Abstract
Ligand-activated nuclear receptors, including peroxisom e proliferator-activated receptor alpha (PPARα), pregnane X receptor, and constitutive androstane receptor, w ere first identified as key regulators of the responses against chemical toxicants. However, numerous studies using mouse disease models and human samples have revealed critical roles for these receptors and others, such as PPARβ/δ, PPARγ, farnesoid X receptor (FXR), and liver X receptor (LXR), in maintaining nutrient/energy homeostasis in part through modulation of the gut-liver-adipose axis. Recently, disorders associated with disrupted nutrient/energy homeostasis, e.g., obesity, metabolic syndrome, and non-alcoholic fatty liver disease (NAFLD), are increasing worldwide. Notably, in NAFLD, a progressive subtype exists, designated as non-alcoholic steatohepatitis (NASH) that is characterized by typical histological features resembling alcoholic steatohepatitis (ASH), and NASH/ASH are recognized as major causes of hepatitis virus-unrelated liver cirrhosis and hepatocellular carcinoma. Since hepatic steatosis is basically caused by an imbalance between fat/energy influx and utilization, abnormal signaling of these nuclear receptors contribute to the pathogenesis of fatty liver disease. Standard therapeutic interventions have not been fully established for fatty liver disease, but some new agents that activate or inhibit nuclear receptor signaling have shown promise as possible therapeutic targets. In this review, we summarize recent findings on the roles of nuclear receptors in fatty liver disease and discuss future perspectives to develop promising pharmacological strategies targeting nuclear receptors for NAFLD/NASH.
Keywords: Peroxisome proliferator-activated receptor, Energy vector, Steatohepatitis, Liver fibrosis, Hepatocellular carcinoma, Tissue-specific agonist/antagonist
1. Introduction
1.1. Liver as a main regulator of whole-body metabolism
Liver is the largest solid organ in the body playing a crucial role in maintaining energy homeostasis through metabolism of various nutrients. For example, the main symptoms and signs of acute liver failure are jaundice (impaired bilirubin conjugation/excretion), bleeding tendency (impaired synthesis of coagulation factors), and consciousness disturbance (impaired detoxification of ammonia and other neurotoxic metabolites). Patients having liver cirrhosis often exhibit impaired glucose metabolism (insulin resistance and diabetes), protein/amino acid metabolism (decreased albumin and branched-chain amino acids and increased aromatic amino acids), and lipid metabolism (hypocholesterolemia). These clinical findings mirror a key role of the liver in whole-body metabolism.
Infection with hepatotropic viruses and parasites, autoimmunity, in-take of ethanol and certain drugs/medications, and exposure to occupational and environmental toxicants cause liver damage. In Asia, hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are among the main causes for chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC). Recent advances in antiviral therapies, such as nucleoside analogues for HBV and pegylated interferon/ribavirin and direct-acting antiviral agents for HCV, have improved the quality of life and survival for HBV- or HCV-infected patients. Further improvements in preventive/therapeutic strategies will lead to reduced incidence and mortality of patients having hepatitis virus-related diseases.
Metabolic derangements also cause chronic liver disease, such as fatty liver disease, glycogen storage disease, and hemochromatosis. Fatty liver disease refers to a pathological spectrum ranging from lipid accumulation in hepatocytes (steatosis) to the development of accompanying hepatocyte degeneration (ballooning, Mallory-Denk body) and hepatic inflammation (steatohepatitis), eventually leading to liver fibrosis/cirrhosis, portal hypertension, decompensated liver failure, and HCC (Cohen, Horton, & Hobbs, 2011). Although the mechanism of fatty liver disease may vary according to the etiologies, the liver pathology is often indistinguishable. Therefore, fatty liver diseases are classified according to their etiology/cause, i.e., long-term excess ethanol consumption [alcoholic liver disease/steatohepatitis (ALD/ASH)], over-nutrition, visceral obesity, and metabolic syndrome without ethanol in-take [non-alcoholic fatty liver disease/steatohepatitis (NAFLD/NASH)], occupational/environmental chemical exposure [toxicant-associated fatty liver disease/steatohepatitis], and others (e.g., drug-induced steatohepatitis and steatohepatitis following gastrointestinal surgery).
1.2. NAFLD/NASH - emerging liver disease
The worldwide spread of sedentary lifestyle and diet westernization has increased a prevalence of NAFLD in many countries among wider generations. In Japan, approximately 30% of Japanese upon annual health checkups were found to have NAFLD (Kojima, Watanabe, Numata, Ogawa, & Matsuzaki, 2003; Eguchi et al., 2012), which extrapolates to an estimated 20 million NAFLD patients nationwide. The prevalence of NAFLD in junior high school students was also estimated as approximately 4% in certain areas of Japan (Tsuruta et al., 2010). NAFLD is associated with obesity, insulin resistance, diabetes, hypertension, dyslipidemia, atherosclerosis, and systemic inflammation, representing hepatic manifestation of metabolic syndrome. Although it remains controversial whether NAFLD is a cause or a result of glucose intolerance and insulin resistance, a prospective study demonstrated higher risk of diabetes and cardiovascular events in non-diabetic humans with NAFLD compared with those without NAFLD (Heianza et al., 2014). Additionally, liver with significant steatosis is more susceptible for hepatotoxicants and retards/impairs regeneration following partial hepatectomy (Kele et al., 2013). Therefore, NAFLD is considered as a detrimental condition necessitating appropriate therapeutic interventions.
Dr. Ludwig, a pathologist in Mayo Clinic, proposed a term non-alcoholic steatohepatitis (NASH) in 1980 (Ludwig, Viggiano, McGill, & Oh, 1980). He described 20 non-alcoholic patients having histological findings as compatible with ASH, such as fatty changes, focal necrosis, ballooned hepatocytes with Mallory-Denk bodies, lobular inflammation, and perisinusoidal/perivenular fibrosis. Clinically, most of these patients were obese and 25% had diabetes. At present, NAFLD is classified into two categories according to liver pathology: non-alcoholic fatty liver (NAFL, previously designated as simple steatosis) and NASH. NASH is de-fined by the presence of hepatocyte ballooning, lobular inflammation, and/or fibrosis in addition to macrovesicular steatosis, and NAFL is characterized as macrovesicular steatosis without ballooned hepatocytes (Hashimoto, Tokushige, & Ludwig, 2015). This pathology-based classification stems from the concept that NASH can progress into advanced liver fibrosis and the prognosis is poorer than that of NAFL and exhibited the clinical outcome different from NAFL. Indeed, in our NASH cases with obesity, diabetes, hypertension, and dyslipidemia, ballooned hepatocytes were detected in the initial biopsied samples, in which liver fibrosis apparently progressed in 5 years (Fig. 1). Matteoni et al. identified that the outcomes of cirrhosis and liver-related death were more frequent in NAFLD patients with ballooned hepatocytes than in those without ballooned hepatocytes (Matteoni et al., 1999). Others reported that the survival of NASH patients, but not NAFL patients, was significantly lower than an age- and sex-matched reference population (Ekstedt et al., 2006). Based on these findings, the notion that NASH is a serious and progressive type of NAFLD has generally been accepted. The diagnosis of NASH and evaluation of histological severity of NAFLD are performed by the pathological findings of the liver, but liver biopsy is somewhat invasive and costly. Additionally, sampling errors and differences in diagnostic accuracy between independent pathologists can sometimes be problematic. Therefore, less invasive and more accurate strategies to discriminate between NAFL and NASH and predict actual steatosis/inflammation/fibrosis instead of liver biopsy have been evaluated (Fujimori et al., 2016; Hatta et al., 2010; Kitabatake et al., 2017; Matsubara et al., 2012; Tanaka, Ichijo, et al., 2006; Tanaka, Tanaka, et al., 2006; Tanaka, Matsubara, Krausz, Patterson, & Gonzalez, 2012; Tsutsui et al., 2010). Recent studies demonstrated that the presence of fibrosis, but not hepatocyte ballooning, was a determinant of poor prognosis in NAFLD patients (Angulo et al., 2015; Loomba & Chalasani, 2015). Indeed, such a case of NAFLD with careful 27-year follow-up was examined (Nagaya et al., 2008). This patient was diagnosed as having NAFL at the first liver biopsy but gradually developed into cirrhosis and HCC over 20 years. This case teaches us that NAFL is not always benign. Additionally, HCC may occur from NAFL regardless of the absence of advanced fibrosis, past HBV infection, and regular ethanol consumption (Kimura et al., 2017). Although key factors affecting clinical course and outcome of NAFLD and methods to predict fibrosis progression and HCC development have not been identified, attenuating steatosis, hepatic injury, and inflammation and inhibiting fibrosis progression are promising strategies to improve the prognosis of NAFLD/NASH patients.
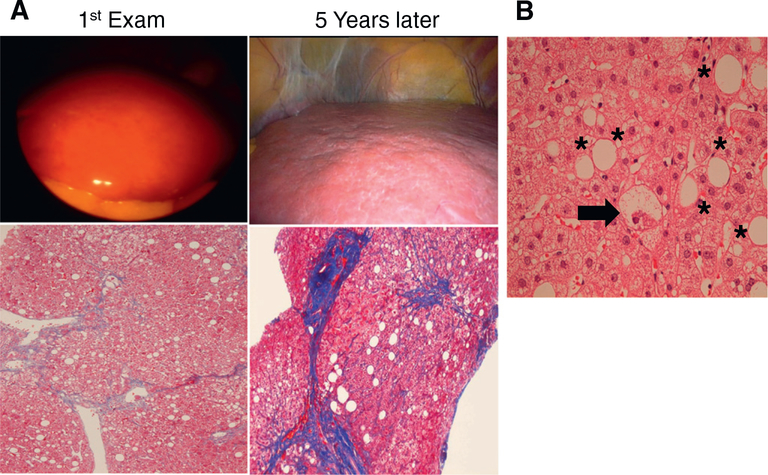
Fig. 1.
Clinical course of NAFLD/NASH. A. The patient had obesity, diabetes, hypertension, dyslipidemia, and persistent elevation of serum alanine aminotransferase (ALT) levels. Initial laparoscopic examination revealed yellowish enlarged liver with smooth surface and soft consistency (1st Exam, upper panel), while liver biopsy section showed steatosis without significant fibrosis (1st Exam, lower panel). Serum ALT levels did not improve and the laparoscopic examination carried out at 5 years after the initial biopsy revealed whitish liver with rough surface (5 years later, upper panel). Dense fibrotic bands were found in biopsied specimen, indicative of pre-cirrhotic phase (5 years later, lower panel). The sections in the lower panel are stained by the Azan-Mallory method, and collagen fiber are indicated as blue. B. Careful pathological examination of the first biopsied specimen detected hepatocyte ballooning (arrow) in addition to macrovesicular steatosis (*), leading to the diagnosis of steatohepatitis. This section is stained by the hematoxylin and eosin method.
Understanding NAFLD/NASH pathogenesis is mandatory for developing novel therapeutic intervention strategies. Insulin resistance and diabetes were reportedly associated with NAFLD with more advanced fibrosis, while impaired glucose metabolism and insulin signaling aggravated liver fibrogenesis and NAFLD, in turn, worsening diabetes and driving systemic inflammation. A ‘two-hit’ model has been proposed to explain why some, but not all, individuals with steatosis develop steatohepatitis (Day & James, 1998). Besides diabetes/insulin resistance, many other second-hit mechanisms, such as lipotoxicity due to saturated fatty acid (FA), free cholesterol, and ceramide, endotoxins from gut and gum, oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, and iron overload, have been extensively reviewed. Additionally, contribution of other organs to the development of NAFLD/NASH should be considered (Jiang, Xie, Li, et al., 2015; Jiang, Xie, Lv, et al., 2015; Tanaka et al., 2014). Recently, a term ‘multiple-hit theory’ has been widely accepted because of close interconnections among these hits (Buzzetti, Pinzani, & Tsochatzis, 2016).
1.3. Nuclear receptors as principal regulators of energy/nutrient homeostasis
More recently, the adverse outcome pathway framework has been used to contextualize the role of nuclear receptors in hepatosteatosis (Mellor, Steinmetz, & Cronin, 2016; Willett et al., 2014). In humans, there are 48 nuclear receptors categorized into 7 subfamilies designated as NR0-NR6 (Evans & Mangelsdorf, 2014) (Table 1). Nuclear receptors are ligand-activated transcription factors regulating the expression of several genes through direct modulation of the transcriptional activities and epigenetic changes. Notably, nuclear receptors in the NR1 subfamily are associated with energy/nutrient control, which may play an important role in the pathogenesis of fatty liver disease. These NR1 nuclear receptors are NR1C1-3: peroxisome proliferator-activated receptor (PPAR) α, β/δ, and γ, NR1H2-3: liver X receptor (LXR) α and β, NR1H4: farnesoid X receptor (FXR), NR1I2: pregnane X receptor (PXR), and NR1I3: constitutive androstane receptor (CAR) (Table 1). These nuclear receptors are mainly activated by binding with ligands, form a heterodimer with retinoid X receptor (RXR) α, β, and γ (NR2B1-3), and exhibit their function as transcription factors. The ligands of NR1 subfamily include nuclear pore-permeable lipophilic endogenous substances mainly derived from nutrients [e.g., FAs, eicosanoids, oxysterols, and bile acids (BAs)] and exogenous chemicals. When the ligands are unbound, the activity of NR1 receptors is suppressed by binding to co-repressors. When either NR1 nuclear receptor or its heterodimeric partner RXR is liganded, these receptors release corepressors and recruit co-activators. However, the transcriptional signal is amplified when both heterodimeric receptors are liganded. The boosting effect of liganded RXR allows these NR1 receptors to significantly increase their transcriptional activities. Not only the ligands of RXR, but also the presence of RXR itself are important for the function of NR1 receptors. Indeed, loss of hepatocyte-specific RXRα disrupts the basal functions of NR1 receptors and alters nutrient metabolism (Anderson et al., 2004).
Table 1.
Classification of nuclear receptors.
| Nomenclature | Abbreviation | Name |
|---|---|---|
| NR0 | ||
| NR0B1 | DAX-1 | Dosage-sensitive sex reversal-adrenal hypoplasia congenital critical region on the X chromosome, gene 1 |
| NR0B2 | SHP | Short heterodimeric partner |
| NR1 | ||
| NR1A1 | TRα | Thyroid hormone receptor α |
| NR1A2 | TRβ | Thyroid hormone receptor β |
| NR1B1 | RARα | Retinoic acid receptor α |
| NR1B2 | RARβ | Retinoic acid receptor β |
| NR1B3 | RARγ | Retinoic acid receptor γ |
| NR1C1 | PPARα | Peroxisome proliferator-activated receptor α |
| NR1C2 | PPARβ/δ | Peroxisome proliferator-activated receptor β/δ |
| NR1C3 | PPARγ | Peroxisome proliferator-activated receptor γ |
| NR1D1 | REV-ERBα | Reverse-Erb α |
| NR1D2 | REV-ERBβ | Reverse-Erb β |
| NR1F1 | RORα | RAR-related orphan receptor α |
| NR1F2 | RORβ | RAR-related orphan receptor β |
| NR1F3 | RORγ | RAR-related orphan receptor γ |
| NR1H2 | LXRβ | Liver X receptor β |
| NR1H3 | LXRα | Liver X receptor α |
| NR1H4 | FXRα | Farnesoid X receptor α |
| NR1H5 | FXRβ | Farnesoid X receptor β (pseudogene) |
| NR1I1 | VDR | Vitamin D receptor |
| NR1I2 | PXR | Pregnane X receptor |
| NR1I3 | CAR | Constitutive androstane receptor |
| NR2 | ||
| NR2A1 | HNF4α | Hepatocyte nuclear factor 4α |
| NR2A2 | HNF4γ | Hepatocyte nuclear factor 4γ |
| NR2B1 | RXRα | Retinoid X receptor α |
| NR2B2 | RXRβ | Retinoid X receptor β |
| NR2B3 | RXRγ | Retinoid X receptor γ |
| NR2C1 | TR2 | Testicular orphan receptor 2 |
| NR2C2 | TR4 | Testicular orphan receptor 4 |
| NR2E1 | TLX | Tailless homolog orphan receptor |
| NR2F1 | COUB-TFα | Chicken ovalbumin upstream promoter-transcription factor α |
| NR2F2 | COUB-TFβ | Chicken ovalbumin upstream promoter-transcription factor β |
| NR2F6 | COUB-TFγ | Chicken ovalbumin upstream promoter-transcription factor γ |
| NR3 | ||
| NR3A1 | ERα | Estrogen receptor α |
| NR3A2 | ERβ | Estrogen receptor β |
| NR3B1 | ERRα | Estrogen related receptor α |
| NR3B2 | ERRβ | Estrogen related receptor β |
| NR3B3 | ERRγ | Estrogen related receptor γ |
| NR3C1 | GR | Glucocorticoid receptor |
| NR3C2 | MR | Mineralocorticoid receptor |
| NR3C3 | PR | Progesterone receptor |
| NR3C4 | AR | Androgen receptor |
| NR4 | ||
| NR4A1 | NGF1-β | Nerve-growth-factor-induced gene B |
| NR4A2 | NURR1 | Nur-related factor 1 |
| NR4A3 | NOR-1 | Neuron-derived orphan receptor 1 |
| NR5 | ||
| NR5A1 | SF-1 | Steroidogenic factor 1 |
| NR5A2 | LRH-1 | Liver receptor homolog-1 |
| NR6 | ||
| NR6A1 | GCNF | Germ cell nuclear factor |
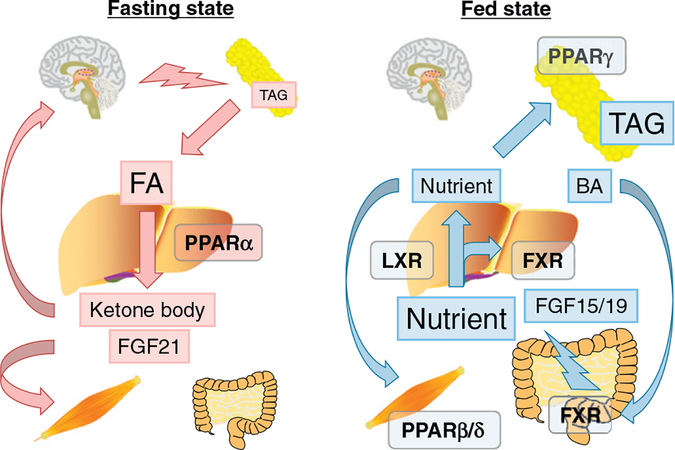
To understand the physiological role of NR1 nuclear receptors, the concept of “energy vector” was proposed (Evans & Mangelsdorf, 2014; Fig. 2). In the fasting state, triacylglycerol (TAG) stored in white adipose tissue (WAT) is subjected to lipolysis and released into the circulation as FAs. FAs are used in many organs as an energy source. In the liver, FAs activate PPARα and enhance FA catabolism, resulting in the production of ATP, ketone bodies, and hepatokine fibroblast growth factor (FGF) 21. Ketone bodies are consumed as an energy source in the brain and FGF21 serves as a stress messenger to prepare other organs for energy deprivation. In the fed state, energy flux is reversed and FXR, LXR, PPARβ/δ and PPARγ are mainly involved in nutrient absorption from the gut and distribution from gut/liver to peripheral tissues, such as WAT and muscle. After meals, BAs activate intestinal FXR, promoting nutrient absorption and maintaining a barrier to the gut microbiome. Absorbed dietary lipids are transported into the circulation as chylomicron and its remnant. Hepatic FXR promotes post-prandial TAG-rich lipoprotein clearance. Excess cholesterol is removed from the body by reverse cholesterol transport under the control of the FXR-stimulated enterokine FGF19 (FGF15 in rodents) and/or activation of hepatic LXR by oxysterols. Fecal elimination of cholesterol is the last step in the reverse cholesterol transport pathway. FGF19 increases hydrophilicity of the bile salt pool and stimulates transintestinal cholesterol excretion (de Boer et al., 2017). FGF15/19 also attenuates post-prandial hyperglycemia through enhancing hepatic glycogenesis. Consequently, excess nutrients are either consumed in muscle or stored in WAT due to PPARβ/δ and PPARγ, respectively. Post-prandial hepatic activation of PXR and CAR promotes the clearance of toxic dietary metabolites and xenobiotics. Because abnormal energy/nutrient homeostasis is a major cause of NAFLD/NASH, the concept of “dysfunction of energy vectors on the gut-liver-adipose axis” may represent a mechanism on how dysregulated nuclear receptors contributes to NAFLD/NASH development (Fig. 2).
Fig. 2.
Nuclear receptors as energy vectors. In a fasting state, triacylglycerol (TAG) stored in white adipose tissue is subjected to lipolysis and released into the circulation as fatty acids (FAs). FAs are taken into many organs as an energy source. In the liver, FAs activate PPARα and enhance FA catabolism, resulting in the production of ATP, ketone bodies, and hepatokine fibroblast growth factor (FGF) 21. Ketone body is consumed as an energy source in the brain and FGF21 serves as a stress messenger to prepare other organs for energy deprivation. In the fed state, energy flux is reversed and FXR, LXR, PPARβ/δ and PPARγ are mainly involved in nutrient absorption from the gut and distribution from gut/liver to peripheral tissues, such as adipose tissue and muscle. After meals, bile acids (BAs) activate intestinal FXR, promoting nutrient absorption and maintaining a barrier to the gut microbiome. Absorbed dietary lipids are transported into the circulation as chylomicron and its remnant. Hepatic FXR promotes post-prandial TAG-rich lipoprotein clearance. Excess cholesterol is removed from the body by reverse cholesterol transport under the control of the FXR-stimulated enterokine FGF19 (FGF15 in rodents) and/or activation of hepatic LXR by oxysterols. FGF15/19 attenuates post-prandial hyperglycemia through enhancing hepatic glycogenesis. Consequently, excess nutrients are either consumed in muscle or stored in WAT due to PPARβ/δ and PPARγ, respectively. The concept “dysfunction of energy vectors on the gut-liver-adipose axis” may explain the pathogenesis of NAFLD/NASH.
The following sections describe the roles of nuclear receptors, mainly focusing on NR1 receptors, in liver pathophysiology and possible therapeutic strategies for the prevention and treatment of fatty liver disease through targeting these key receptors.
2. PPARα
2.1. PPAR overview
Since the administration of certain chemicals, such as Wy-14643, nafenopin, and fibrate derivatives, to mice induces hepatic peroxisome proliferation (increased peroxisome number and size) and hepatomegaly, these chemicals are called as peroxisome proliferators (PP) (Reddy & Krishnakantha, 1975). Furthermore, long-term PP administration results in HCC without accompanying hepatic fibrosis/inflammation (Reddy, Azarnoff, & Hignite, 1980). Since no apparent genetic mutations have been detected in this process, PPs have been recognized as nongenotoxic hepatocarcinogens (Reddy & Lalwai, 1983). To explain a mechanism of rapid and drastic changes following PP administration, the involvement of transcription factors was assumed and the first PPAR [later defined as PPARα (NR1C1)] was identified in 1990 (Issemann & Green, 1990). Subsequently, two other PPARs, PPARβ/δ (NR1C2) and PPARγ (NR1C3), were discovered (Kliewer et al., 1994). A mouse line lacking the PPARα was establish (Lee et al., 1995), demonstrating not only a crucial role of PPARα for the occurrence of peroxi-some proliferation, hepatomegaly, and HCC following PP treatment, but also the physiological function of PPARα in the body (Boverhof et al., 2011).
The main characteristics of three PPARs are summarized in Table 2. The localization of PPARs is quite different. PPARα is expressed in hepatocytes, cardiomyocytes, proximal renal tubular cells, and brown adipocytes. The expression of PPARβ/δ is more ubiquitous but mainly found in muscle, skin, adipose tissue, and liver. PPARγ has three splicing variant isoforms (γ1, γ2, and γ3) that display differences in tissue localization for each isoform; γ1 (ubiquitous localization), γ2 (localized in adipose tissue), and γ3 (localized in macrophages, colon, and adipose tissue). The expression of PPARα and PPARγ is abundant in FA-consuming and FA-storing tissues, respectively.
Table 2.
PPARs and NAFLD/NASH.
| PPARα | PPARβ/δ | PPARγ | |
|---|---|---|---|
| Main tissue | Liver | Muscle | Adipose |
| Heart | Skin | Macrophage | |
| Natural ligands | FA | FA | Prostaglandin |
| Eicosanoids | Eicosanoids | Eicosanoids | |
| Synthetic ligands | Wy-14643 | GW501516 | Thiazolidinedione |
| Fibrates | GW0742, KD3010 | GW1929, GW2090 | |
| Hepatic steatosis | ↓ | ↓ | ↑ |
| Insulin resistance | ↓ | ↓ | ↓ |
| Obesity | ↓ | ↓ | ↑ |
| Cholesterol metabolism | ↓ | ↓ | ~ |
| Hepatic inflammation | ↓ | ↓ | ↓ |
| Hepatic fibrosis | ↓? | ↓? | ↓ |
| Hepatic cancer | ↓? ↑ (rodents) | ↓? | ↓ |
| Drugs for human NASH | Elafibranor (dual PPARα/δ agonist) | Pioglitazone | |
means controversial or limited data.
The optimal response elements for PPARs are direct repeats of AGGTCA separated by a single nucleotide (AGGTCA-n-AGGTCA), termed direct repeat (DR) 1. Long-chain FAs and eicosanoids are typical endogenous ligands for PPARα and PPARβ/δ (Narala et al., 2010; Yu et al., 1995), while PPARγ is specifically activated by arachidonic acid metabolites belonging to 5-hydroxyeicosatetraenoic acid family, such as 5-oxo-15(S)-hydroxyeicosatetraenoic acid and 5-oxo-eicosatetraenoic acid (Altmann et al., 2007; O’Flaherty et al., 2005). Many synthetic PP agents, including lipid-lowering fibrates, exert PPARα-activating properties in rodents. Several environmental toxicants and endocrine disruptors, such as pesticides (diclofap-methyl, pyrethins, and imazalil), herbicides (2,4-dichlorophenoxyacetic acid), phthalate esters (diethylhexyl phthalate), and aldehyde metabolites of chlorinated solvents (perchloroethylene and trichloroethylene), can activate PPARα (Corton et al., 2014; Takeuchi, Matsuda, Kobayashi, Takahashi, & Kojima, 2006). However, existence of significant species-specific differences concerns the effects of these PPARα activators (Gonzalez & Shah, 2008). Wy-14643 is a potent PPARα activator with weak PPARβ/δ and PPARγ activation potential (Kliewer et al., 1994). GW501516 is a highly-selective PPARβ/δ ligand, and anti-diabetic thiazolidinedione (TZD) derivatives, such as troglitazone, pioglitazone, GW1929, and GW2090 are specific PPARγ activators.
Typical PPARα target genes are those encoding FA β-oxidation enzymes (Mandard, Müller, & Kersten, 2004). When PPARα is activated by FA, FA β-oxidation and ensuing ATP production and ketogenesis are enhanced, rendering PPARα as the main FA utilizer and energy generator under nutrient-deprived state. On the contrary, when PPARγ is activated, several proteins, such as adipocyte-specific FA-binding protein (FABP4, also known as aP2), are up-regulated and FAs are stored in adipocytes as TAG. Thus, different features of PPARs in cell-specific expression, ligands, and target genes clearly demonstrate a major but distinct contributions of the three PPARs to energy/nutrient homeostasis (Fig. 2).
2.2. Hepatic steatosis and PPARα
Early studies revealed that constitutive mitochondrial β-oxidation activity was markedly reduced in Ppara-null mouse livers (~40% of wild-type mice) (Aoyama et al., 1998). Typical PPARα target genes include carnitine palmitoyl-CoA transferase 1α (CPT1A), carnitine-acylcarnitine translocase (SLC25A20), and medium-chain acyl-CoA dehydrogenase (ACADM) (Fig. 3). Since ACADM is a rate-limiting enzyme of mitochondrial FA β-oxidation, and acyl-CoA oxidase 1 (ACOX1), a rate-limiting enzyme of peroxisomal FA β-oxidation (not shown in Fig. 3), is also the target of PPARα, this finding revealed a crucial role for PPARα in FA catabolism and clearance. Under the circumstance in which FAs are predominantly utilized as an energy source, such as fasting, Ppara-null mice cannot augment hepatic FA catabolism in response to increased FA influx, leading to severe steatosis (Hashimoto et al., 2000; Kersten et al., 1999).
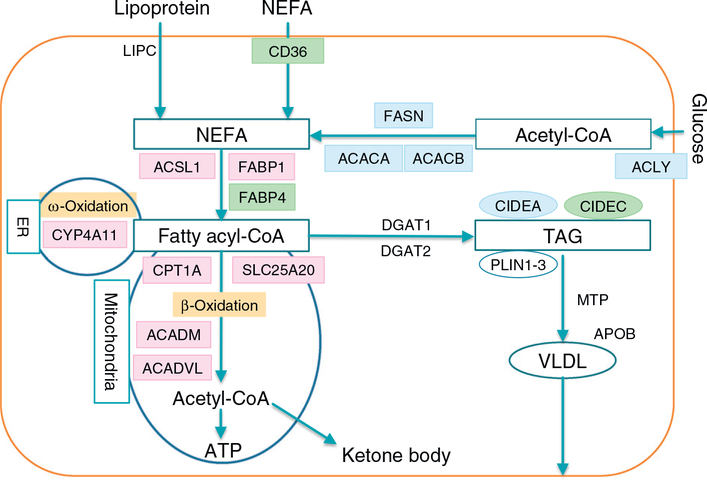
Fig. 3.
FA/TAG metabolism in hepatocytes. Non-esterified fatty acids (NEFAs) are taken from blood into hepatocytes or synthesized from glucose. NEFAs are converted to fatty acyl-CoA esters, then are subjected to β-oxidation (Only mitochondrial β-oxidation pathway is shown for simplification), or generation of triacylglycerol (TAG) and very-low-density lipoprotein (VLDL). TGA is stored in the hepatocytes in the form of lipid droplets, which are coated by perilipin 1–3 and cell death inducing DFFA like effector (CIDE) a-c to prevent excessive lipolysis. The genes regulated by PPARα, PPARγ, and SREBP1c are indicated in pink, green, and blue, respectively. PPARα activation drives FA elimination, but the signaling of PPARγ and SREBP1c stimulates lipogenesis and TAG storage. ACACA, acetyl-CoA carboxylase α; ACACB, acetyl-CoA carboxylase β; ACADM, medium-chain acyl-CoA dehydrogenase; ACADVL, very-long-chain acyl-CoA dehydrogenase; ACLY, ATP citrate lyase; ACSL1, long-chain acyl-CoA synthetase; APOB, apolipoprotein B; CIDEA, cell death inducing DFFA like effector a; CIDEC, cell death inducing DFFA like effector c; CPT1A, carnitine palmitoyl-CoA transferase 1α; CYP, cytochrome P450; DGAT, diacylglycerol acyltransferase; FABP, fatty acid-binding protein; FASN, fatty acid synthase; LIPC, hepatic lipase; MTP, microsomal triacylglycerol transfer protein; NEFA, non-esterified fatty acid; PLIN, perilipin; SLC25A20, carnitineacylcarnitine translocase; TAG, triacylglycerol; VLDL, very-low-density lipoprotein.
In addition to the direct transcriptional activation of genes encoding FA-metabolizing enzymes, the control of energy and nutrient homeostasis via FGF21 is also important means of control. FGF21 is a hepatokine secreted from the liver into blood, binds to a plasma membrane receptor complex on target tissues, mainly the FGF receptor 1 and β-Klotho heterodimer, and enhances expression of glucose transporter 1 in extra-hepatic tissues, leading to improvement of systemic insulin sensitivity and lipid turnover (Kharitonenkov & Adams, 2013; Nishimura, Nakatake, Konishi, & Itoh, 2000). Recombinant FGF21 injection stimulates hepatic FA utilization and attenuates hepatic steatosis in mice, markedly decreases plasma glucose and lipid profiles in diabetic rhesus monkeys (Kharitonenkov et al., 2005, 2007), and improves serum lipid profiles in obese humans with type 2 diabetes (Gaich et al., 2013). Since mice lacking FGF21 gene (Fgf21) are prone to hepatosteatosis and decreased FA catabolism by treatment with a methionine- and choline-deficient (MCD) diet (Tanaka, Takahashi, Zhang, et al., 2015), FGF21 modulates nutrient metabolism to improve hepatic steatosis. Hepatic and serum levels of FGF21 are significantly increased by PPARα activation, while the constitutive levels are markedly lower in Ppara-null mice compared with wild-type mice (Inagaki et al., 2007; Tanaka, Takahashi, Zhang, et al., 2015). These findings suggest that PPARα action for hepatic lipid metabolism is partially mediated by FGF21 (Fig. 4).
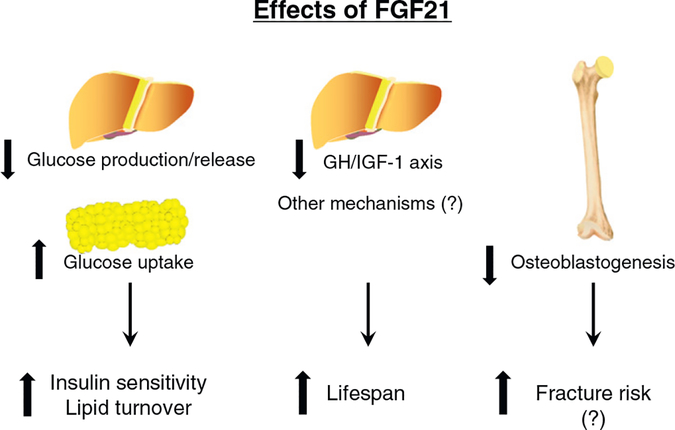
Fig. 4.
Effects of FGF21. A hepatokine FGF21 is secreted into blood in response to various stress, such as fasting and endoplasmic reticulum stress, and activation of PPARα. It binds to a plasma membrane receptor complex, mainly FGF receptor 1 and β-Klotho, and enhances expression of glucose transporter 1 in extra-hepatic tissues, leading to improvement of systemic insulin sensitivity and enhancement of lipid turnover. FGF21 may prolong lifespan through down-regulating growth hormone (GH)-insulin-like growth factor 1 (IGF-1) axis or other unknown mechanisms. FGF21 may inhibit osteoblastogenesis and promote osteoclastogenesis, causing increased fracture risk, while it remains controversial.
Since PPARα is also expressed at the low levels in gut and adipocytes, the contribution of hepatocyte PPARα to whole-body metabolism has not been fully clarified using Ppara-null mice. A hepatocyte-specific Ppara-null mouse line was generated, which exhibited neither induction of hepatocyte proliferation following PP administration nor increases in plasma ketone bodies and FGF21 in a fasting state, similarly to Ppara-null mice (Brocker et al., 2017; Montagner et al., 2016). Additionally, mRNA levels of mitochondrial FA β-oxidation enzymes were basally low in these mouse livers and hepatic TAG was easily accumulated by fasting, aging, and MCD diet (Montagner et al., 2016). These findings corroborate the importance of hepatocyte PPARα in steatogenesis.
2.3. Hepatic inflammation, fibrosis and PPARα
PPARα also has anti-inflammatory properties through counteracting nuclear factor kappa B (NF-κB) at the protein level (Staels et al., 1998). NF-κB is a principal transcription factor regulating inflammatory signaling and its activation induces the expression of genes encoding proinflammatory mediators, such as tumor necrosis factor α (TNFα) and monocyte chemoattractant protein 1 (MCP1). PPARα activation also up-regulates the expression of an inhibitor of NF-κB, resulting in suppression of NF-κB activity. Activation of c-Jun or p65 and their nuclear translocation are also prevented by PPARα (Delerive et al., 1999; Delerive, Gervois, Fruchart, & Staels, 2000; Delerive et al., 2002). Additionally, it is noteworthy that FGF21 induced by PPARα activation has hepatoprotective activity. Acetaminophen overdose-induced liver injury and mortality were significantly aggravated in Fgf21-null mice compared with wild-type mice, likely due to impaired PPARγ coactivator 1α (PGC1α)-nuclear factor erythroid 2-related factor 2 (NRF2)-mediated antioxidant capacity and increased oxidative stress, and acetaminophen-induced hepatotoxicity in Fgf21-null mice were reversed by recombinant FGF21 supplementation (Ye et al., 2014).
However, the anti-inflammatory actions of PPARα may be organ-specific and disease-specific. For example, in mice, PPARα activation by fibrates is protective for acute liver injury (Fang et al., 2017; Patterson, Shah, Matsubara, Krausz, & Gonzalez, 2012), while it is harmful for inflammatory bowel disease (IBD) induced by dextran sulfate sodium (DSS) and 2,4,6-trinitrobenzenesulfonic acid (Qi et al., 2014). Additionally, PPARα activation increases the expression of the peroxisomal β-oxidation enzyme ACOX1, which generates hydrogen peroxide as a byproduct (Kim, Qu, et al., 2014). Therefore, persistent and potent PPARα activation might be harmful for a certain type of cells or tissues.
Attenuated hepatic injury/inflammation by PPARα activation may inhibit fibrosis progression. Indeed, Wy-14643 and fenofibrate attenuated thioacetamide-induced liver fibrosis in rats, which may be associated with the increased expression of catalase, reducing oxidative stress (Toyama et al., 2004). Recently, oleoylethanolamide, an endocannabinoid-like endogenous molecule which binds to PPARα with high affinity, ameliorated thioacetamide-induced hepatic fibrosis by blocking activation of hepatic stellate cells (HSCs) and overexpression of α-smooth muscle actin (αSMA) and collagen matrix in a PPARα-dependent manner (Chen et al., 2015). In vitro studies showed that oleoylethanolamide inhibited transforming growth factor β1-stimulated HSC activation through suppressing SMAD2/3 phosphorylation, but this effect disappeared by co-treatment with the PPARα antagonist GW6571 (Chen et al., 2015). These findings indicate the possibility that PPARα ligands have anti-fibrotic properties.
2.4. Hepatic cancer and PPARα
Administering PPARα ligands to mice induces the expression of cell cycle-regulators and oncogenes, such as cyclin D1/E, cyclin dependent kinase 2/4, and c-Myc in a PPARα-dependent manner (Peters et al., 1998). Prolonged and strong PPARα activation leads to HCC in mice and rats (Furukawa, Numoto, Furuya, Furukawa, & Williams, 1985; Tanaka, Moriya, Kiyosawa, Koike, Gonzalez, et al., 2008; Tanaka, Moriya, Kiyosawa, Koike, & Aoyama, 2008), but not in humans. This phenomenon is mainly due to negative regulation of let-7C, a microRNA important in cell growth, resulting in enhanced expression of c-Myc and the oncogenic mir-17–92 cluster (Qu et al., 2014; Shah, Morimura, et al., 2007). Wy-14643 treatment to mice carrying human PPARA gene enhanced FA metabolism, but neither inhibited let-7C nor induced c-Myc/mir-17 expression and HCC. Based on these findings, let-7C down-regulation and ensuing inductions of c-Myc and mir-17 is one of the mechanisms of murine PP-induced hepatocarcinogenesis (Gonzalez & Shah, 2008). It remains unclear why such species differences exist in the mechanism on how PPARα affects the expression of let-7C and its downstream targets, but the fact that PPARα protein levels are lower in human liver as compared with rodent liver is presumably linked with species differences in responsiveness to PP.
2.5. Targeting PPARα for fatty liver disease
The beneficial effects of PPARα for NAFLD/NASH have been proven in several mouse models by the treatments of high-fat diet, trans-fat-rich diet, and MCD diet (Hu et al., 2017). Using liver tissues from NAFLD/NASH patients, PPARα expression was documented to become low with fibrosis progression (Francque et al., 2015; Nagaya et al., 2010). Considering several hepatoprotective functions of PPARα, therapeutic strategies to restore PPARα expression/activity may be beneficial. FGF21 induction by PPARα activation may also improve insulin sensitivity and attenuate the activity of NAFLD/NASH. Although fibrates have been regarded as available PPARα activators in humans, these agents have not been of clinical benefit in the treatment of NAFLD/NASH. For example, sixteen NASH patients with hypertriglyceridemia were treated with clofibrate 2 g/day for 12 months, but no changes from baseline were found in serum levels of alanine aminotransferase (ALT), TAG, and cholesterol, or in histological grade of steatosis, inflammation, or fibrosis after 12 months of treatment (Laurin et al., 1996). In another study, 48-week treatment with fenofibrate 200 mg/day to sixteen NAFLD patients reduced serum glucose, insulin, and TAG levels and the ratio of the patients having ALT > 45 IU/L, but did not yield any effects on the grade of steatosis, lobular inflammation, fibrosis, or NAFLD activity score (Fernández-Miranda et al., 2008). One possible explanation for these disappointing results is that the degree of PPARα activation is relatively lower in humans as compared with rodents. When clinically-relevant dose of bezafibrate (200–400 mg/day) was administered to humans, the maximum concentration and area under the pharmacokinetic curve were 5–10 μg/mL and 5–40 μg h/mL, respectively (Nakajima et al., 2009). To get similar pharmacokinetic values in mice, the suitable dose of bezafibrate is 10–30 mg/kg/day which does not activate murine PPARα. Therefore, the clinical dose of fibrates might be insufficient to activate PPARα and improve NAFLD/NASH in humans.
Tamoxifen is an estrogen receptor antagonist used for breast cancer treatment, but may cause NAFLD/NASH. Occurrence of liver dysfunction due to tamoxifen-induced NASH may become an obstacle for breast cancer patients. Bezafibrate attenuated hepatosteatosis in spite of continuing tamoxifen intake (Saibara, Onishi, Ogawa, Yoshida, & Enzan, 1999); however, further investigations are needed to determine whether such beneficial effects are actually derived from PPARα activation since bezafibrate can activate PPARα and PPARβ/δ in mice (Peters, Aoyama, Burns, & Gonzalez, 2003).
When a 4% ethanol-containing Lieber-DeCarli diet was given to wild-type and Ppara-null mice, wild-type mice showed only steatosis, but the Ppara-null exhibited the pathological features resembling human ASH, increased oxidative stress, and NF-κB activation (Nakajima et al., 2004). These ethanol-induced abnormalities were attenuated by co-administration of polyenephosphatidylcholine, a naturally-existing antioxidant, suggesting a key role of oxidative stress in the pathogenesis of ASH in Ppara-null mice (Okiyama et al., 2009). Since ethanol reduces PPARα, therapeutic strategies to correct PPARα expression and activity might be useful for the treatment of ALD and ASH.
Urea cycle disorders, such as carbamoyl-phosphate synthase deficiency and ornithine transcarbamoylase deficiency, are often accompanied by diffuse microvesicular steatosis mimicking Reye’s syndrome, but a possible link between urea cycle disorders and disruption of hepatic lipid metabolism remains unclear. SLC25A13 (citrin or aspartate-glutamate carrier 2) is located in the mitochondrial membrane in the liver and its genetic deficiency causes adult-onset type II citrullinemia (CTLN2) (Kobayashi et al., 1999). CTLN2 is one of the urea cycle disorders characterized by sudden-onset hyperammonemia due to reduced argininosuccinate synthase activity and is frequently accompanied by hepatosteatosis in the absence of obesity and ethanol consumption (Komatsu et al., 2008; Saheki et al., 2002; Tanaka, Yazaki, & Kobayashi, 2007). When the expression of genes involved in lipid metabolism was examined using liver samples obtained from CTLN2 patients, the expression of enzymes involved in FA oxidation and PPARα was markedly suppressed in CTLN2 livers (Komatsu et al., 2015). Hypoketonemia in these patients corroborated reduced mitochondrial β-oxidation activity, and hepatic PPARα expression was inversely correlated with the severity of steatosis and circulating ammonia and citrul-line levels. These findings provide a novel link between urea cycle disorder, lipid metabolism, and PPARα, and suggest the possibility that modulation of PPARα might have impacts on impaired urea and ammonia metabolism.
Collectively, PPARα is an intriguing therapeutic target for fatty liver disease; novel agents to efficiently and safely stimulate human PPARα are desired.
3. PPAR β/δ
3.1. Hepatic steatosis and PPARβ/δ
PPARβ/δ is highly expressed in muscle, skin, adipose tissue, and liver. PPARβ/δ levels are far higher in muscle than the other two forms of PPAR in rodents and humans and its activation during the fed state or exercise increases fuel consumption in muscle mainly via enhancement of β-oxidation (Manickam & Wahli, 2017). In obese monkeys, the treatment with PPARβ/δ agonist GW501516 normalizes serum insulin and TAG concentrations, increases high-density lipoprotein (HDL) cholesterol, and decreases low-density lipoprotein cholesterol levels (Oliver et al., 2001). Increased insulin sensitivity and decreased adiposity by PPARβ/δ activation were observed in diet-induced and genetically obese mice (Luquet et al., 2005). Mechanistically, PPARβ/δ activation increases hepatic carbohydrate catabolism by up-regulating glucose-6-phosphate dehydrogenase activity, suppresses hepatic glucose output, inhibits FA release from WAT, and promotes β-oxidation in muscle. Consistently, mice lacking PPARβ/δ gene (Ppard) are metabolically less active and glucose-intolerant (Lee et al., 2006). Although PPARβ/δ might attenuate hepatosteatosis under insulin-resistant state (Wang et al., 2003), the contribution of PPARβ/δ to hepatic lipid metabolism is controversial. Intravenous injection of adenovirus carrying the PPARβ/δ cDNA to db/db mice induced hepatic expression of the insulin-induced gene-1 and suppressed sterol-regulatory element-binding protein (SREBP) 1 activation that up-regulates lipogenic enzymes (showing blue in Fig. 3) and drives de novo lipogenesis in the hepatocytes, thus ameliorating hepatosteatosis (Qin et al., 2008). However, treatment of db/db mice with GW501516, a high-affinity PPARβ/δ ligand, enhanced expression of a lipogenic enzyme acetyl-CoA carboxylase β (ACACB) and increased hepatic TAG contents (Lee et al., 2006). Others reported that GW501516 did not improve hepatosteatosis in high-fat diet-fed mice despite enhancement of the PPARα-PGC1α axis and adenosine monophosphate-activated protein kinase (AMPK) phosphorylation (Barroso et al., 2011). Therefore, the direct impact of PPARβ/δ on hepatic lipid metabolism is presently elusive.
3.2. Hepatic inflammation, fibrosis, cancer, and PPARβ/δ
Ppard-null mice are more susceptible to carbon tetrachloride (CCl4)-induced hepatotoxicity, and the highly specific PPARβ/δ ligand GW0742 attenuates hepatotoxicity in a PPARβ/δ-dependent manner likely through attenuating NF-κB signaling (Shan, Nicol, et al., 2008). Primary hepatocytes and HSC from Ppard-null mice exhibited higher TNFα and αSMA expression, respectively, compared with wild-type mice, but GW0742 treatment did not affect inflammatory signaling in hepatocytes and HSC activation (Shan, Palkar, et al., 2008), suggesting that immune cells, such as Kupffer cells, are responsible for anti-inflammatory effects of PPARβ/δ activators. This view is partially supported by microarray data revealing that various inflammation-related processes, including antigen presentation and natural killer cell-mediated cytotoxicity, were up-regulated in Ppard-null mouse livers (Shan, Nicol, et al., 2008).
On the other hand, the effect of PPARβ/δ activation on liver fibrosis remains controversial. A synthetic PPARβ/δ ligand KD3010 attenuated liver fibrosis induced by CCl4 and bile duct ligation in mice (Iwaisako et al., 2012), but GW501516 promoted it (Kostadinova et al., 2012). Additionally, the relationship between HCC and PPARβ/δ activation has not been documented.
3.3. Targeting PPARβ/δ for fatty liver disease
Although there are limited data on the possible protective role of PPARβ/δ on liver fibrosis and hepatocarcinogenesis, PPARβ/δ might attenuate fatty liver disease through improving insulin sensitivity in muscle and suppressing inflammatory signaling. GW501516 attenuated NASH in MCD diet-treated mice by down-regulating TNFα and MCP1 expression (Nagasawa et al., 2006). Considering weak effects of PPARβ/δ activators on hepatic steatosis and fibrosis, a dual PPARα/β activator, elafibranor, has been developed which showed beneficial effects for human NASH (Ratziu et al., 2016). In this GOLDEN 505 trial, patients with NASH without cirrhosis were randomly assigned to groups given elafibranor 80 mg (n = 93), elafibranor 120 mg (n = 91), or placebo (n = 92) each day for 52 weeks. NASH resolved without exacerbation of fibrosis in a higher proportion of patients in the 120-mg elafibranor group vs the placebo group (19% vs 12%; P = 0.045). Patients with NASH resolution after receiving 120-mg elafibranor had reduced fibrosis stages compared with those without NASH resolution (mean reduction of 0.65 ± 0.61 in responders for the primary outcome vs an increase of 0.10 ± 0.98 in non-responders; P < 0.001). Elafibranor was well tolerated and did not cause weight gain or cardiac events, but did produce a mild, reversible increase in serum creatinine.
Evidence on the association between ALD/ASH and PPARβ/δ is also limited. A cohort of 4% ethanol diet-fed Ppard-null mice exhibited steatosis likely due to increased SREBP1c activity (Goudarzi et al., 2013), while it remains unclear whether PPARβ/δ modulates ethanol-induced oxidative stress, abnormalities in immune responses, and HSC activation. Further studies are needed to address these issues and consider applications of PPARβ/δ activators to humans.
4. PPAR γ
4.1. Hepatic steatosis and PPARγ
PPARγ is highly expressed in adipose tissue and macrophage where it has an important role in energy storage and immune modulation, respectively (Ahmadian et al., 2013). PPARγ expression in hepatocytes is relatively low, but is increased in human steatotic livers and mouse NAFLD/NASH livers. Forced expression of PPARγ in hepatocytes by adenovirus induced hepatosteatosis, and hepatocyte-specific disrUption of PPARγ in ob/ob mice attenuated fatty liver (Matsusue et al., 2003; Yu et al., 2003). PPARγ up-regulates the expression of genes involved in adipogenesis, such as FABP4 and cell death inducing DFFA like effector c [fat-specific protein 27 (FSP27)], a key protein coating lipid droplet in the cells, and hepatic FSP27 expression is associated with the development of fatty liver disease (Matsusue et al., 2008; Tanaka, Takahashi, Matsubara, et al., 2015; Tanaka, Takahashi, Zhang, et al., 2015; Xu, Cai, et al., 2015; Xu, Park, et al., 2015). Therefore, PPARγ-mediated fatty liver is sometimes called as “adipogenic steatosis” (Yu et al., 2003). It remains unclear how PPARγ is ectopically expressed and its expression is regulated in the liver. Hepatic PPARγ expression is down-regulated by hairy and enhancer of split 6 (Hes6), a basic helix-loop-helix transcription repressor (Martinez-Jimenez, Kyrmizi, Cardot, Gonzalez, & Talianidis, 2010), while the promoter of Hes6 gene is activated by retinoic acid receptor (RAR) α (NR1B1). Thus, all-trans retinoic acid, a typical RARα ligand, ameliorated hepatic steatosis in obese mouse models through the RARα-Hes6 axis activation and resulting PPARγ2 down-regulation (Kim, Kim, et al., 2014). Additionally, a novel mechanism that PPARγ expression is negatively regulated by Fos-related antigen 1 (Fra-1) and Fra-2 was recently proposed (Hasenfuss et al., 2014). High-fat diet suppressed hepatic Fra-1 expression causing PPARγ-mediated steatosis, while hepatic Fra-1 overexpression down-regulated PPARγ and reduced TAG accumulation.
While hepatic overexpression/activation of PPARγ is steatogenic, treatment of genetically-obese or diet-induced NAFLD/NASH mice with PPARγ ligands reduces hepatic TAG contents. This discrepant effect of PPARγ is mainly due to adiponectin, an adipokine discovered in 1995 (Scherer, Williams, Fogliano, Baldini, & Lodish, 1995). Circulating adiponectin enhances glucose uptake in peripheral tissues and FA β-oxidation in hepatocytes through activation of AMPK, attenuating hepatic steatosis and improving systemic insulin sensitivity (Ye & Scherer, 2013). Additionally, adiponectin has cytoprotective and anti-cancer effects with yet fully unidentified mechanisms (Ye & Scherer, 2013). Indeed, hypoadiponectinemia is detected in rodents/humans with obesity, diabetes, and/or NAFLD/NASH, and adiponectin attenuates NASH (Fukushima et al., 2009; Hui et al., 2004; Kamada et al., 2007). Since PPARγ activators are potent inducer of adiponectin in adipose tissues, these agents exert anti-steatotic and hepatoprotective effects mainly by increasing adiponectin expression.
Up-regulation of FGF21 is another mechanism for the anti-steatotic effects of PPARγ, as well as PPARα. Expression of FGF21 is significantly increased by rosiglitazone and pioglitazone in mouse livers and primary hepatocytes in a PPARα-independent manner (Oishi & Tomita, 2011).
4.2. Hepatic inflammation, fibrosis, cancer and PPARγ
Similar to other PPAR isoforms, PPARγ attenuates inflammation by suppressing NF-κB activity and increasing circulating anti-inflammatory mediators, such as adiponectin and FGF21. PPARγ activation inhibits the production of TNFα and interleukin (IL) 1β in monocytes and macrophages and primes monocytes into anti-inflammatory M2 macrophages (Bouhlel et al., 2007). In agreement with these findings, macrophage-specific disruption of PPARγ gene (Pparg) in mice resulted in higher levels of necroinflammation/fibrosis, lipid peroxidation, caspase activation, and pro-inflammatory cytokine expression following repeated CCl4 administration compared with hepatocyte-specific Pparg-null and wild-type mice (Morán-Salvador et al., 2013).
Similar exacerbation of hepatic necroinflammation/fibrosis was reported in HSC-specific Pparg-null mice (Morán-Salvador et al., 2013). However, this mouse line used the Cre-LoxP system and Cre recombinase expression under control of the Fabp4 promoter. Since mouse FABP4 is also expressed in adipocytes, this result cannot exclude the influence of adipose PPARγ disruption. PPARγ, predominantly PPARγ2, is highly expressed in quiescent HSC compared as hepatocytes and Kupffer cells, and PPARγ is down-regulated during HSC activation (Miyahara et al., 2000). PPARγ agonists inhibit HSC proliferation and drive activated HSC to apoptosis and the quiescent phenotype, leading to attenuation of liver fibrosis (Bae et al., 2010). However, clinical application of anti-fibrotic effect of PPARγ agonist remains inconclusive. In a trial of an experimental TZD, farglitazar, no efficacy was found on HSC activation or fibrosis in chronic hepatitis C patients (McHutchison et al., 2010).
Several studies using HCC cell lines demonstrated that rosiglitazone/ pioglitazone induced cell cycle arrest and apoptosis in a PPARγ-dependent and -independent mechanism. Mice with heterozygous Pparg disruption were more susceptible to diethylnitrosamine-induced HCC and rosiglitazone administration reduced the incidence of HCC (Yu et al., 2010). These findings imply anti-cancer properties of PPARγ activators.
4.3. Targeting PPARγ for fatty liver disease
The beneficial effect of PPARγ activators has been proven in mouse NAFLD/NASH model. The usefulness of rosiglitazone for human NASH was evaluated in the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) trial in 2008 (Ratziu et al., 2008). Rosiglitazone improved steatosis in correlation with reduced serum ALT and increased insulin sensitivity and adiponectin levels, but with no improvement in other histologic lesions, including fibrosis and NAFLD activity score. In paired biopsied samples from the participants in the FLIRT trial, rosiglitazone treatment raised hepatic expression of MCP1 compared with the baseline (Lemoine, Serfaty, Cervera, Capeau, & Ratziu, 2014). In the Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) trial, NASH patients treated with pioglitazone had dramatically improved steatosis and lobular inflammation, while hepatocellular ballooning and fibrosis were not improved (Sanyal et al., 2010). In a recent randomized double-blinded placebo-controlled trial for pre-diabetic or diabetic NASH patients, 18-month pioglitazone treatment achieved higher NASH histological improvement rate and resolution rate with statistically significant improvement in steatosis, inflammation and ballooning, as well as correction of insulin resistance and hypoadiponectinemia, compared with placebo (Cusi et al., 2016). Pioglitazone reduced the prevalence of NASH patients showing fibrosis progression compared with placebo, but extending the treatment to 36 months did not regress the fibrosis. Collectively, pioglitazone may be beneficial for diabetic NASH patients with none-to-mild liver fibrosis, but it remains unclear whether this agent can reverse advanced fibrosis and improve the outcome in cirrhotic NASH patients.
The efficacy and safety of PPARγ agonists for human ALD/ASH has not been reported. In rats fed an ethanol-containing liquid diet for 6 weeks, pioglitazone significantly attenuated steatosis and lipid peroxidation without altering insulin resistance, likely due to restoring c-Met down-regulation (Tomita et al., 2004). This agent also lowered sensitivity against lipopolysaccharides in Kupffer cells and moderated necroinflammation (Enomoto et al., 2003). Future studies are necessary to assess the usefulness of PPARγ agonists for ALD/ASH patients.
NAFLD sometimes develop after pancreaticoduodenectomy (PD). It was reported that the incidence of newly developed NAFLD following PD (de novo post-PD NAFLD) is 23–37% and some cases are histologically diagnosed as having NASH (Tanaka et al., 2011). Clinicians are concerned about such cases because these patients do not usually have obesity or metabolic syndrome (i.e., non-obese or lean NAFLD/NASH). NAFLD/NASH may be caused by malnutrition due to pancreatic exocrine insufficiency, because hepatic steatosis, body weight, hypoalbuminemia, and liver dysfunction were improved by intensifying pancreatic enzyme supplementation (Tanaka, Horiuchi, Yokoyama, Kawa, & Kiyosawa, 2008; Tanaka et al., 2011). When the expression of genes involved in lipid metabolism was examined using liver samples obtained from post-PD NAFLD/NASH patients, the mRNA levels of the genes encoding PPARγ and its downstream CD36 and FABP4 were markedly enhanced. The gene expression pattern involved in hepatic lipid metabolism in post-PD NAFLD/NASH was different from fibrosis-matched conventional NAFLD/NASH livers, reflecting a distinct mechanism of steatogenesis (Nagaya et al., 2015). These findings provide a novel link among malnutrition, PPARγ, and adipogenic steatosis. Indeed, hepatic steatosis and PPARγ overexpression were detected in non-obese lipodystrophic mice (Tanaka, Takahashi, Matsubara, et al., 2015).
While TZD is a potent insulin sensitizer, several adverse effects, such as body weight gain, body fluid retention, and heart failure, temper the enthusiasm for use of this agent (Ahmadian et al., 2013). In the FLIRT study, weight gain was the main adverse effect (mean gain of 1.5 kg in the rosiglitazone group vs. −1 kg in the placebo group) and painful swollen legs was the main reason for dose reduction/discontinuation. Similar adverse effects were also noted in the PIVENS study. Two independent studies demonstrated that PPARγ activation in the brain contributed to the TZD-induced weight gain (Lu et al., 2011; Ryan et al., 2011). Fluid retention, peripheral edema, anasarca, and congestive heart failure are the major side effects of TZD as well. PPARγ activation in renal distal collecting ducts was reported to alter water/sodium reabsorption ability, causing body fluid retention (Zhang et al., 2005). Further long-term TZD treatment may cause a reduction in bone mineral density and increase fracture risks in humans. A deleterious bone loss caused by PPARγ agonists was prevented in Fgf21-null mice, and Fgf21 transgenic mice and repeated recombinant FGF21-injected mice demonstrated marked trabecular bone loss (Wei et al., 2012). Mechanistically, FGF21 inhibited osteoblastogenesis and stimulated adipogenesis from bone marrow mesenchymal stem cells by potentiating PPARγ activity. While the PPARγ-FGF21 axis might be harmful for bone remodeling (Fig. 4), it remains elusive because of the presence of contradicting results (Li et al., 2017). Based on these observations, the development of new PPARγ agonists with higher specificity to the target tissues/ cells (e.g., adipose-specific PPARγ stimulator) might reduce the incidence of adverse effects while maintaining efficacy.
5. LXR
5.1. LXR overview
There are two LXR isoforms in humans, LXRα (NR1H3) and LXRβ (NR1H2). LXR activates hepatic TAG synthesis and export to peripheral tissues, and stimulates reverse cholesterol transport to moderate cholesterol toxicity in extrahepatic tissues (Hong & Tontonoz, 2014). LXRα is highly expressed in the liver, intestine, kidney and adipose tissue, while LXRβ is ubiquitously expressed (Shinar et al., 1994; Willy et al., 1995).
LXRs bind to DR4, direct repeats of the core sequence AGGTCA spaced by four nucleotides, which is called the LXR response element (Willy et al., 1995). Since the core of this element is identical to that of CAR and PXR, LXR target genes overlap with those of CAR and PXR to some degrees (Quack, Frank, & Carlberg, 2002). The typical endogenous ligands for LXR are oxysterols, which are generated through nonenzymatic auto-oxidation or enzymatic reaction (Gill, Chow, & Brown, 2008). In the former case, oxysterols tend to be ring hydroxylated, while in the enzymatic reaction, oxysterols are predominantly hydroxylated on the side chain to form a stronger activator of LXR, such as 24(S)-hydroxycholesterol, 24(S), 25-epoxycholesterol, and 22(S)-hydroxycholesterol. Additionally, there are several synthetic LXR ligands, such as GW3965 and T0901317; GW3965 is an LXRα/β dual agonist. T0901317 is a selective LXRα agonist, but is also a potent PXR agonist (Shenoy et al., 2004). Some environmental pollutants and endocrine disruptors, including bisphenol A, phthalates, and organophosphates, can interact with LXRα (Mozzicafreddo et al., 2015).
Typical target genes of LXR are those encoding FA synthase (FASN), acetyl-CoA carboxylase, and SREBP1c (Zhang et al., 2012; Zhong et al., 2013) The SREBP1c promoter contains two closely separated DR4 response elements (Fernández-Alvarez et al., 2011). Since SREBP1 regulates the transcriptional levels of genes encoding several lipogenic enzymes, such as ATP citrate lyase (ACLY), acetyl-CoA synthase, FASN, acetyl-CoA carboxylase α (ACACA), and ACACB (Fig. 3), its activation by LXR worsens hepatic TAG accumulation. LXR also induces expression of the ATP-binding cassette transporters (ABC), such as ABCA1 and ABCG1 (Costet, Luo, Wang, & Tall, 2000; Kennedy et al., 2001), which mobilizes cholesterol from the peripheral tissue, enhances HDL formation, and attenuates atherosclerosis.
5.2. Hepatic steatosis and LXR
In humans, hepatic LXR expression was correlated with severity of NAFLD (Ahn, Jang, Jun, Lee, & Shin, 2014). Although LXR stimulation enhances SREBP1-mediated lipogenesis and worsens hepatosteatosis, LXR appears to improve diabetes by decreasing hepatic gluconeogenesis, increasing glucose-stimulated pancreatic insulin secretion, and upregulating adipose glucose transporters including glucose transporter type 4 (Ding et al., 2014). A recent observation revealed that some of the steatotic effects of LXR agonists are modulated in part by the IL-6 responsive gene product, CCAAT/enhancer-binding protein β, since mice lacking this gene exhibited lower levels of steatosis in response to LXR agonists (Rahman et al., 2013).
5.3. Hepatic inflammation, fibrosis, cancer and LXR
LXR tends to suppress inflammation, since SUMOylated LXR can inhibit NF-κB activity (Venteclef et al., 2010). Another important anti-inflammatory mechanism may be derived from elevated expression of the ABCA1 gene and cholesterol depletion. Cholesterol levels in the plasma membrane affect the function of Toll-like receptors (TLRs) and higher ABCA1 expression by LXR activation reduces plasma membrane cholesterol contents and sensitivity of TLRs (Zhu et al., 2010). However, a recent animal study showed a significant reduction in steatosis, inflammation, and collagen disposition by LXR antagonist, SR9238 in high-fat diet-induced NAFLD/NASH mice (Griffett et al., 2015). Relatively less is known about LXR and hepatocarcinogenesis.
5.4. Targeting LXR for fatty liver disease
LXR activation may suppress inflammation and improve atherosclerosis, but promote the development of obesity and hepatosteatosis. LXR antagonism may attenuate hepatosteatosis and ensuing fibrosis. Therefore, liver-specific LXR antagonist might be efficacious for fatty liver disease without giving any impact to reverse cholesterol transport system.
6. FXR
6.1. FXR overview
FXR was identified as a nuclear receptor activated by farnesol pyro-phosphate in 1995 (Forman et al., 1995). FXR exists as two variants in humans, FXRα (NR1H4) and FXRβ (NR1H5), while the latter is a pseudogene (Evans & Mangelsdorf, 2014; Zhang, Cayting, Weinstock, & Gerstein, 2008). FXR is abundantly expressed in the organs involved in BA metabolism and transport, such as liver, intestine, and kidney, but also is present in adipose tissue and adrenal gland. Upon ligand activation, FXR binds to the transcriptional responsive elements as either monomers or heterodimers with RXR (Evans & Mangelsdorf, 2014). BAs are the principal FXR ligands (Makishima et al., 1999), while androsterone, anthracyclines, dihydropyridines, pyrethroids, and vinca alkaloids also activate FXR (Hsu et al., 2014). Obeticholic acid (OCA, also known as INT-747), a 6α-ethyl derivative of chenodeoxycholic acid (CDCA), is the first-in-class selective FXR agonist that is approximately 100-fold potent than CDCA for FXR activation (Pellicciari et al., 2002). Other synthetic agonists, such as GW4064, WAY-362450 (FXR-450 or XL335), and PX-102, have been widely used in animal experiments.
FXR serves as a sensor to regulate BA enterohepatic circulation. In the post-prandial state, intestinal FXR is activated by BAs (de Aguiar Vallim, Tarling, & Edwards, 2013), induces BA-binding protein in enterocytes, and facilitates BA/cholesterol absorption. Additionally, intestinal FXR activation increases the expression of FGF15/19, which is transported via portal circulation to the liver, where it binds to the FGF receptor 4/β-klotho complex and suppresses the expression of cholesterol 7α hydroxylase (CYP7A1), the rate-limiting enzyme of BA synthesis. When hepatocyte FXR is activated by BAs, it inhibits CYP7A1 expression through up-regulated small heterodimer partner (SHP, NR0B2) and increases the expression of ABCB11 (bile salt export pump, BSEP), a major BA transporter from hepatocytes to bile canaliculi and a typical FXR target. A recent study using human precision cut liver slices after OCA treatment revealed that FGF19, NR0B2, ABCB11, SLC51A [organic solute transporter α (OSTα)], and SLC51B (OSTβ) are induced by OCA, corroborating a crucial role of FXR in hepatic BA metabolism in humans (Ijssennagger et al., 2016). FGF21 is also reported to be a transcriptional target of hepatic FXR (Cyphert et al., 2012), while the contribution of FXR to FGF21 induction is weaker than that of PPARα and PPARγ.
6.2. Hepatic steatosis and FXR
Several studies reported that hepatic FXR activation attenuated steatosis in rodents and humans and that Fxr-null mice are prone to hepatosteatosis following high-fat diet (Li, Jadhav, & Zhang, 2013). Since few genes involved in FA/TAG metabolism are directly up-regulated by FXR activation, the mechanism of the anti-steatotic effect of FXR may be indirect, presumably through improvement of insulin resistance and lipoprotein transport.
The role of intestinal FXR in hepatic steatosis is controversial. Activation of FXR by a synthetic agonist, GW4064, strongly induced FGF15/19 expression in the intestine and further inhibited Cyp7a1 gene expression leading to an inhibition of BA synthesis in the liver. Pharmacological administration of FGF15/19 increased the metabolic rate and reversed high fat-induced diabetes while decreasing adiposity (Fu et al., 2004). Intestine-specific FXR activation by fexaramine in mice reduced diet-induced weight gain, steatosis, and hepatic glucose production without activating FXR target genes in the liver, which was mediated via FGF15 signaling (Fang et al., 2015). However, down-regulation of intestinal FXR signaling either with an intestine-specific Fxr-null mice or pharmacologically with an FXR antagonist reduced circulating ceramide levels, inhibited hepatic SREBP1 signaling and attenuated hepatic steatosis (Gonzalez, Jiang, & Patterson, 2016; Jiang, Xie, Li, et al., 2015; Jiang, Xie, Lv, et al., 2015). Therefore, tissue-specific modulation of FXR signaling, e.g., liver-specific FXR agonist, intestine-specific FXR agonist or antagonist, can be of potential value for the treatment of metabolic diseases, including fatty liver and type 2 diabetes (Xie et al., 2017).
6.3. Hepatic inflammation, fibrosis, cancer and FXR
Intestinal FXR plays a crucial role in maintaining small intestine bacterial homeostasis and intestinal barrier function to protect against bacterial translocation (Wiest, Lawson, & Geuking, 2014) and to attenuate hepatic TLR activation. The effects of FXR in hepatic inflammation and fibrosis were demonstrated in mice lacking Fxr, and by activation with synthetic FXR agonists. Fxr-null mice fed a high-fat diet had bacterial overgrowth with increased intestinal permeability and higher levels of pro-inflammatory and pro-fibrogenic factors. FXR activation by GW4064 and CDCA promoted the expression of several genes that have antimicrobial properties, inhibited NF-κB expression, and reduced pro-inflammatory cytokines (Inagaki et al., 2006). FXR activation by GW4064 improved hepatic inflammation and NASH induced by a high fat/high cholesterol diet (Ma, Huang, Yan, Gao, & Liu, 2013). Likewise, another synthetic FXR agonist, WAY-362450, attenuated MCD diet-induced NASH (Zhang, Wang, Liu, & Harnish, 2009).
Compelling evidence suggests that FXR may regulate HCC development. Fxr-null mice develop BA overload and spontaneous HCC by the age of 12–16 months (Kim et al., 2007; Yang et al., 2007). A recent study demonstrated that intestine-specific reactivation of FXR in Fxr-null mice relieved these mice from BA overload and prevented HCC development via the intestinal FXR-FGF15 axis control of BA homeostasis (Degirolamo et al., 2015). While this study suggests that intestinal FXR via the intestine FXR-FGF15 pathway protects against HCC induced by the absence of hepatic FXR, it is not physiologically relevant as there are no scenarios where FXR is deficient in liver in either mice or humans, other than the rare human FXR deficiencies that are lethal (Gomez-Ospina et al., 2016). However, further studies are needed to clarify the role of FXR in hepatocarcinogenesis.
6.4. Targeting FXR for fatty liver disease
Hepatic FXR expression is decreased in NAFLD patients (Yang, Shen, & Sun, 2010). Since FXR activation decreases hepatic lipogenesis, FXR activation may be a promising therapeutic target for NAFLD. In the more recently published study named “The Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment” (FLINT) study, NASH patients were treated with OCA (Neuschwander-Tetri et al., 2015). While obesity, steatosis, inflammation, and fibrosis improved, circulating cholesterol levels, pruritus, and insulin sensitivity worsened. Hypercholesterolemia after OCA treatment may reflect FXR activation and ensuing CYP7A1 down-regulation, inhibiting cholesterol catabolism to BA. While hepatic insulin resistance worsened after OCA treatment, a small study in diabetic patients revealed the improvement of insulin responsiveness, as evidenced by hyperinsulinemic euglycemic clamp method. Aggravated insulin resistance might be an adaptive mechanism in response to long-term OCA treatment. However, FXR activation was also shown to exacerbate weight gain and glucose intolerance (Watanabe et al., 2011). The benefits of FXR activation by synthetic ligands require further investigation, and a phase III clinical trial of OCA (REGENERATE) is underway. Additionally, enhanced tissue specificity of FXR stimulation is an attractive strategy to improve clinical benefit.
While the efficacy of FXR agonists for ALD/ASH is limited, OCA reversed hepatic steatosis and oxidative stress induced by ethanol (Lívero et al., 2014). Thus, OCA may be a promising agent for ALD/ASH.
7. Other NR1 nuclear receptors and fatty liver disease
7.1. PXR
PXR (NR1I2) is a xenobiotic-sensing nuclear receptor that regulates drug metabolism and detoxification. PXR is abundant in the liver and gut, and is expressed not only in hepatocytes, but also in HSC and Kupffer cells (Banerjee, Robbins, & Chen, 2015). PXR recognizes DR3 and DR4 sequences as well as everted repeats separated by 6 or 8 base pairs (Kliewer, Goodwin, & Willson, 2002). A typical target gene of PXR is cytochrome P450 (CYP) 3A4, but the spectrum of PXR targets has expanded to genes involved in energy homeostasis, such as stearoyl-CoA desaturase 1, FA elongase, and the FA transporter CD36 (Wada, Gao, & Xie, 2009; Zhou et al., 2008). PXR also regulates other transcription factors involved in metabolic homeostasis, such as SREBP1 and PPARα (Bitter et al., 2015; Li et al., 2016).
PXR-humanized mice exhibited obesity and glucose intolerance when compared to wild-type mice fed a high fat diet (Spruiell, Richardson, et al., 2014; Spruiell, Jones, et al., 2014). Long-term stimulation of humanized PXR by rifaximin, a non-absorbed antibiotics and PXR ligand in humans, caused hepatosteatosis mainly by up-regulating intestinal FA-binding protein and CD36 expression (Cheng, Krausz, et al., 2012). A clinical study reported insulin resistance in volunteers treated with the PXR agonist rifampin (Rysä et al., 2013). Finally, at least two human NR1I2 polymorphisms, rs7643645/G and rs2461823, were reportedly associated with severe phenotype of NAFLD (Sookoian et al., 2010). Collectively, PXR activation worsens steatosis, obesity, and insulin resistance due to increased hepatic FA uptake and synthesis and decreased β-oxidation (Li et al., 2015; Zhou et al., 2006, 2008).
PXR activation is anti-inflammatory among many tissues through down-regulating NF-κB target genes. Activation of mouse PXR with pregnenolone 16α-carbonitrile was found to be protective against DSS-induced IBD while Pxr-null mice had exaggerated it (Shah, Ma, et al., 2007). Symptoms of DSS-induced IBD were also ameliorated in PXR-humanized mice treated with rifaximin (Cheng et al., 2010; Cheng, Shah, & Gonzalez, 2012). The effects of both mouse and human PXR are due in large part to suppression of NF-κB and decreased inflammation (Cheng, Shah, et al., 2012). Rifaximin was proposed as a treatment for Crohn’s disease (Sartor, 2016), and was approved for the treatment of irritable bowel syndrome, a milder form of IBD (Lucak, Chang, Halpert, & Harris, 2017). The mouse PXR agonist pregnenolone 16α-carbonitrile also attenuated CCl4-induced liver fibrosis in mice (Marek et al., 2005), but anti-fibrotic effect for NASH remains uninvestigated.
The role of PXR in hepatocarcinogenesis was addressed in HCC mediated by aflatoxin B1 (AFB1) and HBV, both often seen in Asian populations. AFB1, a procarcinogenic mycotoxin, activated PXR and increased CYP3A4 expression and the resultant production of carcinogenic epoxide (Ratajewski, Walczak-Drzewiecka, Sałkowska, & Dastych, 2011). Additionally, HBV X protein interacts with the ligand binding domain of PXR and increases the transcriptional activity of PXR at the CYP3A4 promoter (Niu et al., 2013). Therefore, the synergistic effects of AFB1 and HBV on hepatocarcinogenesis might be explained by PXR-dependent mechanisms. However, the role of PXR in hepatocarcinogenesis stemming from fatty liver disease requires further investigation.
7.2. CAR
CAR (NR1I3) is activated through ligand binding or post-transcriptional modifications, such as phosphorylation (Kobayashi, Hashimoto, Honkakoski, & Negishi, 2015; Mutoh et al., 2009). The well-characterized endogenous ligands of CAR include bilirubin, BAs, and androstanes, while phenobarbital and some pesticides are typical xenobiotic ligands. Human CAR is highly expressed in the liver and intestine with lower expression in the heart, muscle, kidneys, and lung. CAR transcriptionally regulates several genes encoding xenobioticsmetabolizing enzymes and drug transporters, including CYP2B6, uridine 5-diphospho-glucuronosyltransferase, sulfotransferase, and MDR1 (ABCB1) (Banerjee et al., 2015; Klaassen & Aleksunes, 2010).
CAR protects against toxic food contaminants or metabolites (Beilke et al., 2009; Uppal et al., 2005). Interestingly, CAR activity was observed to follow a circadian rhythm in mice. CAR is less active during the day, but during the night, when mice eat, CAR is highly active, indicating the interconnection between CAR-mediated xenobiotic metabolism and energy metabolism. Indeed, the expression of CYP2B6 and ABCG2, typical CAR target genes was up-regulated with increasing NAFLD severity (Fisher et al., 2009; Hardwick, Fisher, Canet, Scheffer, & Cherrington, 2011) and this change was thought to be an adaptive response to metabolic stress. Treatment with the CAR agonist TCPOBOP attenuated diet-induced obesity and diabetes in animal models through reducing hepatic gluconeogenesis (Dong et al., 2009; Gao, He, Zhai, Wada, & Xie, 2009). Additionally, CAR activation improved hepatic steatosis by diminishing lipogenesis and inducing β-oxidation in a mouse model (Dong et al., 2009).
CAR has anti-inflammatory and anti-apoptotic properties in NAFLD, but its potential role in hepatic fibrosis and HCC are controversial. Administration of a CAR agonist reduced inflammation and hepatocellular apoptosis, but worsened hepatic fibrosis in MCD diet-fed NASH mice (Yamazaki et al., 2007). CAR activation promoted hepatocarcinogenesis and loss of CAR inhibited NAFLD/NASH-induced HCC in mice (Kettner et al., 2016; Takizawa et al., 2011).
8. Future directions of fatty liver disease treatment targeting nuclear receptors
Pharmacological activation of NR1 is expected to attenuate hepatic steatosis, inflammation, fibrosis, insulin resistance, dyslipidemia and obesity. However, some nuclear receptor agonists exhibited insufficient or paradoxical effects. For example, PPARα activation is basically beneficial for fatty liver disease, but the effects of fibrates for human NAFLD are limited. PPARγ activation improves adipocyte function, but enhances hepatocyte steatosis. Such unexpected findings may be derived from low potency and/or low specificity for the target organ/tissue. In order to increase beneficial effects, the development of more potent and more stable single agonist, and possible combination therapies, such as PPARα agonist + PPARγ agonist, FXR agonist + PPARγ agonist, and PPAR pan-agonist + LXR antagonist, should be examined. For the development of drugs to stimulate/inhibit nuclear receptors more effectively, improvement of organ/tissue specificity should be considered. Furthermore, strategies targeting epigenetic and post-transcriptional modulations and stimulation of nuclear receptor counterparts, such as co-activators and RXRα, might provide promising therapeutics in the future.
9. Conclusion
We reviewed the role of NR1 for the development of fatty liver disease. Nuclear receptor dysregulation contributes to the pathogenesis of NAFLD/NASH by impacting the integrated control of energy/nutrient metabolism thorough the gut-liver-adipose axis and inflammatory signaling. Nuclear receptor-targeted therapies may be beneficial for fatty liver disease, but the effectiveness is still unsatisfactory. Novel pharmacological interventions, such as dual/triple agonists, combination of agonists/antagonists, tissue-specific agonists/antagonists, and nuclear receptor modulators, would be critical to obtain benefits of nuclear receptor activation while minimizing adverse metabolic effects.
Acknowledgment
The authors thank the following collaborators for a lot of helps, advice, instruction, and encouragement for the studies of fatty liver disease: Dr. Michiharu Komatsu, Dr. Tadanobu Nagaya, Dr. Takefumi Kimura, Dr. Naoyuki Fujimori, Dr. Ayumi Sugiura, Dr. Kenji Sano, Dr. Takero Nakajima, Dr. Xiao Hu, Dr. Xiaojing Wang, Dr. Wataru Okiyama, Dr. Goro Tsuruta, Dr. Kan Nakagawa, Dr. Hiroyuki Kitabatake, Prof. Masahide Yazaki, Dr. Yasunari Fujinaga, Dr. Akira Kobayashi, and Prof. Eiji Tanaka (Shinshu University School of Medicine); Dr. Akira Horiuchi (Showa Inan General Hospital); Dr. Takahiro Yamaura (Komachiya Higashi Naika Clinic); Dr. Tsutomu Matsubara and Dr. Shogo Takahashi (National Institutes of Health), and Prof. Etsuko Hashimoto (Tokyo Women’s Medical School).
Abbreviations:
- ABC
ATP-binding cassette transporter
- ACACA
acetyl-CoA carboxylase α
- ACACB
acetyl-CoA carboxylase β, ACLY, ATP citrate lyase
- ACADM
medium-chain acyl-CoA dehydrogenase
- ACOX1
acyl-CoA oxidase 1
- AFB1
aflatoxin B1
- AMPK
adenosine monophosphate-activated protein kinase
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- ASH
alcoholic steatohepatitis
- αSMA
α-smooth muscle actin
- BA
bile acid
- CAR
constitutive androstane receptor
- CCl4
carbon tetrachloride
- CDCA
chenodeoxycholic acid
- CPT1A
carnitine palmitoyl-CoA transferase 1α
- CTLN2
adult-onset type II citrullinemia
- CYP
cytochrome P450
- CYP7A1
cholesterol 7α hydroxylase
- DR
direct repeat
- DSS
dextran sulfate sodium
- FA
fatty acid
- FABP
fatty acid-binding protein
- FASN
fatty acid synthase
- FGF
fibroblast growth factor
- Fra
Fos-related antigen
- FSP27
fat-specific protein 27
- FXR
farnesoid X receptor
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HDL
high-density lipoprotein
- Hes6
hairy and enhancer of split 6
- HSC
hepatic stellate cell
- IBD
inflammatory bowel disease
- IL
interleukin
- LXR
liver X receptor
- MCD
methionine and choline-deficient
- MCP1
monocyte chemoattractant protein 1
- NAFL
non-alcoholic fatty liver
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NF-κB
nuclear factor kappa B
- NRF2
nuclear factor erythroid 2
- OCA
obeticholic acid
- OST
organic solute transporter
- PGC1α
PPARγ coactivator 1α
- PD
pancreaticoduodenectomy
- PP
peroxisome proliferator
- PPAR
peroxisome proliferator-activated receptor
- PXR
pregnane X receptor
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- SHP
small heterodimer partner
- SLC25A20
carnitine-acylcarnitine translocase
- SREBP
sterol-regulatory element-binding protein
- TAG
triacylglycerol
- TLR
Toll-like receptor
- TNFα
tumor necrosis factor α
- TZD
thiazolidinedione
- WAT
white adipose tissue
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- de Aguiar Vallim TQ, Tarling EJ, & Edwards PA (2013). Pleiotropic roles of bile acids in metabolism. Cell Metabolism 17(5), 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, & Evans RM (2013). PPARγ signaling and metabolism: The good, the bad and the future. Nature Medicine 19(5), 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SB, Jang K, Jun DW, Lee BH, & Shin KJ (2014). Expression of liver X receptor correlates with intrahepatic inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Digestive Diseases and Sciences 59(12), 2975–2982. [DOI] [PubMed] [Google Scholar]
- Altmann R, Hausmann M, Spöttl T, Gruber M, Bull AW, Menzel K, … Rogler G (2007). 13-Oxo-ODE is an endogenous ligand for PPARgamma in human colonic epithelial cells. Biochemical Pharmacology 74(4), 612–622. [DOI] [PubMed] [Google Scholar]
- Anderson SP, Dunn C, Laughter A, Yoon L, Swanson C, Stulnig TM, … Corton JC (2004). Overlapping transcriptional programs regulated by the nuclear receptors peroxisome proliferator-activated receptor alpha, retinoid X receptor, and liver X receptor in mouse liver. Molecular Pharmacology 66(6), 1440–1452. [DOI] [PubMed] [Google Scholar]
- Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, … Bendtsen F (2015). Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149(2), 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, & Gonzalez FJ (1998). Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). The Journal of Biological Chemistry 273(10), 5678–5684. [DOI] [PubMed] [Google Scholar]
- Bae MA, Rhee SD, Jung WH, Ahn JH, Song BJ, & Cheon HG (2010). Selective inhibition of activated stellate cells and protection from carbon tetrachloride-induced liver injury in rats by a new PPARgamma agonist KR62776. Archives of Pharmacal Research 33(3), 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Robbins D, & Chen T (2015). Targeting xenobiotic receptors PXR and CAR in human diseases. Drug Discovery Today 20(5), 618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso E, Rodríguez-Calvo R, Serrano-Marco L, Astudillo AM, Balsinde J, Palomer X, & Vázquez-Carrera M (2011). The PPARβ/δ activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1α-Lipin 1-PPARα pathway leading to increased fatty acid oxidation. Endocrinology 152(5), 1848–1859. [DOI] [PubMed] [Google Scholar]
- Beilke LD, Aleksunes LM, Holland RD, Besselsen DG, Beger RD, Klaassen CD, & Cherrington NJ (2009). Constitutive androstane receptor-mediated changes in bile acid composition contributes to hepatoprotection from lithocholic acid-induced liver injury in mice. Drug Metabolism and Disposition 37(5), 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter A, Rümmele P, Klein K, Kandel BA, Rieger JK, Nüssler AK, … Burk O (2015). Pregnane X receptor activation and silencing promote steatosis of human hepatic cells by distinct lipogenic mechanisms. Archives of Toxicology 89(11), 2089–2103. [DOI] [PubMed] [Google Scholar]
- de Boer JF, Schonewille M, Boesjes M, Wolters H, Bloks VW, Bos T, … Groen AK (2017). Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology 152(5), 1126–1138. [DOI] [PubMed] [Google Scholar]
- Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, … Chinetti-Gbaguidi G (2007). PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabolism 6(2), 137–143. [DOI] [PubMed] [Google Scholar]
- Boverhof DR, Chamberlain MP, Elcombe CR, Gonzalez FJ, Heflich RH, Hernández LG, … Gollapudi BB (2011). Transgenic animal models in toxicology: Historical perspectives and future outlook. Toxicological Sciences 121(2), 207–233. [DOI] [PubMed] [Google Scholar]
- Brocker CN, Yue J, Kim D, Qu A, Bonzo JA, & Gonzalez FJ (2017). Hepatocytespecific PPARA expression exclusively promotes agonist-induced cell proliferation without influence from non-parenchymal cells. American Journal of Physiology. Gastrointestinal and Liver Physiology 312(3), G283–G299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzetti E, Pinzani M, & Tsochatzis EA (2016). The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65(8), 1038–1048. [DOI] [PubMed] [Google Scholar]
- Chen L, Li L, Chen J, Li L, Zheng Z, Ren J, & Qiu Y (2015). Oleoylethanolamide, an endogenous PPAR-α ligand, attenuates liver fibrosis targeting hepatic stellate cells. Oncotarget 6(40), 42530–42540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Krausz KW, Tanaka N, & Gonzalez FJ (2012). Chronic exposure to rifaximin causes hepatic steatosis in pregnane X receptor-humanized mice. Toxicological Sciences 129(2), 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Shah YM, & Gonzalez FJ (2012). Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends in Pharmacological Sciences 33(6), 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, … Gonzalez FJ (2010). Therapeutic role of rifaximin in inflammatory bowel disease: Clinical implication of human pregnane X receptor activation. The Journal of Pharmacology and Experimental Therapeutics 335(1), 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, & Hobbs HH (2011). Human fatty liver disease: Old questions and new insights. Science 332(6037), 1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton JC, Cunningham ML, Hummer BT, Lau C, Meek B, Peters JM, … Klaunig JE (2014). Mode of action framework analysis for receptor-mediated toxicity: The peroxisome proliferator-activated receptor alpha (PPARα) as a case study. Critical Reviews in Toxicology 44(1), 1–49. [DOI] [PubMed] [Google Scholar]
- Costet P, Luo Y, Wang N, & Tall AR (2000). Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. The Journal of Biological Chemistry 275(36), 28240–28245. [DOI] [PubMed] [Google Scholar]
- Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, … Portillo-Sanchez P (2016). Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial. Annals of Internal Medicine 165(5), 305–315. [DOI] [PubMed] [Google Scholar]
- Cyphert HA, Ge X, Kohan AB, Salati LM, Zhang Y, & Hillgartner FB (2012). Activation of the farnesoid X receptor induces hepatic expression and secretion of fibroblast growth factor 21. The Journal of Biological Chemistry 287(30), 25123–25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CP, & James OF (1998). Steatohepatitis: A tale of two “hits”? Gastroenterology 114(4), 842–845. [DOI] [PubMed] [Google Scholar]
- Degirolamo C, Modica S, Vacca M, Di Tullio G, Morgano A, D’Orazio A, … Moschetta A (2015). Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology 61(1), 161–170. [DOI] [PubMed] [Google Scholar]
- Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, … Staels B (1999). Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. The Journal of Biological Chemistry 274(45), 32048–32054. [DOI] [PubMed] [Google Scholar]
- Delerive P, De Bosscher K, Vanden Berghe W, Fruchart JC, Haegeman G, & Staels B (2002). DNA binding-independent induction of IkappaBalpha gene transcription by PPARalpha. Molecular Endocrinology 16(5), 1029–1039. [DOI] [PubMed] [Google Scholar]
- Delerive P, Gervois P, Fruchart JC, & Staels B (2000). Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. The Journal of Biological Chemistry 275(47), 36703–36707. [DOI] [PubMed] [Google Scholar]
- Ding L, Pang S, Sun Y, Tian Y, Yu L, & Dang N (2014). Coordinated actions of FXR and LXR in metabolism: From pathogenesis to pharmacological targets for type 2 diabetes. International Journal of Endocrinology 2014 (2014:751859). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, Wakil SJ, … Moore DD (2009). Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proceedings of the National Academy of Sciences of the United States of America 106(44), 18831–18836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, … JSG NAFLD (2012). Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. Journal of Gastroenterology 47(5), 586–595. [DOI] [PubMed] [Google Scholar]
- Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, & Kechagias S (2006). Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44(4), 865–873. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Takei Y, Hirose M, Konno A, Shibuya T, Matsuyama S, … Sato N (2003). Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. The Journal of Pharmacology and Experimental Therapeutics 306(3), 846–854. [DOI] [PubMed] [Google Scholar]
- Evans RM, & Mangelsdorf DJ (2014). Nuclear receptors, RXR, and the big bang. Cell 157(1), 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, … Evans RM (2015). Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nature Medicine 21(2), 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ZZ, Tanaka N, Lu D, Jiang CT, Zhang WH, Zhang C, … Gonzalez FJ (2017). Role of the lipid-regulated NF-κB/IL-6/STAT3 axis in alpha-naphthyl isothiocyanate-induced liver injury. Archives of Toxicology 91(5), 2235–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Alvarez A, Alvarez MS, Gonzalez R, Cucarella C, Muntané J, & Casado M (2011). Human SREBP1c expression in liver is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha). The Journal of Biological Chemistry 286(24), 21466–21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Miranda C, Pérez-Carreras M, Colina F, López-Alonso G, Vargas C, & Solís-Herruzo JA (2008). A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Digestive and Liver Disease 40(3), 200–205. [DOI] [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, & Cherrington NJ (2009). Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metabolism and Disposition 37(10), 2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, … Weinberger C (1995). Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81(5), 687–693. [DOI] [PubMed] [Google Scholar]
- Francque S, Verrijken A, Caron S, Prawitt J, Paumelle R, Derudas B, … Staels B (2015). PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. Journal of Hepatology 63(1), 164–173. [DOI] [PubMed] [Google Scholar]
- Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, … Stewart TA (2004). Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145(6), 2594–2603. [DOI] [PubMed] [Google Scholar]
- Fujimori N, Tanaka N, Shibata S, Sano K, Yamazaki T, Sekiguchi T, … Tanaka E (2016). Controlled attenuation parameter is correlated with actual hepatic fat content in patients with non-alcoholic fatty liver disease with none-to-mild obesity and liver fibrosis. Hepatology Research 46(10), 1019–1027. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Kamada Y, Matsumoto H, Yoshida Y, Ezaki H, Takemura T, … Hayashi N (2009). Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatology Research 39(7), 724–738. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Numoto S, Furuya K, Furukawa NT, & Williams GM (1985). Effects of the hepatocarcinogen nafenopin, a peroxisome proliferator, on the activities of rat liver glutathione-requiring enzymes and catalase in comparison to the action of phenobarbital. Cancer Research 45(10), 5011–5019. [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, … Moller DE (2013). The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metabolism 18(3), 333–340. [DOI] [PubMed] [Google Scholar]
- Gao J, He J, Zhai Y, Wada T, & Xie W (2009). The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. The Journal of Biological Chemistry 284(38), 25984–25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Chow R, & Brown AJ (2008). Sterol regulators of cholesterol homeostasis and beyond: The oxysterol hypothesis revisited and revised. Progress in Lipid Research 47(6), 391–404. [DOI] [PubMed] [Google Scholar]
- Gomez-Ospina N, Potter CJ, Xiao R, Manickam K, Kim MS, Kim KH, … Moore DD (2016). Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nature Communications 7, 10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FJ, Jiang C, & Patterson AD (2016). An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151(5), 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FJ, & Shah YM (2008). PPARalpha: Mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology 246(1), 2–8. [DOI] [PubMed] [Google Scholar]
- Goudarzi M, Koga T, Khozoie C, Mak TD, Kang BH, Fornace AJ Jr., & Peters JM (2013). PPARβ/δ modulates ethanol-induced hepatic effects by decreasing pyridoxal kinase activity. Toxicology 311(3), 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffett K, Welch RD, Flaveny CA, Kolar GR, Neuschwander-Tetri BA, & Burris TP (2015). The LXR inverse agonist SR9238 suppresses fibrosis in a model of non-alcoholic steatohepatitis. Molecular Metabolism 4(4), 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, & Cherrington NJ (2011). Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metabolism and Disposition 39(12), 2395–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss SC, Bakiri L, Thomsen MK, Williams EG, Auwerx J, & Wagner EF (2014). Regulation of steatohepatitis and PPARγ signaling by distinct AP-1 dimers. Cell Metabolism 19(1), 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, & Rao MS (2000). Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. The Journal of Biological Chemistry 275(37), 28918–28928. [DOI] [PubMed] [Google Scholar]
- Hashimoto E, Tokushige K, & Ludwig J (2015). Diagnosis and classification of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: Current concepts and remaining challenges. Hepatology Research 45(1), 20–28. [DOI] [PubMed] [Google Scholar]
- Hatta T, Fujinaga Y, Kadoya M, Ueda H, Murayama H, Kurozumi M, … Tanaka N (2010). Accurate and simple method for quantification of hepatic fat content using magnetic resonance imaging: A prospective study in biopsy-proven nonalcoholic fatty liver disease. Journal of Gastroenterology 45(12), 1263–1271. [DOI] [PubMed] [Google Scholar]
- Heianza Y, Arase Y, Tsuji H, Fujihara K, Saito K, Hsieh SD, … Sone H (2014). Metabolically healthy obesity, presence or absence of fatty liver, and risk of type 2 diabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 20 (TOPICS 20). The Journal of Clinical Endocrinology and Metabolism 99(8), 2952–2960. [DOI] [PubMed] [Google Scholar]
- Hong C, & Tontonoz P (2014). Liver X receptors in lipid metabolism: Opportunities for drug discovery. Nature Reviews. Drug Discovery 13(6), 433–444. [DOI] [PubMed] [Google Scholar]
- Hsu CW, Zhao J, Huang R, Hsieh JH, Hamm J, Chang X, … Xia M (2014). Quantitative high-throughput profiling of environmental chemicals and drugs that modulate farnesoid X receptor. Scientific Reports 4, 6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Tanaka N, Guo R, Lu Y, Nakajima T, Gonzalez FJ, & Aoyama T (2017). PPARα protects against trans-fatty-acid-containing diet-induced steatohepatitis. The Journal of Nutritional Biochemistry 39, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, & George J (2004). Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 40(1), 46–54. [DOI] [PubMed] [Google Scholar]
- Ijssennagger N, Janssen AW, Milona A, Ramos Pittol JM, Hollman DA, Mokry M, … Kersten S (2016). Gene expression profiling in human precision cut liver slices in response to the FXR agonist obeticholic acid. Journal of Hepatology 64(5), 1158–1166. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, … Kliewer SA (2007). Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism 5(6), 415–425. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, … Kliewer SA (2006). Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proceedings of the National Academy of Sciences of the United States of America 103(10), 3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issemann I, & Green S (1990). Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347(6294), 645–650. [DOI] [PubMed] [Google Scholar]
- Iwaisako K, Haimerl M, Paik YH, Taura K, Kodama Y, Sirlin C, … Schnabl B (2012). Protection from liver fibrosis by a peroxisome proliferator-activated receptor δ agonist. Proceedings of the National Academy of Sciences of the United States of America 109(21), E1369–E1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, … Gonzalez FJ (2015). Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. The Journal of Clinical Investigation 125(1), 386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, … Gonzalez FJ (2015). Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nature Communications 6, 10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, … Hayashi N (2007). Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. Journal of Hepatology 47(4), 556–564. [DOI] [PubMed] [Google Scholar]
- Kele PG, van der Jagt EJ, Gouw AS, Lisman T, Porte RJ, & de Boer MT (2013). The impact of hepatic steatosis on liver regeneration after partial hepatectomy. Liver International 33(3), 469–475. [DOI] [PubMed] [Google Scholar]
- Kennedy MA, Venkateswaran A, Tarr PT, Xenarios I, Kudoh J, Shimizu N, & Edwards PA (2001). Characterization of the human ABCG1 gene: Liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. The Journal of Biological Chemistry 276(42), 39438–39447. [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, & Wahli W (1999). Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. The Journal of Clinical Investigation 103(11), 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, … Fu L (2016). Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30(6), 909–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, & Adams AC (2013). Inventing new medicines: The FGF21 story.Molecular Metabolism 3(3), 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, … Shanafelt AB (2005). FGF-21 as a novel metabolic regulator. The Journal of Clinical Investigation 115(6), 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, … Etgen GJ (2007). The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148(2), 774–781. [DOI] [PubMed] [Google Scholar]
- Kim SC, Kim CK, Axe D, Cook A, Lee M, Li T, … Lee YK (2014). All-trans-retinoic acid ameliorates hepatic steatosis in mice by a novel transcriptional cascade. Hepatology 59(5), 1750–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Morimura K, Shah Y, Yang Q, Ward JM, & Gonzalez FJ (2007). Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28(5), 940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Qu A, Reddy JK, Gao B, & Gonzalez FJ (2014). Hepatic oxidative stress activates the Gadd45b gene by way of degradation of the transcriptional repressor STAT3. Hepatology 59(2), 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Kobayashi A, Tanaka N, Sano K, Komatsu M, Fujimori N, … Tanaka E (2017). Clinicopathological characteristics of non-B non-C hepatocellular carcinoma without past HBV infection. Hepatology Research 47(5), 405–418. [DOI] [PubMed] [Google Scholar]
- Kitabatake H, Tanaka N, Fujimori N, Komatsu M, Okubo A, Kakegawa K, … Tanaka E (2017). Association between endotoxemia and histological features of nonalcoholic fatty liver disease. World Journal of Gastroenterology 23(4), 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, & Aleksunes LM (2010). Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacological Reviews 62(1), 1–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, … Evans RM (1994). Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America 91(15), 7355–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, & Willson TM (2002). The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocrine Reviews 23(5), 687–702. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hashimoto M, Honkakoski P, & Negishi M (2015). Regulation of gene expression by CAR: An update. Archives of Toxicology 89(7), 1045–1055. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Sinasac DS, Iijima M, Boright AP, Begum L, Lee JR, … Saheki T (1999). The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nature Genetics 22(2), 159–163. [DOI] [PubMed] [Google Scholar]
- Kojima S, Watanabe N, Numata M, Ogawa T, & Matsuzaki S (2003). Increase in the prevalence of fatty liver in Japan over the past 12 years: Analysis of clinical background. Journal of Gastroenterology 38(10), 954–961. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kimura T, Yazaki M, Tanaka N, Yang Y, Nakajima T, … Aoyama T (2015). Steatogenesis in adult-onset type II citrullinemia is associated with down-regulation of PPARα. Biochimica et Biophysica Acta 1852(3), 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Yazaki M, Tanaka N, Sano K, Hashimoto E, Takei Y, … Kobayashi K (2008). Citrin deficiency as a cause of chronic liver disorder mimicking non-alcoholic fatty liver disease. Journal of Hepatology 49(5), 810–820. [DOI] [PubMed] [Google Scholar]
- Kostadinova R, Montagner A, Gouranton E, Fleury S, Guillou H, Dombrowicz D, … Wahli W (2012). GW501516-activated PPARβ/δ promotes liver fibrosis via p38-JNK MAPK-induced hepatic stellate cell proliferation. Cell & Bioscience 2(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, … McGill DB (1996). Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: A pilot study. Hepatology 23(6), 1464–1467. [DOI] [PubMed] [Google Scholar]
- Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, … Evans RM (2006). PPARdelta regulates glucose metabolism and insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America 103(9), 3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, … Gonzalez FJ (1995). Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Molecular and Cellular Biology 15(6), 3012–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine M, Serfaty L, Cervera P, Capeau J, & Ratziu V (2014). Hepatic molecular effects of rosiglitazone in human non-alcoholic steatohepatitis suggest long-term pro-inflammatory damage. Hepatology Research 44(12), 1241–1247. [DOI] [PubMed] [Google Scholar]
- Li CY, Cheng SL, Bammler TK, & Cui JY (2016). Editor’s highlight: Neonatal activation of the xenobiotic-sensors PXR and CAR results in acute and persistent down-regulation of PPARα-signaling in mouse liver. Toxicological Sciences 153(2), 282–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jadhav K, & Zhang Y (2013). Bile acid receptors in non-alcoholic fatty liver disease. Biochemical Pharmacology 86(11), 1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li H, Garzel B, Yang H, Sueyoshi T, Li Q, … Wang H (2015). SLC13A5 is a novel transcriptional target of the pregnane X receptor and sensitizes drug-induced steatosis in human liver. Molecular Pharmacology 87(4), 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Stanislaus S, Asuncion F, Niu QT, Chinookoswong N, Villasenor K, … Xu J (2017). FGF21 is not a major mediator for bone homeostasis or metabolic actions of PPARα and PPARγ agonists. Journal of Bone and Mineral Research 32(4), 834–845. [DOI] [PubMed] [Google Scholar]
- Lívero FA, Stolf AM, Dreifuss AA, Bastos-Pereira AL, Chicorski R, de Oliveira LG, … Acco A (2014). The FXR agonist 6ECDCA reduces hepatic steatosis and oxidative stress induced by ethanol and low-protein diet in mice. Chemico-Biological Interactions 217, 19–27. [DOI] [PubMed] [Google Scholar]
- Loomba R, & Chalasani N (2015). The hierarchical model of NAFLD: Prognostic significance of histologic features in NASH. Gastroenterology 149(2), 278–281. [DOI] [PubMed] [Google Scholar]
- Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, … Olefsky JM (2011). Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nature Medicine 17(5), 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucak S, Chang L, Halpert A, & Harris LA (2017). Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: Evidence-based treatment in practice. Therapeutic Advances in Gastroenterology 10(2), 253–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J, Viggiano TR, McGill DB, & Oh BJ (1980). Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clinic Proceedings 55(7), 434–438. [PubMed] [Google Scholar]
- Luquet S, Gaudel C, Holst D, Lopez-Soriano J, Jehl-Pietri C, Fredenrich A, & Grimaldi PA (2005). Roles of PPAR delta in lipid absorption and metabolism: A new target for the treatment of type 2 diabetes. Biochimica et Biophysica Acta 1740(2), 313–317. [DOI] [PubMed] [Google Scholar]
- Ma Y, Huang Y, Yan L, Gao M, & Liu D (2013). Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance. Pharmaceutical Research 30(5), 1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, … Shan B (1999). Identification of a nuclear receptor for bile acids. Science 284(5418), 1362–1365. [DOI] [PubMed] [Google Scholar]
- Mandard S, Müller M, & Kersten S (2004). Peroxisome proliferator-activated receptor alpha target genes. Cellular and Molecular Life Sciences 61(4), 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickam R, & Wahli W (2017). Roles of peroxisome proliferator-activated receptor β/δ in skeletal muscle physiology. Biochimie 136, 42–48. [DOI] [PubMed] [Google Scholar]
- Marek CJ, Tucker SJ, Konstantinou DK, Elrick LJ, Haefner D, Sigalas C, … Wright MC (2005). Pregnenolone-16alpha-carbonitrile inhibits rodent liver fibrogenesis via PXR (pregnane X receptor)-dependent and PXR-independent mechanisms. The Biochemical Journal 387(Pt 3), 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Jimenez CP, Kyrmizi I, Cardot P, Gonzalez FJ, & Talianidis I (2010). Hepatocyte nuclear factor 4alpha coordinates a transcription factor network regulating hepatic fatty acid metabolism. Molecular and Cellular Biology 30(3), 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Tanaka N, Krausz KW, Manna SK, Kang DW, Anderson ER, … Gonzalez FJ (2012). Metabolomics identifies an inflammatory cascade involved in dioxin- and diet-induced steatohepatitis. Cell Metabolism 16(5), 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, … Gonzalez FJ (2003). Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. The Journal of Clinical Investigation 111(5), 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, & Gonzalez FJ (2008). Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metabolism 7(4), 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, & McCullough AJ (1999). Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 116(6), 1413–1419. [DOI] [PubMed] [Google Scholar]
- McHutchison J, Goodman Z, Patel K, Makhlouf H, Rodriguez-Torres M, Shiffman M, … Farglitizar Study Investigators (2010). Farglitazar lacks antifibrotic activity in patients with chronic hepatitis C infection. Gastroenterology 138(4), 1365–1373. [DOI] [PubMed] [Google Scholar]
- Mellor CL, Steinmetz FP, & Cronin MT (2016). The identification of nuclear receptors associated with hepatic steatosis to develop and extend adverse outcome pathways. Critical Reviews in Toxicology 46(2), 138–152. [DOI] [PubMed] [Google Scholar]
- Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF Jr., Motomura K, … Tsukamoto H (2000). Peroxisome proliferator-activated receptors and hepatic stellate cell activation. The Journal of Biological Chemistry 275(46), 35715–35722. [DOI] [PubMed] [Google Scholar]
- Montagner A, Polizzi A, Fouché E, Ducheix S, Lippi Y, Lasserre F, … Guillou H (2016). Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 65(7), 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán-Salvador E, Titos E, Rius B, González-Périz A, García-Alonso V, López-Vicario C, … Clària J (2013). Cell-specific PPARγ deficiency establishes anti-inflammatory and anti-fibrogenic properties for this nuclear receptor in non-parenchymal liver cells. Journal of Hepatology 59(5), 1045–1053. [DOI] [PubMed] [Google Scholar]
- Mozzicafreddo M, Cuccioloni M, Bonfili L, Cecarini V, Palermo FA, Cocci P, … Angeletti M (2015). Environmental pollutants directly affect the liver X receptor alpha activity: Kinetic and thermodynamic characterization of binding. The Journal of Steroid Biochemistry and Molecular Biology 152, 1–7. [DOI] [PubMed] [Google Scholar]
- Mutoh S, Osabe M, Inoue K, Moore R, Pedersen L, Perera L, … Negishi M (2009). Dephosphorylation of threonine 38 is required for nuclear translocation and activation of human xenobiotic receptor CAR (NR1I3). The Journal of Biological Chemistry 284(50), 34785–34792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, … Shibata N (2006). Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. European Journal of Pharmacology 536(1–2), 182–191. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Tanaka N, Kimura T, Kitabatake H, Fujimori N, Komatsu M, … Tanaka E (2015). Mechanism of the development of nonalcoholic steatohepatitis after pancreaticoduodenectomy. BBA Clinical 3, 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya T, Tanaka N, Komatsu M, Ichijo T, Sano K, Horiuchi A, … Tanaka E (2008). Development from simple steatosis to liver cirrhosis and hepatocellular carcinoma: A 27-year follow-up case. Clinical Journal of Gastroenterology 1(3), 116–121. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Tanaka N, Suzuki T, Sano K, Horiuchi A, Komatsu M, … Aoyama T (2010). Down-regulation of SREBP-1c is associated with the development of burned-out NASH. Journal of Hepatology 53(4), 724–731. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, … Aoyama T (2004). Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology 40(4), 972–980. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Tanaka N, Kanbe H, Hara A, Kamijo Y, Zhang X, … Aoyama T (2009). Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in mice: A novel peroxisome proliferator-activated receptor alpha-independent mechanism. Molecular Pharmacology 75(4), 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narala VR, Adapala RK, Suresh MV, Brock TG, Peters-Golden M, & Reddy RC (2010). Leukotriene B4 is a physiologically relevant endogenous peroxisome proliferator-activated receptor-alpha agonist. The Journal of Biological Chemistry 285(29), 22067–22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, … NASH Clinical Research Network (2015). Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 385(9972), 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nakatake Y, Konishi M, & Itoh N (2000). Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochimica et Biophysica Acta 1492(1), 203–206. [DOI] [PubMed] [Google Scholar]
- Niu Y, Wu Z, Shen Q, Song J, Luo Q, You H, … Qin W (2013). Hepatitis B virus X protein co-activates pregnane X receptor to induce the cytochrome P450 3A4 enzyme, a potential implication in hepatocarcinogenesis. Digestive and Liver Disease 45(12), 1041–1048. [DOI] [PubMed] [Google Scholar]
- O’Flaherty JT, Rogers LC, Paumi CM, Hantgan RR, Thomas LR, Clay CE, … Morrow CS (2005). 5-Oxo-ETE analogs and the proliferation of cancer cells. Biochimica et Biophysica Acta 1736(3), 228–236. [DOI] [PubMed] [Google Scholar]
- Oishi K, & Tomita T (2011). Thiazolidinediones are potent inducers of fibroblast growth factor 21 expression in the liver. Biological & Pharmaceutical Bulletin 34(7), 1120–1121. [DOI] [PubMed] [Google Scholar]
- Okiyama W, Tanaka N, Nakajima T, Tanaka E, Kiyosawa K, Gonzalez FJ, & Aoyama T (2009). Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress. Journal of Hepatology 50(6), 1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver WR Jr., Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, … Willson TM (2001). A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proceedings of the National Academy of Sciences of the United States of America 98(9), 5306–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Shah YM, Matsubara T, Krausz KW, & Gonzalez FJ (2012). Peroxisome proliferator-activated receptor alpha induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology 56(1), 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, … Willson TM (2002). 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. Journal of Medicinal Chemistry 45(17), 3569–3572. [DOI] [PubMed] [Google Scholar]
- Peters JM, Aoyama T, Burns AM, & Gonzalez FJ (2003). Bezafibrate is a dual ligand for PPARalpha and PPARbeta: Studies using null mice. Biochimica et Biophysica Acta 1632(1–3), 80–89. [DOI] [PubMed] [Google Scholar]
- Peters JM, Aoyama T, Cattley RC, Nobumitsu U, Hashimoto T, & Gonzalez FJ (1998). Role of peroxisome proliferator-activated receptor alpha in altered cell cycle regulation in mouse liver. Carcinogenesis 19(11), 1989–1994. [DOI] [PubMed] [Google Scholar]
- Qi Y, Jiang C, Tanaka N, Krausz KW, Brocker CN, Fang ZZ, … Gonzalez FJ (2014). PPARα-dependent exacerbation of experimental colitis by the hypolipidemic drug fenofibrate. American Journal of Physiology. Gastrointestinal and Liver Physiology 307(5), G564–G573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Xie X, Fan Y, Tian J, Guan Y, Wang X, … Wang N (2008). Peroxisome proliferator-activated receptor-delta induces insulin-induced gene-1 and suppresses hepatic lipogenesis in obese diabetic mice. Hepatology 48(2), 432–441. [DOI] [PubMed] [Google Scholar]
- Qu A, Jiang C, Cai Y, Kim JH, Tanaka N, Ward JM, … Gonzalez FJ (2014). Role of Myc in hepatocellular proliferation and hepatocarcinogenesis. Journal of Hepatology 60(2), 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quack M, Frank C, & Carlberg C (2002). Differential nuclear receptor signalling from DR4-type response elements. Journal of Cellular Biochemistry 86(3), 601–612. [DOI] [PubMed] [Google Scholar]
- Rahman SM, Choudhury M, Janssen RC, Baquero KC, Miyazaki M, & Friedman JE (2013). CCAAT/enhancer binding protein β deletion increases mitochondrial function and protects mice from LXR-induced hepatic steatosis. Biochemical and Biophysical Research Communications 430(1), 336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajewski M, Walczak-Drzewiecka A, Sałkowska A, & Dastych J (2011). Aflatoxins upregulate CYP3A4 mRNA expression in a process that involves the PXR transcription factor. Toxicology Letters 205(2), 146–153. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, … LIDO Study Group (2008). Rosiglitazone for nonalcoholic steatohepatitis: One-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology 135(1), 100–110. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, … GOLDEN-505 Investigator Study Group (2016). Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 150(5), 1147–1159. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Azarnoff DL, & Hignite CE (1980). Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature 283(5745), 397–398. [DOI] [PubMed] [Google Scholar]
- Reddy JK, & Krishnakantha TP (1975). Hepatic peroxisome proliferation: Induction by two novel compounds structurally unrelated to clofibrate. Science 190(4216), 787–789. [DOI] [PubMed] [Google Scholar]
- Reddy JK, & Lalwai ND (1983). Carcinogenesis by hepatic peroxisome proliferators: Evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Critical Reviews in Toxicology 12(1), 1–58. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, & Seeley RJ (2011). A role for central nervous system PPAR-γ in the regulation of energy balance. Nature Medicine 17(5), 623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysä J, Buler M, Savolainen MJ, Ruskoaho H, Hakkola J, & Hukkanen J (2013). Pregnane X receptor agonists impair postprandial glucose tolerance. Clinical Pharmacology and Therapeutics 93(6), 556–563. [DOI] [PubMed] [Google Scholar]
- Saheki T, Kobayashi K, Iijima M, Nishi I, Yasuda T, Yamaguchi N, … Li MX (2002). Pathogenesis and pathophysiology of citrin (a mitochondrial aspartate glutamate carrier) deficiency. Metabolic Brain Disease 17(4), 335–346. [DOI] [PubMed] [Google Scholar]
- Saibara T, Onishi S, Ogawa Y, Yoshida S, & Enzan H (1999). Bezafibrate for tamoxifen-induced non-alcoholic steatohepatitis. Lancet 353(9166), 1802. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, … NASH CRN (2010). Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England Journal of Medicine 362(18), 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor RB (2016). Review article: The potential mechanisms of action of rifaximin in the management of inflammatory bowel diseases. Alimentary Pharmacology & Therapeutics 43(Suppl. 1), 27–36. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, & Lodish HF (1995). A novel serum protein similar to C1q, produced exclusively in adipocytes. The Journal of Biological Chemistry 270(45), 26746–26749. [DOI] [PubMed] [Google Scholar]
- Shah YM, Ma X, Morimura K, Kim I, & Gonzalez FJ (2007). Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. American Journal of Physiology. Gastrointestinal and Liver Physiology 292(4), G1114–G1122. [DOI] [PubMed] [Google Scholar]
- Shah YM, Morimura K, Yang Q, Tanabe T, Takagi M, & Gonzalez FJ (2007). Peroxisome proliferator-activated receptor alpha regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Molecular and Cellular Biology 27(12), 4238–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W, Nicol CJ, Ito S, Bility MT, Kennett MJ, Ward JM, … Peters JM (2008). Peroxisome proliferator-activated receptor-beta/delta protects against chemically induced liver toxicity in mice. Hepatology 47(1), 225–235. [DOI] [PubMed] [Google Scholar]
- Shan W, Palkar PS, Murray IA, McDevitt EI, Kennett MJ, Kang BH, … Peters JM (2008). Ligand activation of peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) attenuates carbon tetrachloride hepatotoxicity by downregulating proinflammatory gene expression. Toxicological Sciences 105(2), 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SD, Spencer TA, Mercer-Haines NA, Alipour M, Gargano MD, Runge-Morris M, & Kocarek TA (2004). CYP3A induction by liver x receptor ligands in primary cultured rat and mouse hepatocytes is mediated by the pregnane X receptor. Drug Metabolism and Disposition 32(1), 66–71. [DOI] [PubMed] [Google Scholar]
- Shinar DM, Endo N, Rutledge SJ, Vogel R, Rodan GA, & Schmidt A (1994). NER, a new member of the gene family encoding the human steroid hormone nuclear receptor. Gene 147(2), 273–276. [DOI] [PubMed] [Google Scholar]
- Sookoian S, Castaño GO, Burgueño AL, Gianotti TF, Rosselli MS, & Pirola CJ (2010). The nuclear receptor PXR gene variants are associated with liver injury in nonalcoholic fatty liver disease. Pharmacogenetics and Genomics 20(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Spruiell K, Jones DZ, Cullen JM, Awumey EM, Gonzalez FJ, & Gyamfi MA (2014). Role of human pregnane X receptor in high fat diet-induced obesity in premenopausal female mice. Biochemical Pharmacology 89(3), 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruiell K, Richardson RM, Cullen JM, Awumey EM, Gonzalez FJ, & Gyamfi MA (2014). Role of pregnane X receptor in obesity and glucose homeostasis in male mice. The Journal of Biological Chemistry 289(6), 3244–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, … Tedgui A (1998). Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature 393(6687), 790–793. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Matsuda T, Kobayashi S, Takahashi T, & Kojima H (2006). In vitro screening of 200 pesticides for agonistic activity via mouse peroxisome proliferator-activated receptor (PPAR)alpha and PPARgamma and quantitative analysis of in vivo induction pathway. Toxicology and Applied Pharmacology 217(3), 235–244. [DOI] [PubMed] [Google Scholar]
- Takizawa D, Kakizaki S, Horiguchi N, Yamazaki Y, Tojima H, & Mori M (2011). Constitutive active/androstane receptor promotes hepatocarcinogenesis in a mouse model of non-alcoholic steatohepatitis. Carcinogenesis 32(4), 576–583. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, … Tanaka E (2011). Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. Journal of Gastroenterology 46(6), 758–768. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Horiuchi A, Yokoyama T, Kawa S, & Kiyosawa K (2008). Pancreatic exocrine insufficiency: A rare cause of nonalcoholic steatohepatitis. The American Journal of Gastroenterology 103(1), 245–246. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Ichijo T, Okiyama W, Mutou H, Misawa N, Matsumoto A, … Kiyosawa K (2006). Laparoscopic findings in patients with nonalcoholic steatohepatitis. Liver International 26(1), 32–38. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Matsubara T, Krausz KW, Patterson AD, & Gonzalez FJ (2012). Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 56(1), 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Moriya K, Kiyosawa K, Koike K, & Aoyama T (2008). Hepatitis C virus core protein induces spontaneous and persistent activation of peroxisome proliferator-activated receptor alpha in transgenic mice: Implications for HCV-associated hepatocarcinogenesis. International Journal of Cancer 122(1), 124–131. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, & Aoyama T (2008). PPARalpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. The Journal of Clinical Investigation 118(2), 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Takahashi S, Fang ZZ, Matsubara T, Krausz KW, Qu A, & Gonzalez FJ (2014). Role of white adipose lipolysis in the development of NASH induced by methionine- and choline-deficient diet. Biochimica et Biophysica Acta 1841(11), 1596–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Takahashi S, Matsubara T, Jiang C, Sakamoto W, Chanturiya T, … Gonzalez FJ (2015). Adipocyte-specific disruption of fat-specific protein 27 causes hepatosteatosis and insulin resistance in high-fat diet-fed mice. The Journal of Biological Chemistry 290(5), 3092–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Takahashi S, Zhang Y, Krausz KW, Smith PB, Patterson AD, & Gonzalez FJ (2015). Role of fibroblast growth factor 21 in the early stage of NASH induced by methionine- and choline-deficient diet. Biochimica et Biophysica Acta 1852(7), 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Tanaka E, Sheena Y, Komatsu M, Okiyama W, Misawa N, … Kiyosawa K (2006). Useful parameters for distinguishing nonalcoholic steatohepatitis with mild steatosis from cryptogenic chronic hepatitis in the Japanese population. Liver International 26(8), 956–963. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Yazaki M, & Kobayashi K (2007). A lean man with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology 5(3), A32. [DOI] [PubMed] [Google Scholar]
- Tomita K, Azuma T, Kitamura N, Nishida J, Tamiya G, Oka A, … Ishii H (2004). Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology 126(3), 873–885. [DOI] [PubMed] [Google Scholar]
- Toyama T, Nakamura H, Harano Y, Yamauchi N, Morita A, Kirishima T, … Okanoue T (2004). PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochemical and Biophysical Research Communications 324(2), 697–704. [DOI] [PubMed] [Google Scholar]
- Tsuruta G, Tanaka N, Hongo M, Komatsu M, Horiuchi A, Hamamoto K, … Tanaka E (2010). Nonalcoholic fatty liver disease in Japanese junior high school students: Its prevalence and relationship to lifestyle habits. Journal of Gastroenterology 45(6), 666–672. [DOI] [PubMed] [Google Scholar]
- Tsutsui M, Tanaka N, Kawakubo M, Sheena Y, Horiuchi A, Komatsu M, … Sano K (2010). Serum fragmented cytokeratin 18 levels reflect the histological activity score of nonalcoholic fatty liver disease more accurately than serum alanine amino-transferase levels. Journal of Clinical Gastroenterology 44(6), 440–447. [DOI] [PubMed] [Google Scholar]
- Uppal H, Toma D, Saini SP, Ren S, Jones TJ, & Xie W (2005). Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology 41(1), 168–176. [DOI] [PubMed] [Google Scholar]
- Venteclef N, Jakobsson T, Ehrlund A, Damdimopoulos A, Mikkonen L, Ellis E, … Treuter E (2010). GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes & Development 24(4), 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Gao J, & Xie W (2009). PXR and CAR in energy metabolism. Trends inEndocrinology and Metabolism 20(6), 273–279. [DOI] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, & Evans RM (2003). Peroxi-some-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 113(2), 159–170. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Horai Y, Houten SM, Morimoto K, Sugizaki T, Arita E, … Auwerx J (2011). Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. The Journal of Biological Chemistry 286(30), 26913–26920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, … Wan Y (2012). Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proceedings of the National Academy of Sciences of the United States of America 109(8), 3143–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest R, Lawson M, & Geuking M (2014). Pathological bacterial translocation in liver cirrhosis. Journal of Hepatology 60(1), 197–209. [DOI] [PubMed] [Google Scholar]
- Willett C, Caverly Rae J, Goyak KO, Landesmann B, Minsavage G, & Westmoreland C (2014). Pathway-based toxicity: History, current approaches and liver fibrosis and steatosis as prototypes. ALTEX 31(4), 407–421. [DOI] [PubMed] [Google Scholar]
- Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, & Mangelsdorf DJ (1995). LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes & Development 9(9), 1033–1045. [DOI] [PubMed] [Google Scholar]
- Xie C, Jiang C, Shi J, Gao X, Sun D, Sun L, … Gonzalez FJ (2017). An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66(3), 613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MJ, Cai Y, Wang H, Altamirano J, Chang B, Bertola A, … Gao B (2015). Fatspecific protein 27/CIDEC promotes development of alcoholic steatohepatitis in mice and humans. Gastroenterology 149(4), 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Park JG, So JS, & Lee AH (2015). Transcriptional activation of Fsp27 by the liver-enriched transcription factor CREBH promotes lipid droplet growth and hepatic steatosis. Hepatology 61(3), 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Kakizaki S, Horiguchi N, Sohara N, Sato K, Takagi H, … Negishi M (2007). The role of the nuclear receptor constitutive androstane receptor in the pathogenesis of non-alcoholic steatohepatitis. Gut 56(4), 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Huang X, Yi T, Yen Y, Moore DD, & Huang W (2007). Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Research 67(3), 863–867. [DOI] [PubMed] [Google Scholar]
- Yang ZX, Shen W, & Sun H (2010). Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatology International 4(4), 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, & Scherer PE (2013). Adiponectin, driver or passenger on the road to insulin sensitivity? Molecular Metabolism 2(3), 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Wang Y, Li H, Jia W, Man K, Lo CM, … Xu A (2014). Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology 60(3), 977–989. [DOI] [PubMed] [Google Scholar]
- Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, … Lazar MA (1995). Differential activation of peroxisome proliferator-activated receptors by eicosanoids. The Journal of Biological Chemistry 270(41), 23975–23983. [DOI] [PubMed] [Google Scholar]
- Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, … Reddy JK (2003). Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. The Journal of Biological Chemistry 278(1), 498–505. [DOI] [PubMed] [Google Scholar]
- Yu J, Shen B, Chu ES, Teoh N, Cheung KF, Wu CW, … Sung JJ (2010). Inhibitory role of peroxisome proliferator-activated receptor gamma in hepatocarcinogenesis in mice and in vitro. Hepatology 51(6), 2008–2019. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Breevoort SR, Angdisen J, Fu M, Schmidt DR, Holmstrom SR, … Schulman IG (2012). Liver LXRα expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. The Journal of Clinical Investigation 122(5), 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZD, Cayting P, Weinstock G, & Gerstein M (2008). Analysis of nuclear receptor pseudogenes in vertebrates: How the silent tell their stories. Molecular Biology and Evolution 25(1), 131–143. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang J, Liu Q, & Harnish DC (2009). Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. Journal of Hepatology 51(2), 380–388. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, & Yang T (2005). Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proceedings of the National Academy of Sciences of the United States of America 102(26), 9406–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong D, Huang G, Zhang Y, Zeng Y, Xu Z, Zhao Y, … He F (2013). MicroRNA-1 and microRNA-206 suppress LXRα-induced lipogenesis in hepatocytes. Cellular Signalling 25(6), 1429–1437. [DOI] [PubMed] [Google Scholar]
- Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, … Xie W (2008). Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 134(2), 556–567. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, … Xie W (2006). A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. The Journal of Biological Chemistry 281(21), 15013–15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, … Parks JS (2010). Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. Journal of Lipid Research 51(11), 3196–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]