Abstract
Background: We previously demonstrated that liver resection triggers estradiol production, which, in turn, induces the proliferation of hepatocytes to promote liver regeneration in mice. In this study, we demonstrated estradiol-induced estrogen receptor alpha (ERα) expression.
Methods: To further explore the role of ERα in estradiol-mediated liver regeneration, in the present study, we confirmed impaired liver regeneration ability in ERα knockout mice.
Results: Further analysis during liver regeneration revealed a role for ERα in hepatic steatosis, tumor necrosis factor-alpha and interleukin 6 expression, and nuclear factor-κB and signal transducer and activator of transcription 3 DNA-binding activities.
Conclusion: Moreover, estradiol administration accelerated liver regeneration through ERα, indicating the feasibility of the estrogen-ERα axis as a target for accelerating the rate of liver regeneration.
Keywords: estrogens, estrogen receptor α; ERα, partial hepatectomy, liver regeneration
Introduction
The endogenous hormone estradiol is mainly produced by the ovary, and its production can be induced under certain contexts and stimuli such as during the estrus cycle and in late pregnancy.1–4 Estradiol exerts its multiple functions by binding to GPR30, estrogen receptor (ER) α, or ERβ, which are members of the nuclear receptor super family.1–4
Estradiol production is also induced after liver resection/injury, suggesting this hormone plays a role in liver regeneration.5,6 Several genes that participate in liver regeneration have been identified, including those encoding the inflammatory cytokines tumor necrosis factor alpha (TNFα) and interleukin 6 (IL-6).5,6 In particular, we have previously focused on the molecules essential for triggering liver regeneration after partial hepatectomy (PH) using mouse models.2–4 In addition to these cytokines, the production of the chemical hormone estradiol is also induced in the acute phase of liver injury after PH, via the ovary and testes.2–4,7–9 We have further demonstrated that estrogen induces hepatocyte proliferation after PH, which was delayed by ovariectomy.3,4 This estradiol induction after PH was in turn found to induce ERα expression in the mainly periportal hepatocytes.2–4 Moreover, the WT mice showed transient steatosis during liver regeneration after PH.2–4 Therefore, we hypothesized that PH initially triggers estradiol production, leading to elevated ERα expression, to consequently initiate the processes of β-oxidation enzyme expression for anti-steatosis. In the present study, we tested this hypothesis by analyzing the liver regeneration process in ERα knockout (KO) mice compared with that in their wild-type (WT) littermates. These findings establish the foundation for the therapeutic application of estradiol to accelerate liver regeneration after resection in clinical settings.
Materials and methods
Reagents
β-Estradiol (E2) was purchased from Wako Chemicals (No. 052–04041, Osaka, Japan,2–4). β-Estradiol measurement was performed using the commercial kit (No. 582251, Funakoshi, Tokyo, Japan,2–4). The mouse TNFα ELISA Kit (ab208348) was purchased from Abcam (Cambridge, UK). The mouse IL-6 ELISA kit (KE10007) was purchased from Funakoshi. An anti-BrdU antibody was purchased from Roche Diagnostics Japan (No. 11170376001, Tokyo, Japan,2–4).
Mice
C57BL/6J mice, a common inbred strain of laboratory mice, were purchased from Charles River Japan (Kanagawa, Japan). The ERα knock out (KO) mice were described previously.1–4,10 Liver resection/partial hepatectomy of the left and medium lobes was performed after midventral laparotomy between 8 am and 11 am.10
Hepatocyte proliferation in vivo
The hepatocyte proliferation rates in mice were analyzed using two methods. One is bromodeoxyuridine (BrdU; M0744, Dako Japan, Tokyo, Japan), and another is Ki67 (Funakoshi, Tokyo, Japan) immunohistochemistry (IHC, SK-4105, Vector Laboratories, California, USA).2–4,11,12
Animal study compliance
All animal experiments using wild-type (WT) and knock out mice were performed in accordance with the ethical guidelines for animal care established by the National Center for Geriatrics and Gerontology (NCGG), The study was approved by the Animal Care Committee at NCGG.13,14
Statistical analysis
Experiments were repeated at least three times. The values are represented as the mean ± standard error (n=5–12). Comparisons between multiple groups were assessed using one-way/two-way analysis of variance test (ANOVA) and the Student’s t-test. Differences were considered significant at *p<0.05, **p<0.005, and ***p<0.0001. Non-statistical differences (p>0.05) are denoted as NS (not significant).
Result
ERα is involved in the liver weight recovery after liver resection
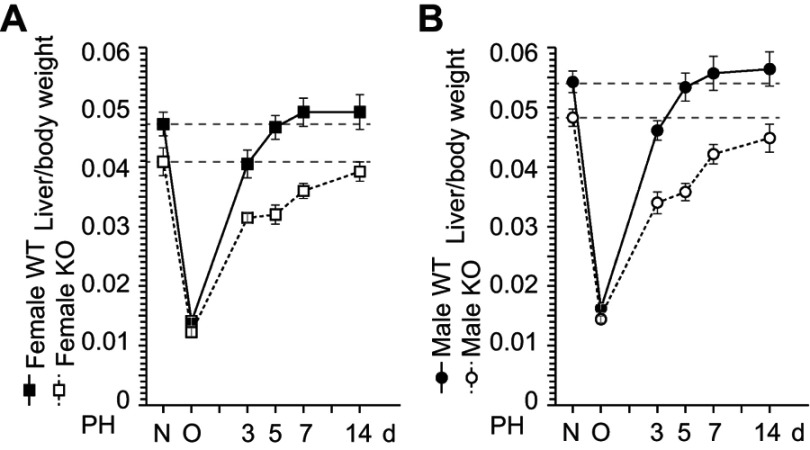
To investigate the role of ERα in liver weight recovery, we performed PH on WT and ERα-KO mice. In WT mice, liver weight recovery was observed 5 d after PH. In contrast, liver weights had still not recovered at up to 14 d in ERα-KO mice of both sexes (Figure 1). This result demonstrates the same delay in resulting from ERα deficiency as previously observed in ovariectomized mice,2–4 indicating that the induction of estrogen production after PH triggers hepatocyte proliferation through ERα. We then performed PH in ERα-KO mice and analyzed liver weight recovery. Liver weight recovery was found to take 5 d in WT mice (Figure 1) and was observed not to recover even after 14 d in ERα-KO mice of both sexes (Figure 1), suggesting that liver weight recovery is delayed in ERα-KO mice.
Figure 1.
ERα is involved in the liver weight recovery after liver resection. (A,B) Wild-type (WT) and ERα knockout (KO) mice were subject to partial hepatectomy (PH), and liver weight was measured at day 3, 5, 7, and 14 after the surgery. N = non-operated, O = operated; n=5–8 per group.
Impaired hepatocyte proliferation after liver resection in the ERα KO mice
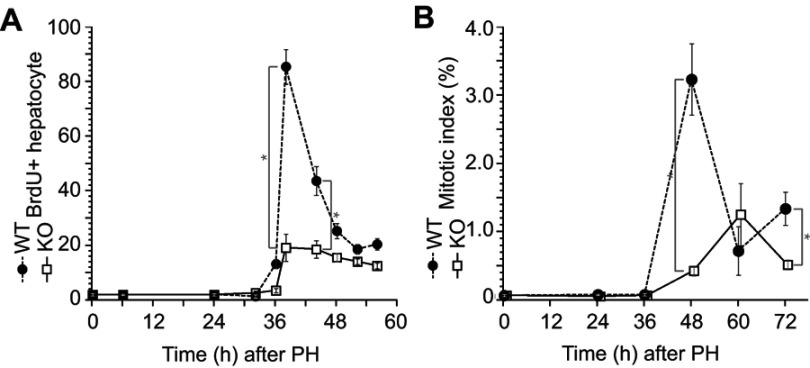
Delayed liver weight recovery in ERα-KO mice was observed after PH; therefore, we analyzed hepatocyte proliferation activity after PH (Figure 1) by immunohistochemistry with BrdU incorporation (Figure 2A) and performed mitotic-phase cell count (Figure 2B). Both data demonstrated impaired and delayed hepatocyte proliferation in ERα-KO mice after PH.
Figure 2.
Impaired hepatocyte proliferation after liver resection in the ERα knockout (KO) mice. Wild-type (WT) and KO mice underwent partial hepatectomy (PH), and the rate of hepatocyte proliferation was assessed for up to 72 h after PH. (A) Bromodeoxyuridine (BrdU) was injected 2 h before dissection. BrdU-positive hepatocytes were counted by immunocytochemistry. (B) Mitotic bodies were counted in liver sections. Values are expressed as the mean ± SEM (n=5–17 per group); *p<0.05.
The ERα is involved in the lipid metabolism after liver resection
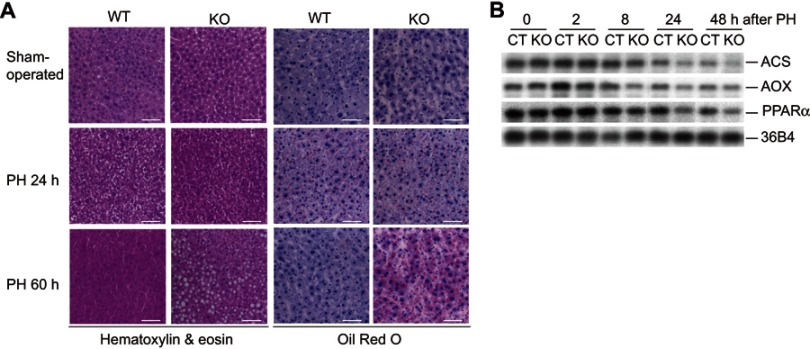
In line with our previous studies, after PH, a slight fatty liver was observed in WT mice after 24 h.2–4 We obtained histological sections after PH and stained them with hematoxylin and eosin (HE) and oil-O-red to detect steatosis. Severe fatty liver was observed at 60 h after PH in the ERα-KO mice (Figure 3A). We analyzed the expression of several β-oxidation-related genes (Figure 3B). A decrease in the number of transcripts of ACS (acyl-CoA synthetase), AOX (acyl-CoA oxidase), and their regulator PPARα was observed in ERα-KO mice after PH, suggesting that ERα regulates PPARα transcription and PPARα regulates ACS and AOX after PH.
Figure 3.
The ERα is involved in the lipid metabolism after liver resection. (A) Wild-type (WT) and knockout (KO) male mice were operated with partial hepatectomy (PH) or sham operation, and their livers were analyzed by hematoxylin and eosin or Oil Red O staining. (B) Reduced transcripts of ACS (acyl-coA synthetase), AOX (acyl-coA oxidase) and their regulator PPARα were observed in ERα KO male mice after PH, suggesting that ERα regulates β-oxidation enzyme gene expression.
ERα is involved in the TNFα and IL-6 production after liver resection
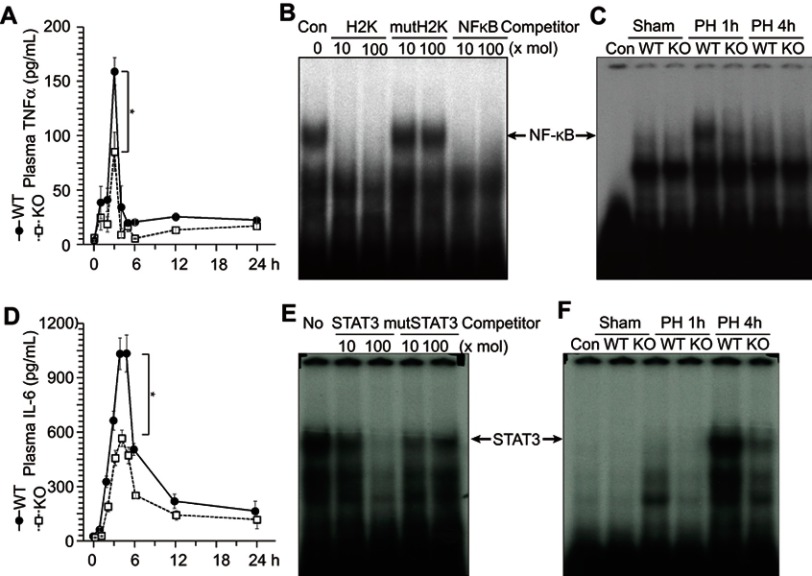
TNFα and IL-6 are known to trigger liver regeneration;5,6 therefore, we analyzed circulating TNFα and IL-6 concentration after PH (Figure 4A and D). ERα-KO mice showed approximately 50% lower levels of circulating TNFα and IL-6 compared with WT mice after PH (Figure 4A and D). TNFα signaling reaches the hepatocytes through TNFαR and stimulates nuclear factor-κB (NF-κB) activity (confirmed by competition assay as shown in Figure 4B and described in6). In the liver extract of WT mice, NF-κB activity was observed at 1 h after PH, whereas no NF-κB activity was observed in the KO mice (Figure 4C), suggesting that ERα is involved in TNFα production after PH. Under IL-6, signal transducer and activator of transcription 3 (STAT3) activity (confirmed by competition assay, as shown in Figure 4E and described in6) was increased at 4 h after PH in WT mice, but reduced in ER-KO mice (Figure 4F).
Figure 4.
ERα is involved in the TNFα and IL-6 production after liver resection. Wild-type (WT) and knockout (KO) male mice underwent partial hepatectomy (PH or were sham-operated. (A) Circulating TNFα concentrations were analyzed by enzyme-linked immunosorbent assay at indicated times after PH. (B and C) ERα is involved in NF-κB activation after PH. NF-κB DNA-binding activity was analyzed with an electrophoretic mobility shift assay using liver extracts. NF-κB activity was confirmed by competition with other NF-κB binding sites (H2K and KF-κB, [B]). (D) Circulating IL-6 concentrations analyzed by enzyme-linked immunosorbent assay at indicated times after PH. (E and F) ERα is involved in STAT3 activation after PH. STAT3 DNA-binding activity was analyzed via an electrophoretic mobility shift assay using liver extracts. STAT3 activity was confirmed by competition with other STAT3 binding sites (E).
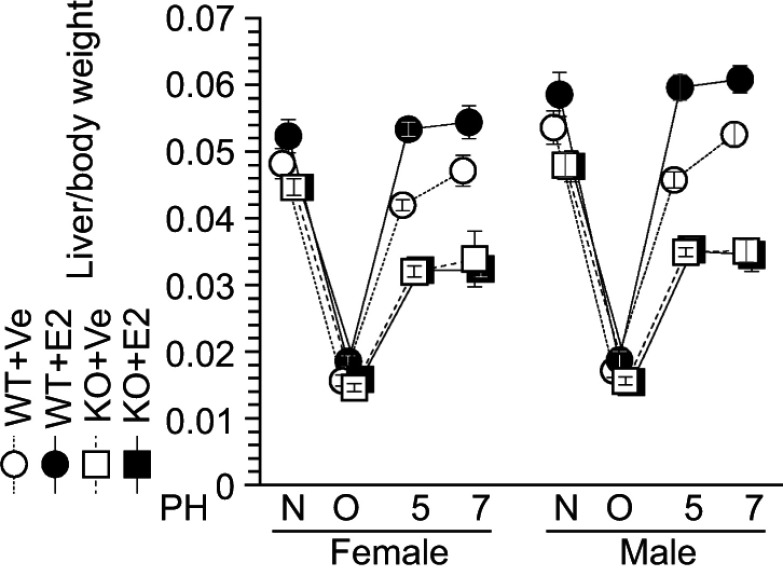
Impaired E2-induced liver weight recovery in ERΑ KO mice
Our previous studies demonstrate the feasibility of estradiol as an accelerator of liver regeneration in WT mice.2–4 Consistent with this, estradiol injection stimulated liver weight recovery in ERα-KO and WT littermates after PH (Figure 5); this was noted after only 5 days in contrast with the 7 days required for liver weight recovery in vehicle-injected WT mice (Figure 5). In contrast, severe impaired liver weight recovery was observed in both vehicle- and estradiol-injected ERα-KO mice, indicating that estradiol-ERα signaling is indispensable for liver regeneration (Figure 5).
Figure 5.
Impaired estradiol (E2)-induced liver weight recovery in ERα knockout (KO) mice. Estradiol (E2) induced liver weight recovery after liver resection in wild-type (WT) mice, but not in KO mice. N = non-operated, O = operated; 5, 7=5 or 7 days after PH; n=5–8 per group.
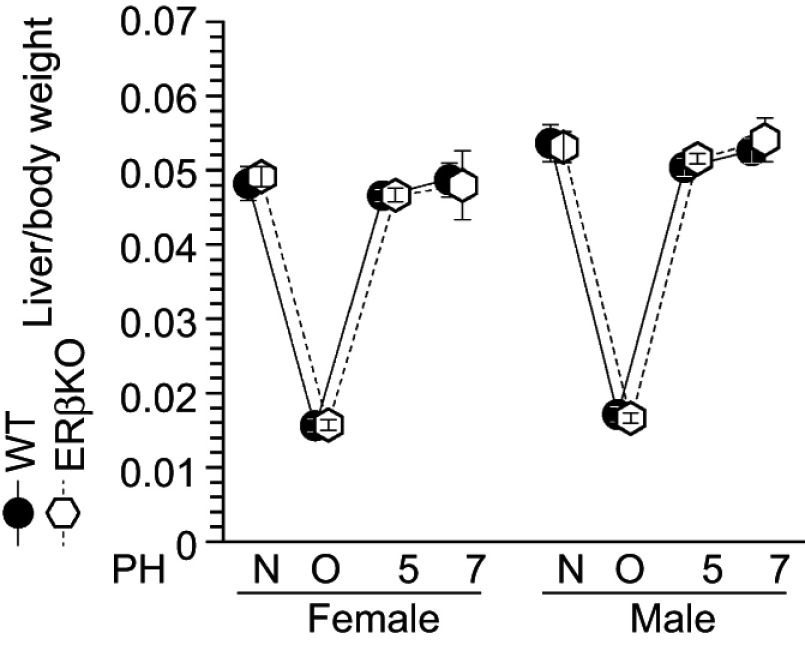
Finally, ERβ-KO mice were also subjected to the same procedures. However, in contrast to the results for ERα-KO mice, there were no differences in liver weight recovery between ERβ-KO and WT mice after liver resection (Figure 6). These data indicate that ERα is specifically involved in estradiol-induced liver regeneration after PH, and that the estradiol-ERα axis stimulates hepatocyte proliferation.
Figure 6.
Normal liver weight recovery in ERβ-knockout (KO) mice. Liver weight recovery in wild-type (WT) and ERβ-KO mice; N = non-operated, O = operated; 5/7=5 or 7 days after PH; n=5–8 per group.
Discussion
Estradiol is widely recognized as a stress signaling molecule,2,3,7–9,15 and PH has been shown to induce estradiol production as an initial response to resection prior to hepatocyte proliferation.2,3,7–9 Conversely, reduced estradiol production and delayed liver regeneration were observed in ovariectomized and orchidectomized mice.2,3 These previous findings suggested that estradiol is involved in triggering hepatocyte proliferation after PH, highlighting its clinical potential to promote liver recovery.
Endogenous estradiol production is altered in the estrus phase during the estrous cycle, after estradiol injection, and in late pregnancy.4 In parallel, the transcription of ERα is also increased in maternal periportal hepatocytes during late pregnancy.2–4 Thus, increased estradiol production in the estrus phase induces ERα expression in the periportal hepatocytes, thereby stimulating their proliferation. Here, we showed that ERα deficiency delayed the rate of liver weight recovery and hepatocyte proliferation, resulted in more severe fatty liver, and suppressed TNFα and IL-6 production, resulting in NF-κB and STAT3 activation (Figures 4 and 5). Moreover, estradiol administration promoted liver regeneration in WT but not ERα-KO mice. Collectively, these findings demonstrate an essential role for ERα in liver regeneration.
Conclusions
Using a gene KO strategy in mice, we demonstrated that ERαplays an important role liver regeneration after liver resection, and estradiol administration stimulates liver regeneration via binding to ERα in both sexes. These data indicate that estradiol might serve as a useful stimulant to accelerate liver regeneration in both sexes.
Acknowledgments
We are grateful to our department members at the National Center for Geriatrics and Gerontology (NCGG) for their helpful discussions. This work was supported by a Grant-in-Aid from NCGG (28-25 and 19-27), the Japan Science and Technology Agency (A-STEP-AS2312036G and FY2013-SICP), and the Ministry of Education, Culture, Sports, Science and Technology (MEXT 18659493).
Data sharing statement
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. [DOI] [PubMed] [Google Scholar]
- 2.Uebi T, Umeda M, Imai T. Estrogen induces estrogen receptor alpha expression and hepatocyte proliferation in the livers of the male mice. Genes Cells. 2015;20:217–223. doi: 10.1111/gtc.12214 [DOI] [PubMed] [Google Scholar]
- 3.Umeda M, Hiramoto M, Imai T. Partial hepatectomy induces delayed hepatocyte proliferation and normal liver regeneration in ovariectomized mice. Clin Exp Gastroenterol. 2015;8:175–182. doi: 10.2147/CEG.S80212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsugawa Y, Hiramoto M, Imai T. Estrogen induces estrogen receptor α expression and hepatocyte proliferation in late pregnancy. Biochem Biophys Res Commun. 2019;511:592–596. doi: 10.1016/j.bbrc.2019.02.119 [DOI] [PubMed] [Google Scholar]
- 5.Steer CJ. Liver regeneration. FASEB J. 1995;9:1396–1400. doi: 10.1096/fasebj.9.14.7589980 [DOI] [PubMed] [Google Scholar]
- 6.Michalpoules GK, Defrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60 [DOI] [PubMed] [Google Scholar]
- 7.Francavilla A, Eagon PK, DiLeo A, et al. Sex hormone-related functions in regenerating male rat liver. Gastroenterology. 1986;91:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francavilla A, Polimeno L, DiLeo A, et al. The effect of estrogen and tamoxifen on hepatocyte proliferation in vivo and in vitro. Hepatology. 1989;9:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francavilla A, Gavaler JS, Makowka L, et al. Estradiol and testosterone levels in patients undergoing partial hepatectomy. Dig Dis Sci. 1989;34:818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai T, Jiang M, Kastner P, Chambon P, Metzger D. Selective ablation of retinoid X receptor α in hepatocytes impairs their lifespan and regenerative capacity. Proc Natl Acad Sci USA. 2001;98:4581–4586. doi: 10.1073/pnas.071056098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uebi T, Umeda M, Maekawa N, Karasawa S, Handa H, Imai T. Prohibitins, novel vitamin K2 target factors in osteoblast. J Biosci Med. 2013;1:1–4. doi: 10.4236/jbm.2013.13001 [DOI] [Google Scholar]
- 12.Imai T, Jiang M, Chambon P, Metzger D. Impaired adipogenesis and lipolysis in the mouse upon cre-ERT2-mediated selective ablation of RXRα in adipocytes. Proc Natl Acad Sci USA. 2001;98:224–228. doi: 10.1073/pnas.011528898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umeda M, Uebi T, Maekawa N, Handa H, Imai T. PGJIFs, new mitochondrial PGJ2 target proteins, regulate cell proliferation. J Biosci Med. 2013;1:11–15. doi: 10.4236/jbm.2013.13003 [DOI] [Google Scholar]
- 14.Tsugawa Y, Handa H, Imai T. Arginine induces IGF-1 secretion from the endoplasmic reticulum. Biochem Biophys Res Commun. 2019;514:1128–1132. doi: 10.1016/j.bbrc.2019.05.044 [DOI] [PubMed] [Google Scholar]
- 15.Newhouse P, Albert K. Estrogen, stress, and depression: a neurocognitive model. JAMA Psychiatry. 2015;75:727–729. doi: 10.1001/jamapsychiatry.2015.0487 [DOI] [PubMed] [Google Scholar]