Abstract
Aims:
To develop a representative, self-report assessment of lower urinary tract symptoms (LUTS) for men and women, the Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index-29 (LURN SI-29).
Methods:
Women and men seeking treatment for LUTS at one of six academic medical centers in the US, were assessed at baseline, 3-month, and 12-month intervals. Twelve-month data on 78 LURN SI-29 items were analyzed among 353 women and 420 men using exploratory factor analysis (EFA), with factor structure confirmed using confirmatory factor analysis (CFA). Internal consistency, reliability and validity of the five developed scales were evaluated by assessing correlations with the American Urological Association Symptom Index (AUA-SI), the Genitourinary Pain Index (GUPI), and the Pelvic Floor Distress Inventory-20 (PFDI-20), and by examining expected sex differences in scores.
Results:
EFA results (n=150 women; 150 men) produced an interpretable eight-factor solution, with three of the factors comprised of dichotomous items addressing LUTS-associated sensations. The remaining five factors, confirmed with CFA in an independent sample of 473 participants, produced five scales: Incontinence, Urgency, Voiding Difficulty, Bladder Pain, and Nocturia. Subscales and total LURN SI-29 scores were correlated as expected with AUA-SI, GUPI, and PFDI-20. LURN SI-29 scores also performed as expected in differentiating men from women based upon clinically expected differences, with men reporting more voiding difficulties and nocturia, and women reporting more Urgency and Incontinence.
Conclusions:
The LURN SI-29 questionnaire has the potential to improve research and clinical outcome measurement for both men and women with LUTS.
Keywords: lower urinary tract symptoms, self-report, urinary incontinence, outcome assessment, questionnaire
Introduction
Lower urinary tract symptoms (LUTS), common among adult men and women,1,2 can adversely affect sleep, mood, daily functioning, and work productivity.3–5 In 2012, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) established the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN), with six tertiary care clinical research sites and a data coordinating center. The charge was to identify and explain the important subtypes of LUTS and improve the measurement of patient experiences of LUTS.6
While there are a number of validated patient reported measures of LUTS available to clinicians and researchers, no instrument captures the full spectrum of LUTS with interpretable scales that can be used for outcome measurement. For example, the LUTS Tool, which captures severity and bother for a comprehensive range of symptoms, provides no scoring system nor information on important differences, making measurement of change over time challenging. The American Urological Association Symptom Index (AUA-SI) was initially developed for men with benign prostatic hyperplasia, provides scoring and interpretation information, but it does not contain questions regarding urinary incontinence (UI). Most other instruments focus on a particular symptom complex such as overactive bladder or UI but do not assess the spectrum of LUTS.
To address this gap, LURN investigators developed a Comprehensive Assessment of Self-Reported Urinary Symptoms (CASUS),7 which assesses a comprehensive set of 93 manifestations of LUTS. The goal of the current research was to reduce this comprehensive set of questions to a representative set of items for use as a clinical or research outcome questionnaire that captures aspects of LUTS relevant to both sexes.7 To develop this clinically-relevant questionnaire, we used factor analyses as well as input from our large group of expert clinicians to ensure that the outcome questionnaire was psychometrically strong and included content appropriate for patients.
Materials and Methods
Study Design
Data were obtained from the LURN Observational Cohort Study.8 Treatment-seeking men and women were recruited between June 2015 and January 2017 and completed in-person clinic visits at baseline, 3 months, and 12 months. At baseline, participants completed a physical exam and questionnaires related to LUTS and other symptoms. Questionnaires were repeated at 3 and 12 months. As LURN CASUS was developed while the Observational Cohort Study was underway, only a subset of participants were administered the full questionnaire at baseline (n=64 women, n=212 men). However, most were administered LURN CASUS at 12 months. This analysis was performed using LURN CASUS responses from 12-month questionnaires, and thus included all participants that had sufficiently complete 12-month LURN CASUS forms (defined as at least 85% of the form completed).
Exploratory Factor Analyses (EFAs)
Candidate subscales were identified using EFA based on a random sample of 300 participants (150 women, 150 men). Sex-specific questions, questions that were suppressed by the survey branching logic, and questions with very low frequency of endorsement were excluded from the analyses, leaving 78 of 93 possible questions available for EFA. Because the response options included dichotomous and polytomous items, we performed multiple EFAs, specifying different factor solutions, using polychoric correlations in order to estimate correlations between the underlying continuous variables. Oblique rotation accounted for the known correlations between factors. For each factor identified, loadings from the individual items were provided as standardized regression coefficients (unlike correlation coefficients, standardized regression coefficients can occasionally exceed 1.0). Scree plots and parallel analysis were used to guide selection of the number of useful factors. Solutions ranging from four to ten factors were reviewed by eight members of the study team, and the final solution was chosen by consensus, based on interpretability. Factor loadings greater than 0.4 were required for inclusion of an item in a designated factor. After the most interpretable factor solution was derived, internal consistency reliability of each scale was assessed using Cronbach’s alpha.
Scale Development
Once the optimal number of factors was chosen, each factor was reviewed by the study team (two urologists; two urogynecologists; one statistician; three outcomes researchers) who nominated the most clinically-relevant items from among those with loadings above 0.4. This process resulted in excluding three factors due to poor interpretability. All remaining factors were reviewed with relevant data, including item content and internal consistency of various item combinations, and the group reached consensus on the final scale composition of the questionnaire. This 29-item questionnaire is referred to as the LURN Symptom Index-29 (LURN SI-29).
Confirmatory Factor Analysis (CFA)
LURN SI-29 response data from a second unique sample of 471 participants (203 women; 268 men) with 12-month data were analyzed to assess the consistency of factor loadings with the EFA results and to evaluate the fit of the response data to the factor solution selected from the EFA process. Confirmatory factor analysis (CFA) was conducted separately for men and women. Fit was assessed using Root Mean Squared Error of Approximation (RMSEA), comparative fit index (CFI), and the non-normed fit index (NNFI).
Scaling and Initial Validation
After establishing interpretable subscales of the LURN SI-29, summed raw scores were transformed to 0–100 scale using linear transformations of each subscale raw sum. A total LURN SI-29 score was computed as the simple raw sum of all responses, also transformed to a 0–100 scale.
Associations between LURN SI-29 Scales and Validated Measures of Urinary Symptoms
Validity testing for the LURN SI-29 scores was performed in the LURN study population with complete 12-month LURN SI-29 questionnaires. Initial validation of the LURN SI-29 was performed by studying its associations with concurrently-administrated questionnaires that capture a wide range of symptoms experienced in people with LUTS, including the American Urological Association Symptom Index (AUA-SI),9 the Genitourinary Pain Index (GUPI),10 the Pelvic Floor Distress Inventory (PFDI-20) (administered in women only),11 and a Urinary Incontinence (UI) Severity Score calculated from the LUTS Tool.12 Multivariable linear regression was used to test for associations between the LURN SI-29 scales and the relevant subscales of AUA-SI, GUPI, UI Severity Score from the LUTS Tool, and the PFDI-20 (women only). Models were run separately for men and women and both standardized and unstandardized regression coefficients were reported. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Demographic Characteristics
Table 1 provides demographic and clinical information for the EFA and CFA samples. Participants in both cohorts were on average 60 years of age old, mostly non-Hispanic whites, and held an associate’s degree or higher. The EFA sample was selected to be 50% female, whereas the CFA sample, unselected for sex, included 43% women. Most participants had AUA-SI scores in the moderate range.
Table 1:
Demographics and baseline data on participants with 12-month LURN SI-29 completed
| EFA Sample (n=300) | CFA Sample (n=473) | |
|---|---|---|
| Age mean (SD) | 59.3 (13.5) | 59.7 (13.4) |
| Gender n (%) | ||
| Male | 150 (50%) | 270 (57%) |
| Female | 150 (50%) | 203 (43%) |
| Race n (%) | ||
| African-American | 33 (11%) | 58 (12%) |
| Other | 25 (8%) | 40 (9%) |
| White | 241 (81%) | 373 (79%) |
| Ethnicity n (%) | ||
| Hispanic/Latino | 17 (6%) | 14 (3%) |
| Non-Hispanic/Non-Latino | 277 (92%) | 448 (95%) |
| Ethnicity unknown | 6 (2%) | 11 (2%) |
| Education n (%) | ||
| < High School/GED | 5 (2%) | 11 (2%) |
| HS Diploma/GED | 24 (8%) | 39 (8%) |
| Some college | 61 (21%) | 105 (23%) |
| Associate’s Degree | 25 (9%) | 36 (8%) |
| Bachelor’s Degree | 87 (30%) | 125 (27%) |
| Graduate Degree | 91 (31%) | 146 (32%) |
| Employment Status n (%) | ||
| Employed part-time | 40 (13%) | 49 (11%) |
| Employed full-time | 115 (39%) | 179 (38%) |
| Unemployed | 9 (3%) | 16 (3%) |
| Not employed | 133 (45%) | 222 (48%) |
| Marital Status n (%) | ||
| Married/civil union | 193 (65%) | 317 (68%) |
| Living with partner | 6 (2%) | 17 (4%) |
| Separated/Divorced | 46 (15%) | 51 (11%) |
| Widowed | 17 (6%) | 15 (3%) |
| Single | 37 (12%) | 68 (15%) |
| BMI median (IQR) | 28.9 (25.7–32.9) | 28.6 (25.1–33.1) |
| Current Tobacco User n (%) | 24 (8%) | 26 (5%) |
| Diabetes n (%) | 46 (15%) | 73 (15%) |
| Functional Comorbidity Index median (IQR) | 2.0 (1.0–4.0) | 2.0 (1.0–3.0) |
| PVR (mL) median (IQR) | 29.5 (10.0–80.0) | 24.0 (0.0–65.0) |
| AUA-SI median (IQR) | 13.0 (8.0–18.0) | 12.0 (8.0–17.0) |
| AUA QOL median (IQR) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) |
Note: LURN SI-29 = Lower Urinary Tract Dysfunction Research Network Symptom Index-29; PVR=Post void residual; EFA: Exploratory factor analysis; CFA: Confirmatory factor analysis; AUA-SI: American Urological Association Symptom Index; QoL: Quality of life; IQR: Interquartile range
EFA and Scaling
From the 78 candidate items, the 8-factor solution provided the most interpretable result (Supplemental Table 1): Incontinence (16 items), pain (16 items), voiding difficulties (12 items), urgency and bother (9 items), nighttime symptoms (3 items), sensations when needing to urinate (4 items), sensations between urinations (8 items), and sensations “that could not be put into words” (2 items). The last three (sensation) factors reflected the LURN effort to characterize, with dichotomous (yes/no) questions, the sensory variability of LUTS for possible diagnostic or phenotyping purposes; as such they were excluded from consideration for the LURN SI-29.
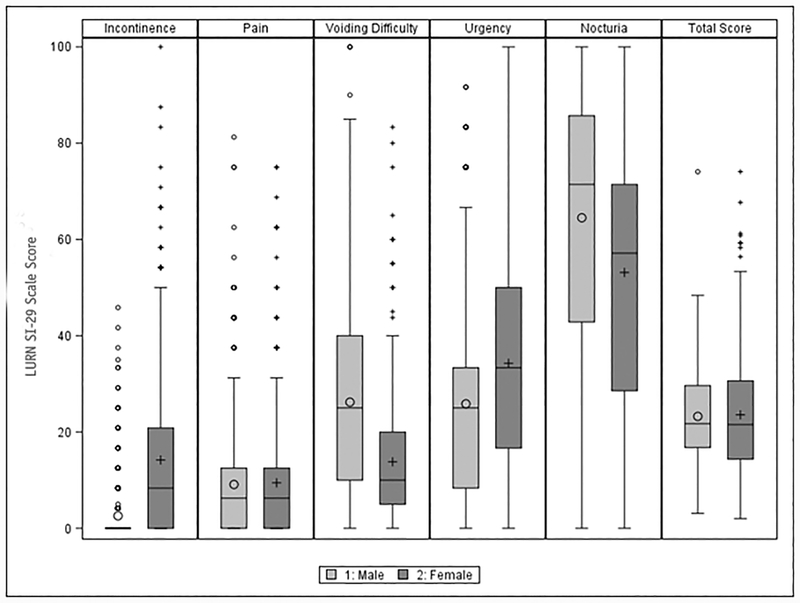
Based on consensus, a six-item Incontinence scale was selected (Supplemental Table 2), including items on leaking at night, completely losing bladder control, leaking with feelings of urgency, leaking when laughing, sneezing, or coughing, and leaking with physical activity. Median scores (0 – 100 range) were 8.3 in women and 0 in men, respectively, with an interquartile range of 0–20.8 (women) and 0–0 (men) and a score range of 0 to 100 in women and 0–45.8 in men (Figure 1a). The very low scores among men is consistent with previous descriptions of this study population.8
Figure 1:
LURN SI-29 Scales by Sex (n=353 females, 420 males)
Note: Large circles are male means and small circles are male outliers (i.e., greater than 1.5 times the interquartile range). Large pluses are female means, and small pluses are female outliers. The line in the box indicates the median, and the lower and upper edges of the box represent the 25th and 75th percentiles, respectively. The whiskers extend to 1.5 times the interquartile range (i.e. the distance between the 25th and 75th percentiles).
The four-item Bladder Pain factor queries the nature, frequency, and intensity of pain and discomfort at different points in the bladder filling and emptying process. The study team review preferred frequency-over intensity-type questions; therefore the final Bladder Pain scale included items related to the frequency of pain and discomfort while the bladder was filling, when it was full, during urination, and right after urination (Supplemental Table 2). The median re-scaled score of this scale was 6.25 (IQR=0–12.5) in both men and women with no participant scoring the maximum possible score of 100 (maximum observed score = 75.0 in women and 81.3 in men, Figure 1b).
After the study team presented two options for the Voiding Difficulty scale, the LURN Steering Committee approved an alternative five-item version (Supplemental Table 2). It consists of items related to straining, hesitancy, intermittency, weak stream, and post-void dribble. Median score for the Voiding Difficulty scale was higher in men than women (25 [IQR=10–40] versus 10 [IQR=5–20]) with scores varying across the full range of the scale in men but not in women (maximum score=83.3, Figure 1c).
The three-item Urgency scale (Supplemental Table 2) assesses how often respondents felt a sudden need to urinate, difficulty in delaying urination, and fear of leaking urine due to a sudden need to rush to urinate. The median score was 33.3 (IQR=16.7–50.0) in women and 25 (IQR 8.33–33.3) in men with scores across the full range of the scale (Figure 1d). A correlated “bother” item was retained by the Steering Committee for clinical relevance but not scaled with Urgency.
A three-item Nocturia scale (Cronbach’s alpha=0.76) originally included the nighttime leakage question; however, the LURN Steering Committee felt it was more clinically appropriate in the incontinence scale and also removed the item related to nighttime urgency from the scale. Thus, the final Nocturia scale (Supplemental Table 2) included items related to number of nighttime voids and frequency of waking up due to a need to void. Scores were higher in men compared to women (median score 71.4 [IQR=42.9–85.7] vs 57.1 [IQR=28.6–71.4], Figure 1e).
Supplemental Table 3 reports inter-subscale correlations. Most of the correlations are below 0.40, indicating that each subscale is measuring a unique symptom domain. The only correlation that consistently exceeded 0.40 was that between urgency and incontinence. Urgency and pain were correlated 0.42 in women but did not exceed 0.40 in men or overall.
Confirmatory Factor Analysis
Confirmatory factor analysis showed that, as with the EFA, all factor loadings remained above 0.4 when examining a second independent dataset. Inspection of fit indices for the five-factor solution indicated good fit based on RMSEA (Supplemental Table 4). CFI and NNFI, however, ranged from 0.813–0.891, suggesting that the data deviate from the hypothesized model, perhaps due to some content diversity in the correlated items comprising the scales.
Additional LURN SI-29 items
The LURN Steering Committee proposed a set of remaining questions, not associated with any of the five factors, to be included on the outcome measure separately from the scales. This list comprised nine items that were deemed clinically relevant: frequency of daily voiding, nighttime urgency, constant urgency, feeling of incomplete emptying, leakage just after voiding, sex-specific questions related to splitting, spraying, or change of direction of urine stream, and overall bother. These questions were not scaled, however, they were included in a total LURN SI-29 score obtained by summing all 29 questions and rescaling from 0 to 100.
Sex Differences
Figure 1 provides LURN SI-29 subscale scores separately by sex, indicating that whereas men and women report similar pain scores, women report more urgency and incontinence, and men report more voiding difficulty and nocturia. Overall, it appears that the average total LUTS burden is comparable for men and women (LURN SI-29 total median score=21.5 for women, 21.7 for men, see Figure 1f), but the variability of scores was significantly greater for women than for men (variance 156.62 vs. 86.00, p<0.001).
Initial Validation
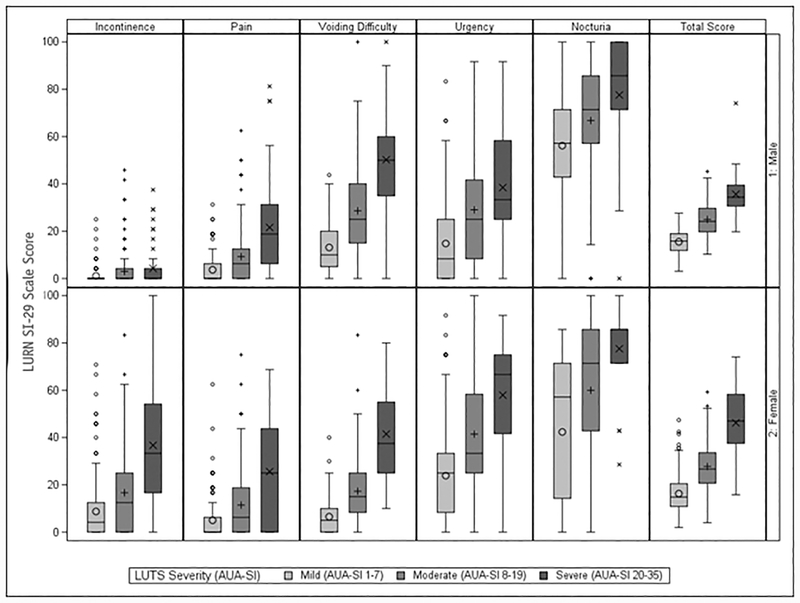
Table 2 demonstrates associations between LURN SI-29 scores and concurrently-administered questionnaires. Multivariable models regressing the AUA-SI on the LURN SI-29 scales showed associations between the AUA-SI and all subscales in women and all subscales except incontinence in men (Table 2). The GUPI was similarly associated with all subscales in men and all subscales except nocturia in women. For men and women, the UI severity score from the LUTS Tool was associated with the incontinence, voiding difficulty, and urgency scales, but not the bladder pain or nocturia scales. In women, the PFDI-20 was associated with all LURN SI-29 scales except nocturia. All associations were positive, i.e. higher (more severe) scores on the LURN SI-29 scales were associated with higher (more severe) scores on the validated scales. When the distribution of the LURN SI-29 scales was considered by AUA-SI severity category (mild [1–7], moderate [8–19], severe [20–35]), all scales showed an increasing trend by severity category (Figure 2), with the exception of incontinence in men, which is not measured by AUA-SI.
Table 2:
Concurrent validity of LURN SI-29 subscales with Validated Instruments
| Females | Males | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Validated PRO | LURN SI-29 Scale | B (SE) | 95% CI | β | p-value | B (SE) | 95% CI | β | p-value |
| AUA-SI (n=396 males, 328 females) | Intercept | 1.77 (0.49) | 0.81–2.74 | 0.00 | <.001 | 0.69 (0.61) | −.50–1.89 | 0.00 | 0.255 |
| Incontinence (per 10 unit increase) | 0.45 (0.15) | 0.15–0.76 | 0.13 | 0.004 | 0.09 (0.38) | −.65–0.83 | 0.01 | 0.802 | |
| Bladder Pain (per 10 unit increase) | 0.43 (0.18) | 0.08–0.78 | 0.10 | 0.016 | 0.93 (0.18) | 0.58–1.28 | 0.18 | <.001 | |
| Voiding Difficulty (per 10 unit increase) | 2.11 (0.16) | 1.79–2.43 | 0.51 | <.001 | 1.92 (0.12) | 1.69–2.15 | 0.54 | <.001 | |
| Urgency (per 10 unit increase) | 0.45 (0.11) | 0.23–0.67 | 0.19 | <.001 | 0.89 (0.12) | 0.66–1.12 | 0.28 | <.001 | |
| Nocturia (per 10 unit increase) | 0.37 (0.08) | 0.22–0.52 | 0.18 | <.001 | 0.40 (0.08) | 0.24–0.56 | 0.16 | <.001 | |
| GUPI (n=331 males, 250 females) | Intercept | 3.11 (0.70) | 1.73–4.49 | 0.00 | <.001 | 2.58 (0.79) | 1.03–4.14 | 0.00 | 0.001 |
| Incontinence (per 10 unit increase) | 0.65 (0.22) | 0.21–1.10 | 0.16 | 0.004 | 1.45 (0.60) | 0.27–2.64 | 0.11 | 0.016 | |
| Bladder Pain (per 10 unit increase) | 2.08 (0.27) | 1.56–2.60 | 0.39 | <.001 | 2.43 (0.23) | 1.98–2.88 | 0.47 | <.001 | |
| Voiding Difficulty (per 10 unit increase) | 1.09 (0.24) | 0.63–1.56 | 0.21 | <.001 | 0.69 (0.15) | 0.39–0.99 | 0.20 | <.001 | |
| Urgency (per 10 unit increase) | 0.75 (0.16) | 0.44–1.06 | 0.26 | <.001 | 0.57 (0.16) | 0.26–0.88 | 0.17 | <.001 | |
| Nocturia (per 10 unit increase) | 0.07 (0.11) | −.15–0.29 | 0.03 | 0.526 | 0.25 (0.11) | 0.03–0.46 | 0.09 | 0.026 | |
| UI Severity Score (LUTS Tool, n=388 males, 305 females) | Intercept | 1.20 (0.17) | 0.87–1.53 | 0.00 | <.001 | 0.77 (0.17) | 0.45–1.10 | 0.00 | <.001 |
| Incontinence (per 10 unit increase) | 0.68 (0.06) | 0.57–0.80 | 0.52 | <.001 | 1.02 (0.11) | 0.81–1.23 | 0.41 | <.001 | |
| Bladder Pain (per 10 unit increase) | 0.04 (0.06) | −.09–0.16 | 0.02 | 0.578 | −.00 (0.05) | −.10–0.09 | −.00 | 0.927 | |
| Voiding Difficulty (per 10 unit increase) | 0.22 (0.06) | 0.11–0.33 | 0.14 | <.001 | 0.15 (0.03) | 0.08–0.21 | 0.18 | <.001 | |
| Urgency (per 10 unit increase) | 0.27 (0.04) | 0.19–0.34 | 0.31 | <.001 | 0.23 (0.03) | 0.17–0.29 | 0.33 | <.001 | |
| Nocturia (per 10 unit increase) | −.00 (0.03) | −.06–0.05 | −.01 | 0.878 | −.01 (0.02) | −.06–0.03 | −.02 | 0.608 | |
| PFDI-20 (345 females) | Intercept | 6.41 (4.13) | −1.7–14.5 | 0.00 | 0.121 | ||||
| Incontinence (per 10 unit increase) | 4.02 (1.34) | 1.39–6.65 | 0.15 | 0.003 | |||||
| Bladder Pain (per 10 unit increase) | 9.12 (1.49) | 6.19–12.1 | 0.27 | <.001 | |||||
| Voiding Difficulty (per 10 unit increase) | 10.4 (1.39) | 7.70–13.2 | 0.30 | <.001 | |||||
| Urgency (per 10 unit increase) | 5.66 (0.93) | 3.84–7.49 | 0.29 | <.001 | |||||
| Nocturia (per 10 unit increase) | −.23 (0.65) | −1.5–1.05 | −.01 | 0.727 | |||||
Note: LURN SI-29 = Lower Urinary Tract Dysfunction Research Network Symptom Index-29; EFA: Exploratory factor analysis; PRO: Patient reported outcome; AUA-SI: American Urological Association Symptom Index GUPI: Genitourinary Pain Index; PFDI-20: Pelvic Floor Distress Inventory-20. B and β represent unstandardized and standardized coefficients, respectively. Unstandardized coefficients (B) can be interpreted as the change in the raw validated PRO per 10 unit increase in the LURN SI-29 scale on its original 0–100 scale. Standardized coefficients (β) represent effect size on the standard deviation scale, i.e. the change validated PRO standard deviations per standard deviation change in the LURN SI-29 scale.
Figure 2:
LURN SI-29 Scales by Sex and LUTS Severity as Measured by AUA-SI severity categories (n=335 females, 399 males). AUA-SI: American Urological Association Symptom Index; LUTS: Lower Urinary Tract Symptoms. AUA-SI scores missing for n=18 females and n=21 males.
Discussion
The goal of this research was to create an outcome measure for clinical research to quantify symptom severity across various types of LUTS. Empirically, we found that items grouped together in a clinically meaningful way, and factor loadings from EFA were replicated in an independent CFA sample. Across the total sample, all of the scales had sufficient internal consistency. Some of the CFA fit indices suggested a mismatch between the observed data and the various factor models. This is most likely due to the unique information captured by certain items within subscales (e.g., for bladder pain, there are questions about pain during urination versus pain between voids). EFA and CFA served as useful guides to scale construction in this study; candidate items selected from EFA were then selected based on clinical relevance, with a specific eye toward avoiding repetition when selecting from among the items loading >0.4 on the respective factor. This may have had an adverse effect on the CFA fit statistics. Nevertheless, the internal consistencies of all scales remained above 0.7 in an independent sample. Thus, it may be helpful for clinicians to consider each item independently when assessing patients, in addition to minding summary scores. The converting of each scale to a 0–100 metric can enable easy comparing across scales and can aid in clinical interpretation.13
Not surprisingly, comparing men and women on responses to the LURN SI-29, women reported more urgency and incontinence, whereas men reported more voiding difficulty and nocturia. There were no sex differences in pain, nor were there differences by sex in the LURN SI-29 Total. Subscales of the LURN SI-29 were associated with relevant scales from the LUTS Tool, AUA-SI, GUPI, and PFDI-20. These high associations with a diverse set of questionnaires offers hope that the LURN SI-29 might have value across different populations, including men and women. Broadly, the data provide support for the LURN SI-29 as a potential outcome tool, so future studies should investigate its feasibility as an endpoint in clinical trials, as well as determine clinically meaningful differences between distinct patient groups, and within patient groups over time.
Strengths of our research include the multicenter study population of treatment-seeking patients with LUTS and our multidisciplinary (including input from clinicians, social scientists, and psychometricians) and rigorous approach to measure development and testing. We also acknowledge limitations. Our sample was not ethnically diverse and was also highly educated, and although our multicenter research enhances generalizability to other treatment-seeking populations, we have not tested the LURN SI-29 in non-treatment-seeking people with LUTS. LURN SI-29 use should be limited to patient populations pending further testing. Finally, data were not available to calculate test-retest reliability. This will be important to establish in future research.
Conclusions
In summary, the LURN SI-29 is a new 29-item patient-reported outcome tool. It was developed from a longer, comprehensive set of urinary symptom items developed by the LURN for use in phenotyping research.7 Five brief scales measuring urgency, incontinence, voiding difficulty, nocturia, and pain, are supplemented with nine individual questions measuring voiding, nighttime urgency, constant urgency, incomplete emptying, leakage just after voiding, splitting, spraying, or change of direction of urine stream, and overall bother, together producing a total score. Internal consistency coefficients of the scales were consistently above the acceptable threshold of 0.70, and the scales were correlated with other commonly used LUTS questionnaires. Further validation and use in LUTS outcomes research is encouraged.
Supplementary Material
Supplemental Table 1: Standardized regression coefficients for LURN SI-29 EFA 8 factor solution with oblique rotation
Supplemental Table 2: Cronbach’s alpha coefficients for LURN SI-29 subscales overall and by sex. Note: LURN SI-29 = Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index-29.
Supplemental Table 3: Correlation between subscales
Supplemental Table 4: CFA in independent sample of 473 participants. * Due to the brevity of Urgency (3 items) and Nocturia (2 items), there are insufficient parameters to estimate the CFA models for those scales. Due to missing item responses, 3–10% of participants were excluded.
Supplemental Table 5: Comparison of standardized factor loadings from EFA and CFA
Supplemental Table 6: Distribution of LURN SI-29 Scales by Sex (n=353 females, 420 males).
Acknowledgements
This is publication number 16 of the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN).
Dr. Siddiqui is supported by grant K23-DK110417 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Duke University, Durham, North Carolina (DK097780): PI: Cindy Amundsen, MD, Kevin Weinfurt, PhD; Co-Is: Kathryn Flynn, PhD, Matthew O. Fraser, PhD, Todd Harshbarger, PhD, Eric Jelovsek, MD, Aaron Lentz, MD, Drew Peterson, MD, Nazema Siddiqui, MD, Alison Weidner, MD; Study Coordinators: Carrie Dombeck, MA, Robin Gilliam, MSW, Akira Hayes, Shantae McLean, MPH
University of Iowa, Iowa City, IA (DK097772): PI: Karl Kreder, MD, MBA, Catherine S Bradley, MD, MSCE, Co-Is: Bradley A. Erickson, MD, MS, Susan K. Lutgendorf, PhD, Vince Magnotta, PhD, Michael A. O’Donnell, MD, Vivian Sung, MD; Study Coordinator: Ahmad Alzubaidi
Northwestern University, Chicago, IL (DK097779): PIs: David Cella, Brian Helfand, MD, PhD; Co-Is: James W Griffith, PhD, Kimberly Kenton, MD, MS, Christina Lewicky-Gaupp, MD, Todd Parrish, PhD, Jennie Yufen Chen, PhD, Margaret Mueller, MD; Study Coordinators: Sarah Buono, Maria Corona, Beatriz Menendez, Alexis Siurek, Meera Tavathia, Veronica Venezuela, Azra Muftic, Pooja Talaty, Jasmine Nero. Dr. Helfand, Ms. Talaty, and Ms. Nero are at NorthShore University HealthSystem.
University of Michigan Health System, Ann Arbor, MI (DK099932): PI: J Quentin Clemens, MD, FACS, MSCI; Co-Is: Mitch Berger, MD, PhD, John DeLancey, MD, Dee Fenner, MD, Rick Harris, MD, Steve Harte, PhD, Anne P. Cameron, MD, John Wei, MD; Study Coordinators: Morgen Barroso, Linda Drnek, Greg Mowatt, Julie Tumbarello
University of Washington, Seattle Washington (DK100011): PI: Claire Yang, MD; Co-I: John L. Gore, MD, MS; Study Coordinators: Alice Liu, MPH, Brenda Vicars, RN
Washington University in St. Louis, St. Louis Missouri (DK100017): PI: Gerald L. Andriole, MD, H. Henry Lai; Co-I: Joshua Shimony, MD, PhD; Study Coordinators: Susan Mueller, RN, BSN, Heather Wilson, LPN, Deborah Ksiazek, BS, Aleksandra Klim, RN, MHS, CCRC
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urology, and Hematology, Bethesda, MD: Project Scientist: Ziya Kirkali MD; Project Officer: Christopher Mullins, PhD; NIH Personnel: Tamara Bavendam, MD, Robert Star, MD
Arbor Research Collaborative for Health, Data Coordinating Center (DK097776 and DK099879): PI: Robert Merion, MD, FACS; Co-Is: Victor Andreev, PhD, DSc, Brenda Gillespie, PhD, Gang Liu, PhD, Abigail Smith, PhD; Project Manager: Melissa Fava, MPA, PMP; Clinical Study Process Manager: Peg Hill-Callahan, BS, LSW; Clinical Monitor: Timothy Buck, BS, CCRP; Research Analysts: Margaret Helmuth, MA, Jon Wiseman, MS; Project Associate: Julieanne Lock, MLitt.
The protocol was approved by the institutional review boards of all participating research sites.
Funding/Support
This study is supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants DK097780, DK097772, DK097779, DK099932, DK100011, DK100017, DK097776, DK099879).
Research reported in this publication was supported at Northwestern University, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declarations of Interest: none
References
- 1.Cox L, Rovner ES. Lower urinary tract symptoms in women: epidemiology, diagnosis, and management. Curr Opin Urol. 2016;26:328–333. [DOI] [PubMed] [Google Scholar]
- 2.Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, Kopp ZS, Chapple CR, Kaplan S, Tubaro A, Aiyer LP, Wein AJ. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU international. 2009;104:352–360. [DOI] [PubMed] [Google Scholar]
- 3.Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS. Effect of Bothersome Overactive Bladder Symptoms on Health-related Quality of Life, Anxiety, Depression, and Treatment Seeking in the United States: Results From EpiLUTS. Urology. 2012;80:90–96. [DOI] [PubMed] [Google Scholar]
- 4.Vrijens D, Drossaerts J, van Koeveringe G, Van Kerrebroeck P, van Os J, Leue C. Affective symptoms and the overactive bladder - a systematic review. J Psychosom Res. 2015;78:95–108. [DOI] [PubMed] [Google Scholar]
- 5.Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Comorbidities and personal burden of urgency urinary incontinence: a systematic review. Int J Clin Pract. 2013;67:1015–1033. [DOI] [PubMed] [Google Scholar]
- 6.Yang CC, Weinfurt KP, Merion RM, Kirkali Z. LURN Study Group. Symptoms of Lower Urinary Tract Dysfunction Research Network. J Urol. 2016; 196:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinfurt KP, Griffith JW, Flynn KE, Cella D, Bavendam T, Wiseman JB, Andreev VP, Lai HH, Liu AB, Kirkali Z, Cameron AP, Bradley CS; LURN Study Group. The Comprehensive Assessment of Self-Reported Urinary Symptoms (CASUS): A New Tool for Research on Subtypes of Patients with Lower Urinary Tract Symptoms. J Urol. 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron AP, Lewicky-Gaupp C, Smith AR, Helfand BT, Gore JL, Clemens JQ, Yang CC, Siddiqui NY, Lai HH, Griffith JW, Andreev VP, Liu G, Weinfurt K, Amundsen CL, Bradley CS, Kusek JW, Kirkali Z. Symptoms of Lower Urinary Tract Dysfunction Research Network Study Group. Baseline Lower Urinary Tract Symptoms in Patients Enrolled in the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN): a Prospective, Observational Cohort Study. J Urol. 2018;199:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry MJ, Fowler FJ Jr., O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. [DOI] [PubMed] [Google Scholar]
- 10.Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM, Landis JR. Urologic Pelvic Pain Collaborative Research Network. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74:983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol 2005;193:103–113. [DOI] [PubMed] [Google Scholar]
- 12.Helmuth ME, Smith AR, Andreev VP, Liu G; LURN Study Group, Lai HH, Cameron AP, Siddiqui NY. Use of Euclidean length to measure urinary incontinence severity based on the lower urinary tract symptoms tool. Am J Obstet Gynecol. 2018;218:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen P, Cohen J, Aiken LS, West SG. The problem of units and the circumstance for POMP. Multivariate Behav Res. 1999;34:315–346. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Standardized regression coefficients for LURN SI-29 EFA 8 factor solution with oblique rotation
Supplemental Table 2: Cronbach’s alpha coefficients for LURN SI-29 subscales overall and by sex. Note: LURN SI-29 = Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index-29.
Supplemental Table 3: Correlation between subscales
Supplemental Table 4: CFA in independent sample of 473 participants. * Due to the brevity of Urgency (3 items) and Nocturia (2 items), there are insufficient parameters to estimate the CFA models for those scales. Due to missing item responses, 3–10% of participants were excluded.
Supplemental Table 5: Comparison of standardized factor loadings from EFA and CFA
Supplemental Table 6: Distribution of LURN SI-29 Scales by Sex (n=353 females, 420 males).