Abstract
Background:
Pathological mutations in cyclin-dependent kinase-like 5 cause CDKL5 deficiency disorder (CDD), a genetic syndrome associated with severe epilepsy, cognitive, motor, visual and autonomic disturbances. CDD is a relatively common genetic cause of early-life epilepsy. A specific severity assessment is lacking, required to monitor clinical course, define the natural history and for clinical trial readiness.
Methods:
A severity assessment was developed based on clinical and research experience from the International Foundation for CDKL5 Research Centers of Excellence consortium and the NIH Rett and Rett-related disorders Natural History Study consortium. An initial draft severity assessment was presented and reviewed at the annual CDKL5 Forum meeting (Boston, 2017). Subsequently it was iterated through four cycles of a modified Delphi process by a group of clinicians, researchers, industry, patient advisory groups and parents familiar with this disorder until consensus was achieved. The revised version of the severity assessment was presented for review, comment and piloting to families at the International Foundation for CDKL5 Research sponsored family meeting (Colorado, 2018). Final revisions were based on this additional input.
Results:
The final severity assessment comprised 51 items that comprehensively describe domains of epilepsy, motor, cognition, behavior, vision, speech and autonomic function. Parental ratings of therapy effectiveness, child and family functioning are also included.
Conclusions:
A severity assessment was rapidly developed with input from multiple stake-holders. Refinement through ongoing validation is required for future clinical trials. The consensus methods employed for the development of the severity assessment may be applicable to similar rare disorders.
Keywords: CDKL5, rare disorder, severity assessment, epilepsy, cortical visual impairment, intellectual disability
Introduction
Pathological mutations in cyclin-dependent kinase-like 5 (CDKL5)[1–5] result in CDKL5 Deficiency Disorder Disorder (CDD, OMIM 300203, 300672, also referred to as CDKL5 Disorder, CDKL5 Syndrome and CDKL5). Previously considered a “Rett variant”, this unique disorder [6, 7], has overlapping features with many of the developmental encephalopathies, disorders defined by genetic or presumed genetic etiology, severe seizures and intellectual/cognitive disability[8]. Incidence varies from ~1:40,000 – 60,000[9–11]; approximately one-half to one-third as common as Dravet syndrome (1:20,000–50,000)[12, 13] or Rett syndrome (1:10,000 female births)[14]. Thus, CDD is a diagnostic consideration in young children with severe, early-onset epilepsy.
CDD is associated with high rates of severe epilepsy as well as cognitive, motor, visual and autonomic disturbances [4, 15–22]. Although surveys have reported the characteristics and frequency of CDD features[6], no clinical severity assessment has integrated CDD’s clinical manifestations. Assessments for Rett Syndrome[23–26], FOXG1[27], tuberous sclerosis[28], and other developmental epileptic encephalopathies[29, 30] incorporate many CDD features, but none provide a focused nor comprehensive assessment of CDD patients. A specific severity CDD assessment targeting all clinical features is lacking and needed for clinicians to evaluate care, define natural history, inform specialist and therapeutic referrals, and with appropriate validation, to assess the outcomes of interventions in clinical trials. Given the recent initiation of human therapeutic trials (CBD[31], Ataluren ClinicalTrials.gov: , ganaxalone ClinicalTrials.gov: , TAK-935 ClinicalTrials.gov: ) and the reversibility of symptoms in CDD animal models[32], a validated assessment is urgently needed for CDD clinical trials.
We established a uniform clinical approach to patients as part of the International Foundation for CDKL5 research (IFCR) Centers of Excellence (COE) at three sites (Children’s Hospital Colorado/University of Colorado School of Medicine, Boston Children’s Hospital and Cleveland Clinic) and sites associated with the NIH-funded Rett and Rett-related disorders Natural History Study (NHS) (U54 HD061222; ClinicalTrials.gov: /). Each site collects clinical or research data on CDD patients. Application of scales and assessments developed for Rett syndrome were not adequate to capture unique features of CDD. The CDD Severity Assessment (CDD-SA) intends to capture unique features of CDD, such as epilepsy severity, cognitive, motor and visual impairment and specific aspects of movement disorder. This assessment needs to be comprehensive but efficient to administer. It must capture the distribution of abilities of CDD patients without saturating. Given the multiple stakeholders with overlapping goals for this type of assessment, we supplemented our clinical research infrastructure by recruiting into our group an international and multi-disciplinary panel of clinicians, researchers and industry professionals outside of the COE and NHS along with parents of patients directly involved in CDD patient advocacy groups. This collaboration provided input to develop and refine the CDD-SA as described here.
Methods
Clinically obtained or research-subject data available under IRB approvals (COMIRB 13–2020, 15–2332, Cleveland Clinic IRB 14–478, need Boston COE IRB P00016602 and UAB NHS parent IRB F150518001) of 111 unique patients with CDD were reviewed. Based on these data, review of available scales and literature noted above, an initial CDD-SA was developed by the principal investigator (PI: TAB) and presented at the annual CDKL5 Forum meeting (Boston, November 2017). This was followed by an open forum allowing input from stakeholders for feedback and queries. Revisions were made based on this input. We questioned whether the CDD-SA should be for clinical or research purposes, the potential domains to assess, the optimal type(s) of response scale to use, and the time-frame of evaluation that is assessed (e.g., birth to present, prior 6 months to present, last month to present and last week to present). Domains considered to be relevant included: overall severity of disorder, epilepsy, cognition, motor function, vision, autonomic disturbances and movement disorders. Response scale that were considered included: 5-point scales (evaluating frequency or severity of a feature), Likert scales (evaluating the appropriateness of a statement) and global impressions of severity or change (caregiver- and clinician global impression scales). We agreed that a clinical component provided by an examination was needed to complement and inform caregiver reported observations, leading to parent and clinician sections of the CDD-SA.
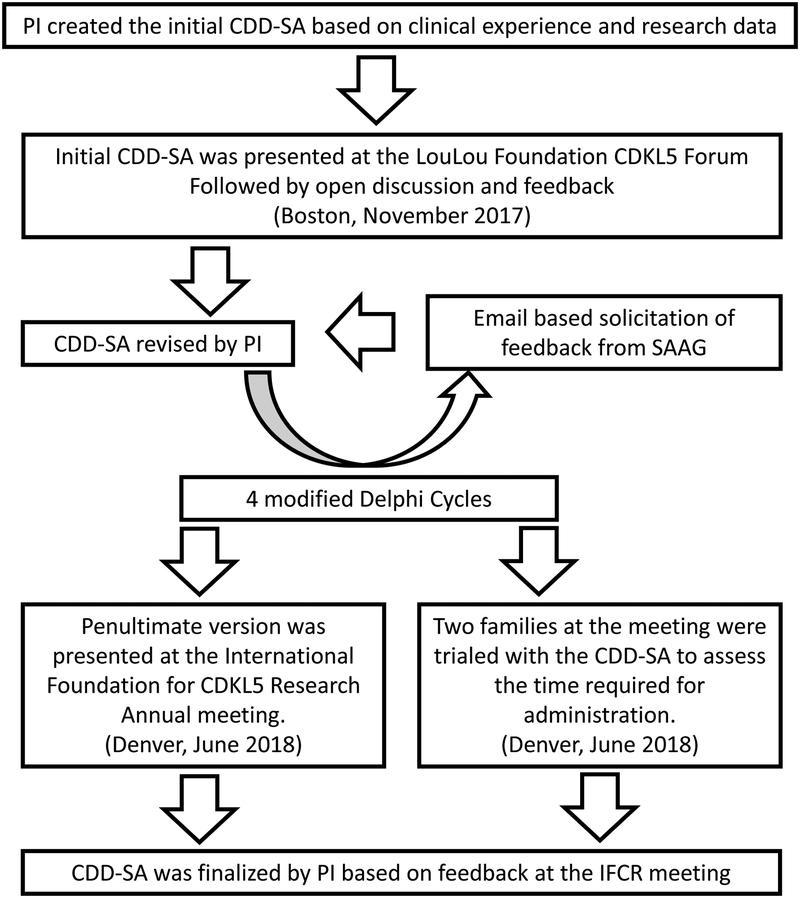
The CDD-SA was then iteratively evaluated through four cycles of anonymous modified Delphi[33] comment and consensus by an international panel of clinicians, researchers, industry, patient advisory groups and parents familiar with CDD (Figure 1). The group grew in numbers from those initially present at the Boston LouLou Foundation CDKL5 Forum to the full CDD-SA advisory group (SAAG, Table 1). Each CDD-SA version was emailed to the group and returned to the PI with comments and suggested changes. The number of questions in each domain, the specific items in each domain and the wording of items were debated and modified to accurately reflect experiences of each group of contributors. The number of items began at 24 and converged by the 3rd round to approximately 50 items, similar to the final. The feasibility of applying the CDD-SA in a clinical setting led to a reduction of items in each domain. The PI reviewed all comments, developed an independently ascertained best consensus from suggested changes, revised the CDD-SA and returned this to the review group with prior anonymous comments to provide historical background from the previous CDD-SA version. This allowed the group to understand the rationale for emerging consensus and provide commentary as to whether the emerging consensus was tracking with the intended changes to the CDD-SA. While this was not a survey-based approach like a traditional Delphi process the overall method of eliciting feedback and creating consensus was similar. The number of participants remained consistent throughout the review period, with no drop outs, providing a representative stakeholder input. The penultimate CDD-SA version was presented by the PI at the IFCR annual meeting to parents of over 100 CDD patients (Denver, June 2018) for review, comment and trial. All families present were provided access to the CDD-SA and comments were solicited and received for a duration of four weeks after the conference. Two families (whose children were not managed by the PI) agreed to trial the CDD-SA at the meeting; the time to administer the CDD-SA was measured and collected. The final revision of the CDD-SA was based on this additional input to result in the current CDD-SA (Figure 2). There was full consensus by SAAG members on the final CDD-SA.
Figure 1: Modified Delphi process for CDD-SA development.
Table 1:
CDD Severity Assessment Advisory Group (SAAG). Affiliations for non-authors noted.
| Sam Amin | Helen Leonard |
| Richard Chin | Eric Marsh |
| J Helen Cross | Lorraine Masuoka (Marinus) |
| Scott Demarest | Jeff Neul |
| Orrin Devinsky | Heather Olson |
| Jenny Downs | Axel Panzer |
| Katheryn Frame | Sumit Parikh |
| Jayne Gershkowitz (Amicus) | Carol-Anne Partridge |
| Femida Gwadry-Sridhar | Alan Percy |
| Joe Horrigan (Amo) | Elia M. Pestana-Knight |
| Amanda Jaksha | Sunny Philp (University of Birmingham, UK) |
| Walter Kaufmann | Robin Ryther (Washington University, USA) |
| Michael Johnson (Imperial College, UK) | Meghan Thorne-Miller (Roche) |
| Omar Khwaja | Karen Utley |
| Denise lasbury (CDKL5-UK) | Judy Weisenberg |
| Dan Lavery (LouLou Foundation) | Ashley Winslow (LouLou Foundation) |
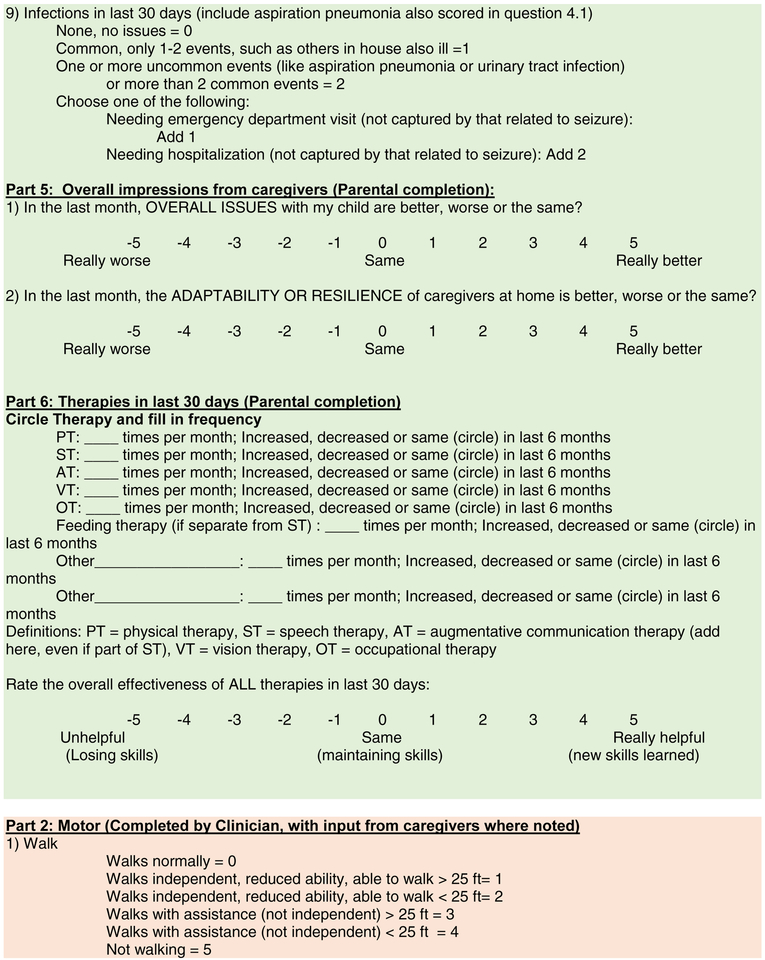
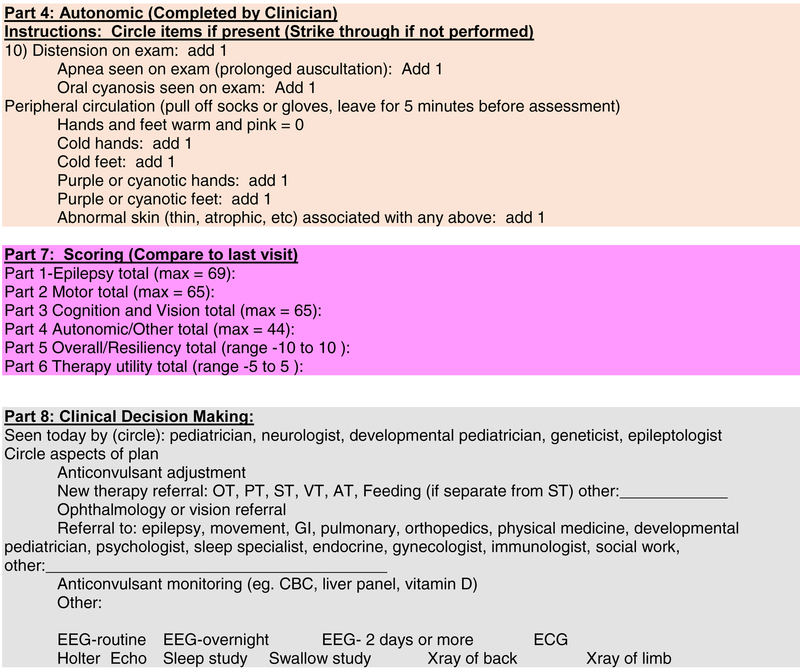
Figure 2: CDD-SA.
The Final CDD-SA with brief instructions on completion.
Results
After multiple revisions by the SAAG, the domains selected were epilepsy, cognition and motor, vision and autonomic function. Movement disorders were included within the motor domain. Clinical examination components were separated from the parent-report section within the cognition, motor, vision, and autonomic domains. This allowed a combination of parent or caregiver-report and a clinician completed portion based on physical exam findings. Parental components would be completed prior to the clinical examination; the time to complete this component has not yet been captured. In a pilot clinical examination, the parent portion was reviewed and the clinical portion was completed in 30 minutes by each of the two volunteer families.
Use of a global impression of severity[24] was rejected by the SAAG because these impression scales may rate self (caregiver)-described and patient-specific features that limit comparisons between patients. Thus the clinical value of a global impression of severity may not translate to research settings and could be a limitation in that context. The 5-point scale (0=normal, 5=most severe), similar to that used in the Rett syndrome Motor-Behavioral Assessment (MBA) [25] was selected, with higher scores more severe. Likert scales were added, as a compromise to deletion of the global impressions scale, for ratings of overall child improvement and parent/caregiver resilience and adaptability (−5=worse, 0 = no change, 5=best possible) and evaluation of therapies (−5=worse, 0 = no change, 5=best possible).
The SAAG determined that the CDD-SA evaluation time-frame should reflect developmental and longitudinal changes[20]. Use of the birth-to-present questions were limited since they could reflect ceiling effects or static assessments that would be insensitive to change. Month-to-present time-frames were considered most likely to reflect accurate changes, though week-to-present time-frames could be substituted if a clinical trial required frequent assessments. Since clinical assessments not part of a clinical trial may occur at 6-monthly intervals, 6-month to present time-frames were also included.
The wording of the items was simplified during the iterations substantially, especially in the epilepsy domain given the complexities of classifying seizures. CDD is associated with multiple seizure types, including prolonged and atypical aura, epileptic spasms, tonic, tonic-clonic, myoclonic and atypical absence [18, 19, 22, 34–36]. Further, a single seizure may involve multiple types that evolve, while other seizures can be challenging to characterize even by experts using video EEG [37]. This feature of epilepsy associated with CDD makes traditional seizure counting difficult for parents and caregivers [38, 39]. Rather, estimates of frequency and impact on function were agreed upon instead. While this approach substitutes one subjective assessment for another, it becomes more patient-centered.
The clinical portion was based on features typically evaluated during an exam by a pediatric neurologist. However, certain CDD-SA components would likely add time to the routine visit, especially if that clinical visit includes a discussion of clinical decision making. Regardless of the country and practice considerations, the CDD-SA had to provide relevant data that could be assimilated and utilized at a clinical visit. The final domains and details of the exam were considered recommendations: clinicians would tailor their approach such that not every item within their usual assessment would necessarily be included for all visits or all patients, although the items seek to limit clinician-to-clinician variability. It can be challenging to assess the breadth of features and the functional impact of movement disorders within a clinical visit. Also, any clinical examination is a snap-shot in time, and may not assess some areas captured for which extended observation by a parent or caregiver may be more informative. There are similar challenges when assessing cognition and vision in CDD patients who are often non-verbal and have some degree of visual impairment. Cognition assessment is limited by both exam time and CDD features to assessing choice and visual attention in the CDD-SA.
In summary (Table 2 and Figure 2), the final CDD-SA comprised 4 domains: 1) Epilepsy, 2) Motor, 3) Cognition, Behavior and Vision and 4) Autonomic, that are nearly equally weighted with similar maximum scores (69, 65, 65 and 44, respectively) on items that mostly were scored on a 0 to 5 range. Impressions of overall improvement, parent/caregiver resiliency and therapy utility were each given a −5 to 5 Likert scale. An optional part of the CDD-SA was medical decision making. While no points were assigned to each intervention, the goal was to provide a formulaic framework to track the impact of these when the CDD-SA is used in a primarily clinical setting. Secondary scoring of data to reflect impact could be developed based on features such as patient discomfort and invasiveness, financial impact, impact to parent/caregivers, etc.
Table 2.
Composition of the CDD-SA by domain and source of data
| Domain | By Caregiver | # questions | By Clinicians | # questions | Total # questions |
|---|---|---|---|---|---|
| 1. Epilepsy | Yes | 15 | No | 0 | 15 |
| 2. Motor | No | 0 | Yes | 13 | 13 |
| 3. Cognition and Vision | Yes | 1 | Yes | 12 | 13 |
| 4. Autonomic | Yes | 9 | Yes | 1 | 10 |
| 5. Overall | Yes | 2 | No | 0 | 2 |
| 6. Therapies | Yes | 1 | No | 0 | 1 |
| 7. Scale Scoring | No | - | Yes | ||
| 8. Visit notes | No | - | Yes |
Discussion and Conclusions
Using a modified Delphi process, we developed a new clinically relevant and easily administered severity assessment (SA) for CDD (CDD-SA). With on-going natural history studies such as the NIH-funded NHS and current and planned drug trials specifically for patients with CDD, our CDD-SA offers the ability capture aspects of this disorder that may change with time or in response to interventions. In the first instance, we have provided some evidence for its content validity, basing the CDD-SA on available literature, the clinical and research experience of an international panel of experts and the lived experience of our parent participants. We achieved a consensus across a broad spectrum of international clinicians from multiple specialties and subspecialties, parents, lay organizations and industry professionals to develop this CDD-SA .
A limitation of the process was the lack of a framework with an objective ‘gold-standard’ to validate our CDD-SA. Further, both the stakeholders and the PI could not reliably determine the relative value of specific recommendations, nor the validity of the scale to measure the feature of interest. Bias by the PI in adjudicating disagreements and alternative views could be an inherent limit of this process but was countered by extensive expertise of the investigators and the lived experiences of families in the consultation process. The SAAG input helped ensure the comprehensive and disease appropriate nature of the CDD-SA and it is unlikely that the primary domains will need major alterations in the future. The SAAG-approved SA is being applied in CDD Centers of Excellence and can be applied in other clinical and research settings. This will provide the basis for future validation that will include some refinement of necessary items and language. In addition, qualitative data is needed to validate parental interpretations of questions and refine future versions in order to determine the sensitivity of the CDD-SA. A quantitative dataset with a large sample size will be necessary to determine change with interventions, evaluate interrater reliability, factor analyses, stability and responsiveness over time.
We propose that our clinical assessment will have immediate utility with clinicians who see children with CDD. The CDD-SA is freely available for general use. This methodology could be applied to the development of clinical assessments for other rare genetic disorders and the framework could potentially serve as an early foundation to other constituent organizations. Key aspects that allowed this to happen included an initial framework (COE and NHS) that standardized the identification of clinical features relevant to CDD. Next, those that were outside of the COE and NHS were included in the process. The support of patient advocacy groups and associated parents/caregivers provided mission-critical context. Finally, a willingness to collaborate by the SAAG despite many other commitments and time constraints allowed the process to move forward.
Acknowledgements:
We sincerely thank all of the patients and families that have participated in this research.
Scott Demarest: NIH/NINDS NSADA K12 (1K12NS089417-01), Children’s Hospital Colorado Research Institute and the International Foundation for CDKL5 Research
Elia M. Pestana Knight: nothing to declare.
Jenny Downs: International Foundation for CDKL5 Research, NHMRC #1103745
Heather Olson: International Foundation for CDKL5 Research, NIH/NINDS K23 NS107646–01
Eric D. Marsh: NIH U54 HD061222
Walter E. Kaufmann: International Foundation for CDKL5 Research
Carol-Anne Partridge: nothing to declare
Helen Leonard: NHMRC Senior Research Fellowship #1103741, International Foundation for CDKL5 Research
Femida Gwadry-Sridhar: nothing to declare
Katheryn Elibri Frame: nothing to declare
J. Helen Cross: nothing to declare
Richard F. M. Chin: nothing to declare
Sumit Parikh: International Foundation for CDKL5 Research
Axel Panzer: nothing to declare
Judith Weisenberg: International Foundation for CDKL5 Research
Karen Utley: nothing to declare
Amanda Jaksha: nothing to declare
Sam Amin: nothing to declare.
Omar Khwaja: nothing to declare
Orin Devinsky: nothing to declare
Jeffery L. Neul: NIH U54 HD061222
Alan K. Percy: NIH U54 HD061222; Rett Syndrome Research Trust
Tim A. Benke: International Foundation for CDKL5 Research, Loulou Foundation, NIH U54 HD061222, Children’s Hospital Colorado Foundation Ponzio Family Chair in Neurology Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest:
Scott Demarest: Consulting for Upsher-Smith and BioMarin. All remuneration has been made to his department.
Elia M. Pestana Knight: None
Jenny Downs: Consultancy for Avexis, Anavex, GW and Marinus. Any remuneration has been made to her department.
Heather Olson: None
Eric D. Marsh: Funding from the NIH, Rettsyndrome.org, and Rett Syndrome Research Trust, Site PI on studies from GW pharma, Zogenix Pharma, Marinus pharma, consultant to Stoke therapeutics.
Walter E. Kaufmann: None
Carol-Anne Partridge: None
Helen Leonard: None
Femida Gwadry-Sridhar: None
Katheryn Elibri Frame: None
J. Helen Cross: J Helen Cross has participated as a clinical investigator for Zogenix, GW Pharma, Marinius Pharmaceuticals and Vitaflo. She has been a member of advisory boards and speaker for Eisai, GW Pharma, Nutricia and Zogenix. All remuneration has been made to her department.
Richard F. M. Chin: None
Sumit Parikh: None
Axel Panzer: None
Judith Weisenberg: None
Karen Utley: None
Amanda Jaksha: None
Sam Amin: None.
Omar Khwaja: None
Orin Devinsky: Consultancy/advisory: Privateer Holdings/Tilray, Egg Rock/Papa & Barkley, Receptor Life Sciences, Empatica, Tevard, Engage, Rettco, Pairnomix/Q-state, Zogenix and GW Pharmaceuticals.
Jeffery L. Neul: Funding from the NIH, Consultancy with Acadia, AveXis, Biohaven, GW Pharmaceuticals, Neuren, Newron, Takeda, Teva
Alan K. Percy: Consultancy for Anavex, AveXIs, and GW Pharmaceuticals; Clinical Trial with Newron Pharmaceuticals
Tim A. Benke: Consultancy for AveXis, Ovid, Takeda and Marinus. All remuneration has been made to his department.
References
- 1.Tao J, Van Esch H, Hagedorn-Greiwe M, Hoffmann K, Moser B, Raynaud M, Sperner J, Fryns JP, Schwinger E, Gecz J et al. : Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5/STK9) gene are associated with severe neurodevelopmental retardation. Am J Hum Genet 2004, 75(6):1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaving LS, Christodoulou J, Williamson SL, Friend KL, McKenzie OL, Archer H, Evans J, Clarke A, Pelka GJ, Tam PP et al. : Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. AmJ HumGenet 2004, 75(6):1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer HL, Evans J, Edwards S, Colley J, Newbury-Ecob R, O’Callaghan F, Huyton M, O’Regan M, Tolmie J, Sampson J et al. : CDKL5 mutations cause infantile spasms, early onset seizures, and severe mental retardation in female patients. J Med Genet 2006, 43(9):729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahi-Buisson N, Villeneuve N, Caietta E, Jacquette A, Maurey H, Matthijs G, Van EH, Delahaye A, Moncla A, Milh M et al. : Recurrent mutations in the CDKL5 gene: genotype-phenotype relationships. AmJ MedGenetA 2012, 158A(7):1612–1619. [DOI] [PubMed] [Google Scholar]
- 5.Hector RD, Kalscheuer VM, Hennig F, Leonard H, Downs J, Clarke A, Benke TA, Armstrong J, Pineda M, Bailey MES et al. : CDKL5 variants: Improving our understanding of a rare neurologic disorder. Neurol Genet 2017, 3(6):e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehr S, Wilson M, Downs J, Williams S, Murgia A, Sartori S, Vecchi M, Ho G, Polli R, Psoni S et al. : The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. EurJ HumGenet 2013, 21(3):266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangatt M, Wong K, Anderson B, Epstein A, Hodgetts S, Leonard H, Downs J: Prevalence and onset of comorbidities in the CDKL5 disorder differ from Rett syndrome. Orphanet J Rare Dis 2016, 11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paciorkowski AR, Seltzer LE, Neul JL: Developmental Encephalopathies In: Swaiman’s Pediatric Neurology. Edited by Swaiman KF, Ashwal S, Ferriero DM, Schor NF, Finkel RS, Gropman AL, Pearl PL, Shevell MI, 6 edn Philadelphia: Mosby; 2018: 242–248. [Google Scholar]
- 9.Lindy AS, Stosser MB, Butler E, Downtain-Pickersgill C, Shanmugham A, Retterer K, Brandt T, Richard G, McKnight DA: Diagnostic outcomes for genetic testing of 70 genes in 8565 patients with epilepsy and neurodevelopmental disorders. Epilepsia 2018, 59(5):1062–1071. [DOI] [PubMed] [Google Scholar]
- 10.Kothur K, Holman K, Farnsworth E, Ho G, Lorentzos M, Troedson C, Gupta S, Webster R, Procopis PG, Menezes MP et al. : Diagnostic yield of targeted massively parallel sequencing in children with epileptic encephalopathy. Seizure 2018, 59:132–140. [DOI] [PubMed] [Google Scholar]
- 11.Symonds JD, Zuberi SM, Vincent A, Lang B, Dorris L, Brunklaus A, Ellis R, Jollands A, Joss S, Kirkpatrick M et al. : The Genetic and Autoimmune Childhood Epilepsy (GACE) Study. In: American Epilpesy Society: 2017; Washington, D.C. 2017. [Google Scholar]
- 12.Rosander C, Hallbook T: Dravet syndrome in Sweden: a population-based study. Dev Med Child Neurol 2015, 57(7):628–633. [DOI] [PubMed] [Google Scholar]
- 13.Wu YW, Sullivan J, McDaniel SS, Meisler MH, Walsh EM, Li SX, Kuzniewicz MW: Incidence of Dravet Syndrome in a US Population. Pediatrics 2015, 136(5):e1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurvick CL, de Klerk N, Bower C, Christodoulou J, Ravine D, Ellaway C, Williamson S, Leonard H: Rett syndrome in Australia: a review of the epidemiology. J Pediatr 2006, 148(3):347–352. [DOI] [PubMed] [Google Scholar]
- 15.Buoni S, Zannolli R, Colamaria V, Macucci F, di Bartolo RM, Corbini L, Orsi A, Zappella M, Hayek J: Myoclonic encephalopathy in the CDKL5 gene mutation. Clin Neurophysiol 2006, 117(1):223–227. [DOI] [PubMed] [Google Scholar]
- 16.Bahi-Buisson N, Nectoux J, Rosas-Vargas H, Milh M, Boddaert N, Girard B, Cances C, Ville D, Afenjar A, Rio M et al. : Key clinical features to identify girls with CDKL5 mutations. Brain 2008, 131(Pt 10):2647–2661. [DOI] [PubMed] [Google Scholar]
- 17.Nemos C, Lambert L, Giuliano F, Doray B, Roubertie A, Goldenberg A, Delobel B, Layet V, N’Guyen MA, Saunier A et al. : Mutational spectrum of CDKL5 in early-onset encephalopathies: a study of a large collection of French patients and review of the literature. ClinGenet 2009, 76(4):357–371. [DOI] [PubMed] [Google Scholar]
- 18.Castren M, Gaily E, Tengstrom C, Lahdetie J, Archer H, Ala-Mello S: Epilepsy caused by CDKL5 mutations. Eur J Paediatr Neurol 2011, 15(1):65–69. [DOI] [PubMed] [Google Scholar]
- 19.Melani F, Mei D, Pisano T, Savasta S, Franzoni E, Ferrari AR, Marini C, Guerrini R: CDKL5 gene-related epileptic encephalopathy: electroclinical findings in the first year of life. Developmental Medicine and Child Neurology 2011, 53(4):354–360. [DOI] [PubMed] [Google Scholar]
- 20.Fehr S, Leonard H, Ho G, Williams S, de Klerk N, Forbes D, Christodoulou J, Downs J: There is variability in the attainment of developmental milestones in the CDKL5 disorder. J Neurodev Disord 2015, 7(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fehr S, Downs J, Ho G, de Klerk N, Forbes D, Christodoulou J, Williams S, Leonard H: Functional abilities in children and adults with the CDKL5 disorder. Am J Med Genet A 2016, 170(11):2860–2869. [DOI] [PubMed] [Google Scholar]
- 22.Fehr S, Wong K, Chin R, Williams S, de Klerk N, Forbes D, Krishnaraj R, Christodoulou J, Downs J, Leonard H: Seizure variables and their relationship to genotype and functional abilities in the CDKL5 disorder. Neurology 2016, 87(21):2206–2213. [DOI] [PubMed] [Google Scholar]
- 23.Downs J, Stahlhut M, Wong K, Syhler B, Bisgaard AM, Jacoby P, Leonard H: Validating the Rett Syndrome Gross Motor Scale. PLoS One 2016, 11(1):e0147555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neul JL, Glaze DG, Percy AK, Feyma T, Beisang A, Dinh T, Suter B, Anagnostou E, Snape M, Horrigan J et al. : Improving Treatment Trial Outcomes for Rett Syndrome: The Development of Rett-specific Anchors for the Clinical Global Impression Scale. J Child Neurol 2015, 30(13):1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neul JL, Fang P, Barrish J, Lane J, Caeg EB, Smith EO, Zoghbi H, Percy A, Glaze DG: Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology 2008, 70(16):1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colvin L, Fyfe S, Leonard S, Schiavello T, Ellaway C, de KN, Christodoulou J, Msall M, Leonard H: Describing the phenotype in Rett syndrome using a population database. ArchDisChild 2003, 88(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma M, Adams HR, Seltzer LE, Dobyns WB, Paciorkowski AR: Phenotype Differentiation of FOXG1 and MECP2 Disorders: A New Method for Characterization of Developmental Encephalopathies. J Pediatr 2016, 178:233–240 e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey A, Ploubidis GB, Yates JR, Steinberg T, Bolton PF: The Early Childhood Epilepsy Severity Scale (E-Chess). Epilepsy Res 2008, 79(2–3):139–145. [DOI] [PubMed] [Google Scholar]
- 29.Purusothaman V, Ryther RC, Bertrand M, Harker LA, Jeffe DB, Wallendorf M, Smyth MD, Limbrick DD: Developing the Pediatric Refractory Epilepsy Questionnaire: a pilot study. Epilepsy Behav 2014, 37:26–31. [DOI] [PubMed] [Google Scholar]
- 30.Carpay HA, Arts WF: Outcome assessment in epilepsy: available rating scales for adults and methodological issues pertaining to the development of scales for childhood epilepsy. Epilepsy Res 1996, 24(3):127–136. [DOI] [PubMed] [Google Scholar]
- 31.Devinsky O, Verducci C, Thiele EA, Laux LC, Patel AD, Filloux F, Szaflarski JP, Wilfong A, Clark GD, Park YD et al. : Open-label use of highly purified CBD (Epidiolex(R)) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav 2018, 86:131–137. [DOI] [PubMed] [Google Scholar]
- 32.Trazzi S, De Franceschi M, Fuchs C, Bastianini S, Viggiano R, Lupori L, Mazziotti R, Medici G, Lo Martire V, Ren E et al. : CDKL5 protein substitution therapy rescues neurological phenotypes of a mouse model of CDKL5 disorder. Hum Mol Genet 2018, 27(9):1572–1592. [DOI] [PubMed] [Google Scholar]
- 33.Jorm AF: Using the Delphi expert consensus method in mental health research. Aust N Z J Psychiatry 2015, 49(10):887–897. [DOI] [PubMed] [Google Scholar]
- 34.Moseley BD, Dhamija R, Wirrell EC, Nickels KC: Historic, clinical, and prognostic features of epileptic encephalopathies caused by CDKL5 mutations. PediatrNeurol 2012, 46(2):101–105. [DOI] [PubMed] [Google Scholar]
- 35.Bahi-Buisson N, Kaminska A, Boddaert N, Rio M, Afenjar A, Gerard M, Giuliano F, Motte J, Heron D, Morel MA et al. : The three stages of epilepsy in patients with CDKL5 mutations. Epilepsia 2008, 49(6):1027–1037. [DOI] [PubMed] [Google Scholar]
- 36.Guerrini R, Parrini E: Epilepsy in Rett syndrome, and CDKL5- and FOXG1-gene-related encephalopathies. Epilepsia 2012, 53(12):2067–2078. [DOI] [PubMed] [Google Scholar]
- 37.Klein KM, Yendle SC, Harvey AS, Antony JH, Wallace G, Bienvenu T, Scheffer IE: A distinctive seizure type in patients with CDKL5 mutations: Hypermotor-tonic-spasms sequence. Neurology 2011, 76(16):1436–1438. [DOI] [PubMed] [Google Scholar]
- 38.Goldenholz DM, Rakesh K, Kapur K, Gainza-Lein M, Hodgeman R, Moss R, Theodore WH, Loddenkemper T: Different as night and day: Patterns of isolated seizures, clusters, and status epilepticus. Epilepsia 2018, 59(5):e73–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tharayil JJ, Chiang S, Moss R, Stern JM, Theodore WH, Goldenholz DM: A big data approach to the development of mixed-effects models for seizure count data. Epilepsia 2017, 58(5):835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]