Abstract
Neonatal abstinence syndrome from opioids (NAS-O) is a multisystem disorder resulting in neurological and gastrointestinal symptoms from the abrupt discontinuation of chronic fetal exposure to opioids. Increasing opioid use during pregnancy has led to a five-fold increase in NAS-O nationally over the past decade. Several knowledge gaps in our experiences with opioid-exposed neonates have been identified: 1) universal maternal screening; 2) diagnostic tools for newborn abstinence syndrome; 3) optimal treatment protocols; and 4) long-term neurodevelopmental effects of fetal opioid exposure. This review article gives a broad overview of the issues associated with screening, diagnosis, and management of opioid exposure in newborns as well as the issues with collecting accurate outcomes data to monitor efforts aimed at curbing opioid exposure in children and NAS-O. Data specific to Oklahoma is provided, when available.
Background
The epidemic of overdose deaths from opioid use has become a hot topic across the country. Deaths from prescription opioids in the early 2000s began to rise rapidly as the United States consumed 85% of the oxycodone and 99% of the hydrocodone in the world, despite having less than 5% of the world’s population.1 By 2014, Oklahoma was ranked number one in the abuse of painkiller drugs.1 Just two years later, there were 899 drug overdose deaths in Oklahoma, a 68% increase from 2007.1 Of those, almost half were opioid-related overdose deaths, at a rate of 11.6 deaths per 100,000 persons (compared with the national rate of 13.3 deaths per 100,000).2 In response to the increasing awareness of the problem, The Oklahoma Commission on Opioid Abuse was convened in 2017.
Opioid prescriptions are, by far, the largest contributor to opioid-related overdose deaths in Oklahoma, resulting in a death rate three times higher than that of synthetic opioids or heroin.2 In 2015, Oklahoma healthcare providers wrote the equivalent of one opioid prescription for every Oklahoman, a rate 30% higher than the national average [IMS Health, 2016].2 Once someone can no longer legitimately obtain prescription opioids, they may turn to cheaper and easier-to-obtain alternatives, such as heroin. Because these substances are manufactured illicitly, they may contain ingredients unknown to the user, resulting in a higher risk of death. Between 2012 and 2016, heroin overdose deaths in Oklahoma more than doubled.2
In Oklahoma in 2012, an average of 2.5 opioid prescriptions were filled per pregnant woman, with one woman filling 29 prescriptions and another having 16 different prescribers.3 A national study in 2014 showed that more than one out of five pregnant women filled an opioid prescription during pregnancy, typically for abdominal pain, lower back pain, joint pain, or headaches.4 As of July 2014, the top opioid prescribers for Oklahoma Medicaid patients were in the fields of obstetrics (35%), dentistry (20%), family medicine (17%), and emergency medicine (8%) with prescriptions typically for hydrocodone (63%) or oxycodone (20%).5 There may be prescribers in other fields, such as surgery, orthopedics, or pain management, who prescribe opioids more often but Oklahoma Medicaid typically covers pregnant women and children which leads to the bias in the fields above as the primary prescribers.
With the increasing use of heroin abuse and opioid-associated overdose deaths, it has been determined that treating pregnant women with Medication Assisted Treatment (MAT) using methadone or buprenorphine is safer than continuing illicit drug use. MAT is preferred to stopping or weaning opioids during pregnancy, as abrupt discontinuation of opioids can result in preterm labor or fetal death. Presently, most states cover methadone treatment; however, there are often limits on dose and length of treatment. As a complete μ-opioid receptor agonist, methadone carries a high risk for abuse. Even with safety and efficacy evidence, only 10% of the people who need treatment are receiving it due to many barriers, including lack of both geographical and financial access.1 Patients must be enrolled in a methadone treatment program, which requires physically showing up to the treatment center daily to obtain the medication, making recovery in a rural area difficult to maintain. There are ~700 Oklahomans on waiting lists for inpatient treatment services daily.1 Buprenorphine is a partial μ-opioid receptor agonist with a lower risk for abuse and side effects. Despite this, buprenorphine is not easily obtained because the federal government limits the number of patients that any single healthcare provider can treat. Only about 3% of potential providers registered with the Drug Enforcement Administration are authorized to prescribe these medications for MAT, while, inexplicably, methadone can be prescribed for other indications, such as pain, without limitation.6
As a result of the overall increase in prescription and illicit opioid use in the general population, there are increasing numbers of infants exposed to opioids in-utero. Depending on the duration and specific opioid exposure, more than half of these neonates develop Neonatal Abstinence Syndrome related to opioids (NAS-O), a group of withdrawal symptoms that develop after birth. Symptoms may include a high-pitched cry, inconsolability, diarrhea, voracious appetite, and seizures. In addition, infants with NAS-O have lengthy hospital stays (weeks to months) with medical costs up to ten times those of normal newborns.1 The incidence of NAS (using the ICD-9 code of 779.5 and ICD-10 code of P96.1, both for neonatal withdrawal symptoms from maternal use of [unspecified] drugs of addiction) among Oklahoma Medicaid members increased from about 0.2% of live births in 2008 to 1.1% in 2014, with the highest rates in the northeastern portion of the state.3,5 In comparison, the Centers for Disease Control estimated the national average of NAS to be 0.6% of live births in the same year (CDC, MMWR, 2014).2
While the upstream issues related to opioid addiction and treatment must be addressed in the adult population to truly fix the problem, the pediatric medical community is currently trying to find the best way to treat opioid-exposed neonates in the short- and long-term. Through the National Institutes of Health “Helping to End Addiction Long-term” (HEAL) initiative,7 funding has been provided to support the IDeA States Pediatric Clinical Trials Network,8 a research collaboration of 23 states and Puerto Rico (including Oklahoma), as they develop the Advancing Clinical Trials in Neonatal Opioid Withdrawal Syndrome (ACT NOW) project.9 This project is designed to learn how to best treat and manage opioid withdrawal in neonates, as well as how to determine and manage long-term developmental consequences. Locally, the Oklahoma Perinatal Quality Improvement Collaborative is partnering with the Alliance for Innovation on Maternal Health (AIM) to lead a quality improvement initiative to reduce opioid exposure in pregnant women and their infants.
Diagnosis
The diagnosis of an opioid-exposed neonate requires a history or toxicology consistent with the maternal use of opioids during pregnancy. Obviously, the reported rates of opioid exposure will differ based on the method utilized (maternal interviews versus universal toxicology testing). The American College of Obstetrics and Gynecology (ACOG) recommends universal prenatal screening using validated screening tools, such as SBIRT (Screening, Brief Intervention, and Referral to Treatment). They do not recommend routine drug testing during pregnancy, but rather, targeted maternal urine drug testing if prenatal screening interviews or complications are suggestive of use.10
Obtaining accurate data on the number of opioid-exposed neonates is a challenge that involves appropriate diagnosis, documentation, coding, and data collection. While providers are likely to diagnose infants with symptomatic neonatal abstinence syndrome, the diagnosis of opioid exposure proves more difficult as not all infants will be symptomatic. Universal newborn toxicology testing is not recommended and many hospitals have a protocol recommending which infants should be tested, such as:
Maternal history of drug use/abuse, positive screen on a validated screening tool, or positive toxicology screen during pregnancy
High risk living situations, such as prostitution or homelessness
Involvement with the Departments of Corrections or Child Welfare
Minimal or no prenatal care
Maternal age < 18 years
Unexplained obstetric events (placental abruption, premature labor)
Unexplained infant neurological complications
Unexpected intrauterine growth restriction (IUGR)
Development of drug withdrawal symptoms
If an exposure history is not determined prenatally, the diagnosis may be made when an infant exhibits withdrawal symptoms after delivery. Several abstinence scoring systems have been developed to lend some objectivity to assessing withdrawal symptomology.11–14 The Finnegan scoring system has traditionally been the most widely adopted scoring tool, but its use is limited to infants >37 weeks gestation and <1 month of age.15 Because the Lipsitz tool has only 11 items to score, it may be preferred for NAS screening in newborn nurseries because it requires less time and has good sensitivity for identifying clinically important withdrawal.11 The Eat Sleep Console tool is the newest approach and focuses on the ability of an infant to self-regulate to determine clinically significant withdrawal. A recently conducted study showed that this approach is an effective method for the management of infants with NAS that limits pharmacologic treatment and may lead to substantial reductions in length of stay.14
Once an infant is suspected of exhibiting withdrawal symptoms, toxicology testing is typically employed. Many hospitals have started using umbilical cord testing, as the costs are comparable to the more historical methods of urine and meconium testing, and it offers the advantage of storing umbilical cords for a couple of weeks in case an infant exhibits late signs of withdrawal. Other comparisons between meconium and umbilical cord testing, including cutoff values for positive opioid results, can be seen in Figure 1.
Figure 1:
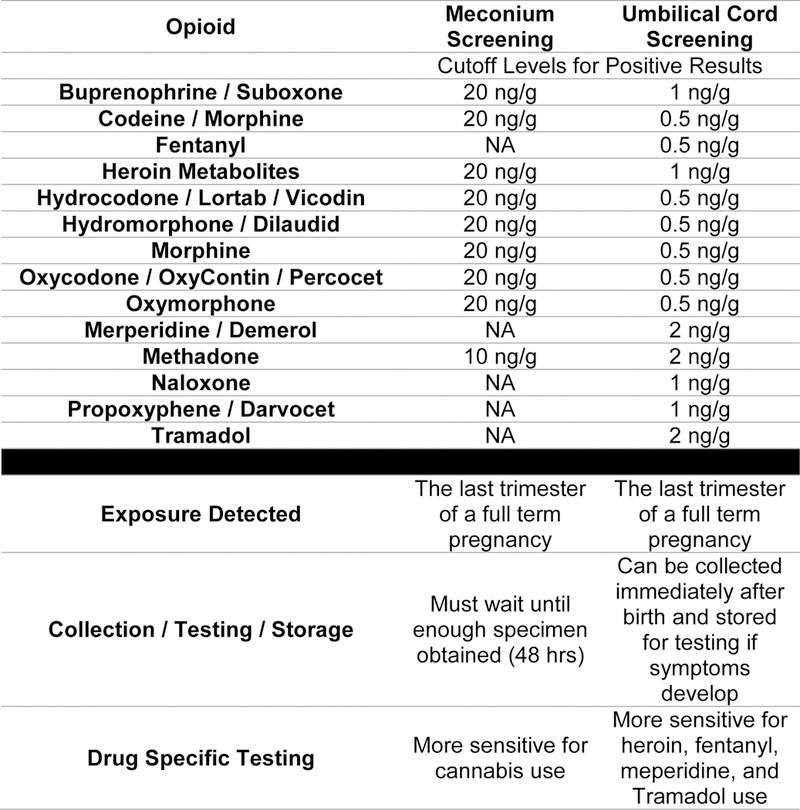
Differences in Toxicology Screening Using Meconium versus Cord Screens37
Note: Routine toxicology screening in newborns is intended for clinical and social support decision making. Chain of command handling of samples has legal implications for Department of Corrections or employment purposes and is not typically utilized in toxicology testing in newborns. If chain of custody is required, a special form should be obtained from the testing lab and the sample must be designated specifically for this specialized documentation and tracking of the collection, handling, and shipping of the specimen.
Because the time to symptomatic withdrawal is dependent on the substance and dose to which the infant is exposed, infants may not show signs of withdrawal while in the hospital during their birth stay.16 The two week period after birth is the prime period for withdrawal symptoms to manifest and pediatric providers should keep NAS-O in their differential for any infant with sepsis symptomology in this timeframe. They should be aware that many birth hospitals may still have the umbilical cord available to send for toxicology testing to confirm the diagnosis. Providers should also be aware that infants who are also exposed to nicotine may exhibit heightened withdrawal symptoms and those exposed to selective serotonin reuptake inhibitors (SSRIs) exhibit a prolonged (up to six months) serotonin syndrome (drug toxicity) with symptoms that mimic opioid withdrawal and do not respond well to the usual opioid treatments.
There are legal and social implications related to a diagnosis of opioid exposure or NAS-O. Infants may exhibit signs of withdrawal even if the substance is taken legally, and most infants born to mothers who are receiving MAT will show signs of withdrawal. While it is controversial whether these mother-infant dyads should be reported to child protective services, as the mothers were receiving medical treatment, most providers would not electively report cases where maternal and infant toxicology were negative for additional drugs of abuse. However, the Oklahoma Statute HB3104 was amended in May 2018 to remove the exception in the definition of a drug-endangered child that included “newborns who test positive for a controlled dangerous substance, with the exception of those substances administered under the care of a physician.”17 The Oklahoma Statute now mandates reporting to the Department of Human Services for any newborn who tests positive for a controlled dangerous substance, as well as those diagnosed with Neonatal Abstinence Syndrome. The burden of mandatory reporting has been broadened to “every physician, surgeon, or other health care professional including doctors of medicine, licensed osteopathic physicians, residents and interns, or any other health care professional or midwife involved in the prenatal care of expectant mothers or the delivery or care of infants shall promptly report to the Department instances in which an infant tests positive for alcohol or a controlled dangerous substance. This shall include infants who are diagnosed with Neonatal Abstinence Syndrome.”17 The preferred method of reporting is by one team member calling the Department of Human Services’ Statewide 24-hour Child Abuse Hotline at (800) 522–3511 with the following information:
The names, addresses, ages and whereabouts of the child and the child’s parents, or other persons responsible for the child’s welfare;
Information pertaining to any safety-related issues child welfare may need to be aware of prior to making contact with the family, such as domestic violence, presence of weapons, or use of illegal substances;
The nature and extent of the abuse;
Any historical information on the family related to the safety and well-being of the children and their parents or other identified caretakers.
Issues Related to Data Collection
Without universal maternal and infant toxicology screening, there will always be underreporting of opioid-exposed newborns. One of the easiest and most efficient ways to collect data is through coding and billing methods. However, this approach relies on the medical coders who depend on providers to document appropriately. Many exposed infants can be treated with non-pharmacological methods, such as limited visitors, low lighting, swaddling, ear muffs, and cuddlers, without the need for pharmacological treatment. None of these interventions are typically documented in the medical record, without the use of a checklist or other deliberate means of documentation. Premature infants are less likely to exhibit symptoms of withdrawal due to a shorter exposure time and greater placental metabolism with more p-glycoprotein transporters to pump the drug back into the maternal circulation. Providers often do not assign an official diagnosis of neonatal abstinence syndrome unless the infant requires pharmacological treatment, missing an opportunity to identify affected babies. The current ICD-10 codes have made the issue even more difficult, as the rate of reported infants depends on which code(s) an organization decides to use in the numerator for affected infants. As of October 2018, new codes will help delineate the substance(s) a newborn may be affected by with the appearance of multiple new codes including P04.14 “Newborn affected by maternal use of opiates” (Figure 2). This new coding scheme will allow a better distinction of opioid-exposed newborns but will still not delineate those exposed from maternal illicit abuse versus medication-assisted therapy. Furthermore, providers and coders will need to be educated regarding the proper coding of P96.1 “Neonatal withdrawal symptoms from maternal use of drugs of addiction” versus the new codes which are exposure codes. An infant could have a code of P04.14 for exposure alone or codes for both P04.14 and P96.1 if they are exposed and exhibit symptomatology.
Figure 2:

New ICD-10 Codes Available in October 201838
Another way to capture data is to utilize insurance claims records. Typically, this is not easily done with private insurers so most data collectors request data from public insurers and extrapolate the results to the entire population. This imposes an inherent bias in data collection that should be recognized. An efficient source of prescription data comes from the prescription monitoring programs (PMP) in each state, such as the one enacted in Oklahoma in November 2015. PMP InterConnect currently allows prescription data transmission between 45 states, including Oklahoma.18 All prescription drug dispensers in Oklahoma must submit information to the system within 24 hours of dispensing a scheduled narcotic. Data from this source may overestimate the number of prescription drug users as not all filled prescriptions are actually utilized, and obviously, not all are abusers. Furthermore, there is also the issue of diversion where an individual may take a medication prescribed to someone else, something not easy to track. These issues make it difficult for states to collect consistent data for comparison and to determine whether quality improvement interventions are effective at decreasing unnecessary prescribing or opioid exposure in newborns and/or symptomology in newborns.
Management
The management goal for infants with NAS-O is to create an optimal environment for the establishment of normal growth and development and the successful integration into their environment and family.19 The American Academy of Pediatrics recommends a two-tiered approach to management, first using non-pharmacological interventions and progressing to pharmacotherapy only when those measures fail.20 Typically, one of the scoring tools mentioned previously will be used to assess an infant assumed to be exhibiting signs of withdrawal, with each tool having a certain threshold for initiating pharmacological treatment.
Non-Pharmacological Interventions
Non-pharmacological interventions should be initiated at birth for all infants with known opioid exposure, and should continue throughout the hospital stay, regardless of the need for pharmacotherapy. The goals of these measures are to provide the infant with an environment that supports their ability to self-regulate and may include:19
Encouragement of parental involvement with skin-to-skin, cuddling, and massage
Swaddling to provide boundaries with the infant’s hands near their face
Providing a low-stimulation environment that includes a darkened room away from the noisy neonatal intensive care area to promote physiologic stability
Encouraging breastfeeding when the mother is not actively using drugs as it may reduce the severity of NAS symptoms and the need for additional pharmacotherapy, reducing length of stay.21,22
Providing small, frequent feedings and higher calorie or lactose-free formula for non-breastfeeding infants, depending on growth and other symptoms, such as diarrhea and/or emesis
Providing rhythmical rocking using swings or vibrating bouncy seats
Giving a warm, swaddled bath
Providing a pacifier to soothe
One of the keys to successful implementation of behavioral interventions is to provide parental support and education so that parents can actively participate in the care of their infants throughout the hospital stay.23 Parents should be taught to understand their child’s cues so they can help the infant to self-soothe. Families should be taught to treat the baby like it has a migraine, as most adults have some experience with this concept (quiet, dark room with decreased stimulation to sleep it off). Hospital staff need to be aware of their own judgements when dealing with these families, as these mothers already feel guilty because their child is withdrawing from a substance that she was taking, even with MAT. Guilt and shame may drive the family away, making it difficult to treat the infant without pharmacological treatment. It is important to praise the mother for maintaining sobriety when toxicology screens are clean and for families to feel empowered to care for their infant as part of their child’s treatment team, with the responsibilities of providing all of the non-pharmacological care. Therefore, the practice of the infant rooming in with the mother is encouraged, as this is one of the contributing factors in reducing length of hospital stay for these patients.24
Pharmacological Interventions
Optimizing non-pharmacological interventions can successfully reduce the need for pharmacotherapy.14 However, due to severity of symptoms, pharmacotherapy cannot be avoided in some cases. The American Academy of Pediatrics recommends drug-specific therapy from the same class as the drug of exposure.20 Morphine and methadone are the two most common agents used for the treatment of NAS-O and are recommended as first-line treatment options in the American Academy of Pediatrics guidelines for NAS.20,25 More recently, buprenorphine has been of interest due to its higher safety profile and a study by Kraft and colleagues had promising results for its use as a potential first-line agent.26 A limited number of studies, mainly retrospective, have compared morphine with methadone as a first-line treatment option for NAS.28–31 One prospective study showed that methadone-treated infants required a shorter length of treatment (14 vs. 21 days, p=0.008) and required fewer rescue doses (6 vs. 9, p=0.64).30 However, both agents were dosed every four hours, potentially underdosing morphine and overdosing methadone due to their differences in half-life. Although a consensus has not been reached regarding the optimal first-line agent for treatment, a recent study demonstrated that use of a protocol-driven weaning taper resulted in significantly shorter duration of treatment (17.7 vs. 32.1 days, p<0.0001).27 When weaning opioid replacement therapies, weight gain should be one of the factors monitored as infants showing severe signs of withdrawal often have poor weight gain related to their increased metabolic rate.32
The authors convened a Task Force on NAS to develop a standardized algorithm for the management of neonates with NAS in our institution. We have opted to use phenobarbital and clonidine as second-line adjunctive therapies for the cases in which NAS symptoms are not controlled, despite maximal opioid replacement. The algorithm can be seen in Figure 3.
Figure 3:
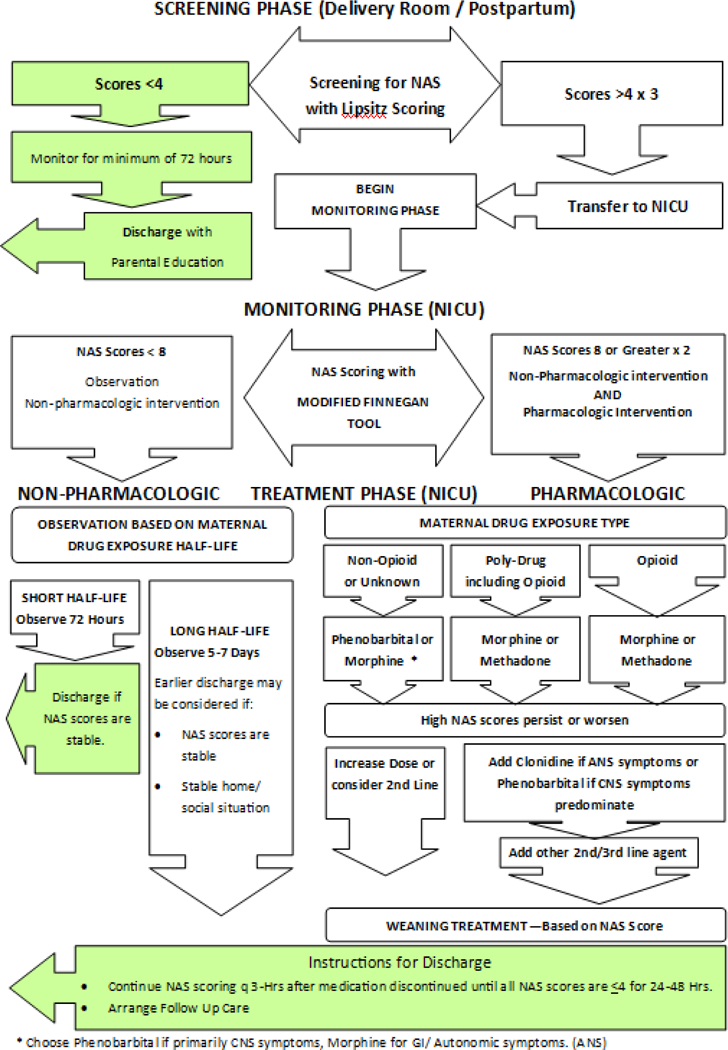
Screening and Treatment Algorithm for Neonatal Abstinence Syndrome for The University of Oklahoma Children’s Hospital Newborn Services
Outpatient Follow-Up
There is controversy in the outpatient management of opioid replacement therapies for NAS-O. The decision to treat on an outpatient basis requires an individual assessment of each situation and a clean maternal toxicology screen (absent prescribed medications). The maternal history, home environment, degree of parental involvement during hospitalization, anticipated length of treatment, and comfort of the follow-up physician are all factors in the decision to treat on an outpatient versus inpatient basis. For infants determined to be appropriate candidates for home therapy, we recommend waiting until the infant is on the lowest dose of opioid-replacement medication (weaning by interval, not dose) and dispensing the medication in individual dosing syringes, as the volumes are very small and difficult to measure.31 Methadone has a longer half-life than morphine and can be a good choice for home use, as dosing times tend to be less rigid than when given by nurses in the hospital.
Providing a thorough discharge summary for a newborn affected by opioid exposure or neonatal abstinence syndrome is important for continuity of care. The summary should include the following:
Maternal history of medication-assisted treatment or substance abuse treatment
Results of any maternal toxicology testing during pregnancy
Length, type, and dose of in-utero opioid exposure (ICD-10 code P04.14 for newborn affected by maternal use of opiates)
- Whether the infant exhibited symptoms of neonatal abstinence syndrome (ICD-10 code P96.1 for neonatal withdrawal symptoms from maternal use of drugs of addiction)
-
○Required treatment of NAS-O with non-pharmacological treatment only
-
○Required pharmacological treatment of NAS-O with type of opioid replacement used, highest dose required (mg/kg/day), length of treatment through the time of discharge
-
○
If the infant is being discharged on home opioid replacement therapy, the weaning plan should be documented and the family should be given a written copy with instructions on what to do if symptoms increase during the wean
A reference that mandatory reporting (including PMP database query for home prescriptions) has taken place with the reporting date
Details on follow-up appointments including recommendations for long-term neurodevelopmental follow-up
Long-Term Neurodevelopmental Follow-Up
Opioid use can have detrimental effects on brain development, as opioid consumption can lead to neuro-apoptosis. Multiple researchers have reported development and behavioral problems in infants with prenatal drug exposure.33–36 Neurodevelopmental outcomes in NAS patients are difficult to predict and depend on the complex interaction of prenatal and early postnatal experiences. Many academic institutions have pediatric developmental clinics that specialize in this population and can provide close multidisciplinary long-term follow-up. Services and consultations related to prenatal substance exposure and child development are available in Oklahoma through A Better Chance program at The University of Oklahoma Health Sciences Center in Oklahoma City (https://www.oumedicine.com/department-of-pediatrics/department-sections/devbehav/child-study-center/programs-and-clinical-services/a-better-chance). Referrals can be made by calling (405) 271–5700.
Key Points.
The rate of opioid-exposed neonates is rising, in part, due to the overprescription of opioids in women.
The diagnosis of an opioid-exposed neonate can usually be made in the first two weeks of life, based on maternal history and screening, infant symptomatology, and toxicology testing.
Oklahoma now has a mandatory reporting statute for all medical professionals who interact with mothers and infants exposed to controlled substances.
Pediatric providers should include neonatal abstinence syndrome in their differential for sepsis in the first two weeks of life and should be aware that cord testing may be available at the infant’s birth hospital for up to two weeks after birth.
The majority of opioid-exposed infants can be treated with non-pharmacologic measures by training the caregivers to respond to the infant’s cues.
For those infants who require pharmacological treatment, adopting a treatment protocol results in shorter treatment duration and hospitalization.
New ICD-10 codes will be available in October 2018 which include P04.14 (newborn exposure to maternal opiates) and P96.1 (newborn withdrawal symptoms
Provider documentation is important for data collection and quality improvement projects in addition to communication between providers and the provision of optimal longitudinal care.
Long-term neurodevelopmental follow-up is important to optimize outcomes for infants who have been prenatally exposed to opioids.
Acknowledgements
The authors wish to acknowledge the contributions of Kathy Kyler, MS, MA, who provided editorial assistance and Jamie Miller, PharmD, who assisted with the development of the Neonatal Abstinence Syndrome pharmacological management protocol (Figure 3) and provided editorial assistance.
Research reported in this publication was supported by the National Institutes of Health under Award Numbers UG1OD024950 and U54GM104938. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Drs. Ernst and Makkar are serving as co-principal investigators for an NIH-funded study for the management of neonatal abstinence syndrome through the IDeA States Pediatric Clinical Trials Network.
Footnotes
Conflicts of Interest and Disclosures
The authors declare no pertinent conflict of interest and did not receive funding for this project.
Dr Ernst serves on a volunteer basis on the Oklahoma Perinatal Quality Improvement Collaborative for neonatal abstinence syndrome. She has been an invited speaker on neonatal abstinence syndrome, including for the Oklahoma Children’s Court Improvement Program. She is a funded site investigator for Fresenius Kabi and Nutrinia for neonatal nutrition clinical trials. She also serves on the Omegaven Advisory Board for Fresenius Kabi and the Neonatal Nutrition Advisory Board for Baxter as a paid consultant.
REFERENCES
- 1.Office of the Oklahoma Attorney General. The Oklahoma commission on opioid abuse final report. http://www.oag.ok.gov/Websites/oag/images/Oklahoma%20Commission%20on%20Opioid%20Abuse%20Final%20Report.pdf. Accessed on August 23, 2018.
- 2.National Institute on Drug Abuse. Oklahoma opioid summary. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-summaries-by-state/oklahoma-opioid-summary. Accessed on August 23, 2018.
- 3.Oklahoma Health Care Authority. Neonatal abstinence syndrome among SoonerCare members. http://www.astho.org/Rx/Profiles/Oklahoma-NAS/. Accessed on August 23, 2018.
- 4.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol 2014; 123:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oklahoma Health Care Authority. Opioids in pregnancy. http://okhca.org/workarea/downloadasset.aspx?id=18409. Accessed on August 23, 2018.
- 6.National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2014/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Accessed on August 23, 2018.
- 7.National Institutes of Health. NIH HEAL Initiative. https://www.nih.gov/research-training/medical-research-initiatives/heal-initiative. Accessed on August 23, 2018.
- 8.National Institutes of Health. Clinical sites for the IDeA States Pediatric Clinical Trials Network. https://www.nih.gov/echo/clinical-sites-idea-states-pediatric-clinical-trials-network Accessed on August 23, 2018.
- 9.National Institutes of Health. NIH-funded study to focus on newborns affected by opioids. https://www.nichd.nih.gov/news/releases/100217-ACTNOW. Accessed on August 23, 2018.
- 10.American College of Obstetricians and Gynecologists. Opioid use and opioid use disorder. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Opioid-Use-and-Opioid-Use-Disorder-in-Pregnancy. Accessed on August 23, 2018.
- 11.Lipsitz PJ. A proposed narcotic withdrawal score for use with newborn infants. A pragmatic evaluation of its efficacy. Clin Pediatr 1975; 14:592–594. [DOI] [PubMed] [Google Scholar]
- 12.Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis 1975; 2:141–158. [PubMed] [Google Scholar]
- 13.Zahorodny W, Rom C, Whitney W, et al. The neonatal withdrawal inventory: a simplified score of newborn withdrawal. J Dev Behav Pediatr 1998; 19:89–93. [DOI] [PubMed] [Google Scholar]
- 14.Grossman MR, Lipshaw MJ, Osborn RR, Berkwitt AK. A novel approach to assessing infants with neonatal abstinence syndrome. Hosp Pediatr 2018; 8(1). [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Pomar E, Christian A, Devlin L, et al. Analysis of the factors that influence the Finnegan Neonatal Abstinence Scoring System. J Perinatol 2017; 37:814–7. [DOI] [PubMed] [Google Scholar]
- 16.Kandall SR, Gartner LM. Late presentation of drug withdrawal symptoms in newborns. Am J Dis Child 1974;127(1):58–61. [DOI] [PubMed] [Google Scholar]
- 17.State of Oklahoma House of Representatives. Enrolled House Bill 3104. http://opqic.org/wp-content/uploads/2018/05/Enrolled-HB3104.pdf. Accessed August 23, 2018.
- 18.National Association of Boards of Pharmacy. NABP PMP InterConnect: the only national network of state-based PMPs https://nabp.pharmacy/initiatives/pmp-interconnect/. Accessed on August 23, 2018.
- 19.Velez M, Jansson LM. The opioid dependent mother and newborn dyad: non-pharmacologic care. J Addict Med. 2008; 2(3):113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudak ML, Tan RC; Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics 2012; 129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e540 [Google Scholar]
- 21.Jansson LM, Choo R, Velez ML, et al. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics 2008; 121(1):106–114. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Latif ME, Pinner J, Clews S, Cooke F, Lui K, Oei J. Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug-dependent mothers. Pediatrics 2006; 117:e1163–e1169. [DOI] [PubMed] [Google Scholar]
- 23.Howard MB, Schiff DM, Penwill N, et al. Impact of parental presence at infants’ bedside on neonatal abstinence syndrome. Hosp Pediatr 2017; 7(2):63–69. [DOI] [PubMed] [Google Scholar]
- 24.Holmes AV, Atwood EC, Whalen B, et al. Rooming-in to treat neonatal abstinence syndrome: Improved family-centered care at lower cost. Pediatrics 2016; 137(6). pii: e20152929. [DOI] [PubMed] [Google Scholar]
- 25.Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in the U.S. neonatal ICUs. N Eng J Med 2015; 28:372:2118–2126. [DOI] [PubMed] [Google Scholar]
- 26.Kraft WK, Adenivi-Jones SC, Chervoneva I, et al. Buprenorphine for the treatment of the neonatal abstinence syndrome. N Engl J Med 2017; 376:2341–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall ES, Wexelblatt SL, Crowley M, et al. A multicenter cohort study of treatments and hospital outcomes in neonatal abstinence syndrome. Pediatr 2014;134:e527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lainwala S, Brown ER, Weinschenk NP, Blackwell MT, Hagadorn JI. A retrospective study of length of stay in infants treated for neonatal abstinence syndrome with methadone versus oral morphine preparations. Adv Neonatal Care 2005; 5(5):265–272. [DOI] [PubMed] [Google Scholar]
- 29.Young ME, Hager SJ, Spurlock D. Retrospective chart review comparing morphine and methadone in neonates treated for neonatal abstinence syndrome. Am J Health Syst Pharm 2015; 72:S162–S167. [DOI] [PubMed] [Google Scholar]
- 30.Brown MS, Hayes MJ, Thornton LM. Methadone versus morphine for treatment of neonatal abstinence syndrome: A prospective randomized clinical trial. J Perinatol 2015; 35:278–283. [DOI] [PubMed] [Google Scholar]
- 31.Ibach BW, Johnson PN, Ernst KD, Harrison D, Miller JL. Initial dosing and taper complexity of methadone and morphine for treatment of neonatal abstinence syndrome. J Pharmacy Technology 2016; 32(5):216–222. [Google Scholar]
- 32.Bada HS, Das A, Bauer CR, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol 2005;25:631–637. [DOI] [PubMed] [Google Scholar]
- 33.Bunikowski R, Grimmer I, Heiser A, et al. Neurodevelopmental outcome after prenatal exposure to opiates. Eur J Pediatr 1998; 157:724–730. [DOI] [PubMed] [Google Scholar]
- 34.van Baar AL, Soepatmi S, Gunning WB, Akkerhuis GW. Development after prenatal exposure to cocaine, heroin and methadone. Acta Paediatr Suppl 1994; 404:40–46. [DOI] [PubMed] [Google Scholar]
- 35.Uebel H, Wright IM, Burns L, et al. Reasons for rehospitalization in children who had neonatal abstinence syndrome. Pediatr 2015; 136(4):e811. [DOI] [PubMed] [Google Scholar]
- 36.Nygaard E, Moe V, Slinning K, Walhovd KB. Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatr Res 2015; 78:330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ARUP Laboratories. Drug test table meconium and umbilical cord. http://ltd.aruplab.com/Tests/Pdf/54. May 2018. Accessed on August 23, 2018.
- 38.Centers for Medicare and Medicaid Services. 2019 ICD-10 PCS. https://www.cms.gov/Medicare/Coding/ICD10/2019-ICD-10-PCS.html. Accessed on August 23, 2018.


