Abstract
Antibody-mediated rejection continues to hinder long-term survival of solid organ allografts. Natural antibodies (Nabs) with polyreactive and autoreactive properties have recently emerged as potential contributors to antibody-mediated graft rejection. This review discusses Nabs, their functions in health and disease, their significance in rejection following kidney, heart and lung transplantation and their implication in serum reactivity to key antigens associated with rejection. Finally, potential Nabs effector mechanisms in the context of transplantation are explored.
INTRODUCTION
Solid organ transplantation is often the only recourse for end-stage organ failure. However, the limited survival of transplanted organs, especially in the long-term, still poses a challenge. Recent data obtained from the nationwide Organ Procurement and Transplantation Network indicate that the one-year survival rate is approximately 90% following kidney transplantation although this number drops to roughly 76% after 5 years. For other organs, such as for lung transplants, the outcome can be far worse with survival rates of approximately 50% after 5 years posttransplant.
One of the main barriers to the longevity of transplanted organs is the development of immune responses directed against the allograft. Both the cellular and humoral arms of the immune system are implicated. On the whole, cellular responses are now well controlled by improved immunosuppressive drugs. The humoral arm, however, is still poorly responsive to immunosuppression and continues to impact long-term outcomes through a complication known as antibody-mediated rejection (AMR).
Antibodies targeting the allogeneic donor graft are thought to contribute to greater than 60% of late failure in solid organs transplants.1 These are predominantly donor-specific human leukocyte antigen (HLA) antibodies (DSA) that can either predate or develop after transplantation (de novo DSA). Antibody-mediated rejection can also occur in the absence of donor-specific antibodies and despite negative B- and T-cell crossmatches,2–5 indicating that other types of antibodies are implicated in humoral rejection. Earlier studies in kidney6 and cardiac rejection patients7 suggested the presence of antibodies that were reactive to antigenic structures distinct from HLA on the graft vascular endothelium. More recently, several of these structures have been identified. Non-HLA antibodies typically target autoantigens expressed on graft donor cells including major histocompatibility complex class I chain-related molecule A (MICA),8 angiotensin II type 1 receptor (AT1R),9 endothelin-1 type A receptors,10 vimentin11 and cardiac myosin.12 These non-HLA antibodies have been attributed pathogenic potential in the context of kidney, heart and lung transplantation.
Often included in the group of non-HLA antibodies are “natural” antibodies (Nabs), however, their unique characteristics largely differ from that of specific autoantibodies. Most notable Nabs include antibodies to ABO blood group antigens or xenoantigens such as α-(1,3)-galactose (α-Gal) and N-glycolylneuraminic acid (Neu5Gc) and are responsible for hyperacute rejection in the context of ABO incompatibility or xenotransplantation, respectively. More recently, studies from our group revealed the development of Nabs following ABO-compatible allotransplantation. However, their impact on the outcomes of transplantation is still unclear. This review will summarize the current evidence supporting a role for Nabs in mechanisms of rejection and identify key areas of uncertainty in need of further investigation.
NATURAL ANTIBODIES AND POLYREACTIVITY
Nabs were recognized over 50 years ago as a group of immunoglobulins implicated in innate defense and homeostasis in humans and other mammalian species.13,14 They are of IgM, IgG and IgA isotypes and their repertoire profiles within an individual are relatively consistent over time.15,16 Most Nabs are polyreactive,14,17 meaning that they are capable of binding to multiple and apparently unrelated antigenic structures. Their inherent polyreactive properties enable them to carry out important functions in health and disease.18,19
Antigenic determinants of Nabs shape their roles in health and disease
Polyreactive Nabs have been reported to maintain healthy host tissue by binding to structures on apoptotic and senescent cells and facilitating their removal, thereby preventing unwanted inflammation. They also bind to various bacterial antigens including phosphorylcholine and lipopolysaccharide (LPS)19–21 and viral proteins such as hemagglutinin,22 forming a first-line defense mechanism against pathogens. Nabs are also able to control the damaging effects of oxidative stress, a consequence of normal cellular processes, by recognizing oxidized epitopes on low-density lipoprotein.23 As such, they are protective against atherosclerosis, a chronic inflammatory disease of the vascular wall.
In contrast, Nabs are also implicated in autoimmune and inflammatory reactions. In lupus, polyreactive DNA-binding antibodies may also react to the N-methyl-D-aspartate receptor.24 Reactivity to DNA deposited in glomeruli25,26 and N-methyl-D-aspartate receptor expressed in neurons24 leads to target cell injury and mediates nephritis and central nervous system disease in lupus, respectively.
Detection of Nabs
Polyreactive Nabs are difficult to define based on their reactivity profile. Perhaps the most direct way to appreciate the breadth of such reactivity is by studying monoclonal polyreactive antibodies. We and others have generated a number of clones from healthy donor blood that bind to multiple targets including LPS, double-stranded and single-stranded DNA, insulin, undefined intracellular structures in Hep-2 cells, apoptotic cells, the oxidation-specific epitope (OSE) malondialdehyde (MDA) as well as numerous proteins in a HEK293 epithelial cell lysate. Monoclonal Nabs generated from solid organ recipient blood or graft infiltrates can also exhibit similar polyreactivity.27,28 One important observation, however, is that no structure appears to be recognized by all monoclonal Nabs. In itself, this broad binding profile and absence of a universally shared target antigen is an obstacle for designing tests to identify all Nabs in clinical specimens.
Conventional methods of identifying polyreactive Nabs use immunoassays to assess reactivity to common autoantigens such as DNA, LPS or insulin.29,30 Monoclonal antibodies binding to multiple antigens are considered polyreactive. Another approach takes advantage of the known reactivity of Nabs to OSEs generated on self-molecules by highly reactive lipid degradation products.31 Studies have used OSE-modified proteins, such as MDA-modified bovine serum albumin, in immunoassays in heart and kidney transplant to detect Nabs.28,32,33 In a similar vein, researchers have measured binding to apoptotic cells as a means to detect Nabs in the transplantation setting.27,34 Another study used a synthetic molecule, dinitrophenol, in their detection assays reasoning that as this molecule is not present in the environment serum antibodies reacting to it must be polyreactive.35
Generation of Nabs
Most of the information on the generation of Nabs comes from mouse studies where B1 B cells from the peritoneal and pleural cavities are the main source. These innate-like cells are present at birth, are self-replenishing, show restricted immunoglobulin heavy chain variable usage and mainly produce germline IgM36 although mutated Nabs have also been observed.37 Subsequent studies indicated that splenic marginal zone B cells and bone marrow precursors also contribute to Nabs generation.38,39
In humans, the presence of IgM,40,41 IgG16,42,43 and IgA44 Nabs is established.31,45 Both germline and somatically mutated sequences coding for polyreactive Nabs have been described.46,47 The exact source of Nabs has not yet been unequivocally identified, however, B cells that produce polyreactive antibodies and express polyreactive receptors are highly abundant in circulation30,48,49 and in the gut mucosa.50,51
Nabs levels can fluctuate depending on age, disease status, and trauma.16,52 Nabs-producing B cells are likely to be stimulated to secrete antibody through B-cell receptor (BCR)-dependent and -independent mechanisms.35,39,43,53 While IgM Nabs are abundant at the steady-state, we have shown IgG Nabs to be elevated in chronic kidney graft rejection and following ventricular assist device implantation in heart transplant recipients.27,32 A possible scenario is that innate-like B cells producing IgM Nabs in normal conditions would undergo class switching to produce IgG Nabs following antigen encounter or in inflammatory situations.
EFFECTOR AND IMMUNOREGULATORY FUNCTIONS OF NABS IN HEALTH AND DISEASE
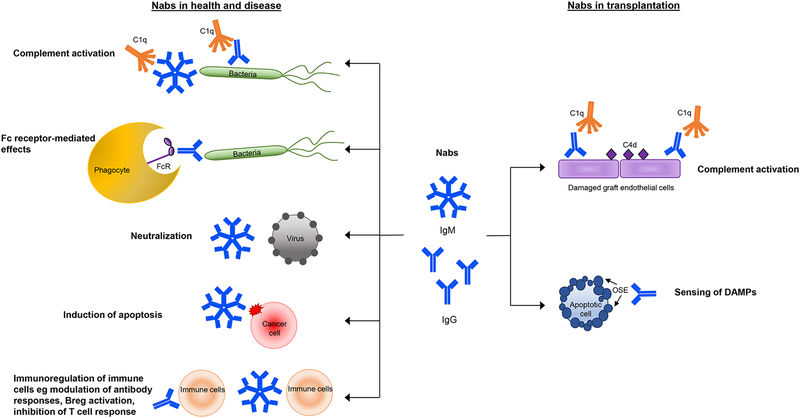
The protective role of Nabs against pathogens, inflammation and autoimmunity is well-documented. Nabs recognize pathogen-associated molecular patterns to exert antibacterial19,54 and antiviral responses.22 IgM Nabs protect against unwanted inflammation, such as in atherosclerosis and autoimmunity, by binding to and preventing the accumulation and subsequent immune responses to apoptotic cells55 and harmful cell debris.56 Nabs may also block pathogenic autoantibodies57 and modulate antibody responses. Conversely, the same Nabs effector mechanisms may also contribute to inflammatory responses in pathogenic autoimmune conditions. Below are a few key Nabs functions in health and disease that could also point to their role after solid organ transplantation (Figure 1).
Figure 1.
Effector and immunoregulatory functions of Nabs in health, disease and transplantation
Complement-dependent mechanisms
Activation of the complement component C1q leads to downstream deposition of C3b on target cells. C3b serves as a signal that promotes the efficient ingestion of opsonized cells by phagocytes. Complement activation can also result in the formation of the membrane attack complex and subsequent lysis of target cells. The binding of Nabs to apoptotic cells55,58,59 or pathogens22,60 recruits C1q and triggers complement activation, leading to their clearance. Mice that are deficient in Nabs exhibit defective apoptotic cell clearance by macrophages31 and dendritic cells61 and are more susceptible to infection.22 These complement-dependent functions can also be pathogenic in conditions conducive to acute tissue damage, such as ischemia reperfusion or spinal cord injury, where the exposure of injury-associated neoantigens are recognized by Nabs to mediate complement-driven damage.62–64
Fc receptor-mediated mechanisms
Nabs are also able to mediate their effects via Fc receptor interactions. Nabs recognizing bacteria opsonized by serum lectins were shown to co-localize with monocyte Fc receptors.54 In a Brucella abortis infection model, passive transfer of Nabs to mice lacking B cells and antibodies contributed to the eliminate the bacteria.65 This protective effect of Nabs was mediated by Fc-receptor signaling as mice deficient in Fcγ receptors demonstrated enhanced bacterial persistence.65
Induction of apoptosis and virus neutralization
In cancer, Nabs recognizing modified carbohydrate structures on malignant cells have anti-tumor reactivity and have been implicated in tumor surveillance. These aberrant oligosaccharides are found on tumor cell glycoproteins such as mucins,66 decay acceleration factor67 as well as glycolipids (reviewed in68). Several such Nabs isolated from patients with different cancers were capable of inducing apoptotic69,70 or necrotic signaling in tumor cells.71 A direct action of Nabs in viral protection is also evident as Nabs are capable of binding and directly neutralizing viruses such as influenza72,73 and HIV in certain circumstances.74,75
Nabs modulate antibody responses
Nabs also exhibit modulatory properties and influence the development of B-cell immunity. For instance, Nabs can enhance IgG responses against the T cell-dependent antigen, keyhole limpet hemocyanin (KLH). Mice deficient in secreted IgM were shown to produce less KLH-specific IgG and these IgG were of lower affinity.76 Similarly, Nabs have been shown to play a part in mouse protective IgG responses against viral infection.77 In these latter studies, the absence of IgM Nabs abrogated antiviral IgG. In contrast, Nabs can also regulate B-cell development and the generation of autoimmunity. Mice that lacked soluble IgM Nabs had elevated IgG autoantibodies to double stranded (ds) DNA, single stranded DNA and nuclear antigens78 and their B2 cells displayed altered development and BCR signaling.79,80 Additionally, the cells that produce Nabs themselves exert immunomodulatory functions. In recent years, a population of B cells with regulatory properties termed B10 B cells or Breg has been identified. In mice, these cells appear to share similarities with the innate-like Nabs-producing B1 B cell population. These cells produce the anti-inflammatory cytokine IL-1081,82 and express polyreactive BCR.83 Following appropriate stimulation, they can produce polyreactive antibodies akin to Nabs.83
ASSOCIATION BETWEEN NABS AND TRANSPLANT REJECTION
Antibodies to blood group antigens and xenoantigens have long been known to promote harmful rejection and precipitate graft loss of ABO-incompatible as well as certain cross-species transplants. These humoral barriers to transplantation are due to natural antibodies targeting carbohydrate structures on the transplanted organs. Early studies demonstrated that xenoreactive antibodies are by nature polyreactive.84 The same appears to be true for blood group-specific antibodies, over 80% of which display polyreactivity.85 Conversely, later studies showed that polyreactive Nabs can also be xenoreactive,86 further supporting the idea that xenoantibodies, ABO antibodies and Nabs belong to the same category of antibodies.
Beyond xenotransplantation and ABO incompatibility, polyreactive Nabs were also investigated in the context of human allogeneic transplantation. We highlight here the most recent studies implicating Nabs in rejection of kidney, heart and lung allografts.
Kidney
Among the earliest reports hinting at a possible contribution of polyreactive antibodies to rejection are studies using protein microarrays to assess the reactivity profile of sera from patients undergoing chronic AMR of kidney allografts. These studies revealed a marked increase in serum reactivity to numerous self-antigens in the rejectors compared to nonrejectors.87 The breadth of the reactivity profiles was unexpected and puzzling as it suggested the development of a significant autoimmune response to self-antigens in these patients. B-cell clones were subsequently generated from the blood of patients experiencing chronic rejection. Some of the clones secreted polyreactive monoclonal antibodies binding to dsDNA, LPS, insulin88 as well as apoptotic cells.27 These polyreactive monoclonal antibodies also reacted to multiple targets on protein microarrays (manuscript in preparation), suggesting that the broad serum reactivity profiles observed in patients with chronic AMR87 could be due to polyreactive Nabs rather than a multitude of monospecific antibodies. A later study by our group correlated higher levels of pretransplant Nabs with worse kidney graft survival in a single-center cohort of 300 patients.34 These polyreactive antibodies were identified as almost exclusively IgG1 and IgG3 and demonstrated the ability to activate complement, resulting in C4d deposition in vitro. These latter findings pointed to their potential in vivo capabilities. A subsequent blinded study investigated Nabs reacting to the OSE MDA in both pretransplant and posttransplant serum samples obtained from a cohort of over 600 well-characterized kidney transplant patients. Posttransplant sera were either protocol samples obtained at one year or for-cause samples collected within the first year. A significant association was found between the development of Nabs in the first-year posttransplant period and graft loss.33 This association was independent of DSA as revealed by multivariable analysis. Strikingly, Nabs generation observed in the year following transplant was also associated with higher grades of Banff-defined histological lesions specific to AMR, including C4d deposition, microvascular inflammation, transplant glomerulopathy, interstitial inflammation and tubulitis and arteriosclerosis.33 Lastly, the overall outcomes were worse in patients with both Nabs and DSA, suggesting an additive detrimental effect of these two types of antibodies on kidney graft survival.
Heart
Cardiac allograft vasculopathy (CAV), characterized by intimal thickening and lumen narrowing of the main coronary arteries, is a major cause of graft loss following heart transplantation. CAV has been associated with intra-graft immune infiltrates28,89–91 containing B- and T-cell clusters together with macrophages and antibody-secreting plasma cells. The plasma cells in these clusters actively secreted IgG and, more rarely, IgM.28,91 Studies by Huibers et al. reported that some of these cells produce DSA in the context of human CAV,92 suggesting a role in local alloresponses. Chatterjee et al. further interrogated the reactivity profile of graft-infiltrating B cells by generating over 100 EBV-immortalized B-cell clones from three explanted heart grafts with CAV.28 No HLA-reactive clones were found in these studies. However, in all cases, approximately half of the clones were polyreactive, i.e. reactive to apoptotic cells, MDA, insulin, dsDNA, LPS and cardiolipin.28 These results suggest an intriguing role for innate B cells and locally secreted polyreactive antibodies in CAV pathogenesis. While their exact functions are currently unknown, it is possible that innate B cells and Nabs promote innate immune responses involving the activation of M2 macrophages. As B cell clusters are also detected during chronic rejection in other organs, it is possible that polyreactive clones and Nabs secreted in situ may also be important in these situations.
The development of Nabs recognizing apoptotic cells and OSE has also been reported following ventricular assist device implant in patients bridged to transplant.32 In this study, elevated Nabs levels at time of transplant were associated with primary graft dysfunction, a serious complication of unclear etiology that represents the leading cause of mortality in the first month following transplant. These findings suggest that very early complications following heart transplant may also be connected to Nabs or to the inflammatory processes that lead to the production of these antibodies.
Lung
In lung transplants, both alloimmunity and autoimmunity, particularly to k-α-tubulin and collagen V, have been implicated in rejection and chronic graft dysfunction.93–95 The involvement of Nabs however, is not so clear. One report looked at pretransplant polyreactive antibodies targeting different types of apoptotic cells.96 The authors found that polyreactive antibodies were elevated in patients with end-stage lung disease and remained high during posttransplant follow-up. However, no correlation to chronic or acute rejection was observed in this study. The discrepant findings between lung and kidney transplant rejection may reflect differences in rejection mechanisms. Alternatively, since most end-stage lung disease patients already had high Nabs levels pretransplant it may be difficult to find an association between these levels and transplant outcome.
POLYREACTIVE ANTIBODIES AND CROSS-REACTIVITY TO IMPORTANT TRANSPLANT ANTIGENS
Studies described in the previous section report situations where Nabs and Nabs-producing innate B cells were directly associated with rejection episodes and graft loss. However, their implication in posttransplant immune reactions may be far broader than what our current knowledge indicates. Below are two instances suggesting that polyreactive Nabs can be responsible for certain serum reactivity wrongly attributed to antigen-specific antibodies. If confirmed, these examples would support the idea that Nabs are more common than initially appreciated but are not always detected as such.
HLA
Advances in laboratory testing methods now allow for the highly sensitive assessment of serum reactivity to specific HLA antigens. Such high sensitivity gives rise to the detection of nonspecific97 or cross-reactive antibodies to nondonor specific antigens, presumably due to shared epitopes between different HLA.98 A recent study by Gao et al, (2016) puts forward the reasoning that polyreactive antibodies could also contribute to serum reactivity to HLA. The authors examined monoclonal Nabs secreted by B-cell clones derived from transplant recipients for binding to self-antigens and HLA.99 Some polyreactive monoclonal Nabs that bound to multiple self-antigens or structures including LPS, dsDNA, insulin, apoptotic cells and HEK293 cell lysate, also recognized multiple HLA class I antigens coated onto Luminex beads routinely used in immunogenetics laboratories. Moreover, reactivity to HLA could be reduced in some serum samples, especially those with high PRA, by adsorption with apoptotic cells, indicating cross-reactivity between HLA on the beads and apoptotic cell determinants.99 These findings strongly suggested that polyreactive Nabs can account for part of serum reactivity to HLA initially attributed to specific antibodies.
AT1R
Preexisting and de novo antibodies to AT1R on endothelial cells are implicated in destructive non-HLA responses against kidney allografts.9 Several groups have correlated these antibodies with increased risk of rejection after kidney,100–102 heart103 and lung transplantation.104 Angiotensin II type 1 receptor antibodies directly affect endothelial cells and smooth muscle cells via extracellular signal-regulated kinase 1/2 signaling, leading to increased binding of nuclear factor κB and proinflammatory chemokine expression.9 These antibodies also appear to act in synergy with DSA.103 Ventricular assist device support prior to heart transplant significantly increased AT1R antibody levels.105 Intriguingly, a recent study using cardiac transplant recipient and healthy donor serum revealed the extent of cross-reactivity between anti-AT1R antibodies, xenoantibodies to Neu5Gc or heterophile antibodies one could also classify as Nabs. These observations raise the possibility that common methods to test for AT1R antibodies may also detect cross-reactive Nabs.106
POSSIBLE NABS EFFECTOR MECHANISMS AND CONCLUDING REMARKS
Collectively, the studies presented above provided converging evidence supporting a role for Nabs in the outcome of solid organ transplants. These studies, however, have not demonstrated a causal link between Nabs and rejection. Several possible effector mechanisms could be envisaged whereby Nabs could influence immune reactions following transplantation and impact on graft survival. Below, we put forward the two most likely mechanisms (Figure 1).
Complement activation
Complement activation is a potential mechanism of Nabs-mediated graft damage. As shown previously, monoclonal Nabs produced by B-cell clones derived from kidney transplant recipients’ specimens have the capacity to activate the complement cascade in vitro resulting in C3d and C4d deposition on target cells.27 Moreover, studies in heart and kidney transplants showed that serum IgG Nabs are predominantly IgG1 and IgG3, the two main complement-activating IgG subclasses.33,34 These polyclonal Nabs could also activate complement in vitro.34 Another observation connecting Nabs and complement comes from a recent study by See et al. Following kidney transplantation, patients who developed Nabs in the first year had increased C4d deposition in biopsies compared to those who did not develop Nabs, even in the absence of DSA.33 These studies uncover Nabs as plausible contributors to complement activation and graft injury.
Sensing ligands from damaged allografts
Danger-associated molecular patterns (DAMPs) are markers of “sterile” tissue injury or cell stress that are recognized by pattern recognition receptors (PRR), the best known being Toll-like receptors on innate immune cells. The ability to sense these danger signals are critical for responses to damaged self.107 The relevance of DAMPs in solid organ transplantation has emerged in recent years, with increasing evidence that immune responses mediated by DAMPS can impact graft inflammation, fibrosis and alloimmunity.108 Damage to the allograft, from ischemia and reperfusion injury to alloimmune responses, results in the release of DAMPs.109 Both animal and clinical studies showed enhanced levels of DAMPs-related elements such as nucleic acids,110,111 high mobility group box 1 protein112 and extracellular components113,114 in kidney, heart and lung transplants. These DAMPS trigger signaling downstream of PRRs and facilitate allograft injury by promoting alloreactive adaptive responses,109,115 fibrosis and up-regulation of major histocompatibility complex expression.116,117
Since DAMPs include a broad range of molecules that are known Nabs targets, including DNA and modified biological molecules such as oxidation epitopes,118 Nabs may be considered as soluble PRRs. It is plausible that Nabs recognize and bind DAMPs released from stressed graft endothelium and amplify their potency by opsonizing them with complement molecules or addressing them to Fc-expressing innate immune cells. Furthermore, graft DAMPs may also activate infiltrating innate B cells through their polyreactive BCR leading to Nabs secretion in situ. Alternatively, the presence of IgG Nabs in graft rejection may signify a protective response, similar to that seen for IgM Nabs in atherosclerosis.23
In conclusion, while the existence of Nabs has been acknowledged for decades, their role in potentiating tissue damage in transplantation is only beginning to emerge. Recent evidence supports a significant, nonredundant role of Nabs in solid organ transplant rejection. Yet, uncertainty remains over their causal link to graft outcomes and mechanisms of action. Further investigations are now necessary to determine their exact function and confirm their importance in transplant rejection.
Funding
this work was supported by NIH grants R01-AI116814 and R01-AI123342
Abbreviations
- AMR
antibody-mediated rejection
- AT1R
angiotensin II type 1 receptor
- BCR
B-cell receptor
- CAV
cardiac allograft vasculopathy
- DAMP
danger-associated molecular pattern
- DNA
deoxyribonucleic acid
- DSA
donor-specific antibodies
- HLA
human leukocyte antigen
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- MICA
major histocompatibility complex class I chain-related molecule A
- Nabs
natural antibodies
- OSE
oxidation-specific epitopes
- PRR
pattern recognition receptors
Footnotes
Disclosure The authors declare no conflicts of interest
REFERENCES
- 1.Cozzi E, Colpo A, De Silvestro G. The mechanisms of rejection in solid organ transplantation. Transfus Apher Sci. 2017;56(4):498–505. [DOI] [PubMed] [Google Scholar]
- 2.Paul LC, Baldwin WM 3rd, van Es LA. Vascular endothelial alloantigens in renal transplantation. Transplantation. 1985;40(2):117–123. [DOI] [PubMed] [Google Scholar]
- 3.Jordan SC, Yap HK, Sakai RS, et al. Hyperacute allograft rejection mediated by anti-vascular endothelial cell antibodies with a negative monocyte crossmatch. Transplantation. 1988;46(4):585–587. [DOI] [PubMed] [Google Scholar]
- 4.Grafft CA, Cornell LD, Gloor JM, et al. Antibody-mediated rejection following transplantation from an HLA-identical sibling. Nephrol Dial Transplant. 2010;25(1):307–310. [DOI] [PubMed] [Google Scholar]
- 5.Djamali A, Kaufman DB, Ellis TM, et al. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohanakumar T, Waldrep JC, Phibbs M, et al. Serological characterization of antibodies eluted from chronically rejected human renal allografts. Transplantation. 1981;32(1):61–66. [DOI] [PubMed] [Google Scholar]
- 7.Brasile L, Zerbe T, Rabin B, et al. Identification of the antibody to vascular endothelial cells in patients undergoing cardiac transplantation. Transplantation. 1985;40(6):672–675. [DOI] [PubMed] [Google Scholar]
- 8.Zou Y, Stastny P, Süsal C, et al. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293–1300. [DOI] [PubMed] [Google Scholar]
- 9.Dragun D, Müller DN, Bräsen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352(6):558–569. [DOI] [PubMed] [Google Scholar]
- 10.Banasik M, Boratyńska M, Kościelska-Kasprzak K, et al. The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes. Transpl Immunol. 2014;30(1):24–29. [DOI] [PubMed] [Google Scholar]
- 11.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71(7):886–892. [DOI] [PubMed] [Google Scholar]
- 12.Kalache S, Dinavahi R, Pinney S, et al. Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients. J Immunol. 2011;187(2):1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyden SV. Natural antibodies and the immune response. Adv Immunol. 1966;5:1–28. [DOI] [PubMed] [Google Scholar]
- 14.Avrameas S Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol Today. 1991;12(5):154–159. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix-Desmazes S, Kaveri SV, Mouthon L, et al. Self-reactive antibodies (natural autoantibodies) in healthy individuals. J Immunol Methods. 1998;216(1–2):117–137. [DOI] [PubMed] [Google Scholar]
- 16.Nagele EP, Han M, Acharya NK, et al. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS One. 2013;8(4):e60726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4(9):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan CP, Berneman A, Pires R, et al. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun. 1997;65(10):3997–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou ZH, Zhang Y, Hu YF, et al. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briles DE, Forman C, Hudak S, et al. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156(4):1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid RR, Prodeus AP, Khan W, et al. Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J Immunol. 1997;159(2):970–975. [PubMed] [Google Scholar]
- 22.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81(7):3487–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binder CJ, Papac-Milicevic N, Witztum JL. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 2016;16(8):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeGiorgio LA, Konstantinov KN, Lee SC, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–1193. [DOI] [PubMed] [Google Scholar]
- 25.Raz E, Brezis M, Rosenmann E, et al. Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol. 1989;142(9):3076–3082. [PubMed] [Google Scholar]
- 26.Kalaaji M, Fenton KA, Mortensen ES, et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 2007;71(7):664–672. [DOI] [PubMed] [Google Scholar]
- 27.Porcheray F, Fraser JW, Gao B, et al. Polyreactive antibodies developing amidst humoral rejection of human kidney grafts bind apoptotic cells and activate complement. Am J Transplant. 2013;13(10):2590–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee D, Moore C, Gao B, et al. Prevalence of polyreactive innate clones among graft--infiltrating B cells in human cardiac allograft vasculopathy. J Heart Lung Transplant. 2018;37(3):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casali P, Notkins AL. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. [DOI] [PubMed] [Google Scholar]
- 30.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. [DOI] [PubMed] [Google Scholar]
- 31.Chou M, Fogelstrand L, Hartvigsen K, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119(5):1335–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.See SB, Clerkin KJ, Kennel PJ, et al. Ventricular assist device elicits serum natural IgG that correlates with the development of primary graft dysfunction following heart transplantation. J Heart Lung Transplant. 2017;36(8):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.See SB, Aubert O, Loupy A, et al. Post-Transplant Natural Antibodies Associate with Kidney Allograft Injury and Reduced Long-Term Survival. J Am Soc Nephrol. 2018;29(6):1761–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao B, Moore C, Porcheray F, et al. Pretransplant IgG Reactivity to Apoptotic Cells Correlates With Late kidney Allograft Loss. Am J Transplant. 2014;14(7):1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunti S, Notkins AL. Polyreactive Antibodies: Function and Quantification. J Infect Dis. 2015;212 Suppl 1:S42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tornberg UC, Holmberg D. B-1a, B-1b and B-2 B cells display unique VHDJH repertoires formed at different stages of ontogeny and under different selection pressures. EMBO J. 1995;14(8):1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimoda M, Inoue Y, Azuma N, et al. Natural polyreactive immunoglobulin A antibodies produced in mouse Peyer’s patches. Immunology. 1999;97(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montecino-Rodriguez E, Dorshkind K. Formation of B-1 B cells from neonatal B-1 transitional cells exhibits NF-κB redundancy. J Immunol. 2011;187(11):5712–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumgarth N The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. [DOI] [PubMed] [Google Scholar]
- 40.Mouthon L, Nobrega A, Nicolas N, et al. Invariance and restriction toward a limited set of self-antigens characterize neonatal IgM antibody repertoires and prevail in autoreactive repertoires of healthy adults. Proc Natl Acad Sci U S A. 1995;92(9):3839–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madi A, Hecht I, Bransburg-Zabary S, et al. Organization of the autoantibody repertoire in healthy newborns and adults revealed by system level informatics of antigen microarray data. Proc Natl Acad Sci U S A. 2009;106(34):14484–14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galili U, Rachmilewitz EA, Peleg A, et al. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984;160(5):1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casali P, Schettino EW. Structure and function of natural antibodies. Curr Top Microbiol Immunol. 1996;210:167–179. [DOI] [PubMed] [Google Scholar]
- 44.Kasaian MT, Ikematsu H, Balow JE, et al. Structure of the VH and VL segments of monoreactive and polyreactive IgA autoantibodies to DNA in patients with systemic lupus erythematosus. J Immunol. 1994;152(6):3137–3151. [PMC free article] [PubMed] [Google Scholar]
- 45.Merbl Y, Zucker-Toledano M, Quintana FJ, et al. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest. 2007;117(3):712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pascual J, Zuckermann A, Djamali A, et al. Rabbit antithymocyte globulin and donor-specific antibodies in kidney transplantation -- a review. Transplant Rev. 2016;30(2):85–91. [DOI] [PubMed] [Google Scholar]
- 47.Fiskesund R, Steen J, Amara K, et al. Naturally occurring human phosphorylcholine antibodies are predominantly products of affinity-matured B cells in the adult. J Immunol. 2014;192(10):4551–4559. [DOI] [PubMed] [Google Scholar]
- 48.Chen ZJ, Wheeler J, Notkins AL. Antigen-binding B cells and polyreactive antibodies. Eur J Immunol. 1995;25(2):579–586. [DOI] [PubMed] [Google Scholar]
- 49.Tiller T, Tsuiji M, Yurasov S, et al. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benckert J, Schmolka N, Kreschel C, et al. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J Clin Invest. 2011;121(5):1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bunker JJ, Erickson SA, Flynn TM, et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science. 2017;358(6361):eaan6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mirilas P, Fesel C, Guilbert B, et al. Natural antibodies in childhood: development, individual stability, and injury effect indicate a contribution to immune memory. J Clin Immunol. 1999;19(2):109–115. [DOI] [PubMed] [Google Scholar]
- 53.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14(5):617–629. [DOI] [PubMed] [Google Scholar]
- 54.Panda S, Zhang J, Tan NS, et al. Natural IgG antibodies provide innate protection against ficolin-opsonized bacteria. EMBO J. 2013;32(22):2905–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Park Y, Patel E, et al. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182(10):6031–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw PX, Hörkkö S, Chang MK, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105(12):1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurez V, Kazatchkine MD, Vassilev T, et al. Pooled normal human polyspecific IgM contains neutralizing anti-idiotypes to IgG autoantibodies of autoimmune patients and protects from experimental autoimmune disease. Blood. 1997;90(10):4004–4013. [PubMed] [Google Scholar]
- 58.Kim SJ, Gershov D, Ma X, et al. I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med. 2002;196(5):655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogden CA, Kowalewski R, Peng Y, et al. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38(4):259–264. [DOI] [PubMed] [Google Scholar]
- 60.Aung KM, Sjöström AE, von Pawel-Rammingen U, et al. Naturally Occurring IgG Antibodies Provide Innate Protection against Vibrio cholerae Bacteremia by Recognition of the Outer Membrane Protein U. J Innate Immun. 2016;8(3):269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y, Khanna S, Goodyear CS, et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183(2):1346–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiser MR, Williams JP, Moore FD Jr, et al. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183(5):2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kulik L, Fleming SD, Moratz C, et al. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182(9):5363–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narang A, Qiao F, Atkinson C, et al. Natural IgM antibodies that bind neoepitopes exposed as a result of spinal cord injury, drive secondary injury by activating complement. J Neuroinflammation. 2017;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rolán HG, Xavier MN, Santos RL, et al. Natural antibody contributes to host defense against an attenuated Brucella abortus virB mutant. Infect Immun. 2009;77(7):3004–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao Y, Blohm D, Ghadimi BM, et al. Mucins (MUC1 and MUC3) of gastrointestinal and breast epithelia reveal different and heterogeneous tumor-associated aberrations in glycosylation. J Histochem Cytochem. 1997;45(11):1547–1557. [DOI] [PubMed] [Google Scholar]
- 67.Hensel F, Hermann R, Schubert C, et al. Characterization of glycosylphosphatidylinositol-linked molecule CD55/decay-accelerating factor as the receptor for antibody SC-1-induced apoptosis. Cancer Res. 1999;59(20):5299–5306. [PubMed] [Google Scholar]
- 68.Durrant LG, Noble P, Spendlove I. Immunology in the clinic review series; focus on cancer: glycolipids as targets for tumour immunotherapy. Clin Exp Immunol. 2012;167(2):206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vollmers HP, Dámmrich J, Ribbert H, et al. Apoptosis of stomach carcinoma cells induced by a human monoclonal antibody. Cancer. 1995;76(4):550–558. [DOI] [PubMed] [Google Scholar]
- 70.Brändlein S, Pohle T, Ruoff N, et al. Natural IgM antibodies and immunosurveillance mechanisms against epithelial cancer cells in humans. Cancer Res. 2003;63(22):7995–8005. [PubMed] [Google Scholar]
- 71.Rodríguez-Zhurbenko N, Martínez D, Blanco R, et al. Human antibodies reactive to NeuGcGM3 ganglioside have cytotoxic antitumor properties. Eur J Immunol. 2013;43(3):826–837. [DOI] [PubMed] [Google Scholar]
- 72.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205(13):3053–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Throsby M, van den Brink E, Jongeneelen M, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One. 2008;3(12):e3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mouquet H, Scheid JF, Zoller MJ, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prigent J, Jarossay A, Planchais C, et al. Conformational Plasticity in Broadly Neutralizing HIV-1 Antibodies Triggers Polyreactivity. Cell Rep. 2018;23(9):2568–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boes M, Esau C, Fischer MB, et al. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160(10):4776–4787. [PubMed] [Google Scholar]
- 77.Baumgarth N, Herman OC, Jager GC, et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen TT, Elsner RA, Baumgarth N. Natural IgM Prevents Autoimmunity by Enforcing B Cell Central Tolerance Induction. J Immunol. 2015;194(4):1489–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsiantoulas D, Kiss M, Bartolini-Gritti B, et al. Secreted IgM deficiency leads to increased BCR signaling that results in abnormal splenic B cell development. Sci Rep. 2017;7:3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Notley CA, Baker N, Ehrenstein MR. Secreted IgM enhances B cell receptor signaling and promotes splenic but impairs peritoneal B cell survival. J Immunol. 2010;184(7):3386–3393. [DOI] [PubMed] [Google Scholar]
- 81.O’Garra A, Chang R, Go N, et al. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22(3):711–717. [DOI] [PubMed] [Google Scholar]
- 82.Margry B, Kersemakers SCW, Hoek A, et al. Activated peritoneal cavity B-1a cells possess regulatory B cell properties. PLoS One. 2014;9(2):e88869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maseda D, Smith SH, DiLillo DJ, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188(3):1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turman MA, Casali P, Notkins AL, et al. Polyreactivity and antigen specificity of human xenoreactive monoclonal and serum natural antibodies. Transplantation. 1991;52(4):710–717. [DOI] [PubMed] [Google Scholar]
- 85.Thompson KM, Sutherland J, Barden G, et al. Human monoclonal antibodies specific for blood group antigens demonstrate multispecific properties characteristic of natural autoantibodies. Immunology. 1992;76(1):146–157. [PMC free article] [PubMed] [Google Scholar]
- 86.Gaca JG, Lee W, Aksoy O, et al. Evidence for polyreactive xenoreactive antibodies in the repertoire of human anti-swine antibodies: the ‘next’ humoral barrier to xenotransplantation? Transpl Immunol. 2001;9(1):19–27. [DOI] [PubMed] [Google Scholar]
- 87.Porcheray F, DeVito J, Yeap BY, et al. Chronic humoral rejection of human kidney allografts associates with broad autoantibody responses. Transplantation. 2010;89(10):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Porcheray F, DeVito J, Helou Y, et al. Expansion of polyreactive B cells cross-reactive to HLA and self in the blood of a patient with kidney graft rejection. Am J Transplant. 2012;12(8):2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thaunat O, Field A, Dai J, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci U S A. 2005;102(41):14723–14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wehner JR, Fox-Talbot K, Halushka MK, et al. B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation. 2010;89(9):1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huibers MM, Gareau AJ, Vink A, et al. The composition of ectopic lymphoid structures suggests involvement of a local immune response in cardiac allograft vasculopathy. J Heart Lung Transplant. 2015;34(5):734–745. [DOI] [PubMed] [Google Scholar]
- 92.Huibers MM, Gareau AJ, Beerthuijzen JM, et al. Donor-specific antibodies are produced locally in Ectopic Lymphoid Structures in Cardiac Allografts. Am J Transplant. 2017;17(1):246–254. [DOI] [PubMed] [Google Scholar]
- 93.Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30(6):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kauke T, Oberhauser C, Lin V, et al. De novo donorspecific anti-HLA antibodies after kidney transplantation are associated with impaired graft outcome independently of their C1q-binding ability. Transpl Int. 2017;30(4):360–370. [DOI] [PubMed] [Google Scholar]
- 95.Safavi S, Robinson DR, Soresi S, et al. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2014;33(12):1273–1281. [DOI] [PubMed] [Google Scholar]
- 96.Budding K, van de Graaf EA, Kardol-Hoefnagel T, et al. Antibodies against Apoptotic Cells Present in End-stage Lung Disease Patients Do Not Correlate with Clinical Outcome after Lung Transplantation. Front Immunol. 2017;8:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Couzi L, Araujo C, Guidicelli G, et al. Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation. 2011;91(5):527–535. [DOI] [PubMed] [Google Scholar]
- 98.Cai J, Terasaki PI, Mao Q, et al. Development of nondonor-specific HLA-DR antibodies in allograft recipients is associated with shared epitopes with mismatched donor DR antigens. Am J Transplant. 2006;6(12):2947–2954. [DOI] [PubMed] [Google Scholar]
- 99.Gao B, Rong C, Porcheray F, et al. Evidence to Support a Contribution of Polyreactive Antibodies to HLA Serum Reactivity. Transplantation. 2016;100(1):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Banasik M, Boratyńska M, Kościelska-Kasprzak K, et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl Int. 2014;27(10):1029–1038. [DOI] [PubMed] [Google Scholar]
- 101.Lee J, Park Y, Kim BS, et al. Clinical implications of angiotensin II type 1 receptor antibodies in antibody-mediated rejection without detectable donor-specific HLA antibodies after renal transplantation. Transplant Proc. 2015;47(3):649–652. [DOI] [PubMed] [Google Scholar]
- 102.Pearl MH, Leuchter RK, Reed EF, et al. Accelerated rejection, thrombosis, and graft failure with angiotensin II type 1 receptor antibodies. Pediatr Nephrol. 2015;30(8):1371–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reinsmoen NL, Lai C, Mirocha J, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014;97(5):595–601. [DOI] [PubMed] [Google Scholar]
- 104.Reinsmoen NL, Mirocha J, Ensor CR, et al. A 3-Center Study Reveals New Insights Into the Impact of Non-HLA Antibodies on Lung Transplantation Outcome. Transplantation. 2017;101(6):1215–1221. [DOI] [PubMed] [Google Scholar]
- 105.Zhang X, Mirocha J, Aintablian T, et al. Revealing a new mode of sensitization induced by mechanical circulatory support devices: Impact of anti-AT1 R antibodies. Clin Transplant. 2018;32(2):e13178. [DOI] [PubMed] [Google Scholar]
- 106.Oaks M, Michel K, Downey FX, et al. Xenoreactive antibodies and latent fibrin formation in VAD and cardiac transplant recipients can confound the detection and measurement of anti-AT1R antibodies. Am J Transplant. 2018;18(11):2763–2771. [DOI] [PubMed] [Google Scholar]
- 107.Tolerance Matzinger P., danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. [DOI] [PubMed] [Google Scholar]
- 108.Todd JL, Palmer SM. Danger signals in regulating the immune response to solid organ transplantation. J Clin Invest. 2017;127(7):2464–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mori DN, Kreisel D, Fullerton JN, et al. Inflammatory triggers of acute rejection of organ allografts. Immunol Rev. 2014;258(1):132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gielis EM, Ledeganck KJ, De Winter BY, et al. Cell-Free DNA: An Upcoming Biomarker in Transplantation. Am J Transplant. 2015;15(10):2541–2551. [DOI] [PubMed] [Google Scholar]
- 111.Bloom RD, Bromberg JS, Poggio ED, et al. Cell-Free DNA and Active Rejection in Kidney Allografts. J Am Soc Nephrol. 2017;28(7):2221–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krüger B, Krick S, Dhillon N, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106(9):3390–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Todd JL, Wang X, Sugimoto S, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189(5):556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shen H, Heuzey E, Mori DN, et al. Haptoglobin enhances cardiac transplant rejection. Circ Res. 2015;116(10):1670–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu H, Noordmans GA, O’Brien MR, et al. Absence of MyD88 signaling induces donor-specific kidney allograft tolerance. J Am Soc Nephrol. 2012;23(10):1701–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matsuoka N, Itoh T, Watarai H, et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120(3):735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shen H, Song Y, Colangelo CM, et al. Haptoglobin activates innate immunity to enhance acute transplant rejection in mice. J Clin Invest. 2012;122(1):383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller YI, Choi S, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]