We report on a 15‐year‐old girl with VAC14 gene‐related juvenile parkinsonism‐dystonia with substantial response to DBS to the globus pallidus (GPi‐DBS).
Case Report
The patient was born in a Middle Eastern country from consanguineous parents. Her past medical and family histories were unremarkable. At 12 years of age, she developed focal dystonia of her left hand, which generalized over 2.5 years (Video 1). She developed bradykinesia and freezing of gait, with intact cognition. Medical treatment was ineffective.
MRI of the brain demonstrated restricted diffusion of the bilateral corpus striatum, and T2 hypointensity with increased susceptibility of the globus pallidus and SN consistent with abnormal mineralization (Fig. 1). A clinically available gene panel evaluating diseases in the spectrum of neurodegeneration with brain iron accumulation (NBIA) was nondiagnostic.
Figure 1.
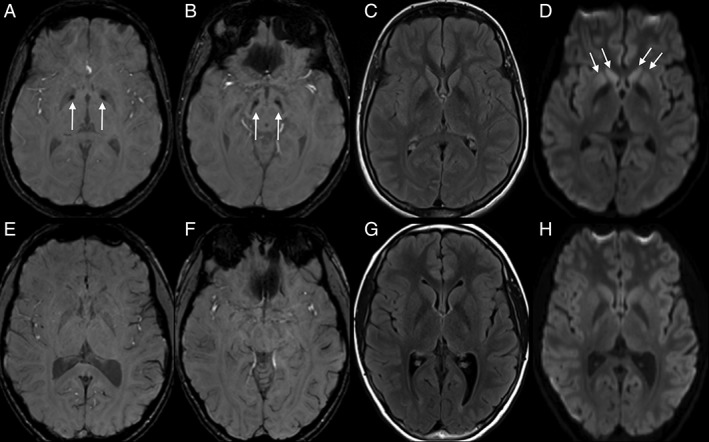
Axial SWI (A,B,E,F), FLAIR (C,G), and diffusion trace (D,H) sequences for the VAC14 proband at 14 years of age (A–D) and an age‐/sex‐matched control evaluated for headache (E–H). SWI imaging demonstrates abnormal susceptibility in the globus pallidus internus (arrows, A) and SN (arrows, B) as well as subtle diffusion abnormality in the corpus striatum (arrows, D). There was no convincing T2/FLAIR hyperintensity in the basal ganglia or SN (C). FLAIR, fluid‐attenuated inversion recovery; SWI, susceptibility‐weighted imaging.
A chromosomal microarray returned with a duplication at 16p13.11 and multiple areas of homozygosity comprising 3% of the genome. The duplication was thought to be an incidental finding, given mismatch between patient and typical phenotype. Upon reviewing the areas of homozygosity, the VAC14 gene stood out as a potential candidate based on previous reports.1, 2, 3
Sequencing of the VAC14 gene identified a homozygous missense variant of unknown significance (p.Lys651Glu, c.1951A > G). The variant has not been previously described in publicly available databases (gnomAD, Exome Variant Server, or 1000 Genomes) and involves a highly conserved amino acid in the protein C‐terminal dimerization domain, where previous pathogenic mutations have been located.1, 4 The alteration is nonconservative from a positively charged amino acid to a negatively charged one. In‐silico analysis with Mutation Taster predicted it to be disease causing, but Align GVGD and SIFT predicted the variant to be benign and tolerated.
The patient's skin fibroblasts were analyzed for abnormal endolysosomal morphology, associated with loss of VAC14 function.5, 6, 7 Patient cells as well as control human fibroblasts were cultured at equal density in full media for 24 hours. Fields of the cells were imaged and scored for the presence of visible perinuclear vacuoles. Cell culture experiments using primary fibroblasts were carried out.1 Briefly, all images were live cells captured using a Leica DMIRB inverted fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Transfections were carried out using Liofectamine 3000 (Thermo Fisher Scientific, Waltham, MA).
The patient's fibroblasts demonstrated evidence of abnormal vacuolization in 76 ± 2% of cells. In a second experiment, normal VAC14 transfected into patient cells rescued the vacuoles, whereas green fluorescent protein (GFP) alone did not (Fig. 2).
Figure 2.
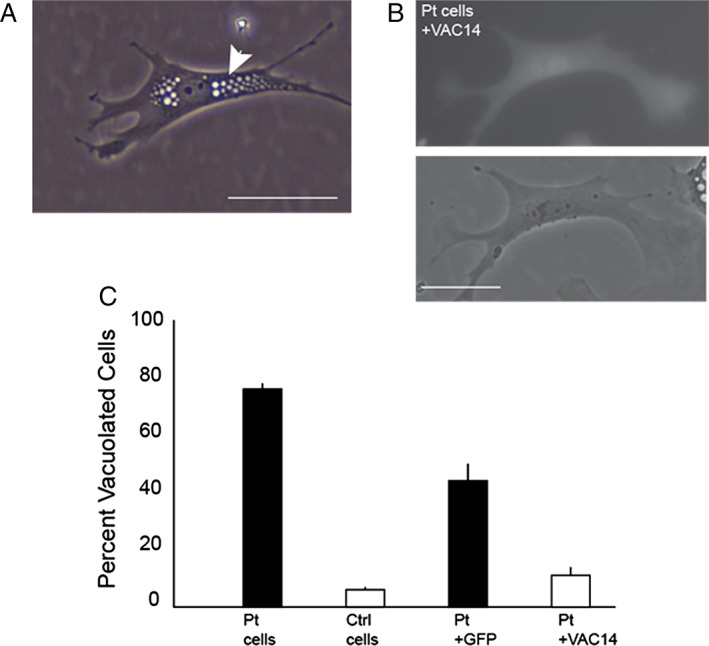
Vacuolization of patient fibroblasts is rescued with VAC14. (A) Live cell image of representative perinuclear vacuolization (arrow) seen in patient skin fibroblasts. (B) Patient fibroblast vacuolization rescued by cotransfection of VAC14 and GFP. (C) Quantification of vacuolization in patient and control fibroblasts as well as rescue of vacuolization following VAC14 transfection. Scale bars, 50 μm; error bars, standard deviation. Pt, patient.
At 15 years old, the patient underwent GPi‐DBS, based on literature supporting benefit of pallidal stimulation in early‐onset dystonia and brain iron accumulation syndromes.8, 9
On initial examination, she had fluctuating tone without rigidity, 1+ hypomimia, 3+ symmetric bradykinesia, and generalized dystonia. There was no resting, postural, or kinetic tremor. She endorsed fatigue, constipation, and joint pain. There was no cognitive change, dysautonomia or rapid eye movement sleep behavior disturbance. After 6 months of DBS, there was improvement in facial animation, 2+ bradykinesia, and recovery of ambulatory capacity. She required five steps to recover from the pull test, with spontaneous retropulsion. Freezing of gait was noted upon turns. Dystonia was much improved. Baseline Burke‐Fahn‐Marsden motor score was 86.5/120; disability score was 27/30. Within 6 months, motor score improved by 76% (to 21) and disability score improved by 56% (to 12; Video 2). The patient also endorsed improvements in pain and fatigue, but these were not formally measured. Medications and programming settings can be seen in Supporting Information Table S1.
Discussion
VAC14 encodes a dimeric scaffold protein that binds the lipid kinase, PIKFYVE, and the phosphatase, FIG 4. All three components are necessary for the synthesis of PI(3,5)P2 in the endolysosomal membrane compartment. The mechanism leading to enlarged endolysosomal vacuoles is not known precisely, but it is postulated that decreased levels of PI(3,5)P2 induce an osmotic effect. Alternatively, it is possible that dysfunction occurs through defects in membrane retrieval or fusion/fission.
Biallelic mutations in the VAC14 gene have been associated with three allelic disorders: childhood striatonigral degeneration, juvenile parkinsonism‐dystonia, and Yunis‐Varon–like syndrome.1 Precise genotype‐phenotype correlation is lacking, although it appears that truncating variants are associated with more severe phenotypes. Mouse models present with neurodegeneration and enlarged endolysosomal vacuoles, recapitulating the neuropathological findings from human subjects.2, 6, 7 In the few patients reported on so far, age of onset seems to range from 1.5 to 13 years, with onset of dystonia in a limb with rapid generalization affecting gait.1, 2, 3 Imaging may demonstrate diffusion restriction in the striatum and susceptibility‐weighed hypointensity in the pallidum and SN1 (Supporting Information Table S2).
We believe that VAC14‐related parkinsonism dystonia should be considered in the differential diagnosis of NBIAs, especially if available genetic panels are negative. To our knowledge, there is no effective treatment. Our patient experienced substantial improvement in dystonia with GPi‐DBS, but it is unclear whether benefit will be sustained. GPi‐DBS may be considered if there is failure of medical therapy.
Author Roles
(1) Research Project: A. Conception; B. Organization, C. Execution; (2) Statistical analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
C.M.dG.: 1A, 1B, 1C, 2A, 3A
S.S.: 1C, 3B
J.L.W.: 1C, 3B
E.Y.: 1C, 3B
G.L.: 1B, 1C, 3B
L.R.: 1A, 1B, 1C, 2A, 2B
Disclosures
Ethical Compliance Statement
The authors confirm that the approval of an institutional review board was not required for this work. Informed consent for videotaping was obtained and it is included in the submitted material for review. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months
Dr. Claudio Melo de Gusmao has grant support from a young investigator award from the National Ataxia Foundation. Dr. Scellig Stone has grant support from Credit Unions Kids at Heart Charitable Organization and received honoraria from Alcyone life sciences on multiple advisory sessions regarding CNS drug delivery devices. He has also consulted for PTC Therapeutics (formerly Agilis Therapeutics). Dr. Jeff L. Waugh has grant support from the American Academy of Neurology (Career Development Award) and the Collaborative Center for X‐linked Dystonia Parkinsonism. In the preceding 12 months, he has received honoraria from the International Parkinson and Movement Disorder Society, the American Academy of Neurology, and Medical Information Systems, Inc. Dr. Guy Lenk has grant support from the NIH (GM24872).
Supporting information
Video S1. Baseline. The patient is nonambulatory, with severe generalized dystonia affecting the neck, trunk, and extremities. Bradykinesia is noted on repeated finger tapping.
Video S2. Segment 1: 3 months after DBS. There is improvement in truncal and appendicular dystonia. Segment 2: 6 months after DBS. Further improvement in dystonia is noted. The patient can walk independently.
Table S1. DBS settings, Burke‐Fahn Marsden scale, and medications.
Table S2. Published reports of VAC14‐related gene disorders, at the time of manuscript submission. Table includes genetic and clinical characteristics. Papers are referenced in the bibliography section of the main manuscript.
Relevant disclosures and conflicts of interest are listed at the end of this article.
Contributor Information
Claudio M. de Gusmao, Email: claudio.degusmao@childrens.harvard.edu.
Lance H. Rodan, Email: lance.rodan@childrens.harvard.edu.
References
- 1. Lenk GM, Szymanska K, Debska‐Vielhaber G, et al. Biallelic mutations of VAC14 in pediatric‐onset neurological disease. Am J Hum Genet 2016;99:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stutterd C, Diakumis P, Bahlo M, et al. Neuropathology of childhood‐onset basal ganglia degeneration caused by mutation of VAC14. Ann Clin Transl Neurol 2017;4:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taghavi S, Chaouni R, Tafakhori A, et al. A clinical and molecular genetic study of 50 families with autosomal recessive parkinsonism revealed known and novel gene mutations. Mol Neurobiol 2018;55:3477–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alghamdi TA, Ho CY, Mrakovic A, et al. Vac14 protein multimerization is a prerequisite step for Fab1 protein complex assembly and function. J Biol Chem 2013;13:9363–9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonangelino CJ, Catlett NL, Weisman LS. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol Cell Biol 1997;17:6847–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Zolov SN, Chow CY, et al. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5‐bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci U S A 2007;104:17518–17523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin N, Chow CY, Liu L, et al. VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P2in yeast and mouse. EMBO J 2008;27:3221–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vidailhet M, Jutras MF, Grabli D, Roze E. Deep brain stimulation for dystonia. J Neurol Neurosurg Psychiatry 2013;84:1029–1042. [DOI] [PubMed] [Google Scholar]
- 9. Castelnau P, Cif L, Valente EM, et al. Pallidal stimulation improves pantothenate kinase‐associated neurodegeneration. Ann Neurol 2005;57:738–741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Baseline. The patient is nonambulatory, with severe generalized dystonia affecting the neck, trunk, and extremities. Bradykinesia is noted on repeated finger tapping.
Video S2. Segment 1: 3 months after DBS. There is improvement in truncal and appendicular dystonia. Segment 2: 6 months after DBS. Further improvement in dystonia is noted. The patient can walk independently.
Table S1. DBS settings, Burke‐Fahn Marsden scale, and medications.
Table S2. Published reports of VAC14‐related gene disorders, at the time of manuscript submission. Table includes genetic and clinical characteristics. Papers are referenced in the bibliography section of the main manuscript.


