ABSTRACT
Background
The BRadykinesia Akinesia INcoordination (BRAIN) tap test is an online keyboard tapping task that has been previously validated to assess upper limb motor function in Parkinson's disease (PD).
Objectives
To develop a new parameter that detects a sequence effect and to reliably distinguish between PD patients on and off medication. In addition, we sought to validate a mobile version of the test for use on smartphones and tablet devices.
Methods
The BRAIN test scores in 61 patients with PD and 93 healthy controls were compared. A range of established parameters captured number and accuracy of alternate taps. The new velocity score recorded the intertap speed. Decrement in the velocity score was used as a marker for the sequence effect. In the validation phase, 19 PD patients and 19 controls were tested using different hardware including mobile devices.
Results
Quantified slopes from the velocity score demonstrated bradykinesia (sequence effect) in PD patients (slope cut‐off −0.002) with 58% sensitivity and 81% specificity (discovery phase of the study) and 65% sensitivity and 88% specificity (validation phase). All BRAIN test parameters differentiated between on and off medication states in PD. Differentiation between PD patients and controls was possible on all hardware versions of the test.
Conclusion
The BRAIN tap test is a simple, user‐friendly, and free‐to‐use tool for the assessment of upper limb motor dysfunction in PD, which now includes a measure of bradykinesia.
Keywords: hypokinesia, Parkinson's disease, digital health, objective measures, ambulatory monitoring
The use of technology to complement the clinical assessment of Parkinson's disease (PD) is growing rapidly. Rating scales are valuable for clinical practice and research but are prone to interrater and intrarater variability.1, 2 To obviate these shortcomings, a range of technologies measuring bradykinesia in PD have been developed.3, 4, 5, 6, 7, 8, 9, 10, 11, 12
The BRadykinesia Akinesia Incoordination (BRAIN) test is a freely available, online keyboard finger‐tapping test that is based on the alternate finger‐tapping task.13, 14 It has previously been shown to differentiate patients with PD from healthy controls and has been used for the longitudinal monitoring of motor function in the PREDICT‐PD study, a large cohort of healthy older individuals stratified for future risk of PD.15
In the present study, we focused on 3 main aspects. First, we developed a new parameter, the velocity score (VS), to quantify an aspect of bradykinesia (which is defined as “slowness of initiation of voluntary movement with progressive reduction in speed and amplitude of repetitive actions”) and known in motor physiology as the sequence effect.16 Second, we used the VS score (and the previously existing BRAIN test parameters) to determine if it could reliably distinguish between patients with PD who were on and off dopaminergic medication. Third, we tested the new parameter in separate patient and control groups and introduced the test to mobile devices.
Methods
Participants
For the first 2 experiments, we assessed 61 patients (mean age 61.3 ± 8.2 years) with mild to moderate stage PD (Hoehn and Yahr <2.5) who were enrolled in the Exenatide‐PD trial at the National Hospital for Neurology and Neurosurgery, Queen Square, London. Inclusion and exclusion criteria for this trial have previously been published.9 Retrospective data from 93 healthy age‐matched controls (60.4 ± 10.7 years) were used for comparison.15
For the third experiment, 20 patients with PD (66.3 ± 6.6 years) were recruited from a movement disorders clinic at the National Hospital and 20 healthy partners (67.4 ± 9.0 years) of the recruited patients acted as the controls.
Experimental Procedure
To assess participants in the off state, the patients were instructed to stop their medications for 12 to 36 hours prior to the study visit. Part III of the Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) was assessed in addition to the performance on the BRAIN tap test. Patients then took their regular medication. Measurements were repeated in the on state. The same neurologist (D.A.) performed all of the clinical ratings.
The BRAIN test experimental task has been described previously,14 and further information can be found in Supplementary File S1 (supplementary material pages S1–S2). Briefly, the users are instructed to strike the “S” and “;” keys on a standard computer keyboard, alternately using 1 index finger as fast and as accurately as possible for 30 seconds. The parameters generated from the test include kinesia score (KS; the number of alternate taps in seconds), akinesia time (AT; the mean dwell time on the keys in milliseconds), incoordination score (IS; a measure of rhythm given by the variance in the traveling times between key presses), and dysmetria score(DS; a measure of the average accuracy of key strikes where the central key scores 1, adjacent keys are 2, and all other keys are 3).
In the third experiment, a smart device version of the test (“TapPD” developed by uMotif Limited for Apple [Cupertino, CA] iPhone and iPad devices) was used in addition to the keyboard test.17, 18 The same device was used for all participants. The participants used their index finger to alternately tap 2 target areas on the screen as fast and as accurately as possible for a period of 30 seconds. The application captured the same measurements as the BRAIN test, but the DS was engineered to incorporate additional capabilities of smart devices. The accuracy of each tap within a hit area was calculated as a decimal, with 0 being at the center of the target (perfect accuracy) and 1 being at the maximum edges of the hit area. DS1 was calculated as the average accuracy during the test. Screenshots of the “TapPD” interface can be viewed in Supplementary File S1 (Fig. S1).
We developed a new parameter, the VS, by measuring the intertap velocity throughout the duration of the test. To look for a sequence effect in patients with PD, the percentage of change in velocity with respect to the initial velocity between the first 2 key taps was computed and plotted as a time‐series graph. Slopes of acceleration/deceleration in the time‐series graphs were compared between the patients with PD in the off state and healthy controls (see Fig. 1). The steeper the slope, the faster the rate in increase/decline of velocity over time. For simplicity, a linear trend line was used for slope quantification. Alternate approaches for calculating a sequence effect using the number of consecutive decrements in dwell and traveling times (for example, 3) were explored and are shown in the Supplementary File S1 (supplementary material pages S2 and S5; Fig. S2).
Figure 1.
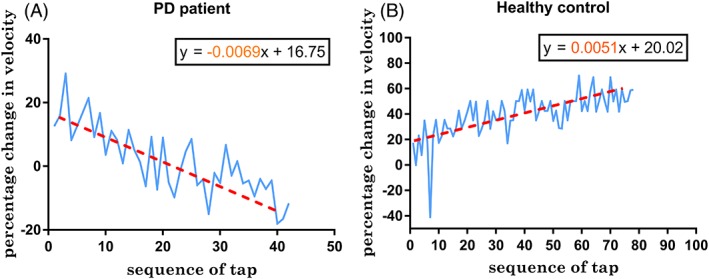
Time‐series analysis of change in velocity for the duration of the test compared to the initial velocity in a patient with Parkinson's disease (PD; left) and healthy control (right). The slope was derived from the regression equation of the linear trendline.
In the third experiment, 2 trials of the BRAIN test were conducted for each hand on the computer keyboard, smartphone, and tablet device.
Statistical Methods
Analyses were performed using GraphPad Prism statistical software (version 7.0; GraphPad Software, San Diego, CA) and IBM SPSS version 24 (SPSS Inc, Chicago, IL). A P value of ≤0.05 was used as cut‐off for determining significance. To reduce the type I error resulting from multiple subgroup analyses, a false discovery rate control for P values was used.19 The BRAIN tap parameters were correlated with total motor MDS‐UPDRS part III score and subscores (rigidity, finger tapping, pronation‐supination, and hand movements) for the on and off states. Pearson's correlation coefficient was used for normally distributed variables and Spearman's rank correlation coefficient for nonparametric correlation. Normality was checked using the D'Agostino Pearson normality test. Receiver operating characteristic curves were used to differentiate the PD patients’ most‐affected side and the controls’ worst performing score on the BRAIN tap test. In addition, Wilcoxon matched pairs signed rank test was used to differentiate between PD patients’ scores on and off medication. A chi‐square test for binary outcome variables was used to compare the epoch analyses for the sequence effect between the patients with PD and controls. The Kruskal‐Wallis test was used to compare the slopes the 3 groups (PD on, PD off, and controls). Fisher's exact test was used to compare decrement in slopes between PD and controls. McNemar's test was used to compare decrement in slopes between PD on and PD off.
In the third experiment, the intraclass correlation coefficient (ICC) for test–retest reliability was calculated. The standard error of measurement, coefficient of variation of the method error, and minimum detectable change were also calculated as agreement parameters. Details of the calculations are shown in the Supplementary File S1 (supplementary material page S3). In addition, the Mann‐Whitney U test was used to compare the distribution of test results between the 2 groups’ performance on the 3 platforms (keyboard, iPad, and iPhone).
Results
The demographic information for the participants is summarized in the Supplementary File S1 (see Tables S1, S2). One PD patient was excluded from MDS‐UPDRS (total and motor subscore) correlation with BRAIN tap scores in the second experiment because of incomplete data. In the third experiment, 1 patient with PD and 1 control were excluded for technical reasons. Sensitivity and specificity cut‐offs for the test parameters are summarized in Supplementary File S1 (Table S3).
Quantifying Bradykinesia Using Slopes
In the discovery phase of the study, the patients with PD differed from the controls (Fisher's exact test P ≤ 0.001) by showing decrements in the slope of the velocity–time graphs (Table 1). Sensitivity and specificity were 58% and 81%, respectively, for a slope cut‐off of −0.002, which is equivalent to a 1% decrement in velocity for every 5 alternate finger taps. Similarly, in the validation phase of the study, the PD patients differed from the controls (P = 0.004), with a sensitivity and specificity of 65% and 88%, respectively, using the same cutoff. A box‐and‐whiskers plot comparing the slopes in the patients with PD and controls is shown in Fig. 2. The cutoff set at −0.002 represents the 10th percentile cut‐off for slopes in controls and the 50th percentile in PD patients with bradykinesia documented during physical examinations.
Table 1.
Combined 2 × 2 contingency tables showing number of patients with Parkinson's disease (PD) and controls with decrement and no decrement in velocity in experiments 1 and 3
| Decrement in Velocity | No Decrement in Velocity | Total | P Value | |
|---|---|---|---|---|
| Experiment 1, discovery | ||||
| PD, most‐affected side | 35 (58%) | 25 (42%) | 60 | <0.001 |
| Controls, non‐dominant side | 11 (19%) | 47 (81%) | 58 | |
| Experiment 3, validation | ||||
| PD, most‐affected side | 11 (65%) | 6 (35%) | 17 | 0.0038 |
| Controls, non‐dominant side | 2 (12%) | 15 (88%) | 17 |
Figure 2.
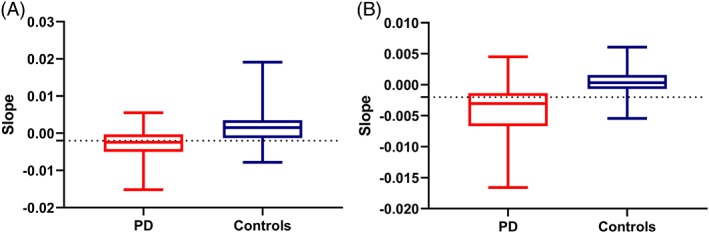
Box‐and‐whisker plot comparing the most‐affected side in patients with Parkinson's disease (PD) in the OFF state with the nondominant side in controls: (A) the first experiment (P < 0.001), (B) the third experiment (P < 0.001).
In addition, the decrement in slopes was useful in differentiating between PD patients off and on medication (McNemar's test P = 0.016; Table 2).
Table 2.
A 2 × 2 contingency table comparing Parkinson's disease (PD) off and on medication for decrement in velocity in experiment 1
| PD On medication | |||
|---|---|---|---|
| PD Off medication | Decrement | No Decrement | Total |
| Decrement | 56 | 34 | 90 |
| No decrement | 16 | 12 | 28 |
| Total | 72 | 46 | 118 |
McNemar's test P = 0.0162.
Time‐Series Analyses of Dwell and Traveling Times for the Sequence Effect in PD
PD patients could not be differentiated from controls on the basis of a sequence effect of dwell and traveling times defined as ≥3 consecutive decrements in time‐series analyses of dwell and traveling times (see Tables S4 in Supplementary File S1).
Differentiation Between PD Patients Off and On Medication
All BRAIN test parameters differentiated PD patients off medication (n = 61) and on medication (see Table 3). When compared with the PD patients’ off state scores, PD patients on medication had higher numbers of alternate taps (55.07 ± 12.46 vs. 49.11 ± 11.34), lower average dwell times (110.4 ± 34.22 vs. 122.5 ± 38.16 msec), and higher average tapping velocity (27.29 ± 6.423 vs. 24.17 ± 5.563 cm/msec).
Table 3.
Mean KS, mean AT, median IS, median DS, mean VS in patients with PD (n = 61) and controls (n = 93) enrolled the second experiment
| Parameter | PD Off | PD On | P Value* | Controls | P Value** |
|---|---|---|---|---|---|
| Mean (95% CI) KS, taps | 49.11 (46.5–51.6) | 55.07 (52.1–57.9) | <0.001 | 60.3 (57.6–63.0) | <0.001 |
| Mean (95% CI) AT, msec | 122.5 (114.1–131) | 110.4 (102.9–118) | <0.001 | 112.1 (101.8–122.3) | 0.0008 |
| Median (IQR) IS, msec2 | 67494 (11275–340854) | 167786 (33318–609816) | 0.0397 | 6758 (4030–16664) | <0.001 |
| Median (IQR) DS, points | 1.044 (1.018–1.136) | 1.097 (1.026–1.205) | 0.0003 | 1.044 (1.013–1.110) | 0.7636 |
| Mean (95% CI) VS, cm/sec | 24.25 (22.99–25.5) | 27.29 (25.77–28.81) | <0.001 | 30.04 (28.31–31.76) | <0.001 |
*P values for the differentiation between on and off medication; **P values for the differentiation between off state and controls.
CI, confidence interval; IQR, interquartile range; PD, Parkinson's disease; KS, kinesia score; AT, akinesia time; IS, incoordination score; DS, dysmetria score; VS, velocity score.
Differentiation Between PD and Controls
In the first experiment, KS, AT, and IS scores differentiated between PD patients who were off medication (n = 61) and healthy controls (n = 93). The same was observed with VS (n = 61) when scores in the off state were compared with controls (n = 40; see Fig. 3). DS did not differentiate between the 2 groups (area under the curve = 0.52, P = 0.7636). IS offered the best discrimination between the 2 groups with sensitivities of 67%, 65%, and 57% for specificities at 80%, 85%, and 90%, respectively. KS and VS were comparable offering sensitivities of 63%, 59%, and 26% and 60%, 48%, and 25% for specificities at 80%, 85%, and 90%.
Figure 3.
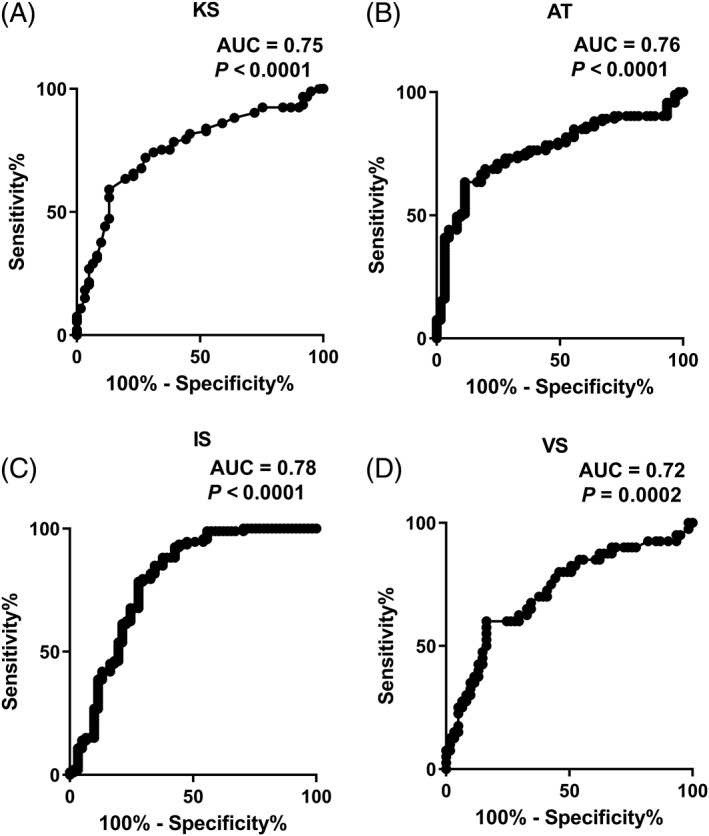
Comparison of KS, AT, IS, and VS (A–D, respectively) between patients with Parkinson's disease (PD) off medication (n = 61) and controls in the first and second experiments (n = 93 for KS, AT, IS and n = 40 for VS) using receiver operating characteristic curves (worse‐affected side in PD was compared to the lowest score of the 2 hands in controls). KS, kinesia score; AT, akinesia time; IS, incoordination score; VS, velocity score; AUC, area under the curve.
Similarly, in the third experiment, the BRAIN test parameters differentiated between PD (n = 19) and controls (n = 19) consistently across the 3 platforms—keyboard, iPad, and iPhone (except for AT parameter on the iPad, P = 0.088; see Table 4). Area under the curve values for receiver operating characteristic curves are shown in Supplementary File S1 (Table S6).
Table 4.
Mean KS, median AT, IS, DS, DS1, and VS in patients with PD (n = 19) and controls (n = 19) enrolled in the third experiment
| Mean (95% CI) KS, taps | Median (IQR) AT, msec | Median (IQR) IS, msec2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Platform | PD | Controls | P Value | PD | Controls | P Value | PD | Controls | P Value |
| Standard keyboard | 51.47 (49.34–53.6) | 64.99 (62.53–67.45) | <0.001 | 107.5 (76.25–138.8) | 81 (62–106) | 0.0002 | 6585 (2870–20744) | 2366 (1328–4790) | <0.001 |
| iPad | 103.1 (96.82–109.5) | 120 (115.2–124.8) | <0.001 | 78.31 (65.7–91.24) | 73.47 (68.23–80.94) | 0.0879 | 1401 (746.7–2422) | 690.7 (483.5–1425) | <0.001 |
| iPhone | 100.6 (94.23–107.1) | 117.1 (112–122.2) | <0.001 | 88.14 (77.49–103.2) | 76.8 (68.75–88.11) | 0.0002 | 1304 (869.1–2863) | 1139 (494.3–2501) | 0.0371 |
| Median (IQR) DS, points | Median (IQR) DS1, points | Mean (95% CI) VS, keys/sec | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Platform | PD | Controls | P Value | PD | Controls | P Value | PD | Controls | P Value |
| Standard keyboard | 1.043 (1.003–1.124) | 1.021 (1–1.046) | 0.0023 | – | – | – | 16.88 (16–17.76) | 21.18 (20.41–21.95) | <0.001 |
| iPad | 1 (1–1.045) | 1 (1–1.008) | 0.0031 | 0.1241 (0.0763–0.1812) | 0.0843 (0.0652–0.1066) | <0.001 | – | – | – |
| iPhone | 1.027 (1.009–1.129) | 1 (1–1.028) | <0.001 | 0.1450 (0.122–0.1952) | 0.1007 (0.0834–0.1404) | <0.001 | – | – | – |
CI, confidence interval; IQR, interquartile range; PD, Parkinson's disease; KS, kinesia score; AT, akinesia time; IS, incoordination score; DS, dysmetria score; DS1, average accuracy during the test; VS, velocity score.
Correlation With Total Motor UPDRS Scores and Subscores
KS and VS showed moderate inverse correlations with the total motor scores of the MDS‐UPDRS and subscores (pronation/supination, finger tapping, hand movements, and upper limb rigidity) in both the on and off states, but the other parameters lacked evidence of an association (see Fig. 4; Tables S7 and S8). Of the 2 parameters, VS showed a marginally stronger correlation than KS.
Figure 4.
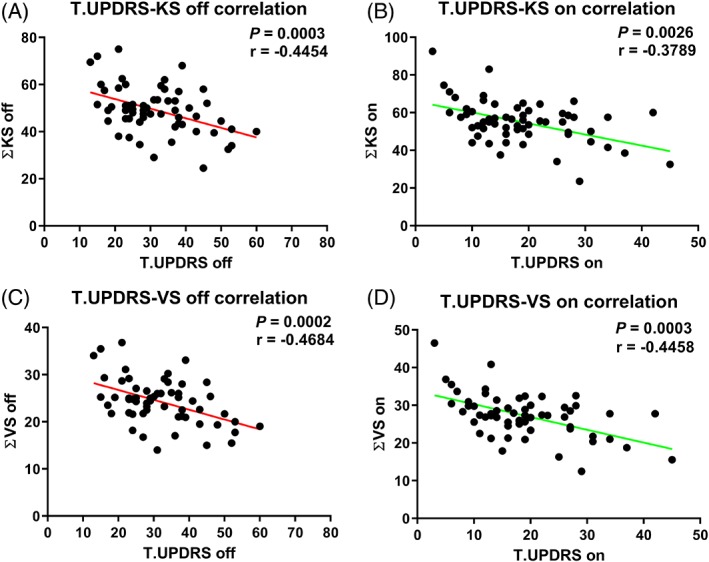
Correlation of ∑KS and ∑VS with total Movement Disorders Society–Unified Parkinson's Disease Rating Scale motor scores in patients (n = 60) off medication (A and C, respectively) and on medication (B and D, respectively). ∑KS, average KS scores for both hands off medication; ∑VS, average VS scores for both hands off medication; T.UPDRS, total motor Unified Parkinson's Disease Rating Scale scores.
Reliability and Agreement
The ICC values are summarized in Supplementary File S1 (Table S9). Using the keyboard version, all BRAIN tap parameters except for IS (poor reliability ICC = 0.141, P = 0.138) achieved good reliability (KS ICC = 0.881, DS = 0.808, VS = 0.883; P < 0.001) and excellent reliability (AT ICC = 0.929; P < 0.001). With the tablet device and smartphone, only KS (ICC = 0.836, P < 0.001) and AT (ICC = 0.760, P < 0.001) achieved good reliability. In addition, measures of agreement (standard error of measurement, minimum detectable change, coefficient of variation of the method error) for the 2 trials are also summarized in Table S9.
Discussion
Here, we report data using the BRAIN test, which address outstanding questions from earlier assessments.13, 14 We demonstrate a new measure for bradykinesia (sequence effect) using the VS that captures a decrement in repetitive movement as opposed to the previous measures, which looked at speed of alternate tapping (KS) and dwell time (AT). The new VS parameter correlated the best of all 5 parameters with established parkinsonian signs and performed similarly to the KS and IS when differentiating patients from controls.
A key finding from this set of experiments was the ability for all BRAIN test parameters to differentiate between a patient on and off dopaminergic medication. This raises the possibility of using the BRAIN test to monitor motor fluctuations and to assist with therapeutic decision‐making. Currently, decisions made clinically regarding the efficacy of treatment depend on clinical examination, records of timing of medication, and patient's subjective reporting of symptoms and ability to perform activities of daily living on self‐scoring diaries.20 The identification of symptoms through history taking is affected by recall bias together with the difficulty experienced by many patients in differentiating between normal, dyskinetic, and bradykinetic states.21
At the chosen cut‐off slope of −0.002, the false positive rate was minimized to ~10% and the detection rate was ~60%. The reason for the suboptimal detection rate in those with established PD may be a result of the nature of alternate tapping and the fact that only proximal sequence effect can be detected in this setting (ie, that which arises from movement at the shoulder/elbow). Adaptation of the test to better capture a distal sequence effect may be beneficial in a future iteration.
The BRAIN tap test parameters correlated only approximately with MDS‐UPDRS part III scores. This could be because BRAIN tap test parameters such as KS (proxy measure for total taps), AT (dwell time), and DS (proxy measure for accuracy) capture aspects of bradykinesia not tested with MDS‐UPDRS subscores (finger tapping, hand movements, pronation/supination), which focus on rhythm, slowing, and decrement in amplitude.2, 22 IS provided the best differentiation between PD patients and controls. However, this was nonspecific as only KS and VS correlated with recognized parkinsonian signs.
We have also introduced a version of the tapping task to smart device platforms such as the iPad (tablet) and iPhone (smartphone). With the exception of AT, the BRAIN tap parameters offered better differentiation between PD patients and controls using standard keyboard when compared to smart devices (see Table S6 in Supplementary File S1). Considering the increasing availability of these technologies, this a further step toward portable domiciliary and clinic‐based testing. The BRAIN test requires no specialized hardware to be purchased and can be accessed online free of charge, requiring 20 seconds for a practice session and 1 minute to perform the test (30 seconds for each hand).
The BRAIN tap test can be used for discriminative as well as evaluative purposes. The high ICC scores reflect the ability of the test to differentiate between respondents by replicating the same ordering on the 2 testing occasions. In addition, measures of agreement summarized in Table S9 can be used as benchmark values for evaluative purposes to differentiate between real change and error in measurement. This is useful to showcase progression when patients are assessed repeatedly.
This study has several limitations. Data mining/exploratory testing has a higher chance of obtaining false positive results when compared with hypothesis‐driven testing. However, this was corrected by using a false discovery rate control for conducting statistical tests.19 PD is a multisystem disease, and motor impairment affecting the lower limb, dyskinesia, rigidity, and tremor are not captured by the test. The BRAIN tap test requires hand–eye coordination, and problems may arise in patients with visual problems or severe tremor. Disparity in the results between iPhone and iPad testing is attributable to the difference in size and the distance between the targets across the 2 devices. Although ICC is dependent on sample heterogeneity that affects variance among the study participants, we have supplemented it with measures of agreement that include the measurement error.
The BRAIN tap test is a simple, sensitive, reliable test of upper limb motor function in PD. It is free to use and has been validated against the accepted gold standard MDS‐UPDRS part III rating scale. It can differentiate between on and off states in individual patients and can quantify the sequence effect using decrement in the VS score, making it a useful adjunctive outcome measure for clinical practice and in clinical trials.
Author Roles
1. Research project: A. Conception, B. Organization, C. Execution
2. Statistical analysis: A. Design, B. Execution, C. Review and Critique
3. Manuscript preparation: A. Writing of the first draft, B. Review and Critique
H.H.: 1C, 2A, 2B, 3A
M.B.: 1C, 2C, 3B
D.S.A.: 1C, 2C, 3B
B.H.: 2C, 3B
B.J.: 2C, 3B
T.W.: 2C, 3B
T.F.: 1A, 1B, 2A, 2C, 3B
G.G.: 2C, 3B
A.J.L.: 2C, 3B
A.J.N.: 1A, 1B, 2A, 2C, 3B
Disclosures
Ethical Compliance Statement
The studies were approved by the Brent National Health Service (NHS) Research Ethics Committee, London (13/LO/1536) and the Queen Square Ethics Committee, London (09/HO716/48). Informed consent was obtained from all participants. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The work presented here had no specific funding, but the Preventive Neurology Unit is funded by the Barts Charity and the Exenatide PD study was funded by the Michael J. Fox Foundation and the Cure Parkinson's Trust. A.J.N. was funded by Parkinson's UK at the time the data were collected and M.B. by the Reta Lila Weston Trust. B.H. and B.J. are directors of uMotif Limited. H.H., M.B., D.S.A., T.W., T.F., G.G., A.J.L., and A.J.N. report no relevant disclosures or conflicts of interest.
Financial Disclosures for the past 12 months
H.H., M.B., B.H., B.J., and D.A. received no specific funding for the past 12 months. A.J.N. holds research grants from Parkinson's UK and Barts Charity. He has also received honoraria from Britannia Pharmaceuticals, Profile Pharma, and GKC Pharmaceuticals. A.J.L. has received funding from the Reta Lila Weston Institute of Neurological Studies, Institute of Neurology, University College London and reports consultancies for Britannia Pharmaceuticals and BIAL Portela. He also reports grants and/or research support from the Frances and Renee Hock Fund, and honoraria from Britannia Pharmaceuticals, Profile Pharma, UCB, Roche, Bial, Nordiclnfu Care, NeuroDerm, Decision Resources, and Windrose Consulting Group. T.F. received grants from MJFF (Michael J. Fox Foundation for Parkinson's research), CPT (Cure Parkinson's Trust), MRC (Medical Research Council), NIHR‐EME (National Institute of Health Research ‐ Efficacy and Mechanism Evaluation), EU‐FP‐7D and honoraria from Boston Scientific, Bial, Profile Pharma, and Oxford Biomedica. T.W. received grants from the Medical Research Council, Reta Lila Weston Medical Trust, Corticobasal Degeneration Solutions Inc., UCLH BRC (University College London Hospital Biomedical Research Centre), ABN (Association of British Neurologists), and honoraria from Britannia Pharmaceuticals. G.G. has received speaker honoraria and consulting fees from Abbvie, Actelion, Atara Bio, Almirall, Bayer Schering Pharma, Biogen Idec, FivePrime, GlaxoSmithKline, GW Pharma, Merck & Co., Merck KGaA, Pfizer Inc, Protein Discovery Laboratories, Teva Pharmaceutical Industries Ltd, Sanofi‐Genzyme, UCB, Vertex Pharmaceuticals, Ironwood, and Novartis and has received research support unrelated to this study from Biogen Idec, Merck & Co., Novartis, and Ironwood.
Supporting information
Figure S1. Supplementary materials, references, and Tables S1‐S9 and Figures S1‐S2.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Post B, Merkus MP, de Bie RMA, et al. Unified Parkinson's Disease Rating Scale Motor Examination: are ratings of nurses, residents in neurology, and movement disorders specialists interchangeable? Mov Disord 2005;20:1577–1584. 10.1002/mds.20640 [DOI] [PubMed] [Google Scholar]
- 2. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 3. Hasan H, Athauda DS, Foltynie T, et al. Technologies assessing limb bradykinesia in Parkinson's disease. J Parkinsons Dis 2017;7:65–77. 10.3233/JPD-160878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horne M, McGregor S, Lynch P, et al. Objective data in Parkinson's disease therapy management–a retrospective analysis of the Parkinson's Kinetigraph (PKG) Database. Value Heal 2015;18:A685 10.1016/j.jval.2015.09.2534 [DOI] [Google Scholar]
- 5. Heldman DA, Giuffrida JP, Cubo E. Wearable sensors for advanced therapy referral in Parkinson's disease. J Parkinsons Dis 2016;6:631–638. 10.3233/JPD-160830 [DOI] [PubMed] [Google Scholar]
- 6. Sanchez‐Ferro A, Elshehabi M, Godinho C, et al. New methods for the assessment of Parkinson's disease (2005 to 2015): a systematic review. Mov Disord 2016;31:1283–1292. 10.1002/mds.26723 [DOI] [PubMed] [Google Scholar]
- 7. Godinho C, Domingos J, Cunha G, et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson's disease. J Neuroeng Rehabil 2016;13:24 10.1186/s12984-016-0136-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rovini E, Maremmani C, Cavallo F. How wearable sensors can support Parkinson's disease diagnosis and treatment: a systematic review. Front Neurosci 2017;11:555 10.3389/fnins.2017.00555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double‐blind, placebo‐controlled trial. Lancet 2017;390:1664–1675. 10.1016/S0140-6736(17)31585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mera TO , Filipkowski DE, Riley DE, et al. Quantitative analysis of gait and balance response to deep brain stimulation in Parkinson's disease. Gait Posture 2013;38:109–114. 10.1016/j.gaitpost.2012.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor Tavares AL, Jefferis GS, Koop M, et al. Quantitative measurements of alternating finger tapping in Parkinson's disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation. Mov Disord 2005;20:1286–1298. 10.1002/mds.20556 [DOI] [PubMed] [Google Scholar]
- 12. Blumenfeld Z, Koop MM, Prieto TE, et al. Sixty‐hertz stimulation improves bradykinesia and amplifies subthalamic low‐frequency oscillations. Mov Disord 2017;32:80–88. 10.1002/mds.26837 [DOI] [PubMed] [Google Scholar]
- 13. Giovannoni G, van Schalkwyk J, Fritz VU, et al. Bradykinesia akinesia inco‐ordination test (BRAIN TEST): an objective computerised assessment of upper limb motor function. J Neurol Neurosurg Psychiatry 1999;67:624–629. 10.1136/jnnp.67.5.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noyce AJ, Nagy A, Acharya S, et al. Bradykinesia‐Akinesia Incoordination test: validating an online keyboard test of upper limb function. PLoS ONE 2014;9:e96260 10.1371/journal.pone.0096260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noyce AJ, Bestwick JP, Silveira‐Moriyama L, et al. PREDICT‐PD: identifying risk of Parkinson's disease in the community: methods and baseline results. J Neurol Neurosurg Psychiatry 2014;85:31–37. 10.1136/jnnp-2013-305420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lakshminarayana R, Wang D, Burn D, et al. Using a smartphone‐based self‐management platform to support medication adherence and clinical consultation in Parkinson's disease. npj Park Dis 2017;3:32 10.1038/s41531-017-0034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. uMotif Limited . 100 for Parkinson's. http://www.100forparkinsons.com/. Accessed September 6, 2018.
- 19. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995;57:289–300. 10.2307/2346101 [DOI] [Google Scholar]
- 20. Giuffrida JP, Rapp EJ. Ambulatory and remote monitoring of Parkinson's disease motor symptoms In: Lai D, Palaniswami M, Begg R, eds. Healthcare Sensor Networks: Challenges Toward Practical Implementation. Boca Raton, FL: CRC Press; 2011:247–282. [Google Scholar]
- 21. Bergquist F, Horne M. Can objective measurements improve treatment outcomes in Parkinson's disease? Eur Neurol Rev 2014;9:27–30. 10.17925/ENR.2014.09.01.27 [DOI] [Google Scholar]
- 22. Wissel BD, Mitsi G, Dwivedi AK, et al. Tablet‐based application for objective measurement of motor fluctuations in Parkinson disease. Digit Biomark 2018;1:126–135. 10.1159/000485468 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Supplementary materials, references, and Tables S1‐S9 and Figures S1‐S2.


