Abstract
Repeated bouts of endurance exercise promotes numerous biochemical adaptations in skeletal muscle fibers resulting in a muscle phenotype that is protected against a variety of homeostatic challenges; these exercise-induced changes in muscle phenotype are often referred to as “exercise preconditioning”. Importantly, exercise preconditioning provides protection against several threats to skeletal muscle health including cancer chemotherapy (e.g., doxorubicin) and prolonged muscle inactivity. This review summarizes our current understanding of the mechanisms responsible for exercise-induced protection of skeletal muscle fibers against both doxorubicin-induced muscle wasting and a unique form of inactivity-induced muscle atrophy (i.e., ventilator-induced diaphragm atrophy). Specifically, the first section of this article will highlight the potential mechanisms responsible for exercise-induced protection of skeletal muscle fibers against doxorubicin-induced fiber atrophy. The second segment will discuss the biochemical changes that are responsible for endurance exercise-mediated protection of diaphragm muscle against ventilator-induced diaphragm wasting. In each section we highlight gaps in our knowledge in hopes of stimulating future research in this evolving field of investigation.
Keywords: skeletal muscle, doxorubicin, endurance exercise, diaphragm, mechanical ventilation, doxorubicin, disuse muscle atrophy, preconditioning
INTRODUCTION
Skeletal muscle comprises approximately 40% of total body mass in healthy young adults and among other functions, muscle plays an essential role in the force generation required for both locomotion and breathing. Moreover, it is established that skeletal muscle is a secretary (endocrine) organ that releases hormones that are collectively termed “myokines”. Numerous myokines are released during exercise and contribute to the many beneficial systemic effects of exercise training on cardiovascular, metabolic, and mental health [24,29,45]. Therefore, it is important to maintain healthy skeletal muscles and engage in regular exercise.
Unfortunately, numerous threats to skeletal muscle health exist; these include age-related loss of skeletal muscle (i.e., sarcopenia), disease-related muscle wasting (e.g., cancer cachexia, sepsis-induced cachexia, etc.), disuse locomotor muscle atrophy (e.g. limb immobilization, etc.), ventilator-induced diaphragm atrophy, and the skeletal muscle myopathy associated with the treatment for cancer using select chemotherapy agents (e.g., doxorubicin) [12,28,79].
Ironically, although prolonged mechanical ventilation (MV) or treatment with doxorubicin (DOX) are often life-saving clinical interventions, both therapies result in significant skeletal muscle wasting. In particular, MV is a life-sparing therapy for many critically ill patients. Unfortunately, prolonged MV promotes the rapid development of diaphragmatic atrophy and contractile dysfunction; this diaphragmatic weakness increases the risk for the inability to wean patients from the ventilator [21,106]. DOX is a potent anticancer drug that is commonly used to treat many human cancers. Regrettably, DOX is also cytotoxic to healthy cells and promotes rapid atrophy of both limb and respiratory muscles resulting in muscle weakness and fatigue in patients [28]. Because of the widespread clinical usage of both doxorubicin and MV, developing strategies to prevent muscle wasting from both of these therapies is important.
It is well-established that regular endurance exercise stimulates numerous adaptations in both limb and diaphragm muscle fibers [11,32,70,77]. In the context of this review, we define endurance exercise as submaximal exercise (i.e., 50–70% VO2 max) performed 4–5 days/week (30–60 minutes per training session). In this regard, treadmill exercise training is the most common exercise modality utilized in rodent endurance exercise studies. It is well-established that repeated bouts of endurance exercise training activates select cell signaling pathways that promote mitochondrial biogenesis, expression of stress proteins, and increased antioxidant capacity in the skeletal muscle fibers recruited during the exercise modality (reviewed in [8]). Collectively, these endurance exercise-induced adaptations in the active limb and respiratory muscles result in fibers that are protected against a variety of stresses; this exercise-induced muscle phenotype is commonly termed “exercise preconditioning”. Importantly, exercise preconditioned muscle fibers are protected against both DOX-induced myopathy and MV-induced diaphragmatic wasting. This review will highlight the experimental evidence that exercise preconditioning protects skeletal muscles against both DOX-induced muscle myopathy and MV-induced diaphragm wasting and debate the putative mechanisms responsible for exercise-induced protection against these myopathies.
EXERCISE TRAINING PROTECTS AGAINST DOXORUBICIN-INDUCED SKELETAL MUSCLE WASTING
DOX is an anthracycline antibiotic that is commonly used as a frontline drug for treating numerous cancers [101]. Although DOX is considered an effective anticancer drug, its usage is limited because of cellular toxicity [31]. Specifically, DOX is cytotoxic in a dose-dependent manner and often results in damage to the heart, skeletal muscles, and other organs [31,100]. In regard to DOX-induced skeletal muscle damage, DOX promotes rapid skeletal muscle fiber atrophy resulting in both muscle weakness and fatigue in patients [100]. This DOX-induced muscle weakness and fatigue is associated with a reduced ability to perform activities of daily living (i.e., impaired quality of life) and a higher risk of morbidity and mortality in cancer patients [60,100]. Therefore, developing countermeasures to protect skeletal muscles against DOX-induced myopathy is important. The next segments discuss the mechanisms responsible for DOX-induced skeletal muscle myopathy, highlight the evidence that endurance exercise training protects muscles against DOX-induced myopathy, and debate the mechanisms responsible for exercise-induced protection against DOX-induced muscle wasting.
DOX-induced muscle weakness: overview and mechanisms
The mechanisms responsible for DOX-induced cardiac and skeletal muscle myopathy have received significant experimental attention during the past two decades and these findings are summarized in recent reviews [54,100]. Therefore, only a brief discussion of the mechanisms responsible for DOX-induced skeletal muscle myopathy will be provided here.
DOX-induced skeletal muscle weakness follows a dose-dependent relationship with higher doses of DOX promoting the greatest muscle weakness [40]. Preclinical studies have shown that this DOX-induced muscle weakness is due to both fiber atrophy and muscle contractile dysfunction (i.e., reduced specific force production); moreover, the DOX-induced muscle fatigue is greatest in skeletal muscles that possess a high percentage of slow fiber types (e.g., soleus muscle in rodents) [20,40].
The molecular basis behind DOX-induced skeletal muscle myopathy remains an active area of research. In this regard, it is established that mitochondrial dysfunction plays a central role in DOX-induced skeletal muscle myopathy [54,59,100].
Although the mechanisms responsible for DOX-induced mitochondrial dysfunction are complex, increases in the production of reactive oxygen species (ROS) are predicted to play a key role in DOX-induced mitochondrial damage [17,84]. Indeed, a single dose of DOX results in a robust increase in ROS emission from skeletal muscle mitochondria [26,27,59]. Although DOX-induced increases in mitochondrial ROS production occurs at several sites within mitochondria, the primary site of elevated ROS production occurs at complex I via the single electron reduction of DOX by mitochondrial NADH-dehyrdrogenase; this reduction of DOX generates an unstable semiquinone that forms a redox cycle to donate an electron to oxygen to form the superoxide radical [16,18].
Another factor that contributes to DOX-induced impairment of mitochondrial energy production is that after entering the mitochondria, DOX forms a complex with the phospholipid, cardiolipin, located on the mitochondrial inner membrane [84]. The formation of the DOX/cardiolipin complex impairs the normal functions of cardiolipin [84]. Moreover, in rats treated with DOX, mitochondrial levels of cardiolipin are also decreased [65,66]. These DOX-induced changes in cardiolipin abundance and function are important because cardiolipin is an essential component of the mitochondrial inner membrane that plays a key role in many mitochondrial processes, including oxidative phosphorylation [19].
Also, preclinical studies reveal that treatment of rodents with DOX results in an accumulation of iron inside the mitochondrion [41]; this is significant because an increase in mitochondrial iron content promotes the production of the highly reactive hydroxyl radical resulting in greater mitochondrial oxidative damage [76]. Indeed, a DOX-induced increase in mitochondrial iron levels accelerates DOX-induced damage to cardiac myocytes [41].
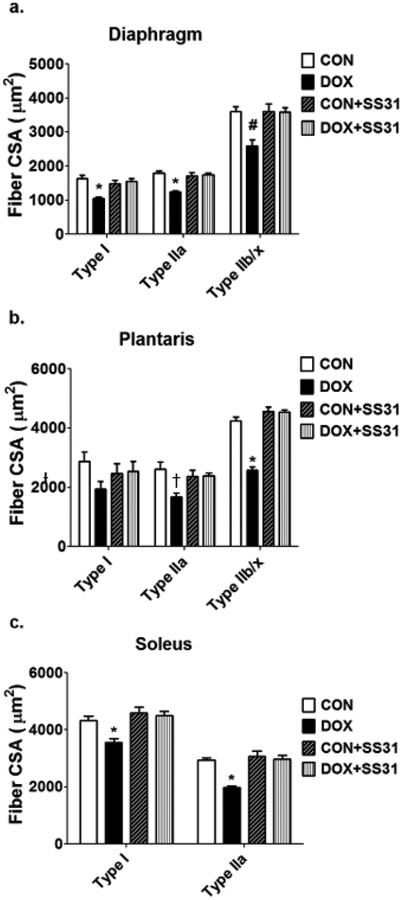
The evidence that increases in mitochondrial ROS emission plays a required role in DOX-induced skeletal muscle myopathy derives from experiments revealing that treatment of animals with a mitochondrial-targeted antioxidant (SS-31) prevents the DOX-induced increase in mitochondrial ROS emission and muscle atrophy [59]. Specifically, SS-31 is a multifunctional mitochondrial protective compound that acts by reducing the DOX-induced increases in ROS production, inhibiting cardiolipin peroxidation, and preserving mitochondrial structure [14]. Indeed, treatment of animals with SS-31 prevented the DOX-induced increase in mitochondrial ROS emission and protected both limb and diaphragm muscle from DOX-induced fiber atrophy (Fig. 1). These findings have been confirmed by a study demonstrating that targeted overexpression of mitochondrial catalase in skeletal muscle prevents DOX-induced mitochondrial ROS emission and protects against both skeletal muscle atrophy and contractile dysfunction [26].
Fig. 1.
Treatment of animals with a mitochondrial-targeted antioxidant (SS-31) protects the (A) diaphragm and limb muscles (i.e., (B) plantaris and (C) soleus) of rodents against DOX-induced muscle fiber atrophy. See the text for more details. Data are from Min et al. [59]. Key: CON = control; DOX = doxorubicin treatment; CON + SS31= control animals treated with SS31; DOX + SS31= animals treated with both DOX and SS31; * = different (P<0.05) from all other groups; # = different (P<0.05) from all CON and CON+SS31; † = different (P<0.05) from CON.
The nexus between increased ROS production (i.e., oxidative stress) and muscle atrophy is well established. In this regard, the first evidence that redox disturbances contribute to muscle atrophy was reported in the early 1990’s [50]. Since this original observation, many studies have confirmed that chronic oxidative stress promotes skeletal muscle atrophy by inhibiting anabolic signaling/muscle protein synthesis and accelerating proteolysis [80].
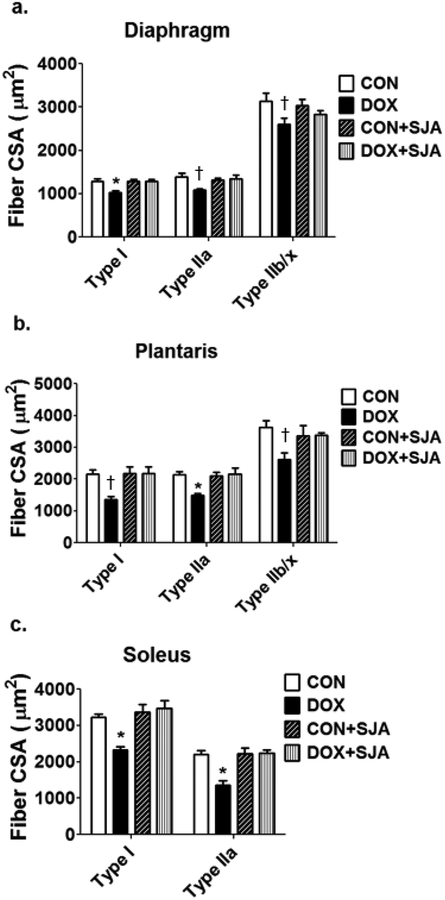
In reference to DOX-induced proteolysis leading to muscle wasting, preclinical studies demonstrate that treatment with DOX activates all of the major proteolytic systems (i.e., calpains, caspase-3, ubiquitin-proteasome system, and autophagy) in skeletal muscles [47,59,94]. Although each of these proteolytic systems likely contribute to DOX-induced muscle weakness, the activation of calpain plays a key role in DOX-mediated muscle atrophy and contractile dysfunction [59]. Indeed, pharmacological inhibition of DOX-induced calpain activation results in significant protection against DOX-induced muscle fiber atrophy (Fig. 2) and partial protection against DOX-mediated muscle contractile dysfunction. Moreover, inhibition of calpain activity in skeletal muscles of DOX-treated animals protects against both caspase-3 activation and myonuclear apoptosis in muscle fibers [59]. The explanation for this finding is likely linked to the regulatory cross-talk that exists between calpain and caspase-3 whereby calpain activation promotes an increase in caspase-3 activity [67].
Fig. 2.
Pharmacological inhibition of the protease calpain protects against DOX-induced atrophy of the rodent (A) diaphragm and locomotor skeletal muscle fibers (i.e., (B) plantaris and (C) soleus). See text for details. Data are from Min et al. [59]. Key: CON = control; DOX = doxorubicin treatment; CON + SJA= control animals treated with the calpain inhibitor SJA; DOX + SS31= animals treated with both DOX and SJA; * = different (P<0.05) from all other groups; † = different (P<0.05) from CON.
Endurance exercise-induced protection against DOX-induced myopathy
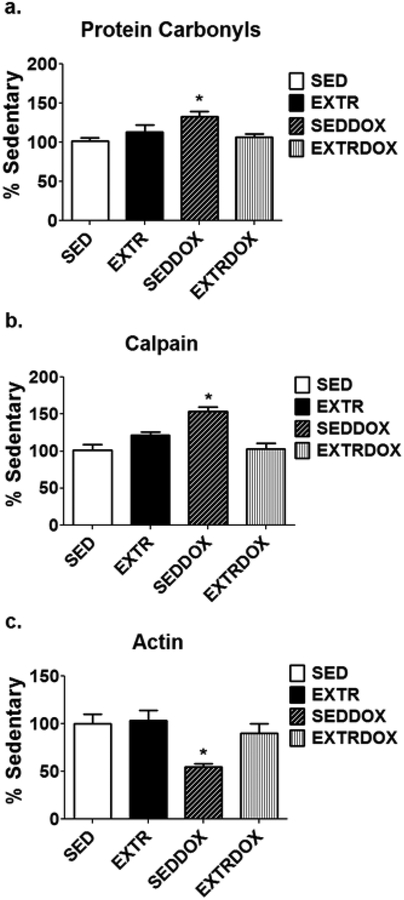
As discussed previously, the use of DOX in human cancer treatment is limited by a dose-dependent cardiotoxicity that can progress to cardiomyopathy and congestive heart failure [108]. The search for therapies to prevent or delay DOX -induced cardiomyopathy has been an ongoing process for several decades. The fact that endurance exercise training promotes numerous cellular changes in both cardiac and skeletal muscles that have the potential to protect against DOX-induced myopathy (e.g., increased antioxidant capacity) has stimulated research to determine if endurance exercise training, performed before or during DOX treatment, confers protection against DOX-induced cardiomyopathy. The first study to investigate the protective effects of exercise on DOX-induced cardiac injury was performed over 30 years ago [44]. Since this original investigation, numerous preclinical studies have confirmed that endurance exercise training results in a cardiac phenotype that is protected against DOX-induced cardiomyopathy. Similar to the studies in the heart, preclinical studies have consistently confirmed that endurance exercise training protects skeletal muscles against DOX-induced oxidative stress, calpain activation, and myofilament degradation [46,47,94] (Fig. 3). Importantly, translational studies in humans also reveal that regular exercise reduces the daily fatigue in female breast cancer patients receiving chemotherapy that includes doxorubicin [87].
Fig. 3.
Endurance exercise training, performed prior to treatment with DOX, protects rodent skeletal muscle fibers against DOX-induced (A) oxidative stress (protein carbonyls), (B) calpain activation, and (C) degradation of the contractile protein actin. Data are from Smuder et al. [94]. Key: SED = sedentary controls; EXTR = endurance exercise trained; SEDDOX= sedentary animals treated with DOX; EXTRDOX= endurance exercise trained and treated with DOX; * = different (P<0.05) from all other groups.
Mechanisms responsible for endurance exercise training-induced protection against DOX-induced skeletal muscle atrophy
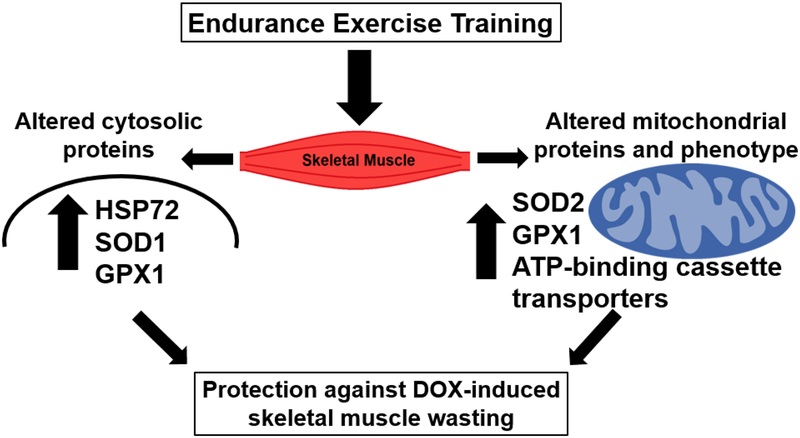
Although it is established that endurance exercise training performed prior to DOX treatment protects against DOX-induced skeletal muscle myopathy, the mechanisms responsible for this exercise-induced protection remains unclear. Specifically, to date, no studies have experimentally confirmed the cause and effect behind exercise-induced protection against DOX-mediated skeletal muscle wasting. Nonetheless, in theory, several endurance exercise-induced changes in skeletal muscles could interact to provide protection against DOX-induced myopathy. For example, DOX-induced toxicity in skeletal muscles is due, at least in part, to the increased generation of ROS and resultant oxidative damage in skeletal muscle fibers [46,47,94]. This DOX-induced oxidative stress has been linked to the activation of all four major proteolytic systems (i.e., calpains, caspase-3, ubiquitin-proteasome system, and autophagy) [46,47,94]. Therefore, an endurance exercise-induced increase in muscle antioxidant capacity has the potential to provide protection against DOX-induced muscle wasting by combatting DOX-induced increases in ROS production. In this regard, endurance exercise training results in increases in both cytosolic and mitochondrial antioxidants. Specifically, compared to sedentary animals, limb muscles of endurance exercise trained animals exhibit a higher abundance of several cytosolic antioxidant enzymes including superoxide dismutase 1 (SOD1) and glutathione peroxidase 1 (GPX1) [94]. The elevation in SOD1 increases the fiber’s ability to eliminate superoxide radicals and the increased abundance of GPX1 enhances the capability to remove hydrogen peroxide [76]. Moreover, endurance exercise training increases cytosolic levels of the cytoprotective stress protein, heat shock protein 72 (HSP72) in the trained limb muscles [53]. Indeed, it is well established that endurance exercise training increases HSP72 gene expression in both the heart and skeletal muscles [78]. Hence, it is feasible that increases in one or more of these aforementioned proteins contribute to the exercise-induced protection against DOX toxicity by reducing oxidative stress and protecting against DOX-induce activation of proteases.
Moreover, endurance exercise training promotes mitochondrial biogenesis in the exercised muscles [34]. Importantly, endurance exercise training also alters mitochondrial phenotype by increasing mitochondrial antioxidant enzymes [76]; this results in a mitochondria phenotype that resists oxidative injury and pro-apoptotic stimuli [98]. Collectively, the exercise-induced increase in mitochondrial biogenesis/turnover and increased abundance of mitochondrial antioxidant enzymes provide skeletal muscles with a healthy population of mitochondria with an improved capacity to resist DOX-induced ROS production.
Further, another mechanism by which endurance exercise training can protect against DOX-induced myopathy is that exercise training increases the expression of mitochondrial-specific ATP-binding cassette transporters that act to reduce the accumulation of DOX in both cardiac and skeletal muscle [62]. Specifically, endurance exercise training increases both the mRNA and protein abundance of four mitochondrial localized ATPase binding cassette (ABC) transporters (i.e., ABC6, ABC7, ABC8, and ABC10) in cardiac myocytes [62]. Hence, exercise training could also protect skeletal muscles by reducing the accumulation of DOX within muscle fibers serving to ameliorate the consequences of a deleterious dose-dependent relationship.
In summary, the precise mechanisms responsible for exercise-induced protection against DOX-induced muscle wasting remain unclear. Nonetheless, it is feasible that endurance exercise-induced protection against myopathy is due to increases in: 1) cytosolic and mitochondrial antioxidant enzymes; 2) HSP72 levels; and 3) expression of mitochondrial-specific ATP-binding cassette transporters to reduce DOX accumulation in muscle fibers (Fig. 4). Regardless of the exact mechanisms responsible for exercise-induced protection of skeletal muscle against DOX-induced myopathy, it is clear that endurance exercise training performed before DOX treatment protects skeletal muscle against DOX-induced atrophy by prevention of both oxidative stress and the activation of skeletal muscle proteolytic systems [47,94].
Fig. 4.
Summary of potential mechanisms responsible for exercise-induced protection against DOX-induced skeletal muscle wasting.
ENDURANCE EXERCISE-INDUCED PROTECTION AGAINST VENTILATOR-INDUCED DIAPHRAGM DYSFUNCTION
As discussed earlier, endurance exercise training results in a muscle fiber phenotype that is “preconditioned” and protected against a variety of stresses [51,107]. This last section of this report will discuss the evidence that endurance exercise training protects against MV-induced atrophy of the primary inspiratory muscle, the diaphragm. We begin with an introduction to the clinical consequence of MV-induced diaphragmatic weakness.
Ventilator-induced diaphragm weakness-clinical significance
MV is a therapy used to maintain pulmonary gas exchange in patients during critical illness, drug overdose, neuromuscular diseases, and surgery; an estimated 15 million patients are treated with MV annually [1]. While MV is a life-saving therapy in many patients, an unfortunate consequence of prolonged MV is the rapid occurrence of respiratory muscle weakness that ensues due to both diaphragmatic atrophy and contractile dysfunction. Collectively, the MV-induced diaphragmatic weakness is termed “ventilator-induced diaphragm dysfunction” (VIDD). VIDD is significant because diaphragmatic weakness is a primary risk factor that contributes to difficulties in removing (i.e., weaning) patients from MV [21,105]. The failure to wean patients from MV is a major clinical problem that results in prolonged hospitalization and increased morbidity and mortality [5,15]. Therefore, developing a therapeutic intervention to protect the diaphragm against VIDD is critical; the prerequisite to designing a therapy to prevent MV-induced diaphragmatic weakness is an understanding of the mechanisms responsible for VIDD. The next two segments discuss the remarkably rapid development of VIDD and highlight the mechanisms responsible for ventilator-induced diaphragm weakness.
MV-induced diaphragmatic atrophy is a unique type of disuse muscle atrophy
Several modes of MV exist (e.g., full and partial ventilator support); nonetheless, all modes result in a reduction in diaphragmatic contractile activity below that required during spontaneous breathing and all modes of MV result in the rapid development of VIDD [82]. Specifically, preclinical studies using a variety of animal models reveal that as few as 12–24 hours of MV promotes atrophy in all muscle fiber types in the diaphragm (i.e., type I, type IIA, type IIx, and type IIB) [2–4,7,9,12,13,23,25,36,85,92,93,47,102]. This rapid rate of MV-induced atrophy in the diaphragm is surprising given that the magnitude of muscle fiber atrophy (i.e., −15–20%) that occurs in the rat diaphragm during 12 hours of MV would require ~96 hours to emerge in rat limb muscles exposed to prolonged disuse (e.g., hindlimb suspension) [104].
Identical to the preclinical studies, reports consistently confirm that prolonged MV also results in the rapid development of VIDD in humans [30,33,35,38,43,86,111]. Indeed, a side-by-side comparison of the human and animal investigations reveals that the temporal pattern of MV-induced diaphragmatic atrophy is comparable between humans and rats, with diaphragmatic atrophy developing within 12–18 hours after initiation of MV [82].
Mechanisms responsible for the rapid development of VIDD
Prolonged MV results in atrophy of diaphragm muscle fibers due to both a decrease in protein synthesis and increased proteolysis [37,91]. Note, however, due to the swift rate of MV-induced diaphragmatic atrophy, it is evident that increased proteolysis is the dominant factor driving diaphragmatic atrophy during the early stage of MV (i.e., first 12–24 hours) [82]. Indeed, all of the four major proteolytic systems are active in the diaphragm during prolonged MV (e.g., calpains, caspase-3, ubiquitinproteasome system, and autophagy) [39,55,36,97,109].
The signaling pathways responsible for MV-induced diaphragmatic atrophy have been studied extensively and several lines of evidence reveal that MV-induced oxidative stress is required for the rapid activation of proteases in diaphragm fibers. For example, treatment of animals with antioxidants (e.g., trolox) before and during MV protects against oxidative stress in the diaphragm and prevents protease activation, resulting in protection against diaphragmatic atrophy [2,6,56,109].
MV-induced oxidative stress in the diaphragm occurs due to both increased oxidant production and decreased muscle fiber antioxidant capacity [22,48,57,58,75]. Although the MV-induced increase in ROS production in the diaphragm fibers originates from three separate oxidant generating systems (e.g., NADPH oxidase, xanthine oxidase, and mitochondria), mitochondria are the primary site of ROS production [22,48,57,58,75]. This MV-induced increase in mitochondrial ROS production occurs rapidly (i.e., within the first 6–12 hours of MV) [48,75]. Moreover, in both humans and rats, this MV-induced increase in mitochondrial ROS emission is accompanied by mitochondrial uncoupling (i.e., decrease in respiratory control ratio) and depressed activities of the electron transport chain complexes (i.e., complexes II, III, and IV) [48,68,103].
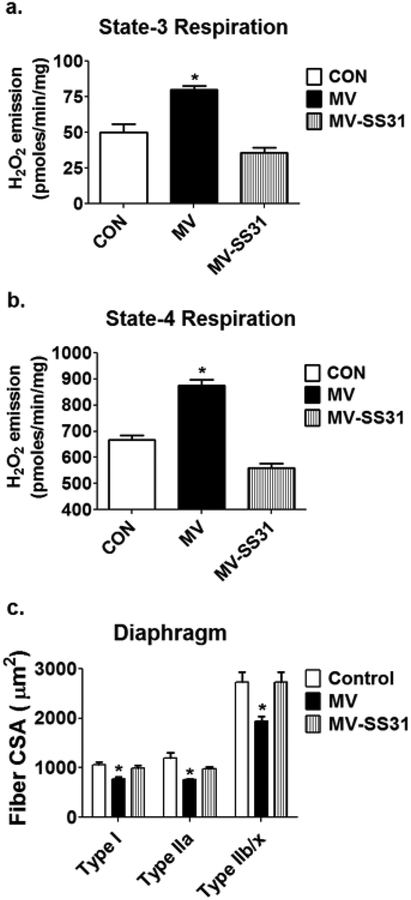
Experimental support for the notion that increased mitochondrial ROS emission is essential for VIDD stems from evidence that treatment of animals with a mitochondrial-targeted peptide that prevents MV-induced increases in mitochondrial ROS emission, protects the diaphragm against MV-induced atrophy by both preventing protease activation and maintenance of protein synthesis (Fig. 5) [81] [37]. For more details about the nexus between oxidative stress and skeletal muscle atrophy see Powers et al. [80,81].
Fig. 5.
Treatment of animals with a mitochondrial-targeted peptide (SS-31) prevents MV-induced increases in mitochondria emission of reactive oxygen species (H2O2) during both (A) state 3 and (B) state 4 respiration and also protects against (C) MV-induced atrophy of diaphragm muscle fibers. Data are from Powers et al. [75]. Key: CON = animals with no experimental treatment; MV = animals exposed to 12 hours of mechanical ventilation; MV-SS31 = animals exposed to 12 hours of mechanical ventilation and treated with the mitochondrial-targeted peptide SS-31; * = different (P<0.05) from all other groups.
Endurance exercise preconditioning protects against VIDD
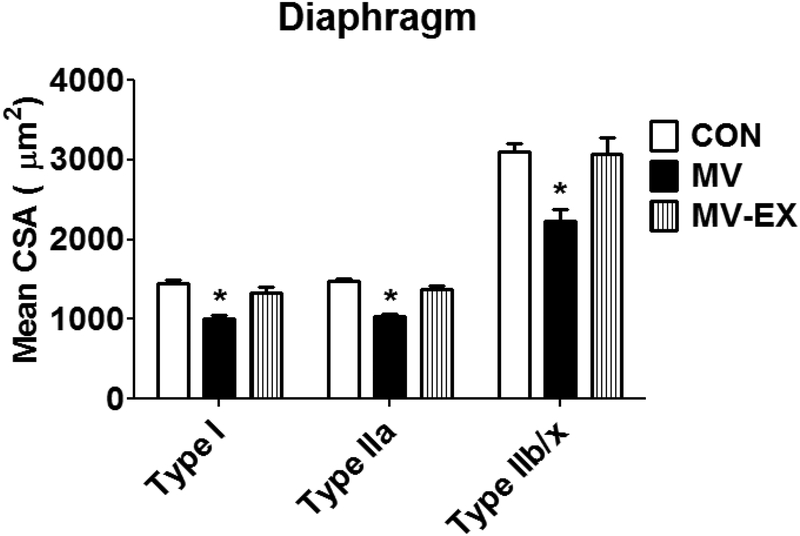
Endurance exercise training promotes numerous alterations in diaphragm muscle fibers that could protect against ventilator-induced diaphragm atrophy [11,71,73,72,74]. Indeed, similar to the endurance exercise-induced protection of limb skeletal muscle fibers against DOX-induced wasting, endurance exercise training performed prior to MV, protects diaphragm fibers from ventilator-induced mitochondrial dysfunction, oxidative stress, protease activation, and fiber atrophy [95]. Specifically, as few as 10 consecutive days of endurance exercise training results in significant protection against ventilator-induced diaphragmatic atrophy (Fig. 6).
Fig. 6.
Endurance exercise training, performed prior to MV, protects the rat diaphragm against MV-induced atrophy of all diaphragmatic fiber types. Data are from Smuder et al. [95]. Key: CON = sedentary animals with no experimental treatment; MV = sedentary animals exposed to 12 hours of mechanical ventilation; MV-EX = endurance exercise training animals exposed to 12 hours of mechanical ventilation; * = significantly different (p<0.05) from all other groups. Data are from Smuder et al. [95].
Interestingly, rats that have been selectively bred to attain a high capacity for aerobic exercise possess a skeletal muscle phenotype similar to muscle fibers from endurance exercise-trained rats [49]. Nonetheless, these untrained animals with a high intrinsic aerobic exercise capacity are not protected against MV-induced diaphragm atrophy [99]. This finding demonstrates that, independent of a genetic predisposition for a high aerobic capacity, endurance exercise training results in unique changes in the phenotype of diaphragm muscle fibers that results in protection against MV-induced diaphragm atrophy [99]. To date, several studies have investigated exercise-induced biochemical changes in diaphragm fibers and recent reports have clarified the mechanisms responsible for exercise-induced protection against VIDD.
Mechanisms responsible for endurance exercise-induced protection against MV-induced diaphragmatic atrophy
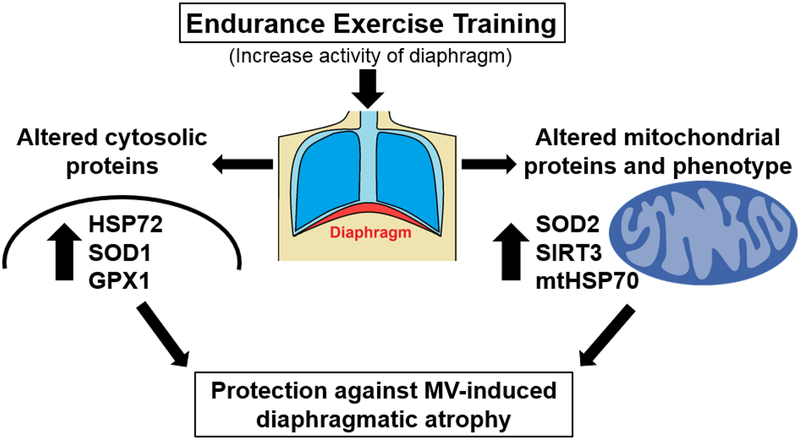
The first study to investigate the impact of endurance exercise training on diaphragm muscle fibers was published over three decades ago [61]. This original investigation demonstrated that, in response to endurance exercise training, diaphragm and limb skeletal muscles undergo similar adaptive responses as evidenced by comparable increases in mitochondrial oxidative enzymes. Since this earliest report, proteomic studies have profiled the exercise-induced changes in the abundance of both cytosolic and mitochondrial proteins in the diaphragm. These studies reveal that endurance exercise promotes changes in >70 cytosolic proteins [95,98] and 30 different mitochondrial proteins [95,98].
Endurance exercise training increases the abundance of three key cytosolic antioxidant enzymes that have the potential to protect the diaphragm against MV-induced oxidative stress and subsequently, ventilator-induced diaphragmatic atrophy [95,98]. Specifically, exercise training increases the abundance of GPX1, SOD1, and catalase in the cytosol [95]. Collectively, these exercise-induced improvements in the diaphragm’s antioxidant capacity could protect diaphragm fibers from both MV-induced oxidative damage and fiber atrophy [80].
Endurance exercise training also elevates the abundance of HSP72 in the diaphragm [95]. This increase in HSP72 is potentially important because overexpression of HSP72 has been demonstrated to protect against inactivity-induced muscle atrophy in locomotor muscles [88,90]. The precise mechanism(s) of how HSP72 protects against disuse muscle atrophy remains uncertain but overexpression of HSP72 can protect against atrophy in at least three ways: 1) Protection of mitochondria against pro-apoptotic stimuli and subsequent mitochondrial permeability transition pore opening [88]; 2) Defense against protein aggregation by HSP72-mediated refolding of damaged proteins in the cytosol [88]; and/or 3) Protection against increased proteolysis via inhibition of the activation of proteolytic transcriptional factors (i.e., forkhead box O3 and nuclear factor kappa beta) that promote muscle atrophy [89,90]. Hence, it is likely that endurance exercise-induced increases in the abundance of HSP72 could protect the diaphragm against ventilator-induced atrophy in multiple ways.
In support of the concept that increases in HSP72 protects the diaphragm against MV-induced atrophy, exposure of sedentary animals to whole body heat stress increases the abundance of HSP72 in the diaphragm and protects against MV-induced diaphragmatic atrophy [42,110]. Nonetheless, this work does not prove cause and effect. In contrast, recent work confirms that endurance exercise-induced increases in diaphragmatic levels of HSP72 plays a required role in exercise-induced protection against MV-induced diaphragmatic atrophy [96]. Specifically, prevention of the endurance exercise-induced increase in HSP72 in the diaphragm attenuates the exercise training-induced protection against MV-induced mitochondrial dysfunction, increased mitochondrial production of ROS, and protease activation [96].
As mentioned previously, endurance exercise training elevates the abundance of at least 30 different mitochondrial proteins [52,98]. The majority of these exercise-induced increases in mitochondrial proteins in the diaphragm fall into three functional categories: 1) anti-apoptotic and fission proteins; 2) chaperone/stress proteins; and 3) antioxidant proteins. Details about exercise-induced changes in the first two categories have been provided previously [69,98] and the current discussion will focus on the importance of exercise-induced changes in mitochondrial antioxidants as a potential protective mechanism against MV-induced diaphragmatic atrophy.
In regard to endurance exercise and mitochondrial antioxidants in the diaphragm, endurance exercise training elevates the abundance of the key mitochondrial antioxidant enzyme superoxide dismutase 2 (SOD2) along with an important positive allosteric regulator of SOD2 activity, sirtuin 3 (SIRT3) [95,98]. Further, endurance exercise training also amplifies mitochondrial levels of GPX1 in the diaphragm [95]. SOD2 is found within the mitochondrial matrix and dismutates superoxide radicals into hydrogen peroxide [76]. An exercise-induced elevation in both SOD2 and SIRT3 is potentially important because SIRT3 decetylates SOD2 and increases SOD2 activity [10,83]; together, these changes improve the capacity of mitochondria to eliminate superoxide radicals. The exercise-induced increase in GPX1 increases the fiber capacity to remove hydrogen peroxide within the mitochondria and collectively, these exercise-induced improvements in the mitochondria’s ability to scavenge ROS could protect the diaphragm against MV-induced mitochondrial uncoupling and oxidative damage [76]. Moreover, endurance exercise training is associated with a mitochondrial phenotype in diaphragm muscle fibers that resists pro-apoptotic stimuli [98]. Indeed, compared to sedentary animals, mitochondria isolated from diaphragms of endurance exercise-trained animals display a resistance to mitochondrial permeability pore opening when confronted with pro-apoptotic stimuli such as oxidants and/or calcium overload [98].
The question now becomes, “what role does the endurance exercise-induced changes in mitochondrial proteins play in protection against MV-induced diaphragmatic atrophy?” A complete answer to this question is not available but recent experiments indicate that an increase in SOD2 is essential to achieve the full benefit of exercise-mediated protection against VIDD [64]. Intriguingly, however, the transgenic overexpression of SOD2 alone in the diaphragm provides limited protection against MV-induced diaphragmatic atrophy [63]. Together, these results indicate that although increased expression of SOD2 plays a role in exercise-induced protection against VIDD, an increase in diaphragmatic SOD2 appears to work in concert with other cytoprotective molecules (e.g., HSP72, SIRT3, etc.) to provide the full exercise-induced protection against VIDD.
In synopsis, studies probing the mechanisms to explain endurance exercise-induced protection against MV-induced diaphragm atrophy reveal that exercise-induced increases in both HSP72 and SOD2 play important roles in this protection (Fig. 7). More specifically, exercise-induced increases in the abundance of HSP72 is essential to achieve exercise-induced protection against MV-induced diaphragmatic atrophy. Finally, although exercise-induced increases in diaphragmatic levels of SOD2 plays a contributory role in exercise-induced protection against VIDD, transgenic overexpression of SOD2 alone in the diaphragm does not protect against MV-induced diaphragm wasting.
Fig. 7.
Summary of the potential mechanisms responsible for exercise-induced protection against ventilator-induced diaphragmatic atrophy.
CONCLUSIONS AND FUTURE DIRECTIONS
Maintaining healthy skeletal muscles is important for locomotion, breathing, and overall health. Numerous threats to skeletal muscle health exist and unfortunately, two of these threats are linked to life-saving medical interventions (i.e., treatment with chemotherapy or prolonged MV). Indeed, prolonged MV promotes the rapid development of diaphragmatic weakness which elevates the risk for problematic weaning of patients from the ventilator. Similarly, although DOX is a life-saving treatment for many cancer patients, DOX is cytotoxic and often results in severe muscle weakness and fatigue in patients. Given the pervasive clinical usage of both MV and DOX, developing strategies to prevent muscle wasting in both of these clinical therapies is important.
Notably, endurance exercise training results in a protective phenotype of skeletal muscle that provides muscle fibers with the “cross tolerance” to withstand a wide variety of harmful challenges such as MV-induced diaphragmatic atrophy and DOX-induced limb muscle weakness. While endurance training protects both diaphragm and locomotor muscles against wasting, the situations foregoing a patients’ need for ventilator support or cancer treatment does not make endurance exercise training a practical countermeasure against muscle wasting in these circumstances. Nonetheless, investigations into the mechanism(s) responsible for exercise-induced protection against skeletal muscle wasting is a powerful investigational instrument to identify molecular targets that can be maneuvered to result in new and beneficial approaches to protect against skeletal muscle wasting.
Investigations into the mechanisms responsible for endurance exercise-induced protection against DOX-induced limb muscle wasting are in the early stages and thus, a complete understanding of the mechanism(s) responsible for exercise-induced protection of skeletal muscles against DOX-induced muscle wasting is not currently available. Clearly, this is an important area for future research as knowledge of the mechanism(s) behind exercise-induced protection of limb muscles against DOX-induced wasting will lead to new therapeutic approaches to protect patients against the cytotoxicity of DOX treatment.
Studies investigating the mechanisms responsible for endurance exercise-induced protection against MV-induced muscle wasting have revealed that exercise-induced protection against MV-mediated diaphragm weakness is linked to increases in both HSP72 and SOD2. Additional mechanistic experiments are required to unravel the interactions between exercise-induced increases in skeletal muscle levels of SOD2, HSP72, and possibly other unidentified molecules. This key information will provide the foundation for the translation of these basic science results into pharmacological interventions that can protect patients against ventilator-induced diaphragm weakness.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (NIH R01 AR064189 awarded to SKP.
References
- 1.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD (2010) Critical care and the global burden of critical illness in adults. Lancet 376:1339–1346. doi: 10.1016/S0140-6736(10)60446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agten A, Maes K, Smuder A, Powers SK, Decramer M, Gayan-Ramirez G (2011) N-Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Critical care medicine 39:777–782. doi: 10.1097/CCM.0b013e318206cca9 [DOI] [PubMed] [Google Scholar]
- 3.Agten A, Maes K, Thomas D, Cielen N, Van Hees HW, Dekhuijzen RP, Decramer M, Gayan-Ramirez G (2012) Bortezomib partially protects the rat diaphragm from ventilator-induced diaphragm dysfunction. Critical care medicine 40:2449–2455. doi: 10.1097/CCM.0b013e3182553a8800003246-201208000-00024 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Anzueto A, Peters JI, Tobin MJ, de los Santos R, Seidenfeld JJ, Moore G, Cox WJ, Coalson JJ (1997) Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Critical care medicine 25:1187–1190 [DOI] [PubMed] [Google Scholar]
- 5.Beduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, Grelon F, Runge I, Nicolas T, Grange S, Barberet G, Guitard PG, Frat JP, Constan A, Chretien JM, Mancebo J, Mercat A, Richard JM, Brochard L, Group WS, the RNdd (2017) Epidemiology of Weaning Outcome according to a New Definition. The WIND Study. American journal of respiratory and critical care medicine 195:772–783. doi: 10.1164/rccm.201602-0320OC [DOI] [PubMed] [Google Scholar]
- 6.Betters JL, Criswell DS, Shanely RA, Van Gammeren D, Falk D, Deruisseau KC, Deering M, Yimlamai T, Powers SK (2004) Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. American journal of respiratory and critical care medicine 170:1179–1184. doi: 10.1164/rccm.200407-939OC [DOI] [PubMed] [Google Scholar]
- 7.Bruells CS, Smuder AJ, Reiss LK, Hudson MB, Nelson WB, Wiggs MP, Sollanek KJ, Rossaint R, Uhlig S, Powers SK (2013) Negative pressure ventilation and positive pressure ventilation promote comparable levels of ventilator-induced diaphragmatic dysfunction in rats. Anesthesiology 119:652–662. doi: 10.1097/ALN.0b013e31829b3692 [DOI] [PubMed] [Google Scholar]
- 8.Camera DM, Smiles WJ, Hawley JA (2016) Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free radical biology & medicine 98:131–143. doi: 10.1016/j.freeradbiomed.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 9.Capdevila X, Lopez S, Bernard N, Rabischong E, Ramonatxo M, Martinazzo G, Prefaut C (2003) Effects of controlled mechanical ventilation on respiratory muscle contractile properties in rabbits. Intensive Care Med 29:103–110 [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, Xiong Y (2011) Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep 12:534–541. doi: 10.1038/embor.2011.65embor201165 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Criswell D, Powers S, Dodd S, Lawler J, Edwards W, Renshler K, Grinton S (1993) High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Medicine and science in sports and exercise 25:1135–1140 [PubMed] [Google Scholar]
- 12.Criswell DS, Powers SK, Herb RA, Dodd SL (1997) Mechanism of specific force deficit in the senescent rat diaphragm. Respiration physiology 107:149–155 [DOI] [PubMed] [Google Scholar]
- 13.Criswell DS, Shanely RA, Betters JJ, McKenzie MJ, Sellman JE, Van Gammeren DL, Powers SK (2003) Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest 124:2302–2308 [DOI] [PubMed] [Google Scholar]
- 14.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS (2014) Mitochondrial oxidative stress in aging and healthspan. Longevity & healthspan 3:6. doi: 10.1186/2046-2395-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S (2015) Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. The Lancet Respiratory medicine 3:544–553. doi: 10.1016/S2213-2600(15)00150-2 [DOI] [PubMed] [Google Scholar]
- 16.Davies KJ, Doroshow JH (1986) Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 261:3060–3067 [PubMed] [Google Scholar]
- 17.Dolinsky VW (2017) The role of sirtuins in mitochondrial function and doxorubicin-induced cardiac dysfunction. Biological chemistry 398:955–974. doi: 10.1515/hsz-2016-0316 [DOI] [PubMed] [Google Scholar]
- 18.Doroshow JH (1983) Anthracycline antibiotic-stimulated superoxide, hydrogen peroxide, and hydroxyl radical production by NADH dehydrogenase. Cancer Res 43:4543–4551 [PubMed] [Google Scholar]
- 19.Dudek J (2017) Role of Cardiolipin in Mitochondrial Signaling Pathways. Frontiers in cell and developmental biology 5:90. doi: 10.3389/fcell.2017.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ertunc M, Sara Y, Korkusuz P, Onur R (2009) Differential contractile impairment of fast- and slow-twitch skeletal muscles in a rat model of doxorubicin-induced congestive heart failure. Pharmacology 84:240–248. doi: 10.1159/000241723 [DOI] [PubMed] [Google Scholar]
- 21.Eskandar N, Apostolakos MJ (2007) Weaning from mechanical ventilation. Crit Care Clin 23:263–274, x. 10.1016/j.ccc.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 22.Falk DJ, Deruisseau KC, Van Gammeren DL, Deering MA, Kavazis AN, Powers SK (2006) Mechanical ventilation promotes redox status alterations in the diaphragm. Journal of applied physiology 101:1017–1024. doi: 10.1152/japplphysiol.00104.2006 [DOI] [PubMed] [Google Scholar]
- 23.Falk DJ, Kavazis AN, Whidden MA, Smuder AJ, McClung JM, Hudson MB, Powers SK (2011) Mechanical ventilation-induced oxidative stress in the diaphragm: role of heme oxygenase-1. Chest 139:816–824. doi: 10.1378/chest.09-2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Febbraio MA (2017) Exercise metabolism in 2016: Health benefits of exercise - more than meets the eye! Nature reviews Endocrinology 13:72–74. doi: 10.1038/nrendo.2016.218 [DOI] [PubMed] [Google Scholar]
- 25.Gayan-Ramirez G, Testelmans D, Maes K, Racz GZ, Cadot P, Zador E, Wuytack F, Decramer M (2005) Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Critical care medicine 33:2804–2809 [DOI] [PubMed] [Google Scholar]
- 26.Gilliam LA, Lark DS, Reese LR, Torres MJ, Ryan TE, Lin CT, Cathey BL, Neufer PD (2016) Targeted overexpression of mitochondrial catalase protects against cancer chemotherapy-induced skeletal muscle dysfunction. American journal of physiology Endocrinology and metabolism 311:E293–301. doi: 10.1152/ajpendo.00540.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilliam LA, Moylan JS, Patterson EW, Smith JD, Wilson AS, Rabbani Z, Reid MB (2012) Doxorubicin acts via mitochondrial ROS to stimulate catabolism in C2C12 myotubes. American journal of physiology Cell physiology 302:C195–202. doi: 10.1152/ajpcell.00217.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilliam LA, St Clair DK (2011) Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxidants & redox signaling 15:2543–2563. doi: 10.1089/ars.2011.3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giudice J, Taylor JM (2017) Muscle as a paracrine and endocrine organ. Current opinion in pharmacology 34:49–55. doi: 10.1016/j.coph.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, Rittayamai N, Lanys A, Tomlinson G, Singh JM, Bolz SS, Rubenfeld GD, Kavanagh BP, Brochard LJ, Ferguson ND (2015) Evolution of Diaphragm Thickness during Mechanical Ventilation. Impact of Inspiratory Effort. American journal of respiratory and critical care medicine 192:1080–1088. doi: 10.1164/rccm.201503-0620OC [DOI] [PubMed] [Google Scholar]
- 31.Gorini S, De Angelis A, Berrino L, Malara N, Rosano G, Ferraro E (2018) Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid Med Cell Longev 2018:7582730. doi: 10.1155/2018/7582730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grinton S, Powers SK, Lawler J, Criswell D, Dodd S, Edwards W (1992) Endurance training-induced increases in expiratory muscle oxidative capacity. Medicine and science in sports and exercise 24:551–555 [PubMed] [Google Scholar]
- 33.Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose K (2012) Diaphragm Muscle Thinning in Mechanically Ventilated Patients. Chest. 10.1378/chest.11-1638 [DOI] [PubMed] [Google Scholar]
- 34.Hood DA (2001) Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. Journal of applied physiology 90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137 [DOI] [PubMed] [Google Scholar]
- 35.Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes AR, Spoelstra de Man AM, Niessen HW, Manders E, van Hees HW, van den Brom CE, Silderhuis V, Lawlor MW, Labeit S, Stienen GJ, Hartemink KJ, Paul MA, Heunks LM, Ottenheijm CA (2015) Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. American journal of respiratory and critical care medicine 191:1126–1138. doi: 10.1164/rccm.201412-2214OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson MB, Smuder AJ, Nelson WB, Bruells CS, Levine S, Powers SK (2012) Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Critical care medicine 40:1254–1260. doi: 10.1097/CCM.0b013e31823c8cc9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudson MB, Smuder AJ, Nelson WB, Wiggs MP, Shimkus KL, Fluckey JD, Szeto HH, Powers SK (2015) Partial Support Ventilation and Mitochondrial-Targeted Antioxidants Protect against Ventilator-Induced Decreases in Diaphragm Muscle Protein Synthesis. PloS one 10:e0137693. doi: 10.1371/journal.pone.0137693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain SN, Cornachione AS, Guichon C, Al Khunaizi A, Leite Fde S, Petrof BJ, Mofarrahi M, Moroz N, de Varennes B, Goldberg P, Rassier DE (2016) Prolonged controlled mechanical ventilation in humans triggers myofibrillar contractile dysfunction and myofilament protein loss in the diaphragm. Thorax 71:436–445. doi: 10.1136/thoraxjnl-2015-207559 [DOI] [PubMed] [Google Scholar]
- 39.Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P (2010) Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. American journal of respiratory and critical care medicine 182:1377–1386. doi: 10.1164/rccm.201002-0234OC-201002-0234OC [pii] [DOI] [PubMed] [Google Scholar]
- 40.Hydock DS, Lien CY, Jensen BT, Schneider CM, Hayward R (2011) Characterization of the effect of in vivo doxorubicin treatment on skeletal muscle function in the rat. Anticancer research 31:2023–2028 [PubMed] [Google Scholar]
- 41.Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H (2014) Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124:617–630. doi: 10.1172/JCI7293172931 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichinoseki-Sekine N, Yoshihara T, Kakigi R, Sugiura T, Powers SK, Naito H (2014) Heat stress protects against mechanical ventilation-induced diaphragmatic atrophy. Journal of applied physiology 117:518–524. doi: 10.1152/japplphysiol.00170.2014 [DOI] [PubMed] [Google Scholar]
- 43.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Matecki S (2011) Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. American journal of respiratory and critical care medicine 183:364–371. 10.1164/rccm.201004-0670OC [DOI] [PubMed] [Google Scholar]
- 44.Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ (1985) Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol 59:1298–1303 [DOI] [PubMed] [Google Scholar]
- 45.Karstoft K, Pedersen BK (2016) Skeletal muscle as a gene regulatory endocrine organ. Current opinion in clinical nutrition and metabolic care 19:270–275. doi: 10.1097/MCO.0000000000000283 [DOI] [PubMed] [Google Scholar]
- 46.Kavazis AN, Smuder AJ, Min K, Tumer N, Powers SK (2010) Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. American journal of physiology Heart and circulatory physiology 299:H1515–1524. doi: 10.1152/ajpheart.00585.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kavazis AN, Smuder AJ, Powers SK (2014) Effects of short-term endurance exercise training on acute doxorubicin-induced FoxO transcription in cardiac and skeletal muscle. Journal of applied physiology 117:223–230. doi: 10.1152/japplphysiol.00210.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK (2009) Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free radical biology & medicine 46:842–850. doi: 10.1016/j.freeradbiomed.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koch LG, Britton SL (2005) Divergent selection for aerobic capacity in rats as a model for complex disease. Integrative and comparative biology 45:405–415. doi: 10.1093/icb/45.3.405 [DOI] [PubMed] [Google Scholar]
- 50.Kondo H, Miura M, Itokawa Y (1991) Oxidative stress in skeletal muscle atrophied by immobilization. Acta physiologica Scandinavica 142:527–528. doi: 10.1111/j.1748-1716.1991.tb09191.x [DOI] [PubMed] [Google Scholar]
- 51.Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. Journal of applied physiology 92:2177–2186. doi: 10.1152/japplphysiol.01267.2001 [DOI] [PubMed] [Google Scholar]
- 52.Lee Y, Min K, Talbert EE, Kavazis AN, Smuder AJ, Willis WT, Powers SK (2012) Exercise protects cardiac mitochondria against ischemia reperfusion injury. Medicine and science in sports and exercise 44:397–405. doi: 10.1249/MSS.0b013e318231c037 [DOI] [PubMed] [Google Scholar]
- 53.Locke M, Noble EG, Atkinson BG (1990) Exercising mammals synthesize stress proteins. The American journal of physiology 258:C723–729. doi: 10.1152/ajpcell.1990.258.4.C723 [DOI] [PubMed] [Google Scholar]
- 54.Marques-Aleixo I, Santos-Alves E, Oliveira PJ, Moreira PI, Magalhaes J, Ascensao A (2018) The beneficial role of exercise in mitigating doxorubicin-induced Mitochondrionopathy. Biochimica et biophysica acta 1869:189–199. 10.1016/j.bbcan.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 55.McClung JM, Kavazis AN, DeRuisseau KC, Falk DJ, Deering MA, Lee Y, Sugiura T, Powers SK (2007) Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. American journal of respiratory and critical care medicine 175:150–159. doi: 10.1164/rccm.200601-142OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK (2007) Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. The Journal of physiology 585:203–215. doi: 10.1113/jphysiol.2007.141119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, Gayan-Ramirez G, Decramer M, DeRuisseau KC, Powers SK (2009) Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Critical care medicine 37:1373–1379. doi: 10.1097/CCM.0b013e31819cef63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McClung JM, Whidden MA, Kavazis AN, Falk DJ, Deruisseau KC, Powers SK (2008) Redox regulation of diaphragm proteolysis during mechanical ventilation. American journal of physiology Regulatory, integrative and comparative physiology 294:R1608–1617. doi: 10.1152/ajpregu.00044.2008 [DOI] [PubMed] [Google Scholar]
- 59.Min K, Kwon OS, Smuder AJ, Wiggs MP, Sollanek KJ, Christou DD, Yoo JK, Hwang MH, Szeto HH, Kavazis AN, Powers SK (2015) Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. The Journal of physiology 593:2017–2036. doi: 10.1113/jphysiol.2014.286518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Watanabe M, Baba H (2015) Negative Impact of Skeletal Muscle Loss after Systemic Chemotherapy in Patients with Unresectable Colorectal Cancer. PloS one 10:e0129742. doi: 10.1371/journal.pone.0129742 PONE-D-15–01055 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore RL, Gollnick PD (1982) Response of ventilatory muscles of the rat to endurance training. Pflugers Arch 392:268–271 [DOI] [PubMed] [Google Scholar]
- 62.Morton AB, Mor Huertas A, Hinkley JM, Ichinoseki-Sekine N, Christou DD, Smuder AJ (2018) Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion. doi: 10.1016/j.mito.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morton AB, Smuder A, Hyatt HW, Hinkley JM, Ichinoseki-Sekine N, Mor Huertas A, Powers S (2018) Overexpression of SOD2 in the diaphragm provides partial protection against ventilator-induced diaphragm atrophy and contractile dysfunction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 32:856–815 [Google Scholar]
- 64.Morton AB, Smuder A, Wiggs MP, Hall SE, Wawrzyniak N, Powers S (2016) Exercise-induced protection against ventilator-induced diaphragm atrophy is dependent upon increased diaphragmatic levels of superoxide dismutase 2. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 30:1244–1210 [Google Scholar]
- 65.Moulin M, Piquereau J, Mateo P, Fortin D, Rucker-Martin C, Gressette M, Lefebvre F, Gresikova M, Solgadi A, Veksler V, Garnier A, Ventura-Clapier R (2015) Sexual dimorphism of doxorubicin-mediated cardiotoxicity: potential role of energy metabolism remodeling. Circulation Heart failure 8:98–108. doi: 10.1161/CIRCHEARTFAILURE.114.001180 [DOI] [PubMed] [Google Scholar]
- 66.Moulin M, Solgadi A, Veksler V, Garnier A, Ventura-Clapier R, Chaminade P (2015) Sex-specific cardiac cardiolipin remodelling after doxorubicin treatment. Biology of sex differences 6:20. doi: 10.1186/s13293-015-0039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelson WB, Smuder AJ, Hudson MB, Talbert EE, Powers SK (2012) Crosstalk between the calpain and caspase-3 proteolytic systems in the diaphragm during prolonged mechanical ventilation. Critical care medicine 40:1857–1863. doi: 10.1097/CCM.0b013e318246bb5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, Godin R, Danialou G, Chaturvedi R, Rygiel K, Matecki S, Jaber S, Rosiers CD, Karpati G, Ferri L, Burelle Y, Turnbull DM, Taivassalo T, Petrof BJ (2012) Mitochondrial Dysfunction and Lipid Accumulation in the Human Diaphragm during Mechanical Ventilation. American journal of respiratory and critical care medicine 186:1140–1149. doi: 10.1164/rccm.201206-0982OC rccm.201206–0982OC [pii] [DOI] [PubMed] [Google Scholar]
- 69.Powers SK (2017) Exercise: Teaching myocytes new tricks. Journal of applied physiology 123:460–472. doi: 10.1152/japplphysiol.00418.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G (1994) Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. The American journal of physiology 266:R375–380. doi: 10.1152/ajpregu.1994.266.2.R375 [DOI] [PubMed] [Google Scholar]
- 71.Powers SK, Criswell D, Lawler J, Martin D, Ji LL, Herb RA, Dudley G (1994) Regional training-induced alterations in diaphragmatic oxidative and antioxidant enzymes. Respiration physiology 95:227–237 [DOI] [PubMed] [Google Scholar]
- 72.Powers SK, Criswell D, Lieu FK, Dodd S, Silverman H (1992) Diaphragmatic fiber type specific adaptation to endurance exercise. Respiration physiology 89:195–207 [DOI] [PubMed] [Google Scholar]
- 73.Powers SK, Criswell D, Lieu FK, Dodd S, Silverman H (1992) Exercise-induced cellular alterations in the diaphragm. The American journal of physiology 263:R1093–1098. doi: 10.1152/ajpregu.1992.263.5.R1093 [DOI] [PubMed] [Google Scholar]
- 74.Powers SK, Grinton S, Lawler J, Criswell D, Dodd S (1992) High intensity exercise training-induced metabolic alterations in respiratory muscles. Respiration physiology 89:169–177 [DOI] [PubMed] [Google Scholar]
- 75.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ (2011) Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Critical care medicine 39:1749–1759. doi: 10.1097/CCM.0b013e3182190b62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiological reviews 88:1243–1276. doi: 10.1152/physrev.00031.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Powers SK, Lawler J, Criswell D, Dodd S, Grinton S, Bagby G, Silverman H (1990) Endurance-training-induced cellular adaptations in respiratory muscles. Journal of applied physiology 68:2114–2118. doi: 10.1152/jappl.1990.68.5.2114 [DOI] [PubMed] [Google Scholar]
- 78.Powers SK, Locke, Demirel HA (2001) Exercise, heat shock proteins, and myocardial protection from I-R injury. Medicine and science in sports and exercise 33:386–392 [DOI] [PubMed] [Google Scholar]
- 79.Powers SK, Lynch GS, Murphy KT, Reid MB, Zijdewind I (2016) Disease-Induced Skeletal Muscle Atrophy and Fatigue. Medicine and science in sports and exercise 48:2307–2319. doi: 10.1249/MSS.0000000000000975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Powers SK, Morton AB, Ahn B, Smuder AJ (2016) Redox control of skeletal muscle atrophy. Free radical biology & medicine 98:208–217. doi: 10.1016/j.freeradbiomed.2016.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Powers SK, Smuder AJ, Criswell DS (2011) Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxidants & redox signaling 15:2519–2528. doi: 10.1089/ars.2011.3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ (2013) Ventilator-induced diaphragm dysfunction: cause and effect. American journal of physiology Regulatory, integrative and comparative physiology 305:R464–477. doi: 10.1152/ajpregu.00231.2013 ajpregu.00231.2013 [pii] [DOI] [PubMed] [Google Scholar]
- 83.Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, Gibson BW (2013) Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci U S A 110:6601–6606. doi: 10.1073/pnas.1302961110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Renu K, V GA, P BT, Arunachalam S (2018) Molecular mechanism of doxorubicin-induced cardiomyopathy - An update. European journal of pharmacology 818:241–253. doi: 10.1016/j.ejphar.2017.10.043 [DOI] [PubMed] [Google Scholar]
- 85.Sassoon CS, Zhu E, Fang L, Sieck GC, Powers SK (2014) Positive end expiratory airway pressure does not aggravate ventilator-induced diaphragmatic dysfunction in rabbits. Crit Care 18:494. doi: 10.1186/s13054-014-0494-0 s13054–014-0494–0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schepens T, Verbrugghe W, Dams K, Corthouts B, Parizel PM, Jorens PG (2015) The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Crit Care 19:422. doi: 10.1186/s13054-015-1141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwartz AL, Mori M, Gao R, Nail LM, King ME (2001) Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Medicine and science in sports and exercise 33:718–723 [DOI] [PubMed] [Google Scholar]
- 88.Senf SM (2013) Skeletal muscle heat shock protein 70: diverse functions and therapeutic potential for wasting disorders. Frontiers in physiology 4:330. doi: 10.3389/fphys.2013.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Senf SM, Dodd SL, Judge AR (2010) FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. American journal of physiology Cell physiology 298:C38–45. doi: 10.1152/ajpcell.00315.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Senf SM, Dodd SL, McClung JM, Judge AR (2008) Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22:3836–3845. doi: 10.1096/fj.08-110163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shanely RA, Van Gammeren D, Deruisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE, Powers SK (2004) Mechanical ventilation depresses protein synthesis in the rat diaphragm. American journal of respiratory and critical care medicine 170:994–999. doi: 10.1164/rccm.200304-575OC [DOI] [PubMed] [Google Scholar]
- 92.Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK (2002) Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. American journal of respiratory and critical care medicine 166:1369–1374. doi: 10.1164/rccm.200202-088OC [DOI] [PubMed] [Google Scholar]
- 93.Smuder AJ, Gonzalez-Rothi EJ, Kwon OS, Morton AB, Sollanek KJ, Powers SK, Fuller DD (2016) Cervical spinal cord injury exacerbates ventilator-induced diaphragm dysfunction. Journal of applied physiology 120:166–177. doi: 10.1152/japplphysiol.00488.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smuder AJ, Kavazis AN, Min K, Powers SK (2011) Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. Journal of applied physiology 111:1190–1198. doi: 10.1152/japplphysiol.00429.2011 [DOI] [PubMed] [Google Scholar]
- 95.Smuder AJ, Min K, Hudson MB, Kavazis AN, Kwon OS, Nelson WB, Powers SK (2012) Endurance exercise attenuates ventilator-induced diaphragm dysfunction. Journal of applied physiology 112:501–510. doi: 10.1152/japplphysiol.01086.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smuder AJ, Morton AB, Hall SE, Ahn B, Wiggs MP, Wawrzyniak N, Powers S (2016) HSP72 is required for exercise-induced protection against ventilator-induced diaphragm dysfunction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 30:769–762 [Google Scholar]
- 97.Smuder AJ, Nelson WB, Hudson MB, Kavazis AN, Powers SK (2014) Inhibition of the ubiquitin-proteasome pathway does not protect against ventilator-induced accelerated proteolysis or atrophy in the diaphragm. Anesthesiology 121:115–126. doi: 10.1097/ALN.0000000000000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sollanek KJ, Burniston JG, Kavazis AN, Morton AB, Wiggs MP, Ahn B, Smuder AJ, Powers SK (2017) Global Proteome Changes in the Rat Diaphragm Induced by Endurance Exercise Training. PloS one 12:e0171007. doi: 10.1371/journal.pone.0171007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sollanek KJ, Smuder AJ, Wiggs MP, Morton AB, Koch LG, Britton SL, Powers SK (2015) Role of intrinsic aerobic capacity and ventilator-induced diaphragm dysfunction. Journal of applied physiology 118:849–857. doi: 10.1152/japplphysiol.00797.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sorensen JC, Cheregi BD, Timpani CA, Nurgali K, Hayes A, Rybalka E (2016) Mitochondria: Inadvertent targets in chemotherapy-induced skeletal muscle toxicity and wasting? Cancer Chemother Pharmacol 78:673–683. doi: 10.1007/s00280-016-3045-3 10.1007/s00280–016-3045–3 [pii] [DOI] [PubMed] [Google Scholar]
- 101.Tacar O, Sriamornsak P, Dass CR (2013) Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x [DOI] [PubMed] [Google Scholar]
- 102.Talbert EE, Smuder AJ, Kwon OS, Sollanek KJ, Wiggs MP, Powers SK (2016) Blockage of the Ryanodine Receptor via Azumolene Does Not Prevent Mechanical Ventilation-Induced Diaphragm Atrophy. PloS one 11:e0148161. doi: 10.1371/journal.pone.0148161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang H, Lee M, Budak MT, Pietras N, Hittinger S, Vu M, Khuong A, Hoang CD, Hussain SN, Levine S, Shrager JB (2011) Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3-Bim axis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 25:2921–2936. doi: 10.1096/fj.11-183798 fj.11–183798 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thomason DB, Biggs RB, Booth FW (1989) Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. The American journal of physiology 257:R300–305 [DOI] [PubMed] [Google Scholar]
- 105.Vassilakopoulos T, Petrof BJ (2004) Ventilator-induced diaphragmatic dysfunction. American journal of respiratory and critical care medicine 169:336–341 [DOI] [PubMed] [Google Scholar]
- 106.Vassilakopoulos T, Zakynthinos S, Roussos C (1996) Respiratory muscles and weaning failure. Eur Respir J 9:2383–2400 [DOI] [PubMed] [Google Scholar]
- 107.Vincent HK, Powers SK, Demirel HA, Coombes JS, Naito H (1999) Exercise training protects against contraction-induced lipid peroxidation in the diaphragm. European journal of applied physiology and occupational physiology 79:268–273. doi: 10.1007/s004210050505 [DOI] [PubMed] [Google Scholar]
- 108.Von Hoff DD, Layard MW, Basa P, Davis HL Jr., Von Hoff AL, Rozencweig M, Muggia FM (1979) Risk factors for doxorubicin-induced congestive heart failure. Annals of internal medicine 91:710–717 [DOI] [PubMed] [Google Scholar]
- 109.Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK (2010) Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. Journal of applied physiology 108:1376–1382. doi: 10.1152/japplphysiol.00098.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoshihara T, Ichinoseki-Sekine N, Kakigi R, Tsuzuki T, Sugiura T, Powers SK, Naito H (2015) Repeated exposure to heat stress results in a diaphragm phenotype that resists ventilator-induced diaphragm dysfunction. Journal of applied physiology 119:1023–1031. doi: 10.1152/japplphysiol.00438.2015 [DOI] [PubMed] [Google Scholar]
- 111.Zambon M, Beccaria P, Matsuno J, Gemma M, Frati E, Colombo S, Cabrini L, Landoni G, Zangrillo A (2016) Mechanical Ventilation and Diaphragmatic Atrophy in Critically Ill Patients: An Ultrasound Study. Critical care medicine 44:1347–1352. doi: 10.1097/CCM.0000000000001657 [DOI] [PubMed] [Google Scholar]