Abstract
Pulmonary sclerosing pneumocytoma (PSP) is pathologically classified as an adenoma and behaves in a benign manner. However, some cases of PSP displayed pathologically malignant behavior, such as lymph node metastasis and necrosis. A 64-year-old woman was referred to our hospital complaining of a cough and breathlessness. Histopathological analysis of the resected specimen by left pneumonectomy and lymph node dissection revealed a large PSP measuring 15 × 14 cm in size, with massive necrosis and vascular invasion. This case was the largest ever reported and suggested that clinico-histological presentation of PSP sometimes showed an aggressive phenotype like advanced lung cancer.
INTRODUCTION
Pulmonary sclerosing pneumocytoma (PSP) is a tumor classified as an adenoma, and the tumor is currently considered to originate from primitive respiratory epithelium based on immunohistochemical data [1–3]. The tumor clinically behaves in a benign manner and has a good prognosis. In some cases, however, PSP reportedly displays pathologically malignant characteristics such as lymph node metastases and necrosis [2, 3]. These heterogenetic cases suggest that PSP has a clinico-pathological diversity. Herein, we present a case of a large PSP with massive necrosis and vascular invasions.
CASE REPORT
A 64-year-old woman presented with a cough and breathlessness. She was referred to our hospital because of an abnormal shadow over the whole left chest area and a mediastinal shift on chest radiography (Fig. 1A). Chest computed tomography (CT) revealed a 15 × 14 × 10 cm mass filling almost the entire left thoracic cavity, which had an extensive low-density area on enhancement (Fig. 1B). There were also multiple nodules in the opposite lung (Fig. 1C). Positron emission tomography (PET)–CT revealed a mild Fluorine-18 deoxyglucose (FDG) accumulation within the tumor in the left thorax and one nodule in the right lung, and maximum standardized uptake values (SUVmax) were 6.5 and 2.3, respectively (Fig. 1D). Although the large tumor size, massive necrosis and the multiple nodules in the opposite lung suggested advanced lung cancer, the mild accumulation of FDG seemed atypical. Furthermore, her general condition was relatively good considering advanced cancer. Subsequent CT-guided tumor biopsy showed a proliferation of round cells with central bland nuclei and low nuclear atypia that were positive for thyroid transcription factor-1 (TTF-1) and low Ki-67 labeling index (<5%). These findings raised PSP as a differential diagnosis in a multidisciplinary board. We, therefore, performed a left pneumonectomy to obtain the definite diagnosis and to palliate the symptoms caused by the rightward mediastinal shift.
Figure 1.
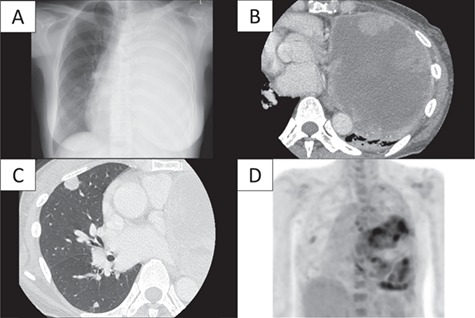
Results of laboratory imaging techniques. (A) Chest radiography and (B) chest CT revealed a large mass measuring 15 × 14 × 10 cm in size present in the left lung with an extensive low-density area in the center of the tumor, thereby raising a suspicion of hemorrhage and necrosis; chest radiography also revealed complete atelectasis of the left lung. (C) Multiple small nodules ranging between 0.5 and 1.3 cm in diameter were also found in the right lung. One nodule was located in the upper lobe, and the others were in the lower lobe. (D) PET–CT confirmed a weak FDG accumulation within the large mass and in only the right upper lung nodule, with SUVmax of 6.5 and 2.3, respectively.
The resected specimen contained a well-demarcated yellowish tumor with massive central necrosis (Fig. 2A). The tumor progressed expansively in the lungs and was well encapsulated. There were a dual population of cuboidal surface cells overlaying stromal round cells (Fig. 2B) with little nuclear atypia (Fig. 2C), showing various proliferation patterns: papillary, solid, sclerotic and hemorrhagic (Fig. 2B and D–F). There was no lymph node metastasis; however, endobronchial locations and vascular invasions were observed (Fig. 3A and B). The Ki-67 immunohistochemistry (IHC) revealed overall labeling index of 5% with partially highly-proliferating areas of up to 30% (Fig. 3C). Tumor cells were positive for TTF-1, epithelial membrane antigen, progesterone receptor and vimentin and focally positive for cytokeratin (AE1/AE3) and cytokeratin-7 (Fig. 3D–F). The patient was consequently diagnosed as atypical PSP with massive necrosis and vascular invasions.
Figure 2.
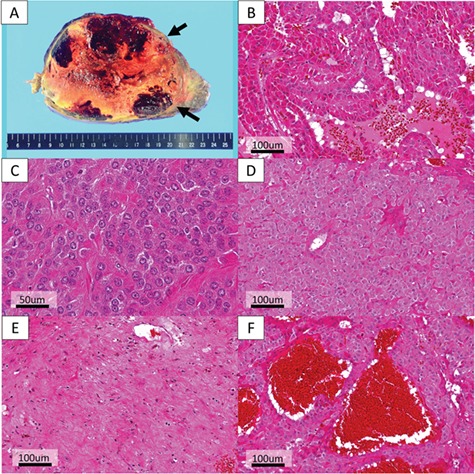
Histopathological findings (B–F: hematoxylin and eosin (HE) method). (A) The resected specimen contained a well-demarcated yellowish tumor with massive central necrosis, encompassing 80% of the tumor mass. (B) Papillary growth shows a dual population of cuboidal surface cells overlaying stromal round cells. (C) Each tumor cell was uniform, round or polygonal, with round bland nuclei having no nucleoli and a fine chromatin. The cytoplasm was eosinophilic, and nuclear fission was scarcely observed. (B, D–F) The tumor proliferated with various growth patterns such as (B) papillary, (D) solid, (E) sclerotic and (F) hemorrhagic.
Figure 3.
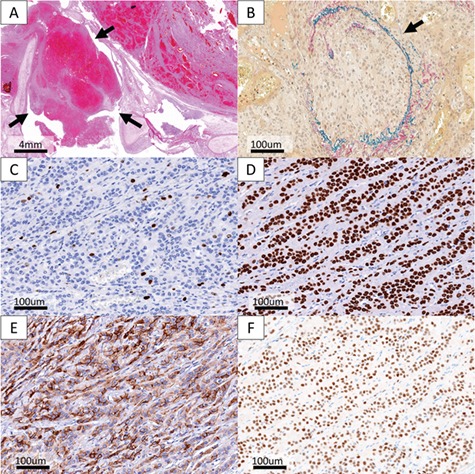
Unusual Pathological findings and IHC findings. (A) Endobronchial lesions were observed in the tumor (hematoxylin and eosin (HE) method). (B) Vascular invasion occurred in the tumor (Verhoeff-van-Gieson (VVG) method). (C) Ki-67 labeling index was totally <5% with partially highly-proliferating areas of up to 30%. (D–F) The tumor cells were positive for (D) TTF-1, (E) epithelial membrane antigen and (F) progesterone receptor.
Six months after the primary surgery, the largest nodule in the right lung was resected because of increase in size from 1.3 to 1.9 cm and was diagnosed as PSP with similar pathological findings to the left lung tumor: dual population of tumor cells with various proliferation patterns, positivity of IHC, such as progesterone receptor, and multiple vascular invasions. We inferred that the nodules were multiple synchronous diseases, but it was difficult to distinguish from metastasis from the left tumor.
DISCUSSION
PSP is relatively rare benign tumor with four distinct histological patterns: solid, papillary, sclerotic and hemorrhagic. According to some recent studies, PSP is considered to originate from primitive respiratory epithelium and is characterized as a tumor of pneumocytic origin with a dual population of cuboidal surface cells and stromal round cells [2–4]. Therefore, PSP was reclassified as adenoma from the previous category of miscellaneous tumors in the 2015 World Health Organization Classification, and its name was changed from ‘sclerosing hemangioma’ [1, 2]. PSP clinically behaves in a benign manner. One study showed that the doubling time of PSP is 660–1250 days, which is slower than that of malignant tumors [5]. The prognosis after surgical resection is generally good, and deaths have not been reported [6].
On the other hand, clinically malignant findings such as lymph node metastasis are reported to occur in approximately 1% [2, 7], which makes difficult to distinguish from poorly differentiated adenocarcinoma (predominantly solid subtype). Histopathological characteristics such as dual population of tumor cells with little nuclear atypia, various proliferation patterns and IHC results confirmed the definitive diagnosis, however, the present case had some atypical characteristics for PSP as follows.
Firstly, the tumor size of 15 cm was noteworthy. Although two previous reports have reported PSPs of more than 10 cm, the present case was the largest one [8, 9]. Secondly, a massive necrosis, endobronchial lesion and multifocal lesions were observed. Previous reports showed that necrosis, multifocal and endobronchial lesion were rarely observed in PSP, and the incidences were 8% 4% and 1%, respectively [2, 3]. Thirdly, vascular invasion was observed, which has not been reported to date.
We did not perform genetic studies because its utility to distinguish synchronous PSPs from metastasis reportedly has not been established at this time. However, recent study of genetic analysis for multiple synchronous PSP identified mutations in genes, which may provide the clues to elucidate the nature of PSP [10]. Further, histopathological and genetic studies are very interesting and may clarify the feature and diversity of PSP.
In conclusion, this case suggested that PSP had a variety of histopathological findings and sometimes could show an aggressive clinical feature like advanced lung cancer.
Acknowledgments
None.
CONFLICT OF INTEREST STATEMENT
None declared.
Funding
None declared.
Ethical Approval
National Cancer Center Hospital IRB approval number: 2017–418.
Consent
We obtained comprehensive informed consent from the patient before this study.
Guarantor
None declared.
References
- 1. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–60. [DOI] [PubMed] [Google Scholar]
- 2. Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000;24:906–16. [DOI] [PubMed] [Google Scholar]
- 3. Chen B, Gao J, Chen H, et al. Pulmonary sclerosing hemangioma: a unique epithelial neoplasm of the lung (report of 26 cases). World J Surg Oncol 2013;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalhor N, Staerkel GA, Moran CA. So-called sclerosing hemangioma of lung: current concept. Ann Diagn Pathol 2010;14:60–7. [DOI] [PubMed] [Google Scholar]
- 5. Sugio K, Yokoyama H, Kaneko S, Ishida T, Sugimachi K. Sclerosing hemangioma of the lung: radiographic and pathological study. Ann Thor Surg 1992;53:295–300. [DOI] [PubMed] [Google Scholar]
- 6. Lei Y, Yong D, Jun-Zhong R, Zhi Y, Zi-Tong W. Treatment of 28 patients with sclerosing hemangioma (SH) of the lung. J Cardiothorac Surg 2012;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adachi Y, Tsuta K, Hirano R, et al. Pulmonary sclerosing hemangioma with lymph node metastasis: a case report and literature review. Oncol Lett 2014;7:997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim KH, Sul HJ, Kang DY. Sclerosing hemangioma with lymph node metastasis. Yonsei Med J 2003;44:150–4. [DOI] [PubMed] [Google Scholar]
- 9. Robbins P, Holthouse D, Newman M. An unusually large pulmonary sclerosing haemangioma. Pathology 2006;38:267–8. [DOI] [PubMed] [Google Scholar]
- 10. Fan X, Lin L, Wang J, et al. Genome profile in a extremely rare case of pulmonary sclerosing pneumocytoma presenting with diffusely-scattered nodules in the right lung. Cancer Biol Ther 2018;19:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


