Abstract
Background and Objective
The role of muscle rigidity as an etiological factor of falls in Parkinson's disease (PD) is poorly understood. Our objective was to determine whether lower leg rigidity was differentially associated with frequent falls in PD compared to upper limb, neck, and total rigidity measures.
Methods
We examined the associations between Unified Parkinson's Disease Rating Scale–Part III (motor) rigidity subscores and the history of monthly or more frequent falls in 216 individuals with PD (age, 66 ± 10 years; 36% female; disease duration, 7 ± 5 years) with logistic regression.
Results
A total of 35 individuals were frequent fallers. Significant associations were identified between lower limb rigidity and frequent falls (P = 0.01) after controlling for age, sex, PD duration, total Unified Parkinson's Disease Rating Scale– Part III score, and presence of freezing of gait. No significant associations (P ≥ 0.14) were identified for total, arm, or neck rigidity.
Conclusion
Lower limb rigidity is related to frequent falls in people with PD. Further investigation may be warranted into how parkinsonian rigidity could cause falls.
Keywords: balance, accidental falls, freezing of gait, cognitive impairment
Balance problems and falls are a major issue in Parkinson's disease (PD).1 Recently, a group of PD patients, caregivers, and health professionals ranked balance problems and falls as a primary research priority.2 Although we have some ability to identify PD patients at high risk for falls based on factors such as fall history,3 many factors are nonmodifiable and hence have little value in preventing falls. Although many studies have attempted to identify the associations between clinical and physiological measures such as including Unified Parkinson's Disease Rating Scale–Part III (UPDRS‐III)4 items and falls,3, 5, 6 rigidity is an understudied factor.5, 7, 8
Parkinsonian rigidity may cause functional balance and gait impairment,9, 10, 11, 12 but its relationship to falls is unclear. Biomechanical simulations13 suggest that rigidity, particularly lower limb rigidity, may be an important contributor to falls. In simulation, increased muscle stiffness such as that identified in rigid patients14 impairs the ability to withstand perturbations to the center of body mass.13 The narrow stance common in PD may compensate for stiffness because of rigidity13 and related problems.15 Although some studies suggest a contribution of axial rigidity to balance impairments and fall risk,16, 17 lower limb rigidity is not commonly considered an independent risk factor.5, 6, 7 Importantly, if lower limb rigidity were a risk factor for falls, it could potentially be modified through standard pharmacotherapy as rigidity responds well to levodopa.9, 18, 19, 20
We used logistic regression to determine whether lower limb rigidity assessed on the UPDRS‐III4 was associated with a history of monthly or more frequent falls in individuals with mild to moderate PD. We reasoned that lower limb rigidity would be associated more with fall history than upper limb or neck rigidity because of the lower limbs’ involvement in locomotion and static balance. We hypothesized that (1) lower limb rigidity scores would be associated with falls and (2) upper limb, neck, and total rigidity scores would not be associated with falls.
Patients and Methods
Data Sources
We used the existing measures of 216 PD patients from observational and rehabilitative studies we conducted from 2011 to 2015. The participants provided written informed consent according to protocols approved by the institutional review boards of Emory University and the Georgia Institute of Technology. All participants met the modified UK Brain Bank diagnostic criteria for PD.21 Exclusion criteria were applied according to the specific protocols of the studies in which they originally enrolled. For most of the participants (143 of 216), these were the following: advanced stage dementia in which patients were unable to perform activities of daily living independently, presence of cerebrovascular disease or extensive white matter disease, findings suggestive of atypical parkinsonism (extraocular movement abnormalities, pyramidal tract signs, ataxia), past neuroleptic use, or past history of multiple head injuries.22 For the remaining participants (73 of 216), the exclusion criteria were a history of neurological insult other than PD, inability to walk ≥3 meters with or without assistance, or other significant musculoskeletal, cognitive, or neurological impairment other than PD as determined by the investigators.23, 24, 25 Beginning with 220 records, 4 records were excluded because of incomplete Freezing of Gait Questionnaire26 or UPDRS‐III4 scores.
Study Variables
The participants were classified as “fallers” if they scored ≥2 on the Gait and Falls Questionnaire item 12,26 which corresponds to monthly or more frequent falls, and were classified as “nonfallers” otherwise. The primary independent variables were the following rigidity subscores assembled from rigidity items of the UPDRS‐III4: total rigidity (/20), the sum of UPDRS‐III items 22a to 22e; lower limb sum (/8), the sum of items 22d to 22e; upper limb sum (/8), the sum of items 22b to 22c; and neck (/4), item 22a. Additional demographic and clinical variables associated with falls in PD included age, female sex, global cognition (assessed with the Montreal Cognitive Assessment [MoCA]27), disease duration,3, 28 and self‐reported presence of freezing of gait (FOG). The participants were classified as freezers if they scored ≥2 on Freezing of Gait Questionnaire26 item 3, which corresponds to weekly or more frequent freezing. When caregivers were present, they were allowed to assist the patients in answering questionnaires. No attempts were made to quantify FOG during motor examination or to query patients as to during which medication state FOG occurred (eg, refs. 22, 29).
Statistical Methodology
The differences in study variables between fallers and nonfallers were assessed with t tests and χ2 tests as appropriate. Satterthwaite's approximation was used for t tests with unequal variance as assessed with folded F tests. Multivariate logistic regressions were performed to identify the associations between rigidity subscores and faller status while controlling for age, gender, PD duration, total UPDRS‐III score, and presence of FOG.28 Associations between rigidity subscores and fall history were expressed as odds ratios (OR) ± 95% confidence interval (CI). To control for overall UPDRS‐III score, the remainder of each UPDRS‐III total score after subtracting rigidity items was entered as a covariate. Statistical tests were performed at α = 0.05 in SAS University Edition 7.2 (SAS Institute, Cary, NC).
Additional Analyses
Additional analyses examined the associations between rigidity subscores for each limb and fall history, the associations between rigidity subscores and annually or more frequent (rather than monthly or more frequent) falls, the impact of postural instability (UPDRS‐III item 30), gait impairment (UPDRS‐III item 29), overall cognition (MoCA score), medication state during examination on associations between rigidity subscores and fall history, the presence of dopamine agonist monotherapy,30 and additional stratified and interaction models examining the potential dependencies of identified associations on FOG status (Supplementary Materials S1).
Results
Demographic and clinical characteristics are presented in Table 1. Among the study sample, 35 of 216 patients (16%) fell monthly or more often. The majority of patients (73%) were classified as nonfreezers by self‐report. Consistent with previous results,3, 28 when compared with nonfallers, fallers had longer disease duration (P < 0.01) and worse performance on the UPDRS‐III (P << 0.01), the Gait and Falls Questionnaire (P << 0.01), and the Freezing of Gait Questionnaire (P << 0.01). Among the fallers, the prevalence of FOG and female sex were also higher by ≈3 times (P << 0.01) and ≈1.5 times (P = 0.04), respectively. No significant differences were observed in age, global cognition (MoCA), education, or age at PD onset. Modified Hoehn and Yahr stages were recorded for a subset of patients (22 of 216). Among those, Hoehn and Yahr stage ranged from 1.5 to 3, with a mean value of 2.3 ± 0.5. The average Hoehn and Yahr stage was significantly higher among fallers (2.9 vs. 2.2; P < 0.01).
Table 1.
Demographic and clinical features of the study population overall and stratified on presence of previous monthly falls
| Characteristic | All Participants, N = 216 | Nonfallers, n = 181 | Fallers, n = 35 | P Value |
|---|---|---|---|---|
| Age, yr | 65.7 ± 9.7 | 65.5 ± 9.6 | 67.1 ± 10.3 | 0.35 |
| Sex | ||||
| Female | 78 (36) | 60 (33) | 18 (52) | 0.04 |
| Male | 138 (64) | 121 (67) | 17 (48) | |
| MoCA (/30) | 24.7 ± 3.6a | 24.8 ± 3.6b | 24.2 ± 4.0c | 0.37 |
| Education, y | 16.1 ± 2.2d | 16.1 ± 2.3e | 16.2 ± 1.7 | 0.92 |
| Disease duration, yr | 7.4 ± 4.5 | 6.9 ± 4.1 | 9.9 ± 5.7 | <0.01 |
| Age at onset, yr | 58.3 ± 10.6 | 58.6 ± 10.1 | 57.3 ± 12.9 | 0.58 |
| UPDRS‐III score (/108) | 22.0 ± 10.0 | 20.6 ± 9.2 | 29.5 ± 10.5 | <<0.01 |
| FOG‐Q total (/24) | 4.5 ± 4.6 | 3.5 ± 3.9 | 9.6 ± 4.9 | <<0.01 |
| FOG‐GF total (/64) | 8.6 ± 9.0 | 6.1 ± 6.2 | 21.1 ± 10.9 | <<0.01 |
| Freezing of gait | ||||
| Freezer | 59 (27) | 35 (19) | 24 (69) | <<0.01 |
| Nonfreezer | 157 (73) | 146 (81) | 11 (31) |
Values are shown as either mean ± standard deviation or N (%). P values reflect univariate tests of central tendency (t tests or χ2 tests) between fallers and nonfallers.
N = 194;
N = 163;
N = 31;
N = 215;
N = 180.
MoCA, Montreal Cognitive Assessment; UPDRS‐III, Unified Parkinson's Disease Rating Scale–Part III: motor exam; FOG‐Q, Freezing of Gait Questionnaire; FOG‐GF, Gait and Falls Questionnaire.
Univariate analyses demonstrated that rigidity subscores for the lower limbs were significantly higher in fallers (P value range, 0.004–0.025). In contrast, no statistically significant differences were found for upper limb (P value range, 0.193–0.245) or neck (P = 0.085) rigidity scores (Fig. 1A).
Figure 1.
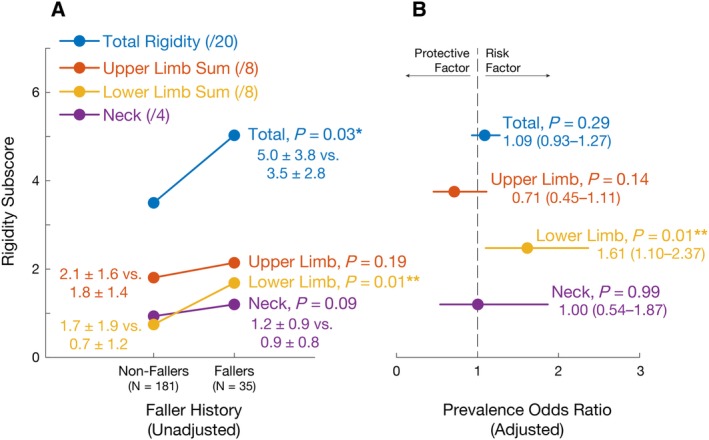
Associations between rigidity and falls. (A) Differences in rigidity subscores between patients with Parkinson's disease with and without histories of monthly or more frequent falls. (B) Associations between rigidity subscores and fall history (expressed as odds ratio ± 95% confidence interval) after adjusting for age, sex, total Unified Parkinson's Disease Rating Scale‐III, Parkinson's disease duration, and presence of freezing of gait.
Multivariate logistic regression models identified significant associations between lower limb rigidity and fall history (P = 0.014; Fig. 1B), but no significant associations for total (P = 0.289), arm (P = 0.135), or neck rigidity (P = 0.991) after controlling for age, gender, PD duration, total UPDRS‐III score, and the presence of FOG. In these models, a total lower limb rigidity score of 2 (the 75th percentile score in the sample) was associated with an OR for monthly or more frequent falls of 2.6 (95% CI, 1.2–5.6), and a total score of 6 (the maximum score in the study sample) was associated with an OR of 17.7 (95% CI, 1.8–175.7). Consistent with the literature,3, 28 logistic regression models also identified significant associations between female sex (OR [95% CI], 3.2 [1.3–8.1]; P = 0.013), FOG (OR [95% CI], 7.2 [2.9–17.9]; P << 0.01), disease duration (OR [95% CI], 1.1 [1.0–1.2]; P = 0.022), and total UPDRS‐III score (OR [95% CI], 1.1 [1.0–1.2]; P = 0.003) and fall history.
Additional Analyses
Associations between lower limb rigidity and history of frequent falls were largely unaltered in additional analyses (Supplementary Materials S1) that stratified participants by medication state during examination or that controlled for the presence of postural instability (UPDRS‐III item 305), gait impairment (UPDRS‐III item 29), reduced cognition (MoCA score), or dopamine agonist monotherapy. These models yielded ORs very similar to those identified in the main model, with changes in lower limb rigidity ORs of −4.3%, +3.7%, −8.1%, +4.3%, and 0.0%, respectively. When a less‐stringent definition of fall history (annual or more frequent rather than monthly or more frequent falls) was used, the associations between lower limb rigidity and fall history were significantly reduced in magnitude (−21.7%), suggesting that lower limb rigidity is associated primarily with frequent falls. The associations between lower limb rigidity and fall history were decreased in magnitude, but remained statistically significant when a less‐stringent definition for freezer status was used (OR decrease of −11.2%). Models that included separate rigidity scores from each limb identified a strong association between rigidity on the more rigid side and a history of frequent falls (OR increase of +23.6%).
Discussion
To our knowledge, this is the first study to demonstrate an association between lower limb rigidity and falls in PD. We found that lower limb rigidity, unlike upper limb or neck rigidity, was associated with history of monthly or more frequent falls, even after controlling for common risk factors including age, sex, total UPDRS‐III, PD duration, and presence of FOG. Additional analyses confirmed that the identified associations between lower limb rigidity and falls were not confounded by coexisting postural instability, gait impairment, or cognitive impairment in lower limb‐rigid patients, as explicitly controlling for these factors altered ORs only minimally. These results suggest that understanding and treating rigidity may be important in reducing fall risk.
In addition to common features on exam that raise concerns to neurologists that falls may be impending for a patient, such as FOG, postural instability, and axial rigidity,5, 6 lower limb rigidity may be a clinically observable—and potentially modifiable—parkinsonian feature associated with falls. Notably, lower limb rigidity may be important to consider independent of axial rigidity,9, 10, 11, 17, 31 which is measured with specialized equipment9, 11 uncommon in clinical use. Although appendicular and axial rigidity are often thought of as having a common etiology, these signs likely reflect distinct underlying pathophysiology and respond differently to dopaminergic drugs such as levodopa9, 18, 20, 32 and apomorphine.19 Here, because most patients were on medications, whether rigidity could be further reduced is unknown. Yet the potential remains that rigidity could be considered during medication changes along with concerns such as wearing off or fluctuations.33, 34, 35
How might lower limb rigidity contribute to falls? Although abnormal deep tendon reflexes are not a key parkinsonian feature,36 rigid patients exhibit increased long‐latency electromyographic responses to passive joint movements37, 38 and possibly increased tonic muscle activity,39 both of which may increase joint stiffness. A previous simulation study of standing balance13 demonstrated that, as the stiffness of the hip joints is increased beyond a certain amount, the neuro‐musculoskeletal system becomes increasingly unstable as a result of response delays. This makes it increasingly more difficult to maintain balance without stepping during perturbations. The implication is that a person with lower limb rigidity may have decreased ability to control the center of mass with the feet in place, requiring frequent steps to maintain balance. We hypothesize that because anticipatory postural adjustments40, 41 and stepping reaction time and accuracy are impaired in PD,42 lower limb rigidity could therefore cause falls. However, the underlying biomechanical and physiological mechanisms of rigidity remain unclear.
These results should be considered in light of a few limitations to the study design. One primary limitation was that fall history was taken via self‐report. Based on average MoCA score (24.7 ± 3.6), cognitive impairments were likely present in the sample and could have affected accuracy. To limit this, we used a stringent criterion to identify fallers—monthly or more frequent falls. We found that the association between lower limb rigidity and falls was significantly attenuated in logistic regression models using a less‐stringent criterion (Supplementary Materials S1). Based on this, we speculate that lower limb rigidity may be uniquely related with very frequent falls in PD; however, prospective studies are required for confirmation. If prospective studies demonstrate that lower limb rigidity is associated with future falls, patients with this sign could potentially be referred to interventions to reduce risk.43, 44, 45, 46, 47, 48
A second important consideration is that because of the retrospective nature of the study, many clinical variables potentially relevant to fall risk were not available for analysis. We could not rigorously control for common comorbidities such as visual and orthopedic problems or for specific parkinsonian features such as the presence of dyskinesias, all of which could potentially be unequally distributed among the groups and therefore influence fall prevalence.17 In particular, detailed medication dosages were not available, prohibiting the use of total levodopa dose49 as a covariate, or the verification that the dopaminergic medication dosage was stable during the lookback period. Finally, the questionnaires were used to assess freezing status; however, for the most reliable assessment, FOG classification should be based on objective confirmation by an experienced observer during clinical assessment,50 particularly because participants with FOG in different medication states (eg, refs. 22, 29) may experience related fall risk at different times. These results suggest that these and other variables could potentially be very important during future prospective studies of fall risk.
Although many studies have attempted to identify the associations between clinical and physiological assessments and fall risk;3, 5, 6 lower limb rigidity is an understudied risk factor.5, 7 This may be in part because of one impactful early study6 that found no association between the combined presence of lower limb/neck rigidity and fall risk, suggesting that combining these sources of rigidity may distort associations with falls. These results suggest that prospective studies of the relationships between rigidity and fall risk in PD could provide new information.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
J.L.M.: 1A, 1B, 1C, 2A, 2B, 3A
M.E.H.: 1A, 1B, 1C, 2C, 3A, 3B
S.A.F.: 1A, 1B, 1C, 2C, 3B
L.H.T.: 1A, 1B, 1C, 2C, 3A, 3B
Disclosures
Ethical Compliance Statement: The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The authors confirm that the institutional review boards of Emory University and the Georgia Institute of Technology approved this study and that informed consent was obtained from all subjects prior to participation.
Funding Sources and Conflict of Interest: This study was supported in part by National Institutes of Health Grants K25HD086276, R01HD046922, R21HD075612, UL1TR002378, and UL1TR000454; Department of Veterans Affairs R&D Service Career Development Awards E7108M and N0870W, The Consolidated Anti‐Aging Foundation, and the Sartain Lanier Family Foundation. The authors report no conflict of interest related to this study.
Financial Disclosures for the previous 12 months: J.L.M. has received research funding or support from the National Institutes of Health. M.E.H. has received research funding or support from the Veteran's Health Administration, National Institutes of Health, National Science Foundation, and the Parkinson's Foundation. S.A.F. has received research funding or support from the National Institutes of Health, the Michael J. Fox Foundation, the CHDI Foundation, Ipsen, Jazz Pharmaceuticals, Medtronic, Sunovion, Teva, US World Meds, Vaccinex Inc., and Voyager Therapeutics; has received honoraria from Acadia, Adamas, Biogen, Lundbeck, Neurocrine, Prexton Therapeutics, Sunovion, and Teva; and has received royalties from Blackwell Futura, Demos, and Uptodate. L.H.T. has received research funding or support from the National Institutes of Health and the National Science Foundation.
Supporting information
Materials S1. Secondary analyses including Tables S1 to S10.
Data S1. Data used for statistical analyses.
J. Lucas McKay and Madeleine. E. Hackney contributed equally to this work.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Bloem BR, Grimbergen YAM, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. J Neurol 2001;248(11):950–958. [DOI] [PubMed] [Google Scholar]
- 2. Deane KH, Flaherty H, Daley DJ, et al. Priority setting partnership to identify the top 10 research priorities for the management of Parkinson's disease. BMJ Open 2014;4(12):e006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paul SS, Canning CG, Sherrington C, Lord SR, Close JC, Fung VS. Three simple clinical tests to accurately predict falls in people with Parkinson's disease. Mov Disord 2013;28(5):655–662. [DOI] [PubMed] [Google Scholar]
- 4. Fahn S, Elton RL, Members of the UPDRS Development Committee. The Unified Parkinson's Disease Rating Scale In: Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163. [Google Scholar]
- 5. Paul SS, Allen NE, Sherrington C, et al. Risk factors for frequent falls in people with Parkinson's disease. J Parkinsons Dis 2014;4(4):699–703. [DOI] [PubMed] [Google Scholar]
- 6. Latt MD, Lord SR, Morris JG, Fung VS. Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. Mov Disord 2009;24(9):1280–1289. [DOI] [PubMed] [Google Scholar]
- 7. Gray P, Hildebrand K. Fall risk factors in Parkinson's disease. J Neurosci Nurs 2000;32(4):222–228. [DOI] [PubMed] [Google Scholar]
- 8. Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry 2002;72(6):721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson's disease: direct measurements of trunk and hip torque. Exp Neurol 2007;208(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol 1996;75(6):2380–2396. [DOI] [PubMed] [Google Scholar]
- 11. Schenkman M, Morey M, Kuchibhatla M. Spinal flexibility and balance control among community‐dwelling adults with and without Parkinson's disease. J Gerontol A Biol Sci Med Sci 2000;55(8):M441–M445. [DOI] [PubMed] [Google Scholar]
- 12. Franzen E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson's disease. Exp Neurol 2009;219(2):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bingham JT, Choi JT, Ting LH. Stability in a frontal plane model of balance requires coupled changes to postural configuration and neural feedback control. J Neurophysiol 2011;106(1):437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marusiak J, Jaskolska A, Budrewicz S, Koszewicz M, Jaskolski A. Increased muscle belly and tendon stiffness in patients with Parkinson's disease, as measured by myotonometry. Mov Disord 2011;26(11):2119–2122. [DOI] [PubMed] [Google Scholar]
- 15. Kim S, Horak FB, Carlson‐Kuhta P, Park S. Postural feedback scaling deficits in Parkinson's disease. J Neurophysiol 2009;102(5):2910–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cano‐de‐la‐Cuerda R, Vela‐Desojo L, Miangolarra‐Page JC, Macias‐Macias Y. Axial rigidity is related to the risk of falls in patients with Parkinson's disease. NeuroRehabilitation 2017;40(4):569–577. [DOI] [PubMed] [Google Scholar]
- 17. van der Marck MA, Klok MP, Okun MS, et al. Consensus‐based clinical practice recommendations for the examination and management of falls in patients with Parkinson's disease. Parkinsonism Relat Disord 2014;20(4):360–369. [DOI] [PubMed] [Google Scholar]
- 18. Weinrich M, Koch K, Garcia F, Angel RW. Axial versus distal motor impairment in Parkinson's disease. Neurology 1988;38(4):540–545. [DOI] [PubMed] [Google Scholar]
- 19. Bartolic A, Pirtosek Z, Rozman J, Ribaric S. Postural stability of Parkinson's disease patients is improved by decreasing rigidity. Eur J Neurol 2005;12(2):156–159. [DOI] [PubMed] [Google Scholar]
- 20. Zach H, Dirkx M, Pasman JW, Bloem BR, Helmich RC. The patient's perspective: the effect of levodopa on Parkinson symptoms. Parkinsonism Relat Disord 2017;35:48–54. [DOI] [PubMed] [Google Scholar]
- 21. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Factor SA, Scullin MK, Sollinger AB, et al. Freezing of gait subtypes have different cognitive correlates in Parkinson's disease. Parkinsonism Relat Disord 2014;20(12):1359–1364. [DOI] [PubMed] [Google Scholar]
- 23. Zafar M, Bozzorg A, Hackney ME. Adapted Tango improves aspects of participation in older adults versus individuals with Parkinson's disease. Disabil Rehabil 2016:1–8. [DOI] [PubMed] [Google Scholar]
- 24. McKay JL, Ting LH, Hackney ME. Balance, body motion, and muscle activity after high‐volume short‐term dance‐based rehabilitation in persons with Parkinson disease: a pilot study. J Neurol Phys Ther 2016;40(4):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang KC, Hackney ME, Ting LH, McKay JL. Antagonist muscle activity during reactive balance responses is elevated in Parkinson's disease and in balance impairment. PLoS ONE 2019;14(1):e0211137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord 2000;6(3):165–170. [DOI] [PubMed] [Google Scholar]
- 27. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009;73(21):1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKay JL, Lang KC, Ting LH, Hackney ME. Impaired set shifting is associated with previous falls in individuals with and without Parkinson's disease. Gait Pos 2018;62:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amboni M, Stocchi F, Abbruzzese G, et al. Prevalence and associated features of self‐reported freezing of gait in Parkinson disease: the DEEP FOG study. Parkinsonism Relat Disord 2015;21(6):644–649. [DOI] [PubMed] [Google Scholar]
- 30. Chou KL, Elm JJ, Wielinski CL, et al. Factors associated with falling in early, treated Parkinson's disease: the NET‐PD LS1 cohort. J Neurol Sci 2017;377:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steiger MJ, Thompson PD, Marsden CD. Disordered axial movement in Parkinson's disease. J Neurol Neurosurg Psychiatry 1996;61(6):645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prochazka A, Bennett DJ, Stephens MJ, et al. Measurement of rigidity in Parkinson's disease. Mov Disord 1997;12(1):24–32. [DOI] [PubMed] [Google Scholar]
- 33. Patel T, Chang F, Parkinson Society Canada. Parkinson's disease guidelines for pharmacists. Can Pharm J (Ott) 2014;147(3):161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dietrichs E, Odin P. Algorithms for the treatment of motor problems in Parkinson's disease. Acta Neurol Scand 2017;136(5):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson's disease (2001): treatment guidelines. Neurology 2001;56(11 suppl 5):S1–S88. [DOI] [PubMed] [Google Scholar]
- 36. Hammerstad JP, Elliott K, Mak E, Schulzer M, Calne S, Calne DB. Tendon jerks in Parkinson's disease. J Neural Transm Park Dis Dement Sect 1994;8(1‐2):123–130. [DOI] [PubMed] [Google Scholar]
- 37. Berardelli A, Sabra AF, Hallett M. Physiological mechanisms of rigidity in Parkinson's disease. J Neurol Neurosurg Psychiatry 1983;46(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tatton WG, Lee RG. Evidence for abnormal long‐loop reflexes in rigid Parkinsonian patients. Brain Res 1975;100(3):671–676. [DOI] [PubMed] [Google Scholar]
- 39. Marusiak J, Jaskolska A, Koszewicz M, Budrewicz S, Jaskolski A. Myometry revealed medication‐induced decrease in resting skeletal muscle stiffness in Parkinson's disease patients. Clin Biomech 2012;27(6):632–635. [DOI] [PubMed] [Google Scholar]
- 40. Petrucci MN, Diberardino LA, Mackinnon CD, Hsiao‐Wecksler ET. A neuromechanical model of reduced dorsiflexor torque during the anticipatory postural adjustments of gait initiation. IEEE Trans Neural Syst Rehabil Eng 2018;26(11):2210–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peterson DS, Lohse KR, Mancini M. Relating anticipatory postural adjustments to step outcomes during loss of balance in people with Parkinson's disease. Neurorehabil Neural Repair 2018;32(10):887–898. [DOI] [PubMed] [Google Scholar]
- 42. Caetano MJD, Lord SR, Allen NE, et al. Stepping reaction time and gait adaptability are significantly impaired in people with Parkinson's disease: implications for fall risk. Parkinsonism Relat Disord 2018;47:32–38. [DOI] [PubMed] [Google Scholar]
- 43. Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L. Falls in Parkinson's disease: a complex and evolving picture. Mov Disord 2017;32(11):1524–1536. [DOI] [PubMed] [Google Scholar]
- 44. Morris ME, Menz HB, McGinley JL, et al. A randomized controlled trial to reduce falls in people with Parkinson's disease. Neurorehabil Neural Repair 2015;29(8):777–785. [DOI] [PubMed] [Google Scholar]
- 45. Sparrow D, DeAngelis TR, Hendron K, Thomas CA, Saint‐Hilaire M, Ellis T. Highly challenging balance program reduces fall rate in Parkinson disease. J Neurol Phys Ther 2016;40(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med 2012;366(6):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology 2010;75(14):1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henderson EJ, Lord SR, Brodie MA, et al. Rivastigmine for gait stability in patients with Parkinson's disease (ReSPonD): a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Neurol 2016;15(3):249–258. [DOI] [PubMed] [Google Scholar]
- 49. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 50. Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non‐freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord 2012;18(2):149–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials S1. Secondary analyses including Tables S1 to S10.
Data S1. Data used for statistical analyses.


