Abstract
Background:
Dysphagia is common in hospitalized patients post-extubation and associated with poor outcomes. Laryngeal sensation is critical for airway protection and safe swallowing. However, current understanding of the relationship between laryngeal sensation and aspiration in post-extubation populations is limited.
Methods:
Acute respiratory failure patients requiring intensive care unit admission and mechanical ventilation received a Flexible Endoscopic Evaluation of Swallowing (FEES) within 72 hours of extubation. Univariate and multivariable analyses were performed to examine the relationship between laryngeal sensation, length of intubation, and aspiration. Secondary outcomes included pharyngolaryngeal secretions, pneumonia, and diet recommendations.
Results:
One-hundred and three patients met inclusion criteria. Fifty-one patients demonstrated an absent laryngeal adductor reflex (LAR). Altered laryngeal sensation correlated with the presence of secretions (p = 0.004). There was a significant interaction between the LAR, aspiration, and duration of mechanical ventilation. Altered laryngeal sensation was significantly associated with aspiration on FEES only in patients with a shorter length of intubation (p = 0.008). Patients with altered laryngeal sensation were prescribed significantly more restricted liquid (p = 0.03) and solid (p = 0.001) diets. No relationship was found between laryngeal sensation and pneumonia.
Conclusions:
There is a high prevalence of laryngeal sensory deficits in mechanically ventilated patients post-extubation. Altered laryngeal sensation was associated with secretions, aspiration, and modified diet recommendations especially in those patients with a shorter length of mechanical ventilation. These results demonstrate that laryngeal sensory abnormalities impact the development of post-extubation dysphagia.
Keywords: Deglutition, Deglutition Disorders, Laryngeal Sensation, FEES, Acute Respiratory Failure, Critical Illness
Background
Acute respiratory failure (ARF) is a common, heterogeneous disorder requiring invasive mechanical ventilation and admission to an intensive care unit (ICU) (1). Dysphagia post-extubation occurs in up to 62% of patients (2) and can result in aspiration, which is associated with increased rates of pneumonia, re-intubation, mortality, and hospital length of stay (3). Dysphagia symptoms can persist up to six months after hospital discharge (4). Multiple mechanisms may contribute to the development of post-extubation dysphagia including neuromyopathy (5), impaired mentation (6), gastroesophageal reflux (7), disrupted coordination of breathing and swallowing (8, 9), direct trauma to laryngeal structures including edema, erythema, ulceration, and granulation tissue (10, 11), and reduced oropharyngeal (12) and laryngeal sensation (12, 13). The role of laryngeal sensation in the development of post-extubation dysphagia, however, is relatively unknown.
Laryngeal sensation is critical for airway protection and is tested through elicitation of the laryngeal adductor reflex (LAR) during Flexible Endoscopic Evaluation of Swallowing (FEES) (14). In healthy adults, tactile stimulation of each arytenoid with the tip of the endoscope triggers a brainstem-mediated LAR, as evidenced by a brief adduction of the true vocal folds (15). Afferent input from the internal branch of the superior laryngeal nerve provides sensory feedback to central neural circuits to ensure timely glottic closure (16, 17). Reduced laryngeal sensation can place patients at greater risk for aspiration of food and liquids (13, 18, 19), as well as pneumonia (20). To date, only one study has reported laryngeal sensory impairments in post-extubation populations. Scheel et al. (11) found no relationship between laryngeal sensory deficits and aspiration; however, this study was limited due to a relatively small sample size.
Despite the impact of aspiration on health outcomes in critically ill patients, little is known about the relationship between laryngeal sensory deficits and aspiration. We sought to examine this relationship, as well as the effect of length of intubation, in a large cohort of post-extubated ARF patients. Secondary outcomes investigated included pharyngolaryngeal secretions, pneumonia, and diet recommendations. Our hypothesis was that laryngeal sensory deficits would be associated with higher rates of aspiration, secretions, pneumonia, and modified diet recommendations.
Methods
Study Design
This study was part of a National Institutes of Health (NIH) funded, prospective, multi-site cohort study examining aspiration in ARF patients. Patients were eligible if they were at least 18 years old, admitted to an ICU, and mechanically ventilated with an endotracheal tube for longer than 48 hours. Exclusionary criteria included contraindications to enteral nutrition, pre-existing or acute primary, central, or peripheral neuromuscular disorder, pre-existing history of dysphagia or head and neck cancer or surgery, presence of a tracheostomy, coagulopathy resulting in uncontrolled nasal or pharyngeal bleeding, and altered mentation. Patients found to have vocal fold immobility during FEES were excluded from this sub-study analysis since the LAR would not be present due to impairment of the motor limb of the reflex (20).
Data Collection
Experienced speech-language pathologists (SLPs) performed FEES on all patients within 72 hours of extubation. Both fiberoptic and distal chip endoscopes were used across study sites. FEES is standard of care at two of the four study sites. All boluses were dyed green and additional white food coloring was added to thin liquid bolus trials. Boluses were administered in the following order: ½ and full teaspoon of ice chips; 5 ml, 15 ml, and 2 oz nectar-thick liquid (Thick & Easy®, Hormel Health Labs, Austin, MN); 5 ml and 10 ml puree; 5 ml, 15 ml, and 2 oz thin liquid; ¼ piece of graham cracker; 3 oz water swallow test. If aspiration was visualized on a small bolus (i.e., 5 ml), then the larger bolus within that consistency was skipped and the next consistency was presented. The 3 oz water swallow test was not performed if aspiration was visualized on any prior thin liquid bolus. Bolus presentations were stopped if the clinician perceived significant risk to the patient. Stopping rules for each bolus consistency and for the entire study optimized both the delivery of diverse bolus consistencies and patient safety. Following PO trials, the clinician assessed laryngeal sensation by advancing the endoscope tip to briefly contact each arytenoid. If the laryngeal adductor reflex was not readily apparent on one side, a second attempt was made. Dietary recommendations were made by the SLP upon the conclusion of the FEES.
Data & Statistical Analysis
An independent rater with 30 years of experience rating FEES judged airway safety on the penetration–aspiration scale (PAS) (21) and the presence or absence of secretions and the LAR. Aspiration was defined as a PAS score of 6 or greater on any consistency (21). Presence of the LAR was defined as adduction of the vocal folds or arytenoids. Absent LAR was defined as a unilateral or bilateral absent reflex. Secretions were grouped into four categories: no secretions, secretions pooled outside the laryngeal vestibule, secretions pooled inside the laryngeal vestibule, and secretions pooled both inside and outside the laryngeal vestibule. All incidences of hospital-acquired pneumonia, as diagnosed by the medical team, were recorded. An independent otolaryngologist assessed vocal fold mobility during a speech task (“eee-sniff”), which elicited vocal fold abduction and adduction.
Categorical variables were compared with Pearson’s chi-square test. Cramer’s V was used to examine the strength of associations for chi-squared analyses. In order to examine demographic predictors of laryngeal sensory deficits, a multivariable logistic regression was performed. Variables included age, gender, and baseline demographic characteristics that were statistically significantly associated with laryngeal sensation in univariate analyses, including race, history of myocardial infarction, and history of diabetes mellitus. To examine the effects of duration of mechanical ventilation on the relationship between altered laryngeal sensation and aspiration, a second multivariable logistic regression adjusting for gender, endotracheal tube size, and duration of mechanical ventilation (log normalized) was performed. In subsequent univariate analyses, patients were categorized into four groups based on length of mechanical ventilation (long/short) and LAR (absent/present). Length of mechanical ventilation was stratified by < or ≥ 100 hours. Alpha was set at 0.05 for all analyses.
Results
Patient Characteristics
A total of 141 patients were enrolled in the study. Laryngeal sensation was performed on 110 patients (bilateral testing on 97; unilateral testing on 13). Seven of these patients were excluded: six patients presented with vocal fold immobility and one did not provide adequate visibility during FEES. Therefore, 103 patients met inclusion criteria and were included in the analysis.
Univariate analyses revealed no statistically significant difference in baseline characteristics of patients who did and did not have laryngeal sensation testing performed (Table 1). Intact bilateral LAR was present in 52 of the 103 patients (50.5%). In the 51 patients who exhibited LAR deficits, 23 demonstrated a unilateral absent LAR (45%), and 28 patients demonstrated bilaterally absent LAR (55%). In univariate analyses, history of myocardial infarction (χ2 = 3.21, p = 0.04, V = 0.18), diabetes mellitus (χ2 = 4.64, p = 0.04, V = 0.15), and non-white race (χ2 = 9.37, p = 0.001, V = 0.30) were statistically significantly associated with an absent LAR (Table 2). In a multivariable logistic regression, race was the only variable that remained statistically significantly associated with an absent LAR (p = 0.02, OR = 2.06, 95% CI: 1.21 – 7.22).
Table 1:
Baseline Characteristics Between Patients With and Without Laryngeal Sensory Testing
| LAR performed (n = 110) |
LAR not performed (n = 31) |
p value | |
|---|---|---|---|
| Age | 56.2 ± 15 | 61.1 ± 15 | 0.11 |
| Gender (% male) | 65% | 59% | 0.58 |
| Service (% MICU) | 72% | 81% | 0.43 |
| Length of intubation (hours) | 147 ± 92 | 153 ± 86 | 0.76 |
| Frequency of aspiration | 36% | 46% | 0.36 |
LAR = laryngeal adductor reflex; MICU = medical intensive care unit
Table 2:
Demographic Characteristics
| LAR present (n = 52) | LAR absent (n =51) | p value | |
|---|---|---|---|
| Age (years) | 54 ± 14 | 57 ± 16 | 0.30 |
| Gender (% male) | 65% | 51% | 0.14 |
| Race (% Caucasian) | 71% | 29% | 0.001 |
| Height (in) | 67 ± 4 | 66 ± 4 | 0.22 |
| Weight (kg) | 85 ± 24 | 92 ± 32 | 0.23 |
| Primary service (% MICU) | 81% | 63% | 0.04 |
| Length of time from admission to intubation (hours) | 7.5 [0.25 – 57] | 20 [0 – 72] | 0.54 |
| Length of intubation (hours) | 116 [70 – 187] | 153 [72 – 216] | 0.23 |
| Length of time from extubation to FEES (hours) | 36 [1 – 73] | 36 [3 – 71] | 0.91 |
| Re-intubation | 0.99 | ||
| None | 79% | 78% | |
| Elective | 8% | 8% | |
| Urgent | 13% | 14% | |
| History of Myocardial Infarction | 12% | 27% | 0.04 |
| History of Congestive Heart Failure | 19% | 24% | 0.59 |
| History of Peripheral Vascular Disease | 8% | 12% | 0.49 |
| History of COPD | 29% | 27% | 0.87 |
| History of Connective Tissue Disease | 2% | 6% | 0.30 |
| History of Liver Disease | 21% | 27% | 0.46 |
| History of Diabetes Mellitus | 19% | 37% | 0.04 |
| History of Renal Disease | 21% | 20% | 0.85 |
| History of Solid Tumor | 6% | 6% | 0.98 |
LAR = laryngeal adductor reflex; in = inches; kg = kilograms; MICU = medical intensive care unit; ETT = endotracheal tube; FEES = flexible endoscopic evaluation of swallowing
Laryngeal Sensation and Aspiration
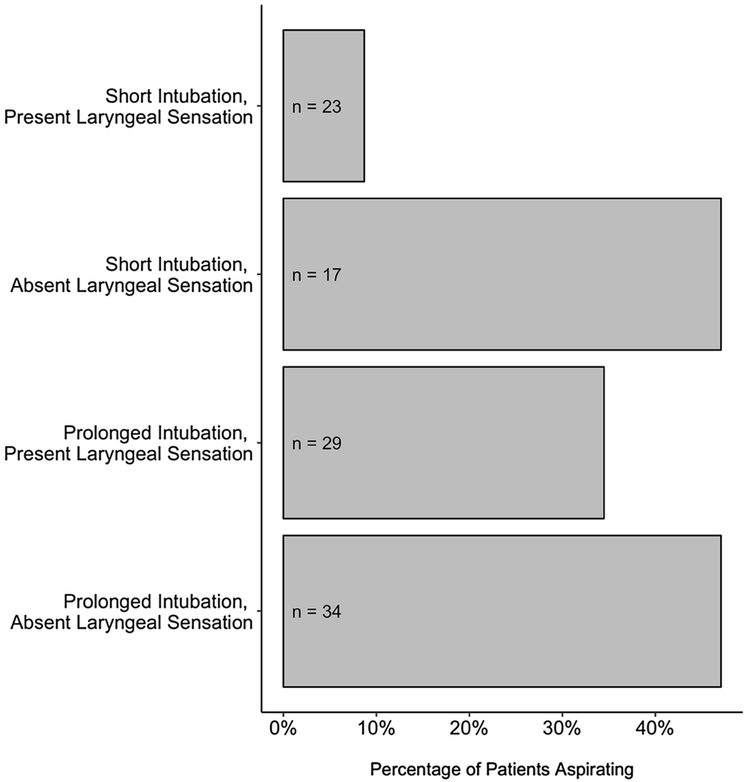
A significant relationship was found between absent LAR and aspiration (χ2 = 5.50, p = 0.01, V = 0.25). Forty-seven percent of patients who demonstrated laryngeal sensory deficits aspirated on their FEES examination, compared to 23% of patients with an intact LAR. Aspiration occurred in 39% of patients with a bilaterally absent LAR compared to 57% of patients with a unilateral absent LAR, though there were no statistically significant differences between patients with a unilateral and bilaterally absent LAR (χ2 = 1.51, p = 0.22, V = 0.17). Of the 35 patients who aspirated, 91% aspirated thin liquid and 29% aspirated without a sensory response. Silent aspiration occurred in 16% of patients with an absent LAR compared to 6% with an intact LAR. An absent LAR was not associated with higher rates of silent aspiration compared to patients with an intact LAR (χ 2 = 1.71, p = 0.19, V = 0.16). A significant interaction between duration of mechanical ventilation and the LAR response and their associations with aspiration was found (p = 0.01). In a stratified analysis of those patients with a shorter duration of mechanical ventilation, an absent LAR was significantly associated with aspiration. Specifically, 47% (8/17) of patients with an absent LAR aspirated compared to only 9% (2/23) in patients with a present LAR (p = 0.005). For those patients with a longer duration of mechanical ventilation, an absent LAR was not associated with aspiration. Specifically, 47% (16/34) of patients with an absent LAR aspirated compared to 35% (10/29) in those patients with a present LAR (p = 0.31; Figure 1).
Figure 1:
Laryngeal Sensation, Length of Intubation, and Aspiration
Laryngeal Sensation and Secretions, Pneumonia, and Diet Recommendations
Absent LAR was significantly associated with the presence of secretions, (χ2 = 8.32, p = 0.004, V = 0.29; Table 3), whereas the length of mechanical ventilation did not reach significance, (χ 2 = 1.08, p = 0.30, V = 0.10). Absent LAR was not associated with hospital-acquired pneumonia after FEES, (χ 2 = 0.10, p = 0.75, V = 0.02). The absence of an LAR was also associated with modified liquid (χ 2 = 9.02, p = 0.03, V = 0.17) and solid (χ 2 = 16.10, p = 0.001, V = 0.28) diet recommendations. Furthermore, patients with longer durations of mechanical ventilation were significantly more likely to have modified liquid (χ 2 = 5.96, p = 0.02, V = 0.24) and solid (χ 2 = 5.84, p = 0.02, V = 0.24) diet recommendations.
Table 3:
Laryngeal Sensation and Secretions, Pneumonia, and Diet Recommendations
| LAR present (n = 52) | LAR absent (n =51) | p value | |
|---|---|---|---|
| Secretions | 0.02 | ||
| Normal/No excess | 67% | 38% | |
| Outside laryngeal vestibule | 25% | 48% | |
| Inside laryngeal vestibule | 0% | 8% | |
| Inside & outside laryngeal vestibule | 8% | 6% | |
| Hospital-Acquired Pneumonia | 10% | 8% | 0.75 |
| FEES liquid diet recommendations | 0.03 | ||
| NPO | 10% | 12% | |
| Honey-thick | 0% | 6% | |
| Nectar-thick | 25% | 43% | |
| Thin | 65% | 39% | |
| FEES solid diet recommendations | 0.001 | ||
| NPO | 10% | 12% | |
| Puree | 8% | 22% | |
| Mechanical soft | 21% | 43% | |
| Regular | 61% | 23% |
FEES = flexible endoscopic evaluation of swallowing; NPO = nothing by mouth
Discussion
Though dysphagia post-extubation is common and associated with poor health outcomes, current understanding of its underlying mechanisms is limited. Laryngeal sensation is critical for airway protection and safe swallowing, but its relationship with aspiration post-extubation is unknown. The primary aim of this prospective study was to examine the relationship between laryngeal sensation and aspiration in a large cohort of patients with ARF. Secondary aims included examining secretions, pneumonia, and diet recommendations, as well as the effect of duration of mechanical ventilation.
Laryngeal sensory deficits were prevalent within 72 hours after extubation and associated with aspiration. This finding is consistent with prior studies demonstrating a relationship between laryngeal sensory impairments and aspiration in stroke, chronic obstructive pulmonary disease, and other heterogeneous populations (13, 18, 22). Though studies have predominantly focused on aspiration, there are multiple changes in swallowing physiology that may contribute to dysphagia after extubation. An increased length of time to achieve laryngeal closure and reopening after the swallow has been observed, which could be caused by altered laryngeal sensation (23). These results suggest that routine assessment of laryngeal sensation post-extubation could be of clinical benefit when determining aspiration risk.
Several studies have suggested a relationship between length of mechanical ventilation and dysphagia (3, 24-30), though other analyses have not shown this relationship (11, 31-34). There are likely many methodological factors that contribute to this discrepancy, including sample size, timing of evaluation, and lack of objective instrumental evaluations. Studies have yet to examine the role of laryngeal sensation in the relationship between length of intubation and aspiration risk. Interestingly, our results showed that altered laryngeal sensation has a more profound effect on aspiration risk in patients with a short length of mechanical ventilation (< 100 hours). In patients with a longer duration of mechanical ventilation, laryngeal sensory deficits alone do not seem to influence the risk of aspiration. It is likely that there are other underlying mechanisms and types of dysfunction related to their critical illness that contribute to a patient’s aspiration risk if they are intubated for a prolonged length of time. These patients are likely sicker at baseline, resulting in deconditioning, neuromyopathy, impaired mentation, and disrupted coordination of breathing and swallowing. Alternatively, patients with a shorter duration of mechanical ventilation are less critically ill and not subjected to these types of dysfunction known to contribute to dysphagia in this patient population. Altered laryngeal sensation resulting from the local impact of an endotracheal tube affects swallowing physiology in these patients, increasing their risk of aspiration. The underlying pathophysiology is complex and multifaceted, underscoring the importance of accounting for multiple, interacting sensory and motoric mechanisms when evaluating and treating this population.
An absent LAR was not associated with silent aspiration in our cohort, though this might be suspected due to separate innervations of the vagus nerve. Supraglottic sensation is mediated by the internal branch of the superior laryngeal nerve, whereas subglottic sensation is mediated by the recurrent laryngeal nerve. However, the lack of statistical significance observed may be due to low power. Larger studies will be necessary to further determine if there is an association between supraglottic and subglottic sensation in patients post-extubation.
Altered laryngeal sensation was associated with the presence of pharyngolaryngeal secretions. Though we were unable to examine differences in secretion severity due to insufficient sample size, patients with an intact LAR were less likely to have pooling inside the laryngeal vestibule, emphasizing the importance of laryngeal sensation to inform a motoric response and effectively manage secretions. Length of mechanical ventilation, however, was not related to the presence of secretions. Contrary to a prior study examining a heterogeneous group of patients with dysphagia (20), we found no association between hospital-acquired pneumonia and reduced laryngeal sensation. This lack of association might be due to inconsistent diagnostic criteria for pneumonia across sites, a small sample size, or insufficient follow-up after discharge. Furthermore, SLP intervention, including diet modifications, feeding strategies, and oral hygiene, might have minimized the incidence of pneumonia in this population, limiting our ability to detect an effect. Future research studies examining the effect of altered laryngeal sensation on pneumonia in a larger sample are warranted. In our cohort, non-white patients were more likely to have altered laryngeal sensation. This result was unexpected as there is no research, to our knowledge, that would suggest an association. Future studies will be necessary to determine the significance of this finding. Finally, patients with altered laryngeal sensation received more restrictive liquid and solid diet recommendations compared to patients with intact sensation, highlighting the clinical implications of changes in laryngeal sensation post-extubation.
We acknowledge that our study has several limitations. First, the FEES rater was not blinded to laryngeal sensation, aspiration, or secretions, but was an independent rater who did not participate in any of the primary data collection. Furthermore, explicit and objective definitions of each outcome were utilized to minimize bias. Secondly, inter- and intra-rater reliability was not reported. However, variable reliability has been previously reported with the touch method of laryngeal sensation testing (35); so to avoid the problem with variable rating secondary to differing levels of expertise, we chose to use the ratings of a clinician who is the foremost expert in FEES as the “best possible” answer. Thirdly, the present study used the ‘touch method’ of laryngeal sensation testing, which has demonstrated variability in pressures compared to the air pulse method (35, 36). However, the air pulse method has been shown to identify sensory impairments with greater frequency compared to the touch method, though the clinical significance is unclear as these sensory impairments were not associated with penetration or aspiration (37).
An area of interest that we were unable to study is the effect of a bilaterally absent LAR on the development of pneumonia, compared to a unilateral presentation. In our cohort, the pattern was nearly equally divided between unilateral and bilateral presentations. However, insufficient sample size limited our ability to examine this research question. Future studies should continue to explore the underlying mechanisms contributing to post-extubation dysphagia and further identify risk factors for altered laryngeal sensation. Doing so would be important to develop optimal treatment for this complex patient population.
Conclusion
This prospective, multi-site study reports a high prevalence of laryngeal sensory deficits in mechanically ventilated patients post-extubation. Altered laryngeal sensation was associated with aspiration and shown to have a more profound effect on aspiration risk in patients with a short length of mechanical ventilation. The presence of secretions and modified diet recommendations were also correlated with altered laryngeal sensation. These results demonstrate that laryngeal sensory abnormalities impact the development of post-extubation dysphagia and underscore the importance of routine assessment of laryngeal sensation in this patient population.
Acknowledgments
Funding: National Institutes of Health (grant number: R21NR015886) provided funding for this study.
Footnotes
Conflicts of Interest: All authors declare that they have no conflict of interest.
Ethical Approval: All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval was obtained from each site’s Institutional Review Board.
Informed Consent: Informed consent was obtained from all participants prior to enrollment in this research study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–53. [DOI] [PubMed] [Google Scholar]
- 2.Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest. 2010;137(3):665–73. [DOI] [PubMed] [Google Scholar]
- 3.Macht M, Wimbish T, Clark BJ, Benson AB, Burnham EL, Williams A, et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care. 2011;15(5):R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodsky MB, Huang M, Shanholtz C, Mendez-Tellez PA, Palmer JB, Colantuoni E, et al. Recovery from Dysphagia Symptoms after Oral Endotracheal Intubation in Acute Respiratory Distress Syndrome Survivors. A 5-Year Longitudinal Study. Ann Am Thorac Soc. 2017;14(3):376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macht M, Wimbish T, Bodine C, Moss M. ICU-acquired swallowing disorders. Crit Care Med. 2013;41(10):2396–405. [DOI] [PubMed] [Google Scholar]
- 6.Leder SB, Suiter DM, Lisitano Warner H. Answering orientation questions and following single-step verbal commands: effect on aspiration status. Dysphagia. 2009;24(3):290–5. [DOI] [PubMed] [Google Scholar]
- 7.Metheny NA, Clouse RE, Chang YH, Stewart BJ, Oliver DA, Kollef MH. Tracheobronchial aspiration of gastric contents in critically ill tube-fed patients: frequency, outcomes, and risk factors. Crit Care Med. 2006;34(4):1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross RD, Atwood CW, Ross SB, Olszewski JW, Eichhorn KA. The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(7):559–65. [DOI] [PubMed] [Google Scholar]
- 9.Shaker R, Li Q, Ren J, Townsend WF, Dodds WJ, Martin BJ, et al. Coordination of deglutition and phases of respiration: effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am J Physiol. 1992;263(5 Pt 1):G750–5. [DOI] [PubMed] [Google Scholar]
- 10.Colton House J, Noordzij JP, Murgia B, Langmore S. Laryngeal injury from prolonged intubation: a prospective analysis of contributing factors. Laryngoscope. 2011;121(3):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheel R, Pisegna JM, McNally E, Noordzij JP, Langmore SE. Endoscopic Assessment of Swallowing After Prolonged Intubation in the ICU Setting. Ann Otol Rhinol Laryngol. 2016;125(1):43–52. [DOI] [PubMed] [Google Scholar]
- 12.Su H, Hsiao TY, Ku SC, Wang TG, Lee JJ, Tzeng WC, et al. Tongue weakness and somatosensory disturbance following oral endotracheal extubation. Dysphagia. 2015;30(2):188–95. [DOI] [PubMed] [Google Scholar]
- 13.Aviv JE, Spitzer J, Cohen M, Ma G, Belafsky P, Close LG. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112(2):338–41. [DOI] [PubMed] [Google Scholar]
- 14.Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia. 1988;2(4):216–9. [DOI] [PubMed] [Google Scholar]
- 15.Domer AS, Kuhn MA, Belafsky PC. Neurophysiology and Clinical Implications of the Laryngeal Adductor Reflex. Curr Otorhinolaryngol Rep. 2013;1(3):178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550(Pt 1):287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkmeier JM, Bielamowicz S, Takeda N, Ludlow CL. Modulation of laryngeal responses to superior laryngeal nerve stimulation by volitional swallowing in awake humans. J Neurophysiol. 2000;83(3):1264–72. [DOI] [PubMed] [Google Scholar]
- 18.Onofri SM, Cola PC, Berti LC, da Silva RG, Dantas RO. Correlation between laryngeal sensitivity and penetration/aspiration after stroke. Dysphagia. 2014;29(2):256–61. [DOI] [PubMed] [Google Scholar]
- 19.Murray J, Langmore SE, Ginsberg S, Dostie A. The significance of accumulated oropharyngeal secretions and swallowing frequency in predicting aspiration. Dysphagia. 1996;11(2):99–103. [DOI] [PubMed] [Google Scholar]
- 20.Kaneoka A, Pisegna JM, Inokuchi H, Ueha R, Goto T, Nito T, et al. Relationship Between Laryngeal Sensory Deficits, Aspiration, and Pneumonia in Patients with Dysphagia. Dysphagia. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–8. [DOI] [PubMed] [Google Scholar]
- 22.Donzelli J, Brady S, Wesling M, Craney M. Predictive value of accumulated oropharyngeal secretions for aspiration during video nasal endoscopic evaluation of the swallow. Ann Otol Rhinol Laryngol. 2003;112(5):469–75. [DOI] [PubMed] [Google Scholar]
- 23.Brodsky MB, De I, Chilukuri K, Huang M, Palmer JB, Needham DM. Coordination of Pharyngeal and Laryngeal Swallowing Events During Single Liquid Swallows After Oral Endotracheal Intubation for Patients with Acute Respiratory Distress Syndrome. Dysphagia. 2018;33(6):768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rousou JA, Tighe DA, Garb JL, Krasner H, Engelman RM, Flack JE, et al. Risk of dysphagia after transesophageal echocardiography during cardiac operations. Ann Thorac Surg. 2000;69(2):486–9. [DOI] [PubMed] [Google Scholar]
- 25.Barker J, Martino R, Reichardt B, Hickey EJ, Ralph-Edwards A. Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can J Surg. 2009;52(2):119–24. [PMC free article] [PubMed] [Google Scholar]
- 26.Brown CV, Hejl K, Mandaville AD, Chaney PE, Stevenson G, Smith C. Swallowing dysfunction after mechanical ventilation in trauma patients. J Crit Care. 2011;26(1):108.e9–13. [DOI] [PubMed] [Google Scholar]
- 27.Kwok AM, Davis JW, Cagle KM, Sue LP, Kaups KL. Post-extubation dysphagia in trauma patients: it's hard to swallow. Am J Surg. 2013;206(6):924–7; discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 28.Brodsky MB, González-Fernández M, Mendez-Tellez PA, Shanholtz C, Palmer JB, Needham DM. Factors associated with swallowing assessment after oral endotracheal intubation and mechanical ventilation for acute lung injury. Ann Am Thorac Soc. 2014;11(10):1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordon A, Bokhari R, Sperry J, Testa D, Feinstein A, Ghaemmaghami V. Swallowing dysfunction after prolonged intubation: analysis of risk factors in trauma patients. Am J Surg. 2011;202(6):679–82; discussion 82-3. [DOI] [PubMed] [Google Scholar]
- 30.Skoretz SA, Yau TM, Ivanov J, Granton JT, Martino R. Dysphagia and associated risk factors following extubation in cardiovascular surgical patients. Dysphagia. 2014;29(6):647–54. [DOI] [PubMed] [Google Scholar]
- 31.Ajemian MS, Nirmul GB, Anderson MT, Zirlen DM, Kwasnik EM. Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: implications for management. Arch Surg. 2001;136(4):434–7. [DOI] [PubMed] [Google Scholar]
- 32.El Solh A, Okada M, Bhat A, Pietrantoni C. Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med. 2003;29(9):1451–5. [DOI] [PubMed] [Google Scholar]
- 33.Barquist E, Brown M, Cohn S, Lundy D, Jackowski J. Postextubation fiberoptic endoscopic evaluation of swallowing after prolonged endotracheal intubation: a randomized, prospective trial. Crit Care Med. 2001;29(9):1710–3. [DOI] [PubMed] [Google Scholar]
- 34.Marvin S, Thibeault S, Ehlenbach WJ. Post-extubation Dysphagia: Does Timing of Evaluation Matter? Dysphagia. 2018. Advance online publication. doi: 10.1007/s00455-018-9926-3 [DOI] [PubMed] [Google Scholar]
- 35.Kaneoka A, Pisegna JM, Krisciunas GP, Nito T, LaValley MP, Stepp CE, et al. Variability of the Pressure Measurements Exerted by the Tip of Laryngoscope During Laryngeal Sensory Testing: A Clinical Demonstration. Am J Speech Lang Pathol. 2017;26(3):729–36. [DOI] [PubMed] [Google Scholar]
- 36.Hammer MJ. Design of a new somatosensory stimulus delivery device for measuring laryngeal mechanosensory detection thresholds in humans. IEEE Trans Biomed Eng. 2009;56(4):1154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneoka A, Krisciunas GP, Walsh K, Raade AS, Langmore SE. A comparison of 2 methods of endoscopic laryngeal sensory testing: a preliminary study. Ann Otol Rhinol Laryngol. 2015;124(3):187–93. [DOI] [PubMed] [Google Scholar]