Abstract
AIMS
In the human brain, supplementary motor area (SMA) is involved in the control of pelvic floor muscles. SMA dysfunction has been implicated in several disorders involving pelvic floor muscles, including urinary incontinence and urologic pain. Here we aimed to provide a proof-of-concept study to demonstrate the feasibility of modulating resting pelvic floor muscle activity (tone) as well as SMA activity with non-invasive stimulation of SMA.
METHODS
We studied six patients (3 women + 3 men) with Urologic Chronic Pelvic Pain Syndrome. Repetitive transcranial magnetic stimulation (rTMS) was applied to SMA immediately after voiding. We tested two rTMS protocols: high-frequency (HF-rTMS) which is generally excitatory, and low-frequency (LF-rTMS) which is generally inhibitory. Pelvic floor muscle activity was measured during rTMS using electromyography. Brain activity was measured immediately before and after rTMS using functional magnetic resonance imaging.
RESULTS
The rTMS protocols had significantly different effects on resting activity in pelvic floor muscles (p = 0.03): HF-rTMS decreased and LF-rTMS increased pelvic floor tone. SMA activity showed a clear trend (p = 0.06) toward the expected differential changes: HF-rTMS increased and LF-rTMS decreased SMA activity.
CONCLUSIONS
We interpret the differential effects of rTMS at the brain and muscle level as novel support for an important inhibitory influence of SMA activity on pelvic floor tone after voiding. This preliminary study provides a framework for designing future studies to determine if neuromodulation of SMA could augment therapy for chronic urologic conditions.
Keywords: cortical neuromodulation, rTMS, pelvic floor muscle tone, supplementary motor area, pelvic pain
INTRODUCTION
Dysfunction in a brain region known as the supplementary motor area (SMA) has been described in disorders of pelvic floor muscle (PFM) activity, such as chronic pelvic pain and urinary incontinence.1–8 Recent brain morphology and resting state neuroimaging studies have shown that patients with interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), collectively referred as Urologic Chronic Pelvic Pain Syndrome (UCPPS), display significant differences in SMA gray matter volume,4 white matter integrity,7 local activity,5 and functional connectivity compared to healthy controls.5,6 It has been, also, proposed that interaction between the periaqueductal gray (PAG) and SMA is altered in incontinence patients.1–3 Furthermore, the extent of SMA dysfunction has been suggested to predict treatment response.3,8 We and others have shown that PFM are strongly represented in SMA, meaning that SMA contains motor neurons that can directly control activity in PFM.9,10 In addition, we have shown that the PFM representation in SMA interacts with brain regions such as the insular and cingulate cortices,11 known to be important for automatic control of continence.1,2,12–15 If activity in SMA could be modulated in a way that modifies PFM activity, this may augment benefits of existing therapies when treatment response is not adequate.
The motor cortex, including SMA, has been suggested as a target for non-invasive therapeutic neuromodulation, such as repetitive transcranial magnetic stimulation (rTMS).8,16–18 However, the effect of rTMS applied to the PFM representation in SMA has not been systematically tested. To begin to examine its effect, we applied two distinct rTMS protocol to SMA. High-frequency rTMS (HF-rTMS) involves pulses applied in bursts at 10 Hz, and generally produces a transient excitatory effect on the stimulated region of cortex.17 Low-frequency rTMS (LF-rTMS) involves pulses applied continuously at 1 Hz, and generally produces a transient inhibitory effect.17 The aim of the current pilot study was to evaluate the role of excitatory and inhibitory rTMS protocols on SMA activity as a proof of concept and necessary step to develop protocols to evaluate the role of rTMS on lower urinary symptoms. If exciting SMA increases PFM tone and inhibiting SMA decreases PFM tone, SMA is likely exerting an excitatory influence on resting PFM tone. On the other hand, if exciting SMA reduces PFM tone and inhibiting SMA increases PFM tone, SMA is likely exerting an inhibitory influence on resting PFM tone. We expect that our preliminary study may provide hypotheses for further studies that seek to reduce PFM tone (e.g., urologic pain) or increase PFM tone (e.g., incontinence), including studies that seek to produce lasting effects on symptoms with repeated rTMS sessions.16–20
MATERIALS AND METHODS
Participant Population
Participants included 3 women age of 55 ± 17 years (mean ± SD, range: 35–68) and 3 men age 35 ± 8 (range: 25–40). Participants were included if they were older than 18 years of age, able to participate in the informed consent process, safe to be scanned by magnetic resonance imaging, safe to receive transcranial magnetic stimulation, and diagnosed with Urologic Chronic Pelvic Pain Syndrome (UCPPS) using previously published criteria (briefly: bladder and/or pelvic pain, pressure or discomfort present the majority of the time over the past 3 months).4–7,21 Female participants were deferred if they were in their menstrual phase. All aspects of the study conformed to the principles described in the Declaration of Helsinki and were approved by our Institutional Review Board. All participants provided informed consent.
Repetitive Transcranial Magnetic Stimulation (rTMS) Protocols
Each participant received two rTMS protocols (HF-rTMS and LF-rTMS) with a one week wash-out period, which has been suggested to be sufficiently long for a single rTMS session.17 Both rTMS protocols were applied to the PFM representation in SMA, as we have defined previously (X: −2, Y: −16, Z: 68 in the Montreal Neurological Institute [MNI] coordinate system).5,10,11,22 TMS coil guidance to this location was performed using a neuronavigation system (Brainsight Frameless, Rogue Research). Both rTMS protocols were applied with an intensity of 80% of resting motor threshold for a finger muscle in the hand area of motor cortex, as reported previously.8 rTMS protocols were performed, with the participant resting in a supine position, using the repetitive mode of a biphasic magnetic stimulator (Magstim Rapid2, Magstim) and a 70 mm figure-of-eight air-cooled coil (Magstim). HF-rTMS, an excitatory protocol, consisted of twenty 10-second trains at 10 pulses per second with a 50 second pause between trains (2000 pulses total). LF-rTMS, an inhibitory protocol, consisted of 1 pulse per second delivered continuously (2000 pulses total).
Pelvic floor muscle (PFM) activity measure
During each rTMS protocol, synchronized electromyography (EMG) signals from PFM and rTMS pulse timing were recorded, as the EMG system of the Brainsight Frameless System (Rogue Research) was integrated with the stimulation system. Brainsight EMG system had an overall EMG amplification of 2500, an input range of 8 millivolt peak-to-peak, an overall bandwidth of 16–470 Hz, and a sampling rate of 3000 Hz per channel. We collected EMG signals from PFM using a rectal EMG sensor (Pathway Rectal EMG Sensor, The Prometheus Group, Dover, NH), which provided a bipolar recording from two bar electrodes – 12 mm apart, with dimensions 30 mm by 7 mm – mounted longitudinally along a cylindrical plug-type applicator. The rectal EMG sensor likely recorded an aggregate signal from the PFM that included the anal sphincter and levator ani, with possible small contributions from more distant muscles.23 To assess the acute effect of rTMS on resting PFM activity (tone) during the application of each protocol, first, we calculated a time-series of changes in resting PFM activity for each participant (Figure 1.A, Figure 2.B). The peak-to-peak difference of the EMG signal during an 80-millisecond window starting 10 milliseconds after each rTMS pulse was recorded (Figure 1.B), and its value was expressed as a percentage of the initial peak-to-peak EMG. The EMG values (2000, one per each rTMS pulse) were smoothed with a 500-pulse moving average to create the time-series of resting PFM activity. Finally, a resting PFM activity outcome measure was calculated, for each participant and each rTMS protocol, by averaging the time series between 500 and 2000 pulses, allowing the first 500 pulses of the rTMS protocol to take effect.17
Figure 1. Electromyographic (EMG) assessment of the effects of repetitive transcranial magnetic stimulation (rTMS).
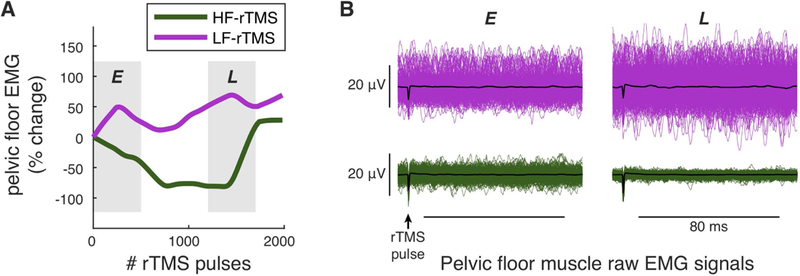
A. Example of estimated time-series of changes in resting pelvic floor muscle (PFM) activity during high frequency (HF-rTMS) or low frequency rTMS (LF-rTMS). B. Four panels of raw demeaned PFM EMG signals, during an earlier (E) or later (L) stage of each rTMS protocol. Each panel shows 500 EMG signals, focusing on the 80-millisecond window after each rTMS pulse where the peak-to-peak difference of each signal was recorded and used to create a time-series of resting PFM activity, as shown in A. In each panel, the mean of the 500 signals is plotted in black, showing no evidence of consistent motor evoked potentials during rTMS. Comparing L to E shows an increase in EMG activity during LF-rTMS (top panels in B) and a decrease in EMG activity during HF-rTMS (bottom panels in B). Data in A and B are from the same participant (P2).
Figure 2. Effects of repetitive transcranial magnetic stimulation (rTMS) on resting pelvic floor muscle (PFM) activity.
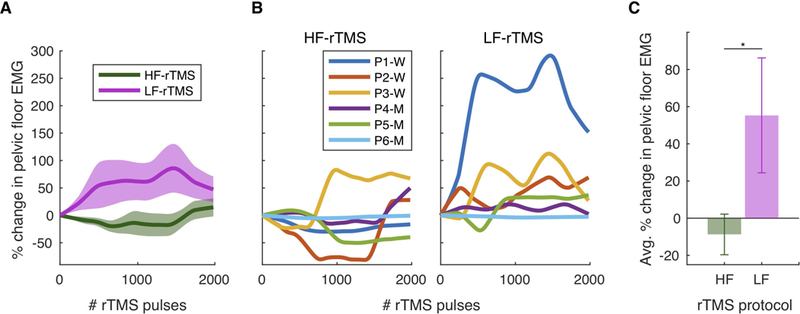
A. Group EMG data showing changes in resting PFM activity (mean and standard error), as measured from EMG immediately after each rTMS pulse, during either high frequency (HF-rTMS) or low frequency rTMS (LF-rTMS). P = participant. W = women. M = men. B. Individual EMG data showing changes in resting PFM activity during either HF-rTMS or LF-rTMS. P2 received LF-rTMS then HF-rTMS, opposite to the other 5 participants who received HF-rTMS then LF-rTMS. Data in B were used to create group results in A and C. C. The effects of HF-rTMS and LF-rTMS on the resting PFM activity outcome measure were significantly different.
Supplementary motor area (SMA) activity measure
We collected resting-state functional magnetic resonance imaging (rs-fMRI) data before and after each rTMS session in each participant using a 3 Tesla MRI scanner (Siemens Magnetom Prisma). To spatially align the rs-fMRI data, we also acquired a structural image with a T1-weighted magnetization prepared rapid gradient-echo sequence (MP-RAGE): repetition time 2300 milliseconds, echo time 2.98 milliseconds, slice thickness 1 mm, 240 slices, 256×256 mm voxel matrices, 1×1×1 mm voxel size. The resting scans were acquired with the participant resting in a supine position, eyes closed, for 10 minutes in 36-slice whole-brain volumes with repetition time 2000 milliseconds, echo time 28 milliseconds, slice thickness 4 mm, 220×220 mm voxel matrices, 3.4×3.4×4.0 mm voxel size, and flip angle 77 degrees. Post-rTMS resting scans were performed 19.5 ± 5.4 minutes (mean ± SD, range: 12–28) after the rTMS protocol ended. We preprocessed each participant’s rs-fMRI data using the FMRIB Expert Analysis Tool (FEAT, http://www.fmrib.ox.ac.uk),24 which included skull extraction using the brain extraction tool (BET) in FSL (FMRIB Software Library), slice timing correction, motion correction, and spatial smoothing using a Gaussian kernel with full-width half-maximum of 5 mm and nonlinear high-pass temporal filtering (150 seconds). Preprocessed data were spatially normalized to the MNI template, using the structural images, to extract brain maps of the fractional amplitude of low frequency fluctuations (fALFF), a standard measure of local brain activity.5,25 We computed an SMA activity outcome measure, for each rTMS protocol and each participant, by averaging fALFF (in the 0.01–0.027 Hz band) across all voxels within 6 mm of the rTMS target location. Since decreases in fALFF have been associated with increased activity, we created a final SMA activity outcome measure [−100 × (post-rTMS fALFF – pre-rTMS fALFF)/(pre-rTMS fALFF)]. This measure is expected to go up if the rTMS protocol increases SMA activity and down if the rTMS protocol decreases SMA activity.
Statistical Analysis
We tested the hypothesis that SMA activity modulates resting PFM activity by testing whether the two rTMS protocols (excitatory or inhibitory) had differential effects on PFM activity as well as differential effects on SMA activity. The outcome measures for PFM and SMA activity were analyzed separately, using a non-parametric sign-rank test and pairing changes induced by HF-rTMS and LF-rTMS within each participant. Due to the small number of participants in this preliminary study, resting PFM activity was analyzed as the primary outcome measure.
RESULTS
Our hypothesis that supplementary motor area (SMA) activity modulates resting pelvic floor muscle (PFM) activity was confirmed, as we observed differential effects of excitatory and inhibitory cortical repetitive transcranial magnetic stimulation (rTMS) protocols on resting PFM activity and activity in the PFM representation in SMA in six participants with Urologic Chronic Pelvic Pain Syndrome (UCPPS). Both rTMS protocols were of the same intensity since the motor threshold of the finger muscle that was used to calculate stimulation intensity did not change between the two sessions.
Effects of rTMS on resting PFM activity
As we initially hypothesized, the two rTMS protocols had opposite effects on resting PFM activity. Surprisingly, as shown in Figure 2, resting PFM activity decreased during the excitatory high-frequency rTMS protocol (HF-rTMS) and increased during the inhibitory low-frequency rTMS protocol (LF-rTMS). At the group level (Figure 2.A) and the individual level (Figure 2.B), we observed opposite changes in the resting PFM activity during the length of each rTMS protocol. In Figure 2.C, when assessing the effect of each rTMS protocol on the resting PFM activity outcome measure, resting PFM activity decreased during HF-rTMS (mean = −8.73%, range = −40.55% to 36.73%, SD = 26.77, SEM = 10.93, effect size = 0.33) and increased during LF-rTMS (mean = 55.29%, range = −2.56% to 202.93%, SD = 75.72, SEM = 30.91, effect size = 0.73). The effects of these two rTMS protocols on the resting PFM activity outcome measure were significantly different (signrank, p = 0.03; Figure 2.C). Finally, our results suggest that the order of applying the two rTMS protocols, with one week wash-out period, does not influence how these protocols affect resting PFM activity. In Figure 2.B, although P1, P3, P4, P5, and P6 received HF-rTMS then LF-rTMS and P2 received LF-rTMS then HF-rTMS, P2 EMG time series during both HF-rTMS and LF-rTMS followed the same group trends observed in Figure 2.A. Also, the resting PFM activity outcome measure for P2 decreased (by 40.55 %) during HF-rTMS and increased (by 40.65 %) during LF-rTMS, following the same group trends observed in Figure 2.C.
Effects of rTMS on resting SMA activity
Applying HF-rTMS and LF-rTMS to pelvic-specific SMA (Figure 3.A) had opposite effects on resting brain activity in the local region surrounding stimulated SMA (Figure 3.B, 3.C). At the group level (Figure 3.B), we observed opposite changes in resting SMA activity after either HF-rTMS or LF-rTMS. In Figure 3.C, when assessing the effect of each rTMS protocol on the SMA activity outcome measure, resting SMA activity increased during HF-rTMS (effect size = 0.59) and decreased during LF-rTMS (effect size = 0.78). These differential effects of the two rTMS protocols on the SMA activity outcome measure clearly trended toward statistical significance (signrank, p = 0.06; Figure 3.C). Finally, these changes in SMA activity validated that the rTMS protocols we used were able to affect brain activity in the expected directions suggested by previous studies:17,26 HF-rTMS was excitatory and increased SMA activity while LF-rTMS was inhibitory and decreased SMA activity.
Figure 3. Effects of repetitive transcranial magnetic stimulation (rTMS) on resting brain activity around the representation of pelvic floor muscles (PFM) in supplementary motor area (SMA).
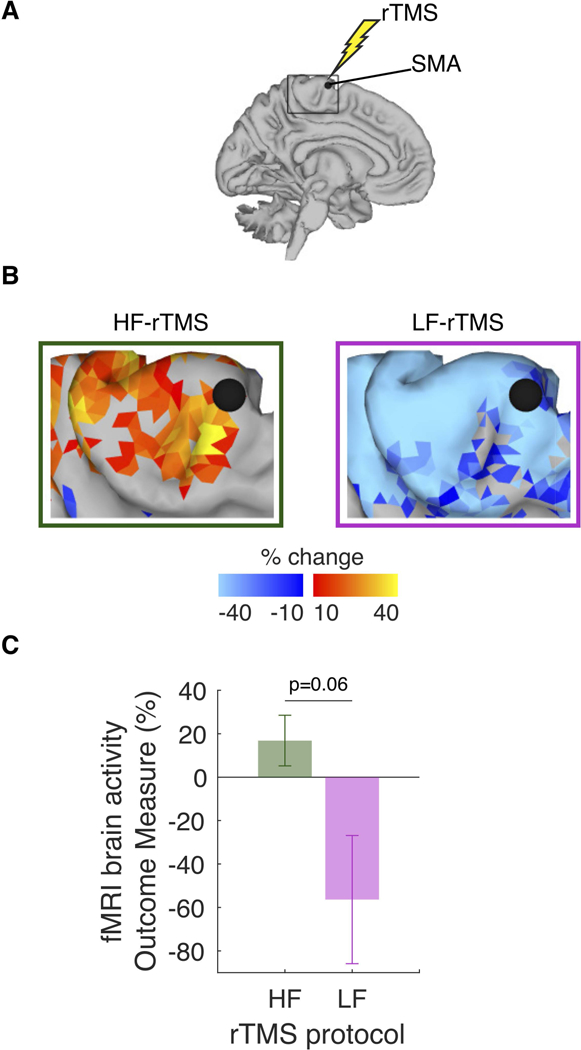
A. In each participant, high-frequency rTMS (HF-rTMS) and low-frequency rTMS (LF-rTMS) targeted the same PFM representation in SMA, with one-week wash-out period. B. Group resting-state functional magnetic resonance imaging (rs-fMRI) data. Brain maps of rTMS-induced changes, as assessed using fALFF in the 0.01–0.027 Hz frequency band before and after either HF-rTMS or LF rTMS. C. The effects of HF-rTMS and LF-rTMS on the resting SMA activity outcome measure trended toward statistical significance.
DISCUSSION
This is the first report, to our knowledge, of how non-invasive brain stimulation of supplementary motor area (SMA) may transiently modulate pelvic floor muscle (PFM) activity. Since modulating PFM activity is a common therapeutic goal across several urologic conditions, our results have broad potential utility in design of clinical research studies. Emerging evidence suggests that SMA function may play a role in both urologic pain and incontinence;1–8 and therefore, SMA stimulation may provide a means to target fundamental sources of dysfunction. A large body of research suggests that the neural control of the lower urinary tract (LUT) is complex, yet achieved by a few neural circuits in the forebrain, including SMA, together with parts of the brainstem.1,2,14,27 This complexity and the fact that both urologic chronic pelvic pain and incontinence involve LUT symptoms, dysfunction in any of these circuits can possibly result in the onset and/or persistence of these symptoms. Therefore, our results may ultimately lead to an adjunctive complementary brain stimulation therapy that can optimize recovery and management in several urologic conditions.
An important finding of this study is that rTMS can both increase and decrease SMA activity, which in turn affords the ability to both increase and decrease PFM activity. This finding increases the potential clinical utility because both increasing and decreasing PFM activity are therapeutic goals depending on the clinical condition. One limitation of our study is that we cannot currently explain the apparent asymmetry between HF-rTMS and LF-rTMS: LF-rTMS appeared to have a greater impact on both SMA and PFM activity than did HF-rTMS. While the protocols can be fairly compared because they consist of the same number of pulses delivered to the same scalp location with the same stimulation intensity, it is possible that the precise temporal pattern of stimulation pulses contributes to the effect size differences we observed. This possibility is because within-individual responses to different rTMS protocols are known to be heterogenous, as a result of the dynamic interaction between the characteristics of the externally applied rTMS pulses and the cortical state.17 Future studies can resolve this question by examining the effect of dose, as well as by examining other excitatory and inhibitory rTMS protocols (e.g., theta burst stimulation).
Since we did not study healthy individuals, our ability to infer basic principles of brain control of urine storage from this report is limited. However, several important basic questions emerge from our research. The neural control of the LUT includes a known excitatory control of PFM activity by SMA during increased urgency – regardless of pathology,1,2,14,27 and our results may suggest an inhibitory control of PFM activity by SMA at minimal bladder volumes. However, whether this inhibitory control exists in healthy individuals and how the nervous system would transition between both control strategies are still unanswered but important questions. Future studies are needed to examine the role of SMA in modulating PFM activity in healthy individuals to establish normative function. Establishing normative function can then enhance our ability to infer whether dysfunction is directly related to motor control strategies, as in case of decreased inhibition by SMA that could be related to local inhibitory interneurons and their inputs to the excitatory pyramidal neurons in SMA. Additionally, we can infer whether dysfunction is of a sensory-motor nature, where sensory inputs to the brain may mistakenly imply increasing urgency, even at minimal bladder volumes, as in case of increased excitation by SMA that could be related to distal excitatory inputs to the excitatory pyramidal neurons in SMA.
The results of the current study provide preliminary guidance for the design of future clinical studies. Cortical centers involved in salience evaluation and control of the LUT can be therapeutically targeted for symptom remission in patients with pelvic floor dysfunction.1,2,14,27 Recently, targeting some of these centers – the primary motor cortex in the area corresponding to the pelvic region 8,18 and the prefrontal cortices 20– with multi-session rTMS provided proof-of-concept evidence of promising therapeutic effects. Specific to UCPPS and of the rTMS protocols tested in the current study, a protocol compromised of multi sessions of the low-intensity HF-rTMS applied to SMA should be studied for its possible effectiveness in treating symptoms of UCPPS, when taking into consideration the observed changes in muscle and brain activity in the current study and structural and functional changes in SMA observed in UCPPS patients.4–7 Studies targeting an increase of PFM activity, such as those attempting to improve the therapeutic outcome of physical therapy for incontinence, may be best accomplished using LF-rTMS of SMA. Studies targeting a decrease of PFM activity, such as those attempting to relieve tight PFM in pelvic pain conditions, may be best accomplished using HF-rTMS. In addition, when additional evidence of altered SMA activity is available, such as for women with IC/BPS,5 this information can be used to guide rTMS protocol selection – HF-rTMS would be expected to be more beneficial if the goal is to increase SMA activity, whereas LF-rTMS would be expected to be more beneficial if the goal is to decrease SMA activity.
CONCLUSION
Non-invasive brain stimulation can be selected to either increase or decrease activity in brain areas directly controlling pelvic floor tone, which may have implications for designing new therapies for different urologic conditions.
ACKNOWLEDGMENTS
The contents of this research paper were developed under support from the Interstitial Cystitis Association, and support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) - National Institutes of Health (NIH) (R01DK110669).
REFERENCES
- 1.de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Comprehensive Physiology. 2015;5(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths D Neural control of micturition in humans: a working model. Nature reviews Urology. 2015;12(12):695. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths D, Clarkson B, Tadic SD, Resnick NM. Brain mechanisms underlying urge incontinence and its response to pelvic floor muscle training. The Journal of urology. 2015;194(3):708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kairys AE, Schmidt-Wilcke T, Puiu T, et al. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2015;193(1):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilpatrick LA, Kutch JJ, Tillisch K, et al. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2014;192(3):947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutch JJ, Yani MS, Asavasopon S, et al. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP: Research Network Neuroimaging Study. NeuroImage: Clinical. 2015;8:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodworth D, Mayer E, Leu K, et al. Unique microstructural changes in the brain associated with urological chronic pelvic pain syndrome (UCPPS) revealed by diffusion tensor MRI, super-resolution track density imaging, and statistical parameter mapping: a MAPP network neuroimaging study. PloS one. 2015;10(10):e0140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louppe J-M, Nguyen J-P, Robert R, et al. Motor cortex stimulation in refractory pelvic and perineal pain: Report of two successful cases. Neurourol Urodyn. 2013;32(1):53–57. [DOI] [PubMed] [Google Scholar]
- 9.Schrum A, Wolff S, Van der Horst C, Kuhtz-Buschbeck J. Motor cortical representation of the pelvic floor muscles. The Journal of urology. 2011;186(1):185–190. [DOI] [PubMed] [Google Scholar]
- 10.Yani MS, Wondolowski JH, Eckel SP, et al. Distributed representation of pelvic floor muscles in human motor cortex. Scientific reports. 2018;8(1):7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rana M, Yani MS, Asavasopon S, Fisher BE, Kutch JJ. Brain connectivity associated with muscle synergies in humans. Journal of Neuroscience. 2015;35(44):14708–14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig A Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences. 2011;1225(1):72–82. [DOI] [PubMed] [Google Scholar]
- 13.Kuehn E, Mueller K, Lohmann G, Schuetz-Bosbach S. Interoceptive awareness changes the posterior insula functional connectivity profile. Brain Structure and Function. 2016;221(3):1555–1571. [DOI] [PubMed] [Google Scholar]
- 14.DasGupta R, Kavia RB, Fowler CJ. Cerebral mechanisms and voiding function. BJU international. 2007;99(4):731–734. [DOI] [PubMed] [Google Scholar]
- 15.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nature Reviews Neuroscience. 2008;9(6):453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein MM, Treister R, Raij T, et al. Transcranial magnetic stimulation of the brain: guidelines for pain treatment research. Pain. 2015;156(9):1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefaucheur J-P, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology. 2014;125(11):2150–2206. [DOI] [PubMed] [Google Scholar]
- 18.Cervigni M, Onesti E, Ceccanti M, et al. Repetitive transcranial magnetic stimulation for chronic neuropathic pain in patients with bladder pain syndrome/interstitial cystitis. Neurourology and Urodynamics. 2018;37(8):2678–2687. [DOI] [PubMed] [Google Scholar]
- 19.Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature medicine. 2017;23(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nizard J, Esnault J, Bouche B, Suarez Moreno A, Lefaucheur J-P, Nguyen J-P. Long-term relief of Painful Bladder Syndrome by high-intensity, low-frequency repetitive transcranial magnetic stimulation of the right and left dorsolateral prefrontal cortices. Frontiers in neuroscience. 2018;12:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrepf A, Williams DA, Gallop R, et al. Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP Research Network study. Pain. 2018;159(10):2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asavasopon S, Rana M, Kirages DJ, et al. Cortical Activation Associated with Muscle Synergies of the Human Male Pelvic Floor. The Journal of Neuroscience. 2014;34(41):13811–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Floyd W, Walls E. Electromyography of the sphincter ani externus in man. The Journal of Physiology. 1953;122(3):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. [DOI] [PubMed] [Google Scholar]
- 25.Zuo X-N, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gangitano M, Valero-Cabré A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone Á. Modulation of input–output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clinical Neurophysiology. 2002;113(8):1249–1257. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths D, Fowler C. The micturition switch and its forebrain influences. Acta physiologica. 2013;207(1):93–109. [DOI] [PubMed] [Google Scholar]


