Abstract
Background:
Endovascular repair of complex abdominal aortic aneurysms (cAAA) has become increasingly common, but reports have been mostly limited to single centers and single devices.
Methods:
We studied all endovascular repairs of cAAA (zone 6 or caudal) from 2014-2018 in the Vascular Quality Initiative (VQI). This included all commercially available fenestrated (FEVAR), chimney/snorkel repairs, and physician-modified devices (PMEG), exclusive of Investigational Device Exemptions (IDEs) and clinical trial devices. We used inverse probability weighted, multilevel logistic regression to compare rates of perioperative outcomes including death, acute kidney injury (AKI), major adverse cardiac events (MACE-the composite of death/stroke/myocardial infarction), and Cox regression for long-term mortality.
Results:
During the study period, surgeons performed 1396 endovascular cAAA repairs; 1308 (94%) elective, 63 (4.5%) for symptomatic aneurysms, and 25 (1.8%) for rupture. The number of centers performing endovascular cAAA repairs expanded steadily from 39 in 2014 to 81 in 2017. There were 880 FEVAR (63%), 256 PMEG (18%), and 260 chimney/snorkel repairs (19%). In elective cases, 3214 visceral vessels were incorporated and revascularized: 120 repairs (9%) involved one vessel, 481 (38%) repairs involved two vessels, 560 (44%) involved three vessels, and 113 (9%) involved four vessels. The mean number of arteries incorporated was 2.5±0.8, with PMEGs involving the most arteries (3.3 ± 0.8 for PMEG vs. 2.5 ± 0.6 for FEVAR and 1.9 ± 0.9 for chimney/snorkel, P < 0.001). PMEGs were used to treat more extensive aneurysms, and more incorporated the celiac and superior mesenteric arteries. There was no change in aneurysm extent, but the length of proximal seal extended over time. Chimney/snorkel cases employed more arm or neck access, had longer procedure times, and used more contrast. Rates of perioperative death (FEVAR: 3.4% vs PMEG: 2.7% vs Chimney/Snorkel: 6.1%, P = .13), and AKI (17% vs 18% vs 19%, P = .42) were similar, but chimney/snorkel was associated with higher rates of stroke (0.8% vs 0.9% vs 3.3%, P = .03), and MACE (6.1% vs 5.4% vs 11.7%, P = .02). After adjustment, rates of perioperative death, AKI, and overall complications remained similar, but chimney/snorkel was associated with significantly higher odds of stroke (OR 7.3 [1.5 – 36.4], P = .015), MI (OR 18.7 [2.6 – 136.8], P = .004), and MACE (OR 11.1 [2.1 – 58.9], P = .005). Overall survival following elective repair was 91% at one year and 88% at three years, with no difference between repair types in crude or adjusted analysis.
Conclusion:
The VQI provides a unique opportunity to study the real-world application and outcomes of complex endovascular aneurysm repair. Perioperative morbidity appears to be higher following chimney/snorkel repair, but further study is needed to confirm these findings and establish the durability of these novel technologies.
Keywords: Endovascular Repair, Juxtarenal, Abdominal Aortic Aneurysm, EVAR, FEVAR, PMEG, Chimney, Snorkel, VQI, Vascular Quality Initiative, Complex Abdominal Aortic Aneurysm, Pararenal
Here is the edited TOC summary:
This VQI study of 1396 complex abdominal aortic aneurysm (cAAA) repairs found that chimney/snorkel procedures were associated with higher rates of perioperative major adverse cardiac events and stroke than commercially available fenestrated repair or physician modified endografts despite treating less extensive aneurysms.
Introduction:
Since the introduction of endovascular aneurysm repair (EVAR) in 1991, its usage and indications have expanded dramatically.1–3 In the United States, the overwhelming majority of abdominal aortic aneurysms (AAA) are treated with EVAR, and recent data show that even ruptured AAA increasingly undergo EVAR.4,5 The renal-visceral segment of the abdominal aorta remains one of the final frontiers for endovascular repair, but surgeons developed a broad array of techniques to repair juxtarenal, pararenal, and thoracoabdominal aortic aneurysms. Strategies for managing renal and visceral arteries include fenestrated or branched endografts and chimney/snorkel/periscope techniques, known collectively as complex endovascular aneurysm repair. Although trials of off-the-shelf grafts are ongoing in the U.S., most devices are custom-manufactured endografts or physician-modified endografts (PMEG), designed for a patient’s specific anatomy.
After the first fenestrated endograft, the Zenith Fenestrated AAA Endovascular Graft (Cook Medical, Bloomington, IN) was approved by the FDA in 2012, complex endovascular repair has become increasingly common.6,7 In Medicare alone, the number of branched-fenestrated repairs increased from 335 in 2011 to 2,143 in 2013, an increase of over 600%.8 However, this figure likely underrepresents the true number of complex repairs, as it only captures patients enrolled in Medicare, the code for branched-fenestrated repair only recently became available, and many repairs, especially PMEG, are performed as part of clinical trials or physician-sponsored Investigational Device Exemptions (IDE), which would not be captured using Medicare billing data. Most reports of outcomes following complex endovascular repair are limited to clinical trials of devices, and single-center reports from high-volume surgeons.9–14 Consequently, it is difficult to understand how these complex repairs are implemented in real-world practice.
In 2010, the Society for Vascular Surgery formed the Vascular Quality Initiative (VQI), based on the Vascular Study Group of New England’s successful registry.15 The VQI captures data from 412 centers in 46 states as well as Canada. As of 2012, the VQI captured 15% of total AAA repairs in the United States, a number that has risen dramatically in the intervening years.16 Of note, the VQI does not report data on procedures performed under IDEs. This registry has been validated for accuracy and procedure capture, and is even considered a valid method of data reporting for eligibility for the Centers for Medicare and Medicaid Merit-Based Incentive Payments System.17 Hospitals undergo routine audits for data accuracy. Although other long-term outcomes such as re-intervention rates are not always well documented in the VQI, mortality is well captured and validated due to the linkage with the SSDI and Medicare. Consequently, we chose to study the usage patterns of complex endovascular repairs in this real-world registry.
Methods:
Patients:
We identified all patients undergoing endovascular repair of complex aneurysms in the VQI between 2014 and 2018. We defined a complex aneurysm as a proximal extent between the top of the celiac artery and the lowest renal artery, or an aneurysm with a proximal extent below the renal arteries that was repaired with at least one scallop, fenestration, branch, or chimney/snorkel into a renal or visceral artery. The VQI does not release data for patients enrolled in IDE studies or pivotal trials. Only commercially available FEVAR devices are included, unless a device was modified by the physician at the time of implant (PMEG) or chimneys/snorkels placed. The VQI records the number of endograft pieces, the renal and visceral arteries intervened upon, and the type of devices implanted (e.g. bare-metal stent, covered stent, etc., up to two devices per branch). The surgeon documents these variables after each case and enters whether each artery was fenestrated, scalloped, chimney/snorkeled, and whether or not a stent was placed. A PMEG was any repair in which at least one endograft was modified by the surgeon. For the cases in which there was a chimney placed alongside a commercially available FEVAR (n=25) or physician-modified endograft (n=10), we included them as chimneys for the purposes of baseline characteristics but excluded them from the analyses.We excluded hybrid procedures (n=16), complex repairs for indications other than aneurysm such as dissection, penetrating aortic ulcer, or thrombus (n=474), and patients with missing data on incorporation of renal/visceral arteries (n=575). This left us with a final sample of 1396 patients. The VQI records patient demographics, comorbid conditions, perioperative complications, one-year follow-up, and long-term mortality through linkage to the Social Security Death Index through March 7, 2018. The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived the need for patient consent due to the nature of the design and minimal risk to human subjects.
Definitions and Variables:
We calculated the estimated glomerular filtration rate (GFR) by the CKD-EPI formula. This allowed us to stratify chronic kidney disease by the National Kidney Foundation Guidelines into eGFR >60 mL/min/1.73m2, 45 – 60 mL/min/1.73m2, 30 – 44 mL/min/1.73m2, and < 30 mL/min/1.73m2. We classified smoking status as never, former, or current (i.e. within 30 days of the procedure), and stratified chronic obstructive pulmonary disease into none, mild-moderate, and requiring home oxygen. Congestive heart failure (CHF) we categorized as none, mild (NYHA Class I or II), or moderate to severe (NYHA Class III or IV). Age and maximum aortic diameter were divided into quintiles. Based on previous work, we considered preoperative anemia as a hemoglobin < 10 mg/dL.18 We classified postoperative renal dysfunction, or acute kidney injury (AKI), by the RIFLE criteria based on the peak postoperative creatinine value.19 The VQI defines stroke as any new motor or sensory loss, speech abnormality, or documentation of any other neurological symptoms related to the right or left hemisphere, lasting >24 hours. We defined perioperative death as occurring within 30 days or discharge dead from index hospitalization. Myocardial infarction (MI) includes elevated troponins or electrocardiographic changes with symptoms. Re-interventions and conversion to open refers to those performed during the index hospitalization. Access site complications included thrombosis, embolus, hematoma, and infection. The VQI records access site complications as a binary variable, without specifying which site the complication occurred in (arm, neck, groin, etc). We defined any complication as death, re-intervention, AKI, stroke, transient ischemic attack (TIA), access site complication, MI, new arrhythmia, new onset CHF, pneumonia, re-intubation, or mesenteric ischemia. A major adverse coronary event (MACE) was perioperative death, stroke, or MI. Hospital volume was classified into quintiles based on the average annual volume over the study period.
Statistical Analysis:
We compared categorical and continuous variables using chi-squared and ANOVA tests respectively. Case details, complications and survival were compared only for elective cases. To adjust for non-random assignment to each treatment, we calculated propensity scores using multinomial logistic regression models in which the three treatments were the outcome. These propensity scores were used to create inverse probability weights (the inverse of the probability of undergoing the treatment the subject received). We used propensity scores rather than standard multivariable regression as the relatively low absolute rates of the outcomes precluded robust adjustment. Because the number of events dictate the number of covariates allowed in a standard multivariable analysis (usually no less than 10 to 15 events per variable), this can often limit the robustness of multivariable analyses. In contrast, to construct propensity scores, every patient experiences the outcome of interest (in this case the repair type). This allows us to adjust for more covariates without the risk of overfitting. Propensity weighting offers an advantage over propensity matching in that it retains the entire sample size, and therefore may reduce the effect of unmeasured confounding. Covariates included in the propensity score models included age, sex, race, aortic diameter, prior aortic surgery, chronic kidney disease, diabetes, hypertension, COPD, coronary artery disease, prior myocardial infarction, congestive heart failure, smoking, body mass index, anemia, Medicaid/self-pay, aspirin, statin, beta-blocker, ACE or ARB inhibitor use, aneurysm extent, proximal extent of the repair, number of vessels incorporated, and quintiles of hospital volume. For perioperative outcomes, we used propensity-weighted multilevel logistic regression, clustering at the center level, and for long-term outcomes we constructed propensity-weighted Kaplan-Meier curves and used Cox regression. Our primary analysis controlled for the number of vessels incorporated, but as a separate sensitivity analysis we also looked at outcomes stratified by the number of vessels incorporated (excluding four-vessel cases since no commercially available fenestrated cases incorporated four vessels).
Results:
Patients:
During the study period, surgeons performed 1396 endovascular cAAA repairs. Of these, 1308 (94%) were performed in the elective setting, 63 (4.5%) were for symptomatic aneurysms, and 25 (1.8%) for rupture. There were 880 FEVAR (63%), 256 PMEG (18%), and 260 chimney/snorkel repairs (19%). Characteristics of the patient population, both overall and by repair type, are presented in Table IA, and the subset of elective cases is shown in Table IB. The majority of the patients were white males not on Medicaid or self-pay, although females comprised a significantly larger proportion (22%) of the study population than most reports of infrarenal EVAR.2,20–22 Ten percent of the patients had prior aortic surgery, most frequently those undergoing PMEG (25% vs. 6% for FEVAR/BEVAR and 12% for chimney/snorkel, P < .001). Cases involving symptomatic aneurysms more often involved PMEG (12%) or chimney/snorkel (11%), compared to 0.5% for FEVAR (P < .001). Ruptured aneurysms were more often repaired using chimney/snorkel (7%), compared to only 2% for PMEG and 0.3% for FEVAR (P < .001). Patients undergoing chimney/snorkel were more often female, anemic, and had CKD and hypertension, while PMEG patients had larger aneurysms, higher rates of COPD, and more often had prior aortic surgery. Comorbidities were distributed similarly in the elective cases.
Table IA.
Baseline Characteristics of the study population. MI: myocardial infarction. COPD: chronic obstructive pulmonary disease. GFR: glomerular filtration rate. Hb: hemoglobin
| Characteristic | Overall (n=1396) | FEVAR (n=880) | PMEG (n=256) | Chimney/Snorkel (n=260) | P |
|---|---|---|---|---|---|
| Age | 74 ± 8 | 73 ± 8 | 74 ± 8 | 75 ± 8 | 0.001 |
| Male Sex | 78 | 78 | 82 | 72 | 0.013 |
| White Race | 95 | 96 | 95 | 92 | 0.038 |
| Diameter (cm) | 6.1 ± 1.1 | 5.9 ± 1.0 | 6.6 ± 1.4 | 6.4 ± 1.4 | < 0.001 |
| <4.5 | 3 | 3 | 2 | 4 | |
| 4.5-5.0 | 7 | 7 | 4 | 8 | |
| 5.0-5.5 | 24 | 29 | 13 | 20 | |
| 5.5-6.0 | 27 | 30 | 24 | 19 | |
| 6.0-6.5 | 15 | 14 | 17 | 15 | |
| 6.5-7.5 | 14 | 11 | 21 | 15 | |
| 7.5+ | 11 | 6 | 19 | 19 | |
| Urgency | < 0.001 | ||||
| Elective | 94 | 99 | 86 | 82 | |
| Symptomatic | 5 | 0.5 | 12 | 11 | |
| Ruptured | 2 | 0.3 | 2 | 7 | |
| Hypertension | 87 | 85 | 87 | 91 | 0.048 |
| Diabetes | 19 | 20 | 18 | 17 | 0.34 |
| Coronary Artery Disease | 47 | 47 | 43 | 49 | 0.29 |
| Prior MI | 25 | 25 | 26 | 24 | 0.88 |
| Smoking | 0.62 | ||||
| Ever | 89 | 90 | 88 | 87 | |
| Current | 35 | 36 | 32 | 33 | |
| Former | 54 | 53 | 56 | 54 | |
| Congestive Heart Failure | 15 | 14 | 14 | 17 | 0.57 |
| mild-mod | 12 | 12 | 11 | 15 | |
| severe | 2 | 2 | 3 | 1.5 | |
| COPD | 36 | 36 | 40 | 35 | < 0.001 |
| mild-moderate | 30 | 32 | 29 | 27 | |
| on home oxygen | 6 | 4 | 10 | 8 | |
| Body Mass Index (BMI) | 0.19 | ||||
| Underweight | 2 | 2 | 2 | 3 | |
| Normal | 30 | 29 | 28 | 34 | |
| Overweight | 39 | 40 | 37 | 39 | |
| Obese | 26 | 27 | 29 | 22 | |
| Morbidly obese | 3 | 3 | 4 | 1 | 4 |
| Chronic Kidney Disease | 43 | 41 | 42 | 51 | 0.001 |
| GFR 30-60 | 37 | 36 | 34 | 40 | |
| GFR <30 | 5 | 4 | 5 | 8 | |
| Dialysis | 1.3 | 0.5 | 2.7 | 2.7 | |
| Anemic (Hb < 10) | 6 | 4 | 7 | 12 | < 0.001 |
| Medicaid/Self-pay Medications | 2 | 3 | 3 | 2 | 0.61 |
| Aspirin | 68 | 68 | 71 | 65 | 0.36 |
| Statin | 77 | 76 | 81 | 74 | 0.10 |
| Beta Blocker | 60 | 59 | 57 | 67 | 0.043 |
| ACE/ARB | 49 | 50 | 44 | 53 | 0.11 |
| Prior Aortic Surgery | 10 | 6 | 25 | 12 | < 0.001 |
Table IB.
Baseline Characteristics of Elective Cases. MI: myocardial infarction. COPD: chronic obstructive pulmonary disease. GFR: glomerular filtration rate. Hb: hemoglobin
| Characteristic | Overall (n=1274) | FEVAR (n=873) | PMEG (n=221) | Chimney/Snorkel (n=180) | P |
|---|---|---|---|---|---|
| Age | 74 ± 8 | 73 ± 8 | 73 ± 8 | 75 ± 8 | 0.005 |
| Male Sex | 78 | 78 | 82 | 72 | 0.042 |
| White Race | 95 | 96 | 96 | 93 | 0.16 |
| Diameter (cm) | 6.1 ± 1.1 | 5.9 ± 1.0 | 6.4 ± 1.2 | 6.3 ± 1.4 | < 0.001 |
| <4.5 | 3 | 3 | 2 | 3 | |
| 4.5-5.0 | 7 | 7 | 4 | 10 | |
| 5.0-5.5 | 25 | 29 | 14 | 21 | |
| 5.5-6.0 | 28 | 30 | 28 | 18 | |
| 6.0-6.5 | 15 | 14 | 18 | 17 | |
| 6.5-7.5 | 13 | 11 | 21 | 15 | |
| 7.5+ | 9 | 6 | 13 | 16 | |
| Hypertension | 87 | 85 | 88 | 93 | 0.006 |
| Diabetes | 19 | 21 | 17 | 17 | 0.30 |
| Coronary Artery Disease | 47 | 47 | 43 | 50 | 0.33 |
| Prior MI | 25 | 25 | 25 | 24 | 0.97 |
| Smoking | 0.47 | ||||
| Ever | 90 | 90 | 91 | 91 | |
| Current | 35 | 36 | 33 | 32 | |
| Former | 55 | 53 | 58 | 59 | |
| Congestive Heart Failure | 15 | 14 | 14 | 17 | 0.68 |
| mild-mod | 12 | 12 | 11 | 15 | |
| severe | 2 | 2 | 3 | 2 | |
| COPD | 36 | 36 | 40 | 35 | < 0.001 |
| mild-moderate | 31 | 32 | 30 | 28 | |
| on home oxygen | 6 | 4 | 11 | 8 | |
| Body Mass Index (BMI) | 0.27 | ||||
| Underweight | 2 | 2 | 1 | 3 | |
| Normal | 30 | 29 | 28 | 33 | |
| Overweight | 40 | 39 | 38 | 42 | |
| Obese | 22 | 27 | 28 | 22 | |
| Morbidly obese | 1 | 3 | 5 | 1 | |
| Chronic Kidney Disease | 43 | 41 | 44 | 52 | 0.001 |
| GFR 30-60 | 37 | 37 | 35 | 43 | |
| GFR <30 | 4.7 | 4 | 6 | 7 | |
| Dialysis | 1.1 | 0.5 | 3.2 | 1.9 | |
| Anemic (Hb < 10) | 5 | 4 | 5 | 9 | 0.023 |
| Medicaid/Self-pay | 2.2 | 2.4 | 2.7 | 1 | 0.36 |
| Medications | |||||
| Aspirin | 69 | 69 | 72 | 70 | 0.61 |
| Statin | 78 | 76 | 82 | 79 | 0.24 |
| Beta Blocker | 60 | 59 | 55 | 68 | 0.015 |
| ACE/ARB | 50 | 50 | 46 | 55 | 0.16 |
| Prior Aortic Surgery | 10 | 6 | 23 | 12 | < 0.001 |
Centers:
The number of centers performing endovascular cAAA repairs expanded steadily from 39 in 2014 to 81 in 2017 (Figure 1). Including 2018 (a partial year), there were a total of 98 centers where are least one repair occurred during the study period. All three repair types increased in number from 2014 to 2016, but the number of PMEGs leveled off by 2017 whereas the number of FEVAR and chimney/snorkels continued to rise steadily (Figure 2). It is worth noting that PMEGs performed under IDEs are not reported by the VQI, and so this may undercapture PMEGs as several centers obtained IDEs during the study period (e.g a center may report their PMEGs from 2014-2016, but then after obtaining an IDE in 2016, subsequent PMEGs would not be reported). The mean number of repairs performed annually per center was 13 ± 11, with a median of 10 (interquartile range 5 – 19), and a range of 1 to 44. Centers annually performed a median of 6 [2 – 11] FEVAR repairs, 0 [0 – 3] PMEG, and 0 [0 – 3] chimney/snorkels. All but 3 centers performed FEVAR, but only 31 (32%) performed at least one PMEG, and only 45 (46%) at least one chimney. Only 18 centers (18%) performed all three repair types.
Figure 1.
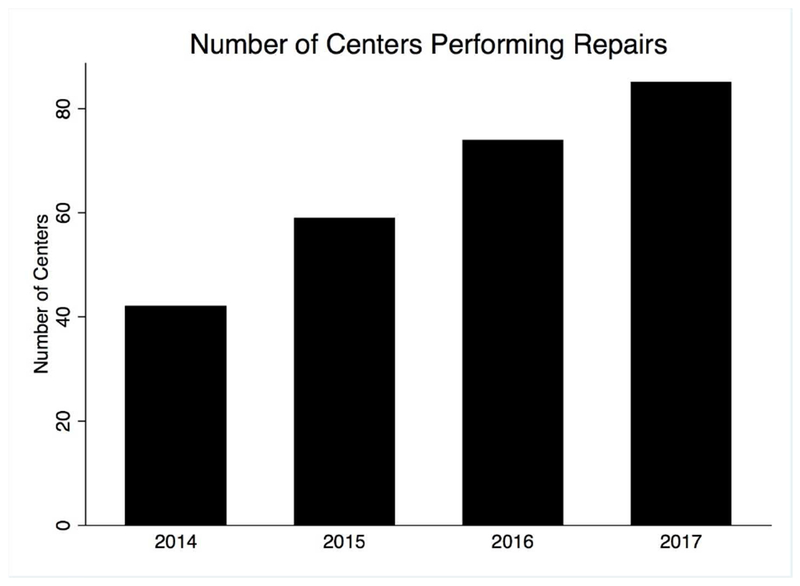
The number of centers recording endovascular complex abdominal aortic aneurysm repairs in the VQI each year.
Figure 2.
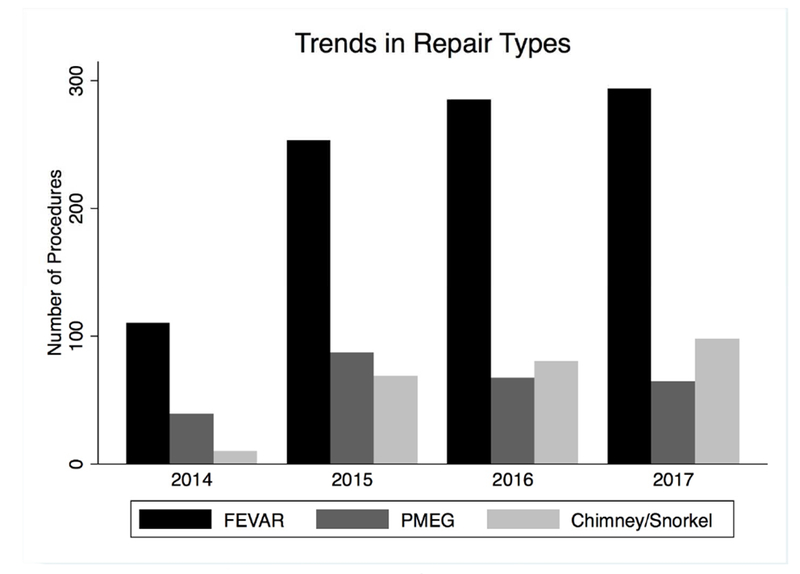
The annual number of repairs by each repair type. FEVAR: fenestrated, PMEG: physician-modified endograft.
Case Details, Elective Cases:
3214 renal/visceral vessels were incorporated and revascularized: 120 repairs (9%) involved one vessel, 481 (38%) repairs involved two vessels, 560 (44%) involved three vessels, and 113 (9%) involved four vessels. Case details for elective cases are presented in Table II. The mean number of arteries incorporated was 2.5 ± 0.8, with PMEGs involving the most arteries (3.3 ± 0.8 for PMEG vs. 2.5 ± 0.6 for FEVAR and 1.9 ± 0.9 for chimney/snorkel, P < .001).
Table II.
Procedural characteristics of the elective cases. Details presented as % or mean ± standard deviation.
| Procedural Characteristics | Overall | FEVAR | PMEG | Chimney/Snorkel | P |
|---|---|---|---|---|---|
| General Anesthesia | 98 | 98 | 98 | 97 | 0.49 |
| At least one side Perc | 65 | 63 | 77 | 61 | < 0.001 |
| Bilateral Percutaneous | 60 | 58 | 73 | 50 | < 0.001 |
| Percutaneous Success | 95 | 94 | 97 | 99 | 0.062 |
| Arm/neck access | 25 | 11 | 19 | 91 | < 0.001 |
| Procedural Time | 242 ±105 | 238 ±100 | 239±118 | 263 ±101 | 0.005 |
| Diameter (cm) | 6.1 ± 1.1 | 5.9 ± 1.0 | 6.4 ± 1.2 | 6.3 ± 1.4 | < 0.001 |
| Vessels incorporated | 2.5 ± 0.8 | 2.5 ± 0.6 | 3.3 ± 0.8 | 1.9 ± 0.9 | < 0.001 |
| Celiac Artery | 11.6 | 1.6 | 55 | 7.2 | < 0.001 |
| SMA | 59 | 55 | 90 | 37 | < 0.001 |
| Right Renal Artery | 91 | 95 | 94 | 69 | < 0.001 |
| Left Renal Artery | 91 | 94 | 91 | 76 | < 0.001 |
| Blood Loss | 411±532 | 397 ± 488 | 458±712 | 416±486 | 0.31 |
| Contrast Volume | 123 ± 73 | 124 ± 65 | 101 ± 69 | 144 ± 95 | < 0.001 |
| Fluoro Time | 69 ± 36 | 70 ± 36 | 65 ± 35 | 69 ± 36 | 0.26 |
Chimney/snorkel cases employed more arm or neck access, had longer procedure times, and used more contrast. PMEG were more often performed through bilateral percutaneous access. Aneurysms treated with PMEG extended more proximally, and landing zones were more proximal as well (Figure 3). FEVAR was used to treat less proximal aneurysms (unsurprising given that the only commercially available fenestrated device is somewhat limited in the proximal extent of disease that fits within IFU). Correspondingly, cases employing PMEGs more often involved revascularization of the celiac and superior mesenteric arteries (Table II). There was no change in aneurysm extent over the course of the study period (p = .48), but landing zones moved more proximally (Figure 4). The mean number of arteries revascularized per case did not change over time. Surgeons landed 9% of their grafts in zone 5 in 2014 compared to 13% in 2017, and the amount of proximal seal increased from half a zone in 2014 to 1.5 zones in 2018 (P < .001). For reference, an aneurysm with a proximal extent in zone 9 (infrarenal) with a proximal landing zone in zone 8 (between the highest and lowest renal arteries) would represent a difference of 1.
Figure 3.
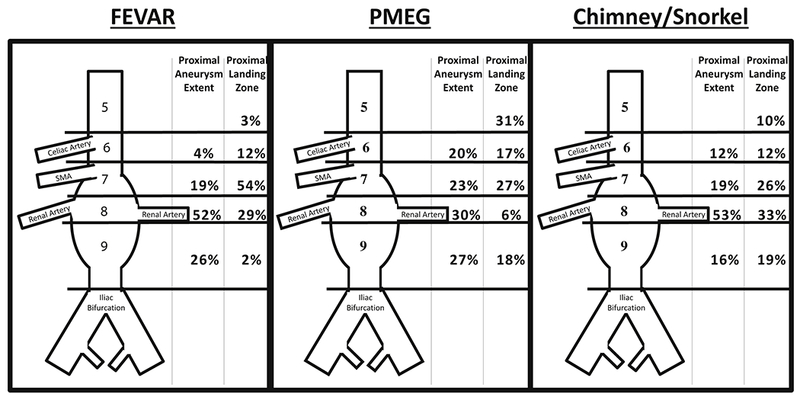
Distribution of Aneurysm Extents and Landing Zones between the Repair Types. FEVAR: fenestrated,. PMEG: physician modified endograft. SMA: superior mesenteric artery.
Figure 4.
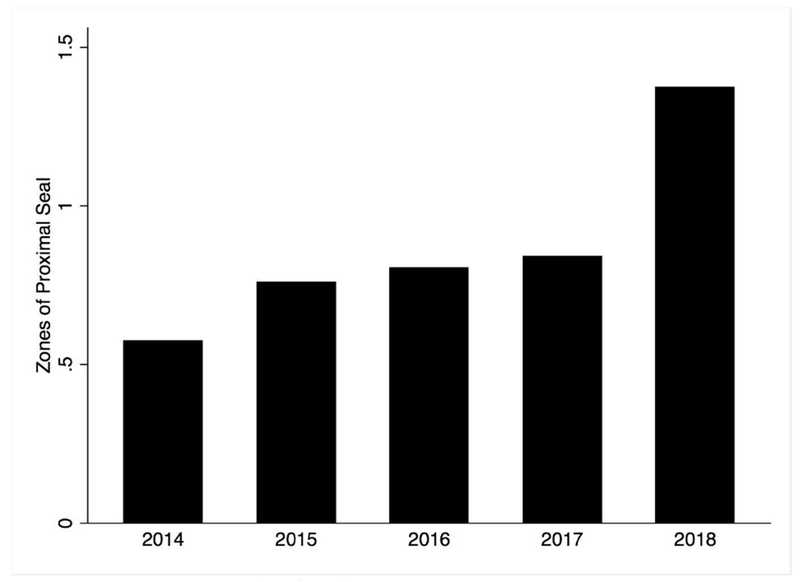
Differences between Proximal Aneurysm Extent and Proximal Landing Zone. For reference, an aneurysm with a proximal extent in zone 9 (infrarenal) with a proximal landing zone in zone 8 (between the highest and lowest renal arteries) would represent a difference of 1. P < .001 for differences over the years.
Perioperative Complications, Elective Cases
Perioperative complications (i.e. in-hospital complications) of elective cases are presented in Table III. In unadjusted analyses, perioperative mortality was similar between repair types (FEVAR 3.4% vs. PMEG: 2.7% vs. Chimney/Snorkel: 6.1%, P = .13), as were rates of AKI (17% vs 18% vs 19%, P = .42) and overall complication rates (27% vs 29% vs 34%, P = .16). However, chimney/snorkel was associated with higher rates of stroke (0.8% vs 0.9% vs 3.3%, P = .027), the composite of stroke/death (4.0% vs 3.6% vs 8.3%, P = .031), and MACE (6.1% vs 5.4% vs 11.7%, P = .017). Cases involving either arm or neck access were associated with higher rates of access site issues (10.2% vs 5.6%, P = .003), as well as higher stroke rates (3.2% vs 0.5%, P < .001). This was true even if the adjunctive access site was only used for a brachial-femoral wire (aka “body-floss”) alone (5.1%).
Table III.
Perioperative Complications in Elective Cases. With the exception of perioperative death, rates refer to in-hospital events. Perioperative death is death within 30-days or within the index hospitalization. MACE: major adverse cardiac event: composite of perioperative death, in-hospital stroke and in-hospital myocardial infarction. ESRD: end-stage renal disease. TIA: transient ischemic attack. Risk, Injury, Failure, Loss of Function and ESRD are defined according to the RIFLE criteria.
| Complication | Overall | FEVAR (n=873) | PMEG (n=221) | Chimney/Snorkel (n=180) | P |
|---|---|---|---|---|---|
| Perioperative Death | 3.8 | 3.4 | 2.7 | 6.1 | 0.13 |
| Stroke/Death | 4.6 | 4.0 | 3.6 | 8.3 | 0.031 |
| MACE | 6.8 | 6.1 | 5.4 | 11.7 | 0.017 |
| Any Complication | 29 | 27 | 29 | 34 | 0.16 |
| Length of Stay | 6.0 ± 25 | 6.4 ± 29 | 4.6 ± 6 | 5.1 ± 5 | 0.54 |
| Acute Kidney Injury | 17 | 17 | 18 | 19 | 0.42 |
| Risk | 7 | 7 | 6 | 4 | |
| Injury | 4 | 4 | 4 | 6 | |
| Failure | 4 | 4 | 6 | 5 | |
| Loss of Function | 1 | 1 | 0.5 | 1.9 | |
| ESRD | 1.5 | 1.3 | 1.4 | 2.3 | |
| Reintervention | 5 | 5 | 2.7 | 7 | 0.25 |
| aneurysm-related | 4 | 4 | 2.7 | 6 | |
| unrelated | 0.9 | 1 | 0 | 1.4 | |
| conversion to open | 0.3 | 0.2 | 0.5 | 0.5 | 0.78 |
| Access Site Issue | 6 | 6 | 6 | 10 | 0.066 |
| Pulmonary | 4 | 3.9 | 4.1 | 6.5 | 0.23 |
| pneumonia | 1.5 | 1.6 | 1.8 | 1.4 | |
| reintubation | 3 | 2.5 | 3.2 | 5.6 | |
| Mesenteric/Colonic Ischemia | 2.5 | 2.5 | 2.2 | 2.3 | 0.89 |
| medical management | 1 | 1.2 | 0.9 | 0.5 | |
| surgical intervention | 1.5 | 1.4 | 1.4 | 1.9 | |
| Cerebrovascular | 1.2 | 0.8 | 0.9 | 3.3 | 0.027 |
| TIA | 0.4 | 0.1 | 0.5 | 1.4 | |
| Stroke | 0.8 | 0.7 | 0.5 | 1.9 | |
| Arrhythmia | 6.7 | 6.9 | 5.0 | 7.8 | 0.49 |
| Myocardial Infarction | 3.4 | 3 | 2.7 | 5.6 | 0.14 |
Adjusted odds ratios are presented in Table IV. In adjusted analysis, rates of perioperative death, AKI, and overall complications remained similar, but chimney/snorkel was associated with significantly higher odds of stroke (OR 7.3 [1.5 – 36.4], P = .015), MI (OR 18.7 [2.6 – 136.8], P = .004), and MACE (OR 11.1 [2.1 – 58.9], P = .005). Results were consistent when stratified by the number of arteries incorporated in the repair.
Table IV.
Adjusted odds ratios for perioperative outcomes.
Models adjusted for: age, sex, race, aortic diameter, prior aortic surgery, chronic kidney disease, diabetes, hypertension, COPD, coronary artery disease, prior myocardial infarction, congestive heart failure, smoking, body mass index, anemia, Medicaid/self-pay, aspirin, statin, beta-blocker, ACE or ARB inhibitor use, aneurysm extent, proximal extent of the repair, number of vessels incorporated, and quintiles of hospital volume
| Outcome | Odds Ratio [95% CI] | P Value |
|---|---|---|
| Death | FEVAR is referent value | |
| PMEG | 0.7 [0.3 - 2.0] | 0.58 |
| Chimney | 1.2 [0.5 - 3.1] | 0.68 |
| AKI | ||
| PMEG | 1.1 [0.7 - 1.9] | 0.62 |
| Chimney | 0.7 [0.3 - 1.7] | 0.39 |
| Any Complication | ||
| PMEG | 1.0 [0.7 - 1.3] | 0.78 |
| Chimney | 2.8 [0.6 - 11.8] | 0.17 |
| Stroke | ||
| PMEG | 0.5 [0.1 - 3.0] | 0.49 |
| Chimney | 7.3 [1.5 - 36.4] | 0.015 |
| MI | ||
| PMEG | 0.8 [0.3 - 2.5] | 0.75 |
| Chimney | 18.7 [2.6 - 136.8] | 0.004 |
| Stroke/Death | ||
| PMEG | 0.8 [0.3 - 1.7] | 0.52 |
| Chimney | 2.3 [0.7 - 8.0] | 0.18 |
| MACE | ||
| PMEG | 0.7 [0.3 - 1.6] | 0.36 |
| Chimney | 11.1 [2.1 - 58.9] | 0.005 |
Complications Based on Arteries Incorporated, Elective Cases
There were 1144 repairs in which both renal arteries were revascularized (83%), and 990 cases involving at least one mesenteric vessel (63%). Excluding dialysis patients, the rate of AKI varied inversely with the number of renal arteries incorporated (no renals: 60%, one renal: 26%, both renals: 16%, P < .001). There were 849 repairs in which are least one mesenteric vessel was incorporated (61%), but rates of postoperative bowel ischemia were similar regardless of visceral vessel incorporation (P = .25).
Medium-Term Survival, Elective Cases
Overall survival following elective repair was 91% at one year and 88% at three years. There was no difference between procedure types (3-year survival: 90% FEVAR vs. 87% PMEG vs. 85% chimney/snorkel, P = .19 (Figure 5). After adjustment, there was no association between procedure type and mortality (P = .40).
Figure 5.
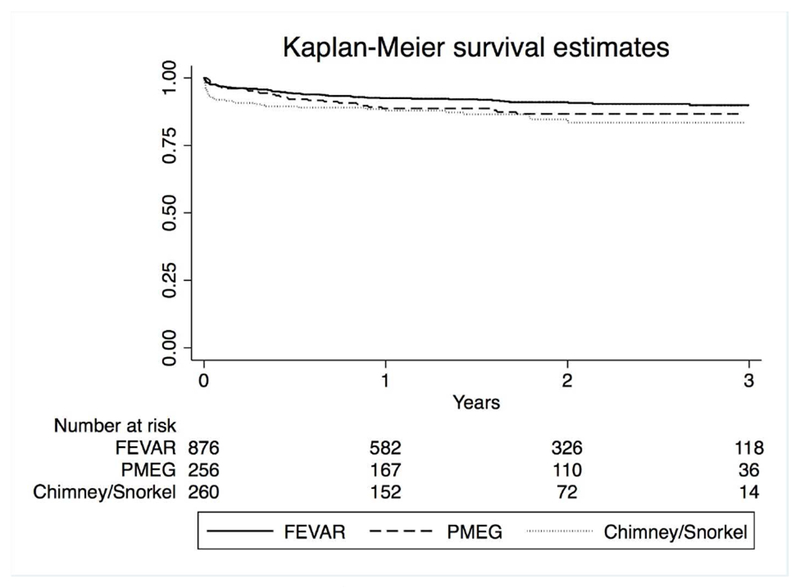
Medium-term survival by repair type. Standard errors < 0.1. P = .19.
Discussion:
This study demonstrates the unique potential of the VQI to study the contemporary application of endovascular therapies for complex aortic pathology. With over 2,000 repairs, this represents the largest registry of complex cases to date. Early results from these complex cases demonstrate acceptable perioperative morbidity and mortality as well as medium-term survival, although chimney/snorkel cases were associated with higher rates of perioperative morbidity.
Endovascular repairs of complex AAAs utilize a relatively new technology, with the first fenestrated endograft approved by the FDA in 2012. Despite this, fenestrated repairs rapidly proliferated, with the number of repairs increasing more than 6-fold between 2011 and 2013.8 As with any novel technology, it is important to rigorously evaluate its outcomes. To date, however, reports have been limited to pivotal trials and single-center reports.7,14,23,24 Although these reports are important, these results do not always translate into real-world practice. Clinical trials enroll carefully selected patients and rigorously adhere to the manufacturer’s instructions for use (IFU). In addition, they are performed by experienced, high-volume surgeons. In contrast, a recent analysis of the only FDA-approved fenestrated device (Cook Zenith Fenestrated-ZFEN, Cook Medical, Bloomington, IN) showed that between 2012 and 2015, 77% of the physicians who attended training sessions ordered less than 6 devices, with the average physician ordering only three devices per year.25 Complex endovascular repairs are more likely to mirror the volume-outcome relationships seen in the more challenging cases such as open surgery rather than more straightforward infrarenal EVAR, so the results from high-volume centers are unlikely to generalize to the population at large.26,27
This study demonstrates the unique potential of the VQI to reveal how trial results translate to the real world. Administrative data sets such as Medicare lack clinical granularity on details such as aneurysm size and extent, as well as important operative details such as the number of vessels incorporated and by what manner (scallop, fenestration, etc). Other registries such as NSQIP have smaller sample sizes and are limited to 30-day outcomes. The VQI provides both the necessary granular detail, and longer-term outcomes which allow for a detailed analysis. Increasingly, the FDA turns to these registries for further evidence of device safety. For example, the FDA recently approved a new indication for transcatheter aortic valve replacement (TAVR) based on data from the Society of Thoracic Surgery/American College of Cardiology’s Transcatheter Valve Therapy (TVT) registry.28–30 Also, the FDA, physicians and industry sponsors initiated a collaboration utilizing the VQI for post-market surveillance of outcomes of thoracic endovascular aneurysm repair (TEVAR) for the treatment of acute and chronic type B aortic dissection.31 Our study reveals the potential for the VQI to provide similar data for other novel technologies such as PMEG and custom-manufactured grafts.
Mortality rates in this study are similar to those from previous reports, with elective mortality of 3-7% depending on repair type.7,10,32–36 Of note, the 2.7% perioperative mortality following PMEG is similar to the initial series reported by Starnes et al. (3.8% and 5.1% mortality in the two series), Scali et al. (6.3%), and the systematic review by Georgiadis et al. (3.2%).11,12,37,38 However, it is worth noting the wide range of aortic pathologies treated in this series, ranging from four-vessel PMEGs to single vessel fenestrations. It is therefore difficult to directly compare the whole of this report to previous case series. Consequently, the primary purpose of this study is not to compare the results in the VQI to previous literature, but rather to demonstrate the potential to use the VQI to perform these comparisons in the future.
Although this report is mostly exploratory and descriptive in nature, the higher morbidity and trend towards higher mortality following chimney/snorkel repairs is worthy of further study. Even after adjustment, chimney/snorkel was associated with eleven-fold the odds of MACE as FEVAR or PMEG cases, and significantly higher odds of stroke and MI. Previous publications on chimney/snorkel repairs have demonstrated mixed results, with 30-day mortality ranging from 0. 4% to the 6.6% in this current study.32,39–44 In the largest systematic review of 28 studies including 1748 patients undergoing FEVAR and 757 undergoing chimney/snorkel, chimney/snorkel procedures were associated with twice the 30-day mortality rate of FEVAR (4% vs 2%).41 However, two earlier systematic reviews as well as a single-center comparison found no significant differences in perioperative mortality.40,43,44 Chimney/snorkel repairs in our report also involved significantly more arm/neck access, with 90% of repairs involving at least one adjunctive access site. Consequently, it is not surprising that these patients suffered higher rates of stroke/TIA, likely due to more wire and catheter manipulation in the aortic arch. Although there was no statistically significant difference, there was a trend towards higher rates of perioperative death following chimney/snorkel repairs that merits further study.
A note of caution is warranted when interpreting the results of this study. These three techniques are not necessarily applicable to every patient, and are not available at every center. For example, commercially available FEVAR devices can only incorporate three vessels, and takes weeks for a graft to be made and delivered. Some centers prohibit the use of PMEG, as it is not an FDA-approved technique. In addition, our sample size and event rates limit our power to detect a difference so further study is needed before any definitive conclusions can be made. Chimney/snorkel repairs remain important options for complex repairs in symptomatic or ruptured patients where custom-made endografts may not be available given time constraints. The aneurysm sizes and extents treated by the three device types in this study are significantly different, and individual centers may vary in their experience and comfort with each repair type. As such, these results should be considered exploratory in nature; even with robust multivariable analyses, we cannot account for all of the important clinical factors that weigh into the choice of repair modality.
This study must be interpreted in the context of its design. As the VQI relies on surgeon reporting of procedural characteristics, as well as the specific intervention, coding errors are always possible. The VQI also does not capture cases performed under IDEs, so we lack data from certain high-volume surgeons and centers. As the VQI is a voluntary quality initiative, we do not know how the results of surgeons who opted to join the VQI generalize to the wider population. It is also worthy of note that once a surgeon’s IDE is approved, their results are no longer released to investigators using the VQI. As a result, the results from experienced, high-volume surgeons that currently have IDEs may be limited to the early part of the learning curve. This is especially true for novel techniques such as PMEG. We also do not know if chimney or snorkel grafts were used as a rescue, and not as a planned parallel graft, which could bias towards inferior outcomes with chimney/snorkel. Additionally, the VQI blinds the specific endograft used, so we cannot make comparisons between different graft types. Although mortality is well captured through the linkage to the Social Security Death Index, data on reinterventions are lacking due to low follow-up rates. Fortunately, the recent linkage to Medicare will improve the capture of these data going forward.
Conclusion:
The VQI provides a unique opportunity to study the real-world application and outcomes of complex endovascular aneurysm repair. Perioperative morbidity and mortality appear to be higher following chimney/snorkel repair, but further study is needed to confirm these findings and establish the durability of these novel technologies.
Key Findings:
This study of 1396 complex abdominal aortic aneurysm (cAAA) repairs found that chimney/snorkel procedures were associated with higher rates of perioperative major adverse cardiac events and stroke than commercially available fenestrated repair or physician modified endografts despite treating less extensive aneurysms.
Take Home Message:
Results of endovascular repair of cAAAs are promising, but longer-term VQI data are needed, especially on chimney/snorkel.
Acknowledgments
TO, SD and PL are supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734-22
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as a poster at the 2018 Society for Vascular Surgery Annual Meeting in Boston, MA
References:
- 1.Parodi JC, Palmaz JC, Barone HD. Transfemoral Intraluminal Graft Implantation for Abdominal Aortic Aneurysms. Ann Vasc Surg. 1991. November;5(6):491–9. [DOI] [PubMed] [Google Scholar]
- 2.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015. July 23;373(4):328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. Open Repair of Abdominal Aortic Aneurysms in the Medicare Population. N Engl J Med. 2008. January 31;358(5):464–74. [DOI] [PubMed] [Google Scholar]
- 4.Bastos Gonqalves F, Ultee KHJ, Hoeks SE, Stolker RJ, Verhagen HJM. Life expectancy and causes of death after repair of intact and ruptured abdominal aortic aneurysms. J Vasc Surg. 2016. March;63(3):610–6. [DOI] [PubMed] [Google Scholar]
- 5.Ultee KHJ, Zettervall SL, Soden PA, Buck DB, Deery SE, Shean KE, et al. The impact of endovascular repair on management and outcome of ruptured thoracic aortic aneurysms. J Vasc Surg. 2017. August;66(2):343–352.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinones-Baldrich WJ, Holden A, Mertens R, Thompson MM, Sawchuk AP, Becquemin J-P, et al. Prospective, multicenter experience with the Ventana Fenestrated System for juxtarenal and pararenal aortic aneurysm endovascular repair. J Vasc Surg. 2013. July;58(1):1–9. [DOI] [PubMed] [Google Scholar]
- 7.Oderich GS, Greenberg RK, Farber M, Lyden S, Sanchez L, Fairman R, et al. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2014. December;60(6):1420–1428.e5. [DOI] [PubMed] [Google Scholar]
- 8.Suckow BD, Goodney PP, Columbo JA, Kang R, Stone DH, Sedrakyan A, et al. National trends in open surgical, endovascular, and branched-fenestrated endovascular aortic aneurysm repair in Medicare patients. J Vasc Surg. 2017. December 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahverdyan R, Majd MP, Thul R, Braun N, Gawenda M, Brunkwall J. F-EVAR does not Impair Renal Function more than Open Surgery for Juxtarenal Aortic Aneurysms: Single Centre Results. Eur J Vasc Endovasc Surg. 2015. October;50(4):432–41. [DOI] [PubMed] [Google Scholar]
- 10.British Society for Endovascular Therapy and the Global Collaborators on Advanced Stent-Graft Techniques for Aneurysm Repair (GLOBALSTAR) Registry. Early Results of Fenestrated Endovascular Repair of Juxtarenal Aortic Aneurysms in the United Kingdom. Circulation. 2012. June 5;125(22):2707–15. [DOI] [PubMed] [Google Scholar]
- 11.Starnes BW, Heneghan RE, Tatum B. Midterm results from a physician-sponsored investigational device exemption clinical trial evaluating physician-modified endovascular grafts for the treatment of juxtarenal aortic aneurysms. J Vasc Surg. 2017. February;65(2):294–302. [DOI] [PubMed] [Google Scholar]
- 12.Starnes BW, Tatum B. Early report from an investigator-initiated investigational device exemption clinical trial on physician-modified endovascular grafts. J Vasc Surg. 2013. August;58(2):311–7. [DOI] [PubMed] [Google Scholar]
- 13.Oderich GS, Fatima J, Gloviczki P. Stent graft modification with mini-cuff reinforced fenestrations for urgent repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2011. November;54(5):1522–6. [DOI] [PubMed] [Google Scholar]
- 14.Mastracci TM, Eagleton MJ, Kuramochi Y, Bathurst S, Wolski K. Twelve-year results of fenestrated endografts for juxtarenal and group IV thoracoabdominal aneurysms. J Vasc Surg. 2015. February;61(2):355–64. [DOI] [PubMed] [Google Scholar]
- 15.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW, et al. A regional registry for quality assurance and improvement: The Vascular Study Group of Northern New England (VSGNNE). J Vasc Surg. 2007. December;46(6):1093–1102.e1. [DOI] [PubMed] [Google Scholar]
- 16.Beck AW, Sedrakyan A, Mao J, Venermo M, Faizer R, Debus S, et al. Variations in Abdominal Aortic Aneurysm Care: A Report From the International Consortium of Vascular Registries. Circulation. 2016. December 13;134(24):1948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI®) Annual Claims Validation Process for 100% Procedure Entry.
- 18.Pothof AB, Bodewes TCF, O’Donnell TFX, Deery SE, Shean K, Soden PA, et al. Preoperative anemia is associated with mortality after carotid endarterectomy in symptomatic patients. J Vasc Surg. 2018. January 16;67(1):183–190.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup the A. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004. August; 8(4):R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karthikesalingam A, Vidal-Diez A, Holt PJ, Loftus IM, Schermerhorn ML, Soden PA, et al. Thresholds for Abdominal Aortic Aneurysm Repair in England and the United States. N Engl J Med. 2016. November 24;375(21):2051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016. November 12;388(10058):2366–74. [DOI] [PubMed] [Google Scholar]
- 22.Powell JT, Sweeting MJ, Ulug P, Blankensteijn JD, Lederle FA, Becquemin J-P, et al. Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm over 5 years. Br J Surg. 2017. February;104(3):166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg RK, Chuter TAM, Sternbergh WC, Fearnot NE, Zenith Investigators. Zenith AAA endovascular graft: intermediate-term results of the US multicenter trial. J Vasc Surg. 2004. June;39(6):1209–18. [DOI] [PubMed] [Google Scholar]
- 24.Farber MA, Eagleton MJ, Mastracci TM, McKinsey JF, Vallabhaneni R, Sonesson B, et al. Results from multiple prospective single-center clinical trials of the off-the-shelf p-Branch fenestrated stent graft. J Vasc Surg. 2017. October;66(4): 982–90. [DOI] [PubMed] [Google Scholar]
- 25.Simons JP, Shue B, Flahive JM, Aiello FA, Steppacher RC, Eaton EA, et al. Trends in use of the only Food and Drug Administration-approved commercially available fenestrated endovascular aneurysm repair device in the United States. J Vasc Surg. 2017. May;65(5): 1260–9. [DOI] [PubMed] [Google Scholar]
- 26.Zettervall SL, Schermerhorn ML, Soden PA, McCallum JC, Shean KE, Deery SE, et al. The effect of surgeon and hospital volume on mortality after open and endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017. March;65(3): 626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landon BE, O’Malley AJ, Giles K, Cotterill P, Schermerhorn ML. Volume-outcome relationships and abdominal aortic aneurysm repair. Circulation. 2010. September 28;122(13):1290–7. [DOI] [PubMed] [Google Scholar]
- 28.Holmes DR, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, et al. Annual Outcomes With Transcatheter Valve Therapy. J Am Coll Cardiol. 2015. December 29;66(25):2813–23. [DOI] [PubMed] [Google Scholar]
- 29.FDA Used Real-World Evidence in Heart Valve Approval | RAPS [Internet]. [cited 2018 Apr 11]. Available from: https://www.raps.org/regulatory-focus™/news-articles/2017/6/fda-used-real-world-evidence-in-heart-valve-approval [Google Scholar]
- 30.Mack MJ, Holmes DR. Rational Dispersion for the Introduction of Transcatheter Valve Therapy. JAMA. 2011. November 16;306(19):2149–50. [DOI] [PubMed] [Google Scholar]
- 31.Beck AW, Lombardi JV, Abel DB, Morales JP, Marinac-Dabic, Wang G, et al. Innovative postmarket device evaluation using a quality registry to monitor thoracic endovascular aortic repair in the treatment of aortic dissection. J Vasc Surg. 2017. May;65(5):1280–6. [DOI] [PubMed] [Google Scholar]
- 32.Donas KP, Lee JT, Lachat M, Torsello G, Veith FJ, PERICLES investigators. Collected World Experience About the Performance of the Snorkel/Chimney Endovascular Technique in the Treatment of Complex Aortic Pathologies. Ann Surg. 2015. September;262(3):546–53. [DOI] [PubMed] [Google Scholar]
- 33.Budtz-Lilly J, Wanhainen A, Eriksson J, Mani K. Adapting to a total endovascular approach for complex aortic aneurysm repair: Outcomes after fenestrated and branched endovascular aortic repair. J Vasc Surg. 2017. November;66(5):1349–56. [DOI] [PubMed] [Google Scholar]
- 34.Oderich GS, Ribeiro M, Reis de Souza L, Hofer J, Wigham J, Cha S. Endovascular repair of thoracoabdominal aortic aneurysms using fenestrated and branched endografts. J Thorac Cardiovasc Surg. 2017. February;153(2):S32–S41.e7. [DOI] [PubMed] [Google Scholar]
- 35.Tsilimparis N, Heidemann F, Rohlffs F, Diener H, Wipper S, Debus ES, et al. Outcome of Surgeon-Modified Fenestrated/Branched Stent-Grafts for Symptomatic Complex Aortic Pathologies or Contained Rupture. J Endovasc Ther. 2017. December 6;24(6):825–32. [DOI] [PubMed] [Google Scholar]
- 36.Schanzer A, Simons JP, Flahive J, Durgin J, Aiello FA, Doucet D, et al. Outcomes of fenestrated and branched endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg. 2017. September;66(3):687–94. [DOI] [PubMed] [Google Scholar]
- 37.Georgiadis GS, van Herwaarden JA, Antoniou GA, Hazenberg CEVB, Giannoukas AD, Lazarides MK, et al. Systematic Review of Off-the-Shelf or Physician-Modified Fenestrated and Branched Endografts. J Endovasc Ther. 2016. February 23;23(1):98–109. [DOI] [PubMed] [Google Scholar]
- 38.Scali ST, Waterman A, Feezor RJ, Martin TD, Hess PJ, Huber TS, et al. Treatment of acute visceral aortic pathology with fenestrated/branched endovascular repair in high-surgical-risk patients. J Vasc Surg. 2013. July;58(1):56–65.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaoguo Y, Zhong C, Lei K, Yaowen X. Treatment of complex aortic aneurysms with fenestrated endografts and chimney stent repair: Systematic review and meta-analysis. Vascular. 2017. February 10;25(1):92–100. [DOI] [PubMed] [Google Scholar]
- 40.Katsargyris A, Oikonomou K, Klonaris C, Topel I, Verhoeven ELG. Comparison of Outcomes With Open, Fenestrated, and Chimney Graft Repair of Juxtarenal Aneurysms: Are We Ready for a Paradigm Shift? J Endovasc Ther. 2013. April;20(2):159–69. [DOI] [PubMed] [Google Scholar]
- 41.Caradu C, Berard X, Sassoust G, Midy D, Ducasse E. Chimney versus fenestrated endovascular aortic repair for juxta-renal aneurysms. J Cardiovasc Surg (Torino). 2016. October 27; [DOI] [PubMed] [Google Scholar]
- 42.Ullery BW, Tran K, Itoga NK, Dalman RL, Lee JT. Natural history of gutter-related type Ia endoleaks after snorkel/chimney endovascular aneurysm repair. J Vasc Surg. 2017;65(4):981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JT, Lee GK, Chandra V, Dalman RL. Comparison of fenestrated endografts and the snorkel/chimney technique. J Vasc Surg. 2014. October;60(4):849–57. [DOI] [PubMed] [Google Scholar]
- 44.Donas KP, Torsello G, Bisdas T, Osada N, Schonefeld E, Pitoulias GA. Early Outcomes for Fenestrated and Chimney Endografts in the Treatment of Pararenal Aortic Pathologies Are Not Significantly Different: A Systematic Review With Pooled Data Analysis. J Endovasc Ther. 2012. December;19(6):723–8. [DOI] [PubMed] [Google Scholar]


