazone | Metformin or LM | 12 months | NAFLD | No | No |
| Aithal et al. 200845 | Blinded, randomized, placebo controlled | 31 | No | Pioglitazone | Placebo | 12 months | NASH | Yes | Yes |
| Idilman et al. 200828 | Open label, randomized | 25 | Mixed | Rosiglitazone | Metformin or LM | 48 weeks | NASH | No | Yes |
| Ratziu et al. 200846 | Blinded, randomized, placebo controlled | 32 | Mixed | Rosiglitazone | Placebo | 12 months | NASH | Yes | Yes |
| Omer et al. 200931 | Open label, randomized | 20 | Yes | Rosiglitazone | Metformin | 12 months | NAFLD | Yes | Yes |
Number of patients who received the study drug.
All patients in the study arm were also placed on lifestyle modifications.
Improvements in ALT or histology are reported if significantly better than comparison group.
ALT=alanine aminotransferase; LM=lifestyle modification; NAFLD=nonalcoholic fatty liver disease; NASH=nonalcoholic steatohepatitis.
Subsequently, larger controlled trials were published using TZDs, which showed greater promise in their efficacy. Belfort et al.43 published the first double-blind, placebo-controlled trial using diet plus pioglitazone compared with diet plus placebo for 6 months in 55 patients with NASH. The 26 patients in the diet plus pioglitazone group showed improved glycemic control, decreased hepatic fat, increased hepatic insulin sensitivity, and improved histologic findings of steatosis and necro-inflammation. There was no significant reduction in fibrosis noted in the pioglitazone group. Aithal et al.45 published a similar trial in patients studied for 1 year. They randomized 74 nondiabetic patients with biopsy-proven NASH to a standard diet, exercise, and either placebo or pioglitazone. Sixty-one patients had posttreatment liver biopsies. Pioglitazone therapy was associated with decreased ALT levels, improved insulin sensitivity, and improved histologic necro-inflammatory markers. Unlike the trial conducted by Belfort and colleagues,43 Aithal et al. revealed that fibrosis was improved in the pioglitazone group.45 The FLIRT (Fatty Liver Improvement with Rosiglitazone Therapy) trial results were recently published examining rosiglitazone or placebo in 63 patients treated for 1 year.46 Patients receiving rosiglitazone did have a statistically significant improvement in aminotransferases, insulin sensitivity, and hepatic steatosis, but did not show improvement in other histologic markers, notably the histologic NAFLD activity score (NAS) or overall fibrosis. In addition, only half of the patients receiving rosiglitazone responded to therapy. This study demonstrates that TZDs alone without lifestyle modifications may not be as effective in NASH. However, the study was limited by low baseline NAS scores, which created little room for improvement.46 Authors reported that randomization to the rosiglitazone arm, absence of diabetes, and higher steatosis score were independent predictors of treatment response.
There have been other recently published, small, open-label trials examining TZD therapy in comparison with metformin and/or lifestyle modifications in NAFLD and NASH. The trial by Akyuz et al.26 included 11 patients with NAFLD who were treated with rosiglitazone for 12 months. ALT normalization occurred in 54.5% at 6 months and in 37.5% at 12 months. No significant change in histology was noted in the eight patients who underwent posttreatment liver biopsies. Idilman et al.28 treated 25 patients in an open-label study with rosiglitazone for 48 weeks. Unlike the patients who were treated with metformin in this trial, the group treated with rosiglitazone did show a significant improvement in ALT. There was also an improvement in steatosis in those patients who underwent a posttreatment liver biopsy.28 In the trial by Omer et al.31 there was a comparison arm of patients treated with rosiglitazone. This study revealed that rosiglitazone led to significant improvements in ALT, HOMA-IR, and in postbiopsy NAFLD scores.
At present, the role for TZD therapy in NASH is promising. However, it is unclear whether TZD-associated increase in adiposity and the resultant weight gain would ultimately limit its benefits in improving long-term health outcomes in NASH patients. In addition to weight gain, practitioners also need to consider other known side effects when considering long-term use of TZDs in NASH, including a potential for increased cardiovascular events and fracture risk. An ongoing US multicenter trial (PIVENS: Pioglitazone Vs. Vitamin E Vs. Placebo for Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis; ) evaluating pioglitazone vs. vitamin E vs. placebo for 96 weeks in nondiabetic patients with NASH, will hopefully help to clarify the long-term usefulness and safety of the TZDs in NASH.47 Clearly, more data are needed regarding the efficacy and safety of the TZD agents in NASH patients without diabetes before recommendations for safe use can be made.
Other Insulin-Sensitizing Agents
Other insulin-sensitizing agents that show promise for clinical effectiveness in NASH are under development or in the early clinical testing phases. The most clinically advanced agent to date is exenatide. Exenatide is a synthetic version of exendin-4, a hormone that was initially isolated from the saliva of the Gila monster. It is a peptide agonist of the glucagon-like peptide-1 (GLP-1) receptor and it functions primarily to stimulate insulin release from the pancreatic β-cells. However, exenatide does not function as a direct insulin sensitizer, but rather induces clinically significant weight loss, which may lead to an insulin-sensitizing effect. In animal studies, administration of exendin-4 significantly reduced glucose levels, improved insulin sensitivity, and reduced hepatic steatosis in ob/ob mice.48 This study suggested that GLP-1 proteins in liver have a novel direct effect on hepatocyte fat metabolism. An open-label, uncontrolled clinical trial using exenatide to assess drug safety in diabetics over an average period of 3.5 years revealed that patients had improved AST and insulin sensitivity.49 In addition, those with elevated ALT at baseline (n=116) had significant reductions in ALT, and 41% achieved normal ALT levels with treatment. Patients with elevated ALT at baseline tended to lose more weight than patients with normal ALT levels at baseline; however, weight change was not correlated with baseline ALT or change in ALT. There has been one case report of a 59-year-old male with type 2 diabetes who was treated with exenatide in addition to metformin monotherapy.50 Following 44 weeks of exenatide therapy, this patient had a normalized ALT, and mean hepatic steatosis measured by liver spectroscopy decline from 15.8% to 4.3%. Exenatide was FDA approved for use in the US in 2005 as adjunctive therapy for type 2 diabetes and is available as a subcutaneous injection. Clearly, exenatide is a promising agent, and two clinical trials have been initiated to study its effect in NASH though no recommendations can be made at this time. Vuppalanchi and colleagues16 have initiated a study to examine its efficacy in NASH patients in an open-label study ().
Other PPAR agonists have been shown to have insulin-sensitizing effects and thus are possible targets in NAFLD. The PPAR-δ agonist GW501516 has been examined in a mouse model of NASH.51 It reduced hepatic triglyceride levels, hepatic fat droplets, inflammatory cells, and decreased the expression of pro-inflammatory markers. PPAR-δ agonist treatment in an ethanol-mediated hepatic injury and steatosis rat model attenuated the severity of ethanol’s adverse effects on hepatic repair by restoring insulin responsiveness.52 Collectively, these findings suggest that PPAR-δ is a potential therapeutic target for insulin resistance and hepatic steatosis.53
Finally, there had been some preliminary promise with the selective cannabinoid type I (CB1) receptor blockers in improving hepatic steatosis and promoting weight loss in NAFLD. The endocannabinoid system is involved in the regulation of food uptake, body weight, and insulin sensitivity. Obesity leads to upregulation of the CB1 receptors, which leads to hepatic lipogenesis, fatty acid synthesis in adipocytes, and decreased adiponectin levels.54 In two large, placebo-controlled clinical trials using the CB1 antagonist rimonabant to study weight loss, metabolic improvements were remarkable with notable improvements in insulin sensitivity.55,56 This is largely thought to be due to weight loss, but the metabolic effects appear to exceed what is directly related to weight loss alone, suggesting a direct action on improvement in insulin sensitivity. Rimonabant (SR141716), taranabant, and otenabant are three CB1 antagonists in clinical development. Rimonabant, the most developmentally advanced agent, was removed from the market in the European Union due to the adverse side effect of psychiatric disturbances, mostly depression.57 All ongoing clinical trials and development programs with these agents has been halted, including ongoing studies in NASH. Hopefully, the safety issues with these agents will be resolved and clinical trials will be developed with select patient populations to allow testing to continue in NAFLD.
SUMMARY
NAFLD is a burgeoning disease with an increasing prevalence worldwide. It has far-reaching consequences due to its association with the metabolic syndrome and cardiac risk factors of hypertension, hyperlipidemia, obesity, and diabetes. Insulin resistance is near universal in this disease and improvements in insulin sensitivity have been shown to correlate with beneficial short-term clinical outcomes in NASH. The impact of lifestyle modifications with weight loss and exercise cannot be overstated, though the long-term effect of this approach is unclear to date. At this time, insulin-sensitizing agents have shown the most promise in altering clinical course and outcomes. However, the published trials to date are in small, heterogeneous populations and it is difficult to make generalized conclusions regarding their mainstream use in NASH at this time. The TZDs have the most robust clinical data and more data from larger trials are expected to be published shortly, which will help to clarify their efficacy and long-term safety in NASH. The clinical data for metformin in NASH is not as robust, and its effect may be largely related to its ability to induce weight loss. However, its long-term use in diabetes has revealed it to be a safe agent in appropriate clinical scenarios. There are no trials to date assessing the cost-effectiveness of lifestyle modifications or the use of insulin-sensitizing agents in NAFLD. Though in the earliest stages of clinical development, there is hope that newer insulin-sensitizing agents will prove to be beneficial in NAFLD.
ACKNOWLEDGMENTS
This work was supported, in part, by the American Gastroenterological Association (AGA) Foundation—Sucampo—ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. Funding was provided by: Atlantic Philanthropies Inc., the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association to R.L.
Contributor Information
Lance L. Stein, Columbia University College of Physicians and Surgeons, Center for Liver Disease and Transplantation, New York Presbyterian Hospital, New York, USA
Mamie H. Dong, Division of Gastroenterology, Department of Medicine, University of California at San Diego, La Jolla, California, USA
Rohit Loomba, Division of Gastroenterology, Department of Medicine and Division of Epidemiology, Department of Family and Preventive Medicine, University of California, at San Diego, 9500 Gilman Drive, MC0063, La Jolla, California, 92093, USA..
REFERENCES
- 1.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology 2007;133:1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom SR, Kuhajda FP, Laher I, et al. The obesity epidemic: pharmacological challenges. Mol Interv 2008;8:82–98. [DOI] [PubMed] [Google Scholar]
- 3.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis 2008;28:386–395. [DOI] [PubMed] [Google Scholar]
- 4.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology 1990;11:74–80. [DOI] [PubMed] [Google Scholar]
- 5.Frantzides CT, Carlson MA, Moore RE, et al. Effect of body mass index on nonalcoholic fatty liver disease in patients undergoing minimally invasive bariatric surgery. J Gastrointest Surg 2004;8: 849–855. [DOI] [PubMed] [Google Scholar]
- 6.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 2009;51:371–379. [DOI] [PubMed] [Google Scholar]
- 7.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999;30: 1356–1362. [DOI] [PubMed] [Google Scholar]
- 8.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98: 960–967. [DOI] [PubMed] [Google Scholar]
- 9.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998;114:842–845. [DOI] [PubMed] [Google Scholar]
- 10.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120:1183–1192. [DOI] [PubMed] [Google Scholar]
- 11.Loomba R, Hwang SJ, O’Donnell CJ, et al. Parental obesity and offspring serum alanine and aspartate aminotransferase levels: the Framingham Heart Study. Gastroenterology 2008;134:953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005;143:722–728. [DOI] [PubMed] [Google Scholar]
- 13.Kang H, Greenson JK, Omo JT, et al. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am J Gastroenterol 2006;101: 2247–2253. [DOI] [PubMed] [Google Scholar]
- 14.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917–923. [DOI] [PubMed] [Google Scholar]
- 15.Bellentani S, Dalle Grave R, Suppini A, Marchesini G. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology 2008;47:746–754. [DOI] [PubMed] [Google Scholar]
- 16.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology 2009;49:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang MA, Greenson JK, Chao C, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol 2005;100:1072–1081. [DOI] [PubMed] [Google Scholar]
- 18.Woods YL, Petrie JR, Sutherland C. Dissecting insulin signaling pathways: individualised therapeutic targets for diagnosis and treatment of insulin resistant states. Endocr Metab Immune Disord Drug Targets 2009;9:187–198. [DOI] [PubMed] [Google Scholar]
- 19.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med 2000;6:998–1003. [DOI] [PubMed] [Google Scholar]
- 21.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet 2001;358:893–894. [DOI] [PubMed] [Google Scholar]
- 22.Nair S, Diehl AM, Wiseman M, Farr GH Jr., Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther 2004;20:23–28. [DOI] [PubMed] [Google Scholar]
- 23.Uygun A, Kadayifci A, Isik AT, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2004;19:537–544. [DOI] [PubMed] [Google Scholar]
- 24.Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 2005;100:1082–1090. [DOI] [PubMed] [Google Scholar]
- 25.Duseja A, Das A, Dhiman RK, et al. Metformin is effective in achieving biochemical response in patients with nonalcoholic fatty liver disease (NAFLD) not responding to lifestyle interventions. Ann Hepatol 2007;6:222–226. [PubMed] [Google Scholar]
- 26.Akyuz F, Demir K, Ozdil S, et al. The effects of rosiglitazone, metformin, and diet with exercise in nonalcoholic fatty liver disease. Dig Dis Sci 2007;52:2359–2367. [DOI] [PubMed] [Google Scholar]
- 27.de Oliveira CP, Stefano JT, de Siqueira ER, et al. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol Res 2008;38:159–165. [DOI] [PubMed] [Google Scholar]
- 28.Idilman R, Mizrak D, Corapcioglu D, et al. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2008;28:200–208. [DOI] [PubMed] [Google Scholar]
- 29.Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther 2008. In press. [DOI] [PMC free article] [PubMed]
- 30.Nar A, Gedik O. The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetol 2009;46:113–118. [DOI] [PubMed] [Google Scholar]
- 31.Omer Z, Cetinkalp S, Akyildiz M, et al. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2009. In press. [DOI] [PubMed]
- 32.Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or nonalcoholic steatohepatitis. Cochrane Database Syst Rev 2007;(1):CD005166. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj M, Suraamornkul S, Piper P, et al. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab 2004;89:200–206. [DOI] [PubMed] [Google Scholar]
- 34.Lutchman G, Promrat K, Kleiner DE, et al. Changes in serum adipokine levels during pioglitazone treatment for nonalcoholic steatohepatitis: relationship to histological improvement. Clin Gastroenterol Hepatol 2006;4:1048–1052. [DOI] [PubMed] [Google Scholar]
- 35.Fryer LG, Parbu-Patel A, Carling D. The antidiabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 2002;277:25226–25232. [DOI] [PubMed] [Google Scholar]
- 36.Coletta DK, Sriwijitkamol A, Wajcberg E, et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia 2009;52:723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dormuth CR, Carney G, Carleton B, Bassett K, Wright JM. Thiazolidinediones and fractures in men and women. Arch Intern Med 2009;169:1395–1402. [DOI] [PubMed] [Google Scholar]
- 38.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471. [DOI] [PubMed] [Google Scholar]
- 39.Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol 2001;96:519–525. [DOI] [PubMed] [Google Scholar]
- 40.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 2003;38:1008–1017. [DOI] [PubMed] [Google Scholar]
- 41.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004;39:188–196. [DOI] [PubMed] [Google Scholar]
- 42.Sanyal AJ, Mofrad PS, Contos MJ, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2004;2:1107–1115. [DOI] [PubMed] [Google Scholar]
- 43.Belfort R, Harrison SA, Brown K, et al. A placebocontrolled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297–2307. [DOI] [PubMed] [Google Scholar]
- 44.Wang CH, Leung CH, Liu SC, Chung CH. Safety and effectiveness of rosiglitazone in type 2 diabetes patients with nonalcoholic fatty liver disease. J Formos Med Assoc 2006;105:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008;135:1176–1184. [DOI] [PubMed] [Google Scholar]
- 46.Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) trial. Gastroenterology 2008;135:100–110. [DOI] [PubMed] [Google Scholar]
- 47.Chalasani NP, Sanyal AJ, Kowdley KV, et al. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials 2009;30:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006;43:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275–286. [DOI] [PubMed] [Google Scholar]
- 50.Tushuizen ME, Bunck MC, Pouwels PJ, van Waesberghe JH, Diamant M, Heine RJ. Incretin mimetics as a novel therapeutic option for hepatic steatosis. Liver Int 2006;26:1015–1017. [DOI] [PubMed] [Google Scholar]
- 51.Nagasawa T, Inada Y, Nakano S, et al. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol 2006;536:182–191. [DOI] [PubMed] [Google Scholar]
- 52.Pang M, de la Monte SM, Longato L, et al. PPAR-delta agonist attenuates alcohol-induced hepatic insulin resistance and improves liver injury and repair. J Hepatol 2009;50:1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reilly SM, Lee CH. PPAR delta as a therapeutic target in metabolic disease. FEBS Lett 2008;582: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nogueiras R, Rohner-Jeanrenaud F, Woods SC, Tschop MH. The endocannabinoid system and the control of glucose homeostasis. J Neuroendocrinol 2008;20(suppl. 1):147–151. [DOI] [PubMed] [Google Scholar]
- 55.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005;365:1389–1397. [DOI] [PubMed] [Google Scholar]
- 56.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 2006;295:761–775. [DOI] [PubMed] [Google Scholar]
- 57.Janero DR, Makriyannis A. Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerg Drugs 2009;14:43–65. [DOI] [PubMed] [Google Scholar]


