Abstract
Introduction
The common predominant clinical features of cholangiopathies such as primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), and biliary atresia (BA) are biliary damage/senescence and liver fibrosis. Curative therapies are lacking, and liver transplantation is only option. An understanding of the mechanisms and pathogenesis is needed to develop novel therapies. Previous studies have developed various disease-based research models and have identified candidate therapeutic targets.
Areas covered
This review summarizes recent studies performed in preclinical models of cholangiopathies and the current understanding of the pathophysiology representing potential targets for novel therapies. A literature search was conducted in PubMed using the combination of the searched term “cholangiopathies” with one or two keywords including “model”, “cholangiocyte”, “animal”, or “fibrosis”. Papers published within five years were obtained.
Expert opinion
Access to appropriate research models is a key challenge in cholangiopathy research; establishing more appropriate models for PBC is an important goal. Several preclinical studies have demonstrated promising results and have led to novel therapeutic approaches, especially for PSC. Further studies on the pathophysiology of PBC and BA are necessary to identify candidate targets. Innovative therapeutic approaches such as stem cell transplantation have been introduced, and those therapies could be applied to PSC, PBC, and BA.
Keywords: cholangiopathy, bile duct, cholangiocyte, inflammation, liver fibrosis
1. Introduction
Cholangiopathies are bile duct disorders that include primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), and biliary atresia (BA). The key clinical features of PSC are chronic biliary inflammation, liver fibrosis, and destruction of intrahepatic/extrahepatic bile ducts [1, 2]. Approximately 70% of PSC patients have concurrent inflammatory bowel disease (IBD) with higher risks of developing cholangiocarcinoma and colorectal cancer [3, 4, 5]. PBC, formerly known as primary biliary cirrhosis, is an autoimmune disorder that is characterized by intrahepatic bile duct destruction, cholestasis, and lymphocyte infiltration. The immune responses triggered in PBC specifically target cholangiocytes [6, 7, 8]. BA is characterized by neonatal cholestasis and jaundice resulting from biliary damage, inflammation or insufficient bile duct development [9, 10, 11]. To date, cholangiopathies typically require liver transplantation for treatment, and hence effective and less invasive therapies are needed.
Establishing research models for the development of novel therapies is critical to understand the pathogenesis of these diseases. There are several preclinical models available for cholangiopathies, and studies using these models have identified signaling pathways and target genes that are associated with the disease development and progression. Drugs that can target these pathways could be a promising therapeutic strategy for the management of cholangiopathies. This review summarizes preclinical research in cholestatic liver disease models and potential candidate therapeutic targets. A literature search was performed using PubMed. The keywords used were “cholangiopathies” or the name of specific cholangiopathies such as “primary sclerosing cholangitis” with one or two other keywords such as “model”, “cholangiocyte”, “bile duct”, “animal”, “fibrosis”, “ductular reaction”, or “proliferation” (e.g., “biliary atresia animal model”). Work published during 2013–2018 were obtained for reading and citation. Highly cited or important literatures were also considered, regardless of published dates.
2. Research models of cholangiopathies
2.1. PSC models
A common strategy for the development of preclinical animal models for cholangiopathies is to mimic genetic and environmental risk factors that generate animals with similar characteristics of human diseases [12, 13, 14]. Patients with PSC typically exhibit bile duct hyperplasia or ductular reaction, cholestasis, and cirrhosis leading to jaundice, hepatocellular injury, and elevated serum bile acid levels [1, 2]. Animal models for PSC have been evaluated based on these characteristics: (i) increased ductular reaction detected by immunohistochemistry for cytokeratin (CK) 7 or CK19 (only expressed by cholangiocytes in the liver); (ii) serum chemistry profiles based upon elevated levels of alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), or bilirubin as a marker of hepatic damage; (iii) hepatic damage and inflammation; (iv) elevated levels of bile acids in serum or plasma as a sign of cholestasis; (v) elevated serum levels of proinflammatory cytokines such as interleukin-6 (IL-6) as a sign of inflammation; or (vi) increased hepatic fibrosis. Table 1 lists common PSC models and expression of these parameters.
Table 1.
Selected PSC models.
| Research model | Background/source | Inflammation (ALP/ALT/AST) | Ductular reaction | Liver fibrosis | References |
|---|---|---|---|---|---|
| BDL | Various | Yes | Yes | Yes | [15, 16] |
| DDC | Various | Yes | Yes | Yes | [18] |
| TAA | Various | Yes | Yes | Yes | [23] |
| CCl4 | Various | Yes | Yes | Yes | [24, 25, 26] |
| Mdr2−/− mice | FVB/NJ mice | Yes | Yes | Yes | [28, 29] |
| Cftr−/− mice | C57BL/6J mice | Yes | Yes | Yes | [35, 36] |
| CDH1ΔL mice | C57BL/6J mice | Yes | Unknown | Yes | [38] |
| Vil2kd/kd mice | Unknown | Yes | Yes | Yes | [48] |
| Primary PSC cholangiocytes | Humans | N/A | N/A | N/A | [51, 52] |
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; BDL=bile duct ligation; CCl4 = carbon tetrachloride; DDC=3,5-deithoxycarbonyl-1,4-dihydrocollidine; TAA=thioacetamide; PSC = primary sclerosing cholangitis.
2.1.1. Bile duct ligation model
Bile duct ligation (BDL) is surgical obstruction of the common bile duct and widely utilized as a model of cholestatic liver injury in rodents [15, 16]. The BDL model is one of the most common animal models of cholangiopathies, as well as biliary fibrosis. Rodent models of BDL mimic some typical liver abnormalities of PSC such as ductular reaction, liver fibrosis and inflammation. BDL allows for the study of characteristics and functional roles of cells or signaling pathways that are associated with the pathogenesis of liver diseases during cholestasis. For example, expression levels of secretin receptor, which is associated with cholangiocyte proliferation and fibrogenesis (see below), are elevated in cholangiocytes following BDL [17]. Although BDL can be performed in rats and mice regardless of their strains and backgrounds representing its flexibility, it requires major surgery and can be technically challenging. The degree of cholestasis and liver damage may vary significantly depending on surgical procedures and individual animal response to ligation. In addition, BDL mimics cholestasis in rodents, but does not mimic the disease state which induces cholestasis in humans. Therefore, researchers have employed less invasive and technically challenging transgenic mouse models in recent studies.
2.1.2. Chemically induced models
Administration of certain chemicals can damage bile ducts and mimic conditions of cholestatic liver injury in rodents. For example, 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) damages bile ducts resulting in cholestasis, liver damage and fibrosis [18]. The DDC model is widely utilized for studies of functional roles of cholangiocytes or other liver cells during liver damage; a previous study has demonstrated that cholangiocytes act as liver stem cells during liver damage induced by DDC with impaired hepatocyte regeneration [19, 20].
Thioacetamide (TAA) is a carcinogen that induces liver fibrosis followed by cholangiocarcinoma in rodents suggesting that TAA is another model leading to the damage of bile ducts in vivo [21, 22]. A previous study using this model has demonstrated that ADP55, an adiponectin-based active short peptide, reduces TAA-induced liver damage and fibrosis in mice [23]. The TAA model can be used as a model for biliary injury and cholangiocarcinoma.
Carbon tetrachloride (CCl4) induces liver damage and fibrosis, both in rats and mice [24, 25]. A previous study using this model has studied the functional role of hepatitis C virus protein using short and long term treatment of CCl4-induced liver damage [26]. These chemically induced models are technically more feasible and provide thesame flexibility as the BDL model. However, administration of these chemicals damages not only cholangiocytes, but also other liver cells such as hepatocytes, indicating that liver conditions induced by these chemicals may not mimic the pathogenesis of human cholestatic liver diseases such as PSC. In addition, the effects of chemicals can vary significantly from case to case depending on the dose and duration of administration. Acute treatment with a high dose may produce different results from chronic treatment with a lower dose, and there are no gold-standard administration procedures for each chemical; hence procedures and their effects in animals may vary depending on studies.
2.1.3. Genetic models
Multi-drug resistance 2 (Mdr2−/−) mice are the most common transgenic animal model utilized as a preclinical model of PSC. Mdr2−/− mice lack MDR2 protein which functions in phospholipid transport from hepatocytes into the bile ducts resulting in accumulation of toxic bile in the liver [27]. Mdr2−/− mice have liver conditions resembling human PSC, such as increased bile duct mass and liver fibrosis [28, 29]. Mdr2−/− mice are widely used to study the pathophysiology of cholestatic liver injury and functional roles of signaling pathways that may lead to the development of novel therapeutic approaches [30, 31].
Cystic fibrosis transmembrane conductance regulator (CFTR) is a chloride transporter located on the apical membrane of cholangiocytes regulating bile secretion. CFTR plays a crucial role in biliary inflammation [32], and functional abnormalities in CFTR are associated with PSC in humans [33, 34]. Cftr−/− mice suffer biliary damage and liver fibrosis similar to Mdr2−/− mice, representing another possibility as a PSC mouse model [35, 36]. Although these knockout mice are powerful research models, they do not exhibit complications or concurrent IBD development similar to human PSC patients. In addition, Mdr2−/− mice can develop hepatocellular carcinoma (HCC), but rarely develop cholangiocarcinoma which is common in patients with PSC [37].
A previous study has generated liver specific E-cadherin knockout mice (CDH1ΔL) and has demonstrated that these mice develop portal inflammation and liver fibrosis resembling human PSC [38]. This study further demonstrated that the loss of E-cadherin in cholangiocytes, but not in hepatocytes, leads to portal inflammation and fibrosis using CK19-Cre-mediated cholangiocyte specific E-cadherin knockout mice. Some previous studies suggest that quiescent cholangiocytes and hepatocytes become reactive and transdifferentiate into fibrogenic myofibroblasts during disease via epithelial-mesenchymal transition (EMT) [39, 40, 41, 42]. The loss of E-cadherin is the hallmark sign of EMT [43, 44]. Therefore, E-cadherin knockout mice may produce more fibrogenic myofibroblasts that are derived from hepatocytes or cholangiocytes in CDH1ΔL mice. However, the role of EMT in liver fibrosis is still unclear and controversial [45, 46, 47]. The detailed mechanisms of inflammatory and fibrogenic conditions of this CDH1ΔL mouse liver are unknown, and hence more studies will be required to use these mice as a PSC model.
Ezrin is a membrane cytoskeletal crosslinker protein that interacts with transporters. Hatano et al. have demonstrated that ezrin knockdown (Vil2kd/kd) mice have decreased expression levels of CFTR and Cl−/HCO3− anion exchanger (AE2) on the apical membrane of cholangiocytes leading to poor bile flow and cholestasis in vivo [48]. These Vil2kd/kd mice had enhanced ductular reaction, liver inflammation, and fibrosis representing characteristics as another model of cholestatic liver injury [48]. Unfortunately, these mice are hard to maintain because of high mortality and only approximately 7% of Vil2kd/kd mice can survive to adulthood [49].
2.1.4. In vitro models
In vitro cell culture models may be a useful counterpart to in vivo animal models for PSC research. Cellular senescence in cholangiocytes is characteristic in PSC patients, and senescent cholangiocytes secrete elevated levels of senescence-associated secretory phenotype (SASP) markers such as IL-1β, IL-6 and IL-8 compared to normal cholangiocytes [50]. Therefore, targeting senescent cholangiocytes may be a strategy to develop novel therapies [31]. Previous studies have established the in vitro PSC model by culturing cholangiocytes isolated from PSC patient’s liver and found that these PSC-derived cholangiocytes are senescent [51, 52]. This in vitro model may produce better understandings of the pathophysiology of senescent cholangiocytes in cholangiopathies as well as drug validation for targeted pathways. There is a limitation in this model; however, since senescent cholangiocytes have a low proliferation rate, making them difficult to obtain and maintain a steady population of cells [51].
Previous studies have transdifferentiated human induced pluripotent stem (iPS) cells into cholangiocyte-like cells, representing another insight for in vitro models [53, 54]. Although this iPS-derived cholangiocyte cell line may allow the testing of drugs or candidate compounds for the treatment of PSC, it is still unclear whether iPS-derived cholangiocyte-like cells are physiologically and functionally identical to human cholangiocytes in vivo. Further studies are required to establish the use of iPS-derived cholangiocyte models in preclinical studies.
2.2. PBC models
PBC is a chronic autoimmune disorder characterized by female dominance, high titer autoantibodies, and bile duct inflammation and destruction leading to cholestasis [55]. PBC models are evaluated primarily on the following parameters: (i) detection of anti-nuclear antibodies (ANA) or anti-mitochondrial antibodies (AMA) as a sign of autoimmune disorder; (ii) abnormal lymphocyte infiltration in portal area as a sign of inflammatory responses; or (iii)the presence of biliary cyst as a sign of cholestasis evaluated histologically. Some PBC patients exhibit bile duct hyperplasia or proliferation, although bile duct destruction is observed in other patients [55]. Since PBC is a progressive disorder, bile duct responses may differ depending on the stage of the disease. The levels of bile duct hyperplasia or dilation followed by bile duct destruction according to PBC stages remain undefined. Table 2 lists common PBC animal models and the presence of human disease characteristics.
Table 2.
Selected PBC models.
| Research model | Background | Female dominance | High titer ANA/AMA | Portal infiltration | Bile duct dilation or proliferation | References |
|---|---|---|---|---|---|---|
| NOD.c3c4 mice | NOD mice | Yes | Yes | Yes | Yes | [56, 57, 58] |
| dnTGFβRII mice | C57BL/6J mice | No | Yes | Yes | Yes | [62, 63] |
| Ae2a,b−/− mice | FVB/NJ mice | No | Yes | Yes | Unknown | [67] |
| ARE-Del−/− mouse | C57BL/6J mice | Yes | Yes | Yes | Unknown | [71, 72] |
| 2-OA immunization | Various | No | Yes | Yes | Unknown | [74] |
| Poly I:C injection | C57BL/6J mice | Unknown | Yes | Yes | Unknown | [78, 79] |
2-OA = 2-octynoic acid; AMA = anti-mitochondrial antibody; ANA = anti-nuclear antibody; Poly I:C = polyinosinic:polycytidylic acid
2.2.1. Genetic models
The use of non-obese diabetic (NOD) mice is a well-established approach for studies on type I diabetes. The NOD.c3c4 mouse model was generated from NOD background with insulin-dependent diabetes-resistant alleles from B6 and B10 mice replacing NOD alleles on chromosome 3 and 4 [56]. NOD.c3c4 mice do not have obesity regardless of their background but do possess PBC-like features such as high titer ANA, biliary cysts, and lymphocyte infiltration into portal areas [56, 57, 58]. Recent studies utilized NOD.c3c4 mice as a PBC model to study the functional role of gut bacteria, natural killer T cells, and a mutation in polycystic kidney and hepatic disease 1 (Pkhd1) [59, 60, 61]. Although these mice have liver conditions resembling human PBC, the association of diabetes with PBC and its pathophysiology is undefined, and hence it is unclear whether this mouse model is suitable to study human PBC.
Mice that express a dominant-negative form of transforming growth factor (TGF)-β receptor type II in CD4+ T cells (dnTGFβRII mice) are another genetic animal model for PBC [62]. These mice exhibit bile duct destruction as well as high serum levels of autoimmune antibodies and proinflammatory cytokines such as IL-6 and tumor necrosis factor alpha (TNFα) indicating biliary damage and inflammation [62, 63]. Previous studies have demonstrated critical roles of gut bacteria and CD8+ T cells in autoimmune cholangitis in this model [64, 65]. However, the limitation of this animal model is that disease conditions are not female dominant, which does not mimic human PBC [62, 63].
The anion exchanger AE2 is located on the apical membrane of cholangiocytes in the liver, andit has been reported that PBC patients have decreased expression of AE2, indicating the association of the loss of AE2 with the pathophysiology of PBC [66]. Ae2a,b−/− mice lack three AE2 isoforms (Ae2a, Ae2b1, and Ae2b2) and have PBC-like features such as portal lymphocyte infiltration, high levels of AMA, and cholestasis followed by high ALP levels [67]. However, this model is difficult to maintain because male Ae2a,b−/− mice are infertile [68].
Interferon (IFN)-γ is known to be associated with autoimmune diseases, including BA [69, 70]. Hodge et al. have generated transgenic mice with the deletion of AU-rich element (ARE) in the 3’-untranslated region (UTR) of the IFN-γ gene leading to high serum levels of IFN-γ [71]. This ARE-Del−/− mouse model has autoimmunity with high ANA levels as well as portal infiltration, liver fibrosis, and female dominance [72]. A recent study using this model has demonstrated that type I IFN signaling plays a key role for the pathophysiology of autoimmunity indicating the potential usefulness of this model [73]. This model may offer a better understanding in the mechanisms and the cause of disease conditions in mice compared to NOD.c3c4 mice, and this model also has female dominance that dnTGFβRII mice and Ae2a,b−/− mice do not.
2.2.2. Chemically induced models
As PBC is an autoimmune disorder, Immunization has been conducted in mice to disrupt immune tolerance against autoantigen leading to autoimmunity. In a previous study, mice were immunized using a chemical xenobiotic, 2-octynoic acid (2-OA) [74, 75]. The 2-OA-immunized mice expressed autoantibodies against the mitochondrial pyruvate dehydrogenase E-2 subunit [74]. This model exhibits high levels of AMA, TNFα, and IFN-γ as well as portal lymphocyte infiltration, resembling PBC [74, 75]. Although this model does not have female dominance, 2-OA immunization can be carried out for various mouse strains and transgenic mice, representing the flexibility of this model [76, 77].
Previous studies have demonstrated that injection of the viral mimetic polyinosinic:polycytidylic acid (poly I:C), which is a type I IFN inducer, causes PBC-like liver conditions in mice including elevated lymphocyte infiltration around the portal area and high serum levels of AMA [78, 79]. Although PBC studies using this model are limited and further studies are required, these findings suggest that chemically induced models could be utilized as an alternative model of transgenic animal PBC models.
2.3. BA models
BA is a form of neonatal cholestasis caused by various factors such as viral infection or insufficient bile duct development [9, 10, 11]. The preclinical animal models available for BA research are limited. Previous studies have performed BDL utilizing 3-week-old rats, which resembled some features of BA [80]. Due to its technical difficulties, BDL is not widely performed in young mice, as should be done in the BA model to better mimic human pathology. It is well known that perinatal viral infection is associated with BA development in children [81]. Injection of rhesus rotavirus into newborn mice is the most established animal model for BA research [82, 83]. Although the effects of virus injection differ depending on the strain of rotavirus, the injected virus can be delivered into cholangiocytes, thereby causing biliary damage, high serum levels of proinflammatory cytokines, and portal lymphocyte infiltration in neonatal mice [82, 83]. This model has progressive jaundice, growth failure and high mortality because of obstruction of extrahepatic bile ducts [84]. This technique can be performed in various strains of mice. Recent studies using this model have demonstrated the functional roles of the gut microbiome and bone marrow-derived mesenchymal stem cells in BA [85, 86]. The viral injection model is the sole well-established BA model currently available, and studies using this model are limited.
3. Candidate signaling pathways of novel therapies for cholangiopathies
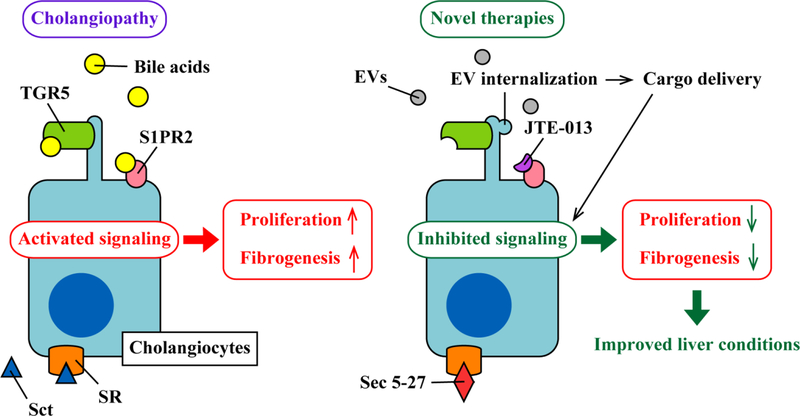
The pathophysiology of cholangiopathies remains largely unknown especially for PBC and BA. However, previous studies for PSC have demonstrated that several signaling pathways play a crucial role in the pathogenesis of PSC resulting in ductular reaction, biliary damage, and liver fibrosis. These candidate pathways may represent novel therapeutic targets for PSC. As described previously, PSC patients generally exhibit ductular reaction, which represents bile duct hyperplasia as well as reactions around bile ducts and the portal area such as lymphocyte infiltration and fibrogenesis. Quiescent cholangiocytes respond to biliary damage and acquire an activated neuroendocrine phenotype [87]. Active cholangiocytes secrete profibrogenic cytokines and factors such as TGF-β1 leading to activation of hepatic stellate cells (HSCs) and portal myofibroblasts that are the primary source of production for extracellular matrix resulting in liver fibrosis [88]. Therefore, it is a strategy for novel therapies to target cholangiocytes regulating excessive and reactive proliferation for the management of disease conditions in cholangiopathies (Figure 1). Although previous studies have identified numbers of signaling pathways in cholangiocytes associated with cholangiocyte proliferation and liver fibrogenesis, this review summarizes recent emerging candidate pathways that could lead to the development of novel therapies for PSC.
Figure 1. The strategy for novel therapies in cholangiopathies.
Cholangiocytes express various membrane receptors such as G protein-coupled bile acid receptor (TGR5), secretin receptor (SR), and sphingosine-1-phosphate receptor 2 (S1PR2). During cholangiopathies, secreted levels of triggering agents including bile acids and secretin (Sct) are increased and they bind to those receptors. This activates specific signaling pathways inducing cholangiocyte proliferation, ductular reaction, and fibrogenesis. Current studies target those signaling pathways to interrupt activation of signaling by administration of drugs binding those receptors. JTE-013 and Sec 5–27 are antagonists for S1PR2 and SR, respectively that bind to these receptors inhibiting downstream signaling. However, functional roles of some receptors such as TGR5 are still undefined during cholangiopathies; therefore, further studies are required to elucidate the potential utilization of agonists/antagonists as novel therapies targeting those receptors. Extracellular vesicles (EVs) can carry mediators such as RNAs and proteins that can be delivered into cholangiocytes. Internalized EVs transfer cargo mediators into recipient cholangiocytes and regulate physiological events leading to improved liver conditions, representing promising potentials of EVs as a tool of novel therapies.
3.1. Secretin and secretin receptor
Secretin (Sct) is a hormone that binds to secretin receptor (SR) thereby regulating cholangiocyte proliferation during cholestatic liver diseases [89, 90]. SR is expressed in the basolateral domain of cholangiocytes in the liver, and the Sct/SR axis plays a critical role in the pathophysiology of cholangiopathies [91]. A recent study using Sct−/−, SR−/−, and Sct−/−SR−/− transgenic mice has demonstrated that these knockout mice have attenuated ductular reaction and liver fibrosis compared to wild-type during BDL-induced liver injury resulting from decreased secretion of TGF-β1 from cholangiocytes [92]. Another study has generated Mdr2−/−SR−/− mice and has demonstrated that these mice have improved liver pathology and reduced fibrosis compared to Mdr2−/− mice [93]. Knockout of SR decreased cellular senescence and secretion of SASP markers from cholangiocytes, such as CCL2 [93]. Administration of an SR antagonist, Sec 5–27, ameliorated ductular reaction and liver fibrosis in Mdr2−/− mice and BDL mice in vivo [94]. In PBC; however, functional roles of Sct may differ from those in PSC. A preliminary study has demonstrated that administration of Sct improves liver conditions by helping cholangiocyte functions in dnTGFβRII mice although effects of Sct may differ between early stage and late stage of PBC and further studies are needed [95]. These studies suggest that inhibition or activation of the Sct/SR axis using inhibitors or agonists could be a novel therapeutic tool for PSC or PBC, respectively.
3.2. Mast cells and histamine signaling
Previous studies have demonstrated that mast cell numbers are increased around the portal area and are associated with liver fibrosis in patients with PSC [96]. Francis and colleagues have demonstrated that mast cell-deficient KitW-sh mice have attenuated liver damage, fibrosis, and ductular reaction following BDL compared to wild-type mice, illustrating an vital role for mast cells in cholangiopathies [97]. Following activation during biliary diseases, mast cells release histamine triggering inflammatory responses. The asthma drug, cromolyn sodium, inhibits histamine release from mast cells. Administration of cromolyn sodium into BDL mice or Mdr2−/− mice decreased histamine secretion and subsequently improved the disease conditions of cholestatic liver damage in vivo [30, 98]. Activation of histamine receptors is associated with cholangiocyte proliferation, and administration of antagonists for histamine receptors decreased liver damage and fibrosis in Mdr2−/− mice [99, 100]. These studies suggest that targeting histamine secretion or histamine receptor signaling could lead to the development of novel therapies for PSC. Although functional roles of mast cells and histamine signaling are undefined in PBC, a previous study has reported increased mast cell infiltration around the portal area in PBC patients, suggesting that similar strategies could be applied to PBC therapies as well as PSC [101].
3.3. Bile acids and bile acid receptors
Bile acids regulate ductular reaction and the response to cholestatic liver injury. Ursodeoxycholic acid (UDCA) inhibits cholangiocyte proliferation and secretion in vivo [102]. UDCA is used as an approved drug for PBC to date, suggesting the potential use for other cholangiopathies including PSC and BA [103]. Hatano et al. have demonstrated that oral administration of UDCA attenuated liver damage and fibrosis in cholestatic Vil2kd/kd mice [104]. A recent study has also reported that UDCA administration inhibits mast cell activation leading to improved liver conditions in Mdr2−/− mice [105]. Although these studies indicate therapeutic potentials of UDCA for PSC and cholestatic liver injury in animal models, trials of UDCA administration for PSC patients provide controversial results. Lindor et al. administered PSC patients with UDCA and reported that UDCA-treated patients did not exhibit improved survival rates compared to untreated patients [106].
TGR5 is a plasma membrane-bound G coupled bile acid receptor located on the primary cilium of cholangiocytes [107]. TGR5 regulates cholangiocyte functions and bile acid signaling, and TGR5 is required for bile acid-dependent cholangiocyte proliferation in vivo, indicating the association of TGR5 with cholangiocyte activation [108, 109]. Hov et al. have sequenced the gene of TGR5 from 276 PSC patients and 274 healthy individuals, and have identified five nonsynonymous mutations [110]. These mutations are associated with the loss of TGR5 functions and PSC. Another study has demonstrated that TGR5−/− mice have exacerbated liver conditions during BDL such as higher ALT and ALP levels and higher concentrations of proinflammatory cytokines including IL-6 and TNFα compared to wild-type mice [111]. Although these findings suggest that TGR5 could be a potential therapeutic target of PSC, it is still unclear whether activation or inhibition of TGR5 leads to inhibition of cholangiocyte proliferation as well as the management of diseased liver conditions during PSC.
Farnesoid X receptor (FXR) is another bile acid receptor highly expressed in the liver and intestine. Similar to TGR5, FXR is associated with bile acid metabolism and secretion. A previous study has demonstrated that PSC patients express decreased levels of FXR in colonic mucosa, indicating the disruption of bile acid metabolism leading to disease conditions in cholangiopathies [112]. Administration of INT-767, which is an agonist for both TGR5 and FXR ameliorated liver inflammation and restored bile flow in Mdr2−/− mice [113]. It has been demonstrated that FXR agonist PX20606 decreases liver fibrosis and expression levels of fibrogenic markers including TGF-β1 and collagen type I using CCl4 model rats [114]. Obeticholic acid is an FXR agonist and may have a potential benefit in patients with cholangiopathies. A trial of obeticholic acid for PBC patients has demonstrated that 12-month administration of obeticholic acid improves serum levels of ALP and bilirubin compared to the placebo group [115]. Another trial has also reported that obeticholic acid administration improves ALP levels and long-term clinical outcomes in PBC patients [116]. A clinical trial of obeticholic acid for PBC patients is currently ongoing (NCT03633227). Obeticholic acid may be beneficial for PSC. In a clinical trial (NCT02177136), administration of obeticholic acid improved serum ALP and bilirubin levels in PSC patients compared to the placebo group. However, Wagner et al. have previously demonstrated controversial results that FXR−/− mice have reduced ductular reaction after BDL while there are no significant differences found in levels of ALT and ALP compared to wild-type mice [117]. Further studies are needed to elucidate whether FXR activation or inhibition will lead to the management of liver conditions in PSC and PBC.
Apical sodium-dependent bile acid transporter (ASBT) is located in the terminal ileum and plays a critical role in the enterohepatic circulation of bile acids. Ileal ASBT determines hepatocellular bile acid reuptake and subsequent biliary bile acid concentrations. A previous study has demonstrated that the ASBT inhibitor, A4250 decreases intestinal bile acid absorption leading to decreased serum bile acid concentrations, indicating the potential therapeutic effects of ASBT inhibition in cholestatic liver diseases [118]. Baghdasaryan et al. have demonstrated that diet supplementation of A4250 decreases ductular reaction, liver damage, and fibrosis in Mdr2−/− mice [119]. A pilot study administered nine PBC patients with A4250 and reported that all nine patients had improved conditions of pruritus in PBC [120]. Although there are only limited studies available to date, these findings suggest that inhibition of ASBT could lead to the development of treatments for both PSC and PBC.
Sphingosine-1-phosphate receptor 2 (S1PR2) is a G protein-coupled receptor that can be activated by bile acids. S1PR2 is expressed in the liver and intestine and is associated with lipid and sterol metabolism in the liver [121]. Wang et al. have demonstrated that cholangiocytes express S1PR2, and its expression levels are elevated during BDL in mice [122]. Knockout of S1PR2 or administration of S1PR2 antagonist JTE-013 decreased ALT and ALP levels, ductular reaction, and liver fibrosis during BDL-induced chronic liver injury, indicating that S1PR2 is another promising therapeutic target for PSC treatments [122]. Together, these studies suggest that administration of agonists/antagonists for bile acid receptors or supplementation of specific bile acids could be utilized to regulate cholangiocyte proliferation and functions leading to the management of liver conditions.
3.4. Melatonin
Melatonin is a hormone secreted from the pineal gland, intestine, and liver. Melatonin has been known to regulate cholangiocyte proliferation [123]. Administration of melatonin decreased BDL-induced liver damage and serum levels of ALT and AST in rats [124, 125]. Renzi et al. have demonstrated that cholangiocytes express melatonin receptors MT1 and MT2, and melatonin administration attenuates ductular reaction and liver fibrosis in BDL rats via activation of MT1 receptor but not MT2 receptor [126]. The expression levels of genes associated with circadian rhythms, such as CLOCK, PER1, CRY1, and BMAL1, were significantly elevated during BDL and were decreased by melatonin administration, suggesting that the circadian rhythm and expression of clock genes may be associated with the pathogenesis of PSC [126]. Patients with PBC often exhibit disturbed circadian rhythms, and this could be related to the pathogenesis of PBC. A pilot study has demonstrated that morning bright light treatment for PBC patients is effective to improve sleep quality and to maintain circadian rhythms although it is still undefined whether bright light treatment improves liver conditions of PBC [127]. Another study has demonstrated that prolonged exposure of BDL rats to darkness induces melatonin production in vivo leading to decreased ductular reaction and liver fibrosis compared to BDL rats in normal housing conditions [128]. Melatonin administration or prolonged darkness improved liver conditions in Mdr2−/− mice [28]. These findings suggest that melatonin supplementation or dark therapy helping melatonin production could represent a novel therapy for cholangiopathies. Further studies are required to elucidate the functional roles of circadian rhythms as well as therapeutic effects of bright light therapy to maintain the circadian rhythm in patients with cholangiopathies.
3.5. Neurokinin-1 receptor
Cholangiocytes express neurokinin-1 receptor (NK-1R) that is a G-protein coupled receptor associated with immune response. Expression levels of NK-1R in cholangiocytes were elevated during BDL-induced liver injury, and NK-1R−/− mice had improved ductular reaction, liver damage and fibrogenesis following BDL compared to wild-type mice [129]. Wan et al. have demonstrated that PSC patients have elevated NK-1R expression in the liver compared to healthy individuals, and administration of NK-1R antagonist L-733,060 attenuates liver fibrosis, cholangiocyte senescence, and SASP marker expression in cholangiocytes from Mdr2−/− mice [130]. Administration of drugs that disrupt NK-1R signaling may be useful for the management of diseased conditions in PSC.
4. Stem cell therapy
Transplantation of stem cells has been utilized to improve conditions in various diseases including liver cirrhosis [131]. Wang et al. have demonstrated that transplantation of bone marrow-derived mesenchymal stem cells significantly decreases serum levels of ALP and AMA, as well as lymphocyte infiltration around portal area compared to control using poly I:C-induced PBC model mice [132]. Lei et al. have utilized rhesus rotavirus BA models and demonstrated that intraperitoneal injection of bone marrow-derived mesenchymal stem cells decreases serum levels of AST and ALT, liver fibrosis, and expression of fibrogenic markers such as TGF-β1 and collagen type I [86].
Cholangiocytes are heterogeneous with small and large cholangiocytes displaying different cell size and functions [133]. Small cholangiocytes have a larger nucleus to cytosol ratio compared to large cholangiocytes, and previous studies have demonstrated that during CCl4-induced large cholangiocyte damage, small cholangiocytes de novo proliferate and differentiate into large to compensate the damaged population of large cholangiocytes indicating the possible stem cell-like features of small cholangiocytes [134, 135]. A previous study has demonstrated that transplantation of small cholangiocytes decreases ductular reaction and liver fibrosis in BDL mice, but large cholangiocyte transplantation exhibits no effects [136]. Although further studies are needed to elucidate whether small cholangiocytes are liver stem cells or have stem cell-like abilities to differentiate into large cholangiocytes or other liver cells, these previous studies suggest that stem cell transplantation could be a therapeutic tool for cholangiopathies.
5. Extracellular vesicle therapy
Extracellular vesicles (EVs), membrane-bound vesicles secreted from various types of cells, contain cargo mediators such as DNAs, RNAs, and proteins. EVs play an important role in cell to cell communications as they can be transferred from one cell to another regulating physiological cell events in recipient cells, indicating the potential use as a drug or mediator carrier to manage disease conditions in liver diseases [137, 138]. A previous study has demonstrated that isolated EVs from rat bile can interact with cholangiocyte cilia and decrease cholangiocyte proliferation in vitro [139]. Another study has isolated EVs from culture media of cholangiocytes treated with lipopolysaccharide (LPS) stimulation and has demonstrated that these LPS-derived EVs drive inflammatory reactions in other cholangiocytes [140]. Chaiyadet et al. isolated EVs from excretory/secretory products of liver fluke Opisthorchis veverrini obtained from experimentally infected hamsters, and those EVs drove cell proliferation and IL-6 expression in human normal cholangiocyte line H69 cells [141]. These studies indicate that secretory EVs can regulate cholangiocyte proliferation and functions. Decreased levels of microRNA (miRNA) let-7 are associated with cholangiocyte proliferation during cholangiopathies [91, 142]. A recent study has demonstrated that injection of liver stem cell-derived EVs, which carry higher levels of let-7 as cargo compared to hepatocyte-derived EVs, attenuates liver damage and fibrosis by delivering cargo let-7 into cholangiocytes of Mdr2−/− mice and inhibits ductular reaction in vivo [143]. These findings suggest that EV injection may be utilized as another treatment for PSC and could be used as a carrier of beneficial drugs or mediators.
6. Conclusion
The majority of cholangiopathy research focuses on PSC because of well-established research models with limited preclinical and therapeutic studies for PBC and BA. However, the techniques and strategies utilized for PSC could potentially be useful for PBC or BA. For example, culturing cholangiocytes isolated from PBC and BA patients may establish in vitro models for these diseases using techniques of the in vitro model for PSC. Current therapeutic approaches utilize agonists or antagonists to regulate signaling pathways in cholangiocytes that are associated with ductular reaction, fibrogenic marker expression such as TGF-β1 and collagen type I that lead to portal fibrosis, senescence-induced SASP marker secretion such as IL-6 and CCL2 that contribute to liver inflammation, and activation of other liver cells by cholangiocytes such as HSCs leading to further liver damage and fibrosis. Recent studies have introduced novel therapeutic tools for cholangiopathies. Injection of stem cells or stem cell-derived EVs leads to improved liver conditions in animal models, and stem cell therapy has demonstrated its therapeutic effects in mouse models of PSC, PBC, and BA, indicating the promising potential as a novel therapy for cholangiopathies. Although further studies are required, various preclinical models and injection therapies could lead to the development of novel treatments for cholestatic liver injuries.
7. Expert opinion
Current therapeutic approaches for cholangiopathies mainly focus on PSC because of limited studies for PBC and BA. Detailed mechanisms of pathophysiology in PBC and BA are not elucidated, and further studies are needed to identify preclinical candidate targets. For PSC, targeting specific pathways or receptors has demonstrated promising therapeutic effects in animal models. Antagonists against SR or NK-1R decreased liver damage and fibrosis, and asthma drug cromolyn sodium or over-the-counter histamine blockers could be used as a drug for PSC. It is critical to understand the pathological mechanisms of the disorder to identify potential targets, and then develop the drugs for those targets. Investigations to find agonists or antagonists targeting receptors that are associated with cholangiocyte activation and proliferation will facilitate the development of treatments for PSC. In other words, therapies for PBC and BA will require more studies to accumulate evidence for identification of targets compared to PSC-targeted therapies. However, methodology and techniques utilized in PSC therapies could also be performed for PBC and BA. Drugs that are developed for PSC to stabilize cholangiocytes could be useful for other disorders. Future studies will try and evaluate promising drugs not only in PSC models, but also using PBC and BA models. Figure 1 represents the strategy for the development of novel cholangiopathy therapies.
Difficulties in studies for PBC and BA are due to the orchestration of various cells in the pathogenesis of the human disorder. PSC is a bile duct disorder and hence cholangiocytes are a primary target. However, previous studies have suggested that not only cholangiocytes but also other liver cells such as Kupffer cells are involved in the pathophysiology of PSC [144]. PBC is an autoimmune disorder and lymphocytes, especially T cells, are critical in pathophysiology of PBC [61, 62, 65]. However, the association between T cells and cholangiocytes is largely unknown, and detailed mechanisms of autoimmunity against bile ducts are unclear. One of the major characteristic features of PBC is female dominance suggesting female hormones such as estrogen may be involved. Future studies may target lymphocytes or other cells, but not cholangiocytes for the development of PBC therapies.
Another difficulty in translational cholangiopathy study is the complexity of its pathophysiology. The cause of the disorder could differ depending on variations of patients, such as genetic factors, bacterial or viral infections, or environmental factors. Secretion levels of hormones vary depending on individuals, and this may also affect the susceptibility of the disorder. In addition, cholangiopathies are progressive disorders and their pathophysiology may differ depending on the stage of the disease. A preliminary study has observed ductular reaction and bile duct hyperplasia in early stage of PBC, but in later stage, bile ducts are destructed and disappeared [95]. In this case, administration of drugs, such as an agonist to SR, may provide different effects at different stages of patients. This indicates that treatments need to be designed or adjusted for each patient according to the disease-causing factors and liver conditions or stages. Future studies may need to consider various factors as the cause as well as individual varieties in disease conditions or progression.
The goal of the studies summarized in this review is to develop novel therapies replacing liver transplantation. It may not be feasible to develop a complete cure for cholangiopathies especially for autoimmune disease PBC, and hence it is important to manage acceptable liver conditions to allow patients to avoid liver transplantation. Access to appropriate research models is one of the biggest challenges in the design of cholangiopathy research. Future studies will try to establish more appropriate models especially for PBC that have resembling liver conditions and fewer difficulties to maintain the strain.
Injection of stem cells or stem cell-derived EVs is another approach for treatments of cholangiopathies. Stem cell transplantation had therapeutic effects for all PSC, PBC, and BA models although previous studies are limited, and mechanisms of the effects are unclear. There will be more studies available in the future to establish injection therapies. EVs contain cargo proteins, mRNAs, and miRNAs, and those cargo mediators could be therapeutic when delivered into the diseased liver. Stem cell-derived EVs may contain specific mediators, such as let-7, resulting in therapeutic effects. This indicates that EV cargo could be designed and modified to create EVs that regulate specific target cell events. Future studies will identify mediators that regulate cholangiocyte functions leading to improved liver conditions and could generate transfected cells to express therapeutic proteins or RNAs and secrete EVs carrying those mediators. An advantage of injection therapy is less possibility of rejection or side effects compared to drug administration. Stem cells or EVs will be isolated from human tissues or cells, and it could be carried out using patients’ own samples. Drug administration could induce side effects, and injected mRNAs or miRNAs could be degraded rapidly before being delivered to the target tissues or cells. EVs could solve these problems by carrying drugs or mediators inside and protect them from degradation or detection by immune cells. Techniques and procedures for EV therapy could be used not only for cholangiopathies but also for other diseases.
Article Highlights.
Cholangiopathies are bile duct disorders characterized by bile duct damage and inflammation
Treatments for cholangiopathies are limited to liver transplantation
Various animal models have been established and are available for researchers
Previous studies have identified candidate signaling pathways that could be targets for novel therapies
Injection of stem cells or extracellular vesicles could be utilized as a novel therapeutic approach for cholangiopathies
Funding
Portions of these discussed studies were supported by Baylor Scott & White Institute, the Hickam Endowed Chair, Gastroenterology, Medicine, Indiana University, a VA Research Senior Career Scientist Award and a VA Merit award to G Alpini (5I01BX000574), H Francis (1I01BX003031), F Meng (1I01BX001724), and S Liangpunsakul (I01CX000361) as well as the NIH grants DK054811, DK076898, DK107310, DK110035, DK062975, DK108959, DK107682, AA025997, AA025157, AA025208, AA026917, and AA026903 to G Alpini, F Meng, H Francis, S Glaser, and S Liangpunsakul. G. Alpini and K Sato were awarded funding from PSC Partners Seeking a Cure, Colorado, USA.
Abbreviations
- 2-OA
2-octynoic acid
- AE2
Cl−/HCO3- anion exchanger
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AMA
anti-mitochondrial antibody
- ANA
anti-nuclear antibody
- ARE
AU-rich element
- ASBT
apical sodium-dependent bile acid transporter
- AST
aspartate aminotransferase
- BA
biliary atresia
- BDL
bile duct ligation
- CCl4
carbon tetrachloride
- CFTR
cystic fibrosis transmembrane conductance regulator
- CK
cytokeratin
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- EMT
epithelial-mesenchymal transition
- EV
extracellular vesicle
- FXR
farnesoid X receptor
- HCC
hepatocellular carcinoma
- HSC
hepatic stellate cell
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- iPS
induced pluripotent stem
- LPS
lipopolysaccharide
- miRNA
microRNA
- NK-1R
neurokinin-1 receptor
- NOD
non-obese diabetic
- PBC
primary biliary cholangitis
- poly I:C
polyinosinic:polycytidylic acid
- PSC
primary sclerosing cholangitis
- S1PR2
sphingosine-1-phosphate receptor 2
- SASP
senescence-associated secretory phenotype
- Sct
secretin
- SR
secretin receptor
- TAA
thioacetamide
- TGF
transforming growth factor
- TNFα
tumor necrosis factor alpha
- UDCA
ursodeoxycholic acid
- UTR
untranslated region
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med. 2016;375:1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sirpal S, Chandok N. Primary sclerosing cholangitis: diagnostic and management challenges. Clin Exp Gastroenterol. 2017;10:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyson JK, Beuers U, Jones DEJ, et al. Primary sclerosing cholangitis. Lancet. 2018;391:2547–2559. [DOI] [PubMed] [Google Scholar]
- 4.Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298–1323. [DOI] [PubMed] [Google Scholar]
- 5.Taghavi SA, Eshraghian A, Niknam R, et al. Diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Expert Rev Gastroenterol Hepatol. 2018;12:575–584. [DOI] [PubMed] [Google Scholar]
- 6.Lleo A, Marzorati S, Anaya JM, et al. Primary biliary cholangitis: a comprehensive overview. Hepatol Int. 2017;11:485–499. [DOI] [PubMed] [Google Scholar]
- 7.Tsuneyama K, Baba H, Morimoto Y, et al. Primary biliary cholangitis: Its pathological characteristics and immunopathological mechanisms. J Med Invest. 2017;64:7–13. [DOI] [PubMed] [Google Scholar]
- 8.Gulamhusein AF, Hirschfield GM. Pathophysiology of primary biliary cholangitis. Best Pract Res Clin Gastroenterol. 2018;34–35: 17–25. [DOI] [PubMed] [Google Scholar]
- 9.Lakshminarayanan B, Davenport M. Biliary atresia: A comprehensive review. J Autoimmun. 2016;73:1–9. [DOI] [PubMed] [Google Scholar]
- 10.Nizery L, Chardot C, Sissaoui S, et al. Biliary atresia: Clinical advances and perspectives. Clin Res Hepatol Gastroenterol. 2016;40:281–287. [DOI] [PubMed] [Google Scholar]
- 11.Govindarajan KK. Biliary atresia: Where do we stand now? World J Hepatol. 2016;8:1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Meyer C, Xu C, et al. Animal models of chronic liver diseases. Am J Physiol Gastrointest Liver Physiol. 2013;304:G449–68. [DOI] [PubMed] [Google Scholar]
- 13.Fickert P, Pollheimer MJ, Beuers U, et al. Characterization of animal models for primary sclerosing cholangitis (PSC). J Hepatol. 2014;60:1290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollheimer MJ, Fickert P. Animal models in primary biliary cirrhosis and primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2015;48:207–17. [DOI] [PubMed] [Google Scholar]
- 15.McMillin M, Frampton G, Grant S, et al. The neuropeptide galanin is up-regulated during cholestasis and contributes to cholangiocyte proliferation. Am J Pathol. 2017;187:819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tag CG, Sauer-Lehnen S, Weiskirchen S, et al. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp. 2015;* Procedures for BDL. BDL is still common in cholangiopathy studies.
- 17.Alpini G, Ulrich CD 2nd, Phillips JO, et al. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. The American journal of physiology. 1994;266:G922–8. [DOI] [PubMed] [Google Scholar]
- 18.Fickert P, Stoger U, Fuchsbichler A, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raven A, Lu WY, Man TY, et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato K, Marzioni M, Meng F, et al. Ductular reaction in liver diseases: Pathological mechanisms and translational significances. Hepatology. 2019;69:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delire B, Starkel P, Leclercq I. Animal models for fibrotic liver diseases: what we have, what we need, and what is under development. J Clin Transl Hepatol. 2015;3:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Minicis S, Kisseleva T, Francis H, et al. Liver carcinogenesis: rodent models of hepatocarcinoma and cholangiocarcinoma. Dig Liver Dis. 2013;45:450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Zhang H, Zhang Z, et al. Adiponectin-derived active peptide ADP355 exerts anti-inflammatory and anti-fibrotic activities in thioacetamide-induced liver injury. Sci Rep. 2016;6:19445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeSage GD, Benedetti A, Glaser S, et al. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology. 1999;29:307–19. [DOI] [PubMed] [Google Scholar]
- 25.Fujii T, Fuchs BC, Yamada S, et al. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010;10:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bansal R, Frelin L, Brenndorfer ED, et al. Hepatitis C virus nonstructural 3/4A protein dampens inflammation and contributes to slow fibrosis progression during chronic fibrosis in vivo. PLoS One. 2015;10:e0128466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauad TH, van Nieuwkerk CM, Dingemans KP, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–45. [PMC free article] [PubMed] [Google Scholar]
- 28.Wu N, Meng F, Zhou T, et al. Prolonged darkness reduces liver fibrosis in a mouse model of primary sclerosing cholangitis by miR-200b down-regulation. FASEB J. 2017;31:4305–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrescu AD, Grant S, Frampton G, et al. Glucocorticoids cause gender-dependent reversal of hepatic fibrosis in the MDR2-knockout mouse model. Int J Mol Sci. 2017;18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones H, Hargrove L, Kennedy L, et al. Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2−/− mice. Hepatology. 2016;64:1202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moncsek A, Al-Suraih MS, Trussoni CE, et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2−/−) mice. Hepatology. 2018;67:247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorotto R, Villani A, Kourtidis A, et al. The cystic fibrosis transmembrane conductance regulator controls biliary epithelial inflammation and permeability by regulating Src tyrosine kinase activity. Hepatology. 2016;64:2118–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheth S, Shea JC, Bishop MD, et al. Increased prevalence of CFTR mutations and variants and decreased chloride secretion in primary sclerosing cholangitis. Hum Genet. 2003;113:286–92. [DOI] [PubMed] [Google Scholar]
- 34.Werlin S, Scotet V, Uguen K, et al. Primary sclerosing cholangitis is associated with abnormalities in CFTR. J Cyst Fibros. 2018;17:666–671. [DOI] [PubMed] [Google Scholar]
- 35.Martin CR, Zaman MM, Ketwaroo GA, et al. CFTR dysfunction predisposes to fibrotic liver disease in a murine model. Am J Physiol Gastrointest Liver Physiol. 2012;303:G474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debray D, El Mourabit H, Merabtene F, et al. Diet-induced dysbiosis and genetic background synergize with cystic fibrosis transmembrane conductance regulator deficiency to promote cholangiopathy in mice. Hepatol Commun. 2018;2:1533–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gossard AA, Lindor KD. Hepatocellular carcinoma: low risk of HCC in patients who have PSC and cirrhosis. Nat Rev Gastroenterol Hepatol. 2014;11:276–7. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa H, Hikiba Y, Hirata Y, et al. Loss of liver E-cadherin induces sclerosing cholangitis and promotes carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada K, Sato Y, Ikeda H, et al. Epithelial-mesenchymal transition induced by biliary innate immunity contributes to the sclerosing cholangiopathy of biliary atresia. J Pathol. 2009;217:654–64. [DOI] [PubMed] [Google Scholar]
- 40.Robertson H, Kirby JA, Yip WW, et al. Biliary epithelial-mesenchymal transition in posttransplantation recurrence of primary biliary cirrhosis. Hepatology. 2007;45:977–81. [DOI] [PubMed] [Google Scholar]
- 41.Zeisberg M, Yang C, Martino M, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. The Journal of biological chemistry. 2007;282:23337–47. [DOI] [PubMed] [Google Scholar]
- 42.Sato Y, Harada K, Ozaki S, et al. Cholangiocytes with mesenchymal features contribute to progressive hepatic fibrosis of the polycystic kidney rat. Am J Pathol. 2007;171:1859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao YL, Zhu RT, Sun YL. Epithelial-mesenchymal transition in liver fibrosis. Biomed Rep. 2016;4:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taura K, Iwaisako K, Hatano E, et al. Controversies over the epithelial-to-mesenchymal transition in liver fibrosis. J Clin Med. 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu AS, Diaz R, Hui JJ, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholten D, Osterreicher CH, Scholten A, et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatano R, Akiyama K, Tamura A, et al. Knockdown of ezrin causes intrahepatic cholestasis by the dysregulation of bile fluidity in the bile duct epithelium in mice. Hepatology. 2015;61:1660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamura A, Kikuchi S, Hata M, et al. Achlorhydria by ezrin knockdown: defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol. 2005;169:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabibian JH, O’Hara SP, Splinter PL, et al. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabibian JH, Trussoni CE, O’Hara SP, et al. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest. 2014;94:1126–33.** This study has developed in vitro PSC model and characererized PSC cholangiocytes.
- 52.Loarca L, De Assuncao TM, Jalan-Sakrikar N, et al. Development and characterization of cholangioids from normal and diseased human cholangiocytes as an in vitro model to study primary sclerosing cholangitis. Lab Invest. 2017;97:1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dianat N, Dubois-Pot-Schneider H, Steichen C, et al. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology. 2014;60:700–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sampaziotis F, de Brito MC, Madrigal P, et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reshetnyak VI. Primary biliary cirrhosis: Clinical and laboratory criteria for its diagnosis. World J Gastroenterol. 2015;21:7683–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koarada S, Wu Y, Fertig N, et al. Genetic control of autoimmunity: protection from diabetes, but spontaneous autoimmune biliary disease in a nonobese diabetic congenic strain. J Immunol. 2004;173:2315–23. [DOI] [PubMed] [Google Scholar]
- 57.Irie J, Wu Y, Wicker LS, et al. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakagome Y, Ueno Y, Kogure T, et al. Autoimmune cholangitis in NOD.c3c4 mice is associated with cholangiocyte-specific Fas antigen deficiency. J Autoimmun. 2007;29:20–9. [DOI] [PubMed] [Google Scholar]
- 59.Schrumpf E, Jiang X, Zeissig S, et al. The role of natural killer T cells in a mouse model with spontaneous bile duct inflammation. Physiol Rep. 2017;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schrumpf E, Kummen M, Valestrand L, et al. The gut microbiota contributes to a mouse model of spontaneous bile duct inflammation. J Hepatol. 2017;66:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang W, Rainbow DB, Wu Y, et al. A novel Pkhd1 mutation interacts with the nonobese diabetic genetic background to cause autoimmune cholangitis. J Immunol. 2018;200:147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. [DOI] [PubMed] [Google Scholar]
- 63.Oertelt S, Lian ZX, Cheng CM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–60. [DOI] [PubMed] [Google Scholar]
- 64.Ma HD, Zhao ZB, Ma WT, et al. Gut microbiota translocation promotes autoimmune cholangitis. J Autoimmun. 2018;95:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang W, Zhang R, Zhang J, et al. Proteomic analysis reveals distinctive protein profiles involved in CD8(+) T cell-mediated murine autoimmune cholangitis. Cell Mol Immunol. 2018;15:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medina JF, Martinez A, Vazquez JJ, et al. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology. 1997;25:12–7. [DOI] [PubMed] [Google Scholar]
- 67.Salas JT, Banales JM, Sarvide S, et al. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134:1482–93. [DOI] [PubMed] [Google Scholar]
- 68.Medina JF, Recalde S, Prieto J, et al. Anion exchanger 2 is essential for spermiogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Funauchi M, Sugishima H, Minoda M, et al. Serum level of interferon-gamma in autoimmune diseases. Tohoku J Exp Med. 1991;164:259–67. [DOI] [PubMed] [Google Scholar]
- 70.Pollard KM, Cauvi DM, Toomey CB, et al. Interferon-gamma and systemic autoimmunity. Discov Med. 2013;16:123–31. [PMC free article] [PubMed] [Google Scholar]
- 71.Hodge DL, Berthet C, Coppola V, et al. IFN-gamma AU-rich element removal promotes chronic IFN-gamma expression and autoimmunity in mice. J Autoimmun. 2014;53:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bae HR, Leung PS, Tsuneyama K, et al. Chronic expression of interferon-gamma leads to murine autoimmune cholangitis with a female predominance. Hepatology. 2016;64:1189–201.** This model has advantages compared to other models and could be the best PBC model.
- 73.Bae HR, Hodge DL, Yang GX, et al. The interplay of type I and type II interferons in murine autoimmune cholangitis as a basis for sex-biased autoimmunity. Hepatology. 2018;67:1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wakabayashi K, Lian ZX, Leung PS, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu SJ, Yang YH, Tsuneyama K, et al. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology. 2011;53:915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wakabayashi K, Yoshida K, Leung PS, et al. Induction of autoimmune cholangitis in non-obese diabetic (NOD).1101 mice following a chemical xenobiotic immunization. Clin Exp Immunol. 2009;155:577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsueh YH, Chen HW, Syu BJ, et al. Endogenous IL-10 maintains immune tolerance but IL-10 gene transfer exacerbates autoimmune cholangitis. J Autoimmun. 2018;95:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okada C, Akbar SM, Horiike N, et al. Early development of primary biliary cirrhosis in female C57BL/6 mice because of poly I:C administration. Liver Int. 2005;25:595–603. [DOI] [PubMed] [Google Scholar]
- 79.Jiang T, Han Z, Chen S, et al. Resistance to activation-induced cell death and elevated FLIPL expression of CD4+ T cells in a polyI:C-induced primary biliary cirrhosis mouse model. Clin Exp Med. 2009;9:269–76. [DOI] [PubMed] [Google Scholar]
- 80.Garrido M, Escobar C, Zamora C, et al. Bile duct ligature in young rats: A revisited animal model for biliary atresia. Eur J Histochem. 2017;61:2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Averbukh LD, Wu GY. Evidence for viral induction of biliary atresia: A review. J Clin Transl Hepatol. 2018;6:410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allen SR, Jafri M, Donnelly B, et al. Effect of rotavirus strain on the murine model of biliary atresia. J Virol. 2007;81:1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mao Y, Tang S, Yang L, et al. Inhibition of the Notch signaling pathway reduces the differentiation of hepatic progenitor cells into cholangiocytes in biliary atresia. Cell Physiol Biochem. 2018;49:1074–1082. [DOI] [PubMed] [Google Scholar]
- 84.Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jee J, Mourya R, Shivakumar P, et al. Cxcr2 signaling and the microbiome suppress inflammation, bile duct injury, and the phenotype of experimental biliary atresia. PLoS One. 2017;12:e0182089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lei J, Chai Y, Xiao J, et al. Antifibrotic potential of bone marrowderived mesenchymal stem cells in biliary atresia mice. Mol Med Rep. 2018;18:3983–3988. [DOI] [PubMed] [Google Scholar]
- 87.Sato K, Meng F, Giang T, et al. Mechanisms of cholangiocyte responses to injury. Biochimica et biophysica acta. 2018;1864:1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glaser SS, Gaudio E, Miller T, et al. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Afroze S, Meng F, Jensen K, et al. The physiological roles of secretin and its receptor. Ann Transl Med. 2013;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guerrier M, Attili F, Alpini G, et al. Prolonged administration of secretin to normal rats increases biliary proliferation and secretin-induced ductal secretory activity. Hepatobiliary Surg Nutr. 2014;3:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glaser S, Meng F, Han Y, et al. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu N, Meng F, Zhou T, et al. The secretin/secretin receptor axis modulates ductular reaction and liver fibrosis through changes in transforming growth factor-beta1-mediated biliary senescence. Am J Pathol. 2018;188:2264–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou T, Wu N, Meng F, et al. Knockout of secretin receptor reduces biliary damage and liver fibrosis in Mdr2−/− mice by diminishing senescence of cholangiocytes. Lab Invest. 2018;98:1449–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu N, Meng F, Invernizzi P, et al. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-β1 biliary secretion in mice. Hepatology. 2016;64:865–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kennedy L, Francis H, Venter J, et al. Secretin-stimulation of bicarbonate secretion reduces biliary damage and liver fibrosis in a model of primary biliary cholangitis (PBC). Gastroenterology. 2017;152:S1060. [Google Scholar]
- 96.Ishii M, Iwai M, Harada Y, et al. A role of mast cells for hepatic fibrosis in primary sclerosing cholangitis. Hepatol Res. 2005;31:127–31. [DOI] [PubMed] [Google Scholar]
- 97.Hargrove L, Kennedy L, Demieville J, et al. BDL-induced biliary hyperplasia, hepatic injury and fibrosis are reduced in mast cell deficient Kitw-sh mice. Hepatology. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kennedy LL, Hargrove LA, Graf AB, et al. Inhibition of mast cell-derived histamine secretion by cromolyn sodium treatment decreases biliary hyperplasia in cholestatic rodents. Lab Invest. 2014;94:1406–18. [DOI] [PubMed] [Google Scholar]
- 99.Kennedy L, Hargrove L, Demieville J, et al. Blocking H1/H2 histamine receptors inhibits damage/fibrosis in Mdr2−/− mice and human cholangiocarcinoma tumorigenesis. Hepatology. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Francis H, Glaser S, Demorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamura A, Yamazaki K, Suzuki K, et al. Increased portal tract infiltration of mast cells and eosinophils in primary biliary cirrhosis. Am J Gastroenterol. 1997;92:2245–9. [PubMed] [Google Scholar]
- 102.Alpini G, Baiocchi L, Glaser S, et al. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 2002;35:1041–52. [DOI] [PubMed] [Google Scholar]
- 103.Gong Y, Huang Z, Christensen E, et al. Ursodeoxycholic acid for patients with primary biliary cirrhosis: an updated systematic review and meta-analysis of randomized clinical trials using Bayesian approach as sensitivity analyses. Am J Gastroenterol. 2007;102:1799–807. [DOI] [PubMed] [Google Scholar]
- 104.Hatano R, Kawaguchi K, Togashi F, et al. Ursodeoxycholic Acid Ameliorates Intrahepatic Cholestasis Independent of Biliary Bicarbonate Secretion in Vil2kd/kd Mice. Biol Pharm Bull. 2017;40:34–42. [DOI] [PubMed] [Google Scholar]
- 105.Meng F, Kennedy L, Hargrove L, et al. Ursodeoxycholate inhibits mast cell activation and reverses biliary injury and fibrosis in Mdr2−/− mice and human primary sclerosing cholangitis. Lab Invest. 2018;98:1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindor KD, Dickson ER, Baldus WP, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–90. [DOI] [PubMed] [Google Scholar]
- 107.Keitel V, Ullmer C, Haussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391:785–9. [DOI] [PubMed] [Google Scholar]
- 108.Masyuk AI, Huang BQ, Radtke BN, et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reich M, Deutschmann K, Sommerfeld A, et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut. 2016;65:487–501. [DOI] [PubMed] [Google Scholar]
- 110.Hov JR, Keitel V, Laerdahl JK, et al. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5:e12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pean N, Doignon I, Garcin I, et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013;58:1451–60. [DOI] [PubMed] [Google Scholar]
- 112.Torres J, Bao X, Iuga AC, et al. Farnesoid X receptor expression is decreased in colonic mucosa of patients with primary sclerosing cholangitis and colitis-associated neoplasia. Inflamm Bowel Dis. 2013;19:275–82. [DOI] [PubMed] [Google Scholar]
- 113.Baghdasaryan A, Claudel T, Gumhold J, et al. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO(−)(3) output. Hepatology. 2011;54:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwabl P, Hambruch E, Seeland BA, et al. The FXR agonist PX20606 ameliorates portal hypertension by targeting vascular remodelling and sinusoidal dysfunction. J Hepatol. 2017;66:724–733. [DOI] [PubMed] [Google Scholar]
- 115.Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–43. [DOI] [PubMed] [Google Scholar]
- 116.Kowdley KV, Luketic V, Chapman R, et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018;67:1890–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wagner M, Fickert P, Zollner G, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–38. [DOI] [PubMed] [Google Scholar]
- 118.Graffner H, Gillberg PG, Rikner L, et al. The ileal bile acid transporter inhibitor A4250 decreases serum bile acids by interrupting the enterohepatic circulation. Aliment Pharmacol Ther. 2016;43:303–10. [DOI] [PubMed] [Google Scholar]
- 119.Baghdasaryan A, Fuchs CD, Osterreicher CH, et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol. 2016;64:674–81. [DOI] [PubMed] [Google Scholar]
- 120.Al-Dury S, Wahlstrom A, Wahlin S, et al. Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis. Sci Rep. 2018;8:6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nagahashi M, Yuza K, Hirose Y, et al. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. J Lipid Res. 2016;57:1636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y, Aoki H, Yang J, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;65:2005–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Glaser S, Han Y, Francis H, et al. Melatonin regulation of biliary functions. Hepatobiliary Surg Nutr. 2014;3:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ohta Y, Kongo M, Kishikawa T. Melatonin exerts a therapeutic effect on cholestatic liver injury in rats with bile duct ligation. J Pineal Res. 2003;34:119–26. [DOI] [PubMed] [Google Scholar]
- 125.Tahan G, Akin H, Aydogan F, et al. Melatonin ameliorates liver fibrosis induced by bile-duct ligation in rats. Can J Surg. 2010;53:313–8. [PMC free article] [PubMed] [Google Scholar]
- 126.Renzi A, Glaser S, Demorrow S, et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol. 2011;301:G634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Turco M, Cazzagon N, Franceschet I, et al. Morning Bright Light Treatment for Sleep-Wake Disturbances in Primary Biliary Cholangitis: A Pilot Study. Front Physiol. 2018;9:1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han Y, Onori P, Meng F, et al. Prolonged exposure of cholestatic rats to complete dark inhibits biliary hyperplasia and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Glaser S, Gaudio E, Renzi A, et al. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wan Y, Meng F, Wu N, et al. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology. 2017;66:528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kwak KA, Cho HJ, Yang JY, et al. Current Perspectives Regarding Stem Cell-Based Therapy for Liver Cirrhosis. Can J Gastroenterol Hepatol. 2018;2018:4197857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang D, Zhang H, Liang J, et al. Effect of allogeneic bone marrow-derived mesenchymal stem cells transplantation in a polyI:C-induced primary biliary cirrhosis mouse model. Clin Exp Med. 2011;11:25–32. [DOI] [PubMed] [Google Scholar]
- 133.Glaser SS, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mancinelli R, Franchitto A, Gaudio E, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mancinelli R, Franchitto A, Glaser S, et al. GABA induces the differentiation of small into large cholangiocytes by activation of Ca2+/CaMK I-dependent adenylyl cyclase 8. Hepatology. 2013;58:251–63. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.McDaniel K, Meng F, Wu N, et al. Forkhead box A2 regulates biliary heterogeneity and senescence during cholestatic liver injury in mice. Hepatology. 2017;65:544–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hirsova P, Ibrahim SH, Verma VK, et al. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology. 2016;64:2219–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sato K, Meng F, Glaser S, et al. Exosomes in liver pathology. J Hepatol. 2016;65:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Masyuk AI, Huang BQ, Ward CJ, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299:G990–9.* This study has demonstrated that cholangiocyte functions are regulated by EVs.
- 140.Sato K, Meng F, Venter J, et al. The role of the secretin/secretin receptor axis in inflammatory cholangiocyte communication via extracellular vesicles. Sci Rep. 2017;7:11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chaiyadet S, Sotillo J, Smout M, et al. Carcinogenic liver fluke secretes extracellular vesicles that promote cholangiocytes to adopt a tumorigenic phenotype. J Infect Dis. 2015;212:1636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McDaniel K, Huang L, Sato K, et al. The let-7/Lin28 axis regulates activation of hepatic stellate cells in alcoholic liver injury. The Journal of biological chemistry. 2017;292:11336–11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McDaniel K, Wu N, Zhou T, et al. Amelioration of ductular reaction by stem cell derived extracellular vesicles in MDR2 knockout mice via let-7 microRNA. Hepatology. 2019;* This study has demonstrated that EV injection could be therapeutic in cholangiopathies.
- 144.Sato K, Hall C, Glaser S, et al. Pathogenesis of kupffer cells in cholestatic liver injury. Am J Pathol. 2016;186:2238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]