Abstract
Background
Vitamin D supplementation during pregnancy may be needed to protect against adverse pregnancy outcomes. This is an update of a review that was first published in 2012 and then in 2016.
Objectives
To examine whether vitamin D supplementation alone or in combination with calcium or other vitamins and minerals given to women during pregnancy can safely improve maternal and neonatal outcomes.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register (12 July 2018), contacted relevant organisations (15 May 2018), reference lists of retrieved trials and registries at clinicaltrials.gov and WHO International Clinical Trials Registry Platform (12 July 2018). Abstracts were included if they had enough information to extract the data.
Selection criteria
Randomised and quasi‐randomised trials evaluating the effect of supplementation with vitamin D alone or in combination with other micronutrients for women during pregnancy in comparison to placebo or no intervention.
Data collection and analysis
Two review authors independently i) assessed the eligibility of trials against the inclusion criteria, ii) extracted data from included trials, and iii) assessed the risk of bias of the included trials. The certainty of the evidence was assessed using the GRADE approach.
Main results
We included 30 trials (7033 women), excluded 60 trials, identified six as ongoing/unpublished trials and two trials are awaiting assessments.
Supplementation with vitamin D alone versus placebo/no intervention
A total of 22 trials involving 3725 pregnant women were included in this comparison; 19 trials were assessed as having low‐to‐moderate risk of bias for most domains and three trials were assessed as having high risk of bias for most domains. Supplementation with vitamin D alone during pregnancy probably reduces the risk of pre‐eclampsia (risk ratio (RR) 0.48, 95% confidence interval (CI) 0.30 to 0.79; 4 trials, 499 women, moderate‐certainty evidence) and gestational diabetes (RR 0.51, 95% CI 0.27 to 0.97; 4 trials, 446 women, moderate‐certainty evidence); and probably reduces the risk of having a baby with low birthweight (less than 2500 g) (RR 0.55, 95% CI 0.35 to 0.87; 5 trials, 697 women, moderate‐certainty evidence) compared to women who received placebo or no intervention. Vitamin D supplementation may make little or no difference in the risk of having a preterm birth < 37 weeks compared to no intervention or placebo (RR 0.66, 95% CI 0.34 to 1.30; 7 trials, 1640 women, low‐certainty evidence). In terms of maternal adverse events, vitamin D supplementation may reduce the risk of severe postpartum haemorrhage (RR 0.68, 95% CI 0.51 to 0.91; 1 trial, 1134 women, low‐certainty evidence). There were no cases of hypercalcaemia (1 trial, 1134 women, low‐certainty evidence), and we are very uncertain as to whether vitamin D increases or decreases the risk of nephritic syndrome (RR 0.17, 95% CI 0.01 to 4.06; 1 trial, 135 women, very low‐certainty evidence). However, given the scarcity of data in general for maternal adverse events, no firm conclusions can be drawn.
Supplementation with vitamin D and calcium versus placebo/no intervention
Nine trials involving 1916 pregnant women were included in this comparison; three trials were assessed as having low risk of bias for allocation and blinding, four trials were assessed as having high risk of bias and two had some components having a low risk, high risk, or unclear risk. Supplementation with vitamin D and calcium during pregnancy probably reduces the risk of pre‐eclampsia (RR 0.50, 95% CI 0.32 to 0.78; 4 trials, 1174 women, moderate‐certainty evidence). The effect of the intervention is uncertain on gestational diabetes (RR 0.33,% CI 0.01 to 7.84; 1 trial, 54 women, very low‐certainty evidence); and low birthweight (less than 2500 g) (RR 0.68, 95% CI 0.10 to 4.55; 2 trials, 110 women, very low‐certainty evidence) compared to women who received placebo or no intervention. Supplementation with vitamin D and calcium during pregnancy may increase the risk of preterm birth < 37 weeks in comparison to women who received placebo or no intervention (RR 1.52, 95% CI 1.01 to 2.28; 5 trials, 942 women, low‐certainty evidence). No trial in this comparison reported on maternal adverse events.
Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D)
One trial in 1300 participants was included in this comparison; it was assessed as having low risk of bias. Pre‐eclampsia was not assessed. Supplementation with vitamin D + other nutrients may make little or no difference in the risk of preterm birth < 37 weeks (RR 1.04, 95% CI 0.68 to 1.59; 1 trial, 1298 women, low‐certainty evidence); or low birthweight (less than 2500 g) (RR 1.12, 95% CI 0.82 to 1.51; 1 trial, 1298 women, low‐certainty evidence). It is unclear whether it makes any difference to the risk of gestational diabetes (RR 0.42, 95% CI 0.10 to 1.73) or maternal adverse events (hypercalcaemia no events; hypercalciuria RR 0.25, 95% CI 0.02 to 3.97; 1 trial, 1298 women,) because the certainty of the evidence for both outcomes was found to be very low.
Authors' conclusions
We included 30 trials (7033 women) across three separate comparisons. Our GRADE assessments ranged from moderate to very low, with downgrading decisions based on limitations in study design, imprecision and indirectness.
Supplementing pregnant women with vitamin D alone probably reduces the risk of pre‐eclampsia, gestational diabetes, low birthweight and may reduce the risk of severe postpartum haemorrhage. It may make little or no difference in the risk of having a preterm birth < 37 weeks' gestation. Supplementing pregnant women with vitamin D and calcium probably reduces the risk of pre‐eclampsia but may increase the risk of preterm births < 37 weeks (these findings warrant further research). Supplementing pregnant women with vitamin D and other nutrients may make little or no difference in the risk of preterm birth < 37 weeks' gestation or low birthweight (less than 2500 g). Additional rigorous high quality and larger randomised trials are required to evaluate the effects of vitamin D supplementation in pregnancy, particularly in relation to the risk of maternal adverse events.
Keywords: Female; Humans; Pregnancy; Pregnancy Outcome; Calcium, Dietary; Calcium, Dietary/administration & dosage; Diabetes, Gestational; Diabetes, Gestational/prevention & control; Dietary Supplements; Pre‐Eclampsia; Pre‐Eclampsia/prevention & control; Pregnancy Complications; Pregnancy Complications/prevention & control; Premature Birth; Premature Birth/prevention & control; Randomized Controlled Trials as Topic; Vitamin D; Vitamin D/administration & dosage; Vitamin D/analogs & derivatives; Vitamins; Vitamins/administration & dosage
Plain language summary
Is vitamin D supplementation beneficial or harmful for women during pregnancy?
What is the issue?
It is not clear if vitamin D supplementation, alone or in combination with calcium or other vitamins and minerals, during pregnancy have benefits or harms to the mother or her offspring.
Why is this important?
Vitamin D is essential for human health, particularly bone, muscle contraction, nerve conduction, and general cellular function. Low concentrations of blood vitamin D in pregnant women have been associated with pregnancy complications. It is thought that additional vitamin D through supplementation during pregnancy might be needed to protect against pregnancy complications.
What was studied in the review?
This is an update of a review that was first published in 2012 and subsequently updated in 2016. This review evaluated the effect of supplementation with vitamin D alone or in combination with other micronutrients for women during pregnancy in comparison to placebo or no intervention, irrespective of dose, duration or time of commencement of supplementation or type of supplementation (oral or by injection).
What evidence did we find?
We searched for evidence (July 2018) and found 30 trials (involving 7033 women) for inclusion in this update.
Evidence from 22 trials involving 3725 pregnant women suggest that supplementation with vitamin D alone during pregnancy probably reduces the risk of pre‐eclampsia, gestational diabetes, and the risk of having a baby with low birthweight compared to placebo or no intervention and may make little or no difference in the risk of having a preterm birth. It may reduce the risk of maternal adverse events, such as severe postpartum haemorrhage, although it should be noted that this result was unexpected and based on a single trial.
Evidence from nine trials involving 1916 pregnant women suggest that supplementation with vitamin D and calcium probably reduces the risk for pre‐eclampsia but may increase the risk of preterm birth. This slight potential harm warrants consideration in women receiving calcium supplementation as part of antenatal care.
Evidence from one study involving 1300 pregnant women suggest that supplementation with vitamin D plus other nutrients may make little or no difference in the risk of most outcomes evaluated.
Data on maternal adverse events were lacking in most trials.
What does this mean?
Supplementing pregnant women with vitamin D alone probably reduces the risk of pre‐eclampsia, gestational diabetes, low birthweight and the risk of severe postpartum haemorrhage. It may make little or no difference in the risk of having a preterm birth < 37 weeks' gestation. Supplementing pregnant women with vitamin D and calcium probably reduces the risk of pre‐eclampsia but may increase the risk of preterm births < 37 weeks (these findings warrant further research). Supplementing pregnant women with vitamin D and other nutrients may make little or no difference in the risk of preterm birth or low birthweight (less than 2500 g) and the effects for gestational diabetes and maternal adverse events are unclear. Additional rigorous high quality and larger randomised trials are required to evaluate the effects of vitamin D supplementation in pregnancy, particularly in relation to the risk of maternal adverse events.
Summary of findings
Summary of findings for the main comparison. Vitamin D supplementation compared to placebo or no intervention for pregnancy and neonatal health outcomes.
| Vitamin D supplementation compared to placebo/control for pregnancy and neonatal health outcomes | ||||||
| Patient or population: pregnant women and their infants. Setting: trials were carried from 1980s to 2015 in countries from Bangladesh, India, Iran, New Zealand and UK. Most trials were conducted outside the tropics and in different seasons. Intervention: vitamin D supplementation. Comparison: placebo or no intervention. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo/control | Risk with vitamin D supplementation | |||||
| Pre‐eclampsia | Study population | RR 0.48 (0.30, 0.79) | 499 (4 RCTs) | ⊕⊕⊕⊝ MODERATE1 | Included trials: Asemi 2013a; Naghshineh 2016; Sablok 2015; Sasan 2017 | |
| 168 per 1000 | 79 per 1000 (49 to 131) | |||||
| Gestational diabetes | Study population | RR 0.51 (0.27 to 0.97) | 446 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | Included trials: Asemi 2013a; Sablok 2015; Shahgheibi 2016; Tehrani 2014 | |
| 127 per 1000 | 65 per 1000 (34 to 123) | |||||
| Maternal adverse events: severe postpartum haemorrhage | Study population | RR 0.68 (0.51 to 0.91) | 1134 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | Included trial: Harvey 2012 | |
| 158 per 1000 | 106 per 1000 (79 to 142) | |||||
| Maternal adverse event: nephritic syndrome | Study population | RR 0.17 (0.01 to 4.06) | 135 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 4,5 | Included trial: Yu 2008 | |
| 22 per 1000 | 4 per 1000 (0 to 90) | |||||
| Maternal adverse event: hypercalcaemia | Study population | Not estimable | 1134 (1 RCT) | ⊕⊕⊝⊝ LOW 3,6 | Included trial: Harvey 2012 | |
| 0 per 1000 | 0 per 1000 | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 0.66 (0.34 to 1.30) | 1640 (7 RCTs) | ⊕⊕⊝⊝ LOW 7,8 | Included trials: Asemi 2013a; Delvin 1986; Grant 2013; Harvey 2012; Mirghafourvand 2013; Roth 2010; Singh 2015 | |
| 87 per 1000 | 57 per 1000 (29 to 113) | |||||
| Low birthweight (less than 2500 g) | Study population | RR 0.55 (0.35 to 0.87) | 697 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 9 | Included trials: Brooke 1980; Bhutta 2011; Marya 1988; Roth 2010; Sablok 2015 | |
| 136 per 1000 | 75 per 1000 (48 to 118) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded (1) level for serious limitations in study design due to one trial being assessed as high risk of bias for several domains and two trials having unclear allocation concealment.
2 We downgraded (1) level for serious limitations in study design due to one trial being assessed as high risk of bias for several domains.
3 We downgraded (2) levels for very serious limitations in study design due to one study being assessed as high risk of other bias because we do not know the impact of the participants who were allowed to continue taking their own multivitamin with 400 IU/d of vitamin D as this was not recorded.
4 We downgraded (1) level for serious limitations in study design due to one study being assessed as high risk of bias for performance and detection bias.
5 We downgraded (2) levels for very serious limitations in imprecision as only one small study, with a small number of events and wide 95% confidence intervals (CI) contributed data.
6 We downgraded (1) level for serious limitations in imprecision due to a single study with zero events contributing data.
7 We downgraded (1) level for serious limitations in study design due to two studies being at unclear risk of selection bias and one study being at high risk of other bias.
8 We downgraded (1) level for serious limitations in imprecision as the 95% confidence interval (CI) was wide and crossed the line of no effect.
9 We downgraded (1) level for serious limitations in study design due to two studies being at unclear risk of selection bias, one study being at high risk of bias for allocation concealment, and three studies being at high risk of attrition bias.
Summary of findings 2. Vitamin D + calcium supplementation compared to placebo or no intervention for pregnancy and neonatal health outcomes.
| Vitamin D + calcium supplementation compared to placebo/control for pregnancy and neonatal health outcomes | |||||||
| Patient or population: pregnant women and their infants.. Setting: trials were carried from 1980s to 2015 in countries from Iran, India, and Brazil. Only the study in Brazil was within the tropics. Most did not report the season in which it was carried out or it was mixed. Intervention: vitamin D + calcium supplementation. Comparison: placebo/control. | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with placebo/control | Risk with vitamin D + calcium supplementation | ||||||
| Pre‐eclampsia | Study population | RR 0.50 (0.32 to 0.78) | 1174 (4 RCTs) | ⊕⊕⊕⊝ MODERATE1 | Included trials: Asemi 2012; Marya 1987; Samimi 2016; Taherian 2002 | ||
| 94 per 1000 | 47 per 1000 (30 to 73) | ||||||
| Gestational diabetes | Study population | RR 0.33 (0.01 to 7.84) | 54 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,3 | Included trial: Asemi 2012 | ||
| 37 per 1000 | 12 per 1000 (0 to 290) | ||||||
| Maternal adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No trials reported on this outcome | |
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 1.52 (1.01 to 2.28) | 942 (5 RCTs) | ⊕⊕⊝⊝ LOW4,5 | Included trials: Asemi 2012; Diogenes 2013, Mirghafourvand 2013, Samimi 2016; Taherian 2002; | ||
| 72 per 1000 | 110 per 1000 (73 to 165) | ||||||
| Low birthweight (less than 2500 g) | Study population | RR 0.68 (0.10 to 4.55) | 110 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW6,7 | Included trials: Diogenes 2013; Samimi 2016 | ||
| 59 per 1000 | 40 per 1000 (6 to 268) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomised controlled trial; RR: Risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1 We downgraded (1) level for serious limitations in study design due to one study being at high risk of attrition and selection bias and three studies being at high risk of performance and detection bias.
2 We downgraded (1) level for serious limitations in study design due to one study being at high risk of performance and detection bias.
3 We downgraded (2) levels for very serious limitations in imprecision due to one small study, with a single event and wide 95% confidence intervals (CI) crossing the line of no effect contributing data.
4 We downgraded (1) level for serious limitations in study design due to three studies being at unclear risk of allocation concealment and three studies being at high risk of performance and detection bias.
5 We downgraded (1) level for serious limitations in imprecision due to wide 95% confidence intervals (CI).
6 We downgraded (1) level for serious limitations in study design due to one study being at unclear risk of allocation concealment and one study being at high risk of attrition bias.
7 We downgraded (2) levels for very serious limitations in imprecision due two small studies, with very few events and wide 95% confidence intervals (CI) crossing the line of no effect contributing data.
Summary of findings 3. Vitamin D + calcium + other vitamins and minerals compared to calcium + other vitamins and minerals (but no vitamin D) for pregnancy and neonatal health outcomes.
| Vitamin D + calcium + other vitamins and minerals compared to calcium + other vitamins and minerals (but no vitamin D) for pregnancy and neonatal health outcomes | ||||||
| Patient or population: pregnant women and their infants.. Setting: the only study included in this comparison was conducted in Bangladesh, which is located outside the tropics and it was conducted in different seasons of the year. Intervention: vitamin D + calcium + other vitamins and minerals. Comparison: calcium + other vitamins and minerals (but no vitamin D). | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with calcium + other vitamins and minerals (but no vitamin D) | Risk with vitamin D + calcium + other vitamins and minerals | |||||
| Pre‐eclampsia | Study population | ‐ | (0 trials) | ‐ | No trials reported on this outcome | |
| see comment | see comment | |||||
| Gestational diabetes | Study population | RR 0.42 (0.10 to 1.73) | 1298 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | Included trial: Roth 2013 | |
| 12 per 1000 | 5 per 1000 (1 to 20) | |||||
| Maternal adverse event: hypercalcaemia | Study population | ‐ | 1298 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2,3 | Included trial: Roth 2013 | |
| 23 per 1000 | 64 per 1000 (28 to 147) | |||||
| Maternal adverse event: hypercalciuria | Study population | 0.25 (0.02 to 3.97) | 1298 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1,2 | Included trial: Roth 2013 | |
| 4 per 1000 | 1 per 1000 (0 to 15) | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 1.04 (0.68 to 1.59) | 1298 (1 RCT) | ⊕⊕⊝⊝ LOW 2,3 | Included trial: Roth 2013 | |
| 93 per 1000 | 96 per 1000 (63 to 147) | |||||
| Low birthweight (less than 2500 g) | Study population | RR 1.12 (0.82 to 1.51) | 1298 (1 RCT) | ⊕⊕⊝⊝ LOW 2,3 | Included trial: Roth 2013 | |
| 162 per 1000 | 182 per 1000 (133 to 245) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded (2) levels for very serious limitations in imprecision with only one trial, with few events, and wide 95% confidence intervals (CI) crossing the line of no effect contributing data.
2 We downgraded (1) level for serious indirectness as there were multiple nutrient interventions in addition to vitamin D.
2 We downgraded (2) levels for very serious limitations in imprecision with only one trial, with zero events, and wide 95% confidence intervals (CI) crossing the line of no effect contributing data.
3 We downgraded (1) level for serious limitations in imprecision due to only one trial with wide 95% confidence intervals (CI) crossing the line of no effect contributing data.
Background
Description of the condition
Vitamin D metabolism
Vitamin D is a fat‐soluble vitamin which comes primarily from exposure to sunlight, and is found naturally only in a few foods, such as fish‐liver oils, fatty fish, mushrooms, egg yolks, and liver (Holick 2007a; Holick 2008). There are two physiologically active forms of vitamin D collectively called calciferol: D2 and D3. Vitamin D2 (also called ergocalciferol) is synthesised by plants while vitamin D3 (also called cholecalciferol) is subcutaneously produced in humans from 7‐dehydrocholecalciferol upon exposure to ultraviolet light B (UVB) radiation (DeLuca 2004). Vitamin D in supplements is found as either vitamin D2 or D3. The latter may be three times more effective than vitamin D2 in raising serum concentrations of vitamin D and maintaining those levels for a longer time particularly during the winter months; also, its metabolites have superior affinity for vitamin D‐binding proteins in plasma (Armas 2004; Logan 2013; McCullough 2007). As vitamin D has a short half‐life, adequate vitamin D intake is necessary in order to ensure sustained circulating levels.
Both D2 and D3 forms share a similar metabolism. They are first hydroxylated in the liver to form 25 hydroxyvitamin D (25(OH)D or calcidiol), and then in the kidney to 1,25 di hydroxyl vitamin D (1,25 (OH)2 D or calcitriol) in response to parathyroid hormone (PTH) levels. Calcitriol is considered an important pre‐hormone with active metabolites that are involved in metabolic processes including bone integrity and calcium homeostasis (Wagner 2008).
The major sites of vitamin D action include the skin, intestine, bone, parathyroid gland, immune system, and pancreas as well as the small intestine and colon in the human fetus (Theodoropoulos 2003). Additionally, vitamin D helps maintain normal levels of glucose in the blood, by binding and activating the vitamin D receptor in the pancreatic beta cells, regulating the release of insulin in response to the level of circulating glucose (Clifton‐Bligh 2008; Maghbooli 2008; Palomer 2008; Xuan 2013). Vitamin D also indirectly affects glucose metabolism via the regulation of calcium homeostasis (Xuan 2013).
There is a unique relationship between vitamin D and calcium. PTH is responsible for raising the calcium concentration in the blood through bone resorption, while calcitriol inhibits PTH and allows an increase of serum calcium concentration from sources other than the bone. In the presence of calcitriol, renal and intestinal calcium and phosphorus absorption is augmented leading to an improved calcium status.
Vitamin D status
Serum calcidiol or 25‐hydroxyvitamin D can be used to assess vitamin D status, as it reflects the sum of the vitamin D produced cutaneously and that obtained from foods and supplements (Jones 2008). This metabolite is difficult to measure, with large variations between methods and among laboratories, even when the same methods are used, which may be explained by differences in sample pretreatment or the solvent extraction system used (Hollis 2004; Lankes 2015).
Recently, the Institute of Medicine (IOM) defined adequate vitamin D status as having serum 25‐hydroxyvitamin D concentrations greater than 50 nmol/L (or 20 ng/mL) in both the general population and pregnant women (IOM 2011). Some investigators propose that concentrations around 80 nmol/L (32 ng/mL) are optimal, since they suppress PTH levels and lead to the greatest calcium absorption and the highest bone mass, reducing the rates of bone loss, falls, and fractures (Dawson‐Hughes 2005; Dawson‐Hughes 2008). It is uncertain whether these higher levels proposed for non pregnant adults are also adequate for pregnant women.
Vitamin D status is affected by factors that regulate its production in the skin (i.e. skin pigmentation, latitude, dressing codes, season, aging, sunscreen use, and air pollution) and by factors affecting its absorption or metabolism (Holick 2007b; Maghbooli 2007). Melanin acts as a filter for ultraviolet (UV) rays hence reducing the production of vitamin D by the skin. Hispanic and black populations in the USA may have a higher melanin content, and thus have reduced vitamin D photosynthesis (endogenous synthesis from exposure to sunlight) (Clemens 1982), explaining the variations in vitamin D concentration among ethnic groups living in the same geographical areas (Brooke 1980; Egan 2008; Ganji 2012; Matsuoka 1991; Nesby‐O'Dell 2002; Rockell 2005). An individual's skin phototype reflects the extent of sun‐burning versus subsequent tanning after an initial moderate sun exposure after a long period of little or no exposure (Gilchrest 2008). Phototypes I and II have rapid vitamin D photosynthesis after a minimal erythematic dose (MED). In contrast, prototype VI has little vitamin photosynthesis following the same MED dose (Clemens 1982). Differences in latitude have also been shown to influence the concentration of vitamin D, and individuals from countries in high and low latitudes have lower vitamin D levels. The importance of UV rays is further shown by the seasonal variation in the concentration of vitamin D between summer and winter, with higher levels during the summer compared with the winter months (Holick 2007b; Levis 2005). Vitamin D metabolism is also affected in obese individuals, as vitamin D is deposited in body fat stores, making it less bioavailable (Arunabh 2003). More recently, this low vitamin D status in obese individuals has been explained by a simple volumetric dilution of vitamin D in the fat mass (Drincic 2012), resulting in a higher prevalence of low levels of 25‐hydroxyvitamin D and these are more prevalent among overweight and obese individuals compared with normal weight individuals (Vilarrasa 2007; Vimaleswaran 2013; Wortsman 2000). In the same context, sedentary activity is also associated with low vitamin D levels as it may be linked with diminished sunlight exposure (Ohta 2009).
Magnitude of vitamin D deficiency
Vitamin D deficiency may be a common health problem worldwide (Bandeira 2006; Palacios 2014; van Schoor 2011). There is a high prevalence of low vitamin D status in infants, children, adolescents, adults and elders worldwide, even in countries with sun exposure all year round (Palacios 2014). The highest reported prevalence has been found in the Middle East, particularly in girls and women, although there is a lack of data in most countries of South America and Africa.
In pregnancy, low concentrations of vitamin D in blood are also common. A review including 17 trials in pregnant and lactating women (two in America, six in Europe, one in Africa, seven in Asia, one in Oceania) (Palacios 2014) found a prevalence of low vitamin D status (defined as concentrations lower than 50 nmol/L) of 33% in the USA and 24% Canadian pregnant women. In Europe, the prevalence of low vitamin D status was 45% in Belgium, 35% in the UK, 44% in the Netherlands, 20% in Spain and 77% in Germany. In addition, prevalence of vitamin D deficiency (defined as concentrations lower than 30 nmol/L) was 12% in Belgium, 4% in England and 23% in the Netherlands. The only study reported in Africa reported a very low prevalence of low vitamin D status (1%) in a sample of 139 pregnant women from Tanzania. In Asia, the prevalence of low vitamin D status in pregnant women was very high: 90% in Turkey, 67% in Iran, 72% in Pakistan, 70% to 83% in Kuwait, 96% in India and 69% in China. Prevalence of vitamin D deficiency was also very high: 50% in Turkey, 45% in Pakistan, 38% to 41% in Kuwait and 60% in India. In Australia, low vitamin D status was found in 48% and vitamin D deficiency was found in 15% of pregnant women.
Most recently, a review including 13 trials from seven countries found prevalence of vitamin D deficiency and insufficiency ranging from 39.4% to 76.5% (van der Pligt 2018). They also reported only vitamin D deficiency and found the highest prevalence among Chinese (100%), Turkish (95.6%), Iranian (89.4%) and Pakistanian (89.0%) women.
Seasonal variation increases the risk of low concentrations of blood vitamin D in pregnancy, with a greater prevalence of lower concentrations of vitamin D in blood during the winter months compared with the summer months (Nicolaidou 2006; O'Riordan 2008). Differences in latitude have also been shown to influence the concentration of vitamin D in a majority of pregnant women (Sloka 2009).
Maternal vitamin D status and health outcomes
Vitamin D status during pregnancy is the most important stage of the lifecycle, as the fetus completely relies on this source during this period for its development. During pregnancy, 1,25‐dihydroxyvitamin D increases early during pregnancy and continues to increase until delivery (Moller 2013). This large increase in 1,25‐dihydroxyvitamin D appears to be dependent on available 25‐dihydroxyvitamin D levels, but independent on calcium metabolism, which is a unique feature of pregnancy that allows such high levels of 1,25‐dihydroxyvitamin D (Pludowski 2013a). Therefore, maintaining high enough levels of 25‐dihydroxyvitamin D are important to sustain the increased levels of 1,25‐dihydroxyvitamin D important during pregnancy. Such levels are still yet to be determined but several trials have shown that maternal vitamin D status is significantly associated with fetal and neonatal vitamin D status (El Koumi 2013; Sachan 2005), and that maternal vitamin D status is associated with health outcomes during pregnancy and neonatal and infant development. These associations will be described below.
Vitamin D status and hypertensive disorders during pregnancy
Maternal vitamin D deficiency in pregnancy has been associated with an increased risk of pre‐eclampsia (new‐onset gestational hypertension and proteinuria after 20 weeks of gestation), a condition associated with an increase in maternal and perinatal morbidity and mortality (Bodnar 2007; Holick 2008; Li 2000b; MacKay 2001; Xiong 1999). A meta‐analysis including eight trials found a significant association between vitamin D deficiency and risk of pre‐eclampsia, which was more evident in those that defined vitamin D deficiency as 25(OH)D 50 nmol/L (20 ng/mL), and in those from the USA (Tabesh 2013). Similarly, another meta‐analysis including 31 trials also found a 78% higher risk of pre‐eclampsia in pregnant women with low vitamin D status (odds ratio (OR) 1.79; 95% confidence interval (CI) 1.25 to 2.58) (Aghajafari 2013). A most recent systematic review including 13 trials from seven countries also found that vitamin D deficiency during pregnancy was associated with pre‐eclampsia in three out of four trials (van der Pligt 2018).
Women with pre‐eclampsia have lower concentrations of 25‐hydroxyvitamin D compared with women with normal blood pressure (Diaz 2002; Frenkel 1991; Halhali 1995; Halhali 2000; Tolaymat 1994). The low levels of urinary calcium (hypocalciuria) in women with pre‐eclampsia may be due to a reduction in the intestinal absorption of calcium impaired by low levels of vitamin D (August 1992; Halhali 1995). Additionally, pre‐eclampsia and low concentrations of blood vitamin D are directly and indirectly associated through biologic mechanisms including immune dysfunction, placental implantation, abnormal angiogenesis, excessive inflammation, and hypertension (Bodnar 2007; Cardus 2006; Evans 2004; Hewison 1992; Li 2002). Vitamin D may influence early placental development and thus, the development of pre‐eclampsia through its role in gene regulation and expression; yet more studies are needed to confirm this.
Vitamin D status and other maternal conditions
Low concentrations of blood vitamin D in early pregnancy has been associated with elevated risk for gestational diabetes mellitus (Farrant 2009; Zhang 2008). A meta‐analysis of 31 observational trials found that low vitamin D levels increased the risk of gestational diabetes in 49% (odds ratio (OR) 1.49; 95% confidence interval (CI) 1.18 to 1.89) (Aghajafari 2013). Similar results were found in another meta‐analysis of 24 observational studies (Wei 2013). Poor control of maternal diabetes in early pregnancy is inversely correlated with low bone mineral content in infants, as is low maternal vitamin D status (Namgunga 2003). Vitamin D deficiency (VDD) may lead to a high bone turnover, bone loss, osteomalacia (softening of the bones) and myopathy (muscle weakness) in the mother in addition to neonatal and infant VDD (El Koumi 2013; Glerup 2000; Lips 2001).
An adequate vitamin D status may also protect against other adverse pregnancy outcomes. For example, maternal vitamin D deficiency has been linked to caesarean section (Merewood 2009; Scholl 2012), but the mechanisms involved are unclear. It has been suggested that vitamin D deficiency during pregnancy may reduce pelvic muscle strength and control (Scholl 2012), but this needs to be confirmed.
Low prenatal and perinatal maternal vitamin D concentrations can affect the function of other tissues, leading to a greater risk of multiple sclerosis, cancer, insulin‐dependent diabetes mellitus, and schizophrenia later in life (McGrath 2001).
Vitamin D status and preterm birth and low birthweight
A potential inverse association between maternal vitamin D status and preterm birth (less than 37 weeks' gestation) has been reported (Dawodu 2011; Morley 2006). Conversely, not all the studies show significant associations between maternal calcidiol levels and any measure of the child's size at birth or during the first months of life (Bodnar 2010; Farrant 2009; Gale 2008; Morley 2006).
A meta‐analysis of 24 observational studies confirmed the association between low vitamin D levels (< 50 nmol/L) and increased risk of preterm birth (OR 1.58; 95% CI 1.08 to 2.31) (Wei 2013). Furthermore, two meta‐analyses also found significant associations between low vitamin D status and small‐for‐gestational age (Theodoratou 2014; Wei 2013). With respect to birthweight, a meta‐analysis including three observational studies found a weak positive association between maternal vitamin D status and birthweight after adjustment for potential confounders (Harvey 2014), but another meta‐analysis including four observational studies did find a significant association between these variables (Theodoratou 2014). A most recent systematic review including 13 studies from seven countries found that vitamin D deficiency during pregnancy was associated with low birthweight in four out of seven studies (van der Pligt 2018).
There is not much information on maternal vitamin D status and low birthweight or preterm birth in children born from HIV‐infected pregnant women (Mehta 2009). Studies have reported a high prevalence of vitamin D deficiency among HIV‐infected pregnant women (Eckard 2013; Mave 2012).
Vitamin D status and postnatal growth
Some observational studies suggest that vitamin D levels during pregnancy influence fetal bone development and children's growth (Bodnar 2010; Brooke 1980; Ioannou 2012; Mahon 2010; Morley 2006). However, there is inconsistent associations between maternal vitamin D status and head circumference, as found in a systematic review of nine observational studies (Harvey 2014). However, a study found that head circumference in children nine years of age was significantly associated with maternal calcidiol levels (Gale 2008). With respect to maternal vitamin D status and infants' bone mass, there are also inconsistent results (Akcakus 2006; Harvey 2014; Javaid 2006; Viljakainen 2010).
It is not clear if maternal vitamin D deficiency leads to neonatal rickets, since rickets is usually identified later in childhood. Early studies indicate a possible risk for neonatal rickets in the offspring of women with osteomalacia, abnormal softening of the bone by deficiency of phosphorus, calcium or vitamin D (Ford 1973). More recent studies have found that vitamin D deficiency (serum levels lower than 25 nmol/L) was identified in 92% of rachitic (having rickets) Arab children and 97% of their mothers compared with 22% of nonrachitic children and 52% of their mothers (Dawodu 2005). A positive correlation was found between maternal and child vitamin D levels.
In addition, analyses using data from pregnant women participating in the Southampton Women's Survey, a prospective longitudinal study, found in fetuses of mothers with low vitamin D status a greater femoral metaphyseal cross‐sectional area and a higher femoral splaying index at 19 and 34 weeks' gestation (Mahon 2010), and a significant association between fetal femur volume and vitamin D status (Ioannou 2012), which has been suggested to be possibly related to early rickets development (Harvey 2014).
Vitamin D status and immune response
Vitamin D has direct effects on both adaptive and innate immune systems (Miller 2010; Walker 2009). In children, vitamin D insufficiency is linked to autoimmune diseases such as type 1 diabetes mellitus, multiple sclerosis, allergies and atopic diseases (Bener 2009; Miller 2010; Pierrot‐Deseilligny 2010). Various studies have also shown that vitamin D deficiency is strongly associated with tuberculosis, pneumonia, and cystic fibrosis (Chocano‐Bedoya 2009; Hall 2010; Nnoaham 2008; Williams 2008), and both prenatal and perinatal vitamin D deprivation might influence early‐life respiratory morbidity as this vitamin is important for lung growth and development (Devereux 2007; Litonjua 2009).
Vitamin D may have positive effects on the immune system by up‐regulating the production of the antimicrobial peptides by macrophages and endothelial cells (Wang 2004), which may inactivate viruses and suppress inflammation (Cantorna 2008), and subsequently reduce the severity of infections.
Vitamin D toxicity
Vitamin D excess leads to hypercalcaemia (calcium levels are 10.5 mg/dL or higher) and hypercalciuria (urinary excretion of calcium exceeds 250 mg/day in women), which is associated with renal and kidney stones (Heaney 2008). Toxicity in adults usually appear at doses of vitamin D higher than 10,000 international units (IU)/day (250 mcg/day), although most of the evidence is based on short‐term exposures (less than six months) (Hathcock 2007; Heaney 2008; IOM 2011; Vieth 1999). Single‐dose supplements providing 7.5 mg (300,000 IU) or more may also be harmful (Roth 2011a).
The potential for vitamin D‐induced teratogeneses (birth defects) and adverse events in the offspring (e.g. growth restriction, delayed ossification, craniofacial hypoplasia) has been suggested by a few studies in rats and rabbits (Ariyuki 1987; Chan 1979; Friedman 1969; Ornoy 1968; Ornoy 1969). However, there are considerable limitations in extrapolating such findings to humans, in whom adverse fetal effects have not reportedly occurred following maternal ingestion of maintenance doses as high as 5 mg (200,000 IU) of vitamin D per day. Overall, animal and human studies show that fetal excess of vitamin D metabolites are unlikely to occur when maternal concentrations are within a normal range (Roth 2011a).
Description of the intervention
The World Health Organization (WHO) currently does not recommend provision of vitamin D supplements during pregnancy as part of routine antenatal care (WHO 2016), mainly due to lack of evidence and only in cases of VDD, which is in alignment with the American Congress of Obstetricians and Gynecologists guidelines (ACOG 2015).
There is ongoing controversy regarding the 25‐hydroxyvitamin D levels that are considered adequate or optimal for overall health. The US Institute of Medicine has determined that concentrations greater than 50 nmol/L or 20 ng/mL are adequate based on the current studies available (IOM 2011), although many investigators consider that optimal levels should be higher (greater than 75 nmol/L or 30 ng/mL) (Dawson‐Hughes 2005; Hollick 2009). Vitamin D recommendations to maintain adequate levels of 25‐hydroxyvitamin D also differ among different organisations. The Recommended Nutrient Intakes (RNI) established by the WHO/Food and Agriculture Organization of the United Nations is 200 IU/day (5 mcg/day) of vitamin D for pregnant women (WHO 2004). The European Food Safety Authority (EFSA) and the Institute of Medicine in the USA recommend 600 IU/day (15 mcg/day) of vitamin D for pregnant women (EFSA 2016; IOM 2011). The Royal College of Obstetricians and Gynaecologists recommend 400 IU/day (10 mcg/day) for all pregnant women (RCOG 2014). For high‐risk women (dark skin, reduced exposure to sunlight, or those who are socially excluded or obese), they recommend at least 1000 IU/day (25 mcg/day). In addition, for women at high risk of pre‐eclampsia, they recommend at least 800 IU/day (20 mcg/day), combined with calcium. An expert panel in Central Europe recommended 1500 to 2000 IU/day (37.5 to 50.0 mcg/day) (Pludowski 2013b).
Recommendations on use of vitamin D supplements during pregnancy also vary, ranging from 200 to 400 IU/day (5 to 10 mcg/day) (Canadian Paediatric Society 2007; UK Department of Health 2009). The American Academy of Pediatrics (Wagner 2008) suggests that healthcare professionals who provide obstetric care should consider monitoring maternal vitamin D status by measuring its concentrations in pregnant women. Different investigators have suggested that a supplemental dose of vitamin D of 1000 to 1600 IU (25 to 40 mcg/day) might be necessary to achieve the optimal level of this vitamin in the body (Dawson‐Hughes 2005). This dose is expected to raise serum 25‐hydroxyvitamin D by 1.2 nmol/L for every mcg (40 IU) of vitamin D3 given orally to individuals with low 25‐hydroxyvitamin D levels; those with higher baseline concentrations would have smaller increments with the same dose (Dawson‐Hughes 2005). Others have suggested that doses around 1000 IU/day may be needed in order for pregnant women to maintain a blood concentration of vitamin D of more than 50 nmol/L (20 ng/mL) (Heaney 2003; Hollis 2004; Hollis 2007; Vieth 2001). Higher doses have also been suggested, such as weekly doses of 5000 IU (125 mcg/week) (Utiger 1998) or a single dose of 200,000 IU (5 mg) or greater (Mallet 1986; Sahu 2009; Yu 2009).
Since vitamin D can also be synthesised by the skin upon exposure to sunlight, increasing casual sun exposure for reaching the optimal serum levels has been recommended (Holick 2002). However, as excessive UV radiation is a carcinogen, it might be worth obtaining additional vitamin D from foods or supplements.
How the intervention might work
Vitamin D supplementation has shown to improve maternal vitamin D status during pregnancy in some studies (Delvin 1986; Yu 2009), which in turn may have a direct influence on the fetal and neonatal supply of vitamin D (Brooke 1980). The potential effect of gestational vitamin D supplementation in preventing preterm birth (less than 37 weeks' gestation) and low birthweight (less than 2500 g) has been suggested (Maxwell 1981), although there is limited information on the additional benefit of vitamin D supplementation over other nutritional interventions during pregnancy such as iron and folic acid supplementation on the risk of low birthweight (Christian 2003). There is also a potential effect of maternal vitamin D supplementation on neonatal growth (Marya 1988). Vitamin D supplementation during pregnancy may be necessary to ensure adequate concentrations of vitamin D in breast milk during lactation (Butte 2002). However, it is important to note that the benefits may be seen if supplementation starts early in pregnancy, as there is evidence to suggests that vitamin D status early pregnancy is an important determinant of maternal and neonatal health outcomes (Karras 2018).
Why it is important to do this review
Currently, most countries do not include vitamin D supplementation as part of their routine antenatal care. As stated by the Working Group convened by the Sackler Institute for Nutrition Science at the New York Academy of Sciences and the Bill & Melinda Gates Foundation (in co‐ordination with a scientific organising committee to assess the global prevalence and disease burden of vitamin D deficiency), vitamin D affects pregnancy and birth outcomes but evidence is conflicting (Roth 2018).
This review updates the previous Cochrane Review on vitamin D supplementation in pregnancy (De‐Regil 2016). The 2016 review included 15 trials (2833 women) and concluded that supplementing pregnant women with vitamin D may reduce the risk of pre‐eclampsia, low birthweight and preterm birth. However, when vitamin D and calcium were combined, it may increase the risk of preterm birth. The present review incorporates new evidence from trials testing the effects and safety of vitamin D supplementation in pregnancy for the well‐being of the mother and her newborn. Results from this review could contribute to establish practice guidelines at the population level.
Information on the most effective and safe dosage, the optimal dosing regimen (daily, intermittent or single doses), the timing of initiation of vitamin D supplementation, and the effect of vitamin D when combined with other vitamins and minerals are also needed to inform policy‐making. In fact, we are conducting another systematic review comparing between doses of vitamin D and its effects on pregnancy and infant outcomes (Palacios 2018).
Objectives
To examine whether vitamin D supplementation alone or in combination with calcium or other vitamins and minerals given to women during pregnancy can safely improve maternal and neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We intended to include randomised and quasi‐randomised trials with randomisation at either individual or cluster level, but we only found randomised controlled trials with individual randomisation. We did not include cross‐over trials or any other observational designs (e.g. cohort or case‐control studies) in this meta‐analysis, but we considered such evidence in the discussion, where relevant. Abstracts were included if they had enough information to extract the data.
Types of participants
Pregnant women of any gestational or chronological age, parity (number of births) and number of fetuses, living in any country. Pregnant women with pre‐existing conditions were excluded.
Types of interventions
Vitamin D supplementation during pregnancy irrespective of dose, duration or time of commencement of supplementation or type of supplementation (oral or by injection). We included trials testing vitamin D alone or in combination with other micronutrients as long as the intervention and the control group were treated similarly. Specifically, we assessed the following comparisons.
Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals)
Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals)
Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals supplementation (but no vitamin D)
Supplementation with vitamin D + calcium versus calcium supplementation (but no vitamin D)
Supplementation with vitamin D + calcium + other vitamins and minerals versus other vitamins and minerals supplementation (but no vitamin D + calcium)
Types of outcome measures
Maternal antenatal clinical and laboratory outcomes and infant clinical and laboratory outcomes as described below.
Primary outcomes
Maternal
Pre‐eclampsia (as defined by trialists).
Gestational diabetes (as defined by trialists).
Adverse events (e.g. hypercalcaemia, kidney stones).
Infant
Preterm birth (less than 37 weeks' gestation).
Low birthweight (less than 2500 g).
Secondary outcomes
Maternal
Impaired glucose tolerance (as defined by trialists).
Caesarean section.
Gestational hypertension (as defined by trialists).
Maternal death (death while pregnant or within 42 days of termination of pregnancy).
Vitamin D concentration at term (25‐hydroxyvitamin D in nmol/L).
Infant
Birth length (cm).
Head circumference at birth (cm).
Birthweight (g).
Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery).
Stillbirth (as defined by trialists).
Neonatal death (within 28 days after delivery).
Apgar score less than seven at five minutes.
Neonatal infection (e.g. respiratory infections within 28 days after delivery).
Very preterm birth (less than 32 weeks' gestation).
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (12 July 2018).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
We also searched the registries at ClinicalTrials.gov and WHO‐hosted International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned trials (12 July 2018) (see:Appendix 1).
Searching other resources
For the identification of ongoing and unpublished studies, we contacted on different institutions including the WHO Departments of Reproductive Health and Research and Nutrition for Health and Development, the WHO regional offices, the United Nations Children's Fund (UNICEF), Nutrition International (NI), the Global Alliance for Improved Nutrition (GAIN) and the US Centers for Disease Control and Prevention (CDC) (15 May 2018)
We did not apply any date or language restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeDe‐Regil 2016.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (LK, CP) independently assessed for inclusion all the references identified through the search. All the papers were assessed in duplicate and we resolved any disagreements through discussion or, if required, we consulted the third review author (JP).
If studies were published only as abstracts, or study reports contained little information on methods, we attempted to contact the authors to obtain further details of study design and results. We were able to screen all the potentially eligible studies.
Data extraction and management
We designed a form to extract data. For included studies, all review authors extracted the data using the agreed form. CP entered data into Review Manager software (RevMan 2014), and JP and LK checked for accuracy.
We analysed dichotomous data in terms of average risk ratio and we analysed continuous data in terms of mean difference. There was no need to use the standard mean difference as trials did not report outcomes in different scales.
Assessment of risk of bias in included studies
Two review authors (CP, LK) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion and consulted the third author (JP).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes);
unclear.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
We classified blinding as 'high risk of bias' if the blinding status of a trial was unclear or the trial was open.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low risk of bias;
high risk of bias;
unclear.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed losses to follow‐up and post‐randomisation exclusions systematically for each trial.
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
low risk of bias;
high risk of bias;
unclear.
We considered follow‐up to be 'low risk of bias' if more than 80% of participants initially randomised in a trial were included in the analysis and any loss was balanced across groups, unclear if the percentage of initially randomised participants included in the analysis was unclear, and 'high risk of bias' if less than 80% of those initially randomised were included in the analysis or if loss was imbalanced in different treatment groups.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We assessed whether each study was free of other problems that could put it at risk of bias: We noted for each included study any important concerns we had about other possible sources of bias:
low risk of further bias;
high risk of further bias;
unclear whether there is a risk of further bias.
(7) Overall risk of bias
We summarised the risk of bias at two levels: within studies (across domains) and across studies.
For the first, we made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and for primary outcomes, we explored the impact of the level of bias through undertaking a Sensitivity analysis.
Assessment of the certainty of the evidence using the GRADE approach
For the assessment across studies, the main findings of the review are set out in the Table 1; Table 2 and Table 3, prepared using GRADE profiler (GRADEpro) Guideline Development Tool (GRADEpro 2015). The primary outcomes for each comparison are listed with estimates of relative effects along with the number of participants and studies contributing data for those outcomes, where available. For each outcome, two review authors independently assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Handbook (GRADE Handbook), which involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias; this results in one out of four levels of certainty (high, moderate, low or very low). This assessment was limited only to the trials included in this review.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as average risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference as the outcomes were measured in the same way between trials; there was no need to use the standardised mean difference to combine trials.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials, but we did not find eligible studies with this design. We planned to adjust the standard errors of the results from cluster‐randomised studies using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), if sufficient information was available to allow for this. We planned to use an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources were used, we planned to report this and to conduct sensitivity analyses to investigate the effect of variation in the ICC.
If we had identified both cluster‐randomised trials and individually‐randomised trials, we would have combined the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit would be considered as unlikely.
Studies with more than two treatment groups
For studies with more than two intervention groups (multi‐arm studies), we combined groups to create a single pair‐wise comparison (Higgins 2011) and included the disaggregated data in the corresponding subgroup category. When the control group was shared by two or more study arms, we divided the control group (events and total population) over the number of relevant subgroup categories to avoid double counting the participants. The details are described in the Characteristics of included studies tables.
Cross‐over trials
We did not consider cross‐over trials eligible for inclusion.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We investigated reporting biases (such as publication bias) by using funnel plots for the primary outcomes with 10 or more studies. We assessed funnel plot asymmetry visually.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We intended to use fixed‐effect meta‐analysis for combining data where it would be reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Since we detected substantial heterogeneity, we used random‐effects meta‐analysis to produce an overall summary of an average treatment effect across trials. We treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
As we used random‐effects analyses, we present the results as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We planned to investigate any substantial heterogeneity on the primary outcomes by using subgroup analyses as follows:
by start of supplementation: less than 20 weeks versus 20 weeks of pregnancy or more versus unknown/mixed;
by pre‐gestational body mass index (BMI) (kg/m2): underweight (lower than 18.5) versus normal weight (18.5 to 24.9) versus overweight (25 or higher) versus unknown/mixed;
by supplementation scheme/regimen: single versus daily versus weekly versus unknown/mixed;
by skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): three or less versus four or more versus mixed/unknown;
by latitude: between the Tropics of Cancer and Capricorn versus north of the Tropic of Cancer or south of the Tropic of Capricorn versus unknown/mixed;
by season at the start of pregnancy: summer versus winter versus mixed/unknown/unreported.
Pragmatically, we decided not to conduct subgroup analyses in those outcomes with three or less trials.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We intended to conducted a sensitivity analysis based on the quality of the studies, however, as only one study was considered of high quality we did not perform this analysis. We considered a study to be of high quality if it was assessed as having low risk of bias in both the randomisation and allocation concealment and additionally a low risk of bias in either blinding or losses to follow‐up. In future updates, we will carry out sensitivity analysis to investigate the effect of the randomisation unit (if appropriate).
Results
Description of studies
Results of the search
We received a total of 111 new reports (after removing duplicates) from the search of Cochrane Pregnancy and Childbirth’s Trials Register, 13 reports from our additional search and we also reassessed the 23 ongoing trials (26 reports) and 27 excluded trials (46 reports) from the previous version of the review (De‐Regil 2016). See: Figure 1.
1.
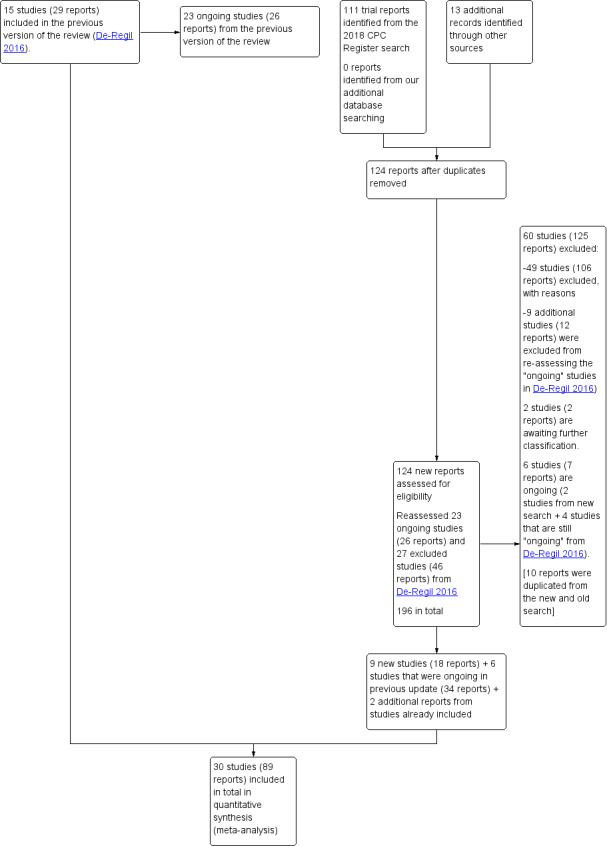
Study flow diagram for this update
A total of 30 trials were included in this update. Fifteen were already included in the previous update (Asemi 2012; Asemi 2013a; Brooke 1980; Delvin 1986; Diogenes 2013; Grant 2013; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Roth 2010; Sablok 2015; Taherian 2002; Yu 2008). We identified nine new trials through our updated search (Kaur 1991, Naghshineh 2016; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Singh 2015; Vaziri 2016) and included six additional trials that were categorised as ongoing in the previous update (Benson 2009; Bhutta 2011; Harvey 2012; Mirghafourvand 2013; Roth 2013; Tehrani 2014).
We identified another study (Qian 2015) that raised concerns with the veracity of the information as there were several outcomes with the same results to another published study (Karamali 2015). We followed the guidelines from the Committee on Publication Ethics (COPE) to investigate the issue with the editors of both journals (Cope 2016) and the publication (Qian 2015) was retracted by the editors on 20 August 2018. Therefore, this trial was moved to excluded.
We excluded a total of 60 trials (125 reports). We identified six ongoing or unpublished trials (Baird 2016; Jelsma 2013; Judkins 2010; Lindqvist 2010; Mosalanejad 2016; Rasmussen 2009). There are two trials awaiting classification as they were available only in the abstract form with not enough information for data extraction (Bimson 2017; Das 2009).
Details of these trials are provided in: Characteristics of included studies; Characteristics of excluded studies; Studies awaiting classification tables.
Included studies
We included 30 trials (involving 7033 women and their infants) in this updated review (Asemi 2012; Asemi 2013a; Benson 2009; Bhutta 2011; Brooke 1980; Delvin 1986; Diogenes 2013; Grant 2013; Harvey 2012; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Roth 2013; Sabet 2012; Sablok 2015; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Singh 2015; Taherian 2002; Tehrani 2014; Vaziri 2016; Yu 2008). Details of these trials are provided in: Characteristics of included studies table.
Settings
The trials included in this review were carried from 1980s to 2015.
Trials were conducted in Australia (Benson 2009), Bangladesh (Roth 2010; Roth 2013), Brazil (Diogenes 2013), China (Li 2000a), France (Delvin 1986; Mallet 1986), India (Kaur 1991;Marya 1987; Marya 1988; Sablok 2015; Singh 2015), Iran (Asemi 2012; Asemi 2013a; Mirghafourvand 2013; Naghshineh 2016; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Taherian 2002; Tehrani 2014; Vaziri 2016), New Zealand (Grant 2013), Pakistan (Bhutta 2011), Russia (Mazurkevich 2013) and the UK (Brooke 1980; Harvey 2012; Yu 2008).
Latitude
Most trials were conducted either above or below the Tropics of Cancer and Capricorn (Asemi 2012; Asemi 2013a; Brooke 1980; Delvin 1986; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Mirghafourvand 2013, Naghshineh 2016; Roth 2010; Roth 2013; Sablok 2015; Taherian 2002; Yu 2008; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Vaziri 2016; Benson 2009; Bhutta 2011; Tehrani 2014; Harvey 2012). Only two trials were conducted between the Tropics of Cancer and Capricorn (Grant 2013; Singh 2015), and one study was conducted just were the tropic of Capricorn lies (Diogenes 2013).
Seasonality
The seasons varied among trials with some trials occurring during the winter‐spring period (Delvin 1986); winter (Mallet 1986; Naghshineh 2016; Tehrani 2014); summer (Roth 2010; Yu 2008); spring‐summer period (Asemi 2013a), fall (Samimi 2016; Vaziri 2016), unknown/unreported in 13 trials (Asemi 2012; Benson 2009; Bhutta 2011; Kaur 1991; Li 2000a; Marya 1987; Marya 1988; Mazurkevich 2013; Sabet 2012; Sasan 2017; Shahgheibi 2016; Singh 2015; Taherian 2002) or mixed (Brooke 1980; Diogenes 2013; Grant 2013; Harvey 2012; Mirghafourvand 2013; Roth 2013; Sablok 2015; Samimi 2017).
Participants
The sample size from all the trials ranged between 40 women (Delvin 1986) and 1560 women (Roth 2013).
Pre‐gestational body‐mass index (kg/m2)
Pre‐gestational body mass index (BMI) of the participants was reported only in five trials (Asemi 2012; Asemi 2013a; Diogenes 2013; Sablok 2015; Taherian 2002). The rest of the trials did not report this. One study stratified for pre intervention BMI (in kg/m2; less than 30 and 30 or more) before randomisation (Asemi 2013a).
Skin pigmentation based on Fitzpatrick skin tone chart
None of the trials used the Fitzpatrick skin tone chart (Fitzpatrick 1988); however, several trials reported the ethnicity/race of participants. Most trials were among women from the Middle East (Asemi 2012; Asemi 2013a; Brooke 1980; Bhutta 2011; Tehrani 2014; Mirghafourvand 2013; Naghshineh 2016; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Taherian 2002; Vaziri 2016) or Asia (Kaur 1991; Li 2000a; Marya 1987; Marya 1988; Roth 2010; Roth 2013; Sablok 2015; Singh 2015). Two trials reported that participants were from mixed ethnicity (Benson 2009; Yu 2008), two trials were on whites (Harvey 2012; Mallet 1986), one among white women or black women (Diogenes 2013), and another among Pacific, European and Maori women (Grant 2013). Two trials did not report the characteristics of the participants in terms of ethnicity or origin (Delvin 1986; Mazurkevich 2013).
Interventions
A total of 22 trials compared provision of vitamin D supplement in comparison with placebo or no intervention (Comparison 1: Asemi 2013a; Benson 2009; Bhutta 2011; Brooke 1980; Delvin 1986; Grant 2013; Harvey 2012; Kaur 1991; Mallet 1986; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sabet 2012; Sablok 2015; Samimi 2017; Sasan 2017; Shahgheibi 2016; Singh 2015; Tehrani 2014; Vaziri 2016; Yu 2008).
A total of nine trials compared provision of oral vitamin D plus calcium supplements versus no intervention or placebo (Comparison 2: Asemi 2012; Asemi 2013a; Diogenes 2013; Li 2000a; Marya 1987; Mazurkevich 2013; Mirghafourvand 2013; Samimi 2016; Taherian 2002). The study by Mirghafourvand 2013 was included in both comparisons as they compared both vitamin D alone and vitamin D plus calcium with placebo.
Only one trial compared oral vitamin D plus calcium, iron and folic acid versus calcium, iron and folic acid but no vitamin D (Comparison 4: Roth 2013).
No trials evaluated the effects of either oral vitamin D plus calcium supplements versus calcium (Comparison 3), nor oral vitamin D + calcium + other vitamins and minerals supplements versus other oral vitamins and minerals supplements (but no vitamin D + calcium) (Comparison 5).
Start of supplementation
A total of seven trials started supplementation before week 20 (Benson 2009; Bhutta 2011; Harvey 2012; Naghshineh 2016; Samimi 2017; Singh 2015; Tehrani 2014). The rest of the trials started supplementation at 20 or more weeks' gestation (Asemi 2012; Asemi 2013a; Brooke 1980; Delvin 1986; Diogenes 2013; Grant 2013; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Mirghafourvand 2013; Roth 2010; Roth 2013; Sabet 2012; Sablok 2015; Samimi 2016; Sasan 2017; Shahgheibi 2016; Taherian 2002; Vaziri 2016; Yu 2008).
Dose of vitamin D used
The dose of vitamin D provided varied in the included trials as well as the regimen.
Trials differed in the frequency of supplementation, with some trials using daily doses, weekly doses, monthly doses or single doses. Some trials had more than one group of vitamin D intervention.
For daily, weekly and monthly dosage, we calculated the total amount in international units (IU) per day. The daily doses used were 200 IU vitamin D in five trials (Asemi 2012; Diogenes 2013; Li 2000a; Mazurkevich 2013; Taherian 2002); 400 IU vitamin D in three trials (Asemi 2013a; Li 2000a; Samimi 2017); 600 IU vitamin D in two trials (Naghshineh 2016; Roth 2013); 800 IU vitamin D in another trial (Yu 2008); 1000 IU vitamin D in six trials (Brooke 1980; Delvin 1986; Grant 2013; Harvey 2012; Mirghafourvand 2013; Mallet 1986); 1200 IU vitamin D in two trials (Kaur 1991; Marya 1987); 2000 IU vitamin D in three trials (Grant 2013; Singh 2015; Vaziri 2016); 2400 IU vitamin D in one trial (Roth 2013); 3333 to 3500 IU vitamin D in five trials (Sabet 2012; Samimi 2016; Sasan 2017; Tehrani 2014); 4000 IU vitamin D in two trials (Bhutta 2011; Roth 2013), and 5000 IU vitamin D in one trial (Shahgheibi 2016). One study started supplementation at 2000 IU per day and if 25(ODH)‐D levels were below 75 nmol/L by week 28 of pregnancy, the dose was doubled to 4000 IU per day (Benson 2009). One study also provided to both groups a gel with 400 mg/day of vaginal progesterone (Samimi 2017). The study by Roth 2013 gave three different doses during pregnancy as mentioned above: 4200 IU per week or 600 IU/day; 16,800 IU per week or 2400 IU/day; 28,000 IU per week or 4000 IU/day. We combined the data from these groups, and on average, this group received 16,333 IU per week or 2333 IU/day.
For single‐dose supplementation of vitamin D, the dose varied from 200,000 IU vitamin D in a group in one study (Yu 2008); 600,000 IU vitamin D in one trial (Marya 1988); and 60,000 IU vitamin D two times (Kaur 1991). There was also one trial that used a monthly dose of 100,000 vitamin D (Sabet 2012); three trials that used a 50,000 dose every two weeks (Tehrani 2014; Samimi 2016; Sasan 2017); and one trial that used a dose of 35,000 IU vitamin D every week (Roth 2010). For the study by Sablok 2015, the dose depended upon the level of serum 25(OH)‐D levels at baseline; it varied from one dose of 60,000 IU (if serum 25(OH)‐D levels were > 50 nmol/L), two doses of 120,000 IU (if serum 25(OH)‐D levels were 25 to 50 nmol/L), or four doses of 120,000 IU (if serum 25(OH)‐D levels < 25 nmol/L).
Overall, the total provision of supplemental vitamin D provided throughout pregnancy varied. Sixteen trials provided 56,000 IU vitamin D or less (Asemi 2012; Asemi 2013a; Benson 2009; Delvin 1986; Diogenes 2013; Grant 2013; Harvey 2012Li 2000a; Mazurkevich 2013; Naghshineh 2016; Roth 2013; Sabet 2012; Samimi 2017; Singh 2015; Taherian 2002; Vaziri 2016); nine trials provided more than 56,000 to 200,000 IU vitamin D (Bhutta 2011;Brooke 1980; Kaur 1991; Mallet 1986; Marya 1987; Mirghafourvand 2013; Roth 2013; Sablok 2015; Yu 2008); and seven trials provided more than 200,000 IU of vitamin D (Marya 1988; Roth 2010; Roth 2013; Sablok 2015; Samimi 2016; Sasan 2017; Tehrani 2014) throughout pregnancy. One study did not specify when the supplementation started, therefore, we were not able to estimate this (Shahgheibi 2016).
Vitamin D form used
The vitamin D was provided in the form of cholecalciferol‐D3 in 20 trials (Asemi 2012; Asemi 2013a; Benson 2009; Delvin 1986; Diogenes 2013; Grant 2013; Harvey 2012; Kaur 1991; Li 2000a; Mazurkevich 2013; Roth 2010; Roth 2013; Sabet 2012; Sablok 2015; Samimi 2016; Samimi 2017; Sasan 2017; Singh 2015; Taherian 2002; Vaziri 2016) and as ergocalciferol‐D2 in three trials (Brooke 1980; Mallet 1986; Yu 2008). Seven trials did not report the vitamin D form used (Bhutta 2011; Marya 1987; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Shahgheibi 2016; Tehrani 2014).
Doses of calcium in the trials providing vitamin D and calcium supplementation
The doses of calcium provided along with the vitamin D ranged from 300 mg (Mirghafourvand 2013), 375 mg (Marya 1987); 500 mg (Asemi 2012; Asemi 2013a; Roth 2013; Taherian 2002); 600 mg calcium (Diogenes 2013; Li 2000a), 1000 mg (Samimi 2016) and 1250 mg (Mazurkevich 2013). All used calcium carbonate.
Health worker cadre
The trials were mostly carried out in the context of antenatal care and the administration of the supplements and the antenatal care was provided by the researchers themselves or through health allied personnel. The outcomes measurements were carried out by different groups according to the nature of the outcome, whether it was clinical, biochemical, anthropometric, or dietary assessments. A more detailed description of the health worker cadre is presented in Characteristics of included studies.
Laboratory methodology for the assessment of vitamin D status
Different laboratory methods were used to measure vitamin D status as serum 25‐hydroxyvitamin D concentrations. Five trials (Asemi 2012; Asemi 2013a; Sabet 2012; Sablok 2015; Samimi 2016) used immunoassay ELISA kit for their determinations; six trials used a chemiluminescent enzyme‐labelled immunometric assay (Benson 2009; Bhutta 2011; Diogenes 2013; Harvey 2012; Singh 2015; Vaziri 2016); another trial used isotope‐dilution liquid chromatography–tandem mass spectrometry (Grant 2013). Two trials used a competitive protein binding assay (Brooke 1980; Mallet 1986); one trial used a radioligand assay (Delvin 1986); and two trials used the Liebermann‐Burchard method (Sasan 2017; Shahgheibi 2016). Only two trials used high‐performance liquid chromatography tandem mass spectroscopy (LCMS/MS) (Roth 2010; Roth 2013). In two trials, the laboratory method was not reported (Samimi 2017; Yu 2008). The other trials did not report on this outcome (Kaur 1991; Li 2000a; Marya 1987; Marya 1988; Mazurkevich 2013; Mirghafourvand 2013; Naghshineh 2016; Samimi 2016; Tehrani 2014).
Funding sources
Trials were funded mainly by research grants from universities, health institutions and non‐government organisations; sometimes in combination. The Vice‐chancellor for research supported Asemi 2012 and Asemi 2013a. The Luke Proposch Perinatal Research Scholarship from the Australian and New Zealand College of Obstetrics and Gynaecology Research Foundation supported Benson 2009. The Pakistan Initiative for Mothers and Newborns (PAIMAN) supported Bhutta 2011. The pathological research fund, St George's Hospital Medical School, and the South‐west Thames Regional Health Authority funded Brooke 1980. Shriners of North America, the France‐Quebec Exchange Program, and INSERM funded Delvin 1986. The Conselho Nacional de Desenvolvimento Cientıfico e Tecnologico and the Fundacao Carlos Chagas Filho de Amparo a` Pesquisa do Estado do Rio de Janeiro supported Diogenes 2013. The Health Research Council of New Zealand and Cure Kids supported Grant 2013. The Arthritis Research UK, Medical Research Council, Bupa Foundation, and National Institute for Health Research supported Harvey 2012. Tabriz University of Medical Sciences funded Mirghafourvand 2013. Isfahan University of Medical Sciences supported Naghshineh 2016. The Thrasher Research Fund supported Roth 2010 and Bill & Melinda Gates Foundation supported Roth 2013. The Research Institute of Endocrine Sciences and the Shahid Beheshti University of Medical Sciences supported Sabet 2012. Kashan University of Medical Sciences funded both Samimi 2016 and Samimi 2017. Research Deputy of Isfahan University of Medical Sciences supported Taherian 2002. Tehrani 2014 did not report funding. The Research Vice‐chancellor of Shiraz University of Medical Sciences supported Vaziri 2016. The Institute of Obstetrics and Gynaecology Trust and the Wolfson and Weston Research Centre for Family Health supported Yu 2008. Sablok 2015 was self‐funded. For Kaur 1991, Li 2000a, Mallet 1986, Marya 1987, Marya 1988, Mazurkevich 2013, , Sasan 2017, and Singh 2015, funding was unknown/unreported. No trials had funding sources of concern, e.g. vitamin D manufacturers or similar. Shahgheibi 2016 reported that they received no funding.
Declarations of interest
The following trials reported that none of the authors had conflict of interests: Asemi 2013a; Benson 2009; Diogenes 2013; Grant 2013; Mirghafourvand 2013; Roth 2010; Roth 2013; Sabet 2012; Sablok 2015; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Tehrani 2014; Vaziri 2016.
The following trials did not include the conflict of interest statement in their publication: Asemi 2012; Bhutta 2011; Brooke 1980; Delvin 1986; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Naghshineh 2016; Singh 2015; Taherian 2002; Yu 2008.
Only one trial reported conflict of interests: Harvey 2012.
SeeCharacteristics of included studies for a detailed description of the trials, including vitamin D doses used and regimens compared.
Excluded studies
We excluded 60 trials. The main reason for exclusion was that the comparisons were among different doses of vitamin D in 26 trials (Baqui 2009; Bhatia 2012; Bhatia 2010; Bisgaard 2009; Dawodu 2013; de Menibus 1984; Gerais 2015; Hashemipour 2014; Kachhawa 2014; Kiely 2015; Lalooha 2012; March 2010; Marya 1981; McLean 2012; Mojibian 2015; Mutlu 2013; Nausheen 2014; Rostami 2018; Shakiba 2013; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014), without placebo or no treatment control. Also, three trials were excluded because the treatment groups differed in other nutrients given in the supplements, other than vitamin D (Asemi 2015; Azami 2017; Pandey 2015), and one trial had no group with vitamin D (Atkinson 2010). In addition, four trials were not randomised trials (Ala‐Houhala 1986; Cockburn 1980; Bhatia 2010; Ito 1994). Four trials (Czech‐Kowalska 2013; Niramitmahapanya 2017; Taheri 2014; von Hurst 2009) were conducted on non pregnant women; 12 trials were carried out in pregnant women with glucose intolerance or with gestational diabetes (Asemi 2013b; Asemi 2014; Jamilian 2016; Jamilian 2017; Karamali 2014; Li 2016; Mozzafari 2010; Razavi 2017; Simsek 2011; Valizadeh 2016; Yazdchi 2016; Zhang 2016) or other chronic conditions (Etemadifar 2015; Shi 2017; Sudfeld 2017), one trial was conducted only among postpartum women (Chandy 2016), and one trial was conducted among couples for fertility purposes (Kermack 2017). One reference referred to a trial registered in 1986 on the Oxford Database of Perinatal Trials and reports the recruitment and follow‐up completed in 1979, but there were no reports available and we were unable to locate the author who registered the trial (MacDonald 1986). One trial was excluded because treatment groups differed more than vitamin D supplementation (Hossain 2012). For more detailed descriptions of excluded trials along with the reasons for exclusion, seeCharacteristics of excluded studies.
Risk of bias in included studies
Allocation
Sequence generation
We assessed 21 trials as having adequate methods for generating the randomisation sequence. Ten trials used computer‐generated random number sequences (Asemi 2013a; Diogenes 2013; Grant 2013; Harvey 2012; Naghshineh 2016; Roth 2010; Roth 2013; Sablok 2015; Samimi 2016; Yu 2008), four used permuted block randomisation (Bhutta 2011; Mirghafourvand 2013; Samimi 2017; Vaziri 2016), and seven trials used a random numbers table (Asemi 2012; Benson 2009; Mallet 1986; Sasan 2017; Shahgheibi 2016; Taherian 2002; Tehrani 2014) to randomise the intervention groups. The other trials reported the trials as randomised but the methods used to generate the sequence were not described (Brooke 1980; Delvin 1986; Kaur 1991; Marya 1987; Marya 1988; Mazurkevich 2013; Sabet 2012; Singh 2015). One trial did not mention that participants were randomly allocated to the treatment groups (Li 2000a).
Allocation concealment
We judged that 13 trials had adequate methods of allocation concealment (Asemi 2013a; Asemi 2012; Benson 2009; Bhutta 2011; Grant 2013; Harvey 2012; Roth 2010; Roth 2013; Samimi 2016; Samimi 2017; Shahgheibi 2016; Tehrani 2014; Yu 2008). It is assumed that allocation concealment did not occur in the following trials as one of the groups did not receive any supplementation: Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Sablok 2015; Singh 2015; Taherian 2002. In Sablok 2015, the intervention dosage depended on the vitamin D status, therefore, there was a selection bias based on status of vitamin D at baseline. In the case of Harvey 2012, participants at 28 weeks had their serum 25‐hydroxyvitamin D measured and if below 75 nmol/L, the dose was doubled to 4000 IU. The others did not report the methods of concealment (Brooke 1980; Delvin 1986; Diogenes 2013; Mirghafourvand 2013; Naghshineh 2016; Sabet 2012; Sasan 2017; Vaziri 2016).
Blinding
Blinding of participants, staff and outcome assessors
Investigators in 15 trials reported that they used a double‐blinded design (Asemi 2013a; Bhutta 2011; Brooke 1980; Grant 2013; Harvey 2012; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Roth 2013; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Tehrani 2014). However, only 10 trials specified that both participants and those conducting the assessments were blinded (Asemi 2013a; Bhutta 2011; Grant 2013; Harvey 2012; Naghshineh 2016; Roth 2010; Roth 2013; Sasan 2017; Shahgheibi 2016; Tehrani 2014). Two trials were reported as single‐blinded, being blinded for participants only (Asemi 2012; Diogenes 2013). The trial by Vaziri 2016 reported that it was a single‐blinded study; however, depression was assessed by blinded nurse although the rest of the assessments were not clear if they were performed by a blinded staff. The rest of the trials reported being single‐blinded but since one of the groups received no supplementation, it was assumed that it was not blinded to participants but to the assessment team: Benson 2009, Delvin 1986; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Sablok 2015; Singh 2015; Taherian 2002; Yu 2008.
Incomplete outcome data
Sixteen trials did not have incomplete data: Asemi 2012; Asemi 2013a; Grant 2013; Harvey 2012; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Roth 2013; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Taherian 2002; Tehrani 2014; Yu 2008). The others did not report on attrition, missing data and lack of intention‐to‐treat analyses.
Selective reporting
We did not have access to study protocols and therefore, formally assessing reporting bias was not possible. Insufficient trials contributed data to allow us to carry out exploration of possible publication bias by using funnel plots.
Other potential sources of bias
This varied in the different trials. For example, Harvey 2012 reported that participants were allowed to continue taking their own multivitamin but they did not specify who took those supplements and who did not take them during the study. The report from Li 2000a is very short, with most details of the methods not available. The trial by Mallet 1986 had groups with notoriously different sample sizes; it is unclear whether the numbers reflect the participants who finished the trial, a non randomised process, or a selection bias in which randomised participants did not receive the intervention. The trial by Shahgheibi 2016 reported different interventions in the abstract to what was described in methods.
We have also included figures that summarise our ’Risk of bias’ assessments (Figure 2; Figure 3).
2.
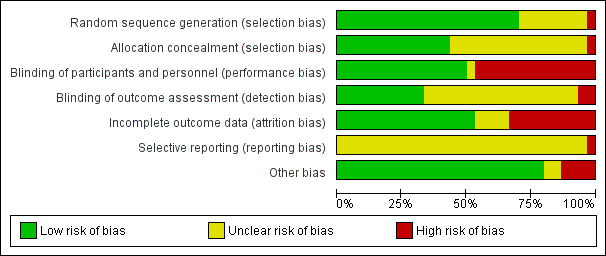
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
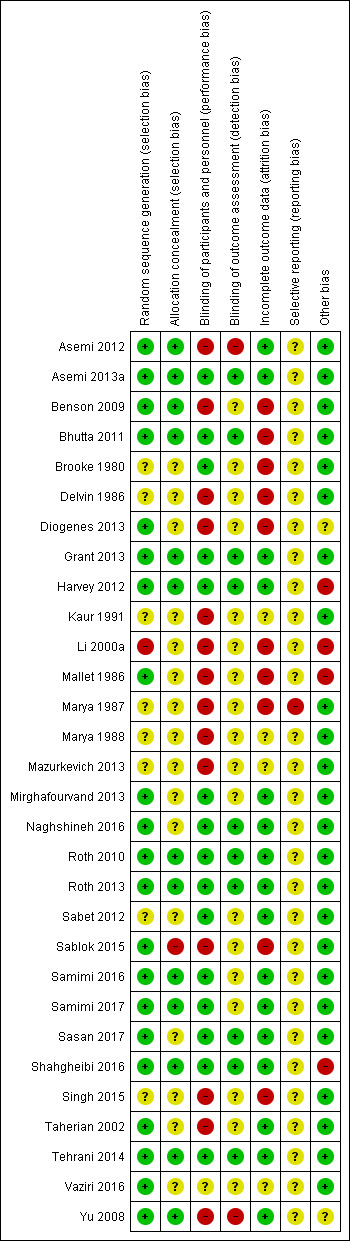
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3
In this updated review we included 30 trials assessing a total of 7033 women. We organised the summary results by comparison and by primary and secondary outcomes.
In the Data and analyses tables, we set up all four prespecified comparisons but outcome data were only available for two of these. We have not added outcomes to those comparisons without data (Comparisons three and four). For the comparisons with data, we set up tables for all primary outcomes (even where no data were available) not only to highlight gaps in the current research evidence, but also to be able to add any data that may become available in future updates.
SeeData and analyses for detailed results on primary and secondary outcomes.
For each of the comparisons, we have indicated the number of trials contributing data and the total number of women recruited in these trials. However, for some outcomes only one or two trials provided data and due to loss to follow‐up, denominators for particular outcomes may have been considerably less than the randomised sample. Therefore, we have indicated the number of trials contributing data and the number of women included in that analysis.
(1) Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals) (22 trials, 3725 women)
Twenty‐two trials involving 3725 pregnant women were included in this comparison (Asemi 2013a; Benson 2009; Bhutta 2011; Brooke 1980; Delvin 1986; Grant 2013; Harvey 2012; Kaur 1991; Mallet 1986; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sabet 2012; Sablok 2015; Samimi 2017; Sasan 2017; Shahgheibi 2016; Singh 2015; Tehrani 2014; Vaziri 2016; Yu 2008). Two trials contributed to both Comparisons 1 and 2 (Asemi 2013a; Mirghafourvand 2013).
The following trials were assessed as having low risk of bias for allocation and blinding: Asemi 2013a; Bhutta 2011; Grant 2013; Harvey 2012; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Samimi 2017; Shahgheibi 2016; Tehrani 2014. The following trials were assessed as having high risk of bias for allocation and blinding: Delvin 1986; Kaur 1991; Mallet 1986; Marya 1988; Sablok 2015; Singh 2015. Benson 2009 and Yu 2008 had high risk for blinding. Sabet 2012 did not report on random sequence generation or on blinding of outcome assessments. Brooke 1980 and Vaziri 2016 did not report on most of these issues, therefore, we could not judge on their risk of bias. In Sasan 2017 allocation concealment was not well described and so was assessed as being at unclear risk of bias.
Primary outcomes
Maternal
Pre‐eclampsia (as defined by trialists)
Data from four trials (Asemi 2013a; Naghshineh 2016; Sablok 2015; Sasan 2017) involving 499 women found that supplementation with vitamin D probably reduces the risk of pre‐eclampsia compared to no intervention or placebo (risk ratio (RR 0.48, 95% CI 0.30 to 0.79); moderate‐certainty evidence; Analysis 1.1). The data from Sablok 2015 were wrongly entered in the previous update (De‐Regil 2016), as it included data for both pre‐eclampsia and gestational hypertension together. We contacted the author and now the data only for pre‐eclampsia are reported here and the data for gestational hypertension are reported in that outcome. It is also important to note that all women in the Sasan 2017 trial had a history of pre‐eclampsia.
1.1. Analysis.
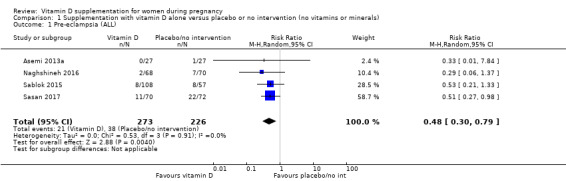
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 1 Pre‐eclampsia (ALL).
Gestational diabetes (as defined by trialists)
Data from four trials (Asemi 2013a;Tehrani 2014; Naghshineh 2016; Sablok 2015) involving 446 women found that supplementation with vitamin D probably reduces the risk of gestational diabetes compared to women receiving no intervention, or in the placebo group (RR 0.51, 95% CI 0.27 to 0.97; moderate‐certainty evidence; Analysis 1.2).
1.2. Analysis.
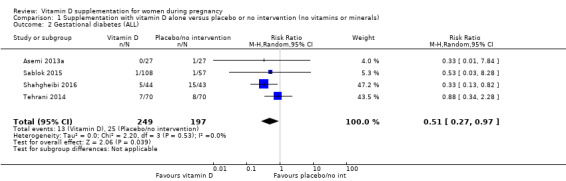
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 2 Gestational diabetes (ALL).
Maternal adverse events Analysis 1.3
One trial reported on severe postpartum haemorrhage in 1134 women (Harvey 2012); vitamin D supplementation appears to reduce the risk of severe postpartum haemorrhage (RR 0.68, 95% CI 0.51 to 0.91; 1 trial, 1134 women, low‐certainty evidence), although it should be noted that this result is based on a single trial and was an unexpected finding that has not been documented before by any other study. Another trial reported on nephritic syndrome in 135 women (Yu 2008); no clear differences were found between groups (just one event in the control group: RR 0.17, 95% CI 0.01 to 4.06; very low‐certainty evidence). In terms of hypercalcaemia, only one trial reported this outcome (Harvey 2012) and there were no cases of hypercalcaemia in any group. Given the scarcity of data in general for maternal adverse events, no firm conclusions can be drawn.
Infant
Preterm birth (less than 37 weeks' gestation)
Data from seven trials (Asemi 2013a; Delvin 1986; Harvey 2012; Grant 2013; Mirghafourvand 2013; Roth 2010; Singh 2015) involving 1640 women suggest that supplementation with vitamin D probably may make little or no difference in the risk of having a preterm birth compared to no intervention or placebo (RR 0.66, 95% CI 0.34 to 1.30; low‐certainty evidence; Analysis 1.4). It is important to note that in Singh 2015, participants in the intervention group had significantly lower 25‐hydroxyvitamin D levels at baseline compared to the control group, therefore, there was an imbalance in study groups. However, the result in lowering preterm birth risk in this trial was consistent with the other trials.
1.4. Analysis.
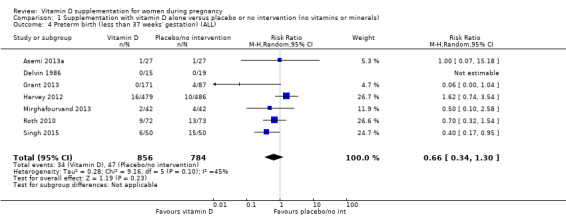
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 4 Preterm birth (less than 37 weeks' gestation) (ALL).
Low birthweight (less than 2500 g)
The data from five trials (Brooke 1980; Bhutta 2011; Marya 1988; Roth 2010; Sablok 2015) involving 697 women suggest that supplementation with vitamin D probably reduces the risk of low birthweight (< 2500 g) compared to no intervention or placebo (RR 0.55, 95% CI 0.35 to 0.87; moderate‐certainty evidence;Analysis 1.5).
1.5. Analysis.
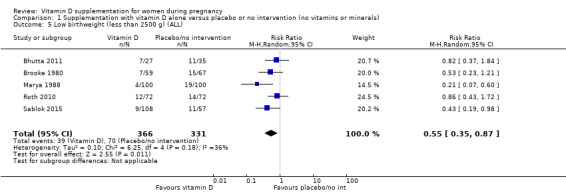
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 5 Low birthweight (less than 2500 g) (ALL).
Subgroup analysis
For preterm birth, results did not differ greatly whether supplementation started before week 20 of pregnancy (three trials, 1149 women) (RR 0.73, 95% CI 0.26 to 2.04), or after 20 weeks of pregnancy (four trials, 491 women) (RR 0.49, 95% CI 0.13 to 1.87; Analysis 1.18).
1.18. Analysis.
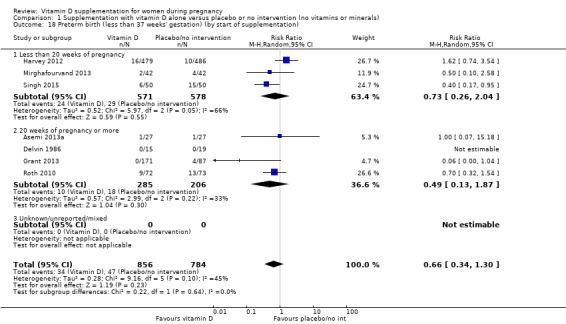
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 18 Preterm birth (less than 37 weeks' gestation) (by start of supplementation).
With respect to the other subgroup analyses, most only had one or two trials (Analysis 1.6, Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16; Analysis 1.17; Analysis 1.19; Analysis 1.20; Analysis 1.21; Analysis 1.22; Analysis 1.23; Analysis 1.24; Analysis 1.25; Analysis 1.26; Analysis 1.27; Analysis 1.28; Analysis 1.29).
1.6. Analysis.
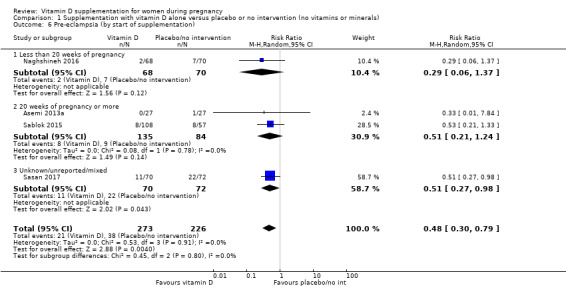
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 6 Pre‐eclampsia (by start of supplementation).
1.7. Analysis.
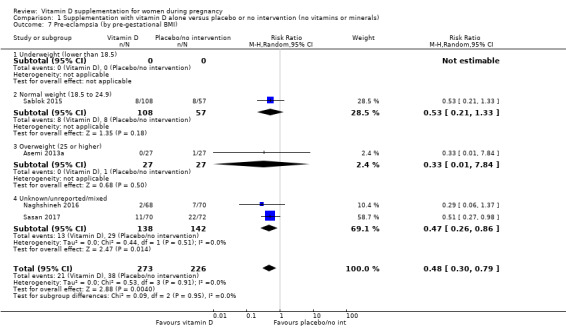
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 7 Pre‐eclampsia (by pre‐gestational BMI).
1.8. Analysis.
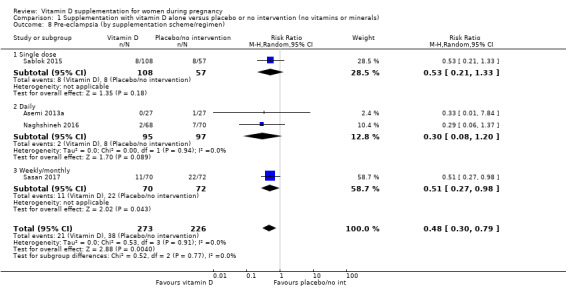
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 8 Pre‐eclampsia (by supplementation scheme/regimen).
1.9. Analysis.
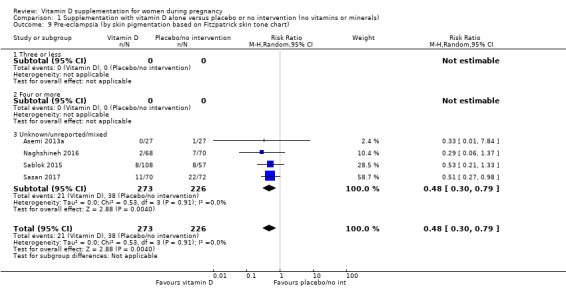
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 9 Pre‐eclampsia (by skin pigmentation based on Fitzpatrick skin tone chart).
1.10. Analysis.
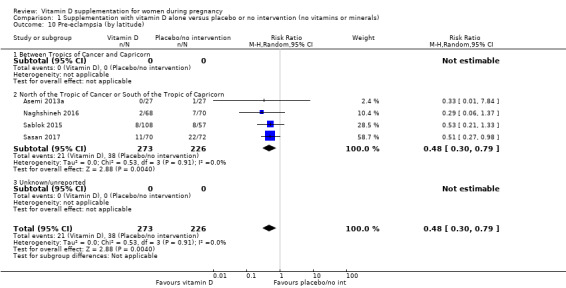
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 10 Pre‐eclampsia (by latitude).
1.11. Analysis.
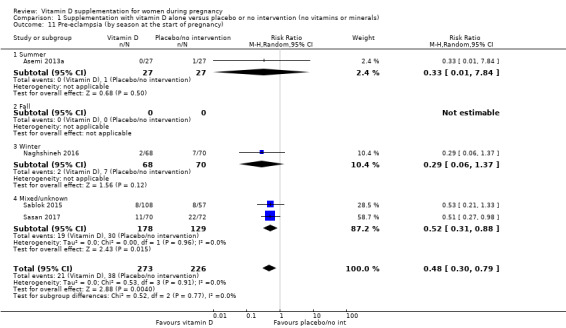
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 11 Pre‐eclampsia (by season at the start of pregnancy).
1.12. Analysis.
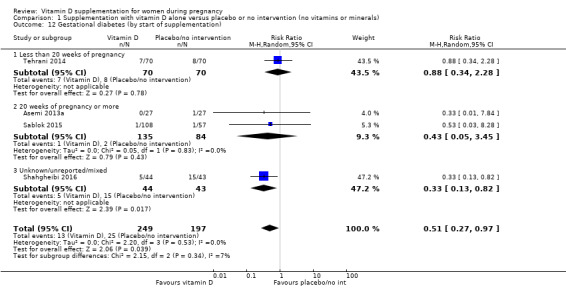
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 12 Gestational diabetes (by start of supplementation).
1.13. Analysis.
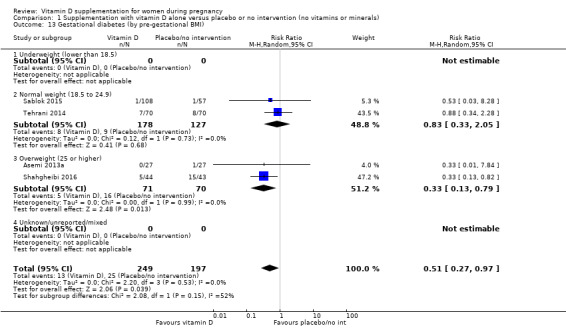
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 13 Gestational diabetes (by pre‐gestational BMI).
1.14. Analysis.
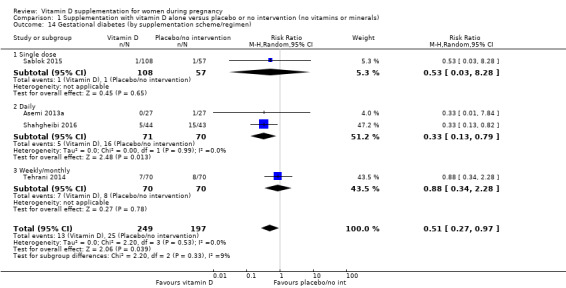
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 14 Gestational diabetes (by supplementation scheme/regimen).
1.15. Analysis.
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 15 Gestational diabetes (by skin pigmentation based on Fitzpatrick skin tone chart).
1.16. Analysis.
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 16 Gestational diabetes (by latitude).
1.17. Analysis.
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 17 Gestational diabetes (by season at the start of supplementation).
1.19. Analysis.
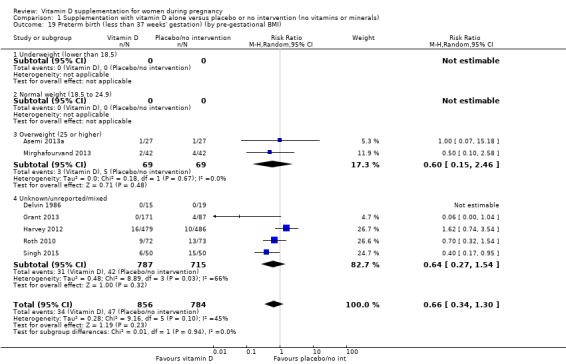
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 19 Preterm birth (less than 37 weeks' gestation) (by pre‐gestational BMI).
1.20. Analysis.
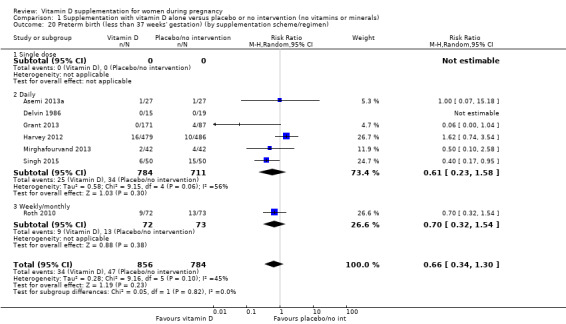
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 20 Preterm birth (less than 37 weeks' gestation) (by supplementation scheme/regimen).
1.21. Analysis.
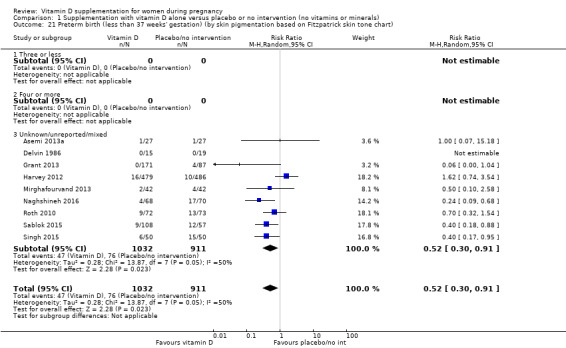
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 21 Preterm birth (less than 37 weeks' gestation) (by skin pigmentation based on Fitzpatrick skin tone chart).
1.22. Analysis.
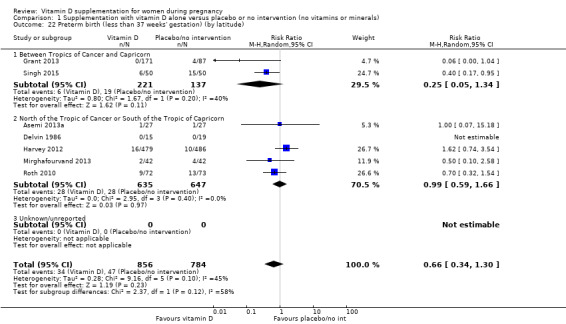
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 22 Preterm birth (less than 37 weeks' gestation) (by latitude).
1.23. Analysis.
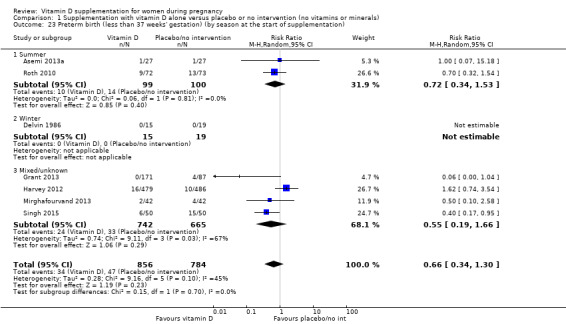
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 23 Preterm birth (less than 37 weeks' gestation) (by season at the start of supplementation).
1.24. Analysis.
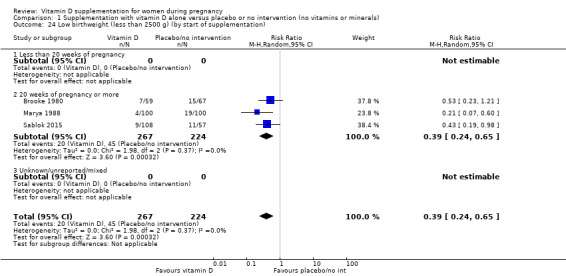
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 24 Low birthweight (less than 2500 g) (by start of supplementation).
1.25. Analysis.
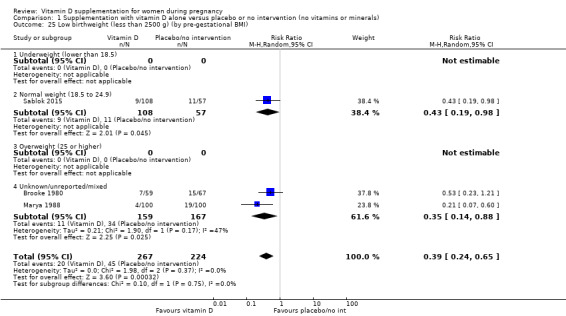
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 25 Low birthweight (less than 2500 g) (by pre‐gestational BMI).
1.26. Analysis.
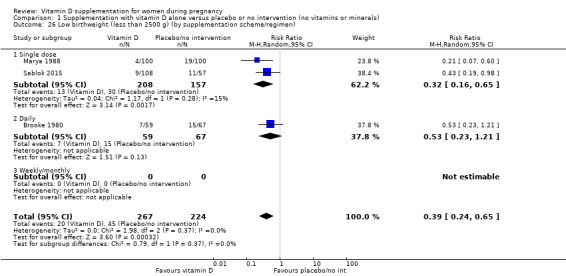
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 26 Low birthweight (less than 2500 g) (by supplementation scheme/regimen).
1.27. Analysis.
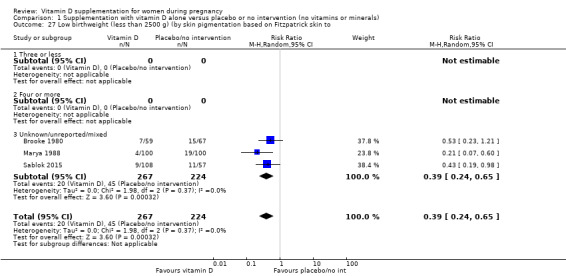
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 27 Low birthweight (less than 2500 g) (by skin pigmentation based on Fitzpatrick skin to.
1.28. Analysis.
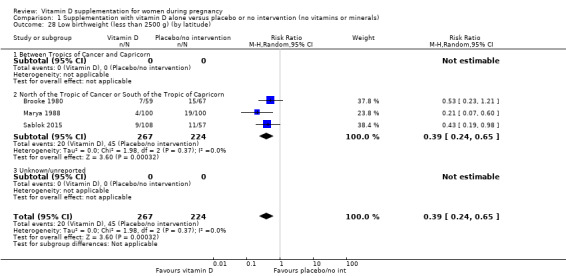
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 28 Low birthweight (less than 2500 g) (by latitude).
1.29. Analysis.
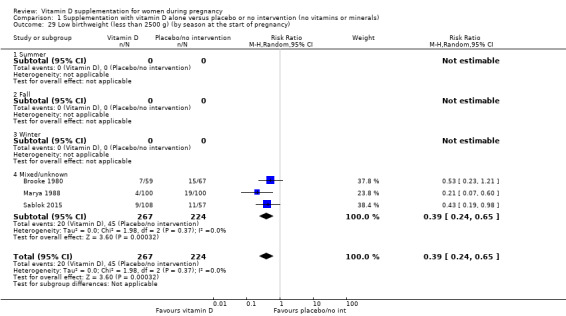
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 29 Low birthweight (less than 2500 g) (by season at the start of pregnancy).
Secondary outcomes
Maternal
Caesarean section
Ten trials including 1104 women reported on this outcome (Asemi 2013a; Delvin 1986; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sablok 2015; Sasan 2017; Shahgheibi 2016; Singh 2015; Yu 2008). The data from this trial suggest that vitamin D supplementation probably makes little or no difference in the risk of caesarean section compared to no supplementation or placebo (RR 0.98, 95% CI 0.80 to 1.21; Analysis 1.30). The trial by Singh 2015 was the only trial detecting a lower risk of caesarean section with the intervention, however, participants in the intervention group had significantly lower 25‐hydroxyvitamin D levels at baseline compared to the control group, therefore, there was an imbalance in study groups. If this trial is removed from the analysis, the results did not change (RR 1.06, 95% CI 0.93 to 1.21).
1.30. Analysis.
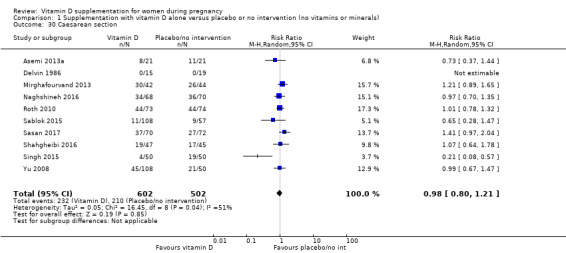
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 30 Caesarean section.
Gestational hypertension
Two trials reported on this outcome in 1130 women (Harvey 2012; Sablok 2015). Vitamin D supplementation probably makes little or no difference in the risk of gestational hypertension compared to no intervention or placebo (RR 0.78, 95% CI 0.41 to 1.49; Analysis 1.31).
1.31. Analysis.
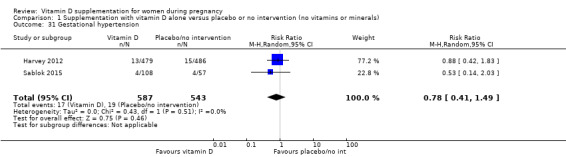
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 31 Gestational hypertension.
Maternal death
One trial reported on this outcome in 180 women (Sablok 2015). No maternal deaths were reported in any of the groups (Analysis 1.32).
1.32. Analysis.
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 32 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL).
Impaired glucose tolerance
No trial reported this outcome.
Maternal vitamin D concentration at term (25‐hydroxyvitamin D in nmol/L)
The data from 14 trials (Asemi 2013a; Benson 2009; Bhutta 2011; Brooke 1980; Delvin 1986; Grant 2013; Harvey 2012; Mallet 1986; Roth 2010; Sabet 2012; Sablok 2015; Samimi 2017; Singh 2015; Vaziri 2016) involving 2470 women show that supplementation with vitamin D probably results in a higher 25‐hydroxyvitamin D concentrations than those women who received no intervention or a placebo. The average mean difference (MD) between groups was 35.66 nmol 25‐hydroxyvitamin D per litre (95% CI 24.19 to 47.13; Analysis 1.33). This result should be interpreted cautiously as the response to supplementation was highly heterogeneous (Tau² = 437.5, I² = 99% and Chi² test for heterogeneity P < 0.00001) and ranged from 16.3 nmol 25‐hydroxyvitamin D per litre (95% CI 13.6 to 19.0) (Mallet 1986) to 152 nmol 25‐hydroxyvitamin D per litre (95% CI 127 to 177) (Brooke 1980). If the trial by Brooke 1980 is removed, heterogeneity remains similar. The trial by Singh 2015 found consistent results with the other trials, even though there was an imbalance in study groups in this outcome at baseline. However, the results are consistent between trials. We also detected funnel plot asymmetry in this outcome (Figure 4); the presence of funnel plot asymmetry suggested that publication bias was a likely source of this heterogeneity.
1.33. Analysis.
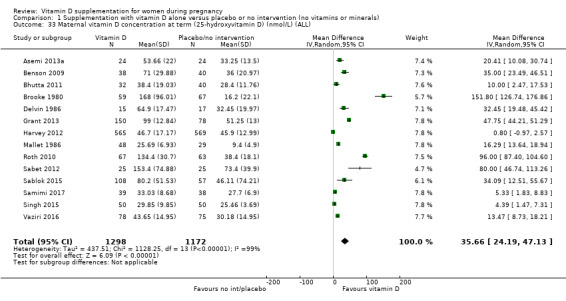
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 33 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).
4.
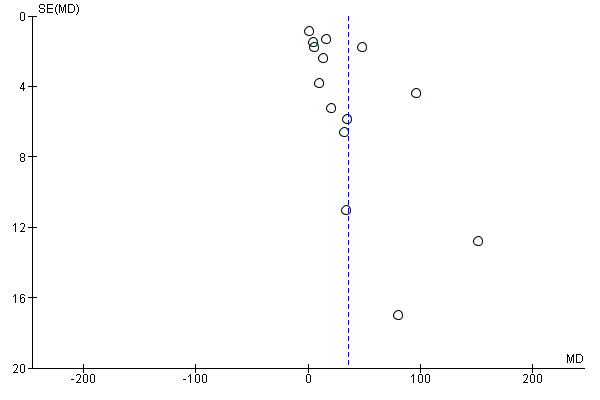
Funnel plot of comparison: 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), outcome: 1.15 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).
Infant
Length at birth (cm)
The data from eight trials (Asemi 2013a; Brooke 1980; Marya 1988; Mirghafourvand 2013; Roth 2010; Sabet 2012; Sablok 2015; Vaziri 2016) involving 931 women probably suggest a longer birth length among infants from women taking vitamin D supplementation during pregnancy compared to women in the no treatment or placebo group (MD 0.57, 95% CI 0.19 to 0.95; Analysis 1.34). There was heterogeneity in the response to the supplementation (Tau² = 0.16; I² = 63% and Chi² test for heterogeneity P = 0.008) and most of these studies did not report if outcome assessments were performed by blinded personnel (Brooke 1980; Marya 1988; Mirghafourvand 2013; Sabet 2012; Sablok 2015; Vaziri 2016). Taking this into consideration and the very small effect, these results should be interpreted with caution.
1.34. Analysis.
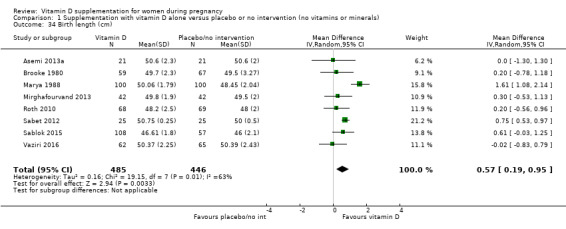
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 34 Birth length (cm).
Head circumference at birth (cm)
Eight trials involving 1841 women (Asemi 2013a; Brooke 1980; Harvey 2012; Marya 1988; Mirghafourvand 2013; Roth 2010; Sablok 2015; Vaziri 2016) reported on this anthropometric measurement. Results suggest that supplementation with vitamin D probably makes little or no difference in head circumference at birth compared to no treatment or placebo (MD 0.11, 95% CI ‐0.21 to 0.44; Analysis 1.35). There was heterogeneity in the response to the supplementation (Tau² = 0.16; I² = 80% and Chi² test for heterogeneity P < 0.00001); therefore, results should be interpreted with caution.
1.35. Analysis.
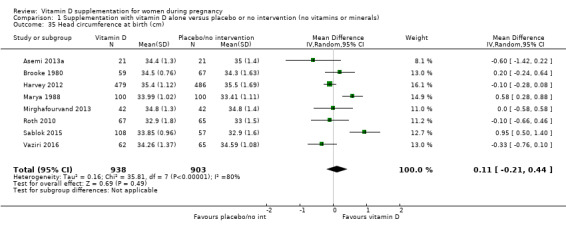
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 35 Head circumference at birth (cm).
Birthweight (g)
Seventeen trials involving 2828 women (Asemi 2013a; Bhutta 2011; Brooke 1980; Grant 2013; Harvey 2012; Kaur 1991; Mallet 1986; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sabet 2012; Sablok 2015; Shahgheibi 2016; Singh 2015; Vaziri 2016; Yu 2008) reported on this outcome. Results suggest that supplementation with vitamin D probably makes little or no difference in birthweight compared to no treatment or placebo (MD 80.30, 95% CI ‐14.40 to 175.00; Analysis 1.36). There was some substantial heterogeneity among trials in terms of the size of the treatment (Tau² = 32319; I² = 92% and Chi² test for heterogeneity P < 0.00001). However, when the trial by Mallet 1986 is excluded from the analysis, heterogeneity is reduced from 92% to 84% and results show that supplementation with vitamin D probably results in a higher birthweight (MD 99.27, 95% CI 16.22 to 182.32). The standard deviations for this study are very small and so we have concerns that these may not be reported correctly. Also, the trial by Singh 2015 found a very different result compared to the other trials and could be explained by the fact that women in the intervention group had significantly lower 25‐hydroxyvitamin D levels at baseline compared to the control group. When this trial is also removed from the analysis, heterogeneity is further reduced to 67%, with no significant differences between groups (MD 59.24, 95% CI ‐1.93 to 120.42).
1.36. Analysis.
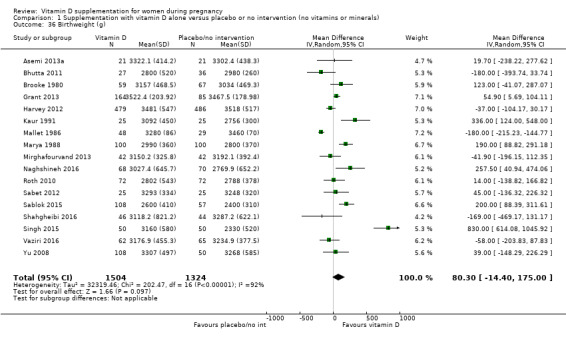
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 36 Birthweight (g).
Stillbirth (as defined by trialists)
Three trials (Grant 2013; Roth 2010; Yu 2008) including 584 women reported this outcome. Vitamin D supplementation probably makes little or no difference in the risk of stillbirth compared to no intervention or placebo (RR 0.35, 95% CI 0.06 to 1.98; Analysis 1.37). In particular, for this outcome, only one case of stillbirth out of 364 was reported in the vitamin D group and three cases out of 220 in the no intervention or placebo group.
1.37. Analysis.
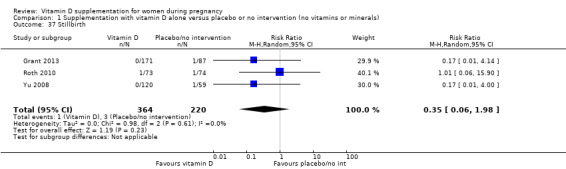
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 37 Stillbirth.
Neonatal death (within 28 days after delivery)
Two trials (Roth 2010; Yu 2008) including 326 women reported this outcome. Vitamin D supplementation probably makes little or no difference in the risk of neonatal death compared to no intervention or placebo (RR 0.27, 95% CI 0.04 to 1.67; Analysis 1.38). Only one neonatal death out of 193 was reported in the vitamin D group and four neonatal deaths were reported out of 133 in the no intervention or placebo group. Given the scarcity of data for this outcome, no firm conclusions can be drawn.
1.38. Analysis.
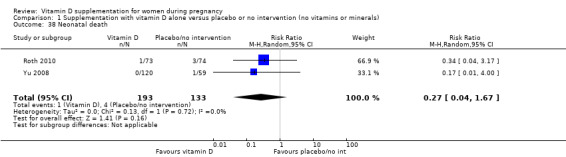
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 38 Neonatal death.
Apgar score less than seven at five minutes
One study including 165 women did not find clear differences in Apgar scores between groups (RR 0.53, 95% CI 0.11 to 2.53; Analysis 1.39).
1.39. Analysis.
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 39 Apgar score less than seven at five minutes.
Other infant secondary outcomes
No trials reported on the other pre‐specified infant secondary outcomes: admission to special care (including intensive care) during the neonatal period (within 28 days after delivery); neonatal infection (e.g. respiratory infections) or very preterm birth (less than 34 weeks' gestation).
(2) Supplementation with vitamin D + calcium versus placebo/no intervention (no vitamin or minerals) (nine trials, 1916 women)
Nine trials involving 1916 women made this comparison (Asemi 2012; Asemi 2013a; Diogenes 2013; Li 2000a; Marya 1987; Mazurkevich 2013; Mirghafourvand 2013; Samimi 2016; Taherian 2002).
The following trials were assessed as having low risk of bias for allocation and blinding: Asemi 2013a; Mirghafourvand 2013; Samimi 2016, while four trials were assessed as having high risk of bias: Diogenes 2013; Li 2000a; Marya 1987; Mazurkevich 2013. The remaining two trials had mixed results, with some components having a low risk, high risk, or unclear risk: Asemi 2012; Taherian 2002.
Primary outcomes
Maternal
Pre‐eclampsia (as defined by trialists)
Four trials (Asemi 2012; Marya 1987; Samimi 2016; Taherian 2002) including 1174 women reported on this outcome. Supplementation with vitamin D probably reduces the risk of pre‐eclampsia compared to no intervention or placebo (RR 0.50, 95% CI 0.32 to 0.78;moderate‐certainty evidence; Analysis 2.1).
2.1. Analysis.
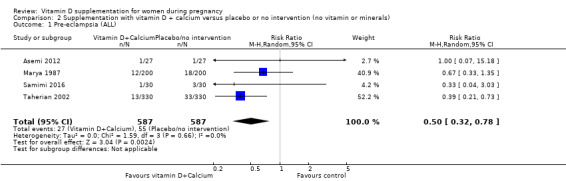
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 1 Pre‐eclampsia (ALL).
Gestational diabetes (as defined by trialists)
A single study including 54 women reported on this outcome (Asemi 2012). It is uncertain whether vitamin D supplementation makes any difference to the risk of gestational diabetes compared to no intervention or placebo (RR 0.33, 95% CI 0.01 to 7.84; very low‐certainty evidence; Analysis 2.2). The scarcity of data for this outcome and the wide CIs means no firm conclusions can be drawn.
2.2. Analysis.
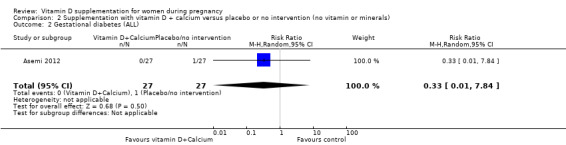
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 2 Gestational diabetes (ALL).
Maternal adverse events
No trial reported this outcome.
Infant
Preterm birth (less than 37 weeks' gestation)
Five trials with 942 women reported on this outcome (Asemi 2012; Diogenes 2013; Mirghafourvand 2013; Samimi 2016; Taherian 2002). Supplementation with vitamin D may increase the risk of preterm birth compared to no intervention or placebo (RR 1.52, 95% CI 1.01 to 2.28; low‐certainty evidence; Analysis 2.3). These results are mostly driven by one trial, which recruited most of the patients and had most of the events (Taherian 2002).
2.3. Analysis.
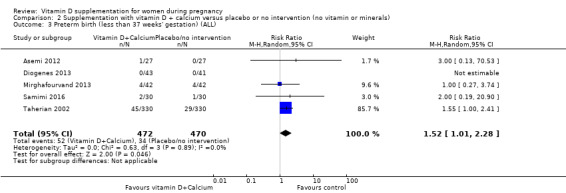
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 3 Preterm birth (less than 37 weeks' gestation) (ALL).
Low birthweight (less than 2500 g)
Two trials with 110 women reported on this outcome (Diogenes 2013; Samimi 2016). We are uncertain whether supplementation with vitamin D makes any difference to the risk of low birthweight compared to no intervention or placebo (RR 0.68, 95% CI 0.10 to 4.55; very low‐certainty evidence; Analysis 2.4). The data were scarce for this outcome and the CIs wide and so, no firm conclusions can be drawn.
2.4. Analysis.
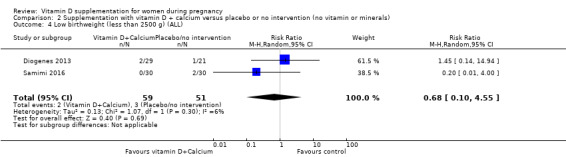
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 4 Low birthweight (less than 2500 g) (ALL).
Secondary outcomes
Maternal
Impaired glucose tolerance
No trial reported this outcome.
Caesarean section
Two trials including 146 women reported on this outcome (Mirghafourvand 2013; Samimi 2016). Vitamin D supplementation probably makes little or no difference in the risk of caesarean section compared to no intervention or placebo (RR 1.16, 95% CI 0.87 to 1.54; Analysis 2.5).
2.5. Analysis.
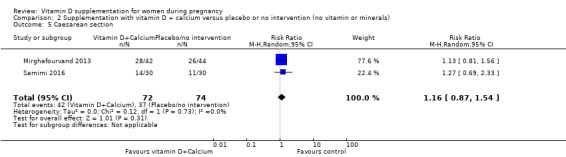
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 5 Caesarean section.
Gestational hypertension
One trial reported on this outcome in 59 women (Li 2000a). Vitamin D supplementation probably makes little or no difference in the risk of gestational hypertension compared to no intervention or placebo (RR 0.26, 95% CI 0.06 to 1.12; Analysis 2.6).
2.6. Analysis.
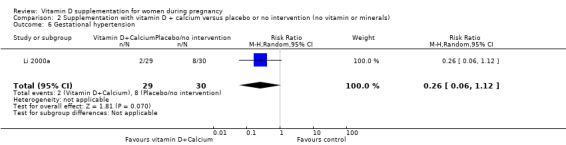
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 6 Gestational hypertension.
Maternal death
No trial reported this outcome.
Maternal vitamin D levels at term (25‐hydroxyvitamin D in nmol/L)
A single study including 60 women reported on this outcome (Samimi 2016). The average MD between groups was 12.50 nmol 25‐hydroxyvitamin D per litre (95% CI 3.80 to 21.20; Analysis 2.7), but given the scarcity of data for this outcome and the wide CIs, no firm conclusions can be drawn.
2.7. Analysis.
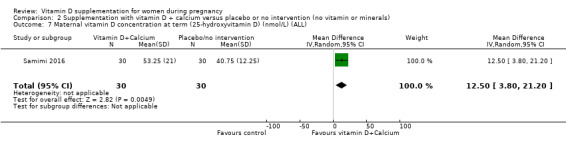
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 7 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).
Infant
Length at birth (cm)
The data from three trials (Diogenes 2013; Mirghafourvand 2013; Samimi 2016) involving 194 women show that vitamin D supplementation probably makes little or no difference in birth length compared to no intervention or placebo (MD ‐0.07, 95% CI ‐0.67 to 0.52; Analysis 2.8).
2.8. Analysis.
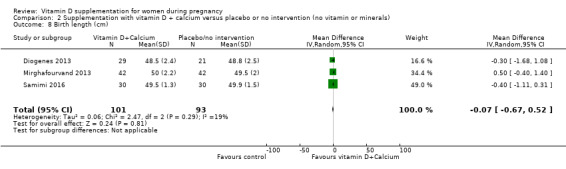
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 8 Birth length (cm).
Head circumference at birth (cm)
Three trials involving 198 women (Diogenes 2013; Mirghafourvand 2013; Samimi 2016) reported on this anthropometric measurement. Vitamin D supplementation probably makes little or no difference in head circumference at birth compared to no intervention or placebo (MD ‐0.03, 95% CI ‐0.39 to 0.33; Analysis 2.9).
2.9. Analysis.
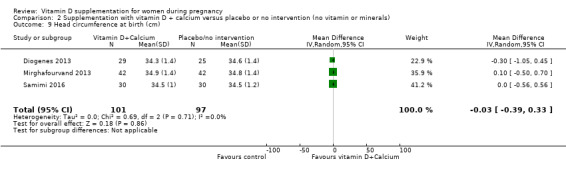
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 9 Head circumference at birth (cm).
Birthweight (g)
Three trials involving 194 women (Diogenes 2013; Mirghafourvand 2013; Samimi 2016) reported on this outcome. Vitamin D supplementation probably makes little or no difference in birthweight compared to no intervention or placebo (MD 42.39, 95% CI ‐86.96 to 171.74; Analysis 2.10).
2.10. Analysis.
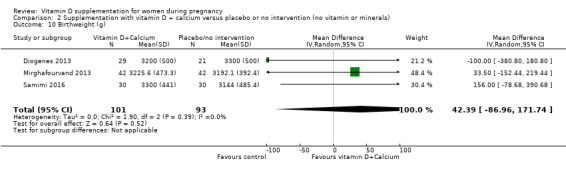
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 10 Birthweight (g).
Neonatal death (within 28 days after delivery)
One trial (Taherian 2002) reported on this outcomes with one death during the study period in the no intervention or placebo group (RR 0.20, 95% 0.01 to 4.15; one study, 660 women; Analysis 2.11).
2.11. Analysis.
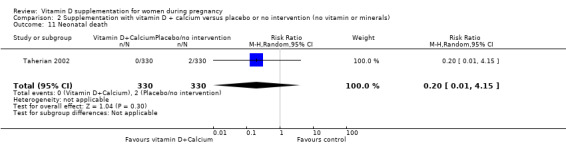
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 11 Neonatal death.
Other infant secondary outcomes
No trials reported on the other pre‐specified infant secondary outcomes: length at birth (cm); head circumference at birth (cm); weight at birth (g); admission to special care (including intensive care) during the neonatal period (within 28 days after delivery); stillbirths (as defined by trialists); Apgar score less than seven at five minutes; neonatal infection (e.g. respiratory infections) or very preterm birth (less than 34 weeks' gestation).
(3) Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D) (one study, 1300 women)
One trial was included in this comparison (Roth 2013). It was assessed as having low risk of bias.
Primary outcomes
Maternal
Pre‐eclampsia (as defined by trialists)
The included study under this comparison did not report on this outcome.
Gestational diabetes (as defined by trialists)
It is unclear whether vitamin D supplementation with calcium and other vitamins and minerals makes any difference in the risk of gestational diabetes compared to no intervention or placebo (RR 0.42, 95% CI 0.10 to 1.73; 1 trial, 1298 women; very low‐certainty evidence;Analysis 3.1) because the certainty of the evidence was found to be very‐low.
3.1. Analysis.
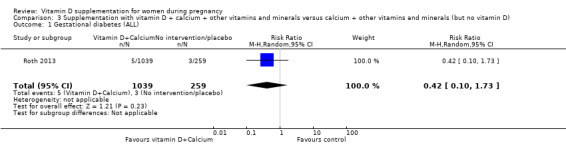
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 1 Gestational diabetes (ALL).
Maternal adverse events
It is unclear whether vitamin D supplementation with calcium and other vitamins and minerals makes any difference in the risk of maternal hypercalciuria (confirmed cases) compared to no intervention or placebo (RR 0.25, 95% CI 0.02 to 3.97; 1 trial, 1298 women; very low‐certainty evidence;Analysis 3.2) because the certainty of the evidence was found to be very‐low. No confirmed cases were reported for maternal hypercalcaemia.
3.2. Analysis.
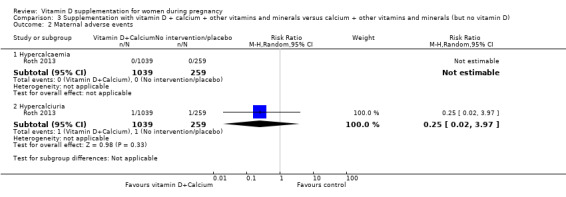
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 2 Maternal adverse events.
Infant
Preterm birth (less than 37 weeks' gestation)
Vitamin D supplementation may make little or no difference in the risk of preterm birth compared to no intervention or placebo (RR 1.04, 95% CI 0.68 to 1.59; 1 trial, 1298 women; low‐certainty evidence;Analysis 3.3).
3.3. Analysis.
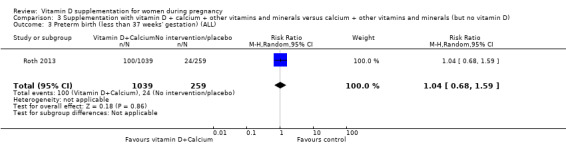
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 3 Preterm birth (less than 37 weeks' gestation) (ALL).
Low birthweight (less than 2500 g)
Vitamin D supplementation probably may make little or no difference in the risk of low birthweight compared to no intervention or placebo (RR 1.12, 95% CI 0.82 to 1.51; 1 trial, 1298 women; low‐certainty evidence;Analysis 3.4).
3.4. Analysis.
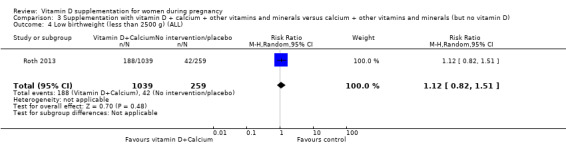
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 4 Low birthweight (less than 2500 g) (ALL).
Secondary outcomes
Maternal
Caesarean section
Vitamin D supplementation probably makes little or no difference in the risk of C‐section compared to no intervention or placebo (RR 1.10, 95% CI 0.95 to 1.27; 1 trial, 1298 women; Analysis 3.5).
3.5. Analysis.
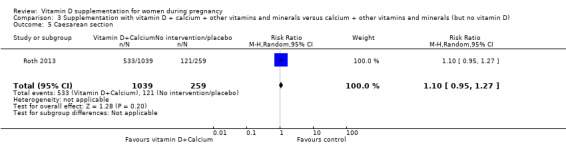
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 5 Caesarean section.
Gestational hypertension
Vitamin D supplementation probably makes little or no difference in the risk of gestational hypertension compared to no intervention or placebo (RR 0.93, 95% CI 0.31 to 2.79; 1 trial, 1298 women; Analysis 3.6).
3.6. Analysis.
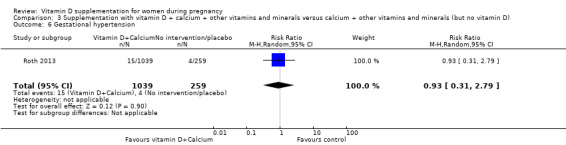
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 6 Gestational hypertension.
Maternal death
Vitamin D supplementation probably makes little or no difference in the risk of maternal death compared to no intervention or placebo (RR 0.25, 95% CI 0.02 to 3.98; 1 trial, 1300 women; Analysis 3.7). Only one maternal death out of 1040 was reported in the vitamin D group and one maternal death was reported out of 260 in the no intervention or placebo group. Given the scarcity of data for this outcome, no firm conclusions can be drawn.
3.7. Analysis.
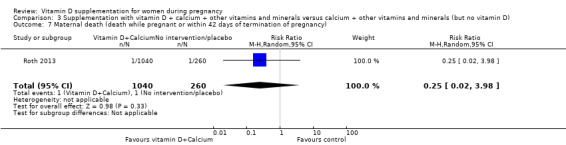
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 7 Maternal death (death while pregnant or within 42 days of termination of pregnancy).
Maternal vitamin D concentration at term (25‐hydroxyvitamin D in nmol/L)
The average MD between groups was 75.17 nmol 25‐hydroxyvitamin D per litre (95% CI 71.97 to 78.37; 1 trial, 635 women; Analysis 3.8) but given the scarcity of data for this outcome and the wide CIs, no firm conclusions can be drawn.
3.8. Analysis.
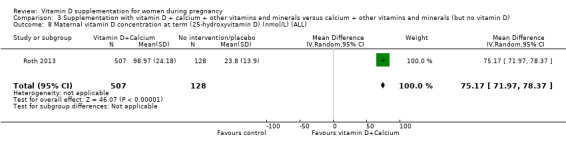
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 8 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).
Infant
Stillbirth (as defined by trialists)
Vitamin D supplementation probably makes little or no difference in the risk of stillbirth compared to no intervention or placebo (RR 0.66, 95% CI 0.29 to 1.46; 1 trial, 1300 women; Analysis 3.12). A total of 21 stillbirths out of 1040 were reported in the vitamin D group and eight stillbirths were reported out of 260 in the no intervention or placebo group. Given the scarcity of data for this outcome, no firm conclusions can be drawn.
3.12. Analysis.
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 12 Stillbirth.
Neonatal death (within 28 days after delivery)
Vitamin D supplementation probably makes little or no difference in the risk of neonatal death compared to no intervention or placebo (RR 0.69, 95% CI 0.22 to 2.14; 1 trial, 1298 women; Analysis 3.13). A total of 11 neonatal deaths out of 1039 were reported in the vitamin D group and four neonatal death were reported out of 259 in the no intervention or placebo group. Given the scarcity of data for this outcome, no firm conclusions can be drawn.
3.13. Analysis.
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 13 Neonatal death.
(4) Supplementation with vitamin D + calcium versus calcium (but no vitamin D) (no trials)
No trials were included in this comparison.
(5) Supplementation with vitamin D + calcium + other vitamins and minerals versus other vitamins and minerals (but no vitamin D + calcium) (no trials)
No trials were included in this comparison.
Discussion
Summary of main results
This review evaluates the effects of vitamin D supplementation alone or in combination with calcium and other vitamins and minerals during pregnancy. It includes 30 trials involving 7033 women, 22 of which compared vitamin D alone versus no treatment or placebo, nine compared vitamin D plus calcium in comparison with no intervention, and one compared vitamin D plus calcium, iron and folic acid compared to no vitamin D. No trials evaluated the effects of vitamin D + calcium + other vitamins and minerals versus other vitamins and minerals (but no vitamin D + calcium).
Supplementation with vitamin D compared to no intervention or a placebo.
Probably reduces the risk of pre‐eclampsia (four trials), the risk of gestational diabetes (four trials), and the risk of having a baby with low birthweight (less than 2500 g) (five trials).
May make little or no difference in the risk of preterm birth (seven trials).
In terms of maternal adverse events, vitamin D supplementation may reduce the risk of severe postpartum haemorrhage, although it should be noted that this result is based on findings from a single trial and was an unexpected finding that has not been documented before by any other study. We are uncertain about the effects in the risk of nephritic syndrome. No trial reported any case of hypercalcaemia. However, given the scarcity of data in general for maternal adverse events, no firm conclusions can be drawn.
For secondary outcomes, supplementation with vitamin D compared to no intervention or a placebo probably results in a higher 25‐hydroxyvitamin D concentrations (14 trials). This result should be interpreted cautiously as the response to supplementation was highly heterogeneous. This could be due to the different doses used in the trials (ranging from 200 IU/day to 4000 IU/day), the different frequencies for the supplementation (i.e. daily, weekly or bolus), the different start of supplementation (i.e. before or after week 20), and also in the difference in methods to assess serum 25‐hydroxyvitamin D. This biomarker is difficult and complex, with high variability in results between methods used (Holick 2008). High performance liquid chromatography mass spectrometry is the best available method (Holick 2005), but only two trials used this method. Therefore, results should be interpreted with caution.
Supplementation with vitamin D + calcium compared to no intervention or a placebo.
Probably reduces the risk of pre‐eclampsia (four trials);
May increase the risk of preterm birth < 37 weeks (five trials). However, the results are driven mainly by only one trial; therefore, results should be interpreted with caution;
We are uncertain about the effects in the risk of gestational diabetes (1 trial) and in the risk of having a baby with low birthweight (two trials);
No trial reported on maternal adverse events.
Only one trial evaluated the supplementation with vitamin D, calcium, iron and folic acid compared to calcium, iron and folic acid but no vitamin D with little or no difference in the risk of preterm birth or low birthweight and the results for other outcomes were unclear due to very‐low certainty evidence.
In general, more data are needed to conclude about the risk of maternal adverse events.
Overall completeness and applicability of evidence
The aim of the present review was to compare trials providing any dose of vitamin D supplementation during pregnancy compared to placebo or no intervention for improving gestational and neonatal outcomes. In this update, the number of trials included doubled in comparison to the previous version (De‐Regil 2016), and some of the results appear to be more consistent. However, there is still a limited number of trials reporting on certain maternal outcomes (adverse events, impaired glucose tolerance, gestational hypertension, or death) as well as infant outcomes (neonatal death, admission to special care in the neonatal period, Apgar score less than seven at five minutes, neonatal infection or very preterm birth).
In general, this review showed that vitamin D supplementation probably reduces the risk of pre‐eclampsia and gestational diabetes, and may reduce the risk of low birthweight, but it may make little or no difference in the risk of having a preterm birth. More information is needed to determine the safety of the intervention.
What is missing from the overview?
More trials are needed for each of the main outcomes, as most outcomes were only reported by a few trials (two to five trials), and with larger sample sizes. Most trials were small to medium size, with 14 trials including less than 100 participants (Asemi 2012; Asemi 2013a; Benson 2009; Bhutta 2011; Delvin 1986; Diogenes 2013; Kaur 1991; Li 2000a; Mallet 1986; Mazurkevich 2013; Sabet 2012; Samimi 2016; Samimi 2017; Shahgheibi 2016), 13 trials including 100 to 500 participants (Brooke 1980; Grant 2013; Marya 1987; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sablok 2015; Sasan 2017; Singh 2015; Tehrani 2014; Vaziri 2016; Yu 2008), and only three trials including more than 500 participants (Harvey 2012; Roth 2013; Taherian 2002). Another missing factor was the lack of specifying pre‐gestational body mass index (BMI) and skin pigmentation, two important determinants of vitamin D status. Also, most trials did not take into account vitamin D status at the beginning of the trial; this is important as the effects of vitamin D supplementation may be more profound among women with vitamin D deficiency. Most trials provided vitamin D alone or with calcium. Only one trial (Roth 2013) compared vitamin D with other nutrients, which is what in practice most women would be taking. This is important to evaluate as there could be interactions between nutrients in dietary supplements that should be tested. Furthermore, more trials are needed starting earlier in pregnancy, as only seven trials started supplementation before week 20 (Benson 2009; Bhutta 2011; Harvey 2012; Naghshineh 2016; Samimi 2017; Singh 2015; Tehrani 2014). The effects of vitamin D may be more important if it starts early in pregnancy as the enzyme 1‐alpha‐hydroxylase, which catalyses the synthesis of 1,25 dihydroxy vitamin D3, has the highest level of expression in the first trimester and it is reduced towards the third trimester, highlighting its possible role early in pregnancy (Zehnder 2002).
Therefore, there is a need for larger trials, starting early in pregnancy and testing the effects of vitamin D in combination with other nutrients on maternal and infant outcomes. Also, these trials need to take into consideration the pre‐pregnancy BMI, skin pigmentation and vitamin D status at baseline, as those who are vitamin D deficient may benefit more. Although the trial by Roth 2013 is the largest so far (> 1500 participants) and tested different vitamin D doses in combination with other nutrients among women with 64% vitamin D deficiency, it started in mid pregnancy. This could explain the lack of significant effects on these health outcomes. As mentioned earlier, the enzyme 1‐alpha‐hydroxylase has the highest level of expression in the first trimester, highlighting its possible role early in pregnancy (Zehnder 2002).
We identified 60 trials that were excluded, mainly because the comparisons were among different doses of vitamin D without a placebo or no supplementation group or undertaken in pregnant women with glucose intolerance, gestational diabetes or other chronic conditions. We did not include trials with different doses and no placebo as most countries do not have the policy to include vitamin D in their prenatal supplementation guidelines. Therefore, it is important to first determine if vitamin D supplementation is beneficial against a placebo or no intervention group.
To the best of our knowledge, there are currently six ongoing trials that, once published, will further increase the body of evidence identified for this updated review. In addition, updates could include the dose‐response of vitamin D supplementation on important pregnancy outcomes. In fact, there is another review that will take into account trials with different doses of vitamin D to determine the best regimen to provide in pregnancy for improvements in prenatal and neonatal health outcomes (Palacios 2018).
Quality of the evidence
The risk of bias was high for allocation and/or blinding in 14 trials and for attrition in 10 trials. In addition, the results varied considerably between trials and this could be related to the variability in vitamin D regimens used. For example, nine trials used doses of about 200 to 600 IU of vitamin D per day. Although these could be considered low doses, these are the recommended doses for pregnancy by several organisations (EFSA 2016; IOM 2011; RCOG 2014; WHO 2004). Twelve studies used medium doses of 800 to 2000 IU vitamin D per day; and eight trials used doses > 2000 IU of vitamin D per day. There was also a large variability in the frequency of the supplementation, with 20 trials providing vitamin D daily; six weekly or monthly; three gave a bolus dose once or twice and one combined daily and bolus. Finally, as mentioned before, only seven trials started supplementation before week 20. The effects of vitamin D may be more important if it starts early in pregnancy as the enzyme 1‐alpha‐hydroxylase, which catalyses the synthesis of 1,25 dihydroxy vitamin D3, has the highest level of expression in the first trimester and it is reduced towards the third trimester, highlighting its possible role early in pregnancy (Zehnder 2002). These differences may have influenced the results observed.
Based on risk of bias and results from the studies, we evaluated the certainty of the body of evidence for the primary outcomes with the GRADE methodology for Comparison 1 (vitamin D alone versus placebo/no intervention; Table 1), Comparison 2 (vitamin D + calcium versus placebo/no intervention; Table 2) and Comparison 3 (vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals but no vitamin D; Table 3). We considered that inconsistency or publication bias were unlikely, but the risk of bias of the trials, and the imprecision resulted in: evidence of moderate certainty for pre‐eclampsia, gestational diabetes, and low birthweight; evidence of low certainty for severe postpartum haemorrhage, hypercalcaemia and preterm birth; and evidence of very‐low certainty for nephritic syndrome in the comparison of supplementation with vitamin D alone versus no intervention or placebo. The certainty of the evidence in the trials assessing supplementation of vitamin D plus calcium was moderate for pre‐eclampsia, low for preterm birth and very low for gestational diabetes and low birthweight. The certainty of the evidence in the trials assessing supplementation of vitamin D + calcium + other vitamins and minerals was low for preterm birth and low birthweight and very‐low for gestational diabetes and maternal adverse events.
Potential biases in the review process
We identified several potential biases in the review process. They were minimised in two ways: (1) eligibility for inclusion and data extraction were assessed independently by two review authors and (2) assessments of risk of bias and data entry were also assessed independently by two review authors. However, when reviewing the assessments of eligibility and risk of bias, this requires that we make a number of subjective judgements. Others may have reached different decisions regarding these issues. We would encourage readers to examine the Characteristics of included studies tables to assist in the interpretation of results.
Agreements and disagreements with other studies or reviews
This review updates the previous Cochrane Review on vitamin D supplementation in pregnancy (De‐Regil 2012; De‐Regil 2016). The 2012 review included six trials with a total of 1023 women and excluded eight trials, and six trials were still ongoing while the 2016 review included 15 trials assessing a total of 2833 women, excluded 27 trials, and 23 trials were still ongoing or unpublished. In the 2016 review, authors concluded that supplementing pregnant women with vitamin D significantly increased serum 25‐hydroxyvitamin D at term and may reduce the risk of pre‐eclampsia, low birthweight and preterm birth. However, when vitamin D and calcium were combined, this may increase the risk of preterm birth. In this update, results are similar but strengthened by the greater number of trials reporting on each outcome. The only difference noted was that in the 2016 review, a higher head circumference in infants born to women who were supplemented with vitamin D during pregnancy was seen but in this update, this was not observed. There are still insufficient data to confirm the effects on other maternal and infant health outcomes, as these were either not reported or assessed in only one or two trials.
Our results are in part in agreement with other similar systematic reviews. For example, Harvey 2014 compared the effects of vitamin D supplementation with placebo or a lower level of vitamin D on maternal and neonatal health outcomes. Among seven trials assessing birthweight, three trials demonstrated significantly greater birthweight in infants from supplemented mothers while the other four did not find a significant effect. For birth length, two trials were identified; one found that supplementation with vitamin D led to greater birth length in infants of women who received supplementation, while the other trial found quote: "no significant association but a trend towards higher birth length in the supplemented group" compared to the control group. In addition, in the two trials assessing offspring head circumference, one found a significantly greater head circumference while the other found a non‐significant trend towards greater head circumference in supplemented mothers. Only one intervention was identified for pre‐eclampsia (no difference in risk between groups) and no interventions were identified for preterm birth, low birthweight, gestational diabetes and caesarean section. Another systematic review and meta‐analysis of 13 trials (n = 2299) compared vitamin D supplementation (with or without calcium) compared to a placebo group, which included the low level of vitamin D (400 IU/day) in pregnancy (Perez‐Lopez 2015). Serum 25‐hydroxyvitamin D at term was significantly higher in the higher vitamin D group compared with the control group (mean difference: 66.5 nmol/L, 95% confidence interval (CI) 66.2 to 66.7; 10 trials; 1468 participants), similar to the present review. However, contrary to the present review, Perez‐Lopez 2015 found that vitamin D supplementation had no effect on pre‐eclampsia (3 trials; 654 participants), gestational diabetes mellitus (3 trials; 384 participants), low birthweight (4 trials; 496 participants) and preterm birth (3 trials; 384 participants), while it significantly increased birthweight (10 trials; 1489 participants) and birth length (6 trials; 866 participants). Consistent with the present review, vitamin D supplementation had no effect on caesarean section (4 trials; 1028 participants). Another review assessed the effect of vitamin D supplementation versus the control group, which also included a low vitamin D dose (400 IU/day) or no intervention during pregnancy for reducing the risk of pre‐eclampsia (Hyppönen 2013). Of the four trials identified, including 5871 women, two studies compared 2000 IU/day versus 4000 IU/day, another compared 1200 IU/day versus no vitamin D, a very old trial compared 450 IU/day versus no vitamin D, and another trial compared several supplements to no supplementation. They found that the intervention significantly reduced the risk of pre‐eclampsia compared with the control group (odds ratio (OR) 0.66; 95% confidence interval (CI) 0.52 to 0.83); however, they used very different trials. The review by Thorne‐Lyman 2012 also included trials with low dose of vitamin D (400I IU/day) in the placebo or control group. No effect was observed in the reduction of preterm birth in the two trials included (529 participants) with vitamin D supplementation; although a 60% lower risk of low birthweight was observed with the supplementation (3 trials; 507 participants). A more recent systematic review by Roth 2017 including 43 trials with a total of 8406 participants compared vitamin D supplementation versus a placebo group (with less than 600 IU/day) or no intervention. Vitamin D supplementation significantly increased maternal 25‐hydroxyvitamin D at term, but the dose‐response effect was weak. Also, women assigned to vitamin D supplementation had infants with higher mean birthweight compared to controls, but no effect was found on the risk of pre‐eclampsia, gestational diabetes, preterm birth, and low birthweight, contrary to our review. However, similar to this review, Roth 2017 also found no effect of vitamin D supplementation on the risk of gestational hypertension, caesarean section, admission to hospital, neonatal death, stillbirth, or other adverse events. This meta‐analysis also found that most trials had high risk of bias. It is important to note that Roth 2017 included trials with low levels of vitamin D in the control group, while we only included those with no vitamin D or no intervention in the control group.
Overall, the available systematic reviews and meta‐analyses have consistently shown that serum 25‐hydroxyvitamin D levels significantly improved with vitamin D supplementation. However, there are important differences in the results. While this review and another (Hyppönen 2013) showed a significant reduction in pre‐eclampsia risk with vitamin D supplementation, two other reviews (Perez‐Lopez 2015; Roth 2017) did not find this. Also, differences were found in the reduction in the risk of preterm birth, gestational diabetes and low birthweight. The main differences found between results in the present review and the aforementioned reviews is the inclusion criteria of trials. We only included trials that compared any dose of vitamin D with a placebo group with 0 IU/day or no intervention. However, the trials described above included trials comparing vitamin D supplementation with a placebo group that could have a low level of vitamin D or no intervention.
With respect to safety, the trials reporting on maternal and infant safety‐related outcomes may suggest that vitamin D supplementation may be safe during pregnancy. However, this was assessed differently in the trials, such as by evaluating haemorrhages, nephritic syndrome, hypercalcaemia and hypercalciuria. Therefore, the safety of this intervention still needs further studies. Also, most secondary outcomes defined in this review (maternal death, neonatal admission to intensive care unit, Apgar score less than seven at five minutes, neonatal infection or very preterm birth) were not reported by any of the trials. The trial by Sablok 2015 reported the Apgar score less than three or seven, with no difference between supplemented or placebo groups. More trials are needed to report on these safety‐related outcomes to have a definite conclusion.
Authors' conclusions
Implications for practice.
In this updated review, new trials have added to the evidence on the effects of vitamin D supplementation alone or with other nutrients during pregnancy for maternal and neonatal health outcomes. Supplementing pregnant women with vitamin D alone probably reduces the risk of pre‐eclampsia, gestational diabetes, low birthweight and may reduce the risk of severe postpartum haemorrhage. However, it may make little or no difference in the risk of having a preterm birth. Supplementing pregnant women with vitamin D plus calcium probably reduces the risk of pre‐eclampsia, but it may increase the risk of preterm births. This slight potential harm merits consideration before routine antenatal supplementation with vitamin D is provided to women who are receiving calcium or iron and folic acid as part of routine antenatal care. With respect to maternal adverse events (e.g. haemorrhages, nephritic syndrome or hypercalcaemia), no firm conclusions can be drawn given the scarcity of data. Supplementing pregnant women with vitamin D and other nutrients may make little or no difference in the risk of preterm birth < 37 weeks' gestation or low birthweight (less than 2500 g) and the findings are uncertain for gestational diabetes and maternal adverse events.
We also found that vitamin D supplementation increased serum 25‐hydroxyvitamin D concentrations during pregnancy. There was large heterogeneity in these results, which could be related to the differences in vitamin D doses and methods used to assess this outcome. However, this large increase in serum 25‐hydroxyvitamin D concentrations in pregnancy may explain the potential health benefits on maternal and neonatal health outcomes.
Implications for research.
Additional rigorous high quality and larger randomised trials are required to evaluate the effects of vitamin D supplementation in pregnancy. It would be helpful if future trials were to evaluate whether the increase of serum 25‐hydroxyvitamin D concentration with the supplementation early in pregnancy is associated with improved maternal and infant outcomes in populations with different degrees of body mass index (BMI), skin pigmentation, vitamin D status and settings. Also, trials are needed evaluating potential interactions between supplements, as many countries currently include several micronutrients as part of their antenatal care and to evaluate systematically maternal adverse events to confirm the safety of the supplementation. Also, the effects of vitamin D supplementation in women with a diagnosis of gestational diabetes or with increased risk of pre‐eclampsia should be assessed. There is also the need to be consistent when reporting maternal adverse events as this was done differently in the trials.
Information on the most effective and safe dosage, the optimal dosing regimen (daily, intermittent or single doses), the timing of initiation of vitamin D supplementation, and the effect of vitamin D when combined with other vitamins and minerals are also needed to inform policy‐making. In fact, we are conducting another systematic review comparing between doses of vitamin D and its effects on pregnancy and infant outcomes (Palacios 2018).
What's new
| Date | Event | Description |
|---|---|---|
| 12 July 2018 | New search has been performed | Search updated. |
| 12 July 2018 | New citation required and conclusions have changed | Supplementation with vitamin D alone (22 trials in total, 13 new trials added in this update) during pregnancy probably reduces the risk of pre‐eclampsia, gestational diabetes and low birthweight. Supplementation with vitamin D + calcium (9 trials in total, three new trials added in this update) during pregnancy probably reduces the risk of pre‐eclampsia but may increase the risk of preterm births. Supplementation with vitamin D + other nutrients (1 trial trial added in this update) in pregnancy may make little or no difference in the risk of preterm birth or low birthweight. In general, more data are needed to conclude about the risk of maternal adverse events. |
History
Protocol first published: Issue 12, 2010 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 30 June 2015 | New search has been performed | Search and methods updated. We included a new comparison to assess the effects of vitamin D + calcium + other vitamins and minerals versus other vitamins and minerals (but no vitamin D + calcium). We also moved adverse effects to primary outcomes. |
| 30 June 2015 | New citation required and conclusions have changed | Nine trials included for this update. The few trials that reported on the effects of vitamin D supplementation during pregnancy on low birthweight and preterm delivery suggest a lower risk on these outcomes with vitamin D in a single or continued dose. However, this result should be interpreted with caution due to the small number of trials and included pregnant women. Also, the quality of the evidence was low in most studies, with high heterogeneity. |
| 10 May 2012 | Amended | Error in 'Plain language summary' corrected: "Data from three trials involving 463 women show a trend for women who receive vitamin D supplementation during pregnancy to less frequently have a baby with a birthweight below 2500 grams than those women receiving no treatment or placebo". |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewer for his time and comments: Zulfiqar A Bhutta, Robert Harding Chair in Global Child Health and Policy, Centre for Global Child Health, The Hospital for Sick Children, Toronto, Canada; and Founding Director, Center of Excellence in Women and Child Health, The Aga Khan University, Karachi, Pakistan.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
The World Health Organization (WHO), Cristina Palacios and Lia Kostiuk retain copyright and all other rights in the manuscript of this updated review as submitted for publication, including any revisions or updates to the manuscript which WHO may make from time to time. We acknowledge the support of Luz‐Maria De‐Regil, Ali Ansary and Regina Kulier in prior versions of this review.
Juan Pablo Peña‐Rosas is currently staff member of the WHO. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the WHO.
We thank Kassam Mahomed for their contribution as an author on previous versions of this review.
Appendices
Appendix 1. Search terms used for additional author searching
Authors searched the ClinicalTrials.gov and the WHO‐hosted International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned trials (12 July 2018) using the terms "vitamin D supplementation and pregnancy".
Data and analyses
Comparison 1. Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia (ALL) | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 2 Gestational diabetes (ALL) | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 3 Maternal adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Severe postpartum haemorrhage | 1 | 1134 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.51, 0.91] |
| 3.2 Nephritic syndrome | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.06] |
| 3.3 Hypercalcaemia | 1 | 1134 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Preterm birth (less than 37 weeks' gestation) (ALL) | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 5 Low birthweight (less than 2500 g) (ALL) | 5 | 697 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.35, 0.87] |
| 6 Pre‐eclampsia (by start of supplementation) | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 6.1 Less than 20 weeks of pregnancy | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.37] |
| 6.2 20 weeks of pregnancy or more | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.24] |
| 6.3 Unknown/unreported/mixed | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.98] |
| 7 Pre‐eclampsia (by pre‐gestational BMI) | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 7.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Normal weight (18.5 to 24.9) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.33] |
| 7.3 Overweight (25 or higher) | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| 7.4 Unknown/unreported/mixed | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.86] |
| 8 Pre‐eclampsia (by supplementation scheme/regimen) | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 8.1 Single dose | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.33] |
| 8.2 Daily | 2 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.08, 1.20] |
| 8.3 Weekly/monthly | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.98] |
| 9 Pre‐eclampsia (by skin pigmentation based on Fitzpatrick skin tone chart) | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 9.1 Three or less | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Four or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Unknown/unreported/mixed | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 10 Pre‐eclampsia (by latitude) | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 10.1 Between Tropics of Cancer and Capricorn | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 10.3 Unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pre‐eclampsia (by season at the start of pregnancy) | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 11.1 Summer | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| 11.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Winter | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.37] |
| 11.4 Mixed/unknown | 2 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.88] |
| 12 Gestational diabetes (by start of supplementation) | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 12.1 Less than 20 weeks of pregnancy | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.28] |
| 12.2 20 weeks of pregnancy or more | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.45] |
| 12.3 Unknown/unreported/mixed | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.82] |
| 13 Gestational diabetes (by pre‐gestational BMI) | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 13.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Normal weight (18.5 to 24.9) | 2 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.33, 2.05] |
| 13.3 Overweight (25 or higher) | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.79] |
| 13.4 Unknown/unreported/mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Gestational diabetes (by supplementation scheme/regimen) | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 14.1 Single dose | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.03, 8.28] |
| 14.2 Daily | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.79] |
| 14.3 Weekly/monthly | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.28] |
| 15 Gestational diabetes (by skin pigmentation based on Fitzpatrick skin tone chart) | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 15.1 Three or less | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Four or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Unknown/unreported/mixed | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 16 Gestational diabetes (by latitude) | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 16.1 Between Tropics of Cancer and Capricorn | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 16.3 Unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Gestational diabetes (by season at the start of supplementation) | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 17.1 Summer | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| 17.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Winter | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.28] |
| 17.4 Mixed/unknown | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.14, 0.82] |
| 18 Preterm birth (less than 37 weeks' gestation) (by start of supplementation) | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 18.1 Less than 20 weeks of pregnancy | 3 | 1149 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.26, 2.04] |
| 18.2 20 weeks of pregnancy or more | 4 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.13, 1.87] |
| 18.3 Unknown/unreported/mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Preterm birth (less than 37 weeks' gestation) (by pre‐gestational BMI) | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 19.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Normal weight (18.5 to 24.9) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Overweight (25 or higher) | 2 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.15, 2.46] |
| 19.4 Unknown/unreported/mixed | 5 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.27, 1.54] |
| 20 Preterm birth (less than 37 weeks' gestation) (by supplementation scheme/regimen) | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 20.1 Single dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Daily | 6 | 1495 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.58] |
| 20.3 Weekly/monthly | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.32, 1.54] |
| 21 Preterm birth (less than 37 weeks' gestation) (by skin pigmentation based on Fitzpatrick skin tone chart) | 9 | 1943 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.91] |
| 21.1 Three or less | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Four or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Unknown/unreported/mixed | 9 | 1943 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.91] |
| 22 Preterm birth (less than 37 weeks' gestation) (by latitude) | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 22.1 Between Tropics of Cancer and Capricorn | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.34] |
| 22.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 5 | 1282 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.59, 1.66] |
| 22.3 Unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Preterm birth (less than 37 weeks' gestation) (by season at the start of supplementation) | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 23.1 Summer | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.34, 1.53] |
| 23.2 Winter | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Mixed/unknown | 4 | 1407 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.19, 1.66] |
| 24 Low birthweight (less than 2500 g) (by start of supplementation) | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 24.1 Less than 20 weeks of pregnancy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 20 weeks of pregnancy or more | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 24.3 Unknown/unreported/mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Low birthweight (less than 2500 g) (by pre‐gestational BMI) | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 25.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Normal weight (18.5 to 24.9) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.19, 0.98] |
| 25.3 Overweight (25 or higher) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.4 Unknown/unreported/mixed | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.14, 0.88] |
| 26 Low birthweight (less than 2500 g) (by supplementation scheme/regimen) | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 26.1 Single dose | 2 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.16, 0.65] |
| 26.2 Daily | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.23, 1.21] |
| 26.3 Weekly/monthly | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Low birthweight (less than 2500 g) (by skin pigmentation based on Fitzpatrick skin to | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 27.1 Three or less | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Four or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Unknown/unreported/mixed | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 28 Low birthweight (less than 2500 g) (by latitude) | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 28.1 Between Tropics of Cancer and Capricorn | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 28.3 Unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Low birthweight (less than 2500 g) (by season at the start of pregnancy) | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 29.1 Summer | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Winter | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.4 Mixed/unknown | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 30 Caesarean section | 10 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.80, 1.21] |
| 31 Gestational hypertension | 2 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 32 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) | 14 | 2470 | Mean Difference (IV, Random, 95% CI) | 35.66 [24.19, 47.13] |
| 34 Birth length (cm) | 8 | 931 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.19, 0.95] |
| 35 Head circumference at birth (cm) | 8 | 1841 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.21, 0.44] |
| 36 Birthweight (g) | 17 | 2828 | Mean Difference (IV, Random, 95% CI) | 80.30 [‐14.40, 175.00] |
| 37 Stillbirth | 3 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.06, 1.98] |
| 38 Neonatal death | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.04, 1.67] |
| 39 Apgar score less than seven at five minutes | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.53] |
1.3. Analysis.
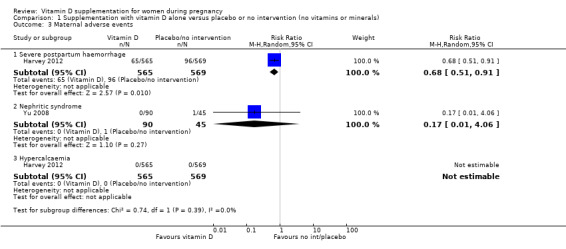
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 3 Maternal adverse events.
Comparison 2. Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia (ALL) | 4 | 1174 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.32, 0.78] |
| 2 Gestational diabetes (ALL) | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| 3 Preterm birth (less than 37 weeks' gestation) (ALL) | 5 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.01, 2.28] |
| 4 Low birthweight (less than 2500 g) (ALL) | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.10, 4.55] |
| 5 Caesarean section | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.87, 1.54] |
| 6 Gestational hypertension | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.12] |
| 7 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 12.5 [3.80, 21.20] |
| 8 Birth length (cm) | 3 | 194 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.67, 0.52] |
| 9 Head circumference at birth (cm) | 3 | 198 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.39, 0.33] |
| 10 Birthweight (g) | 3 | 194 | Mean Difference (IV, Random, 95% CI) | 42.39 [‐86.96, 171.74] |
| 11 Neonatal death | 1 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.15] |
Comparison 3. Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gestational diabetes (ALL) | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.10, 1.73] |
| 2 Maternal adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Hypercalcaemia | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Hypercalciuria | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.02, 3.97] |
| 3 Preterm birth (less than 37 weeks' gestation) (ALL) | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.68, 1.59] |
| 4 Low birthweight (less than 2500 g) (ALL) | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.82, 1.51] |
| 5 Caesarean section | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.95, 1.27] |
| 6 Gestational hypertension | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.31, 2.79] |
| 7 Maternal death (death while pregnant or within 42 days of termination of pregnancy) | 1 | 1300 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.02, 3.98] |
| 8 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) | 1 | 635 | Mean Difference (IV, Random, 95% CI) | 75.17 [71.97, 78.37] |
| 9 Birth length (cm) | 1 | 1297 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 10 Head circumference at birth (cm) | 1 | 1297 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 11 Birthweight (g) | 1 | 1297 | Mean Difference (IV, Random, 95% CI) | ‐7.0 [‐55.95, 41.95] |
| 12 Stillbirth | 1 | 1300 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.29, 1.46] |
| 13 Neonatal death | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.22, 2.14] |
3.9. Analysis.
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 9 Birth length (cm).
3.10. Analysis.
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 10 Head circumference at birth (cm).
3.11. Analysis.
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 11 Birthweight (g).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asemi 2012.
| Methods | Randomised single‐blinded controlled trial with 2 arms: vitamin D plus calcium and placebo. | |
| Participants | 54 pregnant women at risk for pre‐eclampsia, primigravida, aged 18 to 35 years old carrying singleton pregnancy at their third trimester attending maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran (latitude: 33.9889° N, 51.4772° E). Exclusion criteria: maternal severe pre‐eclampsia, IUFD, placenta abortion, preterm delivery and GDM. |
|
| Interventions | Participants were randomly allocated to 1 of 2 groups: group 1 (n = 27): women received 500 mg of carbonate calcium plus 200 IU of vitamin D (cholecalciferol‐D3) daily for 9 weeks; group 2 (n = 27): women received placebo. The intervention lasted 9 weeks overall, starting at 25 weeks of pregnancy until week 34. Participants were asked not to alter their routine PA or usual diets and not to consume any supplement other than the one provided to them by the investigators. Health worker cadre: the trial was carried out in maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran and the investigators provided the supplements to the participants. |
|
| Outcomes | Maternal: body weight and height, BMI, fasting plasma glucose levels, serum total cholesterol, triglycerol concentrations, serum HDL‐cholesterol, serum LDL‐cholesterol levels, dietary intakes, total HDL: cholesterol ratio, gestational diabetes, severe pre‐eclampsia, preterm delivery. Laboratory method used for assessment of vitamin D concentrations: serum 25‐hydroxyvitamin D concentrations were measured using a commercial ELISA kit (Immuno Diagnostic Systems). The inter‐ and intra‐assay coefficient of variation for serum 25(OH)D assays ranged from 5% to 7.5%. |
|
| Notes |
Source of funding: study was funded by research grant from the Vice‐Chancellor for research, KUMS, and Iran. Dates of the study and location: April 2011 to February 2012, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial reported randomisation performed by the use of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Trial reported that the appearance of the placebo capsules, such as colour, shape, size, and packaging, was identical to the vitamin D3 capsules. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial reported that it was single‐blinded. Participants were blinded to the interventions so it is assumed that the research staff were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial is reported as single‐blinded and the methods for concealment of the intervention were described for participants. Therefore, it is assumed that it was not blinded to research staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up of 3 women in the vitamin D group due to preterm delivery (n = 1), IUFD (n = 1), and placental abruption (n = 1). 3 women in the placebo group were also excluded for the following reasons: gestational diabetes (n = 1), preterm delivery (n = 1), and severe pre‐eclampsia (n = 1). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Asemi 2013a.
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial with 2 arms: vitamin D and placebo, during March 2012 to September 2012. | |
| Participants | 48 healthy pregnant women, primigravida, aged 18–40 years old at 25 weeks of gestation and a singleton pregnancy attending maternity clinics affiliated with Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran. Women with pre‐eclampsia, hypertension, GDM, IUFD, or those with a history of rheumatoid arthritis, hepatic or renal failure, metabolic bone disease and malabsorption, or thyroid, parathyroid, or adrenal diseases were excluded from the analysis. Also, smokers and those taking medications including nonsteroidal antiinflammatory drugs and aspirin were excluded. | |
| Interventions | Participants were randomly assigned to receive 1 of 2 groups: group 1 (n = 24) received 400 IU vitamin D (cholecalciferol‐D3) supplements daily; and group 2 (n = 24) received placebo for 9 weeks. Additionally, all participants also consumed 400 mcg (0.4 mg) folic acid daily from the beginning of pregnancy and 60 mg elemental iron (as ferrous sulphate) daily from the second trimester. Health worker cadre: the trial was carried out in maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran and the investigators provided the supplements to the participants. A trained midwife at the maternity clinic performed anthropometric measurements at study baseline and at 6 weeks after the intervention. |
|
| Outcomes | Maternal: weight, height, BMI, systolic blood pressure and diastolic blood pressure, serum calcium concentrations, serum 25‐hydroxyvitamin D [25(OH)D], serum hs‐C‐reactive protein, fasting plasma glucose, serum cholesterol, LDL‐cholesterol, HDL‐cholesterol concentrations, serum insulin, quantitative Insulin sensitivity check index (QUICKI) score, plasma total antioxidant capacity, plasma total glutathione, GDM, preterm delivery, IUFD, placental abruption, severe pre‐eclampsia. Laboratory method used for assessment of vitamin D concentrations: serum 25‐hydroxyvitamin D concentrations were measured using a commercial ELISA kit (Immuno Diagnostic Systems). |
|
| Notes |
Source of funding: study was funded by research grant from the Vice‐chancellor for Research, Kashan University of Medical Sciences, Kashan, Iran. Dates of the study and location: March 2012 to September 2012, Kashan, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was performed by the use of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | A trained midwife at the maternity clinic performed the randomised allocation sequence and assigned participants to the groups. Placebo pills contained microcrystalline cellulose and were packed in identical tablets and coded by the producer to guarantee blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blind to the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measurements of laboratory were performed in a blinded fashion, in duplicate, in pairs (before/after intervention) at the same time, in the same analytical run, and in random order to reduce systematic error and inter assay variability. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 in each group were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Benson 2009.
| Methods | Randomised controlled trial. | |
| Participants | 78 pregnant women between 14 to 18 weeks' gestation at risk, defined as: dark skinned, veiled; with vitamin D deficiency that has not commenced treatment prior to recruitment. Exclusion criteria: women taking barbiturates or anticonvulsants (decreased vitamin D absorption) and severe renal failure. | |
| Interventions | Participants were individually randomised to 1 of 2 groups: group 1 (n = 38): 2000 IU of cholecalciferol orally daily commencing between 14 and 18 weeks' gestation (if still deficient at 28 weeks the dose was doubled to 4000 IU orally daily until birth); group 2 (n = 40): no treatment during pregnancy. The mother received 300,000 IU cholecalciferol orally immediately and the baby 150,000 IU cholecalciferol orally immediately after birth. Health worker cadre: in order to facilitate compliance, encouragement was given from midwifery/medical staff at each 2–4 weekly antenatal visit with additional intervening telephone calls to women with poor compliance. Pill counts were not performed. |
|
| Outcomes | Maternal: vitamin D level. Infant: vitamin D level. Laboratory method used for assessment of vitamin D concentrations: serum 25‐OH vit D concentrations were determined by direct competitive chemiluminescence immunoassay for quantitative determination of total serum 25‐OH vit D (LIAISON®) Diasorin 25‐OH vitamin D assay (Stillwater, MN,USA). |
|
| Notes |
Source of funding: J.E. Benson was a recipient of the Luke Proposch Perinatal Research Scholarship from the Australian and New Zealand College of Obstetrics and Gynaecology Research Foundation enabling her to undertake this research. Study was funded by research grant. Dates of the study and location: between 2008 and 2009, Melbourne, Australia. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): the authors have no conflict of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated (envelopes in a tamper proof box, ratio 1:1). |
| Allocation concealment (selection bias) | Low risk | Envelopes in a tamper‐proof box, ratio 1:1. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial reported that it was single‐blinded. It is assumed that it was not blinded to participants as one of the groups did not receive any supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Single‐blinded study but authors did not specify if staff performing assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 57.9% intervention and 57.5% control data reported. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Bhutta 2011.
| Methods | Randomised, parallel assignment, double‐blind trial. | |
| Participants | 115 pregnant females from 12 to 20 weeks of gestation who agreed to participate in the study with presence of at least 20 natural teeth in mouth excluding third molars. For controls: non pregnant, healthy females matched with pregnant women with respect to age and education. Exclusion criteria: pregnant females with high vitamin D levels, women with metabolic diseases such as diabetes (type 1 or 2), presence of acute dental or periodontal disease, presence of systemic disease and/or medication affecting the periodontium; receipt of systemic antibiotic treatment or dental prophylaxis in the previous 3 months and those who do not provide informed consent. | |
| Interventions | Participants were individually randomised to 1 of 2 groups: group 1 (n = 36): vitamin D3 4000 mg per day (given as 1 tablespoon syrup per day); and group 2 n = (49): placebo (given as 1 table spoon syrup per day,) for approximately 6 months. Health worker cadre: CHWs were responsible for the delivery of supplementation to the study participants. The CHWs were assigned to visit study participants, on a fortnightly basis. The first supplementation was provided by the physician at the time of recruitment; later on, the CHWs continued to replenish the supply fortnightly. |
|
| Outcomes | Maternal: Periodontal Probing Depth, Interleukin 6 (IL‐6), IL‐2, IL‐4, IL‐10, TNF, IFN‐ɣ and IL‐17 levels. Laboratory method used for assessment of vitamin D concentrations: vitamin D levels were analysed on DiaSorin‐LIASON Inc, kit. |
|
| Notes |
Source of funding: the study was supported with a research grant from Pakistan Initiative for Mothers and Newborns (PAIMAN). Dates of the study and location: launched in 2004, Jhelum, Pakistan. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study participants were randomised in blocks. |
| Allocation concealment (selection bias) | Low risk | Allocation codes for vitamin D and placebo were kept in a sealed envelope in a locked cabinet at the Aga Khan University until the completion of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators, study staff, and the participants were blinded about the group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Allocation codes for vitamin D and placebo were kept in a sealed envelope in a locked cabinet at the Aga Khan University until the completion of the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only recorded birthweight from 63/85 (74.1%) participants. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Brooke 1980.
| Methods | Randomised double‐blind controlled trial; 2‐arm design with individual randomisation. | |
| Participants | 126 Asian pregnant women 28 to 32 weeks of gestation attending the antenatal clinic at St George's Hospital, London, UK (latitude: 51°30'N, north of Tropic of Cancer). All pregnant women were first‐generation immigrants mostly from India, Pakistan, Bangladesh, Sri Lanka, Mauritius and east Africa. Exclusion and elimination criteria: preterm deliveries, congenital malformations and maternal illnesses likely to affect fetal growth (such as diabetes) although these data are not presented. |
|
| Interventions | Participants were randomly allocated to 1 of 2 groups: group 1 (n = 59) received daily 1000 IU vitamin D (ergocalciferol‐D2) daily until term (estimated total dose: 56,000 to 84,000 IU); and group 2 (n = 67) received a placebo until term. Start of supplementation: 28 to 32 weeks gestation. Length of the intervention/follow‐up: 8 to 12 weeks from supplementation to term. Health worker cadre: St George's Hospital Medical School, London, UK. Medical doctors that were part of the team conducted the measurements and provided the supplements. |
|
| Outcomes | Maternal: maternal weight gain, dietary vitamin D intake, 25‐hydroxyvitamin D (25‐OHD) concentrations in cord blood and at term. Plasma calcium (adjusted for albumin concentration), inorganic phosphate, bilirubin, albumin concentrations and total alkaline phosphatase activity, alanine transaminase and ʏ‐glutamyl transferase activities, vitamin D binding globulin concentration, compliance. Infant: weight, crown‐heel length, crown‐rump length, rump‐heel length, occipitofrontal head circumference, forearm length, lower leg length, triceps and subscapular skinfold thickness, fontanelle area, plasma cholecalciferol at day 3 and day 6. weight, length and head circumference at 3, 6, 9 and 12 months. Laboratory method used for assessment of vitamin D concentrations: Serum 25‐hydroxyvitamin D was measured by competitive protein binding assay after chromatographic purification of lipid extracts of serum. |
|
| Notes |
Source of funding: the pathological research fund, St George's Hospital Medical School, and the South‐west Thames Regional Health Authority. This study was funded by a combination of a research grant and non governmental organisations. Dates of the study and location: autumn and winter 1977, the whole of 1978 and spring and summer 1979, London, UK. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial reported random allocation to the groups, although the method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | The trial reported that it was double‐blinded but the method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial reported that it was double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial reported that it was double‐blinded but they did not specify if those performing the assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear number of randomised participants. Preterm deliveries, congenital malformations, and maternal illnesses likely to affect fetal growth (such as diabetes) were eliminated from the trial. There is not complete documentation of the exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. There were no significant baseline differences between the groups in maternal age, parity, height, vegetarian: non‐vegetarian ratio or the distribution of the various countries of origin. |
Delvin 1986.
| Methods | Randomised trial; 2‐arm design with individual randomisation. | |
| Participants | 40 pregnant women attending their compulsory visit during the third month of pregnancy at the Obstetrical Unit of the Hopital Edouard Herriot, Lyon, France (latitude: 45° 45' 0" N north of Tropic of Cancer). Inclusion criterion: singleton pregnancy at term and uneventful vaginal deliveries. Pre‐gestational BMI and skin pigmentation not reported. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups at the time of the compulsory visit: group 1 (n = 20): women received daily 1000 IU vitamin D (cholecalciferol‐D3) (estimated total dose: 55,000 IU) and group 2 (n = 20): women received no supplement, during the last trimester of pregnancy for 12 weeks from start of supplementation to term. Health worker cadre: compliance was verified by a weekly visit by a midwife. |
|
| Outcomes | Maternal: serum (during last trimester of pregnancy) and cord blood immunoreactive PTH, 25‐hydroxyvitamin D (25‐OHD), 1‐alfa,25‐dihydroxyvitamin D (1,25(OH)2D), total calcium, ionised calcium, magnesium, inorganic phosphate. Infant: immunoreactive PTH, 25‐hydroxyvitamin D (25‐OHD), 1‐alfa,25‐dihydroxyvitamin D (1,25(OH)2D), total calcium, ionised calcium, magnesium, inorganic phosphate at 4 days of age. Laboratory method used for assessment of vitamin D concentrations: Serum 25‐hydroxyvitamin D and 1,25‐dihydroxyvitamin D levels were measured by radioligand assays with slight modifications. With sample volumes of 0.75 mL to 1.5 mL, the inter assay variation coefficient for the 2 assays were 8% and 10%, respectively. |
|
| Notes |
Source of funding: Shriners of North America, the France‐Quebec Exchange Program, and INSERM Grant 121023. This study was funded by a combination of research grant and non governmental organisations. Dates of the study and location: not reported dates, Lyon, France. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial reported as randomised but the method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial reported that women were assigned, by a blind randomisation process, to 1 of 2 groups at the compulsory visit in the third month of pregnancy. It is assumed that it was not blinded to participants as one of the groups did not receive any supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant from the control group (5%) and 5 (25%) from the vitamin D supplemented group were lost. Laboratory methods reported for 25 to 30 participants (depending on the outcome) out of 40 originally randomised. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Diogenes 2013.
| Methods | Randomised, placebo‐controlled trial; 2‐arm design with individual randomisation. | |
| Participants | 84 pregnant adolescents (13 to 19 years of age) primigravidae (pregnant for the first time) with singleton pregnancies and 23 to 29 weeks of gestation attending prenatal care at the Maternidade Escola, Universidade Federal do Rio de Janeiro, Brazil (latitude: 22.9083° S, 43.1964° W) from September 2009 to June 2011 and intending to exclusively or predominantly breast feed. Women with chronic health problems, pregnancy complications, smokers, users of nutritional supplements besides iron plus folate supplements provided during standard prenatal care, and mothers who decided not to breast feed were excluded from the study. |
|
| Interventions | Participants were randomly assigned to: 1 of 2 groups: group 1 (n = 43) received a commercially available supplement (Rexall Sundown®) containing 600 mg calcium (as calcium carbonate) plus 200 IU vitamin D (cholecalciferol‐D3) daily and group 2 (n = 41) received placebo (capsules of microcrystalline cellulose and corn starch; Quintessencia) daily. The protocol allowed pregnant women to continue with their iron and folate supplements, as part of their standard prenatal care. The use and composition of these supplements was not provided. Health worker cadre: capsules of calcium plus vitamin D or placebo were provided monthly to participants by a member of the research team during prenatal visits. Compliance was controlled by counting the remaining capsules at each visit and by telephone reminders. Calcium and vitamin D dietary intake was assessed by at least 3 24‐hour dietary recall questionnaires applied by a trained nutritionist. Standing height and body weight were measured by using a stadiometer (Seca) and a calibrated electronic scale (Filizola), respectively. The same operator performed all scanning and calibration. |
|
| Outcomes | Maternal: 1 measurement at 5 and 20 weeks postpartum, serum 25(OH)D, PTH, insulin‐like growth factor (IGF‐I), lumbar spine PA, bone mineral content, serum prolactin and oestradiol. Laboratory method used for assessment of vitamin D concentrations: serum 25‐hydroxyvitamin D, intact PTH, and IGF‐I were analysed by using a chemiluminescent enzyme‐labelled immunometric assay. |
|
| Notes |
Source of funding: Conselho Nacional de Desenvolvimento Cientıfico e Tecnologico [grant 471872/2008‐3 (to CMD) and a doctoral fellowship (to MELD)] and the Fundacao Carlos Chagas Filho de Amparo a` Pesquisa do Estado do Rio de Janeiro (grant E‐26/102.759/2008; to CMD), Brazil. This study was funded by a combination of Government programmes and non‐governmental organisations (NGOs). Dates of the study and location: September 2009 to June 2011, Rio de Janeiro, Brazil. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none of the authors had a conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was done by a member of the research team in a 1:1 ratio within permuted blocks of size 10. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report the method of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial reported that it was single‐blinded, only participants were blinded to the assigned groups. It is assumed that the assessment team was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Out of 43 women in the intervention group, 13 did not complete the study. Out of 41 women in the placebo group, 14 did not complete the study. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | The study appears to be free of other sources of bias. |
Grant 2013.
| Methods | Randomised, double‐blind, placebo‐controlled multi‐arm parallel study. | |
| Participants | 260 pregnant women 26 to 30 weeks' gestation, with a singleton pregnancy attending community based primary care maternity clinic in Auckland, New Zealand (latitude 36°S) from April 2010 to July 2011 and then their infants, from birth to age 6 months. Women already taking vitamin D supplementation 200 IU per day, a history of renal stones or hypercalcaemia, or any serious pregnancy complication at enrolment were excluded from the study. |
|
| Interventions | Participants were randomly assigned to 1 of 3 mother/infant groups: group 1 (n = 87) women received placebo from 26 to 30 weeks of pregnancy until parturition and their infants also received placebo from 0‐6 months of age; group 2 (n = 87) women received 1000 IU vitamin D (cholecalciferol‐D3) from 26 to 30 weeks of pregnancy until parturition and their infants received 400 IU vitamin D from 0 to 6 moths of age; group 3 (n = 86) women received 2000 IU vitamin D (cholecalciferol‐D3) from 26 to 30 weeks of pregnancy until parturition and their infants received 800 IU from birth to 6 months of age. Data from groups 2 and 3 were combined for our analysis. Health worker cadre: the study was conducted by the research team but it is not reported who provided the supplements or measured the outcomes. |
|
| Outcomes | Maternal: serum 25(OH)D concentration. Infant: serum 25(OH)D concentration. Laboratory method used for assessment of vitamin D concentrations: serum 25‐hydroxyvitamin D concentration was measured using isotope‐dilution liquid chromatography–tandem mass spectrometry in a Vitamin D External Quality Assurance Scheme–certified laboratory. |
|
| Notes |
Source of funding: Health Research Council of New Zealand, grant number 09/215R. Dr Mitchell is supported by Cure Kids. Study medicine was prepared by the Ddrops Company (Woodbridge, Ontario, Canada). This study was funded by a combination of government programmes and non‐governmental organisations (NGOs). Dates of the study and location: April 2010 to July 2011, Auckland, New Zealand. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): the authors have indicated they have no potential conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial reported computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed from research staff involved in recruitment. Trial reported randomly allocated treatment to each participant and labelled identical study medicine bottles such that study staff and participants were unaware of the treatment status. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study statistician randomly allocated a treatment to each participant and labelled identical study medicine bottles such that study staff and participants were unaware of the treatment status. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study staff and participants were unaware of the treatment status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported compliance did not differ between groups. In the placebo group, 6 did not complete the study; in the lower dose vitamin D group, 6 did not complete the study. In the higher vitamin D dose group, 6 did not complete the study. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Harvey 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 1200 pregnant women living in the UK, aged 18 years old and older, with a singleton pregnancy with less than 17 weeks' gestation at first assessment (based on last menstrual period and dating scan), aiming to give birth at local maternity hospital, and with serum 25‐hydroxyvitamin D is 25 to 100 nmol/L at nuchal fold/dating scan (10 to 17 weeks' gestation). | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 565): received 1000 IU cholecalciferol orally daily and group 2 (n = 569): received placebo, starting from 14 weeks' gestation until delivery. Health worker cadre: the medication was blister packed in a single box for each woman for the duration of pregnancy. Study medication (active/placebo) was supplied to the local pharmacy pre‐randomised by the manufacturer (1:1, unstratified by centre) and sequentially numbered for storage and dispensing. Code break envelopes were supplied to the lead pharmacist, but were not available to the investigative team. Emergency code break access was available through the local principal investigator and on call pharmacist. A single pack for each participant was issued sequentially (containing all pills for duration of the study). Each pack was individually prescribed for each participant. The trials pharmacist allocated a pack to that prescription, documenting both the pack number and the MAVIDOS participant ID; these were checked again by the research nurse on collection, and documented in the participant’s notes; the medication pack came with a tear‐off adhesive label, which was placed in the participant’s notes as an added safeguard against errors in pack allocation. The research nurse collected the medication pack for all participants attending to the clinic that day and issued to the participants directly. |
|
| Outcomes | Infant: whole body bone mineral content of the neonate adjusted for gestational age and age at neonatal DXA scan, whole body bone area, bone mineral density, and size corrected bone mineral density (BMC adjusted for BA, length and weight), body composition adjusted for gestational age and age at DXA scan. Laboratory method used for assessment of vitamin D concentrations: A blood sample was taken and plasma was stored at ‐80°C for measurement of 25(OH)‐vitamin D, vitamin D binding protein (DBP), calcium, bone specific alkaline phosphatase and albumin centrally (MRC Human Nutrition Research, Cambridge, UK) at the end of the study. |
|
| Notes |
Source of funding: Arthritis Research UK, Medical Research Council, Bupa Foundation, and National Institute for Health Research. Study was funded by a combination of research grants, government programmes, non‐governmental organizations (NGOs). Dates of the study and location: October 2008 to February 2014, Southampton, Sheffield, Oxford, the UK. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): CC reports personal fees, consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, Amgen, Eli Lilly, GlaxoSmithKline, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, and Takeda, outside the submitted work. NCH reports personal fees, consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, AMGen, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare, and Internis Pharma, outside the submitted work. NJB reports remuneration from Internis Pharmaceuticals, outside the submitted work. ATP reports grants from the Arthritis Research Council, during the conduct of the study. NKA has received honoraria, held advisory board positions (which involved receipt of fees), and received consortium research grants from Merck, grants from Roche, Bioiberica, and Novartis, personal fees from Smith & Nephew, Nicox, Flexion, Bioventus, and Freshfields, outside the submitted work. KMG reports reimbursement for speaking at Nestle Nutrition Institute conferences, and grants from Abbott Nutrition and Nestec, outside the submitted work. KMG also has a patent pending for phenotype prediction, a patent pending for predictive use of CpG methylation, and a patent pending for maternal nutrition composition, not directly related to this work. HMI reports grants from the Medical Research Council (MRC), Arthritis Research UK, and European Union’s Seventh Framework Programme, during the conduct of the study; and while not directly receiving funding from other bodies, members of her team have received funding from the following companies from other work: Danone, Nestec, and Abbott Nutrition. RE reports grants and personal fees from Amgen and Alexion; grants from the Department of Health, AstraZeneca, ARUK/MRC Centre for Excellence in Musculoskeletal Ageing Research, National Institute for Health Research, MRC/AZ Mechanisms of Diseases Call, and the MRC; grants, personal fees, and non‐financial support from Immunodiagnostic Systems; grants and membership of a clinical and scientific committee from the National Osteoporosis Society; grants, personal fees, and advisory board membership from Roche; personal fees from Otsuka, Novartis, Merck, Bayer, Johnson & Johnson, Fonterra Brands, Janssen Research, Ono Pharma, Alere (Unipath), Chronos, Teijin Pharma Limited, D‐STAR, and GSK Nutrition; personal fees and advisory board membership from Eli Lilly, and CL Biosystems; and advisory board membership from the European Calcifi ed Tissue Society, IOF CSA, and the American Society for Bone and Mineral Research, outside the submitted work. MKJ reports personal fees from Stirling Anglia, Consilient Health, and Internis, outside the submitted work. All other authors declare no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence in randomly permuted blocks of 10. |
| Allocation concealment (selection bias) | Low risk | The treatments were blister packed in a single box for each woman for the duration of pregnancy and supplied to the local pharmacy pre‐randomised by the manufacturer (1:1, unstratified by centre) and sequentially numbered for storage and dispensing. The lead pharmacist was the only one with access to the code break envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, matched pills, only lead pharmacist knew about pills. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessments were double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94% of sample had vitD assessment at term. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | Participants were allowed to continue taking their own multivitamin with 400 IU/d of vitamin D but this was not recorded. |
Kaur 1991.
| Methods | Randomised controlled trial. | |
| Participants | 50 pregnant women with similar socioeconomic conditions in India. | |
| Interventions | Participants were randomised into 2 groups: group 1 (n = 25) received orally 2 pharmacological doses of vitamin D˜ (60,000 IU each) in 6th and 7th month of pregnancy; group 2 (n = 25) did not receive any vitamin supplement and served as controls. Health worker cadre: not specified. |
|
| Outcomes | Infant: mean birthweight, placental weight and DNA content, total protein and RNA, protein/DNA and RNA/DNA ratios. Laboratory method used for assessment of vitamin D concentrations: not applicable. |
|
| Notes |
Source of funding: unknown/unreported. Dates of the study and location: not reported dates, India. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors only mentioned that women were randomly selected for trial initially. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report the method of concealment. It is assumed that no method was used as one of the groups did not receive any supplementation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial did not report if it was blinded. It is assumed that it was not blinded to participants as one of the groups did not receive any supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number lost to follow‐up was not reported in results. |
| Selective reporting (reporting bias) | Unclear risk | The total number of participants that completed the study was not specified in results. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Li 2000a.
| Methods | Clinical controlled trial with 3 arms. | |
| Participants | 88 pregnant women with a predisposition to pregnancy‐induced hypertension, at 20 to 24 weeks' gestation, a BMI index of lower than 24, and an arterial pressure of < 11.3 kPa attending an outpatient clinic and labour ward of the First Afilliated Hospital of Xi’an Medical University, Xi’an, China. | |
| Interventions | Participants were divided into 3 groups: group 1 (n = 29) received a daily dose of a tablet containing 600 mg of calcium and 200 IU of vitamin D (Caltrate‐D) daily from 20 to 24 weeks until deliver; group 2 (n = 29) received 1200 mg of calcium and 400 IU vitamin D (Caltrate‐D) daily from 20 to 24 weeks until deliver; group 3 (n = 30) received no intervention from 20 to 24 weeks until delivery. Health worker cadre: not specified. |
|
| Outcomes | Maternal: blood pressure, ionised calcium and platelet intracellular calcium, incidence rates of pregnancy‐induced hypertension. Laboratory method used for assessment of vitamin D concentrations: not applicable. |
|
| Notes |
Source of funding: unknown/unreported. Dates of the study and location: August 1996 to December 1998, China. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The trial did not report if participants were randomly allocated to the treatment groups. It is unclear if it was random or not. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report the method of concealment. It is assumed that it was not conceal as one of the groups did not receive any supplementation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial did not report if it was blinded. It is assumed that it was not blinded to participants as one of the groups did not receive any supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not mention if the study was single or double blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to make a judgement. |
| Other bias | High risk | The report is very short, with most details of the methods not available. |
Mallet 1986.
| Methods | Randomised controlled trial; 3‐arm design with individual randomisation. | |
| Participants | 77 white pregnant women 18 to 36 years of age in the last trimester of pregnancy living in Northwest of France (latitude: 49° 26' 0" N north of Tropic of Cancer). Pre‐gestational BMI not reported. | |
| Interventions | Participants were randomly assigned to 1 of 3 groups: group 1 (n = 21) women received daily 1000 IU of vitamin D (ergocalciferol‐D2) for the last 3 months of pregnancy (estimated total dose throughout pregnancy: 90,000 IU); group 2 (n = 27) women received a single dose of 200,000 IU (5 mg) vitamin D at the 7th month of pregnancy; group 3 (n = 29) women received no supplement and served as controls. Length of the intervention/follow‐up: 12 weeks from start of supplementation to term. Health worker cadre: the study was conducted by the research team at the maternity of Balvedere, Rouen, France but the roles are not described. It is unclear who provided the supplements and measured the outcomes. |
|
| Outcomes | Maternal: 24‐hour urinary calcium excretion after 6 weeks supplementation, calcium, 25‐hydroxyvitamin D (25‐OHD) and1‐alfa,25‐dihydroxyvitamin D (1,25(OH)2D) metabolites of vitamin D from serum and cord during labour and delivery. Infant: serum calcium levels at days 2 and 6 of life, birthweight. Laboratory method used for assessment of vitamin D concentrations: for 25‐hydroxyvitamin D and 1,25‐dihydroxyvitamin D determinations the following techniques were used: extraction with chloroform‐methanol‐water according to Preece, double step purification, first on a Sephadex LH 20 column with chloroform hexan 45 to 55 vol/vol as solvent, then on a high‐pressure liquid pression system according to Shepard. Plasma metabolites were measured by competitive assay using rat protein for 25 OHD and chicken intestine cytosol for 1,25 (OH)2 D according to Jongen. Assay sensitivity for 1,25 (OH)2 D was 5 pmol/tube and for 25 OHD was 25 pmol/tube. |
|
| Notes |
Source of funding: unknown/unreported. Dates of the study and location: January 1979 to December 1982, France. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted by random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report the method of concealment. It is assumed that it was not conceal as one of the groups did not receive any supplementation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial did not report if it was blinded. It is assumed that it was not blinded to participants as one of the groups did not receive any supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not report if it was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is unclear if there was attrition, but given the uneven number of participants reported, it is likely that there were losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | Groups are reported with notorious different sample sizes. It is unclear whether the numbers reflect the participants who finished the trial (unclear and uneven losses to follow‐up); a non randomised process; or a selection bias in which randomised participants did not receive the intervention. |
Marya 1987.
| Methods | Randomised controlled trial; 2‐arm design with randomisation at individual level. | |
| Participants | 400 pregnant women 20 to 35 years of age, attending the antenatal clinic of Medical College Hospital in Rohtak, India (latitude: 76° 34' 0' north of Tropic of Cancer). Pre‐gestational BMI and skin pigmentation not reported. | |
| Interventions | Participants were allocated to 1 of 2 groups: group 1 (n = 200) received a daily supplement containing 1200 IU vitamin D and 375 mg calcium (estimated total dose from week 20 to 24 of gestation to term:134,400‐168,000 IU); group 2 (n = 200) received no supplement from 20 to 24 weeks of pregnancy until delivery and served as controls. Length of the intervention/follow‐up: 20 to 24 weeks from start of supplementation to term. Health worker cadre: not specified. |
|
| Outcomes | Maternal: pre‐eclampsia (defined as blood pressure of 140 mmHg or higher systolic and/or 90 mmHg diastolic along with proteinuria higher than 300 mg/24 hours); systolic and diastolic blood pressure at 24, 28, 32 and 36 weeks of gestation. Serum calcium and creatinine. Laboratory method used for assessment of vitamin D concentrations: not applicable. |
|
| Notes | Biochemical analyses were made for those who developed pre‐eclampsia (n = 12) and also in a group of women with no pre‐eclampsia (n = 25) and a control group of non pregnant women. The results of the stratified analysis are not reported in this review.
Source of funding: unknown/unreported. Dates of the study and location: not reported dates, India. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial reported that participants were randomly allocated to the intervention groups but they did not report the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report the method of concealment. It is assumed that they did not conceal the allocation as one of the groups did not receive any supplementation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial did not report if the study was blinded. It is assumed that it was not blinded to participants as one of the groups did not receive any supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not report if the research staff was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only data on biochemical were reported for those who developed pre‐eclampsia and some of those with no pre‐eclampsia and a group of non pregnant controls. |
| Selective reporting (reporting bias) | High risk | Outcomes reported for some subgroups only. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Marya 1988.
| Methods | Randomised clinical trial; 2‐arm design with individual randomisation. | |
| Participants | 200 pregnant women, aged 22 to 35 years old, attending the antenatal clinic of the Medical College Hospital, Rohtak, India (latitude: 76° 34' 0' north of Tropic of Cancer). Inclusion criterion: uncomplicated single pregnancy. Exclusion criteria: pre‐eclampsia, antepartum haemorrhage, premature delivery. Pre‐gestational BMI and skin pigmentation not reported. | |
| Interventions | Participants were allocated to 1 of the following groups: group 1 (n = 100) women received 2 doses of 600,000 IU (each dose at 7th and 8th month of pregnancy (estimated total dose: 1,200,000 IU); group 2 (n = 100) women received no intervention and served as controls. Length of the intervention/follow‐up: 12 weeks from start of supplementation to term. Health worker cadre: not specified. |
|
| Outcomes | Maternal: venous and cord serum calcium, serum proteins, inorganic phosphate, alkaline phosphatase, weight. Radiological examination on women with abnormal biochemistry or osteomalacia symptomatology. Side effects: back age, leg‐pains, general weakness, cramps. Infant: birthweight, LBW, crown‐heel length, head circumference, mid‐arm circumference within 24 hours after birth. Skinfold thickness (triceps and infrascapular). Laboratory method used for assessment of vitamin D concentrations: not applicable. |
|
| Notes |
Source of funding: unknown/unreported. Dates of the study and location: not reported dates, India. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial reported that participants were randomly allocated to the intervention groups but they did not report the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report the method of concealment. It is assumed that they did not conceal the allocation as one of the groups did not receive any supplementation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial did not report if the study was blinded. It is assumed that it was not blinded to participants as one of the groups did not receive any supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not report if the research staff was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up are not documented although exclusions included pregnancy complications. Results tables mention that each arm was comprised of 100 women, a number that corresponds to that described for the treatment allocation. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Mazurkevich 2013.
| Methods | Randomised control trial. | |
| Participants | 72 pregnant women with physiological pregnancy aged 18 to 35 years with low alimentary consumption of calcium (< 600 mg/day) who attended to Moscow State University of medicine and dentistry, department of obstetrics and gynaecology. (Latitude: 55.7500° N, 37.6167° E). | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 43) received 1250 mg of calcium carbonate and 200 IU of vitamin D (cholecalciferol‐D3) from the second pregnancy trimester until term, in 2 takes a day; group 2 (n = 29) did not receive any treatment and served as controls. Health worker cadre: not specified. |
|
| Outcomes | Maternal: resistance of uterine arteries, resistance of umbilical arteries, uterine‐placental circulation. Infant: fetal‐placental circulation, intrauterine growth retardation, assessed by dopplerometry. Laboratory method used for assessment of vitamin D concentrations: not applicable. |
|
| Notes |
Source of funding: unknown/unreported. Dates of the study and location: not reported dates, Moscow, Russia. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial reported that participants were randomly allocated to the intervention groups but they did not report the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report the method of concealment. It is assumed that they did not conceal the allocation as one of the groups did not receive any supplementation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial did not report if the study was blinded. It is assumed that it was not blinded to participants as one of the groups did not receive any supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not report if research staff was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Mirghafourvand 2013.
| Methods | Triple‐blind randomised controlled clinical trial. | |
| Participants | 126 pregnant women, aged 18 to 39 years with gestational age of 25 to 30 weeks referring to Tabriz health centres, Iran in 2013 to 2014. | |
| Interventions | Participants were allocated to 3 groups using a randomised block design with block sizes of 3 and 6 with the allocation ratio 1:1:1: group 1 (n = 40) Calcium‐vitamin D group (300 mg carbonate calcium plus 1000 units of vitamin D supplements; group 2 (n = 42) vitamin D group (1000 units of vitamin D supplements; and group 3 (n = 42) received placebo. To hide the allocation, each participant received 2 small envelopes, each with enough medicine for 3 weeks, inside a large matte‐coloured envelope of the same shape that were serially numbered. Each participant received 1 pill every day for 42 days. All pills were of the same shape, size, and weight. Health worker cadre: not specified. |
|
| Outcomes | Maternal: gestational age, mode of delivery based on gestational age. food consumption, in terms of calcium and vitamin D content, pre‐pregnancy BMI, BMI during pregnancy Infant: weight, height, and head circumference, birthweight, height, head circumference. Laboratory method used for assessment of vitamin D concentrations: not specified. |
|
| Notes |
Source of funding: this study was funded by a research grant of Tabriz University of Medical Sciences (Project number: 388). Dates of the study and location: July 2013 to April 2014, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to 3 groups using a randomised block design with block sizes of 3 and 6 with the allocation ratio 1:1:1. |
| Allocation concealment (selection bias) | Unclear risk | To hide the allocation, each participant received 2 small envelopes, each with enough medicine for 3 weeks, inside a large matte‐coloured envelope of the same shape that were serially numbered. Each participant received 1 pill every day for 42 days. Pills were of the same shape, size, and weight. However, authors did not mention how this was concealed from study staff. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to the study treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial reported that it was double‐blinded but they did not specify if those performing the assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were documented. No missing data and no participant was eliminated from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Naghshineh 2016.
| Methods | Double‐blind randomised controlled trial. | |
| Participants | 140 nulliparous pregnant women who had been referred to “Shahid Beheshti” hospital in Isfahan, Iran. Pregnant women at less than 16 weeks' gestation from outpatient clinics at “Shahid Beheshti” hospital were eligible if they did not have any sign of vitamin D deficiency, did not using aspirin and had no diagnosis of chronic hypertension, gestational diabetes, renal disease or systemic lupus erythematous. | |
| Interventions | Subjects were randomly divided into 2 groups: Group 1 (n = 70) received supplementation with 600 IU daily of vitamin D at 16 weeks' gestation until labour; Group 2 (n = 70) received daily supplementation free of vitamin D and followed until labour (placebo group). Women were unaware of the treatment allocation. Health worker cadre: women were followed up monthly by a doctor who was blinded to the study groups. |
|
| Outcomes | Maternal: age and gestational age at delivery, pre‐eclampsia Infant: birthweight Laboratory method used for assessment of vitamin D concentrations: not applicable. |
|
| Notes |
Source of funding: this study was funded by a research grant. Financial support was provided by the Isfahan University of Medical Sciences (Grant 392004); Isfahan, Iran. Dates of the study and location: May 2012 to January 2012, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using random‐maker software “Random Allocation”, participants were randomly divided into 2 groups: intervention and placebo. |
| Allocation concealment (selection bias) | Unclear risk | Women were unaware of the treatment allocation but no other information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research staff was unaware of the treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up visits was done by blinded study personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants for the intervention group were missing, but it was explained in the results section that they did not want to continue in the study. Total of 138 participants (68 cases and 70 controls) were analysed and described |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Roth 2010.
| Methods | Randomised placebo‐controlled trial (AViDD‐2 trial) | |
| Participants | 160 pregnant women aged 18 < 35 years old, attending to the International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh (latitude: 23.7000° N, 90.3750° E, north of the Tropic of Cancer). Inclusion criteria: women with residence in Dhaka, with plans to have the delivery performed at the Shimantik maternity centre, and to stay in Dhaka throughout the pregnancy and 1 month past the delivery, with gestational age of 26th to 29th (inclusive), estimated based on the first day of the last menstrual period. Exclusion criteria: use of any dietary supplement containing more than 400 IU/day (10 mcg/day) of vitamin D within the month prior to enrolment, or refusal to stop taking supplemental vitamin D at any dose after enrolment, current use of anti‐convulsant or anti‐mycobacterial (tuberculosis) medications, severe anaemia (haemoglobin concentration < 70 g/L), complicated medical or obstetric history: cardiovascular disease, uterine haemorrhage, placenta praevia, threatened abortion, hypertension, pre‐eclampsia, preterm labour, or multiple gestation), prior history of delivery of an infant with a major congenital anomaly, birth asphyxia, or perinatal death (stillbirth or death within first week of life). | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 80): women received vitamin D (cholecalciferol‐D3) 35,000 IU per week, started at 26 to 29 weeks' gestation, until delivery; group 2 (n = 80): women received placebo control administered weekly from 26 to 29 weeks' gestation until delivery. Health worker cadre: supplement doses were measured in disposable plastic syringes and orally administered by study personnel. |
|
| Outcomes | Maternal: serum 25‐hydroxyvitamin D concentration, serum calcium concentration, urine Ca:Cr ratio. Infant: immune function, infant growth, postnatal vitamin D status, serum calcium. Laboratory method used for assessment of vitamin D concentrations: Serum 25‐hydroxyvitamin D was quantified by high‐performance liquid chromatography tandem mass spectroscopy (LCMS/MS) in the Department of Pathology and Laboratory Medicine at the Hospital for Sick Children. |
|
| Notes |
Source of funding: this study was funded by a Non‐governmental organization (NGO). The Thrasher Research Fund, Salt Lake City, USA. Dates of the study and location: August 2010 to January 2011, Dhaka, Bangladesh. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): the authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial reported computer‐generated randomisation list for the randomisation procedures. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was prepared by International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh personnel not otherwise involved in the study, and was concealed from investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial reported that participants were blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial reported that research staff (including lab personnel) were blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 160 participants recruited and randomly assigned to intervention or placebo, 13 were lost to follow‐up prior to delivery (6 in the placebo group and 7 in the vitamin D group), all because of having left the Dhaka area. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Roth 2013.
| Methods | Randomised, double‐blind, placebo‐controlled trial (MDIG trial). | |
| Participants | 1300 generally‐healthy pregnant women between 17 and 24 weeks of gestation. | |
| Interventions | Participants were randomly assigned at enrolment to 1 of 5 groups: group 1 (n = 260) received placebo throughout the prenatal period and for 26 weeks postpartum; group 2 (n = 260) received 4200 IU per week prenatally and no supplementation postpartum; group 3 (n = 260) received 16,800 IU per week prenatally and no supplementation postpartum; group 4 (n = 260) received 28,000 IU per week prenatally and no supplementation postpartum; and group 5 (n = 260) received 28,000 IU per week prenatally and during the postpartum for 26 weeks. Data from groups 2‐5 were combined into the intervention group for this analysis. All participants received calcium (500 mg per day), iron (66 mg per day), and folic acid (350 mcg per day) throughout the intervention phase. Health worker cadre: trial personnel contacted participants weekly from enrolment until 26 weeks postpartum, and infants were further assessed at 9 months and 12 months of age. Visits were conducted in the home or at a clinic and included the use of standardized questionnaires, point‐of‐care tests, anthropometric measurements, and specimen collection. |
|
| Outcomes | Maternal: maternal serum 25‐hydroxyvitamin D and calcium concentration, urinary calcium excretion, and maternal PTH concentrations. Infant: length‐for‐age, birth outcomes, morbidity and serum 25‐hydroxyvitamin D and calcium concentrations. Laboratory method used for assessment of vitamin D concentrations: point‐of‐care tests. |
|
| Notes |
Source of funding: this study was funded by the Bill & Melinda Gates Foundation. Dates of the study and location: March 2014 to September 2015, Dhaka, Bangladesh. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): no potential conflict of interest was reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted by computer‐generated, simple randomisation scheme created independently by the trial statistician. |
| Allocation concealment (selection bias) | Low risk | Concealment of trial‐group assignments was ensured with the use of pre‐labelled and sequentially numbered but otherwise identical supplement vials. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial reported that a master list linking participants to supplementation groups was not accessible to trial personnel until final group assignments were revealed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial reported that a master list linking participants to supplementation groups was not accessible to trial personnel until final group assignments were revealed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Among 1164 infants assessed at 1 year of age (89.5% of 1300 pregnancies), < 5% of participants withdrew or were excluded after randomisation until birth. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | There is no any evidence of other bias. |
Sabet 2012.
| Methods | Randomised, double‐blind trial | |
| Participants | 50 pregnant women, in their third trimester, who were scheduled to deliver at Mahdieh Hospital in Tehran. | |
| Interventions | Participants were randomly allocated to 1 of 2 groups: Group 1 (n = 25) received oral vitamin D3 100,000 IU monthly, 3 times; Group 2 (n = 25) control (placebo); until term. Health worker cadre: not specified. |
|
| Outcomes | Maternal: the final maternal 25(OH) serum concentrations at delivery, cord 25(OH) vitamin D concentration serum 25(OH), maternal serum iPTH and cord blood iPTH concentration mean PTH concentration Infant: serum vitamin D lower than30 ng/mL in newborn infants Laboratory method used for assessment of vitamin D concentrations: Serum 25 (OH) D concentrations were measured by EIA using the 25(OH) Vit D kit (Immune diagnostic system Ltd, Bolden, UK). |
|
| Notes |
Source of funding: this study was funded by a research grant from the Research Institute of Endocrine Sciences, Shahid Beheshti University of Medical Sciences. Dates of the study and location: 2009 to 2010, Tehran, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): no conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to the treatment or placebo but methods describing this process were not reported. |
| Allocation concealment (selection bias) | Unclear risk | The trial reported that it was double‐blinded, but no methods of describing the process were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial reported that it was double‐blinded, but no methods of describing the process were reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial reported that it was double‐blinded but they did not specify if those performing the assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all 50 participants. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Sablok 2015.
| Methods | Randomised controlled trial with 2 arms, with randomisation at the individual level from years 2010 to 2012. | |
| Participants | 180 primigravidae women with singleton pregnancy at 14 to 20 weeks in the Department of Obstetrics and Gynaecology in Safdarjung Hospital, New Delhi, India (28°38′08″ N, 77°13′28″ E north of Tropic of Cancer). Pregnant women with pre‐existing osteomalacia, known hyperparathyroidism, renal, liver dysfunction, tuberculosis, sarcoidosis and women not willing to comply to the study protocol were excluded. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 60) did not receive any supplementation of vitamin D; group 2 (n = 120) received vitamin D (cholecalciferol‐D3) supplementation in dosages depending upon the level of serum 25(OH)‐D levels estimated at entry into the study. Participants from this second group with sufficient levels of vitamin D (serum 25(OH)‐D levels > 50 nmol/L), received only 1 dose of 60,000 IU vitamin D (cholecalciferol‐D3) at 20 weeks; participants with insufficient levels of vitamin D (serum 25(OH)‐D levels 25–50 nmol/L) received 2 doses of 120,000 IU vitamin D (cholecalciferol‐D3) at 20 weeks and 24 weeks; and participants with deficient levels of vitamin D status (serum 25(OH)‐D levels < 25 nmol/L) received 4 doses of 120,000 IU vitamin D cholecalciferol‐D3) at 20, 24, 28 and 32 weeks. Independently of the dose, all participants in group 2 were grouped and compared to group 1 for this analysis. Health worker cadre: unclear what the roles of the researchers and other workers in the health worker cadre. |
|
| Outcomes | Maternal: preterm labour, pre‐eclampsia, gestational diabetes, serum 25(OH)‐D concentration, serum calcium, phosphorus and serum ALP levels.
Infants: Apgar score, birthweight, LBW, 25(OH)‐D concentration in cord blood, small‐for‐gestational age; appropriate for gestational age. Laboratory method used for assessment of vitamin D concentrations: serum 25‐hydroxyvitamin D was quantified by sandwich ELISA. |
|
| Notes |
Source of funding: self‐funded. Dates of the study and location: 2010 to 2012, India. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): all the authors have nothing to disclose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer‐generated random number tables. |
| Allocation concealment (selection bias) | High risk | As participants were assigned to either no intervention or intervention and the intervention dosage depended on the vitamin D status, there was a selection bias based on status of vitamin D at baseline. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial did not report if the study was blinded. It is assumed that it was not blinded to participants as one of the groups did not receive any supplementation and the other groups received different doses of vitamin D at different times. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | At the time of delivery, both the groups underwent clinical evaluations and complete anthropometric assessment of the neonate, but it was not reported if staff was blinded to the intervention groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The level of attrition was different in groups 1 and 2: 3/60 (5%) participants in group 1 and 12/120 (10%) participants in group 2 were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other evident sources of bias. |
Samimi 2016.
| Methods | Prospective, double‐blind, placebo‐controlled trial. | |
| Participants | 60 primigravida pregnant women, aged 18–40 years old, who were at risk for pre‐eclampsia, and lived approximately 20 km or less from the clinic and hospital. Women ‘at‐risk’ for pre‐eclampsia were recognised by laboratory tests including free β‐human chorionic gonadotrophin, inhibin a dimeric, unconjugated oestriol and maternal serum α‐foetoprotein, and haemodynamic assessment of uterine artery Doppler wave form at 16 to 20 weeks of gestation. | |
| Interventions | Participants were randomly allocated into 2 groups: Group 1 (n = 30) received 50,000 IU vitamin D3 every 2 weeks plus 1000 mg day 1 calcium supplements (as calcium carbonate); Group 2 (n = 30) received placebos at the same times; from 20 to 32 weeks of gestation. Health worker cadre: an investigator with no clinical involvement in the present study packed cholecalciferol, calcium supplements and placebos into numbered bottles based on the random list. Anthropometric measurements of pregnant women at maternity clinic were measured by a trained midwife at baseline and then after 12 weeks of intervention. |
|
| Outcomes | Maternal: serum 25(OH)D concentrations, fasting plasma glucose, serum insulin concentrations, homeostasis model assessment (HOMA)‐B, inQUICKI score, serum HDL‐cholesterol, plasma GSH concentrations, systolic blood pressure, diastolic blood pressure, lipid profiles and inflammatory markers, pre‐eclampsia. Infant: LBW (< 2500 g), newborn’s birth size (newborn’s weight, length and head circumference) and prevalence of preterm delivery (< 37 weeks). Laboratory method used for assessment of vitamin D concentrations: Serum 25‐hydroxyvitamin D concentrations was determined using a commercial enzyme‐linked immunosorbent assay (ELISA) kit (IDS, Boldon, UK) with inter‐ and intra‐assay coefficients of variation (CVs) of 4.5–7.0%, respectively. |
|
| Notes |
Source of funding: the study was supported by a research grant from Kashan University of Medical Sciences. Dates of the study and location: September 2014 to February 2015, Kashan, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): the authors declare that there are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Parallel, balanced randomisation (1:1). |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation were concealed from both researchers and participants until the statistical analysis was completed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only the person responsible for the distribution of drugs knew how the women were allocated to the treatment groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial reported that it was double‐blinded but they did not specify if those performing the assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported for all participants. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Samimi 2017.
| Methods | Double‐blind randomised and controlled clinical trial. | |
| Participants | 80 women aged 18 to 35 years were examined from November 2013 to March 2015 at the Shabihkhani Maternity Hospital in Kashan, Iran. | |
| Interventions | Participants were randomised into 2 groups: Group 1 (n = 40) received a pill of vitamin D3 400 IU/day and Group 2 (n = 40) received a placebo tablet that was similar to vitamin D3, with no active ingredient as a placebo. Both groups received standard treatment with vaginal progesterone (Behvarzan, Iran) at a dose of 400 mg per day. The serum levels of vitamin D3 were evaluated in the tenth and twentieth weeks to prevent any possible poisoning. If so, the patient was excluded from the study. Health worker cadre: all participants in the study received antenatal care and were given folic acid and ferrous sulphate at least 1 month prior to pregnancy, under the supervision of a gynaecologist. They were checked by monitoring serum β‐hCG level levels and abdominal ultrasound until the confirmation of pregnancy, after which the mothers were divided into 2 groups of intervention and control using permuted block randomisation with twenty blocks of size 4. Only the person responsible for the distribution of drugs knew how the women were allocated to the treatment groups. |
|
| Outcomes | Maternal: the serum level of vitamin D3, serum level of IL‐23, serum levels of vitamin D3 and IL‐23, spontaneous abortions. Laboratory method used for assessment of vitamin D concentrations: not specified. |
|
| Notes |
Source of funding: this study was funded by a research grant of the Kashan University of Medical Sciences. Dates of the study and location: November 2013 to March 2015, Kashan, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): the authors declare that there is no conflict of interests regarding the publication of this paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were divided into 2 groups of intervention and control using permuted block randomisation with 20 blocks of size 4. |
| Allocation concealment (selection bias) | Low risk | Only the person responsible for the distribution of drugs knew how the women were allocated to the treatment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only the person responsible for the distribution of drugs knew how the women were allocated to the treatment groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial reported that it was double blinded but they did not specify if those performing the assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported for all participants. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Sasan 2017.
| Methods | Randomised controlled clinical trial. | |
| Participants | 142 women who were referred to the obstetrical clinic in Besat Hospital of Sanandaj City, Kurdistan Province, Iran, who were receiving prenatal care and had a history of pre‐eclampsia in previous pregnancies. | |
| Interventions | The participants were randomly placed into 2 groups: Group 1 (n = 70) received 50,000 IU pearl vitamin D3 once every 2 weeks; and Group 2 (n = 72) received placebo. Vitamin D or placebo was given until the 36th week of pregnancy. Health worker cadre: not specified. |
|
| Outcomes | Maternal: level of vitamin D, pre‐eclampsia. Laboratory method used for assessment of vitamin D concentrations: level of vitamin D was determined through Liebermann–Burchard method. |
|
| Notes |
Source of funding: unknown/unreported. Dates of the study: not reported dates, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): the authors announce that there are no conflicts of interest between different individuals and organisations involved in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 140 pockets of drug and placebo were randomly (by using table of random numbers). |
| Allocation concealment (selection bias) | Unclear risk | 140 pockets of drug and placebo were randomly offered and both study staff and participants did not know about administration of the treatments. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both study staff and participants did not know about administration of the treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both study staff and participants did not know about administration of the treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all participants. |
| Selective reporting (reporting bias) | Unclear risk | Although participant's level of 25‐hydroxy vitamin D was used to determine study eligibility, serum levels of vitamin D were not reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Shahgheibi 2016.
| Methods | Randomised double‐blind placebo‐controlled trial. | |
| Participants | 90 pregnant women with at least 1 risk factor for gestational diabetes including BMI (BMI; kg/m2) more than 25, history of macrosomic neonate, positive family history for diabetes and gestational diabetes, history of gestational diabetes in previous pregnancies, and glycosuria. | |
| Interventions | Participants were randomised into 1 of 2 groups: group 1 (n = 46) received 5000 units of vitamin D weekly; and group 2 (n = 44) received placebo. Both groups were treated until the 26th week of pregnancy. Then the glucose challenge test (GCT) and the glucose tolerance test (GTT) were performed to evaluate GDM. Health worker cadre: not specified. |
|
| Outcomes | Maternal: vitamin D levels and GCT, incidence of diabetes. Laboratory method used for assessment of vitamin D concentrations: vitamin D level was determined in a laboratory by the Liebermann–Burchard method, in which the patient should fast for 12 hours and not have a fatty dinner. |
|
| Notes |
Source of funding: no funding. Dates of the study and location: 2013, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): the authors have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were included and randomly divided into 2 groups using permuted block randomisation of size 2. |
| Allocation concealment (selection bias) | Low risk | Blinding was carried out in which drugs and placebos were prepared to be completely similar in appearance and taste and put in numbered pockets based on a randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both study staff and participants did not know about administration of the treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both study staff and participants did not know about administration of the treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all participants. |
| Selective reporting (reporting bias) | Unclear risk | Apgar score was taken but is not in results. |
| Other bias | High risk | Intervention described in abstract does not match intervention described in methods. In abstract: participants in the intervention group took 5000 units of vitamin D daily. In methods: participants in the intervention group took 5000 units of vitamin D weekly. |
Singh 2015.
| Methods | Randomised controlled trial | |
| Participants | 100 healthy, pregnant women, primigravida with a singleton pregnancy, gestational age: 12‐16 weeks in Sawangi, Meghe, Wardha. | |
| Interventions | Participants were randomised into 2 groups: Group 1 (n = 50): received 2000 IU of vitamin D3 per day from 12‐16 weeks of gestation of pregnancy; and Group 2 (n = 50) received no supplementation and served as controls. 25‐hydroxyvitamin D [25(OH)D]in maternal blood was measured by chemiluminescence immunoassay, at recruitment and at the time of delivery and a serum 25(OH)D level lower than30 nmol/L was defined as deficiency. Health worker cadre: not specified. |
|
| Outcomes | Maternal: deficiency of vitamin D, mean gestational age, preterm birth. Laboratory method used for assessment of vitamin D concentrations: Mean serum 25(OH)D levels were measured by Roche diagnostic ELECSYS (Electrochemiluminescence immunoassay) 2010 Cobase E 411 Analyser Immunoassay System Germany. |
|
| Notes |
Source of funding: no funding sources reported. Dates of the study and location: October 2012 to September 2014, India. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial reported that participants were randomly allocated to the treatment or no supplementation but methods describing this process were not reported. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report this; however, since one group did not receive any supplementation, it is assumed that the intervention was not concealed to participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial did not report this; however, it is assumed that participants were not blinded as one group did not receive any supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not report if study staff was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is assumed that each group had 50 participants as stated in the abstract but the trial did not report final number of participants that completed the trial or the sample size in any of the tables. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other evident sources of bias. |
Taherian 2002.
| Methods | Randomised controlled study with 3 arms. | |
| Participants | 990 nulliparous women attending antenatal outpatient clinics of Isfahan Health Centers (32.6333° N, 51.6500° E north of Tropic of Cancer) between April 1998 and March 2001, with singleton pregnancies, first prenatal visit before 20 weeks of gestation, systolic/diastolic blood pressure lower than 130/80 mmHg, and no proteinuria detectable by a dipstick. Women with history of cardiovascular, renal or endocrinologic problems, medical or obstetric complications and those with known hazardous condition (multifetal gestation, hydatidiform mole) were excluded. |
|
| Interventions | Participants were randomly assigned to 1 of 3 groups: Group 1 (n = 330) received 75 mg aspirin each day from 20th week of gestation until delivery; Group 2 (n = 330) received a tablet containing 500 mg calcium carbonate + 200 IU vitamin D (cholecalciferol‐D3) daily from 20th week of gestation until delivery; and group 3 received no intervention (n = 330). All cases received standard prenatal care. Health worker cadre: the women were examined by trained staff every 4 weeks through the 28 weeks of gestation, and every 2 weeks through the 36th week and weekly thereafter. Blood pressure was measured by a certified examiner. |
|
| Outcomes | Maternal: blood pressure, bodyweight, BMI, maternal height, urine protein measurements, maternal weight gain, duration of gestation. Infant: neonatal weight at birth, the presence of respiratory distress syndrome, sepsis, jaundice and intrauterine growth retardation, fetal or neonatal death. Laboratory method used for assessment of vitamin D concentrations: not applicable. |
|
| Notes |
Source of funding: this study was funded by a research grant of the Research Deputy of Isfahan University of Medical Sciences grant (No: 76085). Dates of the study and location: April 1998 to March 2001, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report the methods for allocation concealment. It is unclear whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. It is assumed that it was not conceal as one group did not receive any treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial did not mention if it was blinded. It is assumed that it was not blinded to participants as one group did not receive any treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not mention if it was blinded to those conducting the assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other evident sources of bias. |
Tehrani 2014.
| Methods | Single‐arm study, not blinded. | |
| Participants | 210 pregnant women referring to obstetric clinic of Shahid Beheshti and Alzahra hospital in Esfahan city in 2012. Inclusion criteria: patient satisfaction; normal BMI; gestational age below 16 weeks; no history of diabetes mellitus type 2 or GDM; no family history of diabetes mellitus type 1 in first degree relatives. Exclusion criteria: patient dissatisfaction; incorrect consumption of vitamin D supplementation; follow‐up discontinuation. | |
| Interventions | Participants will be individually randomised to 1 of 2 groups: Group 1 (n = 70) received vitamin D supplementation with dose of 50,000 unit every 2 weeks for 10 weeks; Group 2 received a placebo. Pregnant women with levels of above 25 nmol/L were selected as the normal healthy control group and were the ones who received placebo. Health worker cadre: not specified. |
|
| Outcomes | Maternal: gestational blood sugar level, serum vitamin D level. Laboratory method used for assessment of vitamin D concentrations: not specified. |
|
| Notes |
Source of funding: this study was funded by a non‐governmental organization, Sponsor: Isfahan University of Medical Sciences. Dates of the study and location: January 2013 to January 2014, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Researcher, the distributors of the drug and the women, did not know which group was taken the vitamin D or placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Researcher, the distributors of the drug and the women, did not know which group was taken the vitamin D or placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researcher, the distributors of the drug and the women, did not know which group was taken the vitamin D or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other evident sources of bias. |
Vaziri 2016.
| Methods | Randomised clinical trial. | |
| Participants | 169 pregnant women, both nulliparous and multiparous, aged 18 years or older, no history of mental illness and internal diseases such as hyper/hypothyroidism, no addiction to any kind of narcotic drugs or alcohol, not divorced or widowed, no pregnancy complications such as pre‐eclampsia, gestational diabetes, ruptured membranes and suspicion of preterm birth, no previous caesarean sections, with a live fetus singleton pregnancy, and gestational age of 26 to 28 weeks based on ultrasound results. who were under prenatal care in Hafez teaching hospital in Shiraz, Iran. | |
| Interventions | Participants were assigned to 2 groups through block randomisation design: Group 1 (n = 78) received 2000 IU of vitamin D3 per day; and Group 2 (n = 75) received placebo. Both groups received their assigned treatments from 26 to 28 weeks of gestation until childbirth. Maternal serum 25‐hydroxyvitamin D concentrations were measured at baseline and childbirth. Besides, depression scores were evaluated 4 times: at 26 to 28 and 38 to 40 weeks of gestation, and finally at 4 and 8 weeks after birth. Participants were allowed to use prescribed supplementation outside this study’s protocol. Usually, pregnant women in Iran are prescribed iron and folic acid, which may be consumed as part of the multivitamin supplementation with 200–400 IU of vitamin D. They reported that 55 participants in the intervention and 69 in the control used other supplements but they did not provide the composition of the supplements. Health worker cadre: at first, a research team member who was responsible for data collection visited the prenatal care clinic of the hospital daily and based on the inclusion criteria, invited the mothers to participate in the study. The consumption of pills were assessed in later prenatal care visits and over the phone. |
|
| Outcomes | Maternal: baseline 25‐hydroxy vitamin D concentrations and at childbirth, depression score. Infant: vitamin D concentrations, anthropometric measurements of their infants at birth, 4th and 8th weeks of birth. Laboratory method used for assessment of vitamin D concentrations: Serum 25‐hydroxyvitamin D was measured with the Chemiluminescence immunoassay (CLIA) method. |
|
| Notes |
Source of funding: the study was financially supported by a research grant from the Research Vice‐chancellor of Shiraz University of Medical Sciences. Dates of the study and location: November 2014 to October 2015, Iran. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): the authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a block randomisation design. |
| Allocation concealment (selection bias) | Unclear risk | The trial reported that it was a single‐blinded study, but it is not clear who was blinded and they did not report the allocation concealment. It is assumed that participants were blinded as the control group also received 2 pills, similar to the intervention group. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial reported that it was a single‐blinded study. However, data collection related to depression was done by a trained midwife outside the research team, who was blinded to group allocations but the rest of the assessments were not clear if they were performed by a blinded staff. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not report if study staff conducting the assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 143 women began study and 136 finished. Serum vit D checked for 130 women, but does not specify how many women were in each group. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other evident sources of bias. |
Yu 2008.
| Methods | Randomised controlled trial; 4 x 3 block design with randomisation at individual level. | |
| Participants | 180 pregnant women (45 Indian Asians, 45 Middle Eastern, 45 Black and 45 Caucasian) women at 27 weeks' gestation attending the routine antenatal clinic at St Mary’s Hospital, London, the UK (latitude: 51°30'N north of tropic of Cancer). Exclusion criteria: pre‐existing sarcoidosis, osteomalacia, renal dysfunction and tuberculosis. Pre‐gestational BMI and skin pigmentation (in addition to ethnicity) not reported. The study took place between April 2007 and November 2007. As well, a follow‐up trial of the infants of these trial participants. All of the offspring of the 180 mothers recruited in this trial were eligible and were invited to participate in a follow‐up study when their children were 3 years of age. | |
| Interventions | Participants were randomised in blocks of 15 within each of the 4 ethnic groups to 3 groups; Group 1 (n = 60) received a daily dose of vitamin D (ergocalciferol D2) at 800 IU; Group 2 (n = 60) received a one dose of 200,000 IU of calciferol; Group 3 (n = 60) received no treatment and served as controls. All groups received the intervention for 13 weeks, from start of supplementation to term. Data from groups 1 and 2 were collapsed for this analysis. Health worker cadre: each woman collected her tablets directly from the hospital pharmacy department or her local pharmacy. |
|
| Outcomes | Maternal: maternal and cord 25‐hydroxyvitamin D levels at delivery, maternal PTH and corrected calcium levels at delivery, adverse events. Infant: small‐for‐gestational age was defined as birthweight less than the 10th percentile after adjustments for gestation at delivery, infant sex, maternal ethnicity, parity, height and weight. Wheezing episode in the first 3 years of life, measured at 36 to 48 months, use of inhaled bronchodilators in the last 12 months, doctor‐diagnosed rhinitis, any wheezing episode in the preceding 12 months, doctor‐diagnosed asthma, doctor‐diagnosed eczema, doctor‐diagnosed food allergy, positive skin prick test responses, 25‐hydroxyvitamin D levels, bronchodilator responsiveness, exhaled nitric oxide level (in parts per billion), nasal secretions for inflammatory mediators, pulmonary airflow resistance and reactance at a range of frequencies using impulse oscillometry, total number of all wheezing episodes since birth and total number of upper and lower respiratory tract infections since birth, at 36 to 48 months. Laboratory method used for assessment of vitamin D concentrations: not specified. |
|
| Notes | Women who did not speak English were only included if a health advocate was able to interpret and a leaflet was provided in their language;
Source of funding: this study was funded by a research grant from the Institute of Obstetrics and Gynaecology Trust, Wolfson and Weston Research Centre for Family Health, Imperial College, Du Cane Road, Hammersmith Hospital, London W12 0NN, UK. Dates of the study and location: April 2007 to November 2007, London, UK. Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number lists were drawn up by an independent researcher, with randomisation in blocks of 15. |
| Allocation concealment (selection bias) | Low risk | The research staff allocating participants used the next available number on entry to the trial, and each woman collected her tablets directly from the hospital pharmacy department or her local pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study personnel and participants were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study personnel was not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 loss to follow‐up on group 3. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | Women were randomised within each ethnic group. It is not clear if the ethnicity can be clearly established as it was self reported. Women who did not speak English were included only if a health advocate was able to interpret and a leaflet was provided in their language (English, Arabic, Bengali and Farsi) although the ability to read was not clearly established. |
β‐hCG: beta human chorionic gonadotropin BMI: body mass index CHW: community health workers ELISA: enzyme‐linked immunosorbent assay GDM: gestational diabetes mellitus GSH: glutathione HDL: high‐density lipoprotein IGF‐I: insulin‐like growth factor IU: international units IUFD: intrauterine fetal death LBW: low birthweight LDL: low‐density lipoprotein mcg: microgram PA: physical activity PTH: parathyroid hormone 25 (OH)D: 25‐hydroxycholecalciferol
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ala‐Houhala 1986 | 49 healthy, well‐nourished mothers delivering in January 1984 in the maternity wards and outpatient clinic of the Department of Paediatrics of the University Central Hospital of Tampere, Finland (latitude 61°N) and exclusively breastfeeding their infants, were divided in succession into 3 groups: group 1 (n = 17): mothers were given 2000 IU vitamin D3 a day, infants not supplemented; group 2 (n = 16): mothers were given 1000 IU vitamin D3 a day, infants not supplemented; group 3 (n = 16): mothers were not supplemented, and their breast fed infants were given 400 IU of vitamin D2 a day. This is not a randomised trial and the intervention includes mothers at postpartum and their infants. |
| Asemi 2013b | 54 pregnant women aged 18 to 40 years diagnosed with GDM by a 100‐g oral glucose‐tolerance test at 24 to 28 weeks' gestation attending maternity clinics affiliated with Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran. Participants were randomly assigned to 1 of 2 groups: group 1 (n = 27), women received capsules containing 50,000 IU vitamin D (cholecalciferol‐D3) (D‐Vitin 50000; Zahravi Pharm Co) 2 times during the study (at baseline and at day 21 of the intervention): group 2 (n = 27), women received 2 placebos (Barij Essence Co) at the same times. All pregnant women in the study had a diagnosis of gestational diabetes. The type of participant is outside the scope of this review. |
| Asemi 2014 | 56 pregnant women 18 to 40 years of age with gestational diabetes and 24 to 28 weeks' gestation attending prenatal care at maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Iran were randomly assigned to 1 of 2 groups: group 1 (n = 28) received 1000 mg calcium per day and a 50,000 U vitamin D (cholecalciferol‐D3) pearl twice during the study (at study baseline and on day 21 of the intervention); group 2 (n = 28) received 2 placebos at the same times. Participants were pregnant women with diagnosis of gestational diabetes. The type of participant is outside the scope of this review. |
| Asemi 2015 | 46 women at risk for pre‐eclampsia at 27 weeks' gestation with positive roll‐over test were randomly assigned to receive either the multi mineral‐vitamin D supplements (n = 23) or the placebo (n = 23) for 9 weeks. Study was conducted in Kashan, Iran, during November 2013 to May 2014. Multi mineral‐vitamin D supplements were containing 800 mg calcium, 200 mg magnesium, 8 mg zinc, and 400 IU vitamin D3. Fasting blood samples were taken at baseline and after 9‐week intervention to measure related factors. Newborn's outcomes were determined. This type of intervention is outside the scope of this review. |
| Atkinson 2010 | 120 African American or Caucasian primigravidae women 19 to 40 years of age in their first trimester of pregnancy in Children’s Hospital & Research Center Oakland, California, USA were included in this study. Women who were smokers, had a pre‐pregnancy BMI higher than 30, had a medical condition that affected bone or taking a medication that affected bone were excluded. Participants were randomly assigned to 1 of 3 groups: group 1: 1000 mg of calcium; group 2: 2000 IU vitamin D; group 3: placebo. The intervention started at week 16 of pregnancy until delivery. The type of intervention is outside the scope of this review. |
| Azami 2017 | 90 pregnant women,with least 1 of the risk factors for PE were randomly divided into 3 groups according to randomised selection: Group A received 1 ferrous sulphate tablet (Rooz daru©, Iran) + 1 Claci‐care multimineral‐vitamin D tablet [(VitanePharma©, Germany) contained 800 mg Ca, 200 mg Mg, 8 mg Zn and 400 IU vitamin D3)]per day; Group B received 1 ferrous sulphate tablet (Rooz daru©, Iran,) + 250 mg vitamin C and 55 mg vitamin E, and control group only received Ferrous sulphate daily. This type of intervention is outside the scope of this review. |
| Baqui 2009 | 28 pregnant women were enrolled at a maternal health clinic in inner‐city Dhaka, Bangladesh Aged 18 to 34 years; at 27 to 30 weeks of pregnancy. Participants were randomly assigned to 1 of 2 groups: group 1 (N = 14) were assigned to receive a single dose of vitamin D3 70,000 IU (1.75 mg, where 1 mg = 40,000 IU) on day 0 followed by vitamin D3 35,000 IU (0.875 mg) per week starting on day 7 and continuing until delivery); Group 2 (N = 14), were assigned to receive vitamin D3 14,000 IU (0.350 mg) per week starting on day 0 and continuing until delivery. All participants received vitamin D supplementation in different regimens. The type of intervention is outside the scope of this review. |
| Bhatia 2010 | 150 consecutive pregnant women pregnant women during their second trimester from 6 villages of a poor socio‐economic region in district Barabanki (latitude 26.8 ºN), Uttar Pradesh, north India. The participants were initially randomised to receive either no dose or 1 dose of 60,000 IU cholecalciferol under observation in the 5th gestational month. This is not a randomised trial and the comparisons are outside the scope of this review. |
| Bhatia 2012 | 299 pregnant women with 12 and 24 weeks of gestation of lower‐middle and middle socio‐economic groups attending the antenatal clinic in Queen Mary Hospital, Chhatrapati Sahuji Maharaj, India, were randomly assigned to 1 of 2 groups, group 1: received 1500 mcg cholecalciferol at induction into the study, or group 2 3000 mcg cholecalciferol at induction as well as at 28 weeks of gestation. All were prescribed 1 g of elemental calcium daily as calcium carbonate without vitamin D. This type of intervention is outside the scope of our review. |
| Bisgaard 2009 | 623 women were recruited at 24 weeks of pregnancy. Women were randomised 1:1 to a daily dose of 2400 IU vitamin D3 supplementation or matching placebo tablets (Camette A/S) from pregnancy week 24 to 1 week postpartum. In addition, all women were instructed to continue supplementation of 400 IU of vitamin D3 during pregnancy as recommended by the Danish National Board of Health; thus, the study is a dose comparison of 2800 IU/day vs 400 IU/day of vitamin D3 supplementation. Both groups received vitamin D. This type of intervention is outside the scope of our review. |
| Chandy 2016 | 230 infants and mothers giving birth in 2 maternity units of the institution, who intended to continue exclusive breast‐feeding until the first 6 months and come to the hospital of birth for immunisation, were eligible, from September 2012 and June 2014 at the King George Medical University, and Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow (latitude 26°N). After maternal blood sample was collected for serum 25(OH)D 2 to 4 days after delivery, mother–infant pairs were randomly assigned at birth to 1 of 3 treatment regimens described below, to be followed for 9 months. Intervention was postpartum. |
| Cockburn 1980 | 1139 pregnant women were assigned to 1 of 2 wards: group 1 (n = 506) Caucasian pregnant women assigned to 1 ward of the Simpson Memorial Maternity Pavilion, Edinburgh, UK during the 9 months from September to May, were given a daily dietary supplement of 400 IU of vitamin D2 from about the 12th week of pregnancy until delivery; group 2 women (n = 633) were assigned to another ward over the same period and were given a placebo containing no vitamin D. This is not a randomised trial. |
| Czech‐Kowalska 2013 | 174 healthy postpartum women who had delivered babies at term in Poland, were randomised to 1 of 2 groups: group 1 (n = 70) received 1200 IU/day vitamin D (cholecalciferol‐D3 as 800 IU/day alone + 400 IU/day from a multiple micronutrient supplements; group 2 (n = 67) received 400 IU/day vitamin D (cholecalciferol‐D3 as placebo + 400 IU/day from multiple micronutrient supplements) during 6 months of lactation. Participants from both groups received vitamin D supplements. The participants were postpartum women. The type of participant and the type of interventions are outside the scope of this review. |
| Das 2010 | 200 consecutive pregnant women attending antenatal clinic of at Queen Mary Hospital in CSMMU (former KGMC) were enrolled into the study after taking informed consent and randomly allocated to 1 of 3 groups: (1) Intervention group 1 vitamin D 1,20,000IU in 3 doses each 8 weeks apart + calcium carbonate containing 500 mg elemental calcium with 250 IU vitamin D twice a day, daily throughout pregnancy; (2) Intervention group 2 vitamin D 60,000IU in 3 doses each 8 weeks apart + calcium carbonate containing 500 mg elemental calcium with 250 IU vitamin D twice a day, daily throughout pregnancy; (3) Comparator agent group 3 calcium carbonate containing 500 mg elemental calcium with 250 IU vitamin D twice a day, daily throughout pregnancy. This is a registry for an ongoing study (open to recruitment). The type of intervention is outside the scope of this review. |
| Dawodu 2013 | 192 Arab women between 12–16 weeks of gestation after their last menstrual period or by ultrasound assessment who had a singleton pregnancy; and planned to receive prenatal and delivery care in primary healthcare clinics affiliated with Tawam Hospital, Al Ain, United Arab Emirates. All participants received vitamin D supplementation in different regimens. The type of intervention is outside the scope of this review. |
| de Menibus 1984 | 77 mother‐child couples were divided into 3 groups, according to whether women were not receiving vitamin D (29 couples) during the last 3 months of pregnancy ending in winter or taking 1000 units of vitamin D in the form of uvesterol (21 pairs) or a single dose of 200,000 units of vitamin D at 7 months (27 pairs). This type of intervention, with no placebo group, is outside the scope of this review. |
| Etemadifar 2015 | 45 pregnant women with confirmed multiple sclerosis who attended an outpatient clinic in Isfahan University of Medical Sciences, Iran aged 20 to 40 years with low serum 25‐hydroxyvitamin D (25(OH)D) levels were randomly allocated to 2 groups in an open‐label randomised, controlled clinical Phase I/II pilot study. 1 group received 50,000 IU/week vitamin D3 (n = 21) or routine care (n = 22) from 12 to 16 weeks of gestation till delivery. This type of participant is outside the scope of this review. |
| Gerais 2015 | 88 women were recruited at different gestational age, the incidence of vitamin D deficiency about 66%. A single daily dose ranging from 1000 IU to 2000 IU according to the level of deficiency were given to the patient for 6 weeks. In our study 34.1% of women had a level below 10 ng/mL This type of intervention, with no placebo group, is outside the scope of this review. |
| Hashemipour 2014 | 160 pregnant women (24 to 26 weeks of gestation) who attended an obstetric clinic in Qazvin, Iran, from December 2011 to March 2012 were randomised, and included in 2 arms. Women in the control group received a multivitamin containing 400 IU vitamin D3 plus 200 mg elemental calcium each day until delivery. Women in the intervention group received a weekly dose of 50,000 IU oral vitamin D3 for 8 weeks (from 26 to 28 weeks of pregnancy) as well as the drug regimen (multivitamin and elemental calcium) given to the control group. Both groups received vitamin D and calcium. This type of intervention is outside the scope of our review. |
| Hossain 2012 | 200 pregnant women who attended the Department of Obstetrics & Gynaecology unit 3, Dow University and Civil Hospital Karachi, Pakistan aged between 18 and 40 years were randomised, and included in 2 arms. Participants were allocated to 1 of 2 groups: group 1 (n = 100) received along with ferrous sulphate, 4000 IU of vitamin D3; group 2 (n = 100) received routine antenatal care (ferrous sulphate and calcium). Both groups received above medications from 20 weeks of pregnancy until delivery. This type of intervention is outside the scope of our review. |
| Ito 1994 | 876 singleton pregnant women with blood pressure lower than 140/90 mmHg at 20 weeks’ gestation, and no evidence of proteinuria, who were attending the obstetric clinic of Kumamoto University Hospital, Japan were divided into 2 groups: group 1 (n = 666) women received conventional antenatal care; group 2 (n = 210 women) were managed under a protocol for the prediction of pre‐eclampsia with an angiotensin sensitivity test and prevention of the condition by calcium supplementation. This is not a randomised trial and the type of intervention is outside the scope of this review. |
| Jamilian 2016 | 60 participants with GDM were divided into 2 groups of either 1000 IU vitamin D3 and 1000 mg EPO or placebo for 6 weeks. At the beginning and end of the study, fasting blood samples were obtained from the participants to measure related variables. The type of participant is outside the scope of this review. |
| Jamilian 2017 | 140 GDM patients. Participants were randomly divided into 4 groups to receive: (1) 1000 mg omega‐3 fatty acids containing 360 mg eicosapentaenoic acid and 240 mg docosahexaenoic acid (DHA) twice a day + vitamin D placebo (n = 35); (2) 50,000 IU vitamin D every 2 weeks + omega‐3 fatty acids placebo (n = 35); (3) 50,000 IU vitamin D every 2 weeks + 1000 mg omega‐3 fatty acids twice a day (n = 35), and (4) vitamin D placebo + omega‐3 fatty acids placebo (n = 35) for 6 weeks. The type of participant is outside the scope of this review. |
| Kachhawa 2014 | 243 pregnant patients attending the antenatal clinic, between 18 to 40 years old and with gestational age between 12 to 16 weeks were randomised into 4 groups in a ratio of 1:1:1:1. Group 1 active control group received 600 units of vitamin D per day, group 2: 1000 units/day, group 3: 2000 units/day and group 4: 4000 units per day. This type of intervention is outside the scope of our review. |
| Karamali 2014 | 60 women in Arak, Iran, with GDM were divided into 2 groups to receive Ca + vitamin D supplements or placebo. Individuals in the Ca + vitamin D group (n 30) received 1000 mg Ca/day and 2 pearls containing 1250 μg (50 000 IU) of cholecalciferol (vitamin D3) during the intervention (one at study baseline and another at day 21 of the intervention); those in the placebo group (n 30) received 2 placebos of vitamin D at the mentioned times and placebos of Ca every day for 6 weeks. The type of participant is outside the scope of this review. |
| Karamali 2015 | 60 pregnant women Arak, Iran at risk for pre‐eclampsia according to abnormal uterine artery Doppler waveform were randomly divided into 2 groups to receive 50,000 IU vitamin D supplements (n = 30) or receive placebo (n = 30) every 2 weeks from 20 to 32 weeks of gestation. All pregnant women were also taking 400 μg/day folic acid from the start of pregnancy, 60 mg/day ferrous sulphate from the second trimester, and a multivitamin mineral capsule (containing 400 IU vitamin D) from the second half of pregnancy. The type of participant is outside the scope of this review. |
| Kermack 2017 | 111 couples were recruited for a 6‐week intervention prior to oocyte retrieval consisted of a daily drink, containing 2 g of DHA plus EPA and 10 mcg of vitamin D, and olive oil and olive oil spreads, all in unmarked containers. The control group received a placebo drink and sun flower oil and spreads, again in unmarked containers. 55 couples were randomised to the treatment group and 56 to placebo. Following IVF, embryos were cultured in an Embryoscope and validated morphokinetic markers of embryo quality were recorded; day 3 and 5 KIDScores (Known Implantation Data Score) were calculated for individual embryos. This type of intervention is outside the scope of our review. |
| Kiely 2015 | 144 pregnant women aged older than 18 years of age, with no more than 18 weeks' gestation, in good general health, with low‐risk pregnancy and not consuming > 10 mcg/day vitamin D from supplements were randomised in a 3‐arm, parallel, double‐blind, placebo‐controlled dose‐response intervention study with vitamin D. Group 1 received 10 mcg (400 IU) vitamin D3 once daily taken from baseline visit (approximately 15 weeks' gestation) until endpoint (delivery). Group 2 received 20 mcg (800 IU) vitamin D3 once daily from baseline visit (approximately 15 weeks' gestation) until endpoint (delivery). Group 3 (placebo) received a placebo capsule containing 0 mcg (0 IU) of vitamin D3 taken from baseline visit (approximately 15 weeks' gestation) until endpoint (delivery). The primary outcome was serum 25‐hydroxyvitamin D in pregnant women and cord blood. Women were permitted to continue with self‐administration of antenatal supplements containing ≤ 10 μg vitamin D per day. This type of intervention is outside the scope of our review. |
| Lalooha 2012 | Participants were randomly assigned to 1 of 2 groups: group 1: vitamin D capsule 50,000 U weekly for 8 weeks from 28 gestational age and multivitamin tablet include 400 IU vitamin D daily till termination; group 2: multivitamin tab include 400 IU vitamin D daily till termination. This type of intervention is outside the scope of our review. |
| Li 2016 | 103 pregnant GDM women were eligible to participate, using a permuted block randomisation method stratified according to their baseline 1‐hour OGTT results, all participants in their second trimester were randomly assigned to consume 2 servings (100 g per serving) of either plain yogurt (PY) drink (‘PY’ without any vitamin D3 supplement, from Mengniu Dairy, Hohhot, China) or VDY drink (‘PY’ supplemented with 500 IU vitamin D3, from Mengniu Dairy, Hohhot, China), with 1 serving at breakfast and the other 1 at dinner, on a daily basis for a period of 16 weeks. The type of participant is outside the scope of this review. |
| MacDonald 1986 | This trial was registered in 1986 on the Oxford Database of Perinatal Trials and reports the recruitment and follow‐up completed in 1979. The registration form reports a randomised controlled trial to assess the efficacy of calcium and vitamin D supplementation versus placebo in the prevention of maternal and fetal hypocalcaemia. The reports indicates that the sample size was 55 Asian women with morbidity and laboratory results as primary outcomes but no further information is available. |
| March 2010 | 226 healthy pregnant women from Greater Vancouver, British Columbia, Canada, from 13 to 24 weeks of gestation were randomly allocated to 10, 25, or 50 mg vitamin D/d from 13 to 24 weeks of gestation until 8 weeks postpartum, with no infant supplementation. Mother and infant blood was collected at 8 weeks postpartum (n = 76, n = 76, and n = 74, respectively). The overall study retention rate from beginning to end was 76% (n = 172). This type of intervention, with no placebo group, is outside the scope of this review. |
| Marya 1981 | 45 Hindu pregnant women were randomly assigned to 1 of 2 groups: group 1 (n = 25) received tablets containing 1200 IU vitamin D and 375 mg calcium daily throughout the 3rd trimester; group 2 (n = 20) received oral single dose of 600,000 IU vitamin D2 once during 7th month and 8th month (total 2 doses). This group was compared with group 3 (n = 75) who had not received vitamin D supplements during pregnancy. The results were also compared with data from 25 non pregnant, non‐lactating healthy women. The randomised study compares 2 doses of vitamin D supplementation. The type of study, type of participants and types of interventions are outside the scope of this review. |
| McLean 2012 | Pregnant women, aged 18 years or more, with less than 20 weeks’ gestation at recruitment. Participants will be randomly assigned to 1 of 2 groups: group 1: received high‐dose vitamin D supplementation (5000 IU/day); group 2: standard dose pregnancy vitamin supplementation (400 IU vitamin D daily), administered as an oral capsule, from the time of the first antenatal clinic visit (around 12 weeks’ gestation) until delivery. This type of intervention is outside the scope of this review. |
| Mojibian 2015 | 500 women with gestational age 12 to 16 weeks and serum 25 hydroxy vitamin D (25 (OH) D ) less than 30 ng/mL randomly categorized in 2 groups: group A received 400 IU vitamin D daily and group B 50,000 IU vitamin D every 2 weeks orally until delivery. Maternal and neonatal outcomes were assessed in 2 groups. This intervention had no placebo group. |
| Mozzafari 2010 | Women between 20 to 45 years old with gestational diabetes at their recent pregnancy, from the list of GDM Diabetes Research Center of Yazd University, and without thyroid disease, kidney disease, bone disease, PCO, liver disease and not using anti‐epilepsy drugs, glucocorticoids, and statins. Exclusion criteria: risk of any illness that requires medication and lack of any willingness to co‐operate, were randomly assigned to 1 of 2 groups: group 1: intramuscular injection of vitamin D with 300,000 IU dose; group 2: control: not receive any intervention. The type of participant is outside the scope of this review. |
| Mutlu 2013 | 91 pregnant women aged 16 to 42 years were admitted to Kocaeli Maternity and Children Hospital between April 2011 and April 2012. The participants were randomly divided into 3 groups: 600 IU/d (control group; n = 31); 1,200 IU/d (n = 31), and 2,000 IU/d (n = 32) of vitamin D. All groups received vitamin D supplements. This type of comparison is outside the scope of our review. |
| Nausheen 2014 | 315 pregnant women aged 15 to 45 years with less than 16 weeks of gestation in a hospital in Pakistan. Pregnant women with pre‐existing type 1 or type II diabetes, pre‐existing hypertension, multiple fetuses,babies (twins, triplets) or with a diagnosis of pregnancy with a fetal anomaly in scan will be excluded. Participants were randomly assigned to 1 of 3 groups: groups 1 received a dose of 400 IU/day till the time of delivery; group 2 received 2000 IU/day till the time of delivery; group 3 received 4000 IU/day till the time of delivery. This type of intervention is outside the scope of this review. |
| Niramitmahapanya 2017 | 76 Thai lactating mothers and their breast‐fed infants were studied with maternal 25 hydroxyvitamin D 25 (OH) D levels of 10‐30 ng/mL determined using Liquid Chromatography Mass Spectrometry Tandem (LC‐MS/MS). 1 group received vitamin D3 1800 IU/day supplementation for 6 weeks, and members of the other group were given a placebo. 25 (OH) D level of colostrum and 6‐week serum from breast‐fed milk were measured by High Performance Liquid Chromatography (HPLC). The data from the 2 groups were analysed and compared. The type of participant is outside the scope of this review. |
| Pandey 2015 | 20 women with 25 (OH) D < 20 ng/mL & Hb = 8‐10 g/dL were randomised into groups using a computerised program (8 patients in iron alone group and 12 pregnant mothers in iron + vitamin D group). Recruited pregnant mothers received group iron + vitamin D: tablets containing fixed dose combination of vitamin D (1000 IU) + ferrous ascorbate (100 mg of elemental iron) + folic acid (1 mg) + vit B12 (7.5 mcg) (1 tablet/day) for 12 weeks. Group iron alone: tablets containing fixed dose combination of ferrous ascorbate (100 mg of elemental iron) + folic acid (1.1 mg) (1 tablet/day) for 12 weeks. This type of intervention is outside the scope of this review. |
| Qian 2015 | 60 pregnant women at risk for pre‐eclampsia, experiencing their first pregnancy, aged between 20 and 32 years, between 18 and 20 weeks' gestation, and pregnant with a single fetus were eligible. Each pregnant woman selected for the study showed the following abnormalities on uterine artery Doppler, qualifying them as high‐risk: average resistance index (RI) > 0.67, pulsatility index (PI) > 1.65, and incisura at early diastole phase. Pregnant women were randomised into 2 groups to take either cholecalciferol supplements (n = 30) or placebo (n = 30). This study was retracted due to having data very similar to another paper. |
| Razavi 2017 | 120 women with GDM were randomly divided into 4 groups to receive: 1) 1000 mg omega‐3 fatty acids containing 180 mg eicosapentaenoicacid (EPA) and 120 mg docosahexaenoic acid (DHA) twice a day + vitamin D placebo (n = 30); 2) 50,000 IU vitamin D every 2 weeks + omega‐3 fatty acids placebo (n = 30); 3) 50,000 IU vitamin D every 2 weeks + 1000 mg omega‐3 fatty acids twice a day (n = 30) and 4) vitamin D placebo + omega‐3 fatty acids placebo (n = 30) for 6 weeks. The type of participant is outside the scope of this review. |
| Rostami 2018 | 1600 and 900 first trimester pregnant women, aged 18 to 40 years, with gestational age < 14 weeks, with singleton pregnancy in Masjed‐Soleiman, Khuzestan province,Iran were randomised by levels of vitamin D in serum to: Moderate deficiency
Severe deficiency
Pregnant women with normal vitamin D status were the controls. All women were allowed to consumed multivitamins containing no more than 400 IU per day of vitamin D3. This type of intervention is outside the scope of this review. |
| Shakiba 2013 | 51 healthy pregnant women from the beginning of their second trimester of pregnancy during the autumn and winter of 2009 in recruited from 2 primary care clinics in Yazd (31°53’50”N/54°22’04”E), Iran. Participants were distributed in 3 groups according to their serum 25(OH)D at the beginning of the second trimester of pregnancy. Participants with low concentrations (25(OH)D levels < 20 ng/mL) (n = 17) were treated with 200,000 IU (50,000 IU/week for 4 weeks) of vitamin D (as (cholecalciferol‐D3), followed by supplementation with 50,000 IU/month vitamin D (cholecalciferol‐D3). The other 34 participants were randomly assigned to 1 of 2 groups: group 1 received 50,000 IU/month vitamin D (cholecalciferol‐D3); group 2 received 100,000 IU/month vitamin D (50,000 IU every 2 weeks) of vitamin D (cholecalciferol‐D3) supplementation. All participants received vitamin D supplements. The type of study design and the type of intervention are outside the scope of this review. |
| Shi 2017 | 602 women with singleton pregnancy who were diagnosed of pre‐eclampsia in Cangzhou Central Hospital participated in the present trial and were divided into 2 groups using stratified permuted‐block randomisation method with diastolic blood pressure as a factor: (1) nifedipine + VD group (n = 298), given 1 capsule containing nifedipine (10 mg per capsule) and VD (200 IU per capsule) every 15 min orally, up to 4 doses, until blood pressure was equal to or below 150/100 mmHg; (2) nifedipine + placebo group (n = 304), given 1 capsule containing nifedipine (10 mg per capsule) plus glucose (20 mg per capsule) as placebo every 15 minutes orally, up to 4 doses, until blood pressure was equal to or below 150/100 mmHg. The type of participant is outside the scope of this review. |
| Simsek 2011 | Women with gestational diabetes, defined by the WHO criteria: fasting glucose ≥ 7.0 mmol/L or; oral glucose tolerance test: 75 g glucose, 2‐hour glucose ≥ 7.8 mmol/L recognised during pregnancy with a written informed consent, aged between 18 to 42 years. Participants will be randomly assigned to 1 of 2 groups: group 1: cholecalciferol 15,000 IU once a week during pregnancy; group 2: placebo. Per communication with the author, this study was not completed due to low recruitment. |
| Soheilykhah 2013 | 120 women with gestational age less than 12 weeks without gestational diabetes, history of PCO, BMI less than 30 kg/m2 before pregnancy, no vitamin D supplementation in the past 6 months were randomised into 2 groups: supplementation with 50,000 IU of vitamin D monthly (2000 IU daily) or 50,000 IU every 2 weeks (4000 IU daily). Maternal and neonatal outcomes were assessed in 2 groups. This intervention had no placebo group. |
| Stephensen 2011 | Pregnant women less than 20 weeks' gestation and over 18 years of age with no use of medications known to affect vitamin D metabolism, diagnosis of type 1 diabetes, history of thyroid, renal, or liver disease, problems with digestion or absorption participated in the study at USDA Western Human Nutrition Research Center and clinicians at UC Davis Medical Center. They were distributed into 2 groups, receiving: either 400 IU or 2000 IU of vitamin D per day for the duration of their pregnancy. Both groups received vitamin D supplements. This type of intervention is outside the scope of our review. |
| Sudfeld 2017 | 2300 HIV‐infected pregnant women receiving triple‐drug ART under Option B+ in Dar es Salaam Tanzania. HIV‐infected pregnant women of 12 to 27 weeks' gestation were randomised to either: 1) 3000 IU vitamin D3 taken daily from randomisation in pregnancy until trial discharge at 12 months postpartum; or 2) a matching placebo regimen. Maternal participants are followed‐up at monthly clinic visits during pregnancy, at delivery, and then with their children at monthly postpartum clinic visits. The type of participant is outside the scope of this review. |
| Taheri 2014 | 229 women 18 to 35 years old, who were confirmed to be vitamin D deficient (vitamin D < 75 nmol/L), were randomised into the intervention, and control groups and after 15 weeks consumption of the supplement (2000 IU/day oral vitamin D) and placebo. The study was conducted among reproductive women in a high‐risk population for vitamin D deficiency. The participants of the study were not pregnant women. The type of participant is outside the scope of this review. |
| Thiele 2014 | 16 pregnant women at 24 to 28 weeks' gestation were enrolled. The control group (N = 8) received a prenatal vitamin containing 400 IU vitamin D daily, plus a placebo capsule. The experimental group (N = 8) received the same prenatal vitamin with an additional capsule containing 3400 IU vitamin D, for a total of 3800 IU daily. This study had no placebo group, therefore, this type of intervention is outside the scope of this review. |
| Valizadeh 2016 | 96 women with GDM at weeks 12 to 32 of gestation, age > 16 years, singleton pregnancy were randomly assigned to either the intervention (n = 48) or control group (n = 48). Patients were referred from primary health centres affiliated with Zanjan University of Medical Sciences, as well as private obstetric clinics throughout the city. The type of participant is outside the scope of this review. |
| von Hurst 2009 | 235 South Asian women, aged 23 to 68 years, living in Auckland, New Zealand were recruited for the study and those who were insulin resistant ‐ homeostasis model assessment 1 (HOMA1) > 1.93 and had serum 25‐hydroxyvitamin D concentration < 50 nmol/L were randomly assigned to 1 of 2 groups: group 1 (n = 42) received 100 μg (4000 IU) vitamin D(3); group 2 (n = 39) received a placebo daily for 6 months. The study participants were non‐pregnant women. The type of participant is outside the scope of this review. |
| Wagner 2006a | 494 apparently healthy pregnant women (16 to 45 years of age) with 12 to 16 weeks' gestation of singletons attending prenatal care in Medical University of South Carolina, Charleston, United States were randomised into 1 of 3 groups stratified by race: group 1 received 400 IU vitamin D (cholecalciferol‐D3)/day; group 2 received 2000 IU vitamin D (cholecalciferol‐D3)/day; and group 3 received 4000 IU vitamin D (cholecalciferol‐D3)/day until delivery. All women received daily multiple micronutrients supplements. All women received vitamin D supplementation at different doses. The type of intervention is outside the scope of this review. |
| Wagner 2006b | This is an analysis of data from 2 randomised controlled trials by the same research group (Wagner 2006a; Wagner 2010a). In Wagner 2006a, women were randomised to 400, 2000, or 4000 IU vitamin D (cholecalciferol‐D3)/day, stratified by race. In Wagner 2010a, participants were randomised to 2000 or 4000 IU vitamin D (cholecalciferol‐D3)/day. |
| Wagner 2013 | 258 women, exclusively breastfeeding (n = 201) and formula‐feeding (n = 57) women participating in a prospective, randomised controlled trial of vitamin D supplementation were compared at baseline 1 month postpartum (V1), at 4 months (V4), and 7 months postpartum (V7) on the basis of vitamin D status (measured by total circulating 25(OH)D concentration) and BMI. The type of intervention is outside the scope of this review. |
| Weiss 2009 | 881 pregnant women with either a personal history of asthma or allergies or a similar history in the spouse or partner, between 18 and 40 years of age and at an estimated gestational age between 10 and 18 weeks, were recruited at a scheduled obstetrical prenatal visit at 3 clinical centres: Boston Medical Center (Boston, MA), Washington University at Saint Louis (St. Louis, MO), and Kaiser Permanente Southern California Region (San Diego, CA). Participants were randomised to either vitamin D (cholecalciferol, 4000 IU/day; equivalent to 100 μg/day) or placebo. All pregnant mother participants received prenatal vitamins containing 400 IU (10 μg/day) of cholecalciferol; thus, the vitamin D arm received a total of 4400 IU/day (110 μg/day) and the placebo arm received 400 IU/day (10 μg/day). Both groups received vitamin D supplements. This type of intervention is outside the scope of our review. |
| Yap 2014 | 179 pregnant women 18 years of age or older, with singleton pregnancy, with plasma 25‐hydroxivitamin D (25OHD) concentrations lower than 32 ng/mL, less than 20 weeks of gestation were randomly assigned to 1 of 2 groups: group 1 (n = 89) received 5000 IU/d of vitamin D (cholecalciferol‐D3) until delivery; group 2 (n = 90) received 400 IU/d of vitamin D (cholecalciferol‐D3) until delivery. Outcomes included glycaemia and glucose tolerance, gestational diabetes at 26‐28 weeks of gestation; neonatal 25OHD, maternal hypertension, mode of delivery, prematurity, birthweight, crown‐heel length, occipitofrontal head circumference. All participants received vitamin D supplements at different doses. The type of intervention is outside the scope of this review. |
| Yazdchi 2016 | 76 pregnant women without a prior diagnosis of glucose intolerance in the first trimester were asked to participate in a 75‐g OGTT at 24‐28 weeks of gestation. Diagnosis of GDM was based on the International Association of Diabetes and Pregnancy Study Groups criteria. Patients were recruited from Al‐Zahra Hospital, the academic outpatient centre of the Tabriz University of Medical Sciences, Tabriz, Iran. Participants in the vitamin D group received oral capsules containing 50,000 IU vitamin D 3 (D‐Vitin50000; Zahravi Pharm Co., Iran), once every 2 weeks for 2 months, for a total of 4 capsules. Those in the placebo group received placebos composed of paraffin oil (Dana Pharm Co., Iran) using the same schedule, for a total of 4 placebos. The type of participant is outside the scope of this review. |
| Zhang 2016 | 133 pregnant women with GDM during weeks 24 to 28 of pregnancy. The patients were randomly divided into 4 groups. The control group (n = 20) received a placebo (sucrose; 1 granule/day), the low‐dosage group (n = 38) received the daily recommended intake of 200 IU vitamin D (calciferol) daily, the medium‐dosage group (n = 38) received 50,000 IU monthly (2000 IU daily for 25 days) and the high‐dosage group (n = 37) received 50,000 IU every 2 weeks (4000 IU daily for 12.5 days). The general characteristics and dietary intakes of the patients with GDM were similar between each group. High‐dose vitamin D supplementation (50,000 IU every 2 weeks) significantly improved insulin resistance in pregnant women with GDM. The type of participant is outside the scope of this review. |
BMI: body mass index Ca: calcium DHA: docosahexaenoic DRI: dietary references intakes EPA: eicosapentaenoic acid FPG: fasting plasma glucose GDM: gestational diabetes mellitus Hb: haemoglobin IU: international units IVF: in vitro fertilisation mcg: microgram OGTT: oral glucose tolerance test PCO: polycystic ovary syndrome PTH: parathyroid hormone 25OHD: 25‐hydroxycholecalciferol
Characteristics of studies awaiting assessment [ordered by study ID]
Bimson 2017.
| Methods | Randomised, double‐blind, placebo‐controlled study. |
| Participants | 67 pregnant women with vitamin D (25(OH)D) level lower than 25 ng/mL, English or Spanish literacy, age 18 years and gestational age less than 20 weeks. |
| Interventions | Women were randomised to: group 1 (n = 36) received 50,000 IU vitamin D3 or group 2 (n = 31) received placebo, once a week for 8 weeks in addition to a prenatal vitamin with 400 IU vitamin D3. |
| Outcomes | After 8 weeks of treatment, VD levels were 48.1 19.6 ng/mL and 21.1 5.9 ng/mL (P <.0001) with 84.4% and 3.9% achieving VD sufficiency (> 30 ng/mL) in the TG and PG, respectively (P <.0001). At delivery, VD levels were 28.8 9.8 ng/mL and 20.58.9 ng/mL in the TG and PG, respectively (P < 0.0027). |
| Notes | Mount Sinai, West Hospital, New York, NY, USA |
Das 2009.
| Methods | Randomised controlled trial |
| Participants | 150 pregnant women in 2nd trimester from 6 villages of North India |
| Interventions | All women were educated regarding sunshine exposure & were given 1 g calcium carbonate daily. They were randomised to 3 groups no vitamin D, 60,000 IU vitamin D and 2,40,000 IU vitamin D supplementation. |
| Outcomes | Maternal: serum 25 OH D levels at baseline and post delivery |
| Notes | This is an abstract. No information on registry or funding source. |
IU: international units PG: placebo group TG: treatment group VD: vitamin D 25OHD: 25‐hydroxycholecalciferol
Characteristics of ongoing studies [ordered by study ID]
Baird 2016.
| Trial name or title | Southampton PRegnancy Intervention for the Next Generation (SPRING): protocol for a randomised controlled trial |
| Methods | Randomised controlled trial that uses a 2‐by‐2 factorial design |
| Participants | 600 women, with less than 17 weeks' gestation at recruitment based on LMP, aged over 18 years, with a singleton pregnancy and aiming to give birth at local maternity (Princess Anne) hospital |
| Interventions | Healthy conversation skills support plus vitamin D supplementation (1000 IU cholecalciferol) (n = 150); healthy conversation skills support plus placebo (n = 150); usual care plus vitamin D supplementation (n = 150); usual care plus placebo (n = 150) |
| Outcomes | This trial is evaluating 2 approaches to improving maternal diet: a behaviour change intervention and vitamin D supplementation. The factorial design of this trial has the advantage of enabling each intervention be tested separately as well as allowing exploration of the synergistic effect of both interventions on women’s diets and vitamin D levels. |
| Starting date | Registered on 13 September 2013 |
| Contact information | Janis Baird: MRC Lifecourse Epidemiology Unit, University of Southampton,Southampton SO16 6YD, UK. jb@mrc.soton.ac.uk |
| Notes | Sponsor: Medical Research Council,NIHR Southampton Nutrition Biomedical Research Centre and Danone Nutricia Early Life Nutrition. |
Jelsma 2013.
| Trial name or title | DALI: vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention. |
| Methods | Randomised controlled trial with a factorial design. |
| Participants | Pregnant women with gestational age at recruitment < 12 weeks, and more than 18 years of age. Inclusion criteria: pre‐pregnancy BMI (self‐reported weight, measured height) is >= 29 kg/m2), sufficiently fluent in major language of the country of recruitment, being able to be moderately physically active, giving written informed consent, agree to give birth in 1 of the participating hospitals. Exclusion criteria: pre‐existing diabetes, diagnosed with (gestational) diabetes mellitus before randomisation, defined as fasting glucose ≥ 5.1 mmol/L and/or 1‐hour glucose ≥ 10 mmol/L and/or 2‐hour glucose ≥ 8.5 mmol/L at baseline, not able to walk at least 100 metres safely, requirement for complex diets, advanced chronic conditions (e.g. valvular heart disease), significant psychiatric disease, unable to speak major language of the country of recruitment fluently, known abnormal calcium metabolism (hypo/hyperparathyroidism, nephrolithiasis, hypercalciuria) or hypercalciuria detected at screening (0.6 mmol/mmol creatinine in spot morning urine) and twin pregnancy. |
| Interventions | The design is that of 2 trials with a factorial design: PA, diet, PA & diet, control, vitamin D, PA & diet and placebo, vitamin D & PA & diet, placebo; to compare the impact of increased PA, enhanced nutrition and vitamin D supplementation either alone or in combination on maternal glucose tolerance, maternal weight gain and insulin sensitivity. The doses of vitamin D that will be tested in the dosing study are 500, 1000 and 1500 IU/day. One of these doses will be used in the trial. |
| Outcomes |
Maternal: weight gain during pregnancy, fasting plasma glucose, HbA1c, fasting C peptide, leptin, triglycerides, free fatty acids, high density lipoprotein cholesterol (HDL‐C) and low density lipoprotein cholesterol (LDL‐C), adiponectin 2. 3 beta‐hydroxybutyrate, blood pressure, C‐reactive protein. Infant: neonatal growth, adiposity, adipo‐insular axis, glucose‐insulin axis, electrolyte concentrations, clinical outcomes and hypoxia exposure at birth, biparietal diameter, head circumference, abdominal circumference, femur length and determinants of fetal body composition variables (lean body mass and fat body mass, C‐peptide, glucose, leptin, triglycerides, 3‐ beta‐hydroxybutyric acid, pH and erythropoietin, jaundice, hypocalcaemia, hypomagnesaemia. |
| Starting date | 21/11/2011. |
| Contact information | Dr David Simmons Addenbrooke's Treatment Centre Hills Road, Cambridge CB2 0QQ UK |
| Notes | Sponsor: European Union (EU) (Belgium) ‐ Seventh Framework Programme (FP7). |
Judkins 2010.
| Trial name or title | A randomised double‐blinded interventional trial to determine the effect of 50,000 IU vitamin D supplementation monthly or twice monthly from 20 weeks' gestation. |
| Methods | Randomised double‐masked clinical trial with randomisation at the individual level. Method of sequence generation: serial tossing of a coin. Allocation will be not concealed. |
| Participants | Pregnant women seeking maternity care with midwifery services involved in the study. Exclusion criteria: antenatal vitamin D level is > 75 nmol/L when enrolling in study. |
| Interventions | Participants will be assigned to 1 of 2 groups: group 1: will receive 50,000 IU tablets twice monthly, 2 weeks apart; group 2: will receive 50,000 IU monthly and a placebo monthly, 2 weeks apart from 20 weeks' gestation until delivery of baby. The placebo tablet contains lactose monohydrate, acacia, calcium carbonate, castor oil, maize starch, povidone, sucrose, purified talc, hydrated silica, powdered cellulose, magnesium stearate, shellac, gelatin, beeswax white, titanium dioxide and prepared theobroma. |
| Outcomes | Infant: vitamin D levels taken from the cord blood samples at delivery. If emergencies at delivery prevent a cord blood sample being taken then a maternal venous blood sample will be taken for analysis. |
| Starting date | Status: not yet recruiting participants. |
| Contact information | Dr Annie Judkins Newtown Union Health Service 14 Hall Ave, Newtown,. Wellington 6021, New Zealand Email: annie.judkins@nuhs.org.nz |
| Notes | Sponsors: Royal New Zealand College of GP's, New Zealand and Wellington Medical Research Foundation, New Zealand. ACTR Number: ACTRN12610001044011. |
Lindqvist 2010.
| Trial name or title | Vitamin D supplementation for prevention of placenta mediated pregnancy complications. |
| Methods | Randomised, controlled trial. |
| Participants | Pregnant women > 18 years of age, from 3 maternal healthcare units who agree to participate in the study. Exclusion criteria: < 18 years of age, hyperparathyroidism and sarcoidosis. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1: will receive vitamin D, oral drops; group 2: will receive placebo. |
| Outcomes |
Maternal: pre‐eclampsia, blood loss at delivery. Infant: blood flow in umbilical artery, growth restriction, and prematurity. |
| Starting date | 06/04/2011. |
| Contact information | N/A |
| Notes | Sponsor: Karolinska University Hospital. EudraCT Number: 2010‐019483‐37. |
Mosalanejad 2016.
| Trial name or title | Compare the effect of vitamin D and calcium plus vitamin D on pregnancy outcomes in pregnant women |
| Methods | Randomised single‐blind controlled clinical trial |
| Participants | 460 pregnant women between 20 to 40 ages, with gestational age less than 10 weeks of gestation; with no history of diabetes; HTN; a history of PCO syndrome; lack of family history of diabetes in first‐degree relatives; no family history of high blood pressure in first‐degree relatives; tend the sick; the vitamin D < 30 ng/L in women 10 to 16 weeks of pregnancy; BMI between 19 to 26; lack of vitamin D during the last 6 months; singleton pregnancy; gestational first to third who referred to women clinic of Shariati Hospital of Bandarabas, Iran from 2015‐2016 |
| Interventions | In intervention group is used vitamin D3 1000 unit oral daily since 16 weeks until the end of pregnancy. They also prescribed as routine prenatal care multivitamin that has 400 units vitamin D‐ca since 16 weeks of pregnancy. The control group is used multivitamin that has 400 unit vitamin D daily until the end of pregnancy |
| Outcomes | Vitamin D concentration in the serum, diabetes, pre‐eclampsia, preterm delivery |
| Starting date | Registration date: 2016‐12‐26, 1395/10/06 |
| Contact information | Najmehsadat Mosalanejad, email: mosalanejad@hums.ac.ir |
| Notes | Sponsor: Hormozgan University of Medical Sciences |
Rasmussen 2009.
| Trial name or title | Effects of vitamin D supplement before and during and after pregnancy on complications, birthweight and bone mineral density during lactation. |
| Methods | Double‐blind, randomised, placebo‐controlled trial. |
| Participants | 400 apparently healthy women 30 to 35 years of age, all with concentrations of P‐25‐hydroxyvitamin D‐ lower than 50 nmol/L. All women included attempts to get pregnant. Visits take place at Clinic of Osteoporosis, Department of Endocrinology, at Aarhus University Hospital, Aarhus, Denmark. Women with infertility, an intake of 400 IU or more vitamin D/day, cancer, history of alcohol or drug abuse, calcium metabolic disturbances or spontaneous abortion within last 6 months will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 3 groups: group 1: will receive 35 mcg per day cholecalciferol; group 2: will receive 70 mcg per day cholecalciferol; group 3: will receive placebo. All women will receive 2 tablets daily from baseline until 16 weeks after delivery. Intervention with cholecalciferol or placebo starts before pregnancy is achieved and continues until 4 months after the women has given birth. |
| Outcomes | Primary: Infant: birthweight. Maternal: none. Secondary: Infant: weight, crown‐heel length and head circumference, and infections within 16 weeks after birth. Concentration of 25‐hydroxyvitamin D in umbilical cord and venous sample 16 weeks after birth. Maternal: postpartum effects of vitamin D supplement on maternal bone mineral density, concentration of 25‐hydroxyvitamin D in mothers milk, incidence of pre‐eclampsia and abortions. |
| Starting date | Date of start: 12/2009. Status: recruiting participants. Estimated study completion date: December 2011. |
| Contact information | Gitte Bloch Rasmussen, MD Department of Endocrinology, Aarhus University Hospital University of Aarhus Tel: +45 89 4976 81 Email: gittebr@ki.au.dk |
| Notes | Sponsor: University of Aarhus, Denmark. |
BMI: body mass index DRI: dietary references intakes GDM: gestational diabetes mellitus HTN: hypertension IU: international units LMP: last menstrual period mcg: microgram PA: physical activity PCO: polycystic ovary
Differences between protocol and review
In comparison with the previous version (De‐Regil 2016), this 2019 updated review has the following difference.
The contact person (and guarantor) for this review has changed from Luz Maria De‐Regil to Cristina Palacios. Two prior co‐authors left the review team at the full review stage.
Contributions of authors
For this update, Lia Kostiuk and Juan Pablo Peña‐Rosas assessed eligibility of the new trials and extracted the data in duplicate. Any differences were discussed and resolved with Cristina Palacios. All contributed to the preparation of the updated review.
Sources of support
Internal sources
-
Evidence and Programme Guidance, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Dr Juan Pablo Peña‐Rosas is full time staff of the World Health Organization.
External sources
-
The Bill & Melinda Gates Foundation, USA.
WHO thanks the Bill & Melinda Gates Foundation for their financial support to the Department of Nutrition for Health and Development for conducting systematic reviews on nutrition‐specific and nutrition‐sensitive interventions.
-
Evidence and Programme Guidance, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Dr Cristina Palacios and Dr Lia Kostiuk Lombardo received partial funding for this work.
Declarations of interest
The other authors have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
Cristina Palacios received funds from the World Health Organization (WHO) to pay for travel expenses to present and meet with co‐authors and to write the review.
Lia Kostiuk ‐ received partial financial support from the WHO for this commissioned work.
Juan Pablo Peña‐Rosas ‐ the WHO receives partial financial support from the Bill & Melinda Gates Foundation, US Agency for International Development and Nutrition International to support its work in the area of nutrition, including the commissioning of systematic reviews of interventions for health throughout the life course. Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process, including the composition of research questions, membership of the guideline groups, conduct and interpretation of systematic reviews, or formulation of recommendations.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Asemi 2012 {published data only}
- Asemi Z, Tabassi Z, Heidarzadeh Z, Khorammian H, Sabihi SS, Samimi M. Effect of calcium‐vitamin D supplementation on metabolic profiles in pregnant women at risk for pre‐eclampsia: a randomized placebo‐controlled trial. Pakistan Journal of Biological Sciences 2012;15(7):316‐24. [DOI] [PubMed] [Google Scholar]
Asemi 2013a {published data only}
- Asemi Z, Samimi M, Siavashani MA, Mazloomi M, Tabassi Z, Karamali M, et al. Calcium‐vitamin D co‐supplementation affects metabolic profiles, but not pregnancy outcomes, in healthy pregnant women. International Journal of Preventive Medicine 2016;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asemi Z, Samimi M, Tabassi Z, Shakeri H, Esmaillzadeh A. Vitamin D supplementation affects serum high‐sensitivity C‐reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. Journal of Nutrition 2013;143(9):1432‐8. [DOI] [PubMed] [Google Scholar]
Benson 2009 {published data only}
- Benson J. A randomised controlled trial on the effects of antenatal vitamin D supplementation to improve vitamin D levels in the maternal and cord blood at birth in vitamin D deficient pregnant women. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83375 first received 4 February 2009.
- Rodda CP, Benson JE, Vincent AJ, Whitehead CL, Polyakov A, Vollenhoven B. Maternal vitamin D supplementation during pregnancy prevents vitamin D deficiency in the newborn: A randomised controlled trial. Osteoporosis International 2011;Suppl 4:S521‐S559. [DOI] [PubMed] [Google Scholar]
- Rodda CP, Benson JE, Vincent AJ, Whitehead CL, Polyakov A, Vollenhoven B. Maternal vitamin D supplementation during pregnancy prevents vitamin D deficiency in the newborn: An open label randomised controlled trial. Clinical Endocrinology 2015;83(3):363‐8. [DOI] [PubMed] [Google Scholar]
Bhutta 2011 {published data only}
- Bhutta ZA. Evaluation of the effectiveness of vitamin D supplementation to pregnant women and their infants in Pakistan. clinicaltrials.gov/ct2/show/NCT01229189 (first received 27 October 2010).
- Bhutta ZA. Study of vitamin D supplementation on improvement of gums health. clinicaltrials.gov/ct2/show/NCT01422122 (first received 23 August 2011).
- Khan F. A randomized controlled trial of oral vitamin D supplementation in pregnancy to improve maternal periodontal health and birth weight. Journal of International Oral Health 2016;8(6):657‐65. [Google Scholar]
Brooke 1980 {published data only}
- Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. British Medical Journal 1980;1:751‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. British Medical Journal 1981;283:1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IR, Brooke OG, Cleeve HJ. Changes in mineral metabolism in the human foetus and newborns associated with maternal vitamin d supplements [proceedings]. Biochemical Society Transactions 1980;8(1):136‐7. [DOI] [PubMed] [Google Scholar]
- Maxwell JD, Ang L, Brooke OG, Brown IR. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. British Journal of Obstetrics and Gynaecology 1981;88:987‐91. [DOI] [PubMed] [Google Scholar]
Delvin 1986 {published data only}
- Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: effect on neonatal calcium homeostasis. Journal of Pediatrics 1986;109:328‐34. [DOI] [PubMed] [Google Scholar]
Diogenes 2013 {published data only}
- Bezerra F. Calcium plus vitamin d supplementation during pregnancy of adolescent mothers: effects on maternal and infant bone mass, calcium and bone metabolism and breast milk composition. clinicaltrials.gov/ct2/show/NCT01732328 (first received 22 October 2012).
- Diogenes ME, Bezerra FF, Rezende EP, Donangelo CM. Calcium plus vitamin D supplementation during third trimester of pregnancy in adolescents accustomed to low calcium diets did not affect infant bone mass at early lactation in a randomized controlled trial. Journal of Nutrition 2015;145:1515‐23. [DOI] [PubMed] [Google Scholar]
- Diogenes ME, Bezerra FF, Rezende EP, Taveira MF, Pinhal I, Donangelo CM. Effect of calcium plus vitamin D supplementation during pregnancy in Brazilian adolescent mothers: a randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2013;98(1):82‐91. [DOI] [PubMed] [Google Scholar]
- Normando P, Diogenes ME, Cabello PH, Cabello GM, Donangelo CM, Bezerra FF. Calcium plus vitamin D supplementation during pregnancy interacts with polymorphisms in the promoter region of the VDR gene to affect postpartum bone mass of Brazilian adolescent mothers: a randomized controlled trial. Nutrition 2016;32(10):1068‐74. [DOI] [PubMed] [Google Scholar]
Grant 2013 {published data only}
- Grant C. Randomised placebo controlled study of vitamin D during pregnancy and infancy. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=320842 (first received 2 February 2010).
- Grant C, Milne T, Knight J, Sinclair J, Camargo C. Vitamin D supplementation during pregnancy and infancy reduces food allergen sensitisation and parental‐reported food allergy: a randomised controlled trial. European Journal of Pediatrics 2016;175(11):1438. [DOI] [PubMed] [Google Scholar]
- Grant C, Stewart A, Scragg R, Milne T, Rowden J, Ekeroma A, et al. What dose of vitamin D supplementation is required in pregnancy and infancy to increase infants' serum 25‐hydroxyvitamin D concentration > 20ng/ml?. Pediatric Academic Societies Annual Meeting; 2013 May 4‐7; Washington DC, USA. 2013.
- Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitisation: a randomised controlled trial. Allergy 2016;71(9):1325‐34. [DOI] [PubMed] [Google Scholar]
- Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: A randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2016 April 30‐May 3; Baltimore, USA. 2016:1650.8. [DOI] [PubMed]
- Grant CC, Kaur S, Waymouth E, Mitchell EA, Scragg R, Ekeroma A, et al. Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: a randomised‐controlled trial. Acta Paediatrica 2015;104(4):396‐404. [DOI] [PubMed] [Google Scholar]
- Grant CC, Stewart AW, Scragg R, Milne T, Rowden J, Ekeroma A, et al. Vitamin D during pregnancy and infancy and infant serum 25‐hydroxyvitamin D concentration. Pediatrics 2014;133(1):e143‐53. [DOI] [PubMed] [Google Scholar]
- Wall CR, Stewart AW, Camargo CA Jr, Scragg R, Mitchell EA, Ekeroma A, et al. Vitamin D activity of breast milk in women randomly assigned to vitamin D3 supplementation during pregnancy. American Journal of Clinical Nutrition 2016;103:382‐8. [DOI] [PubMed] [Google Scholar]
Harvey 2012 {published data only}
- Barker M, D'Angelo S, Ntani G, Lawrence W, Baird J, Jarman M, et al. The relationship between maternal self‐efficacy, compliance and outcome in a trial of vitamin D supplementation in pregnancy. Osteoporosis International 2017;28(1):77‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook E, Curtis EM, Krstic N, D'Angelo S, Crozier SR, Moon RJ, et al. Perinatal DNA methylation at the RXRA promoter is associated with gestational vitamin d supplementation: results from the mavidos trial. Rheumatology 2017;56(Suppl 2):ii112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. A randomised double‐blind placebo‐controlled trial of vitamin D supplements for pregnant women with low levels of vitamin D in early pregnancy. isrctn.com/ISRCTN82927713 (first received 25 February 2008).
- Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double‐blind, randomised placebo‐controlled trial. Lancet Diabetes and Endocrinology 2016;4(5):393‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C, Harvey NC, Javaid MK, Bishop NJ, Kennedy S, Papageorghiou AT, et al. Effectiveness of maternal Vitamin D supplementation: A multicentre randomised, double‐blind, placebo controlled trial (MAVIDOS). Osteoporosis International 2015;26(1 Suppl 1):S40. [Google Scholar]
- Curtis E, D'Angelo S, Crozier S, Moon R, Inskip H, Cook E, et al. Dna methylation at the rxra promoter at birth is associated with gestational vitamin d supplementation: results from the mavidos trial. Osteoporosis International 2017;28(Suppl 1):S72‐S73. [Google Scholar]
- Harvey N, Moon R, D'Angelo S, Crozier S, Inskip H, Schoenmakers I, et al. Predictors of maternal 25(OH)‐vitamin D response to antenatal vitamin d supplementation: results from the mavidos trial. Osteoporosis International 2016;27(2 Suppl):S628‐S629. [Google Scholar]
- Harvey NC. Influence of diet and lifestyle during pregnancy on offspring bone mass and body composition. The Power of Programming 2014: International Conference on Developmental Origins of Adiposity and Long‐Term Health; 2014 March 13‐15; Munich, Germany. 2014:27.
- Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, Fraser R, et al. O16 Maternal gestational vitamin d supplementation results in greater bone mass for offspring born during the winter months: the mavidos multicentre randomized, double‐blind, placebo‐controlled trial. Rheumatology 2016;55(Suppl 1):i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NC, Javaid K, Bishop N, Kennedy S, Papageorghiou AT, Fraser R, et al. MAVIDOS Maternal Vitamin D Osteoporosis Study: Study protocol for a randomized controlled trial. The MAVIDOS Study Group. Trials 2012;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NC, Moon RJ, D'Angelo S, Crozier S, Schoenmakers I, Bishop NJ, et al. Determinants of the maternal response to vitamin D supplementation during pregnancy: the MAVIDOS trial. Osteoporosis International 2016;27(Suppl 1):S49‐S50, Abstract no: OC19. [Google Scholar]
- Ioannou C, Cavallaro A, D'Angelo S, Javaid MK, Cooper C, Harvey NC, et al. Antenatal vitamin D supplementation does not affect fetal femur volume: secondary outcome of a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2017;124:28. [Google Scholar]
- Moon RJ, Harvey NC, Cooper C, D'Angelo S, Crozier SR, Inskip HM, et al. Determinants of the maternal 25‐hydroxyvitamin D response to vitamin D supplementation during pregnancy. Journal of Clinical Endocrinology and Metabolism 2016;101(12):5012‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RJ, Harvey NC, Cooper C, D'Angelo S, Curtis EM, Crozier SR, et al. 25‐Hydroxyvitamin D response to gestational cholecalciferol supplementation is associated with common vitamin D related genetic variants: findings from the mavidos trial. Rheumatology 2017;56(Suppl 2):ii153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RJ, Harvey NC, Cooper C, D'Angelo S, Curtis EM, Crozier SR, et al. 25‐hydroxyvitamin d response to gestational cholecalciferol supplementation is associated with common vitamin d related genetic variants: findings from the mavidos trial. Osteoporosis International 2017;28(Suppl 1):S70‐1. [Google Scholar]
- Moon RJ, Harvey NC, Cooper C, D'Angelo S, Curtis EM, Crozier SR, et al. Response to antenatal cholecalciferol supplementation is associated with common vitamin d related genetic variants. Journal of Clinical Endocrinology and Metabolism 2017;102(8):2941‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaur 1991 {published data only}
- Kaur J, Marya RK, Rathee S, lal H, Singh GP. Effect of pharmacological doses of vitamin D during pregnancy on placental protein status and birth weight. Nutrition Research 1991;11(9):1077‐81. [Google Scholar]
Li 2000a {published data only}
- Li X, Gou W. Study on prevention of pregnancy induced hypertension and effect of platelet intracellular free ca˜(2+) by calcium supplementation [补钙预防妊高征及对血小板细胞内游离钙浓度的影响]. Journal of Xi'an Medical University 2000;21(1):46‐8. [Google Scholar]
Mallet 1986 {published data only}
- Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstetrics & Gynecology 1986;68:300‐4. [DOI] [PubMed] [Google Scholar]
Marya 1987 {published data only}
- Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecologic and Obstetric Investigation 1987;24(1):38‐42. [DOI] [PubMed] [Google Scholar]
Marya 1988 {published data only}
- Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian Journal of Medical Research 1988;88:488‐92. [PubMed] [Google Scholar]
Mazurkevich 2013 {published data only}
- Mazurkevich M, Doronin G, Firsova T. State of placental complex during physiological pregnancy during correction of mineral insufficiency. Journal of Perinatal Medicine 2013;41(Suppl 1):Abstract no:1213. [Google Scholar]
Mirghafourvand 2013 {published data only}
- Mansouri A, Mirghafourvand M, Charandabi SM, Najafi M. The effect of vitamin d and calcium plus vitamin d on leg cramps in pregnant women: a randomized controlled trial. Journal of Research in Medical Sciences 2017;22(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirghafourvand M. The effect of vitamin D and calcium plus vitamin D for leg cramps in pregnant women: a randomised controlled trial. en.irct.ir/trial/10789 (first received 4 May 2013). [DOI] [PMC free article] [PubMed]
- Mirghafourvand M, Mohammad‐Alizadeh‐Charandabi S, Mansouri A, Najafi M, Khodabande F. The effect of vitamin D and calcium plus vitamin D on sleep quality in pregnant women with leg cramps: A controlled randomized clinical trial. Journal of Isfahan Medical School 2015;32(320):2444‐53. [Google Scholar]
- Mohammad‐Alizadeh‐Charandabi S, Mirghafourvand M, Mansouri A, Najafi M, Khodabande F. The effect of vitamin d and calcium plus vitamin d during pregnancy on pregnancy and birth outcomes: a randomized controlled trial. Journal of Caring Science 2015;4(1):35‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naghshineh 2016 {published data only}
- Naghshineh E, Sheikhaliyan S. Effect of vitamin D supplementation in the reduce risk of preeclampsia in nulliparous women. Advanced Biomedical Research 2016;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2010 {published data only}
- Akhtar E, Mily A, Haq A, Al‐Mahmud A, El‐Arifeen S, Hel Baqui A, et al. Prenatal high‐dose vitamin D3 supplementation has balanced effects on cord blood Th1 and Th2 responses. Nutrition Journal 2016;15(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D, Perumal N, Yazdanpanah M, Al Mahmud A, Baqui AH, Adeli K, et al. Maternal‐fetal‐infant dynamics of the C3‐epimer of 25‐hydroxyvitamin D. Clinical Biochemistry 2014;47(9):816‐22. [DOI] [PubMed] [Google Scholar]
- Dimitris MC, Perumal N, Craig‐Barnes HA, Leadley M, Mahmud AA, Baqui AH, et al. Effect of weekly high‐dose vitamin D3 supplementation on serum cholecalciferol concentrations in pregnant women. Journal of Steroid Biochemistry and Molecular Biology 2016;158:76‐81. [DOI] [PubMed] [Google Scholar]
- Harrington J, Perumal N, Al Mahmud A, Baqui A, Roth DE. Vitamin D and fetal‐neonatal calcium homeostasis: findings from a randomized controlled trial of high‐dose antenatal vitamin D supplementation. Pediatric Research 2014;76(3):302‐9. [DOI] [PubMed] [Google Scholar]
- Perumal N, Al Mahmud A, Baqui A, Raqib R, Roth D. Effect of high‐dose vitamin D supplementation on blood pressure during the third trimester of pregnancy: A randomized controlled trial in Bangladesh. American Journal of Epidemiology 2012;176(1):80, Abstract no: 2. [Google Scholar]
- Perumal N, Al Mahmud A, Baqui AH, Roth DE. Prenatal vitamin d supplementation and infant vitamin d status in Bangladesh. Public Health Nutrition 2017;20(10):1865‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal N, Mahmud A, Baqui A, Roth D. Prenatal vitamin D supplementation and infant vitamin D status in Bangladesh. FASEB Journal 2014;28(1 Suppl 1):[Abstract no. 256.4]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R, Ly A, Akhtar E, Mily A, Perumal N, Al‐Mahmud A, et al. Prenatal vitamin D3 supplementation suppresses LL‐37 peptide expression in ex vivo activated neonatal macrophages but not their killing capacity. British Journal of Nutrition 2014;112(6):908‐15. [DOI] [PubMed] [Google Scholar]
- Roth D. Antenatal vitamin D3 supplementation in Bangladesh: randomized controlled trial (AViDD‐2). clinicaltrials.gov/ct2/show/NCT01126528 (first received 19 May 2010).
- Roth DE, Al Mahmud A, Perumal N, Pezzack B, Raqib R, Baqui AH. Effect of prenatal third‐trimester vitamin D3 supplementation (35,000 IU/week) on fetal and infant linear growth in Bangladesh: the AViDD trial. Pediatric Academic Societies Annual Meeting; 2013 May 4‐7; Washington DC, USA. 2013.
- Roth DE, Al Mahmud A, Raqib R, Akhtar E, Perumal N, Pezzack B, et al. Randomized placebo‐controlled trial of high‐dose prenatal third‐trimester vitamin D3 supplementation in Bangladesh: the AViDD trial. Nutrition Journal 2013;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DE, Al Mahmud A, Raqib R, Baqui AH. Effects of high‐dose antenatal 3rd‐trimester vitamin D supplementation (35,000 IU/week) on maternal and newborn vitamin D status: A randomized placebo‐controlled trial in Dhaka, Bangladesh. FASEB Journal 2012;26:[Abstract no. 392.3]. [Google Scholar]
- Roth DE, Al‐Mahmud A, Arifeen S, Raqib R, Black RE, Baqui AH. Randomized pilot trial of two oral vitamin d3 supplementation regimens during the third trimester of pregnancy in Bangladeshi women: effects on neonatal vitamin d status and safety. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:1411.154.
- Roth DE, Perumal N, Al A, Baqui AH. Maternal vitamin D3 supplementation during the third trimester of pregnancy: Effects on infant growth in a longitudinal follow‐up study in Bangladesh. Journal of Pediatrics 2013;163(6):1605‐11. [DOI] [PubMed] [Google Scholar]
Roth 2013 {published data only}
- Morris SK, Pell LG, Rahman MZ, Dimitris MC, Mahmud A, Islam MM, et al. Maternal vitamin D supplementation during pregnancy and lactation to prevent acute respiratory infections in infancy in Dhaka, Bangladesh (MDARI trial): protocol for a prospective cohort study nested within a randomized controlled trial. BMC Pregnancy and Childbirth 2016;16(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. Maternal vitamin D for infant growth (MDIG) trial. https://clinicaltrials.gov/ct2/show/NCT01924013 (first received August 2013).
- Roth D, Mahmud AA, Morris S, Zlotkin S, Gernand A, Tahmeed A, et al. Maternal vitamin D supplementation during pregnancy and lactation to promote infant growth in Dhaka, Bangladesh (mdig trial): a randomized controlled trial. Annals of Nutrition and Metabolism 2017;71(Suppl 2):571. [Google Scholar]
- Roth DE, Gernand AD, Morris SK, Pezzack B, Islam MM, Dimitris MC, et al. Maternal vitamin D supplementation during pregnancy and lactation to promote infant growth in Dhaka, Bangladesh (MDIG trial): study protocol for a randomized controlled trial. Trials 2015;16:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DE, Morris SK, Zlotkin S, Gernand AD, Ahmed T, Shanta SS, et al. Vitamin D supplementation in pregnancy and lactation and infant growth. New England Journal of Medicine 2018;379(6):535‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabet 2012 {published data only}
- Sabet Z, Ghazi AA, Tohidi M, Oladi B. Vitamin D supplementation in pregnant Iranian women: Effects on maternal and neonatal vitamin D and parathyroid hormone status. Acta Endocrinologica 2012;8(1):59‐66. [Google Scholar]
Sablok 2015 {published data only}
- Sablok A, Batra A, Thariani K, Batra A, Bharti R, Aggarwal AR, et al. Supplementation of vitamin D in pregnancy and its correlation with feto‐maternal outcome. Clinical Endocrinology 2015;83(4):536‐41. [DOI] [PubMed] [Google Scholar]
Samimi 2016 {published data only}
- Samimi M, IRCT201102135444N3. Effects of calcium and vitamin D co‐supplementation on pre‐eclampsia parameters, metabolic status and biomarkers of oxidative stress in pregnant women at risk for pre‐eclampsia. en.search.irct.ir/view/5288 (first received 31 January 2015).
- Samimi M, Kashi M, Foroozanfard F, Karamali M, Bahmani F, Asemi Z, et al. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre‐eclampsia. Journal of Human Nutrition and Dietetics 2016;29(4):505‐16. [DOI] [PubMed] [Google Scholar]
Samimi 2017 {published data only}
- Samimi M, Foroozanfard F, Amini F, Sehat M. Effect of vitamin D supplementation on unexplained recurrent spontaneous abortion: a double‐blind randomized controlled trial. Global Journal of Health Science 2017;9(3):95. [Google Scholar]
Sasan 2017 {published data only}
- Sasan SB, Zandvakili F, Soufizadeh N, Baybordi E. The effects of vitamin d supplement on prevention of recurrence of preeclampsia in pregnant women with a history of preeclampsia. Obstetrics and Gynecology International 2017;2017:8249264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahgheibi 2016 {published data only}
- Shahgheibi S, Farhadifar F, Pouya B. The effect of vitamin D supplementation on gestational diabetes in high‐risk women: Results from a randomized placebo‐controlled trial. Journal of Research in Medical Sciences 2016;21(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2015 {published data only}
- Singh J, Hariharan C, Bhaumik D. Role of vitamin D in reducing the risk of preterm labour. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2015;1:86‐93. [Google Scholar]
Taherian 2002 {published data only}
- Taherian AA, Taherian A, Shirmani A. Prevention of preeclampsia with low‐dose aspirin or calcium supplementation. Archives of Iranian Medicine 2002;5(3):151‐6. [Google Scholar]
Tehrani 2014 {published data only}
- Tehrani HG. Comparison of effectiveness of vitamin D supplementation in decreasing the development of the gestational diabetes mellitus in pregnant women. en.irct.ir/trial/7997 (first received 3 January 2014).
- Tehrani HG, Mostajeran F, Banihashemi B. Effect of vitamin d supplementation on the incidence of gestational diabetes. Advanced Biomedical Research 2017;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaziri 2016 {published data only}
- Vaziri F, Dabbaghmanesh MH, Samsami A, Nasiri S, Shirazi PT. Vitamin D supplementation during pregnancy on infant anthropometric measurements and bone mass of mother‐infant pairs: A randomized placebo clinical trial. Early Human Development 2016;103:61‐8. [DOI] [PubMed] [Google Scholar]
- Vaziri F, Nasiri S, Tavana Z, Dabbaghmanesh MH, Sharif F, Jafari P. A randomized controlled trial of vitamin D supplementation on perinatal depression: in Iranian pregnant mothers. BMC Pregnancy and Childbirth 2016;16:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2008 {published data only}
- Goldring S. Effects of prenatal vitamin D supplementation on respiratory and allergic phenotypes and bone density in the first three years of life. UKCRN (http://public.ukcrn.org.uk) [accessed 6 April 2011] 2010.
- Goldring ST, Griffiths CJ, Martineau AR, Robinson S, Yu C, Poulton S, et al. Prenatal vitamin D supplementation and child respiratory health: a randomised controlled trial. PLOS One 2013;8(6):e66627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M, Goldring S, Griffiths C, Shaheen SO, Martineau A, Cross L, et al. Effects of pre‐natal vitamin D supplementation with partial correction of vitamin D deficiency on early life healthcare utilisation: A randomised controlled trial. PLOS One 2015;10(2):e0145303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Newton L, Robinson S, Teoh TG, Sethi M. Vitamin D deficiency and supplementation in pregnant women of four ethnic groups. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa68. [Google Scholar]
- Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology 2009;70(5):685‐90. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ala‐Houhala 1986 {published data only}
- Ala‐Houhala M, Koskinen T, Terho A, Koivula T, Visakorpi J. Maternal compared with infant vitamin D supplementation. Archives of Disease in Childhood 1986;61:1159‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asemi 2013b {published data only}
- Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double‐blind randomized controlled clinical trial. American Journal of Clinical Nutrition 2013;98(6):1425‐32. [DOI] [PubMed] [Google Scholar]
- Asemi Z, Karamali M, Esmaillzadeh A. Favorable effects of vitamin d supplementation on pregnancy outcomes in gestational diabetes: a double blind randomized controlled clinical trial. Hormone and Metabolic Research 2015;47(8):565‐70. [DOI] [PubMed] [Google Scholar]
Asemi 2014 {published data only}
- Asemi Z, Karamali M, Esmaillzadeh A. Effects of calcium‐vitamin D co‐supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: a randomised placebo‐controlled trial. Diabetologia 2014;57(9):1798‐806. [DOI] [PubMed] [Google Scholar]
Asemi 2015 {published data only}
- Asemi Z, Esmaillzadeh A. The effect of multi mineral‐vitamin D supplementation on pregnancy outcomes in pregnant women at risk for pre‐eclampsia. International Journal of Preventive Medicine 2015;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkinson 2010 {published data only}
- Atkinson SA, Moore C. Bone Health in Pregnancy (B‐Hip). clinicaltrials.gov/ct2/show/NCT01693510 (first received 26 September 2012).
Azami 2017 {published data only}
- Azami M, Azadi T, Farhang S, Rahmati S, Pourtaghi K. The effects of multi mineral‐vitamin D and vitamins (C+E) supplementation in the prevention of preeclampsia: An RCT. International Journal of Reproductive Biomedicine (Yazd, Iran) 2017;15(5):273‐8. [PMC free article] [PubMed] [Google Scholar]
- Azami M, IRCT2016062528617N1. The effects of multi minerals (Zn, Mg and Ca) and vitamins (C and E) supplementation in the prevention of preeclampsia. en.search.irct.ir/view/31125 (first received 24 September 2016).
Baqui 2009 {published data only}
- Baqui A. Antenatal vitamin d supplementation to improve neonatal health outcomes in Dhaka, Bangladesh: preliminary dose‐finding and safety study. clinicaltrials.gov/ct2/show/NCT00938600 (first received 14 July 2009). [NCT00938600]
- Roth DE, Al A, Raqib R, Akhtar E, Black RE, Baqui AH. Pharmacokinetics of high‐dose weekly oral vitamin D3 supplementation during the third trimester of pregnancy in Dhaka, Bangladesh. Nutrients 2013;5(3):788‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DE, Al Mahmud A, Raqib R, Black RE, Baqui AH. Pharmacokinetics of a single oral dose of vitamin D3 (70,000 IU) in pregnant and non‐pregnant women. Nutrition Journal 2012;11(114):doi: 10.1186/1475‐2891‐11‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DE, Al‐Mahmud A, El S, Raqib R, Black RE, Baqui AH. Randomized open‐label trial of two weekly oral vitamin D3 supplementation regimens during the third trimester of pregnancy in Bangladeshi women: Effects on maternal vitamin D status and safety. FASEB Journal 2011;25(Suppl):236.6. [Google Scholar]
Bhatia 2010 {published data only}
- Bhatia V. A study of the effect of vitamin D supplementation in pregnant women, on the growth and biochemical features of the newborn baby and infant. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=1674&EncHid=&modid=&compid= (first received 19 May 2010). [CTRI/2010/091/000499]
- Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, et al. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. British Journal of Nutrition 2012;108(6):1052‐8. [DOI] [PubMed] [Google Scholar]
- Sahu M, Das V, Aggarwal A, Rawat V, Saxena P, Bhatia V. Vitamin D replacement in pregnant women in rural north India: a pilot study. European Journal of Clinical Nutrition 2009;63(9):1157‐9. [DOI] [PubMed] [Google Scholar]
Bhatia 2012 {published data only}
- Bhatia V. Vitamin D supplementation in pregnancy: regimens and long term effects on offspring. Clinical Trials Registry ‐ India CTRI/2012/02/002395 2012. [CTRI/2012/02/002395]
- Bhatia V, Katam K, Agarwal A, Das V, Ramesh V. Vitamin D supplementation in pregnancy and its effect on cord blood 25 hydroxycholecalciferol and anthropometry of the newborn. Hormone Research in Paediatrics 2013;80(Suppl 1):220. [Google Scholar]
- Sahoo SK, Katam KK, Das V, Agarwal A, Bhatia V. Maternal vitamin d supplementation in pregnancy and offspring outcomes: a double‐blind randomized placebo‐controlled trial. Journal of Bone and Mineral Metabolism 2017;35(4):464‐71. [DOI] [PubMed] [Google Scholar]
Bisgaard 2009 {published data only}
- Bisgaard H. Vitamin D supplementation during pregnancy for prevention of asthma in childhood (ABCvitaminD). clinicaltrials.gov/ct2/show/NCT00856947 (first received 6 March 2009).
- Chawes B, Bønnelykke K, Stokholm J, Heickendorff L, Brix S, Rasmussen M, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomised clinical trial. Clinical and Translational Allergy 2016;6:6 [OP10]. [DOI] [PubMed] [Google Scholar]
- Chawes BL, Bonnelykke K, Stokholm J, Vissing NH, Bjarnadottir E, Schoos AM, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA 2016;315(4):353‐61. [DOI] [PubMed] [Google Scholar]
Chandy 2016 {published data only}
- Chandy DD, Kare J, Singh SN, Agarwal A, Das V, Singh U, et al. Effect of vitamin D supplementation, directly or via breast milk for term infants, on serum 25 hydroxyvitamin D and related biochemistry, and propensity to infection: a randomised placebo‐controlled trial. British Journal of Nutrition 2016;116(1):52‐8. [DOI] [PubMed] [Google Scholar]
Cockburn 1980 {published data only}
- Cockburn F, Belton NR, Purvis RJ, Giles MM, Brown JK, Turner TL, et al. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. British Medical Journal 1980;281(6232):11‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czech‐Kowalska 2013 {published data only}
- Czech‐Kowalska J, Latka‐Grot J, Bulsiewicz D, Jaworski M, Pludowski P, Chazan B, et al. Influence of vitamin D supplementation on vitamin D status and bone mass during lactation ‐ double blinded randomized control trial. Annals of Nutrition & Metabolism 2013;63(Suppl 1):796, Abstract no: PO1128. [Google Scholar]
Das 2010 {published data only}
- Das V. Vitamin D and calcium nutrition in pregnancy‐evaluation of optimal supplementation dose of vitamin D during antenatal period. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1553 (first received 2 June 2010). [CTRI/2010/091/000352]
Dawodu 2013 {published data only}
- Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. Journal of Clinical Endocrinology & Metabolism 2013;98(6):2337‐46. [DOI] [PubMed] [Google Scholar]
de Menibus 1984 {published data only}
- Menibus CH, Mallet E, Henocq A, Lemeur H. [Should vitamin d supplements be given to pregnant women?]. Bulletin De L'academie Nationale De Medecine 1984;168(7‐8):909‐16. [PubMed] [Google Scholar]
Etemadifar 2015 {published data only}
- Etemadifar M, Janghorbani M. Efficacy of high‐dose vitamin D3 supplementation in vitamin D deficient pregnant women with multiple sclerosis: Preliminary findings of a randomized‐controlled trial. Iranian Journal of Neurology 2015;14(2):67‐73. [PMC free article] [PubMed] [Google Scholar]
Gerais 2015 {published data only}
- Gerais AS, Hussein S. Vitamin D deficiency in pregnancy and pregnancy outcome. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E352. [Google Scholar]
Hashemipour 2014 {published data only}
- Abotorabi S, Hashemi poor S, Esmailzadehha N, Ziaee A, Khoeiniha MH. Effect of treatment with Vitamin D on maternal and neonatal indices in pregnant women with hypocalcemia: a randomized controlled trial. International Journal of Pediatrics 2017;5(9):5733‐9. [Google Scholar]
- Hashemipour S. Effect of treatment of vitamin D deficiency during pregnancy on hypocalcemia. clinicaltrials.gov/ct2/show/NCT02021864 (first received 27 December 2013).
- Hashemipour S, Ziaee A, Javadi A, Movahed F, Elmizadeh K, Javadi EH, et al. Effect of treatment of vitamin D deficiency and insufficiency during pregnancy on fetal growth indices and maternal weight gain: a randomized clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2014;172:15‐9. [DOI] [PubMed] [Google Scholar]
Hossain 2012 {published data only}
- Hossain N, Kanani F, Khanani R, Ayaz S, Pal L. Effect of maternal supplementation with vitamin D during pregnancy on neonatal serum vitamin D levels and anthropometric measurements. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S372. [Google Scholar]
- Hossain N, Kanani FH, Ramzan S, Kausar R, Ayaz S, Khanani R, et al. Obstetric and neonatal outcomes of maternal vitamin D supplementation: results of an open‐label, randomised controlled trial of antenatal vitamin D supplementation in Pakistani women. Journal of Clinical Endocrinology and Metabolism 2013;99(7):2448‐55. [DOI] [PubMed] [Google Scholar]
Ito 1994 {published data only}
- Ito M, Koyama H, Ohshige A, Maeda T, Yoshimura T, Okamura H. Prevention of preeclampsia with calcium supplementation and vitamin D3 in an antenatal protocol. International Journal of Gynecology & Obstetrics 1994;47(2):115‐20. [DOI] [PubMed] [Google Scholar]
Jamilian 2016 {published data only}
- Jamilian M, Karamali M, Taghizadeh M, Sharifi N, Jafari Z, Memarzadeh MR, et al. Vitamin D and evening primrose oil administration improve glycemia and lipid profiles in women with gestational diabetes. Lipids 2016;51(3):349‐56. [DOI] [PubMed] [Google Scholar]
Jamilian 2017 {published data only}
- Jamilian M, Samimi M, Ebrahimi FA, Hashemi T, Taghizadeh M, Razavi M, et al. The effects of vitamin D and omega‐3 fatty acid co‐supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. Journal of Clinical Lipidology 2017;11(2):459‐68. [DOI] [PubMed] [Google Scholar]
Kachhawa 2014 {published data only}
- Gupta T, Kachhawa G, Sharma H, Bajpai J, Kulshreshtha V, Khadgawat R, et al. A randomized double blind controlled trial to investigate the effects of vitamin D supplementation on maternal and new‐born baby's vitamin D status in Asian‐Indian subjects. Indian Journal of Endocrinology and Metabolism 2017;21(8 Suppl 2):S44‐S45. [Google Scholar]
- Kachhawa G. A randomized controlled trial to investigate the effects of vitamin D supplementation on maternal and new‐born baby's vitamin D status in Asian‐Indian subjects ‐ VIDIMAN. http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=8544&EncHid=&modid=&compid=%27,%278544det%27 (first received 13 June 2014).
Karamali 2014 {published data only}
- Karamali M, Asemi Z, Ahmadi‐Dastjerdi M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects pregnancy outcomes in gestational diabetes: randomized, double‐blind, placebo‐controlled trial. Public Health Nutrition 2016;19(1):156‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamali M, IRCT201407115623N23. Effect of vitamin D plus calcium co‐supplementation on pregnancy outcomes in gestational diabetes. en.search.irct.ir/view/19118 (first received 21 July 2014).
Karamali 2015 {published data only}
- Karamali M, Beihaghi E, Mohammadi AA, Asemi Z. Effects of high‐dose vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre‐eclampsia. Hormone and Metabolic Research 2015;47(12):867‐82. [DOI] [PubMed] [Google Scholar]
Kermack 2017 {published data only}
- Kermack AJ, Calder PC, Macklon NS, Fisk HL, Houghton FD, Lowen PK, et al. Prepare trial: a randomised double blinded controlled trial of a preconception omega 3 and vitamin d rich dietary supplement in couples undergoing assisted reproduction treatment. Human Reproduction 2017;32:i290. [Google Scholar]
Kiely 2015 {published data only}
- Kiely M. Randomized controlled trial to determine the nutritional requirement for vitamin d for prevention of deficiency during pregnancy and in the early neonatal period (D‐MAT). clinicaltrials.gov/ct2/show/NCT02506439 (first received first received 23 July 2015). [NCT02506439]
- O'Callaghan K, Hennessy A, Dowling KG, Hull G, Kenny LC, Cashman KD, et al. Estimation of the dietary requirement for vitamin D in pregnancy: a dose‐response, double‐blind, randomized placebo‐controlled trial. Annals of Nutrition and Metabolism 2017;71(Suppl 2):624. [Google Scholar]
- O'Callaghan K, Hennessy A, Kiely M, Kenny LC. Habitual calcium and vitamin D intakes in pregnant Irish women; preliminary data from the DMAT randomised controlled trial. Proceedings of the Nutrition Society 2016;75(OCE3):E191. [Google Scholar]
- O'Callaghan KM, Hennessy A, Hull GL, Healy K, Ritz C, Kenny LC, et al. Estimation of the maternal vitamin D intake that maintains circulating 25‐hydroxyvitamin D in late gestation at a concentration sufficient to keep umbilical cord sera >/=25‐30 nmol/L: a dose‐response, double‐blind, randomized placebo‐controlled trial in pregnant women at northern latitude. American Journal of Clinical Nutrition 2018;108(1):77‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lalooha 2012 {published data only}
- Lalooha F. The effect of vitamin D supplementation during pregnancy on newborn's anthropometric index. en.irct.ir/trial/10079IRCT (first received 16 August 2012).
Li 2016 {published data only}
- Li Q, Xing B. Vitamin D3‐supplemented yogurt drink improves insulin resistance and lipid profiles in women with gestational diabetes mellitus: a randomized double blinded clinical trial. Annals of Nutrition & Metabolism 2016;68(4):285‐90. [DOI] [PubMed] [Google Scholar]
MacDonald 1986 {published data only}
- MacDonald HN. Fetal and maternal benefits from calcium and vitamin D supplementation of pregnant Asians. Personal communication 1986.
March 2010 {published data only}
- Anon. Erratum for March et al. Maternal vitamin D3 supplementation at 50 μg/d protects against low serum 25‐hydroxyvitamin D in infants at 8 wk of age: a randomised controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. Am J Clin Nutr 2015;102:402–10. American Journal of Clinical Nutrition 2016;104(5):1491. [DOI] [PubMed] [Google Scholar]
- Chen NN, March K, Innis SM, Shand A, Dadelszen P, Lyon P, et al. The effect of vitamin D supplementation during pregnancy and lactation on maternal and infant 25‐hydroxyvitamin D (25OHD) concentration. FASEB Journal 2013; Vol. 27, issue Suppl 1.
- March K. Vitamin D dose‐response study throughout pregnancy and lactation. clinicaltrials.gov/ct2/show/NCT01112891 (first received 29 April 2010).
- March KM, Chen NN, Karakochuk CD, Shand AW, Innis SM, Dadelszen P, et al. Maternal vitamin D3 supplementation at 50 ug/d protects against low serum 25‐hydroxyvitamin D in infants at 8 wk of age: a randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. American Journal of Clinical Nutrition 2015;102(2):402‐10. [DOI] [PubMed] [Google Scholar]
Marya 1981 {published data only}
- Marya RK, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecologic and Obstetric Investigation 1981;12:155‐61. [DOI] [PubMed] [Google Scholar]
McLean 2012 {published data only}
- McLean M. Effect of high‐dose versus low‐dose vitamin D supplementation in pregnancy on maternal glucose metabolism and the risk of gestational diabetes. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=308236 (first received 30 October 2012).
Mojibian 2015 {published data only}
- Mojibian M. The effect of 50000 IU Vitamin D supplement administered two weekly on neonatal and pregnant women outcome. en.irct.ir/trial/7525 (first received 27 February 2012). [IRCT201107237097N1]
- Mojibian M, Soheilykhah S, Fallah Zadeh MA, Jannati Moghadam M. The effects of vitamin D supplementation on maternal and neonatal outcome: a randomized clinical trial. Iranian Journal of Reproductive Medicine 2015;13(11):687‐96. [PMC free article] [PubMed] [Google Scholar]
Mozzafari 2010 {published data only}
- Hosseinzadeh‐Shamsi‐Anar M, Mozaffari‐Khosravi H, Salami MA, Hadinedoushan H, Mozayan MR. The efficacy and safety of a high dose of vitamin d in mothers with gestational diabetes mellitus: A randomized controlled clinical trial. Iranian Journal of Medical Sciences 2012;37(3):159‐65. [PMC free article] [PubMed] [Google Scholar]
- Mozaffari H. Effect of vitamin D supplementation on glucose status, lipid profiles and inflammatory factors in mothers with a history of gestational diabetes. en.irct.ir/trial/3967 (first received 21 May 2010).
- Mozaffari‐Khosravi H, Hosseinzadeh‐Shamsi‐Anar M, Salami MA, Hadinedoushan H, Mozayan MR. Effects of a single post‐partum injection of a high dose of vitamin D on glucose tolerance and insulin resistance in mothers with first‐time gestational diabetes mellitus. Diabetic Medicine 2012;29(1):36‐42. [DOI] [PubMed] [Google Scholar]
Mutlu 2013 {published data only}
- Mutlu GY, Ozsu E, Kalaca S, Yuksel A, Cizmecioglu FM, Hatun S. The evaluation of vitamin D supplementation dose during pregnancy in a high‐risk population. Hormone Research in Paediatrics 2013;80(Suppl 1):92. [DOI] [PubMed] [Google Scholar]
- Mutlu GY, Ozsu E, Kalaca S, Yuksel A, Pehlevan Y, Cizmecioglu F, et al. Evaluation of vitamin D supplementation doses during pregnancy in a population at high risk for deficiency. Hormone Research in Paediatrics 2014;81(6):402‐8. [DOI] [PubMed] [Google Scholar]
Nausheen 2014 {published data only}
- Nausheen S. Assessment of dose effectiveness of vitamin D supplementation during pregnancy. clinicaltrials.gov/ct2/show/NCT02215213 (first received 13 August 2014).
Niramitmahapanya 2017 {published data only}
- Niramitmahapanya S, Kaoiean S, Sangtawesin V, Bordeerat NK, Deerochanawong C. Correlation of 25‐hydroxyvitamin d levels in serum vs breastmilk in vitamin d‐supplementation breastfeeding women during lactation: randomized double blinded control trial. Endocrine Reviews 2017; Vol. 38, issue 3. [PubMed]
Pandey 2015 {published data only}
- Pandey U, Divakar H. Role of vitamin D in iron metabolism, a pilot study. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2015;4(5):1494‐8. [Google Scholar]
Qian 2015 {published data only}
- Qian L, Wang H, Wu F, Li M, Chen W, Lv L. Vitamin D3 alters toll‐like receptor 4 signaling in monocytes of pregnant women at risk for preeclampsia. International Journal of Clinical and Experimental Medicine 2015;8(10):18041‐9. [PMC free article] [PubMed] [Google Scholar] [Retracted]
Razavi 2017 {published data only}
- Razavi M, Jamilian M, Samimi M, Afshar Ebrahimi F, Taghizadeh M, Bekhradi R, et al. The effects of vitamin d and omega‐3 fatty acids co‐supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutrition & Metabolism 2017;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rostami 2018 {published data only}
- Rostami M, Ramezani Tehrani F, Simbar M, Bidhendi Yarandi R, Minooee S, Hollis BW, et al. Effectiveness of prenatal vitamin D deficiency screening and treatment program: a stratified randomized field trial. Journal of Clinical Endocrinology and Metabolism 2018; Vol. 103, issue 8:2936–48. [DOI] [PubMed]
- Rostami M, Ramezani Tehrani F, Simbar M, Hosseinpanah F, Alavi Majd H. Rationale and design of Khuzestan vitamin D deficiency screening program in pregnancy: a stratified randomized vitamin D supplementation controlled trial. JMIR Research Protocols 2017;6(4):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakiba 2013 {published data only}
Shi 2017 {published data only}
- Shi DD, Wang Y, Guo JJ, Zhou L, Wang N. Vitamin d enhances efficacy of oral nifedipine in treating preeclampsia with severe features: a double blinded, placebo‐controlled and randomized clinical trial. Frontiers in Pharmacology 2017;8:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simsek 2011 {published data only}
- Simsek S. Vitamin D supplementation in gestational diabetes mellitus. trialregister.nl/trialreg/admin/rctview.asp?TC=3158 (first received 21 November 2011).
Soheilykhah 2013 {published data only}
- Soheilykhah S. Effect of different doses of vitamin D on insulin resistance in pregnant women attending in Shahid Sadoughi and Mojibian prenatal clinics. en.irct.ir/trial/3385 (first received 23 May 2011). [IRCT138811203312N1]
- Soheilykhah S, Mojibian M, Moghadam MJ, Shojaoddiny‐Ardekani A. The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecological Endocrinology 2013;29(4):396‐9. [DOI] [PubMed] [Google Scholar]
Stephensen 2011 {published data only}
- Stephensen CB. Effects of vitamin D supplementation during pregnancy on clinical outcomes and immune function. clinicaltrials.gov/ct2/show/NCT01417351 (first received August 2011).
- Zerofsky M, Jacoby B, Pedersen TL, Stephensen CB. Effects of a randomized, controlled trial of daily vitamin D3 supplementation during pregnancy on regulatory immunity and inflammation. FASEB Journal 2016;30(Suppl 1):296.7. [DOI] [PubMed] [Google Scholar]
- Zerofsky M, Jacoby B, Stephensen C. A randomized controlled trial of vitamin D supplementation in pregnancy: Effects on vitamin D status and clinical outcomes. FASEB Journal 2014;28(1 Suppl 1):[Abstract no. 1041.5]. [Google Scholar]
- Zerofsky MS, Jacoby BN, Pedersen TL, Stephensen CB. Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized controlled trial. Journal of Nutrition 2016;146(11):2388‐97. [DOI] [PubMed] [Google Scholar]
Sudfeld 2017 {published data only}
- Sudfeld CR, Manji KP, Duggan CP, Aboud S, Muhihi A, Sando DM, et al. Effect of maternal vitamin d3 supplementation on maternal health, birth outcomes, and infant growth among hiv‐infected tanzanian pregnant women: study protocol for a randomized controlled trial. Trials 2017;18(1):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taheri 2014 {published data only}
- Taheri M, Baheiraei A, Rahimi A, Modarres M. Resolving vitamin d deficiency in the preconception period among high‐risk reproductive women: A randomized controlled trial. Iranian Red Crescent Medical Journal 2014;16(1):e11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thiele 2014 {published data only}
- Anderson CM, Thiele DK, Ralph JL, Perley D, OHM JE. Vitamin D supplementation and DNA methylation patterns during pregnancy in and lactation in mothers and infants. FASEB Journal 2016;30(Suppl 1):Abstract no: 1028.3. [Google Scholar]
- Keesling Thiele D. The Impact of Continuous Prenatal and Early Postpartum Maternal Vitamin D Supplementation on the Vitamin D status of Exclusively Breastfed Infants [thesis]. University of North Dakota, 2013. [Google Scholar]
- Thiele D, Anderson CM. CS_018. Maternal vitamin D supplementation increases infant vitamin D status at birth but the impact diminishes during breastfeeding. 17th Conference of the International Society for Research in Human Milk and Lactation (ISRHML); 2014 Oct 23‐27; Kiawah Island, South Carolina, USA. 2014:122.
- Thiele DK, Ralph J, El‐Masri M, Anderson CM. Vitamin d3 supplementation during pregnancy and lactation improves vitamin d status of the mother‐infant dyad. Journal of Obstetric, Gynecologic, and Neonatal Nursing : Jognn 2017;46:135‐47. [DOI] [PubMed] [Google Scholar]
Valizadeh 2016 {published data only}
- Valizadeh M, Piri Z, Mohammadian F, Kamali K, Amir Moghadami HR. The impact of vitamin D supplementation on post‐partum glucose tolerance and insulin resistance in gestational diabetes: A randomized controlled trial. International Journal of Endocrinology and Metabolism 2016;14(2):e34312. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Hurst 2009 {published data only}
- Hurst PR. The role of vitamin D in metabolism and bone health. A thesis presented in partial fulfilment of the requirements for the Degree of Doctor of Philosophy in Nutritional Science. Vol. 1, Albany: Massey University, 2009. [Google Scholar]
- Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient ‐ a randomised, placebo‐controlled trial. British Journal of Nutrition 2010;103(4):549‐55. [DOI] [PubMed] [Google Scholar]
- Hurst PR, Stonehouse W, Matthys C, Conlon C, Kruger MC, Coad J. Study protocol‐‐metabolic syndrome, vitamin D and bone status in South Asian women living in Auckland, New Zealand: a randomised, placebo‐controlled, double‐blind vitamin D intervention. BMC Public Health 2008;8:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2006a {published data only}
- Appelgren KE, Nietert PJ, Hulsey TC, Hollis BW, Wagner CL. Analyzing adherence to prenatal supplement: does pill count measure up?. International Journal of Endocrinology 2010;2010:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: Double‐blind, randomized clinical trial of safety and effectiveness. Journal of Bone and Mineral Research 2011;26(10):2341‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL. Evaluation of vitamin D requirements during pregnancy (ongoing trial). clinicaltrials.gov/ct2/show/NCT00292591 (first received 16 February 2006). [NCT00292591]
- Wagner CL, Johnson D, Hulsey TC, Ebeling M, Shary J, Smith PG, et al. Vitamin D supplementation during pregnancy part 2 NICHD/CTSA randomized clinical trial (RCT): outcomes. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Wagner CL, Johnson D, Hulsey TC, Ebeling M, Shary J, Smith PG, et al. Vitamin D supplementation during pregnancy Part I NICHD/CTSA randomized clinical trial (RCT): safety consideration. Pediatric Academic Societies Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Wei W, Shary JR, Garrett‐Mayer E, Anderson B, Forestieri NE, Hollis BW, et al. Bone mineral density during pregnancy in women participating in a randomized controlled trial of vitamin d supplementation. American Journal of Clinical Nutrition 2017;106(6):1422‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2006b {published data only}
- Wagner CL. Vitamin D status of pregnant women and their children in Eau Claire, South Carolina: a prevalence and supplementation model for community health care centers in the U.S. clinicaltrials.gov/ct2/show/NCT00412087 (first received 15 December 2006).
- Wagner CL, McNeil R, Hamilton SA, Davis DJ, Prudgen C, Winkler J, et al. Vitamin D (vitD) supplementation during pregnancy: Thrasher Research Fund RCT in SC community center networks. Pediatric Academic Societies 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. American Journal of Obstetrics and Gynecology 2013;208(2):137.e1‐137.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, et al. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: A combined analysis. Journal of Steroid Biochemistry & Molecular Biology 2013;136:313‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL, Sen S, Ebeling M, Hollis BW. BMI and vitamin D status are inversely related in breastfeeding mothers through 7 months. Breastfeeding Medicine 2016;11(2):A‐75, Abstract no:P‐121. [Google Scholar]
Wagner 2013 {published data only}
- Moore RS, Mulligan JK, Harmon H, Hollis BW, Wagner CL. Immune mediators and vitamin D status in the development of comorbidities of pregnancy. Pediatric Academic Societies Annual Meeting; 2016 April 30‐May 3; Baltimore, USA. 2016:4109.85.
- Schulz EV, Wagner CL, Cruze L, Wei W, Gehris J. Maternal vitamin d sufficiency and reduced placental gene expression in angiogenic biomarkers related to comorbidities of pregnancy. Journal of Steroid Biochemistry and Molecular Biology 2017;173:273‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL. Preventing health disparities during pregnancy through vitamin D supplementation. clinicaltrials.gov/ct2/show/NCT01932788 (first received 30 August 2013).
Weiss 2009 {published data only}
- Al‐Garawi A, Carey VJ, Chhabra D, Mirzakhani H, Morrow J, Lasky‐Su J, et al. The role of vitamin D in the transcriptional program of human pregnancy. PLOS One 2016;11(10):e0163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Garawi A, Carey VJ, Chhabra D, Morrow J, Lasky‐Su J, Koh A, et al. Differentially expressed genes during the course of pregnancy and their correlation with maternal vitamin d levels. American Journal of Respiratory and Critical Care Medicine 2015;191:A5994. [Google Scholar]
- Al‐Garawi A, Carey VJ, Qiu W, Mirzakhani H, Litonjua AA, Weiss ST. Cord blood vitamin D levels and gene expression profiles at birth are associated with wheezing in the first year of life: results from the vitamin D antenatal asthma reduction trial (vdaart). American Journal of Respiratory and Critical Care Medicine 2016;193:A3168. [Google Scholar]
- Bhaskaran K, Smeeth L, Evans S. Prenatal vitamin d and offspring wheezing. JAMA 2016;315(24):2730. [DOI] [PubMed] [Google Scholar]
- Blighe K, Chawes BL, Kelly RS, Mirzakhani H, McGeachie M, Litonjua AA, et al. Vitamin d prenatal programming of childhood metabolomics profiles at age 3 y. American Journal of Clinical Nutrition 2017;106(4):1092‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotegut CA. Prevention of preeclampsia. Journal of Clinical Investigation 2016;126(12):4396‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby E, Pfeffer PE, Hawrylowicz C, Laranjo N, Litonjua AA, Weiss ST, et al. Vitamin d supplementation during pregnancy: effect on the neonatal immune system in a randomized controlled trial. Journal of Allergy and Clinical Immunology 2018;141(1):269. [DOI] [PubMed] [Google Scholar]
- Litonjua A, Weiss T. Prenatal vitamin d and offspring wheezing‐‐reply. JAMA 2016;315(24):2731. [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O'Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA 2016;315(4):362‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, O'Connor GT, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): Rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemporary Clinical Trials 2014;38:37‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein D, D'Amico F. Prenatal vitamin d and offspring wheezing. JAMA 2016;315(24):2731. [DOI] [PubMed] [Google Scholar]
- Mirzakhani H, O'Connor G, Bacharier LB, Zeiger RS, Schatz MX, Weiss ST, et al. Asthma control status in pregnancy, body mass index, and maternal vitamin D levels. Journal of Allergy and Clinical Immunology 2017;140(5):1453‐6.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzakhani H, O'Connor GT, Bacharier L, Zeiger RS, Schatz MX, Weiss S, et al. Asthma control status during pregnancy and its relationship with vitamin D level: an observation from the vitamin D antenatal asthma reduction trial (VDAART). American Journal of Respiratory and Critical Care Medicine 2017; Vol. 195, issue no pagination.
- Sordillo JE, McGeachie MJ, Ziniti J, Lange N, Laranjo N, Carey V, et al. Factors influencing the infant gut microbiome at age 3‐6 months: findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). Journal of Allergy and Clinical Immunology 2017;139(2):482‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NCT. Randomized trial: maternal vitamin d supplementation to prevent childhood asthma (vdaart). clinicaltrials.gov/ct2/show/NCT00920621 (first received 15 June 2009).
- Wolsk HM, Harshfield BJ, Laranjo N, Carey VJ, O'Connor GT, Sandel M, et al. Vitamin d supplementation in pregnant women of different races and the risk of asthma/recurrent wheeze in the child: findings from the vitamin d antenatal asthma reduction trial (vdaart). American Journal of Respiratory and Critical Care Medicine 2016;193:A3169. [Google Scholar]
- Wolsk HM, Harshfield BJ, Laranjo N, Carey VJ, Weiss ST, Litonjua AA, et al. Vitamin d supplementation in pregnancy, prenatal 25(oh)d levels, race, and subsequent asthma or recurrent wheeze in offspring: secondary analyses from the vitamin d antenatal asthma reduction trial. Journal of Allergy and Clinical Immunology 2017;140(5):1423. [DOI] [PubMed] [Google Scholar]
Yap 2014 {published data only}
- Yap C, Cheung NW, Gunton JE, Athayde N, Munns CF, Duke A, et al. Erratum. Vitamin d supplementation and the effects on glucose metabolism during pregnancy: a randomized controlled trial. Diabetes care 2014;37:1837‐1844. Diabetes Care 2015;38(10):1992. [DOI] [PubMed] [Google Scholar]
- Yap C, Cheung NW, Gunton JE, Athayde N, Munns CF, Duke A, et al. Vitamin D supplementation and the effects on glucose metabolism during pregnancy: a randomized controlled trial. Diabetes Care 2014;37(7):1837‐44. [DOI] [PubMed] [Google Scholar]
Yazdchi 2016 {published data only}
- Yazdchi R, Gargari BP, Asghari‐Jafarabadi M, Sahhaf F. Effects of vitamin D supplementation on metabolic indices and hs‐CRP levels in gestational diabetes mellitus patients: a randomized, double‐blinded, placebo‐controlled clinical trial. Nutrition Research and Practice 2016;10(3):328‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang Q, Cheng Y, He M, Li T, Ma Z, Cheng H. Effect of various doses of vitamin D supplementation on pregnant women with gestational diabetes mellitus: a randomized controlled trial. Experimental and Therapeutic Medicine 2016;12(3):1889‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bimson 2017 {published data only}
- Bimson B, Brustman L, Al Ibraheemi Z, Herrera K, Rosenn B. Can weekly vitamin D dosing adequately treat vitamin D deficiency in pregnancy?. American Journal of Obstetrics and Gynecology 2017;216(1):S380‐S381. [Google Scholar]
Das 2009 {published data only}
- Das V, Agarwal A, Bhatia V, Pandey A, Agarwal S, Saxena P, et al. Evaluation of vitamin d status and need for supplementation in pregnant women of a rural area of North India (abstract 0205). International Journal of Gynecology & Obstetrics 2009;107 (Suppl 2):S151. [Google Scholar]
References to ongoing studies
Baird 2016 {published data only}
- Baird J, Barker M, Harvey NC, Lawrence W, Vogel C, Jarman M, et al. Southampton PRegnancy Intervention for the Next Generation (SPRING): protocol for a randomised controlled trial. Trials [Electronic Resource] 2016;17(1):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jelsma 2013 {published data only}
- Jelsma JG, Poppel MN, Galjaard S, Desoye G, Corcoy R, Devlieger R, et al. DALI: Vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: an European multicentre, randomised trial ‐ study protocol. BMC Pregnancy and Childbirth 2013;13:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Judkins 2010 {published data only}
- Judkins A. A randomised double blinded interventional trial to determine the effect of 50,000 IU vitamin D supplementation monthly or twice monthly from 20 weeks gestation. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610001044011 23 November 2010.
Lindqvist 2010 {published data only}
- Lindqvist P. Vitamin D supplementation for prevention of placenta mediated pregnancy complications. ‐ Prevention of pregnancy complications with vitamin D. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2010‐019483‐37 (first received 4 June 2010).
Mosalanejad 2016 {published data only}
- Mosalanejad N, IRCT2016121430612N2. Compare the effect of vitamin D and calcium plus vitamin D on pregnancy outcomes in pregnant women. en.search.irct.ir/view/34642 (first received 26 December 2016).
Rasmussen 2009 {published data only}
- Rasmussen G. Additional information on registered trial. Effects of vitamin D supplement before, during and after pregnancy on complications, birth weight and bone mineral density during lactation (gravita). Personal communication 2011.
- Rasmussen GB. Effects of vitamin D supplement before and during pregnancy on birth weight (gravita). clinicaltrials.gov/ct2/show/NCT01038453 (first received 24 December 2009).
Additional references
ACOG 2015
- American College of Obstetricians and Gynecologists. Vitamin D: screening and supplementation during pregnancy. Committee Opinion No. 495. Obstetrics and Gynecology 2011 (Reaffirmed 2015) 2015;118:197–8. [DOI] [PubMed] [Google Scholar]
Aghajafari 2013
- Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25‐hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta‐analysis of observational studies. BMJ 2013;346:f1169. [DOI] [PubMed] [Google Scholar]
Akcakus 2006
- Akcakus M, Koklu E, Budak N, Kula M, Kurtoglu S, Koklu S. The relationship between birthweight, 25‐hydroxyvitamin D concentrations and bone mineral status in neonates. Annals of Tropical Paediatrics 2006;26(4):267‐75. [DOI] [PubMed] [Google Scholar]
Ariyuki 1987
- Ariyuki F. Growth retardation induced in rat fetuses by maternal fasting and massive doses of ergocalciferol. Journal of Nutrition 1987;117(2):342‐8. [DOI] [PubMed] [Google Scholar]
Armas 2004
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. Journal of Clinical Endocrinology and Metabolism 2004;89(11):5387‐91. [DOI] [PubMed] [Google Scholar]
Arunabh 2003
- Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25‐hydroxyvitamin D levels in healthy women. Journal of Clinical Endocrinology and Metabolism 2003;88(1):157‐61. [DOI] [PubMed] [Google Scholar]
August 1992
- August P, Marcaccio B, Gertner JM, Druzin ML, Resnick LM, Laragh JH. Abnormal 1,25‐dihydroxyvitamin D metabolism in preeclampsia. American Journal of Obstetrics and Gynecology 1992;166(4):1295‐9. [DOI] [PubMed] [Google Scholar]
Bandeira 2006
- Bandeira F, Griz L, Dreyer P, Eufrazino C, Bandeira C, Freese E. Vitamin D deficiency: a global perspective [Deficiência de vitamina D: uma perspectiva global]. Arquivos Brasileiros de Endocrinologia e Metabologia 2006;50(4):640‐6. [DOI] [PubMed] [Google Scholar]
Bener 2009
- Bener A, Alsaied A, Al‐Ali M, Al‐Kubaisi A, Basha B, Abraham A, et al. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetologica 2009;4(2):183‐9. [DOI] [PubMed] [Google Scholar]
Bodnar 2007
- Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. Journal of Clinical Endocrinology and Metabolism 2007;92(9):3517‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bodnar 2010
- Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal serum 25‐hydroxyvitamin D concentrations are associated with small‐for‐gestational age births in white women. Journal of Nutrition 2010;140(9):999‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butte 2002
- Butte NF, Lopez‐Alarcon MG, Garza C. Nutrient Adequacy of Exclusive Breastfeeding of the Term Infant During the First Six Months of life. Geneva: World Health Organization, 2002. [Google Scholar]
Canadian Paediatric Society 2007
- Canadian Paediatric Society, First Nations, Inuit and Métis Health Committee. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatrics & Child Health 2007;12(7):583‐98. [PMC free article] [PubMed] [Google Scholar]
Cantorna 2008
- Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Molecular Aspects of Medicine 2008;29:369‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardus 2006
- Cardus A, Parisi E, Gallego C, Aldea M, Fernandez E, Valdivielso JM. 1,25‐Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF‐mediated pathway. Kidney International 2006;69:1377‐84. [DOI] [PubMed] [Google Scholar]
Chan 1979
- Chan GM, Buchino JJ, Mehlhorn D, Bove KE, Steichen JJ, Tsang RC. Effect of vitamin D on pregnant rabbits and their offspring. Pediatric Research 1979;13(2):121‐6. [DOI] [PubMed] [Google Scholar]
Chocano‐Bedoya 2009
- Chocano‐Bedoya P, Ronnenberg AG. Vitamin D and tuberculosis. Nutrition Reviews 2009;67(5):289‐93. [DOI] [PubMed] [Google Scholar]
Christian 2003
- Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326(7389):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clemens 1982
- Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982;319:74‐6. [DOI] [PubMed] [Google Scholar]
Clifton‐Bligh 2008
- Clifton‐Bligh RJ, McElduff P, McElduff A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabetic Medicine 2008;25(6):678‐84. [DOI] [PubMed] [Google Scholar]
Cope 2016
- Committee on Publication Ethics (COPE). What to do if you suspect plagiarism flowchart. https://publicationethics.org/files/Full%20set%20of%20English%20flowcharts_9Nov2016.pdf 2016.
Dawodu 2005
- Dawodu A, Agarwal M, Sankarankutty M, Hardy D, Kochiyil J, Badrinath P. Higher prevalence of vitamin D deficiency in mothers of rachitic than nonrachitic children. Journal of Pediatrics 2005;147(1):109‐11. [DOI] [PubMed] [Google Scholar]
Dawodu 2011
Dawson‐Hughes 2005
- Dawson‐Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis International 2005;16:713‐6. [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 2008
- Dawson‐Hughes B. Serum 25‐hydroxyvitamin D and functional outcomes in the elderly. American Journal of Clinical Nutrition 2008;88(2):527S‐540S. [DOI] [PubMed] [Google Scholar]
DeLuca 2004
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. American Journal of Clinical Nutrition 2004;80(6 Suppl):1689S‐1696S. [DOI] [PubMed] [Google Scholar]
Devereux 2007
- Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. American Journal of Clinical Nutrition 2007;85(3):853‐9. [DOI] [PubMed] [Google Scholar]
Diaz 2002
- Diaz L, Arranz C, Avila E, Halhai A, Vilchis F, Larrae F. Expression and activity of 25‐hydroxyvitamin D‐1 alpha‐hydroxylase are restricted in cultures of human syncytiotrophoblast cells from preeclamptic pregnancies. Journal of Clinical Endocrinology and Metabolism 2002;87(8):3876‐82. [DOI] [PubMed] [Google Scholar]
Drincic 2012
- Drincic AT, Armas LA, Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring, Md.) 2012;20(7):1444‐8. [DOI] [PubMed] [Google Scholar]
Eckard 2013
- Eckard AR, Leong T, Avery A, Castillo MD, Bonilla H, Storer N, et al. Short communication: high prevalence of vitamin D deficiency in HIV‐infected and HIV‐uninfected pregnant women. AIDS Research and Human Retroviruses 2013;29(9):1224‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
EFSA 2016
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for vitamin D. European Food Safety Authority Journal 2016. [DOI] [PMC free article] [PubMed]
Egan 2008
- Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African‐Americans in the southeastern United States: implications for cancer disparities (United States). Cancer Causes and Control 2008;19(5):527‐35. [DOI] [PubMed] [Google Scholar]
El Koumi 2013
- Koumi MA, Ali YF, Abd El Rahman RN. Impact of maternal vitamin D status during pregnancy on neonatal vitamin D status. Turkish Journal of Pediatrics 2013;55(4):371‐7. [PubMed] [Google Scholar]
Evans 2004
- Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental‐decidual function. Journal of the Society for Gynecologic Investigation 2004;11(5):263‐71. [DOI] [PubMed] [Google Scholar]
Farrant 2009
- Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. European Journal of Clinical Nutrition 2009;63(5):646‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzpatrick 1988
- Fitzpatrick TB. The validity and practicality of sun‐reactive skin types I through VI. Archives of Dermatology 1988;124(6):869‐73. [DOI] [PubMed] [Google Scholar]
Ford 1973
- Ford JA, Davidson DC, McIntosh WB, Fyfe WM, Dunnigan MG. Neonatal rickets in Asian immigrant population. BMJ 1973;3(5873):211‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frenkel 1991
- Frenkel Y, Barkai G, Mashiach S, Dolev E, Zimlichmann R, Weiss M. Hypocalciuria of preeclampsia is independent of parathyroid hormone level. Obstetrics & Gynecology 1991;77:822‐5. [PubMed] [Google Scholar]
Friedman 1969
- Friedman WF, Mills LF. The relationship between vitamin D and the craniofacial and dental anomalies of the supravalvular aortic stenosis syndrome. Pediatrics 1969;43(1):12‐8. [PubMed] [Google Scholar]
Gale 2008
- Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. European Journal of Clinical Nutrition 2008;62(1):68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganji 2012
- Ganji V, Zhang X, Tangpricha V. Serum 25‐hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay‐adjusted data. Journal of Nutrition 2012;142(3):498‐507. [DOI] [PubMed] [Google Scholar]
Gilchrest 2008
- Gilchrest BA. Sun exposure and vitamin D sufficiency. American Journal of Clinical Nutrition 2008;88:570S‐577S. [DOI] [PubMed] [Google Scholar]
Glerup 2000
- Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcified Tissue International 2000;66(6):419‐24. [DOI] [PubMed] [Google Scholar]
GRADE Handbook
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version Accessed 12 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Halhali 1995
- Halhali A, Bourgues H, Carrillo A, Garabedian M. Lower circulating insulin‐like growth factor I and 1,25‐dihydroxyvitamin D levels in preeclampsia. Revista de Investigacion Clinica 1995;47(4):259‐66. [PubMed] [Google Scholar]
Halhali 2000
- Halhali A, Tovar AR, Torres N, Bourgues H, Garabedian M, Larrae F. Preeclampsia is associated with low circulating levels of insulin‐like growth factor I and 1,25‐dihydroxyvitamin D in maternal and umbilical cord compartments. Journal of Clinical Endocrinology and Metabolism 2000;85(5):1828‐33. [DOI] [PubMed] [Google Scholar]
Hall 2010
- Hall WB, Sparks AA, Aris RM. Vitamin D deficiency in cystic fibrosis. International Journal of Endocrinology 2010;2010:218691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harvey 2014
- Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technology Assessment 2014;18(45):1‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hathcock 2007
- Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. American Journal of Clinical Nutrition 2007;85(1):6‐18. [DOI] [PubMed] [Google Scholar]
Heaney 2003
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger‐Lux MJ. Human serum 25‐hydroxycholecalciferol response to extended oral dosing with cholecalciferol. American Journal of Clinical Nutrition 2003;77(1):204‐10. [DOI] [PubMed] [Google Scholar]
Heaney 2008
- Heaney RP. Vitamin D: criteria for safety and efficacy. Nutrition Reviews 2008;66(10 Suppl 2):S178‐S181. [DOI] [PubMed] [Google Scholar]
Hewison 1992
- Hewison M. Vitamin D and the immune system. Journal of Endocrinology 1992;132(2):173‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holick 2002
- Holick MF. Too little vitamin D in premenopausal women: why should we care?. American Society for Clinical Nutrition 2002;76:1, 3‐4. [DOI] [PubMed] [Google Scholar]
Holick 2005
- Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. Journal of Clinical Endocrinology and Metabolism 2005;90(6):3215‐24. [DOI] [PubMed] [Google Scholar]
Holick 2007a
- Holick MF. Vitamin D deficiency. New England Journal of Medicine 2007;357(3):266‐81. [DOI] [PubMed] [Google Scholar]
Holick 2007b
- Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D‐lightful story. Journal of Bone and Mineral Research 2007;22 Suppl 2:V28‐V33. [DOI] [PubMed] [Google Scholar]
Holick 2008
- Holick MF. Vitamin D deficiency: a worldwide problem with health consequences. American Journal of Clinical Nutrition 2008;87(4):1080S‐1086S. [DOI] [PubMed] [Google Scholar]
Hollick 2009
- Holick MF. Vitamin D status: measurement, interpretation and clinical application. Annals of Epidemiology 2009;19:73‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollis 2004
- Hollis BW, Wagner CL. Vitamin D requirements during lactation: high‐dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. American Journal of Clinical Nutrition 2004;80(6 Suppl):1752S‐178S. [DOI] [PubMed] [Google Scholar]
Hollis 2007
- Hollis B. Vitamin D requirement during pregnancy and lactation. Journal of Bone and Mineral Research 2007;22 Suppl 2:V39‐V44. [DOI] [PubMed] [Google Scholar]
Hyppönen 2013
- Hyppönen E, Cavadino A, Williams D, Fraser A, Vereczkey A, Fraser WD, et al. Vitamin D and pre‐eclampsia: original data, systematic review and meta‐analysis. Annals of Nutrition & Metabolism 2013;63(4):331‐40. [DOI] [PubMed] [Google Scholar]
Ioannou 2012
- Ioannou C, Javaid MK, Mahon P, Yaqub MK, Harvey NC, Godfrey KM, et al. The effect of maternal vitamin D concentration on fetal bone. Journal of Clinical Endocrinology and Metabolism 2012;97(11):E2070‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
IOM 2011
- Institute of Medicine (IOM). Dietary Reference Intakes for Calcium and Vitamin D. 2nd Edition. Washington DC: National Academy Press, 2011. [Google Scholar]
Javaid 2006
- Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 2006;367:36‐43. [DOI] [PubMed] [Google Scholar]
Jones 2008
- Jones G. Pharmacokinetics of vitamin D toxicity. American Journal of Clinical Nutrition 2008;88(2):582S‐586S. [DOI] [PubMed] [Google Scholar]
Karras 2018
- Karras SN, Wagner CL, Castracane VD. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018;86:112‐23. [DOI] [PubMed] [Google Scholar]
Lankes 2015
- Lankes U, Elder PA, Lewis JG, George P. Differential extraction of endogenous and exogenous 25‐OH‐vitamin D from serum makes the accurate quantification in liquid chromatography‐tandem mass spectrometry assays challenging. Annals of Clinical Biochemistry 2015;52(Pt 1):151‐60. [DOI] [PubMed] [Google Scholar]
Levis 2005
- Levis S, Gomez A, Jimenez C, Veras L, Ma F, Lai S, et al. Vitamin D deficiency and seasonal variation in an adult south Florida population. Journal of Clinical Endocrinology and Metabolism 2005;90(3):1557‐62. [DOI] [PubMed] [Google Scholar]
Li 2000b
- Li DK, Wi S. Maternal pre‐eclampsia/eclampsia and the risk of sudden infant death syndrome in offspring. Paediatric and Perinatal Epidemiology 2000;14(2):141‐4. [DOI] [PubMed] [Google Scholar]
Li 2002
- Li Y, Kong J, Wei M, Chen ZF, Liu S, Cao LP. 1,25‐dihydroxyvitamin D3 is a negative endocrine regulator of the renin‐angiotensin system. Journal of Clinical Investigation 2002;110(2):229‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lips 2001
- Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrine Reviews 2001;22(4):477‐501. [DOI] [PubMed] [Google Scholar]
Litonjua 2009
- Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Current Opinion in Allergy and Clinical Immunology 2009;9(3):202‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Logan 2013
- Logan VF, Gray AR, Peddie MC, Harper MJ, Houghton LA. Long‐term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25‐hydroxyvitamin D status over the winter months. British Journal of Nutrition 2013;109(6):1082‐8. [DOI] [PubMed] [Google Scholar]
MacKay 2001
- MacKay AP, Berg CJ, Atrash HK. Pregnancy‐related mortality from preeclampsia and eclampsia. Obstetrics & Gynecology 2001;97(4):533‐8. [DOI] [PubMed] [Google Scholar]
Maghbooli 2007
- Maghbooli Z, Hossein‐Nezhad A, Shafaei AR, Karimi F, Madani FS, Larijani B. Vitamin D status in mothers and their newborns in Iran. BMC Pregnancy and Childbith 2007;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maghbooli 2008
- Maghbooli Z, Hossein‐Nezhad A, Karimi F, Shafaei AR, Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes/Metabolism Research and Reviews 2008;24(1):27‐32. [DOI] [PubMed] [Google Scholar]
Mahon 2010
- Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, et al. Low maternal vitamin D status and fetal bone development: cohort study. Journal of Bone and Mineral Research 2010; Vol. 25, issue 1:14‐9. [DOI] [PMC free article] [PubMed]
Matsuoka 1991
- Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW. Racial pigmentation and the cutaneous synthesis of vitamin D. Archives of Dermatology 1991;127(4):536‐8. [PubMed] [Google Scholar]
Mave 2012
- Mave V, Shere D, Gupte N, Suryavanshi N, Kulkarni V, Patil S, et al. SWEN India and Byramjee‐Jeejeebhoy Medical College Clinical Trials Unit Study Team. Vitamin D deficiency is common among HIV‐infected breastfeeding mothers in Pune, India, but is not associated with mother‐to‐child HIV transmission. HIV Clinical Trials 2012;13(5):278‐83. [DOI] [PubMed] [Google Scholar]
Maxwell 1981
- Maxwell JD, Ang L, Brooke OG, Brown IR. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. British Journal of Obstetrics and Gynaecology 1981;88(10):987‐91. [DOI] [PubMed] [Google Scholar]
McCullough 2007
- McCullough M. Vitamin D deficiency in pregnancy: bringing the issues to light. Journal of Nutrition 2007;137:305‐6. [DOI] [PubMed] [Google Scholar]
McGrath 2001
- McGrath J. Does 'imprinting' with low prenatal vitamin D contribute to the risk of various adult disorders?. Medical Hypotheses 2001;56(3):367‐71. [DOI] [PubMed] [Google Scholar]
Mehta 2009
- Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother‐to‐child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. Journal of Infectious Diseases 2009; Vol. 200, issue 7:1022‐30. [DOI] [PMC free article] [PubMed]
Merewood 2009
- Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. Journal of Clinical Endocrinology and Metabolism 2009;94(3):940‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2010
- Miller J, Gallo RL. Vitamin D and innate immunity. Dermatologic Therapy 2010;23(1):13‐22. [DOI] [PubMed] [Google Scholar]
Moller 2013
- Møller UK, Streym S, Mosekilde L, Heickendorff L, Flyvbjerg A, Frystyk J, et al. Changes in calcitropic hormones, bone markers and insulin‐like growth factor I (IGF‐I) during pregnancy and postpartum: a controlled cohort study. Osteoporosis International 2013;24(4):1307‐20. [DOI] [PubMed] [Google Scholar]
Morley 2006
- Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25‐hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. Journal of Clinical Endocrinology and Metabolism 2006;91(3):906‐12. [DOI] [PubMed] [Google Scholar]
Namgunga 2003
- Namgunga R, Tsang RC. Bone in the pregnant mother and newborn at birth. Clinica Chimica Acta 2003;333(1):1‐11. [DOI] [PubMed] [Google Scholar]
Nesby‐O'Dell 2002
- Nesby‐O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988‐1994. American Journal of Clinical Nutrition 2002;76(1):187‐92. [DOI] [PubMed] [Google Scholar]
Nicolaidou 2006
- Nicolaidou P, Hatzistamatiou Z, Papadopoulou A, Kaleyias J, Floropoulou E, Lagona E, et al. Low vitamin D status in mother‐newborn pairs in Greece. Calcified Tissue International 2006;78(6):337‐42. [DOI] [PubMed] [Google Scholar]
Nnoaham 2008
- Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta‐analysis. International Journal of Epidemiology 2008;37(1):113‐9. [DOI] [PubMed] [Google Scholar]
O'Riordan 2008
- O'Riordan MN, Kiely M, Higgins JR, Cashman KD. Prevalence of suboptimal vitamin D status during pregnancy. Irish Medical Journal 2008;101(8):240, 242‐3. [PubMed] [Google Scholar]
Ohta 2009
- Ohta H, Kuroda T, Onoe Y, Orito S, Ohara M, Kume M, et al. The impact of lifestyle factors on serum 25‐hydroxyvitamin D levels: across‐sectional study in Japanese women aged 19‐25 years. Journal of Bone and Mineral Metabolism 2009;27(6):682‐8. [DOI] [PubMed] [Google Scholar]
Ornoy 1968
- Ornoy A, Menczel J, Nebel L. Alterations in the mineral composition and metabolism of rat fetuses and their placentas induced by maternal hypervitaminosis D2. Israel Journal of Medical Sciences 1968;4(4):827‐32. [PubMed] [Google Scholar]
Ornoy 1969
- Ornoy A, Nebel L, Menczel Y. Impaired osteogenesis of fetal long bones.Induced by maternal hypervitaminosis D2. Archives of Pathology 1969;87(6):563‐71. [PubMed] [Google Scholar]
Palacios 2014
- Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem?. Journal of Steroid Biochemistry and Molecular Biology 2014;144(Pt A):138‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palacios 2018
- Palacios C, Lips P, Martinez RX, Lopez‐Perez L, Peña‐Rosas JP, Trak‐Fellermeier MA. Regimens of vitamin D supplementation for women during pregnancy. Prospero CRD42018103763 https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018103763 2018. [CRD42018103763] [DOI] [PMC free article] [PubMed]
Palomer 2008
- Palomer X, González‐Clemente JM, Blanco‐Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes, Obesity and Metabolism 2008;10(3):185‐97. [DOI] [PubMed] [Google Scholar]
Perez‐Lopez 2015
- Pérez‐López FR, Pasupuleti V, Mezones‐Holguin E, Benites‐Zapata VA, Thota P, Deshpande A, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta‐analysis of randomized controlled trials. Fertility and Sterility 2015;103(5):1278‐88. [DOI] [PubMed] [Google Scholar]
Pierrot‐Deseilligny 2010
- Pierrot‐Deseilligny C, Souberbielle JC. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis?. Brain 2010;133(Pt 7):1869‐88. [DOI] [PubMed] [Google Scholar]
Pludowski 2013a
- Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality‐a review of recent evidence. Autoimmunity Reviews 2013;12(10):976‐89. [DOI] [PubMed] [Google Scholar]
Pludowski 2013b
- Płudowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna‐Sokół D, Czech‐Kowalska J, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe — recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynologia Polska 2013;64(4):319‐27. [DOI] [PubMed] [Google Scholar]
RCOG 2014
- Royal College of Obstetricians and Gynaecologists. Vitamin D in Pregnancy. Scientific Impact Paper https://www.rcog.org.uk/globalassets/documents/guidelines/scientific‐impact‐papers/vitamin_d_sip43_june14.pdf 2014; Vol. No. 43:1‐11.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rockell 2005
- Rockell JE, Green TJ, Skeaff CM, Whiting SJ, Taylor RW, Williams SM, et al. Season and ethnicity are determinants of serum 25‐hydroxyvitamin D concentrations in New Zealand children aged 5–14 y. Journal of Nutrition 2005;135:2602‐8. [DOI] [PubMed] [Google Scholar]
Roth 2011a
- Roth DE. Vitamin D supplementation during pregnancy: safety considerations in the design and interpretation of clinical trials. Journal of Perinatology 2011;31(7):449‐59. [DOI] [PubMed] [Google Scholar]
Roth 2017
- Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E. Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ 2017;359:j5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2018
- Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low‐ and middle‐income countries. Annals of the New York Academy of Sciences 2018;1430(1):44‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sachan 2005
- Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. American Journal of Clinical Nutrition 2005;81(5):1060‐4. [DOI] [PubMed] [Google Scholar]
Sahu 2009
- Sahu M, Das V, Aggarwal A, Rawat V, Saxena P, Bhatia V. Vitamin D replacement in pregnant women in rural north India: a pilot study. European Journal of Clinical Nutrition 2009;63(9):1157‐9. [DOI] [PubMed] [Google Scholar]
Scholl 2012
- Scholl TO, Chen X, Stein P. Maternal vitamin D status and delivery by cesarean. Nutrients 2012;4(4):319‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sloka 2009
- Sloka S, Stokes J, Randell E, Newhook LA. Seasonal variation of maternal serum vitamin D in Newfoundland and Labrador. Journal of Obstetrics and Gynaecology Canada: JOGC 2009;31(4):313‐21. [DOI] [PubMed] [Google Scholar]
Tabesh 2013
- Tabesh M, Salehi‐Abargouei A, Tabesh M, Esmaillzadeh A. Maternal vitamin D status and risk of pre‐eclampsia: a systematic review and meta‐analysis. Journal of Clinical Endocrinology and Metabolism 2013;98(8):3165‐73. [DOI] [PubMed] [Google Scholar]
Theodoratou 2014
- Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta‐analyses of observational studies and randomised trials. BMJ 2014;348(g2035):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theodoropoulos 2003
- Theodoropoulos C, Demers C, Delvin E, Ménard D, Gascon‐Barré M. Calcitriol regulates the expression of the genes encoding the three key vitamin D3 hydroxylases and the drug‐metabolizing enzyme CYP3A4 in the human fetal intestine. Clinical Endocrinology 2003;58(4):489‐99. [DOI] [PubMed] [Google Scholar]
Thorne‐Lyman 2012
- Thorne‐Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta‐analysis. Paediatric and Perinatal Epidemiology 2012;26(Suppl 1):75‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tolaymat 1994
- Tolaymat A, Sanchez‐Ramos L, Yergey AL, Vieira NE, Abrams SA, Edelstein P. Pathophysiology of hypocalciuria in preeclampsia: measurement of intestinal calcium absorption. International Journal of Gynecology & Obstetrics 1994;83(2):283. [PubMed] [Google Scholar]
UK Department of Health 2009
- UK Department of Health. Maternal nutrition: Vitamin D. UK Department of Health (http://www.dh.gov.uk/en/Healthcare/Children/Maternity/Maternalandinfantnutrition/Maternalnutrition/index.htm) (accessed 2009).
Utiger 1998
- Utiger RD. The need for more vitamin D. New England Journal of Medicine 1998;338(12):828‐9. [DOI] [PubMed] [Google Scholar]
van der Pligt 2018
- Pligt P, Willcox J, Szymlek‐Gay EA, Murray E, Worsley A, Daly RM. Associations of maternal vitamin D deficiency with pregnancy and neonatal complications in developing countries: a systematic review. Nutrients 2018;10(5):640. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Schoor 2011
- Schoor NM, Lips P. Worldwide vitamin D status. Best Practice & Research. Clinical Endocrinology & Metabolism 2011;25(4):671‐80. [DOI] [PubMed] [Google Scholar]
Vieth 1999
- Vieth R. Vitamin D supplementation, 25‐hydroxyvitamin D concentrations, and safety. American Journal of Clinical Nutrition 1999;69(5):842‐56. [DOI] [PubMed] [Google Scholar]
Vieth 2001
- Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. American Journal of Clinical Nutrition 2001;73(2):288‐94. [DOI] [PubMed] [Google Scholar]
Vilarrasa 2007
- Vilarrasa N, Maravall J, Estepa A, Sánchez R, Masdevall C, Navarro MA, et al. Low 25‐hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. Journal of Endocrinological Investigation 2007;30(8):653‐8. [DOI] [PubMed] [Google Scholar]
Viljakainen 2010
- Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Makitie O, et al. Maternal vitamin D status determines bone variables in the newborn. Journal of Clinical Endocrinology and Metabolism 2010;Epub:1391. [DOI] [PubMed] [Google Scholar]
Vimaleswaran 2013
- Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi‐directional Mendelian randomisation analysis of multiple cohorts. PLOS Medicine 2013;10(2):e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2008
- Wagner CL, Greer FR, American Academy of Pediatrics Section on breastfeeding and American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122(5):1142‐52. [DOI] [PubMed] [Google Scholar]
Walker 2009
- Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatrics Research 2009;65(5 Pt 2):106R‐113R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2004
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25‐dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of Immunology 2004;173:2909‐12. [DOI] [PubMed] [Google Scholar]
Wei 2013
- Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta‐analysis. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(9):889‐99. [DOI] [PubMed] [Google Scholar]
WHO 2004
- World Health Organization and Food and Agriculture Organization. Vitamin and Mineral Requirements in Human Nutrition. 2nd Edition. Geneva: WHO, 2004. [Google Scholar]
WHO 2016
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
Williams 2008
- Williams B, Williams AJ, Anderson ST. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatric Infectious Disease Journal 2008;27(10):941‐2. [DOI] [PubMed] [Google Scholar]
Wortsman 2000
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. American Journal of Clinical Nutrition 2000;72(3):690‐3. [DOI] [PubMed] [Google Scholar]
Xiong 1999
- Xiong X, Mayes D, Demianczuk N, Olson DM, Davidge ST, Newburn‐Cook C, et al. Impact of pregnancy‐induced hypertension on fetal growth. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 1):207‐13. [DOI] [PubMed] [Google Scholar]
Xuan 2013
- Xuan Y, Zhao HY, Liu JM. Vitamin D and type 2 diabetes mellitus (D2). Journal of Diabetes 2013;5(3):261‐7. [DOI] [PubMed] [Google Scholar]
Yu 2009
- Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology 2009;70(5):685‐90. [DOI] [PubMed] [Google Scholar]
Zehnder 2002
- Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, et al. The ontogeny of 25‐hydroxyvitamin D(3) 1alpha‐hydroxylase expression in human placenta and decidua. American Journal of Pathology 2002;161(1):105‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2008
- Zhang C, Qiu C, Hu FB, David RM, Dam RM, Bralley A, et al. Maternal plasma 25‐hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLOS One 2008;3(11):e3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ansary 2010
- Ansary A, Palacios C, De‐Regil LM, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008873] [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2012
- De‐Regil LM, Palacios C, Ansary A, Kulier R, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008873.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2016
- De‐Regil LM, Palacios C, Lombardo LK, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD008873.pub3] [DOI] [PubMed] [Google Scholar]
Mahomed 1999
- Mahomed K, Gülmezoglu AM. Vitamin D supplementation in pregnancy. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD000228] [DOI] [PubMed] [Google Scholar]


