Abstract
Introduction
Tests of ambulatory function are common clinical trial endpoints in Duchenne muscular dystrophy (DMD). The ImagingDMD study has generated a large data set using these tests, which can describe the contemporary natural history of DMD in 5–12.9 year olds.
Methods
92 corticosteroid treated boys with DMD and 45 controls participated in this longitudinal study. Subjects performed the 6 minute walk test (6MWT) and timed function tests (TFTs: 10m walk/run, 4 stairs, supine to stand).
Results
Boys with DMD had impaired functional performance even at 5–6.9 years. Boys older than 7 had significant declines in function over 1 year for 10m walk/run and 6MWT. 80% of subjects could perform all functional tests at 9 years old. TFTs appear to be slightly more responsive and predictive of disease progression than 6MWT in 7–12.9 year olds.
Discussion
This study provides insight into the contemporary natural history of key functional endpoints in DMD.
Keywords: Outcome measures, 6 Minute walk test, Duchenne muscular dystrophy, Functional endpoints, Loss of ambulation, Ambulatory function
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder that causes progressive loss of muscle strength, leading to significant functional impairment and loss of ambulation in the second decade of life.5,6 Glucocorticosteroids are the most frequently used treatment option to modify disease course, and delay loss of ambulation by up to three years9 in boys with DMD. Hence, management of DMD largely targets the maintenance of quality of life and function.7,8
Even though there is currently no cure for DMD, there is a surge in promising therapeutic strategies that are rapidly moving into and beyond clinical trials.10,11 The design of clinical trials has, however, proven challenging, with narrow age- and functional inclusion criteria. Additionally, there are a limited number of natural history studies12–15 available in large patient cohorts characterizing disease progression and permitting a comparison with experimental treatment groups. The heterogeneity of disease progression, lack of prior natural history data, and lack of reliable and sensitive clinical endpoints that adequately span a large age range and ambulatory spectrum of the disease have created significant challenges for the design of clinical trials in DMD.
The primary aim of this study was to collect natural history data in a large cohort of ambulatory boys with DMD across multiple centers, and to examine 1 year longitudinal changes in performance on the functional tests commonly used as endpoints in clinical trials. A secondary aim was to evaluate the ability of each functional test to predict loss of function in the following year and to examine the relationship between baseline six minute walk test (6MWT) performance and functional decline.
Materials and Methods
Study design
The study was approved by the institutional review boards at the University of Florida, Children's Hospital of Philadelphia, and Oregon Health and Science University. Written informed consent and assent were obtained from parents and/or guardians and subjects. The subjects were enrolled in a longitudinal multicenter magnetic resonance imaging natural history study, referred to as ImagingDMD (NCT01484678). All participants in the DMD cohort had a genetic diagnosis of DMD as well as onset of symptoms prior to 5 years of age. Additional inclusion and exclusion criteria are provided in the supplementary materials. Here we present data from a subgroup of subjects, specifically corticosteroid treated boys with DMD aged 5–12.9 years and age-matched unaffected controls.
Functional Measures
At each visit, functional test performance was assessed following magnetic resonance imaging scans (data not presented). Functional evaluators at each site (n=1–3 per site) were trained to perform assessments according to established manuals of operating procedures (MOP). To be certified, each evaluator submitted a video of him or herself performing all tests, which was evaluated by an expert physical therapist at the central site against a standardized evaluation checklist. Following certification, functional evaluators participated in periodic teleconferences as well as annual meetings during which testing procedures were reiterated and MOPs were updated if needed.
All tests were performed without the parent or guardian present, and comfortable walking shoes were worn by subjects for all tests. The functional evaluator provided verbal encouragement during each test. The order of test performance as well as the instructions given to the subject prior to each test were standardized. Subjects first performed timed function tests (TFTs) –10m walk/run, then climbing 4 stairs, then supine to stand (STS). Each timed function test was performed 3 times, and the fastest time was used for analysis. A maximal time of 45 seconds was provided to complete each test. Following TFTs testing, subjects rested for at least 5 minutes and then performed the 6MWT as described by McDonald et al.17 Full details of the instructions for each test can be found in the supplementary material.
Clinical function was assessed using the Modified Brooke Lower Extremity Scale,18 and the ability to complete each test was assessed at each visit. Loss of function for the TFTs was defined as inability to complete the test independently within 45 seconds, while loss of function for the 6MWT was defined as inability to attempt the test or inability to walk without support from a person or wall.
A web interface was used to input data to a secure server during or immediately following functional testing; periodically, all functional test data was inspected by 2 independent reviewers, who examined comments entered by the functional evaluator as well as the time or distance recorded and assigned each data sets a label of valid or invalid.
Data analysis
Non-parametric tests were used for all comparisons. Data are reported as mean ± standard deviation (SD), unless otherwise stated. Significance was set at 0.05 with Bonferroni corrections for all multiple comparisons within functional tests as needed. Velocities were calculated as the reciprocal of the time for the 10m walk/run, climb 4 stairs, and STS tests. A 6 minute walk distance (6MWD) or TFT velocity of 0 was recorded when the subject had lost the ability to perform the test. Percentage predicted 6MWD was calculated based on the Geiger equation (6MWD = 196.72 + (39.81 * Age) − (1.36 * Age2) + (132.28 * Height)).19,20
Annual changes in TFT time or 6MWD were compared with thresholds for clinically important decline as defined by McDonald et al.21 Annual changes in TFT velocity and 6MWD were also used to calculate the standardized response mean (SRM, mean change/standard deviation of the change). Sample sizes for a hypothetical clinical trial of a treatment that either completely stabilized the disease, or slowed disease progression by 50%, were calculated from the change over 12 months in 7–12.9 year old boys using the same methods as Bonati et al.22
Using all available data for each boy with DMD (n=288 visits), receiver operating characteristic curves (ROC) were estimated to investigate loss of function. Sensitivity and specificity are reported at the point on the ROC curve closest to (0, 100%). To properly account for uncertainty in this analysis, given multiple (correlated) time points from the same subject, we used the bootstrap (resampling subjects, not individual data points for a subject) to compute 95% confidence intervals for the area under the curve (AUC), the optimal cutoff, and the sensitivity and specificity.
Results
Demographic Information
Demographic data for affected and unaffected boys at baseline are provided in Table 1. 80 boys with DMD completed a follow-up visit 12.1±0.5 months after baseline, and additional follow up visits were completed in 74 (2 years), 36 (3 years), and 6 boys (4 years) respectively. Genetically, 67% of boys had deletion mutations, 10% had duplication mutations, 21% had point mutation/others and 2% of boys had a genetic diagnosis of DMD per parent report, but a genetic report was not available. Control subjects did not differ in age from boys with DMD (Table 1). Across all tests completed, 98% yielded valid data; 2% were invalid due to compliance or behavioral issues and were not included in analysis. Additionally, ~0.5% of tests had missing data, typically because a subject or parent did not agree to participate in that test.
Table 1.
Demographic information at baseline
| Age group (years) | ||||||
|---|---|---|---|---|---|---|
|
|
|
|
|
|||
| Mean ± SD | Total | 5 – 6.9 | 7 – 8.9 | 9 – 10.9 | 11 – 12.9 | |
|
|
|
|
|
|
|
|
| Age (years) | DMD | 8.8 ± 2.0 (n = 92) | 6.3 ± 0.5 (n = 23) | 8.1 ± 0. 5 (n = 27) | 9.9 ± 0.6 (n = 24) | 11.7 ± 0.6 (n = 18) |
| Control | 8.9 ± 2.0 (n = 45) | 6.1 ± 0.6 (n = 9) | 8.1 ± 0.6 (n = 16) | 10.2 ± 0.5 (n = 13) | 11.7 ± 0.7 (n = 7) | |
| Weight (kg) | DMD | 29.6 ± 10.0† | 21.3 ± 3.4 | 27.1 ± 5.4 | 29.6 ± 6.3 | 43.6 ± 10.4 |
| Control | 32.3 ± 11.2 | 23.2 ± 5.4 | 29.1 ± 6.7 | 36.0 ± 11.7 | 44.6 ± 12.1 | |
| Height (m) | DMD | 1.22 ± 0.10 | 1.11 ± 0.05 | 1.21 ± 0.05 | 1.25 ± 0.08 | 1.33 ± 0.07 |
| Control | 1.36 ± 0.13 | 1.20 ± 0.08 | 1.32 ± 0.06 | 1.44 ± 0.08 | 1.54 ± 0.09 | |
| Body Mass Index (kg/m2) | DMD | 19.5 ± 4.3† | 17.2 ± 1.8 | 18.5 ± 3.2 | 18.9 ± 3.2 | 24.6 ± 5.1 |
| Control | 16.9 ± 3.2 | 15.8 ± 1.4 | 16.7 ± 3.2 | 17.1 ± 3.9 | 18.6 ± 3.1 | |
| Brooke Score | DMD | 1 – 3 | 1 – 2 | 1 – 2 | 1 – 3 | 1 – 3 |
| Control | 1 | 1 | 1 | 1 | 1 | |
Data reported as mean ± standard deviation with a range given for Brooke Score.
indicates data collected in n = 91.
Baseline Functional Performance
At baseline, controls performed TFTs significantly more quickly than boys with DMD and walked significantly farther on the 6MWT (Table 2, Supplementary Figure 1). The magnitude of this difference increased with age. The youngest boys took about twice as long as controls to complete the TFTs, while the older boys took 3–4 times longer. Similarly, at younger ages boys with DMD walked about 75% of the distance measured in controls whereas older boys with DMD walked just over 50% of the distance of their peers. This was true for raw 6MWD as well as % predicted 6MWD, which takes into account stature and age. The distribution of baseline TFT values in boys with DMD can be seen in Supplementary Figure 2. The 6MWT and 10m walk/run test both measure ambulatory function, and as expected, were significantly correlated with each other (Supplementary Figure 3). However, in highly functional boys, the relationship between the two tests is less strong than in boys whose performance is declining.
Table 2.
Velocities of controls and boys with DMD at baseline and change over 1 year in velocities in boys with DMD for each timed function test.
| Age group (years) | |||||
|---|---|---|---|---|---|
| Functional tests | 5 – 6.9 | 7 – 8.9 | 9 – 10.9 | 11 – 12.9 | |
| 10 meter walk/run | Control | 2.96 ± 0.28 | 3.38 ± 0.20 | 3.46 ± 0.49 | 3.71 ± 0.44 |
| DMD BL | 2.16 ± 0.35* | 1.98 ± 0.48* | 1.72 ± 0.52* | 1.46 ± 0.37* | |
| DMD 1 year Change | 0.07 ± 0.32 | −0.33 ± 0.32† | −0.31 ± 0.28† | −0.32 ± 0.44† | |
| 4 Stairs | Control | 0.67 ± 0.09 | 0.76 ± 0.10 | 0.76 ± 0.15 | 0.84 ± 0.21 |
| DMD BL | 0.42 ± 0.15* | 0.31 ± 0.12* | 0.29 ± 0.15* | 0.22 ± 0.08* | |
| DMD 1 year Change | 0.02 ± 0.09 | −0.05 ± 0.06† | −0.06 ± 0.08† | −0.06 ± 0.08 | |
| STS | Control | 0.53 ± 0.10 | 0.70 ± 0.14 | 0.61 ± 0.10 | 0.66 ± 0.21 |
| DMD BL | 0.32 ± 0.09* | 0.24 ± 0.12* | 0.19 ± 0.13* | 0.10 ± 0.08* | |
| DMD 1 year Change | −0.01 ± 0.06 | −0.07 ± 0.06† | −0.05 ± 0.04† | −0.05 ± 0.06 | |
BL = Baseline;
: significant differences between controls and boys with DMD at baseline;
: significant differences over 1 year change in boys with DMD, p <0.05.
Functional Changes over 1 Year
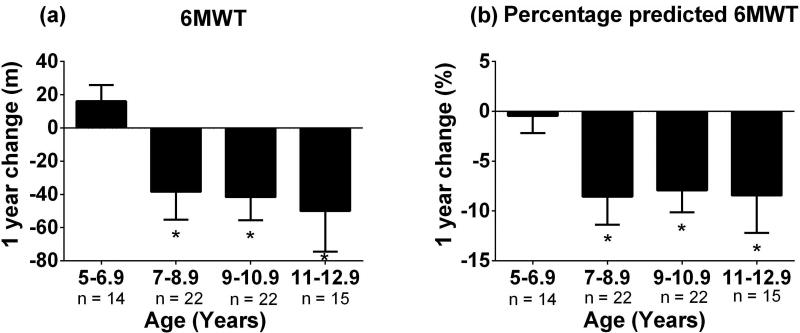
Performance on all functional tests as well as % predicted 6MWD significantly declined over 12 months in boys with DMD aged 7–10 years (Table 2, Figure 1). Significant declines in 10m walk/run and 6MWT performance, but not climbing 4 stairs or STS, were seen in 11–12.9 year old boys. In boys <7 years, no significant changes in function were detected over 1 year. As shown in Supplementary Table 1, the most responsive tests in 7–10.9 year olds were the 10m walk/run and STS tests. The 6MWD has a relatively lower responsiveness with SRM values below 0.70 in all age groups tested.
Figure 1. Decline in 6 minute walk test performance over 1 year across ages.
A significant decline in 6 minute walk test over 1 year was found for boys >7 years of age for both (a) 6 minute walk test and (b) percentage predicted 6 minute walk test, p <0.05.
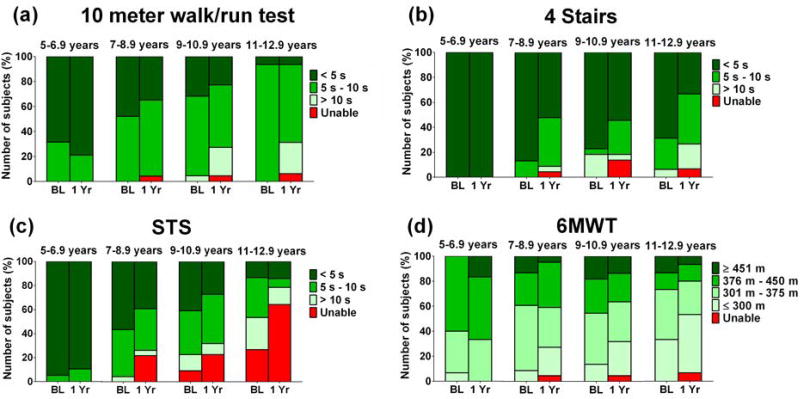
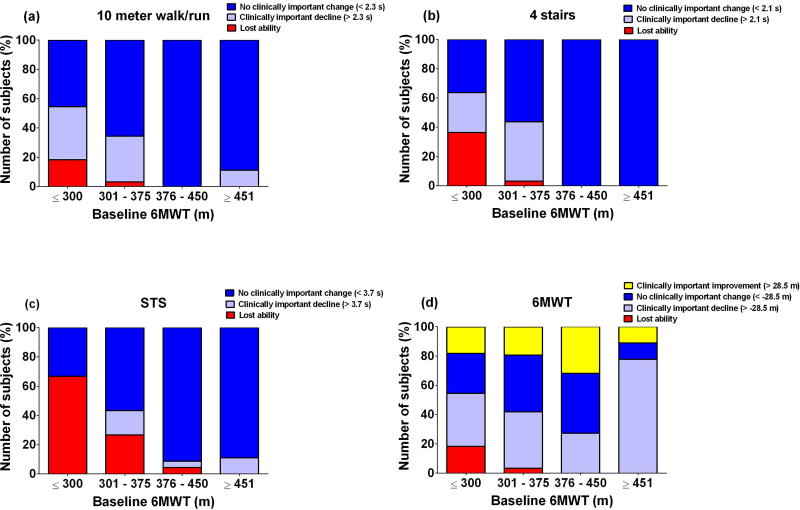
Figure 2 shows that while most of the youngest boys were able to complete the TFTs in <5 s at baseline and 1 year follow up, by 9–10 years a number of boys could no longer complete these tests in less than 10 s. When boys were grouped by baseline 6MWD instead of baseline age (Figure 3), boys with baseline 6MWD <300m were very likely to experience clinically important declines in performance on all functional outcomes over 12 months. Boys with 6MWD ≥376m were highly likely to remain stable as measured by the TFTs and 6MWT over 12 months. Notably, a subset of boys in all baseline 6MWD groups experienced clinically important improvements in 6MWD, but no subjects showed clinically important improvement over 12 months in the TFTs. This was also true when boys walking <350m and ≥350m were compared, a cut-off threshold that has been used in the past15,24 (Supplementary Figure 4).
Figure 2. Change over 1 year for all functional tests for all age groups in boys with DMD. BL= Baseline.
Overall there was a shift to slower times/shorter distance in boys aged >7 years, but not in younger boys. Data represent 19 boys aged 5 – 6.9, 23 boys aged 7 – 8.9, 22 boys aged 9 – 10.9, and 16 boys aged 11 – 12.9. Missing data or invalid data did not exceed 10% for any test in any age group.
Figure 3. Clinically important change in functional tests over 1 year by baseline 6 minute walk distance.
Subjects with lower baseline 6 minute walk test distances were more likely to experience declines in functional performance or loss of functional ability over 1 year on timed function tests but not the 6 minute walk test itself.
Loss of Function
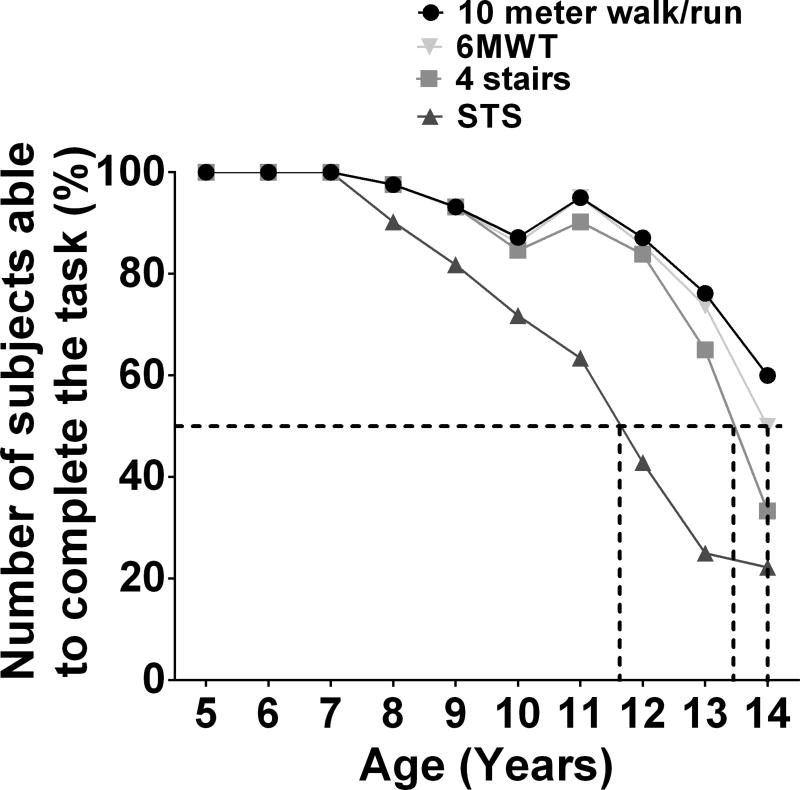
Figure 4 displays the longitudinal trajectory in 6MWD performance as a function of age and baseline 6MWD across all available visits. No subject with baseline 6MWD ≥451m lost ambulation over the course of 4 years, while a substantial proportion of boys with baseline 6MWD <376m had lost ambulation at the 2 year follow-up visit. Figure 5 shows that 80% of boys with DMD maintained the ability to complete all functional tests until 9 years of age. However, after 12 years, functional decline was rapid and by 14 years, only half of subjects could still complete the 6MWT or 10m walk/run.
Figure 4. Heterogeneity in 6 minute walk test performance over 48 months.
As the study progressed, an increasing number of boys lost ambulation. However, some boys were able to maintain performance over up to 4 years, notably all those with baseline 6MWD ≥451m.
Figure 5. Number of subjects who are still able to perform each functional test by age.
At 9 years old, more than 80 % of boys could still perform all functional tests. At 12 years of age, more than 80% subjects could perform functional tests except the supine to stand, which only about 40% could perform. By 14 years old, about half of the boys were unable to perform any of the functional tests.
ROC analysis was used to examine the predictive relationship between functional performance and loss of function (Table 3) and showed that all tests were significantly predictive of loss of function, with slightly higher AUC for the TFTs compared with the 6MWD. The cut-off value that best predicted loss of function was between 6 and ~7.5 s for each of the TFTs, and the cut-off value that best predicted loss of function for the 6MWT was 319m.
Table 3.
Predicting loss of function over 1 year using ROC curves for each timed function test.
| Predicting loss of function in 1 year | ||||
|---|---|---|---|---|
|
| ||||
| Functional tests | Area Under ROC Curve |
Cut-off Time or Distance |
Sensitivity | Specificity |
| 10 meter walk/run | 0.93(0.88–0.97) | >7.6s(7.6s–09.2s) | 93% (79%–100%) | 82% (77%–95%) |
| 4 Stairs | 0.96 (0.92–0.99) | >6.2s (4.5s–7.4s) | 90% (80%–100%) | 91% (79%–97%) |
| STS | 0.96 (0.93–0.99) | >6.6s (6.3s–7.9s) | 97% (91%–100%) | 91% (85%–96%) |
| 6MWT | 0.85 (0.76–0.93) | <319m (277m–335m) | 77% (66%–90%) | 85% (70%–100%) |
95% CI range given in parentheses.
Discussion
Boys with DMD had impaired functional performance on tests of ambulatory function commonly used as clinical trial endpoints even at 5–6.9 years, however only boys 7 years or older showed significant declines in functional test performance over 1 year for 10m walk/run and 6MWT tests. 80% of enrolled subjects remained able to complete all four functional tests until at least 9 years old, and by age 14 approximately 50% of the boys could still complete the 6MWT or 10m walk/run. All functional tests were able to predict the loss of function over 12 months, with the TFTs displaying slightly higher sensitivity and responsiveness to change than the 6MWD. Finally, classification of the subjects based on baseline 6MWD, showed that boys with 6MWD ≤300m are very likely to experience clinically important declines in performance on all the functional tests over 12 months, and boys with 6MWD ≥376m highly likely to remain stable.
The cohort of subjects described in this manuscript is broadly similar in baseline 6MWD performance to other large natural history cohorts,12,23 despite differences in standard of care and functional inclusion criteria. The average decline in 6MWD over the course of 1 year (21m) was smaller than the 44m decline measured over 48 weeks in the placebo treated arm of the ataluren clinical trial.24 Unlike previous studies, this study also included an unaffected age-matched cohort, allowing us to quantify the increasing gap in performance between boys with DMD and their peers with age. All controls completed the 10m walk/run test in 3 s, and the STS and climbing 4 stairs in 1–2 s. These normative data may be helpful in understanding alterations in disease progression with treatment in DMD.
The choice of primary endpoint for a clinical trial is critical to the success of the trial. An ideal primary endpoint is highly sensitive to disease progression and conversely a treatment-related slowing of the disease and can be measured in all participants for the duration of the study. A number of trials to date have used the 6MWT as a primary endpoint, however recently the 6MWT has come under some criticism.16,25 TFTs, including the 10m walk/run, climbing 4 stairs, and STS, offer potentially appealing alternative primary endpoints for use in ambulatory patients.
In this ImagingDMD cohort of ambulatory patients, uniformly treated with corticosteroids, the responsiveness of all tests was low in 5–6.9 year old boys with DMD, necessitating the development of other outcome measures. This is consistent with the known stability or even gains in motor skills observed in boys with DMD at younger ages, followed by a progressive decline. The slightly lower responsiveness of the 6MWT compared to the TFTs, may have important implications for the number of subjects needed to detect a treatment-related stabilization in clinical trials. We estimate that if the 6MWT was used as the primary outcome, a trial would need 50 boys to detect a complete stabilization in the treated cohort over one year, or 256 boys to detect a 50% slowing of disease progression. If the trial used the 10m walk/run, on the other hand, we estimate that 17 boys would be needed to detect complete stabilization, and 68 boys would be needed to detect a 50% slowing of disease progression.
It is important to regulatory authorities that the primary endpoint chosen is related to clinically meaningful disease events. In DMD, loss of the ability to get up from the floor, climb stairs, or walk are important milestones to the patient. Loss of each of these abilities can be predicted from the respective test. In this study, we observed that the TFTs were slightly stronger predictors of loss of function than the 6MWT. This is important information in planning a clinical trial; each subject who loses the ability to complete a test compromises the statistical power and thus the ability to detect a treatment effect. The results of ROC analysis suggest that boys who take less than 6 to ~7.5 seconds to complete the TFTs, or walk more than 319m on the 6MWT, are more likely to retain the ability to complete that test over 12 months. The 10m walk/run threshold estimated here, ~7.5 seconds, is substantially lower than the 10 s threshold previously reported.24 On the other hand, the 6MWT threshold is similar to previous reports.15,24 Age is another important predictor of loss of function, and this study found that at 9 years old, >80% of corticosteroid treated boys could still complete all tests. However, by ~14 years old about half of the boys were unable to complete any of the tests.
Inclusion criteria for a study may target a population in which it is possible to observe differences in the decline in function.26 The 6MWT has shown promise in predicting decline previously.24 In the current study, 6MWD was effective in predicting functional stability or decline in performance on the TFTs, with boys walking ≥376m likely to remain stable. Interestingly the relationship between baseline 6MWD and future change in the 6MWT itself was limited. Maturational improvements in 6MWD in younger boys may make future changes in 6MWD more difficult to predict in a diverse sample.
This large multicenter natural history study provides important information to help guide clinical trial design that 10m walk/run may be slightly more sensitive than the 6MWT in 7–12.9 year old corticosteroid treated boys. We conjecture that the 6MWT may be less sensitive to changes in muscle performance per se as it does not measure muscle power, but is rather reflective of cardiopulmonary function and potentially overall “well being”. In keeping with this, we found that although the overall relationship between the 6MWT and the 10m walk/run was strong, the relationship was less pronounced in highly functional boys, who are most likely to be running during the 10m walk/run. Given the duration of the test, performance on the 6MWT may also be more subject to compensatory strategies and influenced by motivation.27 We propose that the 10m walk/run test offers a valuable alternative, providing high sensitivity, a strong predictive value, and the ability to be performed in a large proportion of the boys (>80%) until the age of 12 years. In contrast, the STS and climbing 4 stairs tests both have good sensitivity to change in 7–10 year old boys with DMD, but do not detect functional progression in older boys, a high proportion of whom have lost the ability to perform these tests. Additionally, the 4 stairs time is susceptible to commonly used compensatory movement strategies, such as leaning on the handrail, to complete the task, reducing its specificity to ambulatory decline.
This study included only corticosteroid treated subjects. Current clinical trials typically specify corticosteroid status as an inclusion criterion, selecting only treated or untreated boys. In contrast, most large natural history studies to date have included both steroid treated and untreated boys, limiting the applicability of their results to clinical trial planning. Although the ImagingDMD study includes both steroid treated and untreated subjects, the volume of data in steroid untreated boys is too limited to include in the current manuscript; a set of these results have been published previously.28
There are some limitations to this study. We did not account for the presence or impact of differences in clinical care, such as age at steroid initiation, use of dietary supplements and medications, or physical therapy treatment. The impact of primary genetic mutation was not examined. Assessments in this study took place annually, and no information is available about the timing of changes in function, such as loss of ambulation, between visits. Finally, the inclusion criteria in this study targeted subjects who were able to walk >100m and climb 4 stairs at baseline, while other natural history studies and clinical trials may have utilized other thresholds for inclusion.12–15
Conclusions
Overall, the results of this study provide compelling evidence supporting the use of TFTs as primary outcomes in clinical trials, particularly the 10m walk/run, which may be especially valuable given potential limitations of the 6MWT.16,25 In addition, we believe that this study offers critical contemporary natural history data in corticosteroid treated boys with DMD as well as normative control data that is of value to many in the DMD community.
Supplementary Material
Acknowledgments
The ImagingDMD study is supported by grant funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (Magnetic Resonance Imaging and Biomarkers in Muscular Dystrophy - R01AR056973). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to acknowledge the subjects and their families for their dedication and participation in ImagingDMD, and we are appreciative of the research staff who assisted in data collection.
Abbreviations
- DMD
Duchenne Muscular Dystrophy
- ROC
Receiver Operating Characteristic
- SD
Standard Deviation
- STS
Supine to Stand
- TFTs
Timed Function Tests
- 6MWT
Six Minute Walk Test
- 6MWD
Six Minute Walk Distance
Footnotes
Ethical publication statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflict of interest: None of the authors has any conflict of interest to disclose.
References
- 1.Aartsma-Rus A. Dystrophin Analysis in Clinical Trials. Journal of Neuromuscular Diseases. 2014;1:41–53. [PubMed] [Google Scholar]
- 2.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Annals of Neurology. 2012;71:304–13. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 4.Emery AE. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscular Disorders : NMD. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 5.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. The Lancet. Neurology. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 6.Sussman M. Duchenne muscular dystrophy. The Journal of the American Academy of Orthopaedic Surgeons. 2002;10:138–51. doi: 10.5435/00124635-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. The Lancet. Neurology. 2010;9:177–89. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 8.Vignos PJ, Jr, Spencer GE, Jr, Archibald KC. Management of progressive muscular dystrophy in childhood. JAMA. 1963;184:89–96. doi: 10.1001/jama.1963.03700150043007. [DOI] [PubMed] [Google Scholar]
- 9.Bello L, Gordish-Dressman H, Morgenroth LP, et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology. 2015;85:1048–55. doi: 10.1212/WNL.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bushby K, Finkel R, Wong B, et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle & Nerve. 2014;50:477–87. doi: 10.1002/mus.24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendell JR, Goemans N, Lowes LP, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Annals of Neurology. 2016;79:257–71. doi: 10.1002/ana.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle & Nerve. 2013;48:343–56. doi: 10.1002/mus.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzone E, Vasco G, Sormani MP, et al. Functional changes in Duchenne muscular dystrophy: a 12-month longitudinal cohort study. Neurology. 2011;77:250–6. doi: 10.1212/WNL.0b013e318225ab2e. [DOI] [PubMed] [Google Scholar]
- 14.Mazzone ES, Pane M, Sormani MP, et al. 24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy. PloS One. 2013;8:e52512. doi: 10.1371/journal.pone.0052512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pane M, Mazzone ES, Sivo S, et al. Long term natural history data in ambulant boys with duchenne muscular dystrophy: 36-month changes. PloS One. 2014;9:e108205. doi: 10.1371/journal.pone.0108205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman EP, McNally EM. Exon-skipping therapy: a roadblock, detour, or bump in the road? Science Translational Medicine. 2014;6:230fs14. doi: 10.1126/scitranslmed.3008873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald CM, Henricson EK, Han JJ, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle & Nerve. 2010;41:500–10. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 18.Brooke MH, Griggs RC, Mendell JR, et al. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle & Nerve. 1981;4:186–97. doi: 10.1002/mus.880040304. [DOI] [PubMed] [Google Scholar]
- 19.Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. The Journal of Pediatrics. 2007;150:395–9. 9.e1–2. doi: 10.1016/j.jpeds.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 20.Henricson E, Abresch R, Han JJ, et al. Percent-predicted 6-minute walk distance in duchenne muscular dystrophy to account for maturational influences. PLoS Currents. 2012;4:Rrn1297. doi: 10.1371/currents.RRN1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle & Nerve. 2013;48:357–68. doi: 10.1002/mus.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonati U, Hafner P, Schädelin S, et al. Quantitative muscle MRI: A powerful surrogate outcome measure in Duchenne muscular dystrophy. Neuromuscul Disord. 2015 doi: 10.1016/j.nmd.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Pane M, Mazzone ES, Sormani MP, et al. 6 Minute walk test in Duchenne MD patients with different mutations: 12 month changes. PloS one. 2014;9:e83400. doi: 10.1371/journal.pone.0083400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle & Nerve. 2013;48:343–56. doi: 10.1002/mus.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shieh PB. Duchenne muscular dystrophy: clinical trials and emerging tribulations. Current Opinion in Neurology. 2015;28:542–6. doi: 10.1097/WCO.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 26.Bushby K, Connor E. Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings. Clinical Investigation. 2011;1:1217–35. doi: 10.4155/cli.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfano LN, Lowes LP, Berry KM, et al. Pilot study evaluating motivation on the performance of timed walking in boys with Duchenne muscular dystrophy. Neuromuscular Disorders. 2014;24:1. [Google Scholar]
- 28.Arpan I, Willcocks RJ, Forbes SC, et al. Examination of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology. 2014;83:974–80. doi: 10.1212/WNL.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henricson E, Abresch R, Han JJ, et al. The 6-Minute Walk Test and Person-Reported Outcomes in Boys with Duchenne Muscular Dystrophy and Typically Developing Controls: Longitudinal Comparisons and Clinically-Meaningful Changes Over One Year. PLoS Currents. 2013;5 doi: 10.1371/currents.md.9e17658b007eb79fcd6f723089f79e06. ecurrents.md.9e17658b007eb79fcd6f723089f79e06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfano LNL LP, Berry KM, Yin H, Dvorchik I, Flanigan KM, Cripe L, Mendell JR. Pilot study evaluating motivation on the performance of timed walking in boys with Duchenne muscular dystrophy. Neuromuscular Disorders. 2014;24:860. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.