Abstract
Nocturnin (NOCT) belongs to the Mg2+ dependent Exonucleases, Endonucleases, Phosphatase (EEP) family of enzymes that exhibit various functions in vitro and in vivo. NOCT is known to function as a de-adenylase, cleaving poly-A tails from mRNA transcripts. Previously, we reported a role for NOCT in regulating bone marrow stromal cell differentiation through its interactions with PPARγ. In this study, we characterized the skeletal and adipose tissue phenotype when we globally overexpressed Noct in vivo. After 12 weeks of Noct overexpression, transgenic male mice had lower fat mass compared to controls, with no significant differences in the skeleton. Based on the presence of a mitochondrial target sequence in NOCT, we determined that mouse NOCT protein localizes to the mitochondria; subsequently we found that NOCT overexpression led to a significant increase in preadipocytes ability to utilize oxidative phosphorylation for ATP (adenosine triphosphate) generation. In summary, the effects of NOCT on adipocytes are likely through its novel role as a mediator of mitochondrial function.
Keywords: Nocturnin, bone mass, adipogenesis and osteogenesis
2). Introduction
Nocturnin (NOCT) was first discovered in the Xenopus retina as a circadian regulated gene (Green and Besharse, 1996). The circadian function of NOCT appears to be conserved, as recent studies have identified curled in Drosophila as being the fly homolog of Nocturnin (Gronke et al., 2009). A more recent study has reported that Demosponge Suberites domuncula (sponge) also has expression of a circadian gene similar to mammalian Noct that can regulate the level of glycogenin (Muller et al., 2012). Previously, NOCT was found to be essential for controlling peripheral circadian output in enterocytes and bone (Douris et al., 2011; Kawai et al., 2010b). Interestingly, Noct expression is nonrythmic in white adipose tissue and its expression becomes rhythmic in this tissue only during restricted feeding in mice (Gilbert et al., 2011). Other studies have shown that Noct null mice (Noct−/−) are resistant to high fat diet induced obesity and hepatic steatosis (Green et al., 2007). This was thought to be mediated through the impaired absorption of dietary lipids in enterocytes, where Noct has been shown to exhibit a circadian regulation (Douris et al., 2011). In addition to those functions, NOCT has been postulated to play a role in regulating mitochondrial related mRNA (Kojima et al., 2015) through its de-adenylase activity, where its endonuclease activity can regulate poly-A tail lengths to reduce the stability of the transcripts (Baggs and Green, 2003). More recently, two independent reports described the crystal structures for human NOCT. In T.Abshire et al in vivo assays have shown that NOCT can regulate mRNA translation and decay whereas Estrella,Du,and Korrennykh suggest that Hnoct is inactive against polyA RNA (E et al., 2018; Estrella et al., 2018). Though it is not exactly clear what the substrates of NOCT are,it is regulated by both environmental and metabolic cues and in turn, NOCT can post-transcriptionally modulate metabolic and skeletal activity.
In bone, Noct is expressed in antiphase with insulin-like growth factor-1 (Igf-1) (Kawai et al., 2010a). Noct is also expressed in bone marrow stromal cells, and mice lacking the Noct gene have higher bone mass at 16 weeks of age (Kawai et al., 2010b). We previously showed that NOCT plays a critical role in regulating bone mass through its actions on PPARɣ; overexpression inhibits osteogenesis but increases adipogenesis in vitro (Kawai et al., 2010b). We have also reported that Noct−/− mice are resistant to rosiglitazone (Rosi) induced bone loss (Guntur et al., 2011a). However, the effects of Rosi on bone could also be direct and independent of PPARɣ. Specifically, Rosi is capable of inhibiting both pyruvate transport into the mitochondria and complex I of the electron transport chain (Dello Russo et al., 2003; Divakaruni et al., 2013). Furthermore, in the absence of NOCT, there is an increase in the poly-A tail lengths of a number of mitochondrial transcripts (Kojima et al., 2015). In the present study, we tested the hypothesis that NOCT could regulate adipogenesis and osteogenesis by directly modulating mitochondrial function. We present the bone and body composition phenotypes of mice overexpressing Noct. We identified a putative mitochondrial target sequence in the murine NOCT amino acid sequence. Expression studies for Flag-tagged NOCT in vitro showed that it can colocalize with mitochondria. Overexpression of NOCT in preadipocytes increased oxidative phosphorylation which we have shown previously is necessary for adipogenesis (Guntur et al., 2018). These novel findings expand on the role of NOCT in regulating bone and energy metabolism.
3). Materials and methods
3.1. Transgenic animals:
Transgenic (TG) mouse lines were generated by pronuclear injection using standard techniques as previously described (Hong et al., 2007). The founder lines for the tetO::NoctWT-FLAG were crossed with mice from the C57BL/6J (B6) inbred strain. We obtained mice expressing the optimized form of the reverse tetracycline transactivator protein (rtTA*M2) under control of the ROSA26 promoter from the Jackson Laboratory (Bar Harbor, ME, USA, JAX stock #6965). Mice containing the tetO::NoctWT-FLAG transgenes were crossed with ROSA26-rtTA mice to obtain ROSA26-rtTA x tetO::NoctWT-FLAG double TG lines. These double TG lines were each backcrossed with mice from the B6 inbred line for at least 8 generations before experimentation. Detection of the transgenes for genotyping information was obtained using the following primers: ROSA26-rtTA transgene, 5’-AAAGTCGCTCTGAGTTGTTAT-3’ (forward primer) and 5’- GCGAAGAGTTTGTCCTCAACC-3’ (reverse primer); ROSA26-rtTA control, 5’-AAAGTCGCTCTGAGTTGTTAT-3’ (forward primer) and 5’-GGAGCGGGAGAAATGGATATG-3’ (reverse primer); tetO::NoctWT-FLAG transgenes are both detected with 5’-CCATTCTAAACAACACCCT-3’ (forward primer) and 5’ CTCCCTACACAACCTGGAAGATCCGGACC-3’ (reverse primer). We assigned Noct WT to refer to animals overexpressing the wildtype Noct. The protocol for treatments and sample collection were approved by the Maine Medical Center Research Institute Institutional Animal Care and Use Committee (IACUC). The animals were housed in a barrier facility with a 14-hour light and 10-hour dark cycle. All samples were collected between 9 am-12 noon. The lights were switched off at 8:00 pm and switched on at 6:00 am in the facility.
3.2. Nocturnin Overexpression:
Noct WT mice were able to freely access water supplemented with either 1 mg/mL saccharin (designated as Sacch) (Sigma, St. Louis, USA) or 2 mg/mL of Dox (Sigma, St. Louis, USA) containing 1 mg/mL of Saccharin (designated as Dox) starting at 4 weeks of age. Sacch and Dox solutions were changed twice a week until mice were harvested at 16 weeks of age, (12 weeks of Sacch or Dox treatment), and tissues were collected for later procedures. For simplicity, Noct WT treated with Dox indicates Noct overexpression.
3.3. Dual-energy X-ray absorptiometry (DXA):
DXA (PIXImus densitometer; GE-Lunar, Fairfield, CT, USA) was performed on the whole body and femora to determine areal bone mineral density (aBMD, g/cm2), areal bone mineral content (aBMC, g) and body composition. Analyses were performed exclusive of the head. Prior to use, the PIXImus was calibrated daily with a phantom provided by the manufacturer.
3.4. Micro-computed tomography (μCT):
Micro-architecture of the trabecular bone and midshaft cortical bone of the femur was analyzed by μCT (resolution 10 μm, VivaCT-40, Scanco Medical AG, Bruttisellen, Switzerland). Bones were scanned at energy level of 55kVp, and intensity of 145 μA. Trabecular bone volume fraction and micro-architecture were evaluated in the secondary spongiosa, starting proximately at 0.6 mm proximal to the distal femoral growth plate, and extending proximally 1.5 mm. Approximately 230 consecutive slices were made at 10.5 um interval at the distal end of the growth plate and extending in a proximal direction, and 180 contiguous slices were selected for analysis. Measurements included bone volume/total volume (BV/TV), trabecular number (Tb.N.), trabecular thickness (Tb.Th.) and trabecular separation (Tb.Sp.). Scans for the cortical region were measured at the mid-point of each femur and used to to calculate total cross-sectional area (Tt.Ar), cortical bone area (Ct.Ar.), medullary area (Ma.Ar.), bone area fraction (Ct.Ar/Tt.Ar.), cortical tissue mineral density (Ct.TMD.), cortical thickness (Ct.Th.) cortical porosity (%), and maximum, minimum, and polar moments of inertia (Imax, Imin, and pMOI) . All scans were analyzed using manufacturer software (Scanco, version 4.05). Acquisition and analysis of μCT data were performed in accordance with published guidelines (Carvalho et al., 2018).
3.5. RNA isolation and quantitative real-time polymerase chain reaction:
Total RNA was isolated using a standard TRIzol extraction (Life Technologies, Carlsbad, CA, USA) method from whole bones that were flash frozen in liquid nitrogen. Five hundred ng of RNA were then reverse-transcribed using a Life Technologies cDNA reverse transcription kit (Life Technologies, Beverly, MA, USA). The cDNA was diluted 1:10 with water. Quantification of mRNA expression was carried out using an iQ SYBR Green Supermix and BioRad iQ™5 multicolor Real-Time PCR detection system (BioRad, Hercules, CA, USA). Primers were designed and tested to be 95–100% efficient by PrimerDesign (South Hampton, UK), Primers used to amplify the Noct (Ccrn4l, P2) P2: 5’-CGGGATTTTGTGGACCTGAG −3’ (forward primer) and 5’-TGTCTTTGCCTTCTCCGAGA −3’ (reverse primer); Additional primer sets were ordered from integrated DNA technologies. Primers used to amplify the Noct WT (P1) close to the Flag Tag, and pTRE2 (Tetracycline response element) Noct 5’ untranslated region (P3) are as follows: P1: 5’-AGCCCCATGAGCTCTTTCTC-3’ (forward primer) and 5’-TAAGGTACCGGGCCCTACTT-3’ (reverse primer); P3: 5’-CGCCTCTCTAACGAATCCCC-3’ (forward primer) and 5’-GCGACTGTAGATCTCCCACG-3’ (reverse primer).
3.6. Immunofluorescence:
Flag mouse monoclonal antibody, 1:100 dilution (Rockland, catlog#200301383) and MitoTracker Red CMXRos (Invitrogen, catlog# M7152) were used to stain for NOCT and the mitochondria respectively. Primary eMSCs and BMSCs were treated with MitoTarcker Red for 1 hour prior to fixation. Cells were fixed in 4% Paraformaldehyde and processed for staining with the Flag antibody primary antibody and Alexa FITC 488 secondary antibody (Invitrogen catlog# A11029). Vectashield with DAPI (Vector Laboratories catlog# H-1200) was used to mount the coverslips and images were scanned using a Leica SP8 confocal microscope (from n=3 coverslips). Confocal microscopy was performed at the core facility at MMCRI following a previously published protocol (Kawai et al., 2010b).
3.7. Serum markers:
IGF-1 levels were measured using a commercially available kit from Immunodiagnostic Systems (Gaithersburg, MD, USA) following the manufacturer’s instructions. The assay sensitivity was 2.8 ng/mL. The intra-assay and inter-assay variation coefficients were 5.8% and 7.8% respectively. All measurements were performed in duplicate. Serum P1NP and CTx levels were measured as described (DeMambro et al., 2015). In brief, samples were measured using commercially available kits from IDS (Gaithersburg, MD, USA) according to the manufacturer’s instructions. The assay sensitivities were 0.7 and 2 ng/mL for P1NP and CTx, respectively. The intra-assay variations were 6.3 and 6.9%, and the inter-assay variations were 8.5 and 12% respectively, for both assays. All measurements were performed in duplicate.
3.8. Histological analysis:
Fat pads including inguinal white adipose tissue and brown adipose tissue were collected at 16 weeks of age and formalin fixed before processing for histology. The fixed tissue was paraffin embedded, processed and sectioned at 5μM on a microtome before staining with Hematoxylin and Eosin.
3.9. Primary Ear mesenchymal stem cell (eMSCs) and Bone marrow stem cell (BMSCs) culture:
Primary eMSCs and bone marrow stem cells (BMSCs) were cultured as previously described in (Maridas et al., 2017), Briefly, isolated cells were processed for confocal microscopy by plating them on cover slips as described above in 3.6 , to induce NOCT expression, 1μg/ml Dox was used for a minimum of three days. Additionally, 1×104 and 2×104 cells were plated in 12 or 6 well plates for isolation of protein lysates. Western blotting was essentially done as described in (Guntur et al., 2011b). The BioRad turbo system was used for gel transfer.
3.10. XF Analysis to measure Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR):
Noct expression was induced in ear mesenchymal stem cells (eMSCs) isolated from Noct WT mice using Dox treatment. Cells were plated at a density of 5×103 cells per well in a 96-well Seahorse culture plate. Cells were cultured in 200 μL of growth media for three days with or without Dox (1μg/ml). Mitostress test assay was performed in XF DMEM media containing 2mM glutamine and supplemented with 10mM glucose and 10mM pyruvate (Guntur et al., 2014). The mitochondrial stress test was described previously (Guntur et al., 2018) with three sequential injections of A) Oligomycin 1.5μM B) FCCP 1μM and C) Antimycin and Rotenone 0.5μM each. The OCR and ECAR raw values were normalized to cell number.
3.11. Statistical Analyses:
All data are expressed as the mean ± standard error of the mean (S.E.M.) unless otherwise noted. Results were analyzed for statistically significant differences using Student’s t-test or two way ANOVA followed by Sidak multiple comparison post hoc test where appropriate. A p-value < 0.05 was considered statistically significant.
4). Results
Validation of the Noct WT transgenic mouse model
To overexpress the Noct WT construct in vivo in a variety of tissues, we used a Tet-on-inducible system with doxycycline. The transgene was engineered to express a Flag epitope at the c-terminus and has seven Tet operator sequences at the 5’ end (Fig1A). To confirm the expression of the transgene, we used immunofluorescence on ear mesenchymal stem cells (eMSCs) from 8 weeks old mice expressing the transgene and treated with Dox in vitro to visualize Flag expression (Fig1B). We also studied Flag (NOCT protein) expression using western blotting and showed a robust increase in protein levels with Dox treatment in bone marrow stromal cells (BMSCs) (Fig1C). Additionally, we analyzed gene expression from whole bones harvested at 16 weeks (12 weeks on Sacch or Dox) animals by using primers targeting three specific regions: P1 primers were designed to amplify the regions flanking the Flag tag, P2 primers amplified the coding region, and P3 primers specifically amplified the 5’ untranslated region of the endogenous transcript. We showed a robust increase in expression of the transgene in the presence of Dox (Fig1D). However, the endogenous gene at 16 week time point was not significantly different suggesting that any phenotypic differences reflect the effects of the transgene expression (Fig1D).
Figure 1: Validation of Noct WT mouse model.
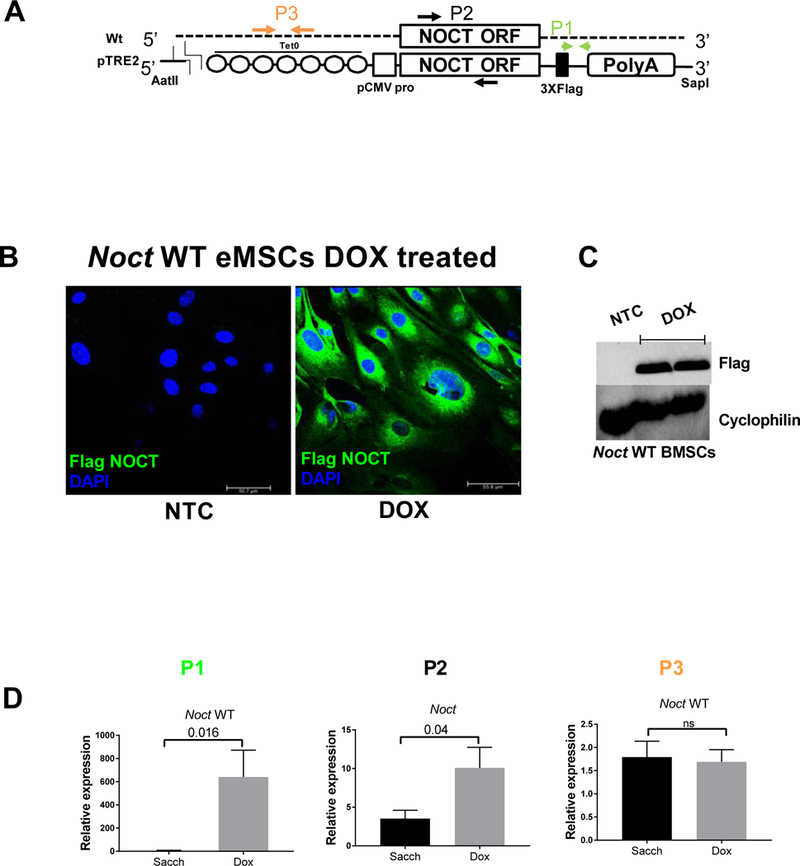
A) Arrangement of the Wildtype (WT) Noct gene and TRE2 Noct transgene (pTRE2 is an expression plasmid with a tetracycline response element) containing the Tet-operator sequences, Noct open reading frame, and 3xFlag tag (not to scale). Primers used to amplify the Noct WT (P1), total Noct containing both WT and pTRE2 Flag (P2), and pTRE2 Noct 5’ untranslated region (P3) are indicated. pTRE2 (plasmid containing the tetracycline response elements), pCMV (cytomegalovirus promoter), TetO (Tetracycline operator), AatII and SapI (Restriction endonucleases used for cloning). B) Confirmation of Noct transgene expression in ear mesenchymal stem cells isolated from Noct WT animals and treated with 1 μg/mL Dox in vitro before fixation and staining for Flag expression. There is robust Flag expression (Green, FITC) showing Dox can induce NOCT-Flag protein expression. Nuclei stained with DAPI are in blue. C) Confirmation of Noct transgene expression in bone marrow stromal cells isolated from Noct WT animals and treated with 1 μg/mL Dox in vitro before western blotting for Flag expression. As can be seen, there is robust Flag expression, the same blot was probed with cyclophilin as a protein loading control. D)Total tibial bone RNA from 16 weeks old Noct WT mice showing expression of Noct using qRT-PCR and primers described in A (12 weeks on Sacch or Dox respectively). Values represent mean ± SEM of n ≥ 3 for qRT-PCR analysis. p values are calculated using student’s t-test. (NTC in fig 1B and 1C, represents no treatment control)
Sacch and Dox treatments for 12 weeks have no effect on bone mass or body composition in B6 mice
Previous studies suggested that long term Dox treatment had minimal impact on bone mass (Fowlkes et al., 2015). To confirm this, we treated B6 with either Sacch or Dox starting at 4 weeks of age. After 12 weeks of treatment (16 weeks of age), there was a significant increase in body weight and total fat mass in mice treated with Dox (Fig2A); however, we observed no changes in bone mineral density by DXA measurements (data not shown). μCT analyses confirmed DXA findings; there were no differences in trabecular or cortical parameters (Fig2B) between Sacch or Dox treated male mice.
Figure 2: Dox treatment increases fat mass, but has no effect on bone microarchitecture in B6 mice.
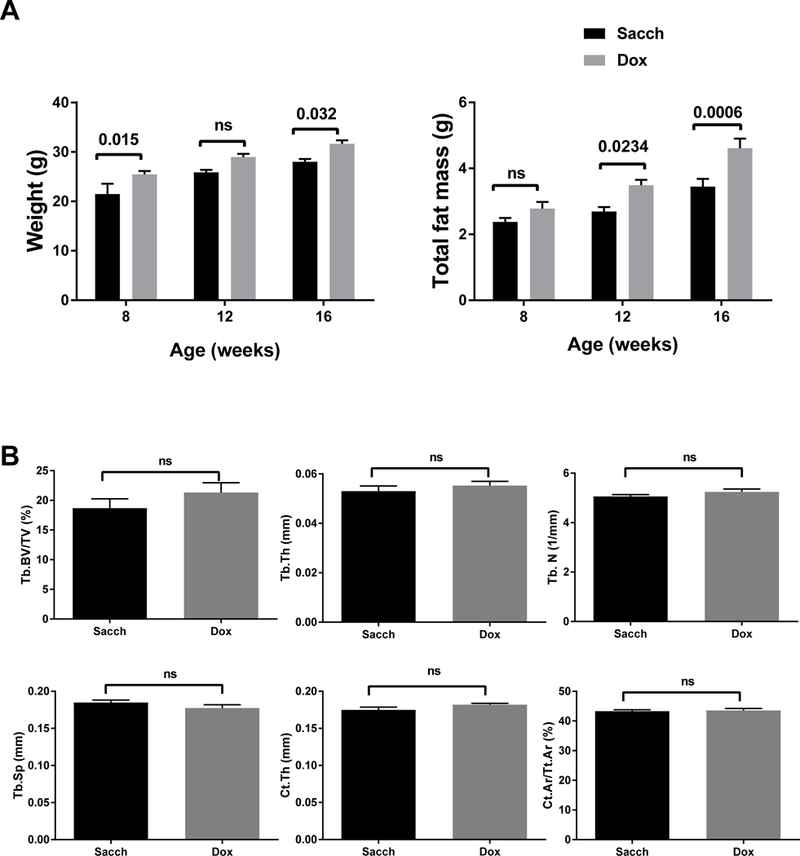
Male mice were treated with either Sacch or Dox beginning at 4 weeks of age and followed till 16 weeks of age (12 weeks on Sacch or Dox). A) Body composition was determined by DXA at 8, 12, and 16 weeks of age (n=5–12); body weight and total fat mass are shown. B) Bone microarchitecture was analyzed in femur by μCT. No significant difference was observed in Tb. BV/TV, Tb.Th, Tb.N, Tb.Sp, Ct.Th, and Ct. Ar/Tt.Ar. Values represent mean ± SEM of n=6–9. p-values are calculated using GraphPad Prism as described in the methods section.
Body composition of Noct WT mice on Sacch and Dox
Although there was no significant change in body weight and lean mass, Noct WT overexpression mice had a significant decrease in total fat mass at 8, 12 and 16 weeks (Fig3A). On the other hand, Noct−/− mice from a previous study were shown to be resistant to high fat diet induced obesity (Green et al., 2007). One of the potential mechanisms may be the circadian expression pattern in the enterocytes resulting in decreased expression of the enzymes involved in formation or transport of chylomicrons into the blood stream. Altered peripheral circadian rhythms could also cause an overall decrease in the circulating triacylglycerols (TAG) in the Noct−/− animals. The phenotype that we observe in the overexpression models in this study mirrors the phenotype observed in the Noct null animals on a high fat diet. Furthermore, histological analysis of inguinal white adipose tissue (iWAT) and brown adipose tissue (BAT) from Noct WT overexpression qualitatively showed smaller adipocytes compared to controls (Fig3B). Which could be based on the constitutive and continuous overexpression resulting in altered circadian regulation of its targets.
Figure 3: Reduction in fat mass by Noct WT overexpression in male mice.
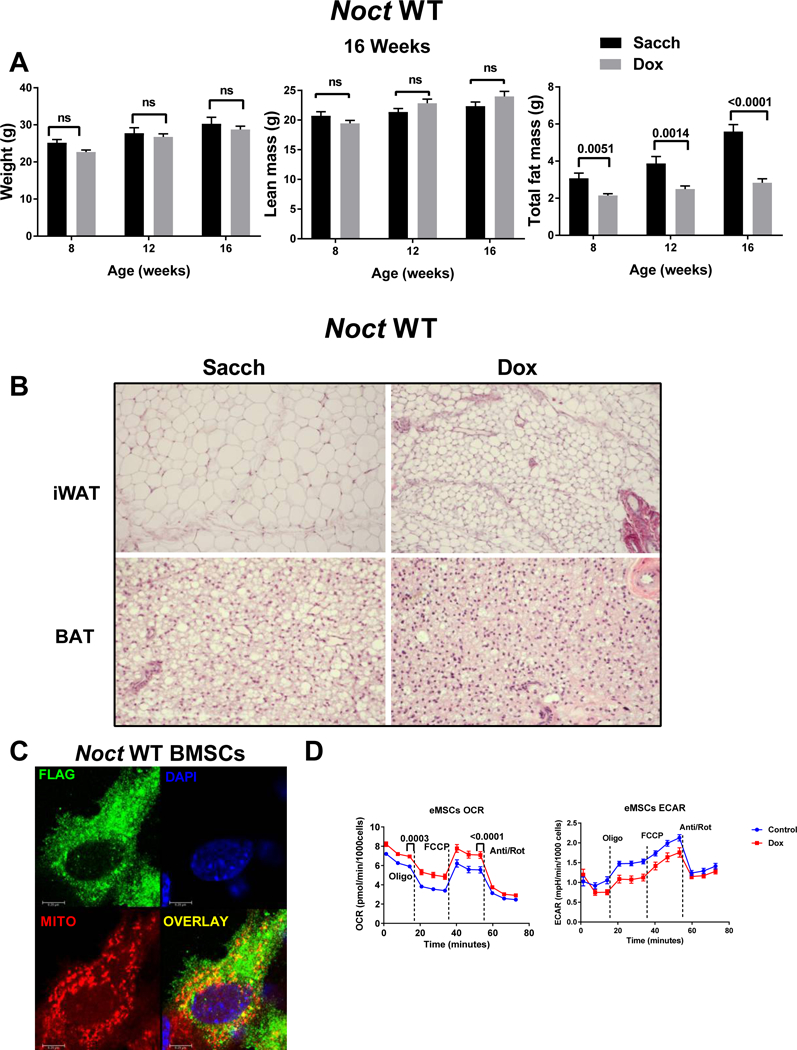
A) Noct WT mice were treated with either Sacch or Dox beginning at 4 weeks and followed till 16 weeks of age (12 weeks on Sacch or Dox). Body composition parameters were measured by DXA at 8, 12, and 16 weeks of age (n=4–12) and body weight, lean mass (fat free mass), and total fat mass are shown. Values represent mean ± SEM. p values are calculated using GraphPad Prism as described in the methods section. B) Histological sections (H&E) of iWAT and BAT from Noct WT mice treated with either Sacch or Dox beginning at 4 weeks and followed till 16 weeks of age (12 weeks on Sacch or Dox). Images were captured using 10X and 20X objective for iWAT and BAT respectively using a Zeiss Axiovert A1 microscope. Images shown are representative of at least three independent samples per group for both iWAT and BAT. C) NOCT-FLAG overexpressed in BMSCs following Dox treatment and stained with FLAG antibody (green), Mitotracker Red (red), and DAPI (blue, nuclear stain). A representative image is shown where the overlay image shows co-localization of Flag-NOCT and mitotracker (yellow). D) Mitochondrial respiration is increased when Noct is overexpressed in eMSCs. Oxygen consumption rate (OCR) in Dox treated cells (red trace) compared to control cells (blue trace) measured by Agilent Extracellular flux analyzer in response to 25 mM glucose, 10 mM pyruvate and 2 mM glutamine as mentioned in the methods section. The data shown is an average of n=43 wells for control and n=34 wells for Dox treated cells. Extracellular acidification rate (ECAR) a surrogate measurement for glycolysis shows that the control cells have an higher oligomycin induced rate compared to the Dox treated cells. Values represent mean ± SEM. p values are calculated using GraphPad Prism using a paired student’s t-test.
Our previous studies showed that Noct−/− mice are protected from Rosi induced bone loss. Though this suggests a role for NOCT and PPARɣ interaction, recent studies have shown Rosi can act as an inhibitor of pyruvate transport into the mitochondria. Mitofate, a mitochondrial pre-sequence and cleavage algorithm, was developed recently and predicts proteins that have a potential mitochondrial localization (Fukasawa et al., 2015). Of 42,217 human proteins, a total of 1847 were predicted to contain the mitochondrial target sequence and 851 were without annotations as mitochondrial proteins (19). We found that human NOCT was predicted to be localized to the mitochondria with a 0.942 probability predication. Similarly, two other mitochondrial target sequence analyzers, MitoprotII (Claros and Vincens, 1996) and TargetP (Emanuelsson et al., 2000) also predicted that both human and mouse NOCT protein sequences have a very high probability of localizing to the mitochondria. Noct has two alternate start sites in its open reading frame, though it is not known which form is transcribed in vivo. To test the mitochondrial co-localization, we utilized bone marrow stromal cells treated with Dox to induce expression of NOCT in vitro and showed that NOCT co-localized with mitotracker red using confocal microscopy (Fig3C). To test if there is a functional consequence to the increase observed in NOCT and its regulation in the mitochondria, we used eMSCs from Noct WT mice treated with Dox and compared oxygen consumption rate on the XF96 analyzer using a mitostress test. Basal respiration and FCCP mediated uncoupled respiration were significantly increased in the Dox treated cells (Fig3D).
Skeletal phenotype of 16 weeks old Noct WT overexpressing mice
To examine the effect of Noct WT overexpression on bone mass, we separated transgenic male mice into two groups and treated them either with Sacch or Dox for 12 weeks starting at 4 weeks of age then assessed their bone density and microarchitecture using DXA and μCT. After 4 and 8 weeks of treatment, there was a significant decrease in aBMD; however, this significant difference in aBMD diminished after 12 weeks of treatment (16 weeks of age) (Fig4A). The decrease seen in aBMD at 8 and 12 weeks of age with no differences in 16 weeks of age with DXA was confirmed by μCT analysis, after 8 weeks of treatment trabecular bone volume fraction in Noct WT overexpressing mice was significantly reduced compared to controls (Supplemental Fig1A) but at 16 weeks there was no differences in both trabecular and cortical bone parameters suggesting a delay in peak bone acquisition with the overexpression of Noct WT (Fig4B and C). Since a previous study identified IGF-1 as a target for the de-adenylase function and IGF-1 has a crucial role in skeletal homeostasis with higher circulating levels being anabolic, we measured serum IGF-1 levels in both the Noct WT and their controls. Though there were trends towards a decrease in IGF-1 levels at 12 weeks and 16 weeks there was no significant difference between groups (supplemental Fig1B). Furthermore, we observed no significant differences in bone formation marker P1NP nor in bone resorption marker CTx in the serum (Supplemental Fig1B).
Figure 4: Overexpression of Noct WT showed no significant changes in both trabecular and cortical bone at 16 weeks with Dox treatment.
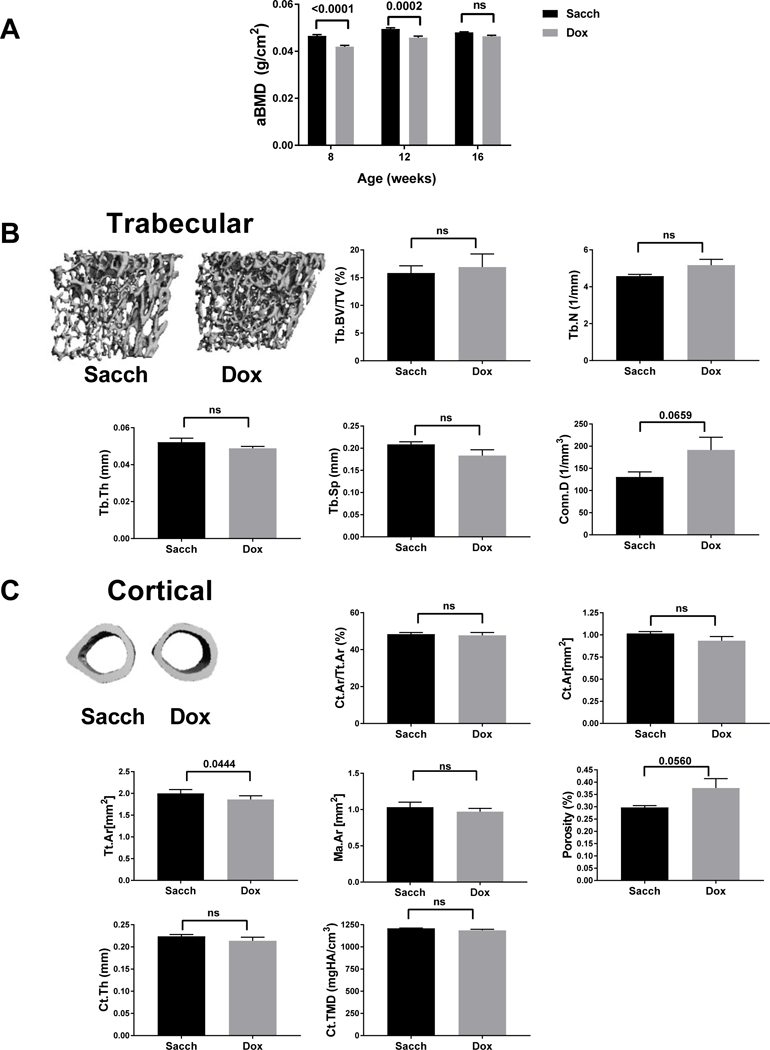
Noct WT mice were treated with either Sacch or Dox beginning at 4 weeks of age and followed till 16 weeks of age. A) Areal Bone mineral density (aBMD) was measured by DXA at 8, 12 and 16 weeks (n=6–12). B) Representative μCT images of trabecular bone (Tb); followed by femoral microarchitecture trabecular parameters measured at 16 weeks. Tb. BV/TV, Tb.Th, Tb.N, Tb.Sp and Conn.D. C) Representative μCT images of cortical bone (Ct.) and femoral microarchitecture cortical parameters measured Ct.Ar/Tt.Ar, Ct.Ar, Tt.Ar, Ma.Ar, Ct.Porosity, Ct.Th and Ct.TMD are shown. Values represent mean ± SEM of n=4–5. p values are calculated as described in methods section for A and using an unpaired students t-test in GraphPad Prism for B,C.
5). Discussion
In this study, we demonstrated that overexpression of Noct impacts body composition and has transient effects on bone mass through both its de-adenylase properties and its mitochondrial actions. The mechanism underlying this effect might be related to the 3’UTR targets of NOCT de-adenylase function. Noct is expressed in several tissues and hence could affect bone mass in a cell autonomous or non-cell autonomous manner when overexpressed. One particularly important target gene that we have previously reported that was regulated by NOCT de-adenylation is Igf-1. We did not observe any differences in circulating levels of IGF-1 when Noct was overexpressed, though there could be a physiological effect of the overall levels observed.
A tight balance between bone formation and resorption maintains skeletal homeostasis. With aging, there is an uncoupling between these two processes leading to a decrease in bone mass and ultimately osteoporosis (Wright et al., 2014). Genes under circadian regulation such as Noct, regulate metabolic processes as well as bone and cartilage development through central and peripheral circadian networks. Loss of brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (Bmal1), a clock related transcriptional factor, has been reported to result in both inhibition of chondrocyte differentiation and a decrease in bone mass (Dudek et al., 2016; Samsa et al., 2016). More recently, deletion of Bmal1 specifically in osteoprogenitors using Osterix-cre (Takarada et al., 2017) suggests that in the absence of Bmal1 there is an increase in RANKL mediated bone resorption resulting in low bone mass.
Noct is regulated in a circadian fashion (Kawai et al., 2010b) and belongs to the EEP family of enzymes. It can regulate the mRNA poly-A tail through its endonuclease activity reducing the stability of the target transcripts (Baggs and Green, 2003). The EEP family includes 2’-phosphodiesterase PDE12, the only currently identified human mitochondrial de-adenylase that can regulate the mRNA poly-A tail of mitochondrial-encoded transcripts (Rorbach et al., 2011). Further evidence implicating NOCT in controlling metabolism was recently shown using a novel poly(A) denylome analysis from Noct−/− liver tissue. It was identified that Noct−/− liver had about 213 transcripts with longer poly-A tails, though whether this is regulated through NOCT’s direct de-adenylase function is not known. Those identified transcripts fall into two major categories: one group involved in the function of ribosomes and another involved in oxidative phosphorylation, further supporting a role for NOCT regulating mitochondrial metabolism (Kojima et al., 2015). Furthermore, Noct was also identified in a genetic screen for genes that suppress the mitochondrial fusion defect observed in dsRNAi Pink1 in Drosophila. In that study, the authors observed that Curled (NOCT) suppresses the mitochondrial fusion defect suggesting that it could be involved in mitochondrial dysfunction mechanisms downstream of the PINK1/PARKIN system (Pogson et al., 2014). Mechanistically, we identified a mitochondrial localization sequence in the NOCT protein, and showed that in vitro NOCT can co-localize with the mitochondria.
In respect to the marked changes in fat mass, we noted that the overexpressing Noct WT showed a marked reduction in fat mass at 16 weeks of age. The decrease in fat mass observed in Noct WT treated with Dox was contrary to what was reported by the resistance to high fat diet induced obesity observed in Noct−/− mice published previously (Green et al., 2007). Conversely, the B6 control animals on Dox gained weight compared to age matched Sacch controls. Although previously we showed that NOCT could regulate PPARɣ transport into the nucleus, the current data suggest that it might play another role in regulating adipogenesis; i.e. via its mediation of mitochondrial function. Indeed, we showed that overexpression of Noct increases oxidative phosphorylation in a cell autonomous manner. Adipocytes require optimal mitochondrial respiration for differentiation; hence this may be one potential mechanism (Zhang et al., 2013) though it is not yet clear what exact role Noct overexpression has on adipocyte respiration.
There are some limitations to this study. First, we induced Noct expression using a ROSA26-rtTA, which leads to overexpression globally. Since we observed that Noct−/− animals have dysfunctional brown adipose tissue (BAT) (Kawai et al., 2010b), we cannot rule out the possibility that some of the effects of Noct overexpression could be mediated through enhanced sympathetic nerve system (SNS). Previous work from our lab had established a link between BAT dysfunction and bone mass through sympathetic tone such that the skeletal effects of Noct overexpression may in part be mediated through other pathways (Motyl et al., 2013). Therefore, the mouse models described in this study will be valuable tools to delineate the central versus peripheral regulation of circadian bone mass. In support of the notion that there could be SNS mediated effects we observed a decrease in both BAT and iWAT adipocyte size when Noct was overexpressed in the Noct WT animals. Second, the full effect of Noct on mitochondrial respiration needs further study since there are likely to be direct and indirect effects, in the preadipocyte cells we observed an increase in oxidative phosphorylation when we overexpressed Noct. Contrary to what was observed in the Noct null animals on a high fat diet, we observe a significant decrease in fat mass and adipocyte size (inguinal WAT and BAT) when we constitutively overexpress Noct suggesting that there could be tissue specific roles. A recent study reported on the critical role for Noct in regulating metabolic amplitude in response to nutrients in a circadian manner (Stubblefield.,2018). Additionally, it is not known if Noct is translated from an alternative start site that could lead to the endogenous expression of Noct devoid of the mitochondrial target sequence. Furthermore, we did not study the localization to the mitochondria of Noct WT transgene in vivo and therefore cannot exclude the possibility of the transgenes expression being altered because of the nature of the constructs lacking the 5’ UTR. Third, we cannot exclude the possibility that Noct regulates mitochondrial respiration through its other actions on PPARɣ, a master transcriptional factor. Fourth, we still do not know the direct targets of Noct’s de-adenylase activity making it difficult to follow up on the effects of overexpression on gene targets.
Finally, there are caveats to Dox-inducible transgenic studies; importantly the potential for Sacch or/and Dox to affect global metabolism and skeletal remodeling, independent of transgene expression. Recently, Parlee et al have shown that Sacch treatment of neonates before weaning leads to an increase in total trabecular and cortical bone parameters in male mice (Parlee et al., 2014). In this same study, the authors also observed a decrease in fat mass in male mice. The long-term treatment of male DBA/2J mice with Dox (10 weeks) in a study by Fowlkes et al also showed that there is no detrimental effect of this treatment on the microarchitecture of the bone (Fowlkes et al., 2015). Our data (Fig 2) on B6 mice show that long-term Dox treatment also did not cause any changes in trabecular and cortical bone parameters, but resulted in a significant increase in fat mass after 8 and 12 weeks of treatment. This set of data is crucial based on a recent study which suggests that the use of tetracycline especially doxycycline can directly induce mitochondrial proteotoxic stress (Moullan et al.). Therefore, our data with regards to the transgenic line suggest that the changes in bone mass and fat mass that we observe are independent of Sacch and Dox treatment, though the time, mode and duration of treatment are different.
In summary, we present an overexpression mouse model suggesting that Noct is crucial for maintaining metabolic homeostasis. Constitutively increased expression of Noct, wildtype form, leads to lower fat mass and transiently disrupts bone mass. Our data provide further evidence for the important interaction between metabolic status and bone mass, particularly through circadian related genes such as Nocturnin.
Supplementary Material
S Fig1 A) Noct WT mice were treated with either Sacch or Dox beginning at 4 weeks of age and followed till 12 weeks of age. Representative μCT images of trabecular bone (Tb.) followed by femoral microarchitecture trabecular parameters measured at 12 weeks for Tb.BV/TV. B) Serum IGF-1 levels, bone formation P1NP marker (N-terminus propeptide of type I collagen), and bone resorption CTx marker (c-terminus telopeptide of type I collagen) were measured using ELISAs in Noct WT (12 weeks, n=3–7, 16 weeks n=6–8).
Acknowledgements
The authors thank Terry Henderson for technical assistance, and members of the Rosen lab for critical review of the manuscript. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R03AR068095 to GAR and NIH P50 MH074924, the Silvio O. Conte Center for Neuroscience Research to JST and CBG. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by the Maine Medical Center Research Institute, Physiology Core which is supported by NIH COBRE in Stem and Progenitor Cell Biology and Regenerative Medicine, P30GM106391 (R. Friesel, PI) and the COBRE in Metabolic Networks P20GM121301 (L. Liaw, PI).
Footnotes
Data Sharing
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure Statement: The authors have nothing to disclose
References
- Baggs JE, Green CB. 2003. Nocturnin, a deadenylase in Xenopus laevis retina: a mechanism for posttranscriptional control of circadian-related mRNA. Current biology : CB 13(3):189–198. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, DeMambro VE, Guntur AR, Le P, Nagano K, Baron R, de Paula FJA, Motyl KJ. 2018. High fat diet attenuates hyperglycemia, body composition changes, and bone loss in male streptozotocin-induced type 1 diabetic mice. Journal of cellular physiology 233(2):1585–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claros MG, Vincens P. 1996. Computational method to predict mitochondrially imported proteins and their targeting sequences. European journal of biochemistry 241(3):779–786. [DOI] [PubMed] [Google Scholar]
- Dello Russo C, Gavrilyuk V, Weinberg G, Almeida A, Bolanos JP, Palmer J, Pelligrino D, Galea E, Feinstein DL. 2003. Peroxisome proliferator-activated receptor gamma thiazolidinedione agonists increase glucose metabolism in astrocytes. The Journal of biological chemistry 278(8):5828–5836. [DOI] [PubMed] [Google Scholar]
- DeMambro VE, Le PT, Guntur AR, Maridas DE, Canalis E, Nagano K, Baron R, Clemmons DR, Rosen CJ. 2015. Igfbp2 Deletion in Ovariectomized Mice Enhances Energy Expenditure but Accelerates Bone Loss. Endocrinology 156(11):4129–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, Henry RR, McDonald WG, Colca JR, Simon MI, Ciaraldi TP, Murphy AN. 2013. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proceedings of the National Academy of Sciences of the United States of America 110(14):5422–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Kojima S, Pan X, Lerch-Gaggl AF, Duong SQ, Hussain MM, Green CB. 2011. Nocturnin regulates circadian trafficking of dietary lipid in intestinal enterocytes. Current biology : CB 21(16):1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek M, Gossan N, Yang N, Im H-J, Ruckshanthi JPD, Yoshitane H, Li X, Jin D, Wang P, Boudiffa M, Bellantuono I, Fukada Y, Boot-Handford RP, Meng Q-J. 2016. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. The Journal of clinical investigation 126(1):365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E TA, Chasseur J, Bohn JA, Del Rizzo PA, Freddolino PL, Goldstrohm AC, Trievel RC. 2018. The structure of human Nocturnin reveals a conserved ribonuclease domain that represses target transcript translation and abundance in cells. Nucleic acids research 46(12):6257–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of molecular biology 300(4):1005–1016. [DOI] [PubMed] [Google Scholar]
- Estrella MA, Du J, Korennykh A. 2018. Crystal Structure of Human Nocturnin Catalytic Domain. Sci Rep 8(1):16294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes JL, Nyman JS, Bunn RC, Cockrell GE, Wahl EC, Rettiganti MR, Lumpkin CK Jr., Thrailkill KM. 2015. Effects of long-term doxycycline on bone quality and strength in diabetic male DBA/2J mice. Bone reports 1:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa Y, Tsuji J, Fu SC, Tomii K, Horton P, Imai K. 2015. MitoFates: improved prediction of mitochondrial targeting sequences and their cleavage sites. Molecular & cellular proteomics : MCP 14(4):1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MR, Douris N, Tongjai S, Green CB. 2011. Nocturnin expression is induced by fasting in the white adipose tissue of restricted fed mice. PloS one 6(2):e17051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Besharse JC. 1996. Identification of a novel vertebrate circadian clock-regulated gene encoding the protein nocturnin. Proceedings of the National Academy of Sciences of the United States of America 93(25):14884–14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Douris N, Kojima S, Strayer CA, Fogerty J, Lourim D, Keller SR, Besharse JC. 2007. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America 104(23):9888–9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke S, Bickmeyer I, Wunderlich R, Jackle H, Kuhnlein RP. 2009. Curled encodes the Drosophila homolog of the vertebrate circadian deadenylase Nocturnin. Genetics 183(1):219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntur AR, Gerencser AA, Le PT, DeMambro VE, Bornstein SA, Mookerjee SA, Maridas DE, Clemmons DE, Brand MD, Rosen CJ. 2018. Osteoblast-like MC3T3-E1 Cells Prefer Glycolysis for ATP Production but Adipocyte-like 3T3-L1 Cells Prefer Oxidative Phosphorylation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntur AR, Kawai M, Le P, Bouxsein ML, Bornstein S, Green CB, Rosen CJ. 2011a. An essential role for the circadian-regulated gene nocturnin in osteogenesis: the importance of local timekeeping in skeletal homeostasis. Annals of the New York Academy of Sciences 1237:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntur AR, Le PT, Farber CR, Rosen CJ. 2014. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology 155(5):1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntur AR, Reinhold MI, Cuellar J Jr., Naski MC. 2011b. Conditional ablation of Pten in osteoprogenitors stimulates FGF signaling. Development (Cambridge, England) 138(7):1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HK, Chong JL, Song W, Song EJ, Jyawook AA, Schook AC, Ko CH, Takahashi JS. 2007. Inducible and reversible Clock gene expression in brain using the tTA system for the study of circadian behavior. PLoS Genet 3(2):e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Delany AM, Green CB, Adamo ML, Rosen CJ. 2010a. Nocturnin suppresses igf1 expression in bone by targeting the 3’ untranslated region of igf1 mRNA. Endocrinology 151(10):4861–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Green CB, Lecka-Czernik B, Douris N, Gilbert MR, Kojima S, Ackert-Bicknell C, Garg N, Horowitz MC, Adamo ML, Clemmons DR, Rosen CJ. 2010b. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation. Proceedings of the National Academy of Sciences of the United States of America 107(23):10508–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Gendreau KL, Sher-Chen EL, Gao P, Green CB. 2015. Changes in poly(A) tail length dynamics from the loss of the circadian deadenylase Nocturnin. Scientific reports 5:17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridas DE, DeMambro VE, Le PT, Mohan S, Rosen CJ. 2017. IGFBP4 Is Required for Adipogenesis and Influences the Distribution of Adipose Depots. Endocrinology 158(10):3488–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl KJ, Bishop KA, DeMambro VE, Bornstein SA, Le P, Kawai M, Lotinun S, Horowitz MC, Baron R, Bouxsein ML, Rosen CJ. 2013. Altered thermogenesis and impaired bone remodeling in Misty mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 28(9):1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moullan N, Mouchiroud L, Wang X, Ryu D, Williams Evan G, Mottis A, Jovaisaite V, Frochaux Michael V, Quiros Pedro M, Deplancke B, Houtkooper Riekelt H, Auwerx J. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell reports 10(10):1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WE, Wang X, Grebenjuk VA, Korzhev M, Wiens M, Schlossmacher U, Schroder HC. 2012. Nocturnin in the demosponge Suberites domuncula: a potential circadian clock protein controlling glycogenin synthesis in sponges. The Biochemical journal 448(2):233–242. [DOI] [PubMed] [Google Scholar]
- Parlee SD, Simon BR, Scheller EL, Alejandro EU, Learman BS, Krishnan V, Bernal-Mizrachi E, MacDougald OA. 2014. Administration of saccharin to neonatal mice influences body composition of adult males and reduces body weight of females. Endocrinology 155(4):1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson JH, Ivatt RM, Sanchez-Martinez A, Tufi R, Wilson E, Mortiboys H, Whitworth AJ. 2014. The complex I subunit NDUFA10 selectively rescues Drosophila pink1 mutants through a mechanism independent of mitophagy. PLoS genetics 10(11):e1004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorbach J, Nicholls TJ, Minczuk M. 2011. PDE12 removes mitochondrial RNA poly(A) tails and controls translation in human mitochondria. Nucleic acids research 39(17):7750–7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsa WE, Vasanji A, Midura RJ, Kondratov RV. 2016. Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone 84:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarada T, Xu C, Ochi H, Nakazato R, Yamada D, Nakamura S, Kodama A, Shimba S, Mieda M, Fukasawa K, Ozaki K, Iezaki T, Fujikawa K, Yoneda Y, Numano R, Hida A, Tei H, Takeda S, Hinoi E. 2017. Bone Resorption Is Regulated by Circadian Clock in Osteoblasts. Journal of Bone and Mineral Research 32(4):872–881. [DOI] [PubMed] [Google Scholar]
- Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. 2014. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 29(11):2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Marsboom G, Toth PT, Rehman J. 2013. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PloS one 8(10):e77077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S Fig1 A) Noct WT mice were treated with either Sacch or Dox beginning at 4 weeks of age and followed till 12 weeks of age. Representative μCT images of trabecular bone (Tb.) followed by femoral microarchitecture trabecular parameters measured at 12 weeks for Tb.BV/TV. B) Serum IGF-1 levels, bone formation P1NP marker (N-terminus propeptide of type I collagen), and bone resorption CTx marker (c-terminus telopeptide of type I collagen) were measured using ELISAs in Noct WT (12 weeks, n=3–7, 16 weeks n=6–8).


